Abstract
Hyaluronan (hyaluronic acid, HA) is a glycosaminoglycan in the extracellular matrix of tissues that plays a role in cellular migration, proliferation and differentiation. Injury to the stratum corneum elicits an epidermal hyperproliferative response, a pathogenic feature in many cutaneous diseases including eczema and psoriasis. Because HA is abundant in the matrix between keratinocytes, we asked whether the presence of HA is required for epidermal hyperplasia to occur in response to barrier injury. Disruption of the stratum corneum, by acetone application on the skin of hairless mice, led to a marked accumulation of HA in the matrix between epidermal basal and spinous keratinocytes, and also within keratinocytes of the upper epidermis. To test whether HA may have a functional role in epidermal hyperplasia, we used Streptomyces hyaluronidase (StrepH), delivered topically, to degrade epidermal HA and blunt the accumulation of epidermal HA after acetone. StrepH signficantly reduced epidermal HA levels, and also significantly inhibited the development of epidermal hyperplasia. This reduction in epidermal thickness was not attributable to any decrease in keratinocyte proliferation, but rather to an apparent acceleration in terminal differentiation (ie, increased keratin 10 and filaggrin expression). Overall, the data show that HA is a significant participant in the epidermal response to barrier injury.
Hyaluronan (hyaluronic acid, HA) is a large glycosaminoglycan (carbohydrate polymer) that is abundant in many tissues, where it acts as a hydrating agent and an organizer of proteins that form structural scaffolding in tissues such as cartilage and dermis.1 Recently, the discovery of new roles for hyaluronan have challenged the older, outmoded view that HA is simply a passive bystander; it now appears that HA is an active regulator of many dynamic cellular processes (reviewed in2–6). HA facilitates cell proliferation and migration during embryonic tissue development,7 wound repair,8,9 cell locomotion,10 and tumor invasion.2 HA in the extracellular matrix binds to cells through specific cell-surface receptors, including CD44 and RHAMM,6 leading to intracellular signaling and modification of cell behavior. HA binds to monocytes and lymphocytes in inflammatory diseases such as ulcerative colitis,11,12 and probably participates in other inflammatory conditions as well.
While HA is a well-known component of connective tissues, eg, the cartilage of joints or the dermis of skin, its importance in epithelial tissues such as the retina of the eye13 or the epidermis of skin14 is only now gaining attention. In human epidermis, HA is actually quite abundant in the matrix between keratinocytes, occurring predominantly in the spinous layers but also in the basal layer15,16 and the stratum corneum.17 Evidence that HA plays a regulatory role in epidermal homeostasis includes the fact that retinoic acid causes HA accumulation in the epidermis,18 whereas hydrocortisone decreases epidermal HA.19 The changes in HA that occur during treatment of skin with retinoic acid versus hydrocortisone, respectively, correlate directly with changes in epidermal proliferation (hyperplasia versus atrophy), and correlate inversely with changes in terminal differentiation (stratum corneum thinning versus thickening). Similarly, a blockade of HA binding to its cell-surface receptor (CD44), using a skin-targeted transgene that expresses antisense CD44,20 causes an atrophic condition resembling lichen sclerosus et atrophicus.21 Further evidence that HA regulates the progression of keratinocyte differentiation is provided by studies using organotypic epidermal cultures, in which the experimental removal of HA (via an exogenous hyaluronidase enzyme) causes an acceleration of terminal differentiation characterized by increased expression of filaggrin-containing keratohyalin granules (KHG).22
Data in the literature, showing that (1) HA is important in cell migration and proliferation (see above), (2) HA accumulates to high levels at the margins of cutaneous wounds,23 and (3) HA accumulates in wound fluids in high amounts,24 strongly suggest that HA may play an important role in cutaneous wound healing. Further evidence is offered by situations wherein cutaneous HA levels are found to be elevated, as in the hoxb13 knockout mouse. Hoxb13 is a transcription factor that regulates a number of differentiation-related genes in the skin, including genes that control the synthesis and/or degradation of HA; mice which lack both hoxb13 gene alleles show a >2-fold increase in HA levels in both the dermis and epidermis.25 In hoxb13 knockout animals, wound healing is accelerated,25 further supporting the notion that elevated HA in the tissues is beneficial for wound healing. However, understanding the mechanisms whereby HA might function during healing, using full-thickness skin wounds, represents a formidable challenge. Reconstitution of a full-thickness skin wound involves many types of cells (fibroblasts, keratinocytes, endothelial cells, and inflammatory cells), which must proliferate and migrate into the wound site before the defect is repaired.26,27 Each cell type may produce and interact with HA in different ways. Also, measurements of changes in HA in the setting of full-thickness wounds are made more difficult due to the constitutively high levels of HA in the dermis. To avoid such complications, we chose to limit our initial studies on HA and cutaneous injury responses to the epithelial compartment, concentrating on an examination of epidermal HA. Shallow abrasion wounding is one example of a single-compartment wounding model that is simpler to interpret than a full-thickness wound because the epidermis is preferentially involved. Even so, epidermal abrasions deep enough to disrupt the basement membrane could still trigger a complex series of events, eg, exposure of dermal collagen and other matrix components will trigger changes in the profile of integrin receptors expressed on keratinocytes, and thereby alter their phenotypic behavior.28 Therefore, we decided to study a relatively simple type of injury, disruption of the stratum corneum (the most superficial cornified layer of the epidermis).
Barrier disruption, ie, injury of the stratum corneum via repeated applications of acetone, detergent, or tape-stripping, is a well-established model for eliciting an epidermal repair response characterized by an increased production of cytokines,29,30 increased DNA synthesis in keratinocytes,31 and pronounced epidermal thickening.29,30,32,33 The hyperplastic response to barrier disruption appears to be a pathogenic feature in many cutaneous diseases including eczema, ichthyosis, and psoriasis.34–38 Because HA is clearly important for keratinocyte proliferation in vitro and in normal skin in vivo, we asked whether the presence of HA may be a requirement for increased keratinocyte proliferation that occurs in response to barrier injury. In the current study, we injured the stratum corneum to induce epidermal hyperplasia in hairless mice. The data establish that barrier disruption leads to a marked accumulation of HA in the epidermis. To test whether the HA present after injury may have a functional role in epidermal hyperplasia, we used a technique whereby an exogenous bacterial enzyme (hyaluronidase from Streptomyces) is delivered under a semi-occlusive dressing, and blunts the accumulation of epidermal HA in the skin in vivo. Our results show that degradation and loss of intact HA in the epidermis inhibits the development of the hyperplastic response to cutaneous barrier disruption.
Materials and Methods
Induction of Hyperplasia in Mouse Skin Using Tape-Stripping or Acetone
Hairless SKH-1 mice, 6- to 8-weeks-old, were obtained from Charles River Breeding Laboratories (Wilmington, MA). To permit reproducible treatments of well-defined areas of skin, tattoos were placed on the animals at least 1 week before the experiment by injecting India Ink intradermally at six sites on the dorsum of a hairless SKH-1 mouse. For each barrier-disruption treatment, a cotton-tipped swab (Q-tip) was soaked in acetone and pressed at each tatoo site for ∼5 seconds, firmly enough to wet the skin but without liquid run-off. This swabbing was repeated with new acetone-soaked Q-tips for as many repetitions as desired (up to nine times in our experiments). For tape-stripping treatment, Scotch tape (3M, St. Paul, MN) was applied firmly at the tatooed skin site, then briskly pulled off; this was repeated six times with fresh pieces of tape. Acetone or tape-strip treatments were done twice daily for 5 days, after which time the animal was euthanized by carbon dioxide inhalation before skin biopsy.
Delivery of Topical Streptomyces Hyaluronidase Enzyme
Streptomyces hyaluronidase (StrepH) was purchased from Seikagaku (Seikagaku Ltd; Tokyo, Japan) and dissolved at 100 units/ml in phosphate-buffered saline (PBS). To create a topical delivery system, the 1-cm diameter piece of gauze from a circular spot Band-Aid (Johnson & Johnson, Skillman, NJ) was removed from its adhesive backing with tweezers, and wetted with 40 μl of StrepH solution. The gauze was carefully placed directly over the tatoo site on the skin, and immediately covered with an 18 × 18-mm square of DuoDERM dressing (DuoDERM extra-thin CGF dressing, Convatec; Princeton, NJ). The dressing was removed after 90 minutes. For control sites, the gauze was soaked with PBS instead of with StrepH before covering with DuoDerm. Note that for each experiment a single mouse with four or six skin treatment sites was used, so that each animal served as its own control to minimize effects of interanimal variability.
Histology and Immunohistology
Skin biopsies were fixed in Histochoice (Amresco Inc., Solon, OH) and embedded in paraffin. Five-μm sections were rehydrated and stained with hematoxylin and eosin (H&E) by standard procedures. Before immunofluorescent staining, slides were warmed for 2 hours at 56°C before deparaffinization and rehydration to improve epitope availability. After deparaffinization, specimens were blocked for 30 minutes with 3% normal donkey or goat serum before application of the primary antisera.
For detection of HA, rehydrated specimens were blocked in 3% normal donkey serum, then overlaid with 10 μg/ml of the biotinylated probe (biotinylated HA-binding protein (bHABP), from Seikagaku Ltd) in PBS and 3% donkey serum overnight at 4°C in a humidified chamber. Cy3-conjugated streptavidin (1:500; Jackson Immunoresearch, West Grove, PA) was used to detect the bHABP. Slides were mounted in 30% glycerol. To check the specificity of bHABP binding, some samples were incubated with hyaluronidase from Streptococcus dysgalactiae (from Seikagaku; 250 mU/ml in 0.1 mol/L ammonium acetate buffer, pH 7.0, for 30 minutes at 37°C) to demonstrate a loss of specific HA signal.
For analysis of cell proliferation, specimens were blocked in 3% normal goat serum, then incubated with a rabbit polyclonal antibody against proliferating cell nuclear antigen (PCNA) (1:50; Santa Cruz Biotech, Santa Cruz, CA) overnight at 4°C. Specimens were rinsed several times in PBS, incubated with a biotinylated secondary antibody (goat anti-rabbit IgG, 1:200, Vector Labs, Burlingame, CA) for 10 minutes at room temperature. The PCNA-positive cells were visualized using streptavidin-peroxidase (R.T.U. Vectastain kit, Vector Labs) as instructed by the manufacturer. Slides were mounted in 30% glycerol. Positively stained nuclei were counted and expressed as a fraction of total nuclei in the basal epidermal layer.
For analysis of differentiation marker expression, specimens were re-hydrated and blocked in 3% donkey serum as above, then incubated in rabbit polyclonal antisera raised against mouse K10 (1:100, BABCO, Berkeley, CA) or against rat filaggrin (1:2000, BABCO) at 4°C overnight, rinsed in PBS, then incubated in Cy3-conjugated donkey anti-rabbit IgG (1:1500; Jackson Immunoresearch) for 4 hours at room temperature. For the double-immunofluorescence images in Figure 2, a FITC-conjugated donkey anti-rabbit antisera (1:500; Jackson Immunoresearch) to visualize the polyclonal anti-filaggrin antibody (1:2000), simultaneously with the Cy3-avidin probe used to visualize bHABP (see above). After PBS rinses, slides were mounted in 30% glycerol.
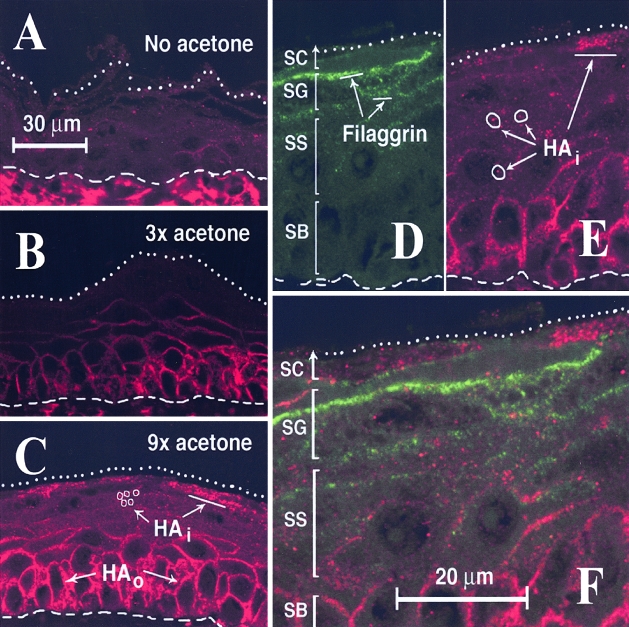
Figure 2-4256.
Barrier disruption induces the accumulation of HA at specific locations in murine epidermis. Sections from the same experiment as in Figure 1 were examined by confocal microscopy to analyze the microanatomic localization of HA in the epidermis after (A) no treatment, (B) three swabs acetone/treatment, or (C) nine swabs acetone/treatment. After acetone-induced barrier disruption, the HA-specific signal (Cy3, red), is markedly increased within the basal layer in the intercellular space outside of the cells (HAo), and is also increased in the upper epidermal layers in granules located inside of cells (HAi). D—F: Co-labeling experiment in which acetone-treated skin (nine swabs) was probed with (D), an antibody to filaggrin (FITC, green) simultaneously with (E), a probe to HA (Cy3, red). From the overlay in (F), no overlap of the two colors can be found, indicating a lack of co-localization. Dashed lines, dermal-epidermal junction. Dotted lines, top of cornified layer, or its visible remnant. SC, stratum corneum; SG, stratum granulosum; SS, stratum spinsosum; SB, stratum basale. Bars, distances shown on the figure.
Image Capture and Quantitative Analysis
Histological specimens, stained with standard H&E or with the immunofluorescent probes, were visualized on an Olympus BX-50 microscope equipped with epifluorescence attachments and a Polaroid DU-DMC2 digital camera. Image processing was done using IPLab Spectrum software (Signal Analytics, Vienna, VA). For all of the analyses described below, at least six images (each containing ∼350 μm of linear epidermis) were evaluated and the average values obtained. For measurement of epidermal thickness, rectangular regions encompassing the living epidermis (bounded by the basement membrane below and the stratum corneum above) were traced on H&E sections using the tracing tool of the software. Epidermal thickness was calculated by dividing the area of the region by the length of the basement membrane, with results expressed as height per unit epidermal length. Pixel values were converted to microns (μm) by a conversion factor calculated from an etched glass calibration slide, photographed at the same time as the tissue specimens. For quantitation of relative changes in K10 expression in Table 1, color digital images of the immunofluorescently stained sections were converted to grayscale. A region encompassing all living epidermal layers within the specimen was drawn with the tracing tool, and the integrated signal intensity within that region was calculated and normalized to epidermal length. Relative changes were expressed as the ratio of integrated signal intensities (StrepH-treated divided by untreated control). To quantify relative changes in the expression of HA or filaggrin expression, a slightly modified method was used because these antigens occupy only a small fraction of the total epidermal area. First, color digital images were converted to grayscale and the intensity threshold that gave an undetectable HA signal in untreated skin was determined. All other images were then set to this same threshold. The software’s paintbrush tool was used to highlight all detectable signal within the living epidermal layers, and the sum of the highlighted areas was normalized to the total area analyzed.
Table 1.
Quantitation of Changes in Markers of Keratinocyte Proliferation and Differentiation in Barrier-Disrupted Skin, in the Absence or Presence of Topically-Applied Streptomyces Hyaluronidase
| Gene marker | Acetone treatment only Mean ± SD (n) | Acetone treatment followed by StrepH Mean ± SD (n) | Difference§ (Student t-test) |
|---|---|---|---|
| PCNA* | 0.46 ± 0.07 (11) | 0.45 ± 0.06 (11) | NS |
| K10† | 1.00 ± 0.14 (10) | 1.64 ± 0.15 (11) | p < 0.05 |
| Filaggrin‡ | 1.00 ± 0.28 (12) | 4.38 ± 0.91 (12) | p < 0.01 |
Values represent measurements of labelling index (PCNA), relative staining intensity (K10), or positive stained area (filaggrin) as described in Materials and Methods. In all cases, data were pooled from two experiments and presented as mean ± standard deviation (n = number of fields analyzed).
Proliferative index, estimated as the relative fraction of PCNA-positive basal keratinocytes relative to total keratinocytes in the basal layer of acetone-treated epidermis. The proliferative index in normal untreated skin was 0.28 ± 0.06 (8).
Keratin 10 expression, estimated by integrated fluorescence intensity per unit area. The control condition (no StrepH) was set at 1.0, and the StrepH-treated value expressed relative to control. Pooled results of two experiments (four mice; five or six images per mouse).
Filaggrin expression, estimated by measuring the area of filaggrin-positive regions within the epidermis (normalized to total area analyzed). Values of StrepH treated skin were expressed relative to controls (no StrepH) set at 1.0. Pooled results of two experiments (four mice, six images per mouse).
The significance of the difference between two conditions (presence versus absence of StrepH) was analyzed by Student t-test, with p-value shown. NS, not significant.
In Figure 2, confocal images were obtained using a Leica TCS-SP laser-scanning confocal microscope (Leica, Heidelberg, Germany) designed with three lasers and photodetectors to facilitate detection of three different fluorochromes.
Results
Selection of Acetone-Swabbing as the Method of Choice for the Studies
The goal of this study was to test the hypothesis that HA in the epidermis may play a functional role in regulating epidermal hyperplasia that occurs in response to superficial barrier disruption. To ask this question, we turned to a well-established model for disrupting the integrity of the stratum corneum, namely, the repeated application of adhesives (tape stripping) or organic solvents (acetone) to the skin for several days, which causes epidermal thickening (ie, callus formation) in mice and humans.31–33,37 To incite epidermal hyperplasia, the skin of hairless mice was either tape-stripped or swabbed with acetone (as described in Materials and Methods) twice daily for 5 days, and then biopsied to assess changes in epidermal and other parameters. Treatment sites were marked by placement of dermal tatoos (India ink) 1 week before the experiment, to allow reproducible placement of acetone or other treatments, and also to ensure that treated locations could be confirmed in subsequent histological sections. In pilot experiments, tape stripping at the tatooed sites produced significant hyperplasia but also led to shallow erosions and ulcerations, apparently from increased irritation and scratching. Acetone applications stimulated hyperplasia but with little or no epidermal breakdown. Therefore, all subsequent experiments were performed with acetone.
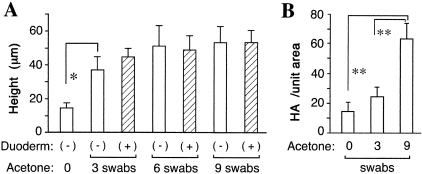
When epidermal thickening (acanthosis) was quantitatively evaluated in H&E-stained sections, we found that three swabs of acetone per treatment gave a highly significant hyperplastic response, with successive increases in thickness observed using six swabs or nine swabs (Figure 1, open bars). Because the degree of acanthosis using six or nine swabs was not statistically different from three swabs, and because the mice began to display cutaneous erosions with the nine-swab regimen, we chose to use six swabs of acetone as our standard method for inducing hyperplasia for most further studies.
Figure 1-4256.
Dose-response analysis for (A) acetone-induced epidermal hyperplasia, and (B) increases in epidermal HA associated with barrier disruption. A single hairless SKH-1 mouse was treated with acetone (twice-daily applications for 5 days) at six tatooed sites on the back as described in Materials and Methods. For each treatment (acetone), an acetone-soaked cotton-tipped applicator was pressed against the skin at duplicate sites, either no times, three times, or nine times in succession. For each acetone condition, one area of skin was covered with Duoderm dressing for 90 minutes following every treatment (hatched bars), while the other area was left uncovered (open bars). Skin biopsies were performed at the end of 5 days. A: Paraffin sections were stained with hematoxylin and eosin, microscopic images photographed digitally, and epidermal thickness (height in μm) evaluated using the imaging processing technique described in Materials and Methods. Each bar represents the mean ± SD of six high-power fields (magnification, ×400). Asterisk, difference from untreated area of skin is statistically significant by Student’s t-test, P < 0.005. B: Sections were stained with the bHABP probe, detected by Cy3-avidin fluorescence on an Olympus microscope equipped for epifluorescence detection, and the intensity of the HA-specific signal estimated by digital image analysis as described in Materials and Methods. Each bar represents the mean ± SD of five high-power fields. Paired comparisons marked with double asterisks are significantly different by Student’s t-test, P < 0.0005.
Disruption of the Stratum Corneum Leads to an Accumulation of Epidermal HA Both Extracellularly and Intracellularly
To determine whether barrier disruption causes any change in the intraepidermal content of HA, mice were subjected to increasing amounts of acetone treatment and the relative levels of HA in skin were examined by confocal microscopy using a specific binding probe to HA (Figure 2). In uninjured skin, high levels of HA were seen in the dermis (below the basement membrane) as expected, whereas HA levels between keratinocytes of the epidermis were barely detectable (Figure 2A). After acetone treatment however, epidermal HA accumulated to high levels (Figure 2, B and C). The pattern of HA staining was essentially the same whether three, six, or nine swabs were applied, but was significantly more intense with the use of nine swabs (Figure 2C; see Figure 1B for quantitation). An interesting dichotomy was observed in the epidermal-staining pattern of HA. In the lower epidermis, intense extracellular staining was observed outside and between keratinocytes of the basal and lower spinous layers (HAo in Figure 2C), while in the upper epidermis an intracellular accumulation of HA in cytoplasmic granules was seen (HAi in Figure 2, C and E). To rule out the possibility that the observed intracellular HA might be associated with keratohyalin granules (the most abundant feature in the stratum granulosum), skin sections were double-stained with the HA-binding probe and an antiserum to filaggrin, the principal component of the keratohyalin granule.39 The results showed a complete lack of colocalization of HA and filaggrin in the epidermis, suggesting that intracellular HA granules are distinct from keratohyalin granules (Figure 2, D to F).
Exogenous Hyaluronidase, Purified from Streptomyces Bacteria, Can Be Used as an Experimental Means to Degrade HA in the Epidermis
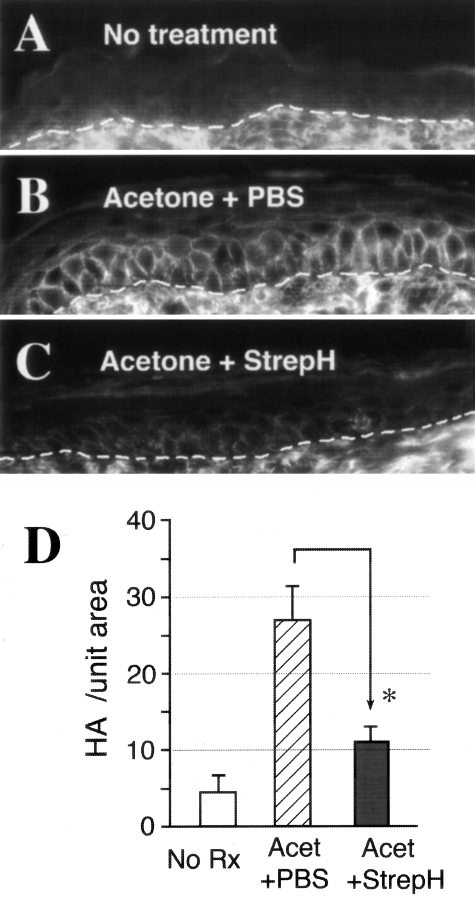
In our previous experimental work using an organotypic epidermal model, we had established that HA in the matrix between keratinocytes can be degraded using the highly HA-specific bacterial enzyme, StrepH.22 In those studies, a concentration of 1 U/ml StrepH in the culture media was sufficient to completely degrade HA. However, for the delivery of StrepH through murine skin in vivo, we predicted that a higher concentration would be needed. Based on the work of Laugier et al,40 in which 10 and 100 U/ml StrepH was reported to permeate the stratum corneum and degrade HA in cultured explants of human skin, we performed pilot experiments in which 10 U/ml and 100 U/ml of StrepH were applied to murine skin under a variety of dressings. The final regimen selected was to place a saline-soaked, 1-cm circular piece of gauze containing StrepH (10 U/ml) against the skin for 90 minutes under a semi-permeable dressing (Duoderm), as described in Materials and Methods. Results are shown in Figure 3. Epidermal HA levels were induced by barrier disruption (compare Figure 3B with 3A), and were markedly reduced when StrepH was applied after acetone treatments (Figure 3C). Quantitative analysis of the HA signal revealed an ∼50% decrease in epidermal HA overall, a difference that was statistically significant (Figure 3D). In StrepH-treated skin, an increased level of HA was sometimes observed in the dermis concurrently with the decrease in HA in the epidermis, an interesting trend but not seen in all experiments.
Figure 3-4256.
Accumulation of HA in acetone-treated epidermis is significantly reduced by topical application of the enzyme StrepH for 90 minutes immediately after each barrier disruption treatment with acetone (six swabs). Paraffin-fixed sections were stained with the HA-specific probe (bHABP) and Cy3-avidin, with the intensity of the fluorescent signal roughly proportional to levels of HA in the specimen. Representative images are shown: (A), untreated skin. (B), skin treated with acetone followed by saline under Duoderm. (C), skin treated with acetone followed by StrepH (10 U/ml) under Duoderm for 90 minutes. (D), quantitative analysis of the HA signal intensity for each condition, performed as described in Materials and Methods. Open bar, no treatment; hatched bar, acetone followed by saline, solid bar, acetone followed by StrepH. Values represent the mean ± SD of seven high-power fields (×400) from each biopsy site on the mouse. Asterisk, difference statistically significant by Student’s t-test, P < 0.005.
Depletion of Epidermal HA via Topical Delivery of Hyaluronidase Inhibits the Epidermal Hyperplastic Response to Barrier Disruption
Before conducting experiments to test the effects of topical StrepH on acetone-induced hyperplasia, it was necessary to ask whether the topical delivery method itself (occlusion with a vapor-retardant dressing) might block the permeability-related stimulus that triggers hyperplasia after barrier disruption, and thereby block epidermal thickening.41 In the pilot experiments shown in Figure 1, we had found that occlusion of the skin with Duoderm immediately after acetone disruption of the permeability barrier did not affect the hyperplastic response of the epidermis (Figure 1, hatched bars). Therefore, the Duoderm-covered-gauze technique was an appropriate means for delivery of StrepH into the epidermis in experiments to test the enzyme’s effect on acetone-induced hyperplasia.
Figure 4, A to D shows the results of a typical experiment in which the acetone-induced hyperplasia response was measured in the presence or absence of the HA-degrading enzyme, StrepH. Compared to a control site that received no acetone (Figure 4A), a large epidermal hyperplastic response (>3-fold) was seen after repeated application (×6) of acetone alone (Figure 4B) or acetone followed by 90 minutes of occlusion with Duoderm/saline (Figure 4C). Interestingly, when acetone was followed by StrepH, epidermal thickening was reduced by half (Figure 4D). The difference in epidermal height between the conditions of Figure 4, C and D was statistically significant.
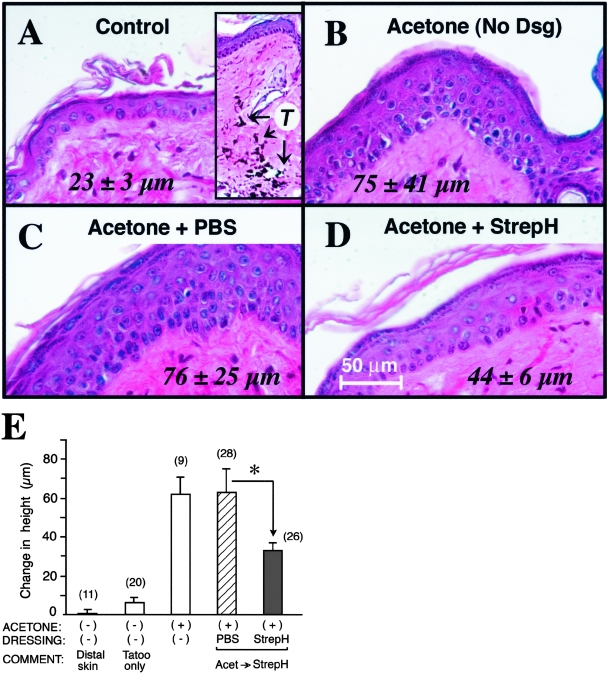
Figure 4-4256.
Epidermal hyperplasia in response to barrier disruption is significantly blunted in the presence of topically-applied StrepH enzyme. Hematoxylin and eoxin-stained sections of skin from a representative experiment. A: No acetone. Inset; low-power view (magnification, ×100) to illustrate the tatoo ink (T) that was visible at every treatment site. B: Acetone without occlusion. C: Acetone followed by PBS under Duoderm. D: Acetone followed by StrepH (10 U/ml) under Duoderm. Numbers in the panels represent epidermal thickness (μm), mean ± SD from seven high-power fields (×400). The difference between mean thickness values in C and D is statistically significant by Student’s t-test, P < 0.005. (E), Epidermal thickness data pooled from three experiments. When skin was occluded under a topical dressing containing PBS control (hatched bars) or StrepH (solid bars), acetone swabbing was followed by application of the topical agents. Data are presented as change in thickness (μm; normal skin value of 13 μm subtracted at each point), mean ± SEM The number in parentheses above each bar is the total number of high-power fields (×400) analyzed per point, pooled from three experiments (total of three skin biopsies per condition). Asterisk, difference is significant by Student’s t-test, P < 0.005. Bar, 50 μm.
Figure 4E displays the combined results from three experiments, with data presented as change in epidermal thickness relative to normal skin. Tatoo placement, required to mark each treatment site, itself induced a slight hyperplasia (∼50% increase over the normal thickness of 13 μm; second bar), but this change was relatively small compared to the thickening caused by acetone alone, or by acetone followed by Duoderm/saline (an ∼500% increase; third and fourth bar). The presence of StrepH under Duoderm for 90 minutes after acetone-swabbing inhibited the epidermal thickening response, a reduction that was highly significant (Figure 4E, compare fourth and fifth bar). Overall, the results suggest that loss of intact HA immediately following barrier disruption interferes in some manner with the epidermal hyperplastic response.
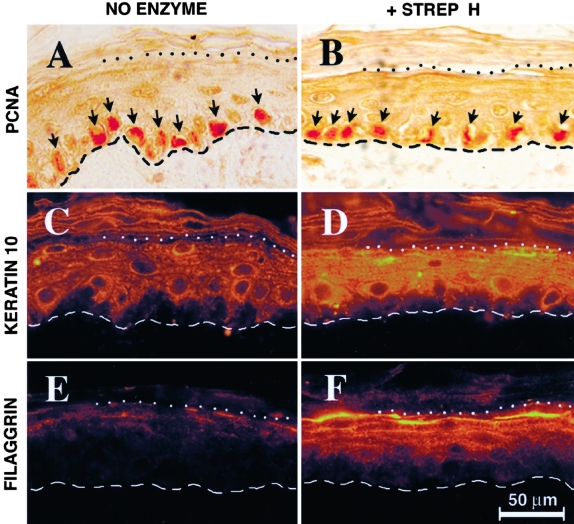
Digestion of HA by StrepH Results in a Thinner Epidermis, Not Due to Any Effect on Cell Proliferation, But Rather through an Enhancement of Terminal Differentiation
In an effort to explain the mechanism whereby acetone-stimulated epidermis appears relatively thinner if treated with topical StrepH, as compared to saline vehicle alone, two formal possibilities warrant consideration. First, StrepH might lessen the rate of epidermal cell proliferation, or second, keratinocytes in the StrepH-treated epidermis might complete their genetic program of upward migration and terminal differentiation sooner, undergoing cell death and desquamation more quickly. Either scenario would result in a thinner epidermis at equilibrium. To distinguish between these possibilities, biopsy specimens from control or StrepH-digested areas of acetone-treated skin were immunostained for markers of proliferation (PCNA), early-stage differentiation (K10), or late-stage differentiation (filaggrin). PCNA-labeling in acetone-injured skin was increased nearly twofold relative to quiescent normal skin (Table 1), as previously shown by others.31 However, when focusing on acetone-stimulated hyperplastic skin, StrepH had no demonstrable effect on keratinocyte proliferation (Figure 5, A and B, and Table 1). In contrast, the presence of StrepH had a strong effect on the staining intensity of an early marker of differentiation, K10, resulting in a statistically significant increase in K10 expression in StrepH-treated skin (Figure 5D) relative to saline controls (Figure 5C; also see Table 1). Similarly, StrepH-treated epidermis displayed a significant increase in the expression of the late terminal differentiation marker, filaggrin, relative to control epidermis (Figure 5, E and F). In conclusion, digestion of epidermal HA by StrepH appears to enhance the expression of terminal differentiation markers in the epidermis, correlating with a probable acceleration in the loss of keratinocytes via desquamation and a thinner epithelial tissue as a result.
Figure 5-4256.
StrepH has no effect on keratinocyte proliferation, but instead enhances terminal differentiation, in acetone-treated skin. Representative immunostained sections are shown in pairs, with acetone/saline controls on the left and acetone/StrepH-treated skin on the right, as follows: A and B, PCNA stain; C and D, keratin 10 stain; E and F, filaggrin stain. Bar, 50 μm. Dashed lines, dermal-epidermal junction. Dotted lines, junction between viable epidermal layers and stratum corneum.
Discussion
In this paper, we have shown that an abundant but previously unappreciated component of the extracellular matrix in mammalian epidermis, a long-chain sugar molecule called hyaluronan (HA), has an important regulatory role in the tissue response to injury that occurs after epidermal barrier disruption. This response, characterized by epidermal hyperplasia, has been previously well-described for mouse and human skin whose stratum corneum has been disrupted by agents that dissolve lipids between the corneocytes (eg, organic solvents, detergents, or adhesive tape).29–33,38,41 The ensuing loss of the vapor permeability barrier is thought to trigger cytokine release from keratinocytes, leading to cell surface receptor-mediated signaling cascades that initiate a hyperproliferative response in the tissue, detectable by an increase in DNA synthesis or PCNA-labeling (as seen for example in Figure 5, A and B of our study). The new aspect described here is a demonstration that changes in the physical state of the extracellular matrix, specifically in the status of HA, can have a profound influence on the behavior of keratinocytes in terms of the cellular physiology involved in generating a normal hyperplastic response.
Much evidence from the literature suggests that HA may be pivotal in controlling epidermal responses to injury, including the previously-described involvement of HA in keratinocyte migration and proliferation in various wound-healing settings (see Introduction). Interestingly, data from recent experiments in which keratinocytes were grown in organotypic lift cultures, a model of re-epithelialization, have shown that removal of intercellular HA from the matrix by digestion with StrepH added to the cell culture medium, leads to an enhancement of keratinocyte terminal differentiation, with little or no effect on cell proliferation.22 Those in vitro studies prompted us to examine the role of HA in epidermal responses to injury in vivo, using the murine barrier-disruption model popularized by Feingold et al.31,41 Since relatively little HA is found in normal, undisturbed murine epidermis (Tammi R, personal communication; and this study), the large accumulation of epidermal HA seen after barrier injury (Figures 1 and 3) is particularly intriguing because such changes imply a role for HA in mediating post-injury events. Such evidence, however, is merely correlative. To directly test for functional involvement of HA in the hyperproliferative response, we adapted the enzymatic technique used previously in the study with the cultured organotypic model,22 and developed a method to deliver the StrepH enzyme topically under an occlusive dressing to degrade intact HA in the native skin. Despite the fact that the StrepH enzyme could not be delivered continuously, but instead was applied in two divided doses for a total of 3 hours per day, a significant ∼50% reduction in intraepidermal HA accumulation was nevertheless observed at the end of the 5-day study period (Figure 3). This relative reduction in HA coincided with a proportional ∼50% reduction in overall epidermal thickening over the same period (Figure 4).
To attempt to explain how the StrepH-mediated loss of HA might be reducing overall acetone-induced epidermal hyperplasia, we examined keratinocyte markers of proliferation and differentiation. Somewhat surprisingly, no reduction in proliferative index (PCNA-labeling) was observed when HA was removed from the matrix. This finding might seem counterintuitive at first, considering that hyperproliferation is a well-known response to acetone-induced barrier injury.31 In our experiments, epidermal keratinocyte proliferation was in fact stimulated in response to acetone, increasing by ∼150% (Table 1, footnote 1). However, with the addition of StrepH after acetone, the resulting loss of HA from the intercellular matrix did not prevent the acetone-induced stimulation of keratinocyte proliferation (Table 1, PCNA). Apparently, the mechanism by which barrier disruption stimulates keratinocyte proliferation does not require the presence of HA, at least not when examined carefully in the epidermis in vivo.
Given that a loss of intercellular HA does not seem to blunt keratinocyte proliferation in response to acetone, then why was the overall epidermal thickness reduced in our experiments? We observed no gross changes in the average size of keratinocytes, nor in the apparent spacing between the cells, that could account for the thinner epidermis in StrepH-treated skin. In search for an answer to the question, we noted that StrepH treatment differentially enhanced the expression of K10 and filaggrin (markers of early and late differentiation, respectively) in the acetone-stimulated epidermis. This provides an interesting potential explanation for the relatively thinner epidermis after StrepH treatment. Specifically, we propose the working hypothesis that in the absence of intact HA, the keratinocyte differentiation program in the acetone-injured epidermis is shifted toward the formation of terminally-differentiated cells, resulting in a new dynamic equilibrium that features a less hyperplastic epidermal tissue. Much more work will be needed to prove the hypothesis, including transit-time measurements of pulse-labeled cells and measurements of differential changes in stratum corneum structure and function, which are beyond the scope of this study.
While provocative, our finding of an important role for HA in modulating keratinocyte differentiation in murine skin agrees nicely with our previous findings in an organotypic epidermal model,22 in which loss of epidermal HA led to an enhanced (premature) differentiation of keratinocytes. K10 and filaggrin are considered early and late markers of differentiation, respectively, because K10 is first expressed in the stratum spinosum42 whereas filaggrin expression occurs much later, in the stratum granulosum.39 In the in vitro epidermal model, StrepH-treated cultures showed a strong enhancement in expression of both K10 and filaggrin, confirmed by two independent methods (immunohistochemistry and Western blot analysis).22 In the present study, murine skin in vivo shows very similar increases in epidermal K10 and filaggrin expression, using the same immunohistochemical methods as before. We conclude that the presence of intact HA is important for the keratinocyte differentiation program in barrier-disrupted skin, and that perturbation of HA in the matrix can have functional consequences (relatively premature differentiation and a less hyperplastic tissue).
How the removal of HA exerts the observed changes in epidermal cell differntiation, and what significance this may have for our understanding of the physiology of HA, remains an open question. For example, it is entirely possible that experimentally produced fragments of HA (cleaved by the StrepH enzyme) could be biologically active,43 perhaps by competing with intact HA for binding to the HA-specific membrane receptor (called CD44) on keratinocytes. StrepH, a microbe-derived hyaluronan lyase that selectively destroys HA with minimal effect on other glycosaminoglycans such as chondroitin sulfate,44,45 may or may not mimic the effects of native hyaluronidases in the epidermis. Three different HYAL enzymes (HYAL-1, -2, and -3) have been described in mammalian tissues,46 and while still incompletely understood, they are known to have a very different mechanism of action (hydrolases, as opposed to lyases), to operate better at an acidic pH, and in some instances to have membrane-bound GPI-anchors, in contrast to the microbial lyases. One of the mammalian enzymes, HYAL2, may be implicated in a mechanism whereby HA is bound to CD44 at the cell surface, cleaved into fragments roughly 20 kd in size, and internalized in vesicles just beneath the plasma membrane before being transported to lysosomes for more complete degradation.47 The HA-positive intracellular structures seen in the upper layers of skin, ie, stratum spinosum, granulosum, and corneum (Figure 2), shown by our study to be distinct from keratohyalin granules, may instead represent the vesicles containing HA-degradation products as described by Tammi et al.47 (Interestingly, HA in the stratum corneum is probably not an artifact, having been confirmed previously using different methods48). On the other hand, new work suggests that under conditions of cellular stress and injury, HA may be synthesized within cells,49 and could thereby accumulate via an internal mechanism. Internal HA synthesis, however, is still controversial, whereas synthesis and extrusion of HA into the extracellular matrix through the action of HA-synthetic enzyme complexes assembled in the plasma membrane, is now well-substantiated.3,50 Three HA-synthases are currently cloned in mammals,50 with HAS2 being the most abundant in keratinocytes.51 Accumulation of HA in the lower epidermis within extracellular spaces, after acetone-induced injury, might conceptually be due to increased HAS2 activity, or to decreased HA turnover. The ability to distinguish between increased synthesis versus decreased degradation of HA must await future studies regarding expression levels and activities of the major epidermal hyaluronan synthases and hyaluronidases.
Acknowledgments
We thank Dr. Hiroko Kawamura for some of the initial pilot work on tape-stripping and tatooing, Dr. Ken Feingold for advice on barrier disruption, and Dr. Vincent Hascall and Dr. Raija Tammi for their helpful discussions on the role of HA in epidermal biology. We also thank Dr. Judy Drazba in the Digital Imaging Core of the Lerner Research Institute for the confocal microscopy images in Figure 2.
Footnotes
Address reprint requests to Edward Maytin, M.D., Ph.D., Biomedical Engineering/ND-20, Cleveland Clinic Foundation, 9500 Euclid Avenue, Cleveland, OH 44195. E-mail: maytine@ccf.org.
References
- Hascall VC, Laurent TC: Hyaluronan: structure and physical properties. Glycoforum/Science of Hyaluronan Review Series. Edited by Hascall VC, Yanagishita M. 1997, http://www.glycoforum.gr.jp/science/hyaluronan/HA01/HA01E.html [Google Scholar]
- Toole BP, Wight TN, Tammi MI. Hyaluronan-cell interactions in cancer and vascular disease. J Biol Chem. 2002;277:4593–4596. doi: 10.1074/jbc.R100039200. [DOI] [PubMed] [Google Scholar]
- Lee JY, Spicer AP. Hyaluronan: a multifunctional, megaDalton, stealth molecule. Curr Opin Cell Biol. 2000;12:581–586. doi: 10.1016/s0955-0674(00)00135-6. [DOI] [PubMed] [Google Scholar]
- Noble PW. Hyaluronan and its catabolic products in tissue injury and repair. Matrix Biol. 2002;21:25–29. doi: 10.1016/s0945-053x(01)00184-6. [DOI] [PubMed] [Google Scholar]
- Tammi MI, Day AJ, Turley EA. Hyaluronan and homeostasis: a balancing act. J Biol Chem. 2002;277:4581–4584. doi: 10.1074/jbc.R100037200. [DOI] [PubMed] [Google Scholar]
- Turley EA, Noble PW, Bourguignon LY. Signaling properties of hyaluronan receptors. J Biol Chem. 2002;277:4589–4592. doi: 10.1074/jbc.R100038200. [DOI] [PubMed] [Google Scholar]
- Toole BP. Hyaluronan in morphogenesis. J Intern Med. 1997;242:35–40. doi: 10.1046/j.1365-2796.1997.00171.x. [DOI] [PubMed] [Google Scholar]
- Longaker MT, Adzick NS, Hall JL, Stair SE, Crombleholme TM, Duncan BW, Bradley SM, Harrison MR, Stern R. Studies in fetal wound healing: VII Fetal wound healing may be modulated by hyaluronic acid stimulating activity in amniotic fluid. J Pediatr Surg. 1990;25:430–433. doi: 10.1016/0022-3468(90)90387-o. [DOI] [PubMed] [Google Scholar]
- Chen WY, Abatangelo G. Functions of hyaluronan in wound repair. Wound Repair Regen. 1999;7:79–89. doi: 10.1046/j.1524-475x.1999.00079.x. [DOI] [PubMed] [Google Scholar]
- Rilla K, Lammi MJ, Sironen R, Torronen K, Luukkonen M, Hascall VC, Midura RJ, Hyttinen M, Pelkonen J, Tammi M, Tammi R. Changed lamellipodial extension, adhesion plaques, and migration in epidermal keratinocytes containing constitutively expressed sense and antisense hyaluronan synthase 2 (Has2) genes. J Cell Sci. 2002;115:3633–3643. doi: 10.1242/jcs.00042. [DOI] [PubMed] [Google Scholar]
- de La Motte CA, Hascall VC, Calabro A, Yen-Lieberman B, Strong SA. Mononuclear leukocytes preferentially bind via CD44 to hyaluronan on human intestinal mucosal smooth muscle cells after virus infection or treatment with poly(IC) J Biol Chem. 1999;274:30747–30755. doi: 10.1074/jbc.274.43.30747. [DOI] [PubMed] [Google Scholar]
- de La Motte CA, Hascall VC, Drazba J, Bandyopadhyay SK, Strong SA. Mononuclear leukocytes bind to specific hyaluronan sturctures on colon mucosal smooth muscle cells treated with polyinosinic acid: polycytidylic acid. Am J Pathol. 2003;163:121–133. doi: 10.1016/s0002-9440(10)63636-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollyfield JG, Rayborn ME, Tammi M, Tammi R. Hyaluronan in the interphotoreceptor matrix of the eye: species differences in content, distribution, ligand binding, and degradation. Exp Eye Res. 1998;66:241–248. doi: 10.1006/exer.1997.0422. [DOI] [PubMed] [Google Scholar]
- Tammi R, Ripellino JA, Margolis RU, Tammi M. Localization of epidermal hyaluronic acid using the hyaluronate binding region of cartilage proteoglycan as a specific probe. J Invest Dermatol. 1988;90:412–414. doi: 10.1111/1523-1747.ep12456530. [DOI] [PubMed] [Google Scholar]
- Tammi R, Tammi M. Correlations between hyaluronan and epidermal proliferation as studied by [3H]glucosamine and [3H]thymidine incorporations and staining of hyaluronan on mitotic keratinocytes. Exp Cell Res. 1991;195:524–527. doi: 10.1016/0014-4827(91)90405-j. [DOI] [PubMed] [Google Scholar]
- Wells AF, Lundin A, Michaelsson G. Histochemical localization of hyaluronan in psoriasis, allergic contact dermatitis, and normal skin. Acta Dermatol Venereol. 1991;71:232–238. [PubMed] [Google Scholar]
- Sakai S, Yasuda R, Sayo T, Ishikawa O, Inoue S. Hyaluronan exists in the normal stratum corneum. J Invest Dermatol. 2000;114:1184–1187. doi: 10.1046/j.1523-1747.2000.00992.x. [DOI] [PubMed] [Google Scholar]
- Tammi R, Ripellino JA, Margolis RU, Maibach HI, Tammi M. Hyaluronate accumulation in human epidermis treated with retinoic acid in skin organ culture. J Invest Dermatol. 1989;92:326–332. doi: 10.1111/1523-1747.ep12277125. [DOI] [PubMed] [Google Scholar]
- Agren UM, Tammi M, Tammi R. Hydrocortisone regulation of hyaluronan metabolism in human skin organ culture. J Cell Physiol. 1995;164:240–248. doi: 10.1002/jcp.1041640204. [DOI] [PubMed] [Google Scholar]
- Kaya G, Rodriguez I, Jorcano JL, Vassalli P, Stamenkovic I. Selective suppression of CD44 in keratinocytes of mice bearing an antisense CD44 transgene driven by a tissue-specific promoter disrupts hyaluronate metabolism in the skin and impairs keratinocyte proliferation. Genes Dev. 1997;11:996–1007. doi: 10.1101/gad.11.8.996. [DOI] [PubMed] [Google Scholar]
- Kaya G, Augsburger E, Stamenkovic I, Saurat JH. Decrease in epidermal CD44 expression as a potential mechanism for abnormal hyaluronate accumulation in superficial dermis in lichen sclerosus et atrophicus. J Invest Dermatol. 2000;115:1054–1058. doi: 10.1046/j.1523-1747.2000.00194.x. [DOI] [PubMed] [Google Scholar]
- Passi A, Sadeghi P, Kawamura H, Anand S, Sato N, White LE, Hascall VC, Maytin EV. Hyaluronan suppresses epidermal differentiation in organotypic cultures of rat keratinocytes. Exp Cell Res. 2004;296:123–134. doi: 10.1016/j.yexcr.2004.01.031. [DOI] [PubMed] [Google Scholar]
- Oksala O, Salo T, Tammi R, Hakkinen L, Jalkanen M, Inki P, Larjava H. Expression of proteoglycans and hyaluronan during wound healing. J Histochem Cytochem. 1995;43:125–135. doi: 10.1177/43.2.7529785. [DOI] [PubMed] [Google Scholar]
- Longaker MT, Chiu ES, Adzick NS, Stern M, Harrison MR, Stern R. Studies in fetal wound healing: V A prolonged presence of hyaluronic acid characterizes fetal wound fluid. Ann Surg. 1991;213:292–296. doi: 10.1097/00000658-199104000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack JA, Abramson SR, Ben Y, Coffin JC, Rothrock JK, Maytin EV, Hascall VC, Largman C, Stelnicki EJ. Hoxb13 knockout adult skin exhibits high levels of hyaluronan and enhanced wound healing. FASEB J. 2003;17:1352–1354. doi: 10.1096/fj.02-0959fje. [DOI] [PubMed] [Google Scholar]
- Clark RAF, editor. Kluwer Academic/Plenum Publishers,; New York: The Molecular and Cellular Biology of Wound Repair, (ed 2) 1996:pp 1–611. [Google Scholar]
- Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999;341:738–746. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- Woodley DT. Reepithelialization. Clark RAF, editor. Kluwer Academic/Plenum Publishers,; New York: The Molecular and Cellular Biology of Wound Repair. 1996:pp 339–350. [Google Scholar]
- Wood LC, Elias PM, Calhoun C, Tsai JC, Grunfeld C, Feingold KR. Barrier disruption stimulates interleukin-1 alpha expression and release from a pre-formed pool in murine epidermis. J Invest Dermatol. 1996;106:397–403. doi: 10.1111/1523-1747.ep12343392. [DOI] [PubMed] [Google Scholar]
- Tsai JC, Feingold KR, Crumrine D, Wood LC, Grunfeld C, Elias PM. Permeability barrier disruption alters the localization and expression of TNF alpha/protein in the epidermis. Arch Dermatol Res. 1994;286:242–248. doi: 10.1007/BF00387595. [DOI] [PubMed] [Google Scholar]
- Proksch E, Holleran WM, Menon GK, Elias PM, Feingold KR. Barrier function regulates epidermal lipid and DNA synthesis. Br J Dermatol. 1993;128:473–482. doi: 10.1111/j.1365-2133.1993.tb00222.x. [DOI] [PubMed] [Google Scholar]
- Taljebini M, Warren R, Mao-Oiang M, Lane E, Elias PM, Feingold KR. Cutaneous permeability barrier repair following various types of insults: kinetics and effects of occlusion. Skin Pharmacol. 1996;9:111–119. doi: 10.1159/000211406. [DOI] [PubMed] [Google Scholar]
- Yang L, Mao-Qiang M, Taljebini M, Elias PM, Feingold KR. Topical stratum corneum lipids accelerate barrier repair after tape stripping, solvent treatment, and some but not all types of detergent treatment. Br J Dermatol. 1995;133:679–685. doi: 10.1111/j.1365-2133.1995.tb02738.x. [DOI] [PubMed] [Google Scholar]
- Elias PM, Friend DS. The permeability barrier in mammalian epidermis. J Cell Biol. 1975;65:180–191. doi: 10.1083/jcb.65.1.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias PM, Wood LC, Feingold KR. Epidermal pathogenesis of inflammatory dermatoses. Am J Contact Dermat. 1999;10:119–126. [PubMed] [Google Scholar]
- Elias PM, Feingold KR. Does the tail wag the dog? Role of the barrier in the pathogenesis of inflammatory dermatoses and therapeutic implications. Arch Dermatol. 2001;137:1079–1081. [PubMed] [Google Scholar]
- Ghadially R, Reed JT, Elias PM. Stratum corneum structure and function correlates with phenotype in psoriasis. J Invest Dermatol. 1996;107:558–564. doi: 10.1111/1523-1747.ep12582813. [DOI] [PubMed] [Google Scholar]
- Jackson SM, Williams ML, Feingold KR, Elias PM. Pathobiology of the stratum corneum. West J Med. 1993;158:279–285. [PMC free article] [PubMed] [Google Scholar]
- Fisher C, Haydock PV, Dale BA. Localization of profilaggrin mRNA in newborn rat skin by in situ hybridization. J Invest Dermatol. 1987;88:661–664. doi: 10.1111/1523-1747.ep12470281. [DOI] [PubMed] [Google Scholar]
- Laugier JP, Shuster S, Rosdy M, Csoka AB, Stern R, Maibach HI. Topical hyaluronidase decreases hyaluronic acid and CD44 in human skin and in reconstituted human epidermis: evidence that hyaluronidase can permeate the stratum corneum. Br J Dermatol. 2000;142:226–233. doi: 10.1046/j.1365-2133.2000.03289.x. [DOI] [PubMed] [Google Scholar]
- Grubauer G, Elias PM, Feingold KR. Transepidermal water loss: the signal for recovery of barrier structure and function. J Lipid Res. 1989;30:323–333. [PubMed] [Google Scholar]
- Maytin EV, Lin JC, Krishnamurthy R, Batchvarova N, Ron D, Mitchell PJ, Habener JF. Keratin 10 gene expression during differentiation of mouse epidermis requires transcription factors C/EBP and AP-2. Dev Biol. 1999;216:164–181. doi: 10.1006/dbio.1999.9460. [DOI] [PubMed] [Google Scholar]
- Tammi R, Saamanen AM, Maibach HI, Tammi M. Degradation of newly synthesized high molecular mass hyaluronan in the epidermal and dermal compartments of human skin in organ culture. J Invest Dermatol. 1991;97:126–130. doi: 10.1111/1523-1747.ep12478553. [DOI] [PubMed] [Google Scholar]
- Ohya T, Kaneko Y. Novel hyaluronidase from Streptomyces. Biochim Biophys Acta. 1970;198:607–609. doi: 10.1016/0005-2744(70)90139-7. [DOI] [PubMed] [Google Scholar]
- Suzuki S: Microbial hyaluronan lyases. Glycoforum/Science of Hyaluronan Review Series. Edited by Hascall VC, Yanagishita M. 2000, http://www.glycoforum.gr.jp/science/hyaluronan/HA14/HA14E.html [Google Scholar]
- Stern R, Csoka AB: Mammalian hyaluronidases. Glycoforum/Science of Hyaluronan Review Series. Edited by Hascall VC, Yanagishita M. 2000, http://www.glycoforum.gr.jp/science/hyaluronan/HA15/HA15E.html [Google Scholar]
- Tammi R, Rilla K, Pienimaki JP, MacCallum DK, Hogg M, Luukkonen M, Hascall VC, Tammi M. Hyaluronan enters keratinocytes by a novel endocytic route for catabolism. J Biol Chem. 2001;276:35111–35122. doi: 10.1074/jbc.M103481200. [DOI] [PubMed] [Google Scholar]
- Sakai S, Yasuda R, Sayo T, Ishikawa O, Inoue S. Hyaluronan exists in the normal stratum corneum. J Invest Dermatol. 2000;114:1184–1187. doi: 10.1046/j.1523-1747.2000.00992.x. [DOI] [PubMed] [Google Scholar]
- de la Motte CA, Drazba J, Banyopadhyay S, Majors A, Hascall VC, Strong SA: Viral stimuli induce novel hyaluronan cable structures on colon smooth muscle cells that bind leukocytes externally and nuclei internally. Conference Proceedings from Hyaluronan 2003, An International Symposium. Edited by Balasz E, Hascall VC. Cleveland OH, in press [Google Scholar]
- Spicer NP, McDonald JA: Eukaryotic hyaluronan synthases. Glycoforum/Science of Hyaluronan Review Series. 1998, http://www.glycoforum.gr.jp/science/hyaluronan/HA07/HA07E.html [Google Scholar]
- Pienimaki JP, Rilla K, Fulop C, Sironen RK, Karvinen S, Pasonen S, Lammi MJ, Tammi R, Hascall VC, Tammi MI. Epidermal growth factor activates hyaluronan synthase 2 in epidermal keratinocytes and increases pericellular and intracellular hyaluronan. J Biol Chem. 2001;276:20428–20435. doi: 10.1074/jbc.M007601200. [DOI] [PubMed] [Google Scholar]