Abstract
During the systemic inflammatory state induced by sepsis, the potential for coagulopathy exists because of up-regulation of natural procoagulants and anti-fibrinolytics, and down-regulation of natural anti-coagulants, with protein C (PC) being a critical example of the latter case. PC functions as an anti-coagulant, profibrinolytic, and anti-inflammatory agent, and, thus, its administration or deficiency may affect the course and outcome of sepsis in patients. In this study, a cecal ligation and puncture model of septic peritonitis was applied to wild-type mice and littermates with a targeted heterozygous deficiency of PC (PC+/−) to characterize the importance of a PC-deficiency on polymicrobial sepsis. An enhanced mortality rate was found to accompany a PC deficiency. Plasma cytokines, as well as organ-specific expression of cytokine transcripts, were elevated in PC+/− mice. No signs of severe disseminated intravascular coagulation (DIC) were observed in wild-type or PC+/− mice, as indicated by an increase in fibrinogen levels and the invariability of platelet counts after cecal ligation and puncture. Consumption of coagulation factors was similar in both genotypes and a decrease in the PC mRNA and protein levels was more prominent in PC+/− mice. Renal and organ muscle damage was enhanced in PC+/− mice, as shown by increases in plasma blood urea nitrogen, creatinine, and creatinine kinase. Hypotension and bradycardia were more enhanced in PC+/− mice than in wild-type mice, thus provoking a more severe septic shock response. Thus, the hemodynamic role of PC during sepsis is of critical importance to the outcome of the disease.
Bacterial infection with resultant sepsis affects ∼700,000 people annually in the United States, with a mortality of ∼30%.1,2 This disease is associated with a response to infection causing systemic inflammatory response syndrome, which involves a variety of symptoms including hypothermia and hyperthermia, tachycardia and tachypnea, and anomalous white blood cell counts.3 As a consequence, the inflammatory system is hyperactive and evokes both cellular and humoral responses, such as the release of cytokines and chemokines that serve as mediators for crosstalk between endothelial cells (ECs) and epithelial cells on one hand, and neutrophils and macrophages on the other.4,5 The inflammatory state is closely related to coagulation through several connecting elements, which include: 1) receptors and membrane proteins, eg, endothelial protein C receptor (EPCR), thrombomodulin (Tm), and protease-activated receptors (PAR); 2) hemostasis-related proteins, eg, tissue factor (TF), factor (F) VII, FXI, and FXII; 3) systems that regulate thrombin formation, such as protein C (PC) system components, tissue factor pathway inhibitor, and anti-thrombin-III; 4) inflammatory mediators, eg, tumor necrosis factor (TNF)-α, interleukin (IL)-1, IL-6, and inducible nitric oxide synthase; and 5) cell adhesion mediators for extravasation of inflammatory cells, such as E-selectin, P-selectin, and integrins.6 All of these pathways function at early stages of the infection in attempts to rid the system of the infectious agent, but undergo a loss of control in severe sepsis, leading to unregulated hemostasis. In many cases, disseminated intravascular coagulation (DIC) is established,7–9 with resultant contributions to presentations of hypotension, hypoperfusion, and multiple organ dysfunction syndrome. This advanced stage of the disease normally culminates in multiple organ failure and death.
Natural anti-coagulant mechanisms, namely the PC pathway, anti-thrombin-III, and tissue factor pathway inhibitor, as well as a functional fibrinolytic system, are needed to maintain hemostasis. However, after systemic inflammatory response syndrome in sepsis, anti-thrombin-III and PC are consumed, and damage to the endothelium impairs the EC-derived Tm- and EPCR-modulated activation of PC.10 In addition, hypofibrinolysis is a typical outcome during advanced stages of the disease,11 thus exacerbating the potential coagulopathy. These findings have led to many approaches for attempting to control the multiple septic responses, and replacement of natural endogenous anti-coagulants was believed to offer potential benefits. However, although clinical trials in severe sepsis with anti-thrombin-III and tissue factor pathway inhibitor replacement therapies have proved disappointing,12–14 suppression of thrombin generation by recombinant human activated PC (aPC) may indeed correlate with improved survival,15 at least in cases of severe sepsis coupled with DIC.
In principal, the aPC system offers many advantages as a combined anti-thrombotic/profibrinolytic/anti-inflammatory therapeutic agent that is capable of down-regulating thrombin generation by attenuating levels of FV and FVIII,16,17 and enhancing clot dissolution by stimulating fibrinolysis directly via plasminogen activator inhibitor-1 (PAI-1) inactivation.18 In addition, an indirect fibrinolytic effect of aPC is observed through reduction of thrombin levels and consequent activation of thrombin activatable fibrinolytic inhibitor (TAFI).19,20In vitro experiments suggest that aPC also serves as an inflammatory modulator through several routes, eg, down-regulation of PAR-mediated thrombin-induced proinflammatory signals,21 inhibition of the generation of cytokines, such as TNF-α,22 modulation of macrophage migration inhibitory factor (MIF),23 and inhibition of the responses of human mononuclear phagocytes to lipopolysaccharide (LPS), interferon-γ, or phorbol ester.22,24 In addition, aPC attenuated ICAM-1 and E-selectin expression,25 and inhibited apoptosis in EC cultures25 through its receptor/co-receptor relationships with EPCR and PAR-1.26 aPC has also been shown to inhibit LPS-induced translocation of nuclear factor (NF)-κB in monocytes,27 to inhibit leukocyte cell adhesion,28 and to attenuate LPS-induced pulmonary vascular injury by inhibiting activated leukocytes,29 all potentially contributing to its anti-inflammatory effectiveness independent of its anti-coagulant activity. In a rat model of endotoxemia, aPC prevented LPS-induced hypotension, an effect that was dependent on its serine protease activity.30
Although administration of aPC appears to be beneficial to a subset of patients with severe septicemia, there is little available evidence to suggest that deficiencies in PC would lead to a more severe host response to the septic state,31 ie, although therapeutically somewhat effective, there is no proof that aPC is on the mechanistic pathway to septic responses. Thus, a new experimental model of PC-deficient mice becomes a valuable tool to study gene associations in this disease, particularly with regard to those genes that might have a reciprocal relationship with the PC pathway. A model of LPS-induced endotoxin shock was recently used in mice heterozygous for a targeted PC deficiency (PC+/−).9 Although the LPS dosages in this case were very high and somewhat mechanistically unrealistic, PC+/− mice showed a higher mortality rate, a more elevated inflammatory response, and a more severe DIC when compared to wild-type (WT) mice.
Various animal models are available for the study of sepsis,32 with LPS bolus injection and cecal ligation and puncture (CLP) among those models in widest use. Each has its advocates and critics. The CLP model has gained wide acceptance in the scientific community because of its polymicrobial nature, and is considered to reproduce the pathophysiology of sepsis closely. In the present study, we used the CLP model to explore the outcome of polymicrobial sepsis in PC+/− mice in a detailed manner. In particular, hemodynamic parameters and gene expression in major organs were analyzed. The current article presents a summary of our findings.
Materials and Methods
Animals
Mice were housed in microisolation cages and given access to food and water ad libitum. The procedures for generation of mice heterozygous for a null PC allele (PC+/−) have been described earlier.33PC+/− mice were bred to the >F10 generation in the C57BL/6J strain. All animal experiments were performed in accordance with protocols approved by the Institutional Animal Care and Use Committee.
Genotypic Analyses
PC genotyping was performed on ear punch or tail-tip DNA by polymerase chain reaction (PCR) methodology using three different primers: 1) a common forward primer (CGTGATGAGTTTCAGGCAGTGAGAG) for both the WT and null alleles, located 300 bp downstream of the beginning of the first intron of the PC gene; 2) a specific reverse primer (GAGATAAGCAGATCCTGTGGATTGC) for the WT allele situated in intron 1 at the 5′ flank of the PC gene at a location 200 bp upstream of the start codon of exon 2; and 3) a reverse primer (ATTCGCAGCGCATCGCCTTCTATC) present in the NEO cassette for the null allele. Amplicons of 1000 bp and 600 bp were obtained for the WT and null alleles, respectively.
The PCR conditions were as follows: samples were initially denatured for 5 minutes at 95°C, followed by 30 cycles of denaturation (95°C, 30 seconds), annealing (64°C, 50 seconds), and extension (72°C, 1 minute and 50 seconds). PCR was concluded with one additional extension period at 72°C for 10 minutes. PCR reactions were performed on a DNA engine Tetrad, PTC-225 (MJ Research, Watertown, MA).
CLP Surgery
For CLP,32,34,35 male WT and PC+/− mice (ages 2 months and 6 months) were sedated with rodent cocktail (9 mg/ml ketamine, 1.8 mg/ml xylazine, 0.3 mg/ml acepromazine in saline) before surgery, which was performed under a surgical microscope. The abdomens of the sedated mice were shaved. A longitudinal 1-cm incision was then made in the peritoneum, the cecum was localized and exposed, and then ligated just below the ileocecal valve with a sterile 5.0 silk ligature. The anti-mesenteric cecal surface was then punctured twice with a 20-gauge needle. The bowel was then replaced into the peritoneal cavity.
Necropsy
Animals were sacrificed at 6 hours or 24 hours after CLP surgery. For experimental purposes, the animals were divided in four different groups. The first group was maintained undisturbed up to 1 week for the survival analysis. The second group was sacrificed by exsanguination via the orbital vein and the blood placed into 3.8% citrate, at a volume ratio of 9:1, blood:citrate. An amount of 25 μl of this sample was diluted immediately in 475 μl of 3% acetic acid (Unopette, Becton Dickinson Vacutainer System; Becton Dickinson, Franklin Lakes, NJ) to lyse red blood cells. White blood cells were then counted on a Neubauer (Fisher Scientific, Pittsburgh, PA) hematocytometer using ×100 magnification under a transmission microscope. Another drop of blood was spotted onto histological slides and smeared to perform white blood cell differentials. The resulting blood smear slides were immediately treated with a fixative spray (CH3OH, HOAc) and stained with Giemsa solution. The remaining plasma that was obtained from the mice was retained for further use in plasma assays. At this point, the mice were disinfected by immersion in 70% alcohol for peritoneal lavage. A 20-gauge sterile venous catheter was connected to a 3-ml syringe containing 2 ml of sterile saline and inserted into the peritoneum. Peritoneal lavage fluid (PLF) was collected in the syringe and dispensed in an Eppendorf tube. PLF (25 μl) was diluted into 475 μl of 3% acetic acid (Unopette microcollection system) for leukocyte counts. Differentials were performed on cytospin slides, as above. The third group of mice was first anesthetized with rodent cocktail at the proposed sacrifice time points. Blood was collected through vena cava puncture into 3.8% citrate at a volume ratio of 9:1, citrate:blood, for plasma assays of coagulation factors. The mice were then perfused through the left ventricle with saline until no additional blood emerged from the right auricule. The liver, kidneys, and spleen were removed and divided for histological analyses, myeloperoxidase (MPO) assays, and RNA isolation, for which the latter samples were stored in RNA Later (Ambion, Austin, TX) at −20°C. The fourth group of mice was used for heart rate and blood pressure (BP) monitoring.
Implantation of the BP and Heart Rate Probes
At a time of 7 to 10 days before CLP, mice were sedated with isoflurane using a ventilator and placed under a surgical microscope. A longitudinal 1-cm incision was made on the neck. The salivary glands were separated and the trachea and left carotid artery were exposed. The distal end of the carotid artery was ligated with a 6.0 silk thread to avoid back-flow or flow-through. The pressure sensor (Transoma Medical-DSI, St. Paul, MN) that is encapsulated in a cannula was filled with a gel provided by the manufacturer. A loose loop was made in the proximal end of the carotid artery with a 6.0 silk thread and the cannula was inserted up to the aortic arch. The proximal loop was tied to restrain the cannula. The remainder of the probe (battery and transmitter) external to the cannula was inserted in a pocket made between the skin and the muscle layer covering the ribs on the left side of the mouse. Finally, the skin was sutured with 5.0 ethicon thread (Harvard Apparatus, Holliston, MA). After CLP surgery (performed under isoflurane anesthesia), the heart rates, along with diastolic and systolic pressures, were continually recorded.
Histochemistry and Immunohistochemistry
Lungs, livers, and kidneys were fixed in 4% paraformaldehyde-phosphate-buffered saline (PBS) for 2 hours, embedded in paraffin blocks, and serially sectioned at 3 to 4 μm thickness. Tissue sections were deparaffinated, rehydrated, and stained with hematoxylin and eosin. In addition, immunostains for fibrin(ogen) and leukocytes (CD45) were performed to complete the analysis of the histopathology.
Colony Forming Unit Assays
Fresh PLF (10 μl) samples were serially diluted up to 1:105 in saline and 10 μl of each dilution was plated on LB plates and incubated at 37°C overnight. After this time, the colonies were counted and the results were expressed as colony-forming units/10 μl.
Plasma Assays
All assays were performed at room temperature.
PAI-1 Levels
PAI-1 levels were determined using a two-antibody-sandwich enzyme-linked immunosorbent assay (ELISA) in 96-well microtiter plates coated with a monoclonal mouse anti-mouse PAI-1 antibody (H34G6; Molecular Innovations, Southfield, MI). The wells were blocked with 5% milk. The coated plates were treated with 100 μl of plasma (1:5 dilution in PBS) or standard (0 to 12.5 ng/ml murine PAI-1; Molecular Innovations), and incubated for 2 hours. After four washes with PBS/Tween-20, 100 μl of 4 μg/ml rabbit anti-mouse PAI-1 polyclonal antibody (Molecular Innovations) in PBS was added to the wells and incubated for 2 hours. The plates were then washed and 100 μl of a 1:2000 diluted goat anti-rabbit IgG-alkaline phosphatase conjugate (Bio-Rad, Richmond, CA) was added and further incubated for 2 hours. After washing 4 times, 100 μl of the colorimetric substrate pNPP (Sigma, St. Louis, MO) was added and incubated for 30 minutes. The absorbance was measured at 405 nm in a plate reader.
PC Assays
PC assays were also performed using ELISA to detect its levels in plasma using an in-house polyclonal monospecific rabbit anti-mouse PC to coat the wells. The plasma sample (100 μl of a 1:10 dilution of plasma in PBS) or standard (0 to 5 nmol/L recombinant murine PC, provided by Dr. Angelina Lay of this laboratory) in PBS was added to the plates and incubated for 2 hours. After suitable washing with PBS/Tween-20, an in-house chicken anti-mouse PC polyclonal antibody in PBS was added to the wells and incubated for an additional 2 hours, followed by addition of goat anti-chicken IgG-horseradish peroxidase conjugate (Bio-Rad), and further incubated for 2 hours. The colorimetric substrate TMB (3,3′,5,5′-tetramethylbenzidine) was used for development. After incubation for 30 minutes in the dark, the absorbance at 540 nm was determined and subtracted from the absorbance at 450 nm.
Fibrinogen Assays
Clottable fibrinogen levels were measured using the Fibri-Prest Automate coagulometric assay kit (Diagnostica Stago, Parsippany, NJ), which consists of the determination of the clotting time of the plasma sample after the addition of an excess amount of thrombin. Calibrations were accomplished with different concentrations of the human plasma Unicalibrator from the assay kit. The assay was performed in a StART 4 coagulometer (Diagnostica Stago).
Activated Partial Thromboplastin Time (APTT) Assays
Activated partial thromboplastin time (APTT) assays were performed on plasma samples that were incubated with an equal volume of PTA reagent (Diagnostica Stago) for 3 minutes at 37°C. Clotting times were determined by coagulometry after the addition of an equal volume of a 25 mmol/L CaCl2 solution.
Prothrombin Time (PT) Assays
Prothrombin time (PT) assays were accomplished by measuring clotting times by coagulometry after the addition of an equal volume of PT reagent (thromboplastin, Diagnostica Stago).
FXI and FXII Levels
FXI and FXII levels were determined on dilutions of plasma samples that were added to FXI- or FXII-deficient plasmas (Diagnostica Stago) and APTT reagent (Diagnostica Stago) for 3 minutes at 37°C. Clotting times were determined by coagulometry after the addition of 25 mmol/L CaCl2. A calibration curve was constructed using different dilutions of artificial plasma, and the results are expressed relative to the average FXI or FXII mouse levels for the resting WT group.
Cytokine Assays
Cytokine assays were determined on plasma and PLF. TNF-α, IL-6, and MIP-2 were determined using Quantikine-M murine kits (R&D Systems, Minneapolis, MN). For the simultaneous detection of other cytokines (IL-1β, IL-2, IL-4, IL-5, IL-10, IL-12p70, and interferon-γ) and chemokines (MIP-1α, KC, Eotaxin, RANTES, TARC, SDF1β, JE, and MCP5), a multiplex ELISA assay was used (Pierce Biotechnology, Rockford, IL). The assay is a traditional quantitative sandwich ELISA, and the difference relies on the fact that each well of a microtiter plate is precoated with an antibody microarray, thus allowing for detection of up to 36 different analytes. In this case, the plates were analyzed using a SearchLight CCD camera (Pierce Biotechnology).
Blood Chemistry Analyses
Blood chemistry analyses were performed on plasma samples prepared from blood collected from the vena cava. Determinations were made of alkaline phosphatase, amylase, aspartate aminotransferase, creatinine kinase, creatinine, γ-glutamyltransferase, glucose, lactate dehydrogenase, lipase, inorganic phosphate, total bilirubin, and urea. The VET-TEST 8008 (IDEXX, Ellisvile, MO) was used for the assays. For 12 tests, 150 μl of plasma was required. Standards were supplied by the manufacturer.
MPO Activities
MPO activities were performed as described earlier36 on samples from livers, kidneys, and spleens from saline-perfused animals, first using a heat step to eliminate spurious peroxidase activity. The MPO assays were performed on lysed perfusate cells in microtiter 96-well plates by adding 50 μl of 0.75 mmol/L H2O2, 80 mmol/L PBS, pH 5.4, 50 μl of sample or standard (in triplicate), and 25 μl of 8 mmol/L TMB in dimethyl sulfoxide. The plates were incubated in the dark for 2 minutes and the reaction was terminated with 50 μl of 1 mol/L HCl. The absorbance at 540 nm was subtracted from the absorbance at 450 nm and the values were converted to units of peroxidase activity using the standard curve that is simultaneously generated during assays of the samples. The standards were prepared using various concentrations (0 to 0.5 U) of horseradish peroxidase (POD; Roche, Indianapolis, IN) in 0.5% CTAB, 50 mmol/L PBS, pH 6.0.
Expression and Quantitation of Hemostasis and Inflammation Transcripts
RNA Isolation
A 15- to 20-mg portion of liver, kidney, or lung was added to 2 ml of DNase/RNase-free screw cap tubes containing 1 g of 1-mm-diameter zirconia beads (Biospec Products, Bartlesville, OK) and 600 μl of RLT buffer (Qiagen, Valencia, CA). The samples were then homogenized in a Fast-Prep FP120 shaker (Thermo Savant, Holbrook, NY). The purification procedure continued using the RNeasy system (Qiagen). Once isolated, the RNA samples were analyzed for purity and quantitation in an Agilent Bioanalyzer 2100 (Agilent, Palo Alto, CA).
Gene Expression Assays
Expression of mRNA of IL-1, IL-6, TNF-α, MPO, TF, PAI-1, TM, and EPCR was studied at the different time points in liver, kidney, and lung by quantitative (Q) reverse transcriptase (RT)-PCR. Expression of coagulation FXI and FXII was quantitated only in liver, and the expression of PC was determined in liver and kidney. Q-RT-PCR was performed in one step in a 7700 ABI PRISM TaqMan real-time PCR apparatus (Applied Biosystems, Foster City, CA).
Each reaction contained 100 ng of total RNA, 2 μl of 10× Master Mix (dNTP mixture with dUTP instead of DTTP), 6 mmol/L MgCl2, 50 nmol/L each primer, 100 nmol/L probe, 16 U RNase inhibitor (Promega, Madison, WI), 2 U Multiscribe reverse transcriptase (ABI), and diethyl pyrocarbonate-treated H2O (20 μl). Thermocycling conditions were, sequentially: 48°C for 30 minutes and 95°C for 10 minutes, 40 cycles at 95°C for 15 seconds, 60°C for 1 minute, with fluorescent readings at the end of each cycle. The amount of mRNA detected was quantitated using individual standard curves. All samples were run in triplicate. FAM fluorescence was measured at the end of each 60°C period. The mRNA levels of the gene of interest were expressed as a ratio to the housekeeping gene, RPL19, and the averages for each experimental group were expressed relative to the WT resting levels for a particular gene.
Statistical Treatment of the Data
For survival analysis, the data were analyzed by the Kaplan-Meyer treatment and the comparison of survival between different genotypes was accomplished using the log-rank test with the NCSS software (NCSS, Kaysville, UT). For data analysis and comparison of single pairs, the Student’s t-test was used. For overall group comparisons, Kruskal-Wallis one-way analysis of variance test was performed. Tukey-Kramer tests were conducted to analyze multipairwise comparisons.
Results
Survival of Mice after CLP Surgery
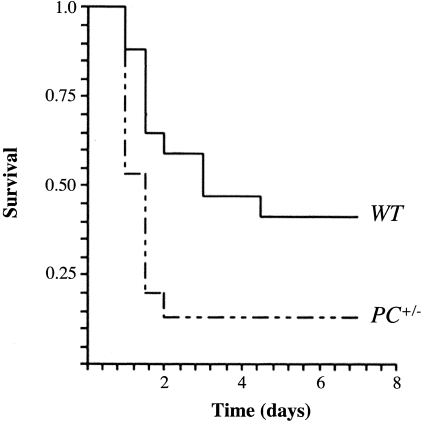
The initial experiments that compared survival of WT versus PC+/− mice after CLP were performed with young mice at 2 months of age. No significant survival differences in these two groups were found by Kaplan-Meier analyses of the data. However, similar experiments conducted with older mice at 6 months of age did indeed show a significant difference in survival between these groups (Figure 1). Additionally, survival at 6 months of age after CLP was significantly lower than the survival presented by 2 months of age mice under the same treatment. Thus, it is clear that there is an age dependency involved in the susceptibility of PC+/− mice in this model of polymicrobial sepsis, and the remainder of the studies described herein was performed on mice at 6 months of age.
Figure 1-4239.
Survival curves of WT and PC+/− male mice after CLP surgery, as evaluated by the Kaplan-Meier product limit. The survival curves were compared by a log-rank test showing a significantly lower survival for 6 months of age in PC+/− mice (n = 15) compared to WT mice (n = 13) of the same age (P = 0.015).
Female mice presented with a lower mortality rate than their male counterparts, and although this trend was clear, the survival difference between WT and PC+/− female mice was not as distinct as in male mice. The issue of gender responses was not a focus of the current study, because such a highly involved study would require a large number of mice and suitable control groups.
Hypotensive Effects after CLP Surgery
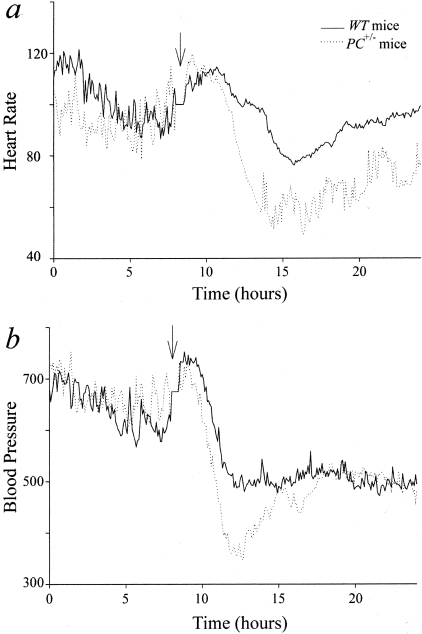
A hallmark feature of the fatal course of sepsis in humans is the severe hypotension and shock that occurs. For this reason, we have measured continual central BP and heart rates of mice after CLP, using a radiotelemetric method after insertion of suitable probes in the central thoracic aortas of WT and PC+/− mice. The probes were placed in the mice (n = 4 in each group) ∼1 week before the CLP procedure in order that the BP and heart rates would stabilize before the experimental procedure. The experiments began after these values returned to normal. Point-by-point average data of synchronized plots are shown in Figure 2. In WT mice, heart rates began to decline at a time ∼5 hours after CLP surgery, and were minimal at 6 to 8 hours after CLP (Figure 2a). Significantly, PC+/− mice were more susceptible to this change than WT mice, with heart rates of this latter group decreasing to ∼50% of their pre-CLP values. The central BP (average of systolic-diastolic pressures) decreased in both groups of mice and paralleled the temporal responses of the heart rates. These values decreased to significantly lower levels in PC+/− mice, relative to the BP of WT mice (Figure 2b). Thus, a more severe sepsis-induced shock state accompanied a PC deficiency, a heretofore unrecognized potential function of PC.
Figure 2-4239.
Point-by-point average of heart rate (a) and BP (b) profiles from WT and PC+/− mice. Central BPs and heart rates were monitored in four WT mice and four PC+/− mice before and after CLP surgery. The time of surgery is indicated by the arrow. The average BPs and heart rates were calculated during the 8-hour interval before CLP and during the shock period, ∼6 hours after the surgery. a:WT rest, 620 ± 20 beats per minute (bpm); PC+/− rest, 580 ± 20 bpm; WT shock, 540 ± 20 bpm; PC+/− shock, 460 ± 20 bpm. b:WT rest, 110 ± 3 mmHg; PC+/− rest, 116 ± 1 mmHg; WT shock, 79 ± 3 mmHg; PC+/− shock, 65 ± 4 mmHg. Although there was no significant difference in the BP and heart rate between WT and PC+/− mice before the surgery, PC+/− mice showed a more dramatic decrease in both parameters (P = 0.026 for BP and P = 0.009 for heart rate).
Leukocyte Analysis
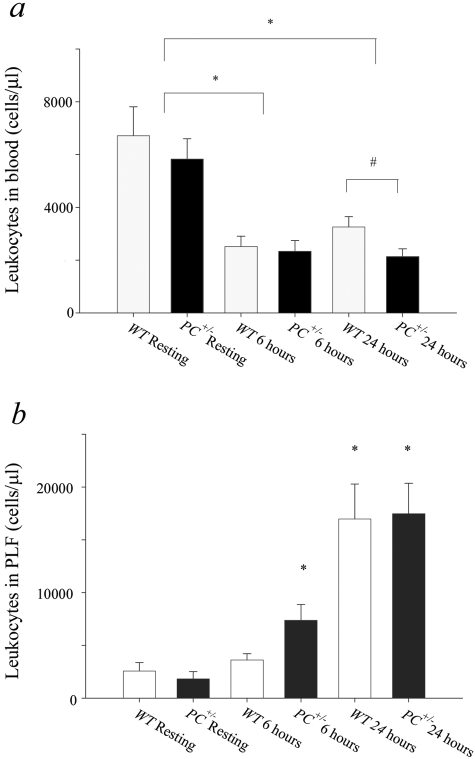
Leukocyte counts decreased steeply and significantly, from values of 6000 to 7000/μl in WT and PC+/− mice in presurgical blood, to 2000 to 2500/μl in both groups 6 hours after CLP (Figure 3a). At this latter time point, there was no significant difference between the decreases in WT and PC+/− mice. However, although circulating leukocytes increase somewhat at 24 hours in WT mice, this does not occur in PC+/− mice, and leads to significantly higher blood leukocyte counts in WT mice as compared to PC+/− mice, both of which remain significantly lower than the values found in presurgical mice. A significantly higher leukocyte count has been found 6 hours after CLP PLF of PC+/− mice, relative to similar WT mice. Large increases in PLF leukocyte counts occur at 24 hours after CLP (Figure 3b), but the differences between the groups seen at 6 hours after surgery are not evident at the 24-hour time point. The PLF is enriched in neutrophils in both groups of mice at 24 hours after CLP, displaying counts of (10,000 ± 3000 neutrophils/μl blood in WT mice and 11,000 ± 2000 neutrophils/μl blood in PC+/− mice, as compared to resting mice (2500 ± 500 neutrophils/μl blood in both WT and PC+/− mice). These data demonstrate the increased neutrophil migration into the peritoneal cavity as a major inflammatory response in this model, as is the case with the disease state in humans.
Figure 3-4239.
Leukocyte levels of WT and PC+/− mice after CLP surgery. a: Total number of leukocytes in blood from WT and PC+/− mice under resting conditions (WT rest, n = 7; PC+/− rest, n = 10), 6 hours after CLP surgery (WT 6 hours, n = 8; PC+/− 6 hours, n = 8), or 24 hours after CLP surgery (WT 24 hours, n = 12; PC+/− 24 hours, n = 11). *, P < 0.002; #, P < 0.02. b: Total number of leukocytes in the PLF from WT and PC+/− mice under resting conditions (WT rest, n = 3; PC+/− rest, n = 3), 6 hours after CLP surgery (WT 6 hours, n = 6; PC+/− 6 hours, n = 3), or 24 hours after CLP surgery (WT 24 hours, n = 25; and PC+/− 24 hours, n = 24). *, P < 0.02 compared with the WT mice under resting conditions.
Histopathology
Histological examination of the tissues did not show severe pathologies at 24 hours after CLP surgery, not unexpected when taking into account the acute nature of the process that led to shock and sudden death. Particularly, analysis of hematoxylin and eosin-stained slides of WT and PC+/− liver sections presented leukocyte accumulation and necrosis in the parenchyma, a typical sign of peritonitis. Lungs showed edemas in the alveolar spaces and leukocyte accumulation in the peripheries of the pulmonary arterioles, an indication of a mild pneumonia. Kidneys displayed a mild proteinurea in the tubuli and the hearts showed no pathological symptoms. Additionally, fibrin(ogen) immunostaining did not show any signs of fibrin(ogen) deposition in organs, with the exception of the edemas found occasionally in lungs. Further, CD45 staining did not reveal evidence of leukocyte infiltration. Overall, no overt differences in histology were found between WT and PC+/− organs.
Coagulation Factor Assays
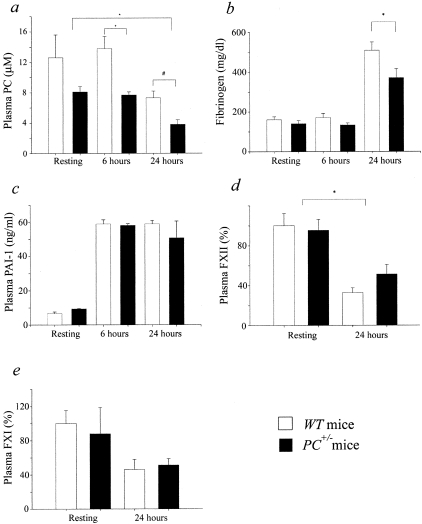
Because a strong potential for coagulopathy and DIC are common features of severe sepsis, and are displayed to various degrees in different animal models of sepsis, assays of critical coagulation factors that might produce this state have been performed. APTTs increased from 37 ± 2 (n = 13) seconds and 57 ± 8 seconds (n = 12) in resting WT and PC+/− mice, respectively, to 70 ± 12 seconds (n = 9) and 91 ± 14 seconds (n = 7) in WT and PC+/− plasmas 24 hours after CLP, respectively. PT values increased from 11.8 ± 0.3 seconds (n = 4) and 11.0 ± 0.8 seconds (n = 4) in resting WT and PC+/− mice, respectively, to 23 ± 3 seconds (n = 4) and 20 ± 3 seconds (n = 4) in WT and PC+/− plasmas 24 hours after CLP, respectively. Additionally, no significant differences were found in these parameters between WT and PC+/− mice at any particular time point. This suggested that consumption of coagulation factors has occurred after septic challenge, thus showing that the coagulation system has been activated. Plasma PC levels did not change from their resting values at 6 hours after CLP in both groups of mice, but were significantly attenuated in each at 24 hours after surgery. At all time points, plasma PC in PC+/− mice maintained its ratio of ∼50% of that of WT plasma (Figure 4a). Plasma fibrinogen levels, determined at 6 hours after CLP, were unchanged from those of resting mice, and were not different between WT and PC+/− mice (Figure 4b). However, at 24 hours after CLP, the plasma fibrinogen levels were significantly elevated by 2- to 2.5-fold, with a significantly larger increase found in WT mice relative to PC+/− mice. These increases at 24 hours most likely reflect the acute phase response of fibrinogen, which more than counterbalanced any possible consumptive coagulopathy resulting from the inflammatory response. Further and substantially contributing to a possible prothrombotic state were the elevations observed in plasma levels of PAI-1, which increased approximately fivefold at 6 hours and 24 hours after CLP, when compared to these same values in plasmas of resting mice of both genotypes (Figure 4c). Lastly, intrinsic pathway components, specifically FXII and FXI, at 24 hours after CLP, declined to ∼50% of the values of WT and PC+/− mice. No significant differences were observed between the two genotypes. This decrease is, most likely, because of the consumption of the coagulation factors because of the hypercoagulable state evident at this time point.
Figure 4-4239.
Plasma coagulation factor assays of WT and PC+/− mice after CLP surgery. a: Plasma PC levels. The plasma PC levels were determined by ELISA before (WT, n = 4; PC+/−, n = 5), and 6 hours (WT, n = 5; PC+/−, n = 7) and 24 hours (WT, n = 5; PC+/−, n = 5) after CLP surgery in WT and PC+/− male mice. *, P < 0.001; #, P < 0.01. b: Plasma fibrinogen levels were determined by a coagulometric assay at 6 hours (WT, n = 4; PC+/−, n = 5) and 24 hours (WT, n = 15; PC+/−, n = 14) after, CLP surgery and in resting (WT, n = 11; PC+/−, n = 9) WT and PC+/− male mice. *, P = 0.016. c: Plasma PAI-1 levels were determined by ELISA before (WT, n = 3; PC+/−, n = 4), and 6 hours (WT, n = 3; PC+/−, n = 7) and 24 hours (WT, n = 5; PC+/−, n = 4) after, CLP surgery in WT and PC+/− male mice. d: Plasma FXII levels relative to the resting WT group, as measured by a coagulometric assay, before (WT, n = 4; PC+/−, n = 3) and 24 hours after (WT, n = 5; PC+/−, n = 3), CLP surgery. *, P = 0.05 (Kruskal-Wallis). e: Plasma FXI levels relative to the resting WT group as measured by a coagulometric assay, before (WT, n = 7; PC+/−, n = 5) and 24 hours after (WT, n = 5; PC+/−, n = 3), CLP surgery. No significant differences were observed.
Cytokine Analyses
For measurement of the plasma cytokine responses in both groups of mice, the 24 hours after CLP surgery was selected. The choice for this particular time point was justified by previous experimental evidence.37 After CLP surgery, cytokine levels keep increasing at ∼8 hours after CLP and reach a plateau at ∼10 hours later. Measurements at earlier times might reflect the early response to the surgery itself (anesthesia and opening of abdomen). Moreover, the 24-hour time point was shown in the past to be effective for these types of measurements after CLP.38–40 Plasma levels of 18 cytokines and chemokines were thus measured in WT and PC+/− mice 24 hours after CLP surgery. Both groups showed low levels (less than 100 pg/ml) of MIP-1α, IL-1β, IL-4, and IL-12, moderate levels (100 to 2000 pg/ml) of TNF-α, interferon-γ, MIP-2, MCP-5, RANTES, TARC, SDF1β, IL-2, and IL-5, and highly elevated levels (more than 2000 pg/ml) of IL-6, IL-10, KC, JE, and eotaxin at this time point. Although differential trends were observed in many of these groups, individual variations between mice were too large to achieve statistical significance with a reasonable number of mice. By pooling plasma samples, this issue can be artificially controlled, because the large individual variations that are observed in many cases would not be recognized, but we did not feel that this was an effective manner of proceeding.
However, levels of several cytokines, namely IL-1, IL-2, IL-5, IL-6, and IL-12, and also possibly TNF-α, were significantly higher in PC+/− mice 24 hours after CLP in comparison to similarly treated WT mice, and these values are listed in Table 1. These cytokines were undetectable in plasmas of untreated or in sham-surgical mice. Additionally, the differences in plasma levels of IL-4, IL-10, and KC approached statistical significance (P < 0.1), being more elevated in PC+/− mice 24 hours after CLP than in similarly treated WT mice. Although these may not be the only cytokines/chemokines that are differentially elevated in this model, very large numbers of mice would be required to achieve significance for many of these other agents, and we felt that this initial screen was ample for highlighting some critical inflammatory components that are possibly differentially affected in this model.
Table 1.
Plasma Cytokine Levels of Mice 24 Hours After CLP
| Genotype | Cytokine (pg/ml) |
|||||
|---|---|---|---|---|---|---|
| IL-1β | IL-2 | IL-5 | IL-6 (×103) | IL-12 | TNF-α | |
| WT | 40 ± 4 | 110 ± 32 | 190 ± 30 | 61 ± 20 | 9 ± 2 | 110 ± 30 |
| n = 7 | n = 8 | n = 5 | n = 7 | n = 8 | n = 8 | |
| PC+/− | 105 ± 33 | 315 ± 90 | 460 ± 110 | 875 ± 435 | 25 ± 7 | 186 ± 51 |
| n = 7 | n = 7 | n = 6 | n = 8 | n = 8 | n = 8 | |
| P value | 0.05 | 0.02 | 0.03 | 0.05 | 0.02 | 0.07 |
Blood Chemistries
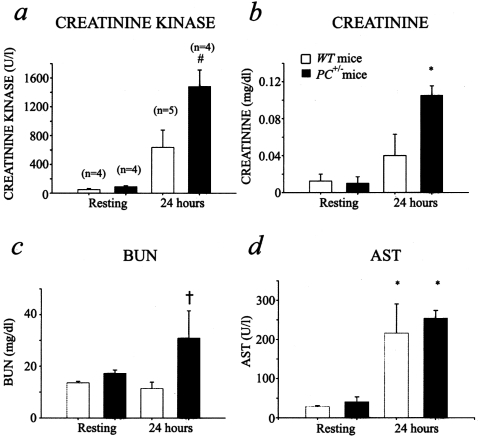
At 24 hours after CLP, blood levels of creatinine kinase (Figure 5a) and creatinine (Figure 5b) were elevated in both WT and PC+/− mice, as compared to control mice. For both of these parameters, the values were significantly higher in the case of treated PC+/− mice, as compared to similar WT mice. With regard to blood urea nitrogen values (Figure 5c), only the PC+/− mice displayed significantly higher values than the other groups tested. Hepatic injury was observed to a similar extent in both WT and PC+/− mice, as shown by the increase in aspartate aminotransferase at 24 hours after CLP surgery (Figure 5d). These results suggest that kidney and heart injury occur to a greater extent in the septic PC+/− mice, at the time point measured, than in WT mice, and this can be a contributing factor to the higher mortality rate of these latter animals after septic challenge.
Figure 5-4239.
Blood chemistry profiles after CLP surgery. Plasma creatinine kinase (a), creatinine (b), BUN (c), and aspartate aminotransferase (d) levels in WT and PC+/− mice under resting conditions and 24 hours after CLP surgery. a:P = 0.005 according to the Kruskal-Wallis (K-W) test (#); b:P = 0.05 (K-W) (*); c:P = 0.13 (K-W) (†). The Tukey-Kramer test showed a significant difference between the 24-hour PC+/− group and the other groups (α = 0.05 for a and b) and between the 24-hour PC+/− and the 24-hour WT (α = 0.1 for c) groups; d:WT and PC+/− resting groups were different from both 24-hour WT and PC+/− groups. Additionally, PC+/− 24 hours is different from the WT resting group. P = 0.05 (K-W) (*).
Gene Expression
The amounts of mRNA from genes related to the coagulation cascade, such as TF, PC, EPCR, TM, PAI-1, FXI, and FXII, or linked inflammatory components, such as MPO, IL-1β, IL-6, and TNF-α, were determined by RT-PCR from liver, kidney, and lung tissues. The primers and probes used for each assay are listed in Table 2.
Table 2.
Gene Expression Primers and Probes
| TNF-α |
| 5′ primer: 5′-CCCCAAAGGGATGAGAAGTTC-3′ |
| 3′ primer: 5′-GCTTGTCACTCGAATTTTGAGAA-3′ |
| Probe: FAM-5′-TCATCAGTTCTATGGCCCAGACCCTCA-3′-TAMRA |
| Amplicon size: 100 bp |
| IL-1β |
| 5′ primer: 5′-TGAAGTTGACGGACCCCAAA-3′ |
| 3′ primer: 5′-TGATGTGCTGCTGCGAGATT-3′ |
| Probe: FAM-5′-CCTTTGACCTGGGCTGTCCTGATGA-3′-TAMRA |
| Amplicon size: 101 bp |
| IL-6 |
| 5′ primer: 5′-CTTCAACCAAGAGGTAAAAGATTTA-3′ |
| 3′ primer: 5′-TAGGAGAGCATTGGAAATTGGGGTAGGAAGG-3′ |
| Probe: FAM-5′-CCTTCCTACCCCAATTTCCAATGCTCTCCTA-3′-TAMRA |
| Amplicon size: 100 bp |
| PAI-1 |
| 5′ primer: 5′-GACACCCTCAGCATGTTCATC-3′ |
| 3′ primer: 5′-AGGGTTGCACTAAACATGTCAG-3′ |
| Probe: FAM-5′-TCCTGCCTAAGTTCTCTCTGGAGACTGAAG-3′-TAMRA |
| Amplicon size: 218 bp |
| TF |
| 5′ primer: 5′-CTCCTCCTCCAGGTGATCG-3′ |
| 3′ primer: 5′-GGGTTGCCACTCCAAAATTG-3′ |
| Probe: FAM-5′-AGGCATTCCAGAGAAAGCGTTTAATTTAACTTGG-3′-TAMRA |
| Amplicon size: 99 bp |
| FXI |
| 5′ primer: 5′-GTATACGACAGCAGAAAGTGGGTATG-3′ |
| 3′ primer: 5′-GGCCGCTGAAAATCTGTGTAA-3′ |
| Probe: FAM-5′-CCCTGTTAAAACTGGAATCAGCCATG-3′-TAMRA |
| Amplicon size: 81 bp |
| FXII |
| 5′ primer: 5′-GAAAGCAAAACCAACAGTTG-3′ |
| 3′ primer: 5′-GCAGGAAGGTGGAGTATTCT-3′ |
| Probe: FAM-5′-GCAGGAAGGTGGAGTATTCT-3′-TAMRA |
| Amplicon size: 154 bp |
| PC |
| 5′ primer: 5′-GCACACCAACAACTATGGCATCTA-3′ |
| 3′ primer: 5′-GCTTCTGGCTCTTAAGGGAGACA-3′ |
| Probe: FAM-5′-CCAAAGTGGGAAGCTACCTCAAATGGATTC-3′-TAMRA |
| Amplicon size: 101 bp |
| MPO |
| 5′ primer: 5′-ACGGCCTCCCAGGATACAA-3′ |
| 3′ primer: 5′-TGCCAACTCCAGGTTCTTCAG-3′ |
| Probe: FAM-5′-CCGAGCTCACCCACTGTGCTAGGCT-3′-TAMRA |
| Amplicon size: 101 bp |
| TM |
| 5′ primer: 5′-TCCATTGCCAGCCTGTCC-3′ |
| 3′ primer: 5′-TGCTTCTTGCGCAGGTGAC-3′ |
| Probe: FAM-5′-TGGTGGTGGCGCTTTTGGCG-3′-TAMRA |
| Amplicon size: 65 bp |
| EPCR |
| 5′ primer: 5′-AAAGGACTTGAGAGCATTTGTG-3′ |
| 3′ primer: 5′-CCAAGTCTATGGTCTGATTTCC-3′ |
| Probe: FAM-5′-CCCACTTCTCAGACCAGCTACACAAAGCA-3′-TAMRA |
| Amplicon size: 82 bp |
| RPL19 |
| 5′ primer: 5′-ATGTATCACAGCCTGTACCTG-3′ |
| 3′ primer: 5′-TTCTTGGTCTCCTCCTCCTTG-3′ |
| Probe: FAM-5′-TTTCGTGCTTCCTTGGTCTTAGACCT-3′-TAMRA |
| Amplicon size: 233 bp |
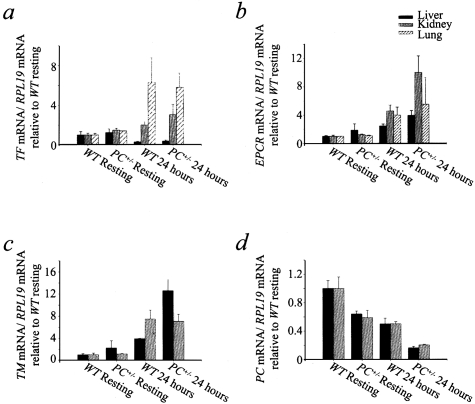
Expression of FXI and FXII remained constant at least to 24 hours after CLP surgery and did not display differences between WT and PC+/− livers (data not shown). This result supports the proposal that the decreases observed in the FXI and FXII plasma levels 24 hours after CLP were because of consumption of the coagulation factors, not decreased synthesis. The expression of TF showed different trends depending on the organ studied (Figure 6a). In livers, TF mRNA levels decreased 24 hours after challenge, whereas lung expression of TF increased significantly at the 24-hour time point. In kidneys, TF expression displayed an increase 24 hours after CLP surgery relative to resting levels. No significant differences were observed in hepatic, pulmonary, or renal TF expression between WT and PC+/− mice at the 24-hour time point. EPCR expression showed a tendency to increase in liver and lungs 24 hours after CLP. Importantly, this temporal increase was more remarkable in kidney, also showing higher EPCR levels in PC+/− mice compared to WT individuals at the late time points (Figure 6b). TM also displayed increases at 24 hours after CLP in kidney and liver. Although there were no significant differences between resting WT and PC+/− mice, higher TM mRNA levels were observed in PC+/− livers, relative to WT livers, at the 24-hour time point (Figure 6c). In resting PC+/− mice, PC gene expression was, ∼60% of the PC mRNA levels of resting WT mice, in both liver and kidney, where PC expression totaled ∼33% of the liver expression. PC mRNA levels decreased at 24 hours after CLP, relative to resting mice. Interestingly, whereas WT individuals displayed a 50% decrease in PC expression in liver and kidney at the 24-hour time point, relative to the resting stage, the decrease in PC expression was more dramatic in PC+/− mice (66% in kidney and 75% in liver) at the same time point (Figure 6d). These results are consistent with the plasma PC levels determined by ELISA.
Figure 6-4239.
Gene expression ratio to RPL-19 of TF (a), EPCR (b), TM (c), and PC (d), relative to the resting WT group (n = 3) in liver, kidney, and lung, as determined by RT-PCR in WT mice 24 hours after CLP (n = 3), PC+/− resting mice (n = 3), and PC+/− mice 24 hours after CLP (n = 4). In all cases: a: in liver, PC+/− and WT mice 24 hours after CLP were different from PC+/− and WT resting mice, respectively (P = 0.039); in kidney, PC+/− and WT mice 24 hours after CLP were different from PC+/− and WT resting mice, respectively (P = 0.07, K-W); in lung, WT resting mice were different from PC+/− mice 24 hours after CLP and WT mice 24 hours after CLP (P = 0.046, K-W). b: In liver, PC+/− mice 24 hours after CLP were different from PC+/− resting and WT resting mice (P = 0.12, K-W); in kidney, PC+/− mice 24 hours after CLP were different from PC+/− resting and WT resting mice (P = 0.02, K-W); in lung, P values show no significant differences (K-W). c: Liver, PC+/− mice 24 hours after CLP were different from WT resting and PC+/− resting mice (P = 0.035, K-W); kidney, PC+/− and WT mice 24 hours after CLP were different from both PC+/− resting and WT resting mice (P = 0.044, K-W). d:PC+/− mice 24 hours after CLP were different from WT resting mice 24 hours after CLP and PC+/− resting mice in both liver (P = 0.02, K-W) and kidney (P = 0.025, K-W). WT mice 24 hours after CLP were different from WT resting mice in liver (P = 0.02, K-W) and kidney (P = 0.02, K-W).
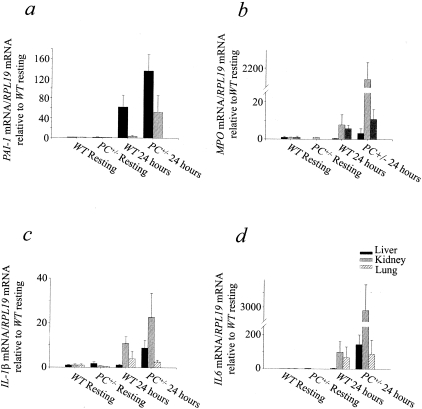
PAI-1 expression showed significant increases in the liver 24 hours after challenge, relative to the resting stage (Figure 7a). At the 24-hour time point, hepatic PAI-1 expression was significantly higher in PC+/− mice, compared to WT individuals.
Figure 7-4239.
Gene expression ratio to RPL-19 of PAI-1 (a), MPO (b), IL-1β (c), and IL-6 (d) relative to the WT resting group (n = 3) in liver, kidney, and lung, as determined by RT-PCR in WT mice 24 hours after CLP (n = 3), PC+/− resting mice (n = 3), and PC+/− mice 24 hours after CLP (n = 4). a: In liver, PC+/− mice 24 hours after CLP were different from PC+/− resting and WT resting groups (P = 0.025, K-W), in kidney, differences between groups were not significant (K-W, multicomparison test). b: In liver, PC+/− resting mice were different from PC+/− mice 24 hours after CLP and WT resting groups (P = 0.055); in kidney, PC+/− mice 24 hours after CLP were different from all of the other groups (P = 0.02, K-W); in lung, PC+/− resting mice were different from PC+/− and WT mice 24 hours after CLP (P = 0.08, K-W). c: In liver, no significant differences between groups were found; in kidney: PC+/− group 24 hours after CLP was different from WT and PC+/− resting groups (P = 0.04, K-W); in lung: the PC+/− group after CLP was not different form the PC+/− resting group (T-K); P = not significant (K-W). d: In liver, PC+/− mice 24 hours after CLP were different from the WT resting group (P = 0.11, K-W); in kidney, PC+/− mice 24 hours after CLP were different from the WT and PC+/− resting groups (P = 0.032, K-W); in lung: no significant differences between the groups were found (K-W).
MPO expression was low both before and after CLP in kidneys, livers, and lungs. There was nonetheless a significant increase in MPO expression (2000-fold) 24 hours after CLP, relative to the resting stage in kidney from PC+/− individuals (Figure 7b). This finding may support the undetectable MPO activity measured by the functional assay (not shown), and provided additional evidence of the poor leukocyte infiltration seen in the histology (not shown). Levels of TNF-α remained low before and after challenge and showed no variation when compared in the different experimental groups (data not presented). Levels of IL-1β mRNA increased 24 hours after challenge relative to resting mice in kidneys and, less significantly, in livers. In both organs, there was a tendency toward higher expression of IL-1β in PC+/− mice, compared to WT mice (Figure 7c). Finally, expression of IL-6 showed a remarkable difference between resting and challenged mice in kidney (3000-fold). In the liver, this difference was less pronounced (100-fold) and less significant. Also, higher levels of IL-6 mRNA were observed in liver and kidney from PC+/− mice, compared to their WT littermates, 24 hours after challenge (Figure 7d). It is believed that the elevated expression of IL-1β, IL-6, and PAI-1 in PC+/− mice was mainly because of EC expression levels and additionally Kupffer cells in the liver, but not expression by leukocytes, based on the histology and MPO results.
Discussion
In the systemic inflammatory state characteristic of sepsis, both the endothelium and leukocytes are activated by proinflammatory cytokines,3 with a consequent up-regulation of coagulation. As a result, vascular permeability is increased and activated leukocytes adhere to the endothelium and extravasate into organs. Additionally, the release of reactive oxygen species by these inflammatory cells could cause further damage to the endothelium with consequent additional up-regulation of coagulation. It is thus obvious that such perturbation of the vascular endothelium could impair the PC pathway, an effect observed in individuals during sepsis.10 In fact, septic patients present with low plasma PC concentrations on admission to intensive care units,41,42 and it is thought that this parameter could have a prognostic value.43,44 Thus, we believed that a congenital PC deficiency would present a more severe septic response to bacterial challenge.
Investigators in a previous report9 used a model of gram-negative endotoxin shock in PC+/− and WT mice after LPS administration (50 mg/kg). A comparatively higher mortality rate in PC+/− mice was attributed to the exacerbation of DIC in PC+/− mice as compared to WT mice. The dose of LPS used in this work was approximately fivefold higher than the dose applied in comparable studies. The administration of such a high level of LPS may cause a response because of the toxicity of the endotoxin per se in terms of its direct interaction with organs, instead of a systemic inflammatory response that would result from a lower dose of this agent. In the present study, CLP was used as a model of polymicrobial sepsis and focused on hemodynamic parameters, organ failure, and focal gene expression. It is believed that CLP mimics sepsis symptoms in terms of the kinetics of cytokine expression much more accurately than the LPS model used in the previous study, which showed differences with clinical studies and with the model used in this work.3,45 As examples, serum TNF-α37,46,47 and LPS levels48 were not elevated in the same kinetic manners in septic patients or CLP-treated mice, as was found in LPS-injected mice. Moreover, the kinetics and magnitude of cytokine expression described by the LPS models do not reproduce appropriately the cytokine profile of sepsis.37 However, CLP may present other types of complications because of its polymicrobial nature, because it is not feasible to control the type and titer of the gastrointestinal flora in different individuals. Because the intestinal microflora is predominantly composed of gram-negative anaerobic bacteria, eg, Escherichia coli, we performed PLF bacterial counts on LB media appropriate for this type of microorganism. There were no significant differences in the colony-forming units found in WT and PC+/− peritoneal lavages at 24 hours after CLP. This result may indicate that the increased mortality in PC+/− mice is not because of bacterial count differences, which had been suggested earlier.49
Among the original goals of the present study, was to conduct a comparative analysis of sepsis between young mice (8 to 12 weeks old) and older mice (26 to 30 weeks old) to evaluate the prognosis of PC+/− mice with age. There was no difference in the survival of WT and PC+/− mice at the younger age. Age dependence on survival after CLP has been previously studied,50,51 and revealed improved survival in mature mice as compared to young mice. Nonetheless, under our experimental conditions, survival was more favorable in the younger group than in the older group, in which PC+/− mice displayed a higher mortality rate than WT mice. The explanation for these observations may be a function of the increase in the risk of thrombosis with age, because of higher expression of procoagulant proteins such as fibrinogen, FVIII, and FIX,52 among other factors. Moreover, individuals with a heterozygous PC deficiency have a higher rise in the propensity to a coagulopathy with age than healthy individuals,53–56 which could explain the higher susceptibility to sepsis of PC+/− mice in the older age group.
Hemodynamic parameters, namely central BPs and heart rates, were continuously monitored before CLP and during the 24 hours after surgery and closely paralleled the phenomena observed in human sepsis. At ∼6 hours after challenge, the mice entered septic shock, displaying clear signs of hypotension and bradycardia. Importantly, the drop in both parameters was remarkably more dramatic in PC+/− mice as compared to WT mice, likely the most significant factor in the higher mortality in PC+/− mice. Moreover, poor tissue irrigation and hypoxia because of hypotension explains the renal damage observed by the elevated plasma levels of BUN and creatinine. Heart or muscle damage, as indicated by the elevated plasma creatinine kinase values, was higher in PC+/− mice compared to WT mice and hepatic failure, noted by the increase in plasma aspartate aminotransferase levels, was equivalent in both WT and PC+/− 24 hours after CLP. Hence, a more severe septic shock state in PC+/− mice supports previous evidence that PC contributes to the regulation of BP.30,57 Importantly, although previous studies described the effect of aPC administration on reduction of hypotension independent of its anti-coagulant properties,30 the present study shows an aggravated hypotensive state in mice expressing ∼50% of normal PC levels. The convergence of the results of these different approaches strongly suggests a mechanistic role of aPC in the prevention of hypotension.
The elevated plasma levels of TNF-α and IL-1β observed throughout the inflammatory state6,58 likely contribute to the up-regulation of tissue factor (TF) that is observed in this report. Furthermore, endothelium damage because of inflammation can activate blood coagulation. The procoagulant state induces the generation of thrombin and thrombin signaling through its PARs on ECs and/or platelets,59 and triggers inducible nitric oxide synthase expression60,61 and NO release. This provides an additional mechanism for hypotension in septic shock. Moreover, this plausible pathway for hypotension in sepsis-induced DIC, and PC signaling in ECs through EPCR and PAR-1 or PAR-2,62 may provide another mechanism for a protection against hypotension. A co-localization of PAR-1 with EPCR can reduce the availability of PAR-1 for thrombin signaling and thus participate in protection against inflammation through PC signaling.63 This protection could be lost in a PC deficiency and the diminution of plasma aPC levels could in this manner be a factor in hypotension. The decrease in the hemodynamic parameters seen after CLP-induced sepsis, which is more dramatic in PC+/− mice compared to WT mice, supports this hypothesis, taking into account that the plasma PC levels in PC+/− mice are approximately one-half of the value in WT mice at the time of shock. Additionally, at later time points, PC+/− mice displayed a more remarkable decrease in plasma PC levels than WT mice (Figure 4a). Furthermore, PC gene expression in liver and kidney showed a more striking drop in PC+/− mice compared to WT mice (Figure 6a).
A statistically significant decrease in blood leukocytes was observed in both WT and PC+/− mice 24 hours after CLP, the effect being more prominent in the later case. However, the total number of leukocytes measured in PLF did not differ between both genotypes at the 24-hour time point, as observed at 6 hours after CLP, in which the leukocyte count was higher in PC+/− as compared to WT PLF. Additionally, there is no evidence of inflammatory cell infiltration in the tissues, as displayed by CD45 staining (not shown) and MPO functional and gene expression assays at the 24-hour time point. Importantly, cellular debris was observed in the cytological analysis of the PLF of both groups at 24 hours after CLP by Giemsa staining. Based on these results, it appears that more significant leukocyte death in the PLF of PC+/− mice may result in the compensation in the leukocyte count at the present time point. A protective role of PC against apoptosis shown recently,25 justifies our assumption.
Interpretation of the hemostasis analyses presented herein indicates mild signs of consumption of factors from both the extrinsic and intrinsic pathways of blood coagulation 24 hours after CLP, as evidenced by the prolongation of the PTs and APTTs, respectively, along with the decrease in FXI and FXII activities. Further, FXI and FXII mRNA levels in the liver remained invariant after the challenge, implying that the decrease in their respective activities is not transcriptionally regulated (not shown). Overall, these results show that the consumption of coagulation factors is similar in WT and PC+/− mice. On the other hand, there was an ∼2.5-fold elevation in fibrinogen levels after CLP. This phenomenon reflects the acute phase response of this protein and possibly counterbalances an effect of a consumptive coagulopathy. Moreover, the blood platelet count remained unchanged 24 hours after the challenge, at ∼500,000 platelets/ml blood in both WT and PC+/− mice. Hemostasis factor differences between WT and PC+/− mice were not evident and did not account for a severe DIC in a PC deficiency. These results are not in accord with the observed aggravation of DIC observed in PC+/− mice after LPS,9 and this likely reflects differences in the models used. Thus, it seems that the PC contribution to amelioration of sepsis does not necessarily depend on a protective role against DIC, although DIC enhances the progress of the disease, but more so as a modulator of hypotension and bradycardia, and on its anti-inflammatory properties.
From the preceding considerations, it is apparent that EPCR-assisted PC activation and signaling are critical for the host defense against septic shock. Expression of EPCR in cell cultures decreased after treatment with mediators of the inflammatory response, eg, TNF-α,64 which is in agreement with the impairment in PC activation seen in septic shock patients. However, up-regulation of EPCR gene expression, and increase in the release of a soluble form of EPCR by shedding from the endothelium, were observed in rats as a result of an endotoxin challenge.65 The same study showed that endotoxin-stimulated EPCR up-regulation was inhibited by hirudin, a thrombin inhibitor. Further incubation of rat ECs with thrombin or a PAR-1 agonist resulted in an increase of EPCR expression. These results imply that EPCR expression after endotoxin treatment occurs through PAR-1-dependent thrombin signaling. An increase in EPCR mRNA expression is observed 24 hours after CLP challenge predominantly in lung and kidney. Additionally, the increase in EPCR mRNA expression after CLP-induced sepsis was higher in PC+/− mice than in WT mice, which is supported by the hypothesis that thrombin levels are more elevated because of the PC deficiency. Thus, there may be a higher level of sEPCR in PC+/− mice compared to WT mice, contributing to impairment of the anti-inflammatory and anti-coagulant activities of PC.
Expression of Tm decreases on vascular endothelium in vitro and in vivo66,67 after treatment with endotoxin via TNF-α up-regulation and signaling. Increased soluble Tm (sTm) values were observed in septic DIC models,68 which supports our observations. Interestingly, it was shown that Tm expression after endotoxin challenge was increased in macrophages but decreased in ECs.69 These observations suggest the importance of this receptor during sepsis in the regulation of coagulation through PC activation in different organs. Tm may serve anti-coagulant or anti-inflammatory purposes elsewhere, as expressed by other cell types. The significant increase in TM mRNA observed in the liver is not surprising, considering that this organ is rich in Kupffer cells, the hepatic macrophages. Further, transcription of TNF-α or other proinflammatory cytokines that are known to down-regulate Tm expression, was not as elevated in liver as compared with the other organs and remained unchanged 24 hours after CLP.
It is believed that transcription factors NF-κB and AP-1 intervene in the expression of TF after septic or traumatic shock70,71 in the vital organs via cytokine-mediated induction. Up-regulation of TF was observed in lung, kidney, liver, and vascular ECs in several inflammation and sepsis studies using different animal models,72,73 which is in agreement with our results in kidney and lung. Nonetheless, in the liver, we found a decrease in TF mRNA. The liver is the last organ to receive venous blood and, therefore, highly affected during the hypotensive shock caused by sepsis, as shown by the elevated plasma aspartate aminotransferase. It appears that in this model of sepsis, the liver was protected from a coagulopathy through lower cytokine expression, compared to other organs, leading to a decrease in TF and an increase in Tm expressions by the Kupffer cells. This observation is supported by the fact that fibrin(ogen) deposition was absent in this organ 24 hours after CLP.
Overall, the presented evidence confirms that individuals with a congenital PC deficiency have lesser chances of survival after sepsis because of a more severe hypotensive state, with organ malfunctioning. Remarkably, PC concentrations of ∼50% of normal are not sufficient to provide protection against systemic inflammation and shock, showing the importance of this protein in vivo. These findings imply that PC therapy would be an excellent choice for the treatment of septic patients with a congenital PC deficiency, in agreement with previous observations.
Acknowledgments
We thank Mayra Sandoval-Cooper for assistance with the histology, Stacey Raje for maintenance of the animal colony, Mr. J. Andrew Martin for design of the Q-RT-PCR designs, Ms. Deborah Donahue for performing the surgeries required for placement of the BP probes, Dr. Harm HogenEsch for assistance with evaluation of the histopathology, and Dr. David Joyce for helpful commentary on the manuscript.
Footnotes
Address reprint requests to Dr. Francis J. Castellino, Department of Chemistry and Biochemistry, 434 Stepan Hall of Chemistry and Biochemistry, University of Notre Dame, Notre Dame, IN 46556. E-mail: fcastell@nd.edu.
Supported by grants from the National Institutes of Health (HL19982 and HL73750 to F.J.C.) and the Kleiderer-Pezold endowed professorship (to F.J.C.).
References
- Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- Riedemann NC, Guo RF, Ward PA. The enigma of sepsis. J Clin Invest. 2003;112:460–467. doi: 10.1172/JCI19523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman JA, Gomez-Cambronero J. Molecular crosstalk between p70S6k and MAPK cell signaling pathways. Biochem Biophys Res Commun. 2002;293:463–469. doi: 10.1016/S0006-291X(02)00238-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedemann NC, Guo RF, Ward PA. Novel strategies for the treatment of sepsis. Nat Med. 2003;9:517–524. doi: 10.1038/nm0503-517. [DOI] [PubMed] [Google Scholar]
- Cicala C, Cirino G. Linkage between inflammation and coagulation: an update on the molecular basis of the crosstalk. Life Sci. 1998;62:1817–1824. doi: 10.1016/s0024-3205(97)01167-3. [DOI] [PubMed] [Google Scholar]
- Levi M, Ten Cate H. Disseminated intravascular coagulation. N Engl J Med. 1999;341:586–592. doi: 10.1056/NEJM199908193410807. [DOI] [PubMed] [Google Scholar]
- Levi M, de Jonge E, van der Poll T, ten Cate H. Disseminated intravascular coagulation. Thromb Haemost. 1999;82:695–705. [PubMed] [Google Scholar]
- Levi M, Dorffler-Melly J, Reitsma PH, Buller HR, Florquin S, Van Der Poll T, Carmeliet P. Aggravation of endotoxin-induced disseminated intravascular coagulation and cytokine activation in heterozygous protein C-deficient mice. Blood. 2003;101:4823–4827. doi: 10.1182/blood-2002-10-3254. [DOI] [PubMed] [Google Scholar]
- Faust SN, Levin M, Harrison OB, Goldin RD, Lockhart MS, Kondaveeti S, Laszik Z, Esmon CT, Heyderman RS. Dysfunction of endothelial protein C activation in severe meningococcal sepsis. N Engl J Med. 2001;345:408–416. doi: 10.1056/NEJM200108093450603. [DOI] [PubMed] [Google Scholar]
- Robbie LA, Dummer S, Booth NA, Adey GD, Bennett B. Plasminogen activator inhibitor 2 and urokinase-type plasminogen activator in plasma and leucocytes in patients with severe sepsis. Br J Hematol. 2000;109:342–348. doi: 10.1046/j.1365-2141.2000.02057.x. [DOI] [PubMed] [Google Scholar]
- Creasey AA, Chang ACK, Feigen L, Wun TC, Taylor FB, Hinshaw LB. Tissue factor pathway inhibitor reduces mortality from Escherichia coli septic shock. J Clin Invest. 1993;91:2850–2860. doi: 10.1172/JCI116529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jonge E, Dekkers PE, Creasey AA, Hack CE, Paulson SK, Karim A, Kesecioglu J, Levi M, van Deventer SJ, van der Poll T. Tissue factor pathway inhibitor does not influence inflammatory pathways during human endotoxemia. J Infect Dis. 2001;183:1815–1818. doi: 10.1086/320723. [DOI] [PubMed] [Google Scholar]
- Opal SM. Protein C levels in severe sepsis. Chest. 2001;120:699–701. doi: 10.1378/chest.120.3.699. [DOI] [PubMed] [Google Scholar]
- Bernard GR, Vincent JL, Laterre PF, LaRosa SP, Dhainaut JF, Lopez-Rodriguez A, Steingrub JS, Garber GE, Helterbrand JD, Ely EW, Fisher CJ., Jr Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344:699–709. doi: 10.1056/NEJM200103083441001. [DOI] [PubMed] [Google Scholar]
- Kisiel W, Canfield WM, Ericsson LH, Davie EW. Anticoagulant properties of bovine plasma protein C following activation by thrombin. Biochemistry. 1977;16:5824–5831. doi: 10.1021/bi00645a029. [DOI] [PubMed] [Google Scholar]
- Vehar GA, Davie EW. Preparation and properties of bovine factor VIII (antihemophilic factor) Biochemistry. 1980;19:401–410. doi: 10.1021/bi00544a001. [DOI] [PubMed] [Google Scholar]
- Taylor FB, Lockhart MS. A new function for activated protein C: activated protein C prevents inhibition of plasminogen activators by releasate from mononuclear leukocytes-platelet suspensions stimulated by phorbol diester. Thromb Res. 1985;37:639–649. doi: 10.1016/0049-3848(85)90042-8. [DOI] [PubMed] [Google Scholar]
- Bajzar L, Nesheim M. Activated protein C (APC) promotes fibrinolysis by inhibiting prothrombinase catalyzed formation of an antifibrinolytic component derived from prothrombin. Thromb Haemost. 1991;65:1199–1207. [Google Scholar]
- Bajzar L, Morser J, Nesheim M. TAFI, or plasma procarboxypeptidase B, couples the coagulation and fibrinolytic cascades through the thrombin-thrombomodulin complex. J Biol Chem. 1996;271:16603–16608. doi: 10.1074/jbc.271.28.16603. [DOI] [PubMed] [Google Scholar]
- Coughlin SR. Thrombin signalling and protease-activated receptors. Nature. 2000;407:258–264. doi: 10.1038/35025229. [DOI] [PubMed] [Google Scholar]
- Grey ST, Tsuchida A, Hau H, Orthner CL, Salem HH, Hancock WW. Selective inhibitory effects of the anticoagulant activated protein C on the responses of human mononuclear phagocytes to LPS, IFN-γ, or phorbol ester. J Immunol. 1994;153:3664–3672. [PubMed] [Google Scholar]
- Schmidt-Supprian M, Murphy C, While B, Lawler M, Kapurniotu A, Voelter W, Smith O, Bernhagen J. Activated protein C inhibits tumor necrosis factor and macrophage migration inhibitory factor production in monocytes. Eur Cytokine Netw. 2000;11:407–413. [PubMed] [Google Scholar]
- Hancock WW, Grey ST, Hau L, Akalin E, Orthner C, Sayegh MH, Salem HH. Binding of activated protein C to a specific receptor on human mononuclear phagocytes inhibits intracellular calcium signaling and monocyte-dependent proliferative responses. Transplantation. 1995;60:1525–1532. doi: 10.1097/00007890-199560120-00026. [DOI] [PubMed] [Google Scholar]
- Joyce DE, Gelbert L, Ciaccia A, DeHoff B, Grinnell BW. Gene expression profile of antithrombotic protein C defines new mechanisms modulating inflammation and apoptosis. J Biol Chem. 2001;276:11199–11203. doi: 10.1074/jbc.C100017200. [DOI] [PubMed] [Google Scholar]
- Riewald M, Petrovan RJ, Donner A, Mueller BM, Ruf W. Activation of endothelial cell protease activated receptor 1 by the protein C pathway. Science. 2002;296:1880–1882. doi: 10.1126/science.1071699. [DOI] [PubMed] [Google Scholar]
- White B, Schmidt M, Murphy C, Livingstone W, O’Toole D, Lawler M, O’Neill L, Kelleher D, Schwarz HP, Smith OP. Activated protein C inhibits lipopolysaccharide-induced nuclear translocation of nuclear factor kappa B (NF-kappa B) and tumour necrosis factor alpha (TNF-alpha) production in the THP-1 monocytic cell line. Br J Haematol. 2000;110:130–134. doi: 10.1046/j.1365-2141.2000.02128.x. [DOI] [PubMed] [Google Scholar]
- Grinnell BW, Hermann RB, Yan SB. Human protein C inhibits selectin-mediated cell adhesion: role of unique fucosylated oligosaccharide. Glycobiology. 1994;4:221–225. doi: 10.1093/glycob/4.2.221. [DOI] [PubMed] [Google Scholar]
- Murakami K, Okajima K, Uchiba M, Johno M, Nakagaki T, Okabe H, Takatsuki K. Activated protein C attenuates endotoxin-induced pulmonary vascular injury by inhibiting activated leukocytes in rats. Blood. 1996;87:642–647. [PubMed] [Google Scholar]
- Isobe H, Okajima K, Uchiba M, Mizutani A, Harada N, Nagasaki A, Okabe K. Activated protein C prevents endotoxin-induced hypotension in rats by inhibiting excessive production of nitric oxide. Circulation. 2001;104:1171–1175. doi: 10.1161/hc3501.093799. [DOI] [PubMed] [Google Scholar]
- Garnier JM, Aillaud MF, Devred P, Juhan-Vague I, Unal D. Septicemia, portal thrombosis and congenital protein C deficiency. Arch Fr Pediatr. 1988;45:119–122. [PubMed] [Google Scholar]
- Wichterman KA, Baue AE, Chaudry IH. Sepsis and septic shock—a review of laboratory models and a proposal. J Surg Res. 1980;29:189–201. doi: 10.1016/0022-4804(80)90037-2. [DOI] [PubMed] [Google Scholar]
- Jalbert LR, Rosen ED, Moons L, Chan JCY, Carmeliet P, Collen D, Castellino FJ. Inactivation of the gene for anticoagulant protein C causes lethal perinatal consumptive coagulopathy in mice. J Clin Invest. 1998;102:1481–1488. doi: 10.1172/JCI3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsukawa A, Hogaboam CM, Lukacs NW, Lincoln PM, Strieter RM, Kunkel SL. Endogenous MCP-1 influences systemic cytokine balance in a murine model of acute septic peritonitis. Exp Mol Pathol. 2000;68:77–84. doi: 10.1006/exmp.1999.2296. [DOI] [PubMed] [Google Scholar]
- Matsukawa A, Hogaboam CM, Lukacs NW, Lincoln PM, Evanoff HL, Strieter RM, Kunkel SL. Expression and contribution of endogenous IL-13 in an experimental model of sepsis. J Immunol. 2000;164:2738–2744. doi: 10.4049/jimmunol.164.5.2738. [DOI] [PubMed] [Google Scholar]
- Daemen MA, Kurvers HA, Kitslaar PJ, Slaaf DW, Bullens PH, Van den Wildenberg FA. Neurogenic inflammation in an animal model of neuropathic pain. Neurol Res. 1998;20:41–45. doi: 10.1080/01616412.1998.11740483. [DOI] [PubMed] [Google Scholar]
- Remick DG, Newcomb DE, Bolgos GL, Call DR. Comparison of the mortality and inflammatory response of two models of sepsis: lipopolysaccharide vs. cecal ligation and puncture. Shock. 2000;13:110–116. doi: 10.1097/00024382-200013020-00004. [DOI] [PubMed] [Google Scholar]
- Benjamim CF, Silva JS, Fortes ZB, Oliveira MA, Ferreira SH, Cunha FQ. Inhibition of leukocyte rolling by nitric oxide during sepsis leads to reduced migration of active microbicidal neutrophils. Infect Immun. 2002;70:3602–3610. doi: 10.1128/IAI.70.7.3602-3610.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart D, Fulton WB, Wilson C, Monitto CL, Paidas CN, Reeves RH, De Maio A. Genetic contribution to the septic response in a mouse model. Shock. 2002;18:342–347. doi: 10.1097/00024382-200210000-00009. [DOI] [PubMed] [Google Scholar]
- Ono S, Ueno C, Seki S, Matsumoto A, Mochizuki H. Interleukin-12 and -18 induce severe liver injury in mice recovered from peritonitis after sublethal endotoxin challenge. Surgery. 2003;134:92–100. doi: 10.1067/msy.2003.189. [DOI] [PubMed] [Google Scholar]
- Fijnvandraat K, Derkx B, Peters M, Bijlmer R, Sturk A, Prins MH, van Deventer SJ, ten Cate JW. Coagulation activation and tissue necrosis in meningococcal septic shock: severely reduced protein C levels predict a high mortality. Thromb Haemost. 1995;73:15–20. [PubMed] [Google Scholar]
- Hesselvik JF, Malm J, Dahlback B, Blomback M. Protein C, protein S and C4b-binding protein in severe infection and septic shock. Thromb Haemost. 1991;65:126–129. [PubMed] [Google Scholar]
- Mesters RM, Helterbrand J, Utterback BG, Yan B, Chao YB, Fernandez JA, Griffin JH, Hartman DL. Prognostic value of protein C concentrations in neutropenic patients at high risk of severe septic complications. Crit Care Med. 2000;28:2209–2216. doi: 10.1097/00003246-200007000-00005. [DOI] [PubMed] [Google Scholar]
- Yan SB, Helterbrand JD, Hartman DL, Wright TJ, Bernard GR. Low levels of protein C are associated with poor outcome in severe sepsis. Chest. 2001;120:915–922. doi: 10.1378/chest.120.3.915. [DOI] [PubMed] [Google Scholar]
- Michie HR. The value of animal models in the development of new drugs for the treatment of the sepsis syndrome. J Antimicrob Chemother. 1998;41(Suppl A):S47–S49. doi: 10.1093/jac/41.suppl_1.47. [DOI] [PubMed] [Google Scholar]
- Michie HR, Manogue KR, Spriggs DR, Revhaug A, O’Dwyer S, Dinarello CA, Cerami A, Wolff SM, Wilmore DW. Detection of circulating tumor necrosis factor after endotoxin administration. N Engl J Med. 1988;318:1481–1486. doi: 10.1056/NEJM198806093182301. [DOI] [PubMed] [Google Scholar]
- Eskandari MK, Bolgos G, Miller C, Nguyen DT, DeForge LE, Remick DG. Anti-tumor necrosis factor antibody therapy fails to prevent lethality after cecal ligation and puncture or endotoxemia. J Immunol. 1992;148:2724–2730. [PubMed] [Google Scholar]
- Newcomb D, Bolgos G, Green L, Remick DG. Antibiotic treatment influences outcome in murine sepsis: mediators of increased morbidity. Shock. 1998;10:110–117. doi: 10.1097/00024382-199808000-00005. [DOI] [PubMed] [Google Scholar]
- Godshall CJ, Scott MJ, Peyton JC, Gardner SA, Cheadle WG. Genetic background determines susceptibility during murine septic peritonitis. J Surg Res. 2002;102:45–49. doi: 10.1006/jsre.2001.6319. [DOI] [PubMed] [Google Scholar]
- Hyde SR, Stith RD, McCallum RE. Mortality and bacteriology of sepsis following cecal ligation and puncture in aged mice. Infect Immunol. 1990;58:619–624. doi: 10.1128/iai.58.3.619-624.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde SR, McCallum RE. Lipopolysaccharide-tumor necrosis factor-glucocorticoid interactions during cecal ligation and puncture-induced sepsis in mature versus senescent mice. Infect Immunol. 1992;60:976–982. doi: 10.1128/iai.60.3.976-982.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkerson WR, Sane DC. Aging and thrombosis. Semin Thromb Hemost. 2002;28:555–568. doi: 10.1055/s-2002-36700. [DOI] [PubMed] [Google Scholar]
- Pabinger I. Clinical relevance of protein C. Blut. 1986;53:63–75. doi: 10.1007/BF00321089. [DOI] [PubMed] [Google Scholar]
- Bovill EG, Bauer KA, Dickerman JD, Callas P, West B. The clinical spectrum of heterozygous protein C deficiency in a large New England kindrid. Blood. 1989;73:712–717. [PubMed] [Google Scholar]
- Marlar RA, Mastovich S. Hereditary protein C deficiency: a review of the genetics, clinical presentation, diagnosis and treatment. Blood Coagul Fibrinolysis. 1990;1:319–330. [PubMed] [Google Scholar]
- Allaart CF, Poort SR, Rosendaal FR, Reitsma PH, Bertina RM, Briet E. Increased risk of venous thrombosis in carriers of hereditary protein C deficiency defect. Lancet. 1993;341:134–138. doi: 10.1016/0140-6736(93)90003-y. [DOI] [PubMed] [Google Scholar]
- Kalil AC, Coyle SM, Um JY, LaRosa SP, Turlo MA, Calvano SE, Sundin DP, Nelson DR, Lowry SF. Effects of drotrecogin alfa (activated) in human endotoxemia. Shock. 2004;21:222–229. doi: 10.1097/01.shk.0000116778.27924.79. [DOI] [PubMed] [Google Scholar]
- Bevilacqua MP, Pober JS, Majeau GR, Cotran RS, Gimbrone MA. Interleukin 1 (IL-1) induces biosynthesis and cell surface expression of procoagulant activity in human vascular endothelial cells. J Exp Med. 1984;160:618–623. doi: 10.1084/jem.160.2.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlin SR. Protease-activated receptors in vascular biology. Thromb Haemost. 2001;86:298–307. [PubMed] [Google Scholar]
- Bevilacqua MP, Gimbrone MA. Inducible endothelial functions in inflammation and coagulation. Semin Thromb Hemost. 1987;13:425–433. doi: 10.1055/s-2007-1003519. [DOI] [PubMed] [Google Scholar]
- De Meyer E, Van Hove CE, Feng XJ, Rampart M, Herman AG. Thrombin triggers the de novo expression of an inducible NO synthase in porcine aortic valve endothelial cells. Eur J Pharmacol. 1995;291:67–72. doi: 10.1016/0922-4106(95)90126-4. [DOI] [PubMed] [Google Scholar]
- Riewald M, Petrovan RJ, Donner A, Ruf W. Activated protein C signals through the thrombin receptor PAR1 in endothelial cells. J Endotoxin Res. 2003;9:317–321. doi: 10.1179/096805103225002584. [DOI] [PubMed] [Google Scholar]
- Ruf W. PAR1 signaling: more good than harm? Nat Med. 2003;9:258–260. doi: 10.1038/nm0303-258. [DOI] [PubMed] [Google Scholar]
- Fukudome K, Esmon CT. Identification, cloning, and regulation of a novel endothelial cell protein C/activated protein C receptor. J Biol Chem. 1994;269:26486–26491. [PubMed] [Google Scholar]
- Gu JM, Katsuura Y, Ferrell GL, Grammas P, Esmon CT. Endotoxin and thrombin elevate rodent endothelial cell protein C receptor mRNA levels and increase receptor shedding in vivo. Blood. 2000;95:1687–1693. [PubMed] [Google Scholar]
- Moore KL, Andreoli SP, Esmon NL, Esmon CT, Bang NU. Endotoxin enhances tissue factor and suppresses thrombomodulin expression of human vascular endothelium in vitro. J Clin Invest. 1987;79:124–130. doi: 10.1172/JCI112772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kume M, Hayashi T, Yuasa H, Tanaka H, Nishioka J, Ido M, Gabazza EC, Kawarada Y, Suzuki K. Bacterial lipopolysaccharide decreases thrombomodulin expression in the sinusoidal endothelial cells of rats—a possible mechanism of intrasinusoidal microthrombus formation and liver dysfunction. J Hepatol. 2003;38:9–17. doi: 10.1016/s0168-8278(02)00324-0. [DOI] [PubMed] [Google Scholar]
- Asakura H, Jokaji H, Saito M, Uotani C, Kumabashiri I, Morishita E, Yamazaki M, Matsuda T. Plasma levels of soluble thrombomodulin increase in cases of disseminated intravascular coagulation with organ failure. Am J Hematol. 1991;38:281–287. doi: 10.1002/ajh.2830380406. [DOI] [PubMed] [Google Scholar]
- Grey ST, Hancock WW. A physiologic anti-inflammatory pathway based on thrombomodulin expression and generation of activated protein C by human mononuclear phagocytes. J Immunol. 1996;156:2256–2263. [PubMed] [Google Scholar]
- Armstead VE, Opentanova IL, Minchenko AG, Lefer AM. Tissue factor expression in vital organs during murine traumatic shock: role of transcription factors AP-1 and NF-kappaB. Anesthesiology. 1999;91:1844–1852. doi: 10.1097/00000542-199912000-00039. [DOI] [PubMed] [Google Scholar]
- Dhainaut JF, Marin N, Mignon A, Vinsonneau C. Hepatic response to sepsis: interaction between coagulation and inflammatory processes. Crit Care Med. 2001;29:S42–S47. doi: 10.1097/00003246-200107001-00016. [DOI] [PubMed] [Google Scholar]
- Arai M, Mochida S, Ohno A, Ogata I, Obama H, Maruyama I, Fujiwara K. Blood coagulation equilibrium in rat liver microcirculation as evaluated by endothelial cell thrombomodulin and macrophage tissue factor. Thromb Res. 1995;80:113–123. doi: 10.1016/0049-3848(95)00157-m. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Yoshimura N, Sano H, Kobayashi Y, Ushigome H, Oka T. Role of tissue factor in ischemia-reperfusion injury: immunohistochemical assessment of tissue factor pathway inhibitor staining of the liver. Transplant Proc. 1998;30:3737–3739. doi: 10.1016/s0041-1345(98)01216-0. [DOI] [PubMed] [Google Scholar]