Abstract
Herpes simplex virus types 1 and 2 (HSV-1 and HSV-2), varicella-zoster virus (VZV), cytomegalovirus (CMV), Epstein-Barr virus (EBV), human herpesvirus 6 (HHV-6), and human herpesvirus 8 (HHV-8) are responsible for a number of clinical manifestations in both normal and immunocompromised individuals. The parent benzimidazole ribonucleosides evaluated in this series, 2-bromo-5,6-dichloro-1-(β-d-ribofuranosyl)benzimidazole (BDCRB) and maribavir (1263W94), are potent and selective inhibitors of human CMV replication. These nucleosides act by two different mechanisms. BDCRB blocks the processing and maturation of viral DNA, whereas 1263W94 inhibits the viral enzyme pUL97 and interferes with DNA synthesis. In the present study, we have evaluated the in vitro antiviral activity of BDCRB, an analog, GW275175X (175X), and 1263W94 against the replication of HSV-1, HSV-2, VZV, CMV, EBV, HHV-6, and HHV-8. By using various methodologies, significant activity was observed against human CMV and EBV but not against HSV-1, HSV-2, VZV, HHV-6, or HHV-8. Plaque reduction assays performed on a variety of laboratory and clinical isolates of human CMV indicated that all strains, including those resistant to ganciclovir (GCV) and foscarnet, were sensitive to all three benzimidazole ribonucleosides, with mean 50% effective concentration values of about 1 to 5 μM compared to that of GCV at 6 μM. The toxicity of these compounds in tissue culture cells appeared to be similar to that observed with GCV. These results demonstrate that the benzimidazole ribonucleosides are active against human CMV and EBV and suggest that they may be useful for the treatment of infections caused by these herpesviruses.
The family Herpesviridae consists of several subfamilies of viruses. Herpes simplex virus types 1 and 2 (HSV-1 and HSV-2) and varicella-zoster virus (VZV) belong to the alphaherpesvirus subfamily. The betaherpesvirus subfamily includes cytomegalovirus (CMV) and human herpesvirus 6 (HHV-6). Epstein-Barr virus (EBV) and human herpesvirus 8 (HHV-8) belong to the gammaherpesvirus subfamily. All of these viruses share the property of remaining latent in their natural host after primary infection. These herpesviruses cause a number of diseases, such as oral and genital herpes, chicken pox, congenital and posttransplant infections, Kaposi's sarcoma, and infectious mononucleosis, that are in need of new drug therapy (34).
Current therapies for human CMV (HCMV) infection include the nucleoside analogs ganciclovir (GCV) (6), its ester prodrug valganciclovir (7), cidofovir (CDV) (14), the pyrophosphate analog foscarnet (PFA) (4), and the antisense phosphorothioate oligonucleotide fomivirsen (24). Although CDV, GCV, and PFA are potent antiviral drugs, they have poor oral bioavailability, and all produce certain toxicities. Because they inhibit viral replication by interacting with virally encoded DNA polymerase, there is also the potential for cross-resistance (9, 10).
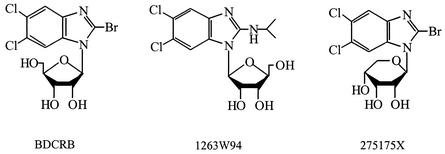
Previous studies with the benzimidazole d-ribonucleosides 2,5,6-trichloro-(1-β-d-ribofuranosyl)benzimidazole (TCRB) and its 2-bromo homolog (BDCRB) (Fig. 1) have established that they are potent and selective inhibitors of HCMV replication (30, 36). These compounds prevent the processing and maturation of head-to-tail concatemeric viral DNA to monomeric genomes (31), and mutations in HCMV genes UL56 and UL89 have been identified as the cause of resistance to TCRB and BDCRB (18, 32). More recent studies have established that BDCRB inhibits the nuclease activity of the protein encoded by gene UL89 (28) and the ATPase activity of pUL56 (29). Although these compounds had good oral bioavailability, their short plasma half-lives (12) led to the design and synthesis of many additional analogs (16, 32), including 1263W94 and 275175X (175X), to obtain more biologically stable, orally active compounds. Compound 175X is an analog of BDCRB in which the sugar (d-ribose) is in the pyranosyl rather than the furanosyl form. In contrast, 1263W94 is an l-ribofuranosyl nucleoside (Fig. 1). Initial studies suggest that 175X acts as an inhibitor of viral DNA processing in a manner similar to that of BDCRB (M. R. Underwood, J. C. Drach, and K. K. Biron, unpublished results).
FIG. 1.
Structures of benzimidazole ribonucleosides.
1263W94 is also very active against HCMV (2) and, surprisingly, has a mechanism of action that is different from that of BDCRB and involves the inhibition of viral DNA synthesis (2, 5). A mutation in the HCMV UL97 protein kinase results in resistance to 1263W94, and this compound strongly inhibits the kinase activity of the viral enzyme (2). Furthermore, 1263W94 inhibits the phosphorylation and accumulation of EBV early antigen D, an essential cofactor in EBV replication (11). 1263W94 is currently in phase II clinical trials for the treatment of HCMV infections (5, 20). Compound 175X has also been used in early clinical trials and its in vitro activity is presented as part of the present study.
We evaluated the antiviral activity of these three benzimidazole nucleosides for their activity against members of the herpesviruses that most commonly produce infections in humans. Because the mechanism of action of these compounds differs from those already approved for treatment of CMV infections, they could play an important role against infections caused by drug-resistant viruses as well as provide oral bioavailability. By using several different antiviral in vitro analyses, including cytopathic effect assay, plaque reduction assay, immunofluorescence assay (IFA), enzyme-linked immunosorbent assay (ELISA), in situ DNA hybridization, and fluorescence-activated cell sorter flow cytometry (FACS), we evaluated the effects of these compounds on the replication of selected herpesviruses. The data obtained indicated that all three compounds were active against a number of HCMV isolates, including those resistant to GCV, and two of the compounds had activity against EBV. None of the compounds inhibited the replication of HSV-1, HSV-2, VZV, HHV-6, or HHV-8.
MATERIALS AND METHODS
Compounds and reagents.
The benzimidazole ribonucleosides, BDCRB, 175X, and 1263W94, were synthesized at GlaxoSmithKline, Inc., Research Triangle Park, N.C. They were reconstituted in 10% dimethyl sulfoxide (DMSO) or 1% carboxymethylcellulose. Acyclovir (ACV), CDV, PFA, and GCV were purchased from the University of Alabama Hospital pharmacy. One of these compounds was used as a positive control in all assay systems. Stock solutions were diluted in minimal essential medium (MEM) containing 2% fetal bovine serum (FBS), l-glutamine, penicillin and gentamicin or RPMI 1640 containing 2 mM l-glutamine, 10% FBS, and 100 μg of gentamicin per ml. All antibodies used were purchased from Chemicon (Temecula, Calif.) or Jackson ImmunoResearch (West Grove, Pa.). Final concentrations of the antibodies were prepared in 1% bovine serum albumin containing 0.5% Tween 20 in phosphate-buffered saline (PBS) or a blocking solution containing 5% FBS, 4% normal goat serum, and 0.5% DMSO.
Cells and viruses.
Human foreskin fibroblast (HFF), mouse embryo fibroblast, rat embryo fibroblast, or guinea pig embryo fibroblast cells were prepared as primary cultures and used in assays with HSV-1 and -2, CMV, or VZV. These cells were propagated in MEM containing 10% FBS, glutamine, and antibiotics as indicated above. Daudi cells (American Type Culture Collection, Manassas, Va.), HSB-2 cells (NIH AIDS Research and Reference Reagent Program, Rockville, Md.), and body cavity-based lymphoma (BCBL-1) cells (NIH AIDS Research and Reference Reagent Program) were propagated for use in EBV, HHV-6, or HHV-8 assays. These cells were cultured in RPMI 1640 containing 10% FBS, glutamine, and antibiotics and split 1:5 twice a week. Cord blood lymphocytes (CBL) were isolated from umbilical cord blood on a Histopaque 1077 (Sigma, St. Louis, Mo.) gradient and used in HHV-6 assays. They were maintained as described above.
All viruses were propagated by using standard virological methods. The source of most virus strains used in these studies has been reported previously (1, 27). Additional HCMV isolates, including ones resistant to GCV, were obtained from K. K. Biron, GlaxoSmithKline.
Adherent cell lines (HFF, MEF, REF, and GPEF).
Cells were seeded into 96-well plates at a concentration of 2.5 × 104 cells/well. After 24 h, the medium was aspirated and 125 μg of drug per ml was added to the first row and serially diluted 1:5. The plates were incubated for 7 days, the medium was aspirated, and the cells were stained with neutral red, all followed by 1 h of incubation. Cells were washed, and solubilizing solution (50% ethanol, 1% glacial acetic acid) was added. Plates were shaken for 15 min on a rotating shaker, and the optical densities were read at 540 nm. Cell proliferation was determined by seeding cells in six-well plates at a concentration of 2.5 × 104 cells/well. After 24 h, the medium was aspirated, and drug that was serially diluted 1:5 was added. The cells were incubated for 72 h at 37°C and then trypsinized and counted with a Beckman Coulter counter. In both assays, values obtained for control cultures were compared with those from drug-treated cultures. The concentration of drug that reduced treated cultures by 50% was calculated by using the MacSynergy II computer program (25, 26).
Nonadherent cell lines (Daudi, HSB-2, SupT-1, CBL, and BCBL-1).
Serial fivefold dilutions of drug starting at 50 μg/ml were prepared in RPMI 1640 and added to tubes containing 106 cells. Controls were prepared by incubating 106 cells in drug-free medium. After an incubation period of 3 to 7 days, depending on the assay system, the total number of cells for each sample was determined. A neutral red cytotoxicity assay was performed with the remaining cells, and the optical density was measured at 540 nm. Daudi cell viability was determined by Trypan blue exclusion and neutral red cytotoxicity. The drug concentration was plotted against the optical density of each sample. By using a linear-regression program, the drug concentration for 50% cytotoxicity value was interpolated from this plot. Likewise, the plot of drug concentration versus the number of cells was used to determine the concentration of drug required for 50% inhibition of cell proliferation for each drug by using the MacSynergy II software as described above.
Plaque reduction assay for HSV-1, HSV-2, HCMV, and VZV.
HFF cells were plated into six-well plates and incubated at 37°C. Two days later, drug was serially diluted 1:5 in MEM with FBS and a 1:500 dilution of human serum (Baxter Health Care, Glendale, Calif.) by using six concentrations of drug with a starting concentration of 100 μg/ml. ACV or GCV was used as a positive control. The virus to be used was diluted in MEM containing 10% FBS to a desired concentration, which yielded 20 to 30 plaques per well. Medium was then aspirated from the wells, and 0.2 ml of virus suspension was added to each well in triplicate with 0.2 ml of medium being added to drug toxicity wells. Plates were then incubated for 1 h with shaking every 15 min, and drug was added to each well. After an incubation period of 3 to 10 days, cells were stained with 1% crystal violet in 20% methanol for HSV-1 and -2 or a 1% neutral red solution in PBS for HCMV and VZV. The stain was aspirated, the wells were washed with PBS, and the plaques were counted with a stereomicroscope. By comparing drug-treated with untreated wells, 50% effective concentration (EC50) values were calculated with MacSynergy II software.
Flow cytometric assay for HHV-6.
Serial fivefold dilutions of drug starting at 50 μg/ml were prepared in RPMI 1640 with 10% FBS. CDV was used as a positive control. Samples for determining antiviral efficacy were prepared by incubating 106 HSB-2 cells for 1 h with sufficient virus to infect approximately 35% of the cells. After infection, the appropriate concentrations of drug were added, and cells were incubated for 4 to 6 days at 37°C. Virus-free controls were prepared by incubating 106 cells in drug-free medium for the designated period, and virus controls were prepared by incubating 106 cells for 1 h with sufficient virus to infect 35% of the cells followed by an incubation in drug-free medium for the designated period. After incubation, cells were rinsed with PBS and permeabilized overnight in methanol at −80°C for use in FACS analysis as described below.
Determination of antiviral drug efficacy against HHV-8.
Lytic HHV-8 infection was induced in BCBL-1 cells (a continuous cell line latently infected with HHV-8) by the addition of 100 ng of Phorbol 12-myristate 13-acetate (TPA) per ml. Serial fivefold dilutions of drug starting at 50 μg/ml were prepared in RPMI 1640. CDV was used as a positive control. Samples used for determining antiviral efficacy were prepared by incubating 106 TPA-induced BCBL-1 cells with the appropriate dilution of drug for 5 days, with new media added on day 2 of incubation. TPA-induced and uninduced controls were prepared by incubating 106 TPA-induced and uninduced BCBL-1 cells in drug-free medium. After incubation, cells were rinsed with PBS and permeabilized by overnight storage in methanol at −80°C for subsequent use in FACS assay.
Determination of antiviral drug efficacy against EBV.
Serial fivefold dilutions of drug starting at 50 μg/ml were prepared in RPMI 1640 medium with 10% FBS. ACV was used as a positive control. Samples for determining antiviral efficacy were prepared by infecting 106 Daudi cells with sufficient EBV P3HR-1 strain to infect 10% of the cells. After infection for 1 h, the appropriate dilutions of drug were added and cells were incubated for 3 days at 37°C. Virus-free controls were prepared by incubating 106 Daudi cells in drug-free medium, and virus controls were prepared by infecting 106 Daudi cells as described above. After incubation, cells were counted, rinsed thoroughly with PBS, and resuspended to a final concentration of 4 × 106 cells/ml. Duplicate sets of slides were spotted with 25 μl of cell suspension for each drug concentration and allowed to dry before staining for IFA or in situ hybridization. Slides for IFA were then fixed in acetone for 10 min at room temperature, rinsed, and stored before staining. For ELISA, 4 × 105 cells were added to three duplicate wells of a 96-well plate and allowed to air dry.
IFAs.
Fixed Daudi cells were incubated with a monoclonal antibody to EBV viral capsid antigen (Chemicon), followed by a secondary fluorescein isothiocyanate-labeled immunoglobulin G (IgG) plus IgM antibody (Jackson ImmunoResearch) at 37°C for 1-h intervals. Slides were rinsed thoroughly with PBS between incubations. Cells were counterstained with 0.1% Evans blue dye (Fisher, Fair Lawn, N.J.) in PBS for 5 min, and coverslips were mounted by using 50% glycerol in PBS. Cells were viewed at a magnification of ×400 with a fluorescence microscope. Five fields were scanned, and 100 cells per field were counted; the percentage of positive cells was calculated at each drug concentration. Drug efficacy was determined by plotting drug concentration versus the percentage of positive cells and then interpolating the concentration of drug required to inhibit 50% of virus replication with the MacSynergy II software.
Flow cytometric assays.
Cells were rinsed thoroughly with PBS, and a blocking solution containing 5% FBS, 4% normal goat serum, and 0.5% DMSO was added. Cells were then incubated with a monoclonal antibody to HHV-6 early nuclear proteins (Chemicon), 101-kDa virion (Chemicon), or KS8.1 (Bala Chandran, University of Kansas Medical Center, Kansas City, Kans.), followed by fluorescein isothiocyanate-labeled IgG plus IgM secondary antibody (Jackson ImmunoResearch). Between each incubation, cells were thoroughly washed with blocking solution. After staining, samples were fixed in 2% paraformaldehyde in PBS and analyzed with a Becton-Dickinson FacsCalibur instrument. Flow cytometry data were analyzed with the WinMDI 2.7 data analysis program, and the EC50 was interpolated from the plot of drug concentration versus the percentage of antigen-positive cells as described above.
In situ hybridization assay.
The Simply Sensitive Horseradish Peroxidase-AEC In Situ Detection System (Enzo Diagnostics, Farmingdale, N.Y.) was used to monitor EBV DNA synthesis in the presence of antiviral compounds and was used according to the manufacturer's instructions. The EC50 value for each drug was interpolated from the plot of drug concentration versus the percentage of positive cells, as described above.
ELISA.
ELISA was performed on cells fixed with 95% ethanol and 5% acetic acid, rinsed with PBS, and incubated first with a monoclonal antibody to EBV viral capsid antigen (Chemicon) followed by an incubation with horseradish peroxidase-labeled goat anti-mouse IgG1 (Southern Biotechnology, Birmingham, Ala.). The antibodies were diluted in a blocking solution of 1% bovine serum albumin containing 0.5% Tween 20 in PBS. The colorimetric reaction was initiated by addition of O-phenylenediamine dihydrochloride in citrate buffer (pH 5.0) and hydrogen peroxide. The reaction was stopped by the addition of 3 N sulfuric acid after approximately 10 min. The EC50 value for each drug was interpolated from the plot of drug concentration versus the average optical density at 492 nm as described above.
RESULTS
Activity against alphaherpesviruses.
To determine the activity of the benzimidazole ribonucleosides against members of the alphaherpesvirus group, the three compounds were tested initially against representative strains of HSV-1, HSV-2, and VZV. The activity and toxicity of the three compounds are presented in Table 1. The compound used as a positive control, ACV, had EC50 values of 1 to 7 μM against HSV-1, HSV-2, and VZV. In contrast, BDCRB, 1263W94, and 175X were all inactive against the three alphaviruses tested. Compared with ACV, the three experimental compounds exhibited slight toxicity against stationary cells, with somewhat greater toxicity shown by 1263W94 against proliferating cells. Similar values were found in other studies for BDCRB (23, 29) and 1263W94 (2) in cell lines and in human progenitor cells.
TABLE 1.
Effect of benzimidazole nucleosides on alphaherpesvirus replication
| Assay for: | 50% inhibitory concn (μM)a
|
|||
|---|---|---|---|---|
| ACV | BDCRB | 1263W94 | 275175X | |
| Virus | ||||
| HSV-1 | 4.1 ± 0.9 | >251 | >53 | >50 |
| HSV-2 | 3.5 ± 3.1 | >251 | >53 | >50 |
| VZV | 0.7 ± 0.3 | >50 | >266 | >50 |
| Cytotoxicity | ||||
| Stationary cellsb | >405 | 170 ± 30 | 243 | 152 |
| Proliferating cellsc | >405 | 184 ± 20 | 59 ± 22 | 143 ± 28 |
Values are expressed as mean ± standard deviation from at least two plaque reduction assays.
Concentration of drug cytotoxic to 50% of HFF cells by neutral red uptake.
Concentrations of drug that inhibit HFF cell growth by 50%.
Activity against betaherpesviruses.
The activity of the benzimidazole nucleosides was also determined against the following betaherpesviruses and strains: HCMV (AD169), HHV-6A (GS), and HHV-6B (Z-29). Results summarized in Table 2 indicate that the AD169 strain of HCMV was inhibited by all three compounds at levels less than that required for GCV. However, none of the HHV-6 strains were inhibited by the benzimidazoles compared with the positive controls, PFA, or CDV.
TABLE 2.
Activity of benzimidazole nucleosides against betaherpesviruses
| Virus (cells) | Drug | 50% inhibitory concn (μM)
|
|||
|---|---|---|---|---|---|
| Antiviral activitya | Cytotoxicity stationary cellsb | Toxicity proliferating cellsb | SIc | ||
| HCMVAD169 (HFF) | GCV | 4.3 ± 0.4 | >361 | 144 | >83 |
| BDCRB | 0.4 ± 0.3 | 170 | 184 ± 20 | 425 | |
| 1263W94 | 19.4 ± 18.6 | 243 | 58.5 ± 22.1 | 13 | |
| 175X | 2.2 ± 0.5 | 152 | 143 ± 28 | 69 | |
| HHV-6AGS (HSB-2) | PFA | 13.7 ± 8.3 | >166 | >166 | >12 |
| BDCRB | >125 | >104 | >125 | — | |
| 1263W94 | >125 | >133 | >133 | — | |
| 175X | >125 | >125 | >126 | — | |
| HHV-6AGS (SupT-1) | CDV | 11.7 ± 8 | >159 | 90 ± 18.1 | >13 |
| BDCRB | >125 | 69 ± 34 | 70 ± 12 | — | |
| 1263W94 | >133 | >108 | >105 | — | |
| 175X | >125 | >104 | >102 | — | |
| HHV-6BZ-29 (CBL) | CDV | 1.6 ± 0.6 | >159 | >159 | >100 |
| BDCRB | >125 | >109 | >87 | — | |
| 1263W94 | >106 | >98 | 70 ± 59 | — | |
| 175X | 95 ± 12 | >122 | 111.8 ± 14 | >1.3 | |
Values are the mean ± standard deviation of at least two assays. See Materials and Methods for a description of the assays.
See footnote to Table 1 for description of toxicity assays.
SI, selective index, cytotoxicity in stationary cells/antiviral activity. —, ≤1.
Because BDCRB, 1263W94, and 175X were all active against the AD169 strain of HCMV, we determined their activity against additional laboratory and clinical isolates of HCMV. The results tabulated in Table 3 indicate that all three compounds were at least as active as, if not more active than, GCV against most of the isolates. BDCRB was more active against AD169 than Towne, a phenomenon also observed by Krosky et al. (19). Of interest was the observation that 1263W94 was generally less active when assayed at 37°C than at 34°C, whereas BDCRB and 175X were not affected by temperature. This phenomenon was first observed with the laboratory strains of HCMV, Towne, and AD169; however, the results in Table 3 indicate that it is true for other HCMV isolates. In addition, 1263W94 was considerably less active against the Towne strain of HCMV than was AD169 in the plaque assay. We have previously noted that 1263W94 is as active against Towne in a yield assay as it is against AD169 in a plaque assay (13), but we have no explanation for why the compound is less active against Towne in a plaque assay. The seven low-passage-number clinical isolates were all susceptible to the three benzimidazole nucleosides at levels that were generally lower than those observed for GCV. In fact, the differences between BDCRB and GCV were statistically significant by the Wilcoxon rank sum test at a P of <0.001.
TABLE 3.
Effect of benzimidazole ribonucleosides on laboratory and clinical isolates of HCMV
| Isolate | 50% inhibitory concn (μM)a
|
||||
|---|---|---|---|---|---|
| BDCRB | 1263W94 (37°C) | 1263W94 (34°C) | 175X | GCV | |
| AD169 | 0.4 ± 0.2 | 19.4 ± 18.6 | 3.0 ± 3 | 2.2 ± 0.5 | 4.3 ± 0.4 |
| Towne | 1.2 ± 0.3 | >44 | 14 ± 9 | 5.2 ± 2.5 | 7.2 ± 3.3 |
| Davis | 0.6 ± 0.1 | 0.6 ± 0.2 | NTb | 1.9 ± 1.1 | 4.3 ± 0.6 |
| Toledo | 1.1 ± 0.1 | >47 ± 7 | 2.1 | 5.3 ± 1.3 | 6.9 ± 2.3 |
| EC | 1.0 ± 0.5 | 11.2 ± 11.7 | 0.9 | 3.8 ± 0.3 | 9.7 ± 5.4 |
| CH | 0.4 | 4.0 | 1.1 | 3.0 | 4.3 ± 0.4 |
| C8708/17-1-1 | 0.7 ± 0.5 | 0.2 ± 0.05 | 0.3 | 2.0 ± 1.3 | 4.3 ± 0.6 |
| Coffman | 1.1 ± 0.4 | 16.5 ± 3.5 | 11 | 3.8 ± 0.4 | 11.5 ± 5.1 |
| C9208/3-3-1 | 0.8 | 15.9 ± 10.6 | 11.2 | 2.0 ± 0.5 | 2.2 ± 0.8 |
| C9208/5-4-2 | 0.8 ± 0.3 | 1.3 | 0.7 | 2.3 | 2.4 |
Values are expressed as the mean ± standard deviation of at least two plaque reduction assays.
NT, not tested.
Since the use of GCV for treatment of chronic HCMV infections often results in the selection of GCV-resistant mutants, we determined the activity of the benzimidazole nucleosides against a panel of GCV-resistant HCMV isolates. The phenotypes and genotypes of many of these isolates were summarized recently (1), and their susceptibility to GCV, BDCRB, 1263W94, and 175X is included in Table 4. All of the GCV-resistant isolates as well as two PFA-resistant isolates were sensitive to all three of the benzimidazoles—providing additional evidence for a different mechanism of action for these compounds than for GCV or PFA.
TABLE 4.
Activity of benzimidazole ribonucleosides on drug-resistant isolates of HCMV
| Isolate | 50% inhibitory concn (μM)a
|
||||
|---|---|---|---|---|---|
| BDCRB | 1263W94 (37°C) | 1263W94 (34°C) | 175X | GCV/PFA | |
| C8805/37-1-1 | 1.4 ± 1.4 | >27 | 15 | 2.8 ± 0.8 | 100 ± 56 |
| 759rD100 | 0.4 ± 0.2 | 3.3 ± 0.6 | 2.1 | 2.1 ± 0.1 | 113 ± 62 |
| GDGrP53 | 2.1 ± 1.0 | 37 ± 2 | 8.5 | 2.9 ± 0.5 | 62.8 ± 27 |
| C8706/13-1-1 | 1.3 ± 1.1 | 0.3 ± 0.2 | 0.8 | 2.1 ± 0.4 | 22 ± 10 |
| C9209/1-4-4 | 1.5 ± 1.0 | 1.0 ± 0 | 0.1 | 3.5 ± 0 | 98 ± 34 |
| C8914-6 | 3.3 ± 0.4 | 24 ± 24 | 0.6 | 10 ± 2.8 | 104 ± 77 |
| VR4760R | 1.7 ± 1.5 | 0.5 ± 0.3 | <0.08 | 2.5 ± 1.5 | 830 ± 28b |
| VR4955R | 2.8 ± 0.3 | 0.7 ± 0.03 | 0.2 | 3.5 ± 0 | 700 ± 183b |
| 1117R | 2.8 ± 0 | 50 ± 5 | 0.4 | 3.5 ± 1.0 | 42 ± 2.2 |
| RCMP97 | 1.0 | 85 | NTc | 2.3 | 35 |
Values are expressed as the mean ± standard deviation of at least two plaque reduction assays.
Control drug for these strains is PFA.
NT, not tested.
HCMV is species specific and does not infect experimental animals (22). Consequently, surrogate animal strains of CMV, such as murine, rat, guinea pig, and (to a lesser extent) rhesus monkey strains, have been utilized as animal model infections for the study of the biology and development of antiviral drugs directed against CMV infection in humans. The activities of BDCRB, 1263W94, and 175X were compared with that of GCV against murine, rat, guinea pig, and rhesus CMV strains (MCMV, RCMV, GpCMV, and RhCMV, respectively), and the results are shown in Table 5. Although there was variability in the toxicity of the compounds in primary mouse, rat, and guinea pig cells, calculation of a selectivity index generally indicated that there was little activity of the three compounds against those three viruses compared to GCV. An exception was BDCRB, which was active against gpCMV. In contrast, both BDCRB and 175X were active against the RhCMV isolate. The lower activity of 1263W94 compared to that of BDCRB is similar to that observed with several strains of HCMV (Table 3) and could be a characteristic of this compound in a plaque assay. Thus, activity of the compounds against the betaherpesviruses tested was seen only with HCMV and, to a lesser extent, RhCMV.
TABLE 5.
Effect of benzimidazole nucleosides against various animal CMV strains
| Virus | Drug | 50% inhibitory concn (μM)a
|
||
|---|---|---|---|---|
| Antiviral activity | Cytotoxicity | SIb | ||
| MCMV | GCV | 2.9 ± 1.4 | >36 | >12 |
| BDCRB | 37 | 151 | 4 | |
| 1263W94 | >11 | 50 ± 4 | <4.5 | |
| 175X | 20 ± 6.5 | 158 ± 7.3 | 7.9 | |
| RCMV | GCV | 31 ± 1.4 | >361 | >12 |
| BDCRB | 26 ± 4.5 | 190 ± 6.5 | 7.3 | |
| 1263W94 | >11 | 31.6 ± 1.3 | <3.0 | |
| 175X | 28.2 ± 3.8 | 117 ± 12 | 4.2 | |
| GPCMV | CDV | 1.6 ± 1.0 | >317 | >198 |
| GCV | 191 ± 15 | >361 | >1.9 | |
| BDCRB | 2.0 ± 7 | 188 ± 28 | 94 | |
| 1263W94 | >53 | 60 ± 9.0 | <1.1 | |
| 175X | 27 ± 8 | 130 ± 4 | 4.8 | |
| RhCMV | GCV | 22 ± 8 | >72 | >3.3 |
| BDCRB | 0.4 | >250 | >625 | |
| 1263W94 | 48 ± 4.5 | 227 ± 3.7 | 4.7 | |
| 175X | 6.3 ± 0.8 | 179 ± 14 | 28 | |
Values are expressed as the mean ± standard deviation of at least two plaque reduction assays.
SI, selective index, cytotoxicity in stationary HFF cells/antiviral activity.
Activity against gammaherpesviruses.
The activity of the benzimidazole nucleosides was also determined against the gammaherpesviruses EBV and HHV-8 (Table 6). The P3HR-1 strain of EBV was tested with three different assays that were reflective of EBV replication. There was generally a good correlation among the results of the three assays, which showed that 1263W94 and 175X were as active as ACV against EBV, whereas BDCRB was not active. No activity was observed with any of the compounds against HHV-8.
TABLE 6.
Effect of benzimidazole nucleosides against the gammaherpesviruses
| Virus | Drug | 50% inhibitory concn (μM)a
|
||||
|---|---|---|---|---|---|---|
| IFA-FACSb | DNA | ELISA | Cytotoxicity | SIc | ||
| EBV | ACV | 17.4 ± 3.6 | 3.6 ± 1.2 | 9.3 ± 0.4 | >187 | >11 |
| BDCRB | >125 | >80 | >74 | >125 | — | |
| 1263W94 | 10 ± 8 | 5.1 ± 4.8 | 4.3 ± 0.8 | >77 | >7.7 | |
| 175X | 18 ± 3 | 10 ± 6.8 | >72 | >94 | >5.2 | |
| HHV-8 | CDV | 10.2 ± 2.5 | NTd | NT | >159 | >16 |
| BDCRB | >125 | NT | NT | >125 | — | |
| 1263W94 | >133 | NT | NT | >80 | — | |
| 175X | >126 | NT | NT | >126 | — | |
Values are expressed the mean ± standard deviation of at least two assays.
IFA was performed in EBV assays and FACS was performed in HHV-8 assays.
SI, selective index, cytotoxicity in Daudi or BCBL-1 cells/antiviral activity determined by IFA-FACS.
NT, not tested. —, ≤1.
Collectively, the results indicate that the three benzimidazole ribonucleosides were active only against human and rhesus CMV isolates of the betaherpesviruses and against EBV of the gammaherpesviruses. No activity was seen against the alphaherpesviruses that were tested. The activity of these compounds against a variety of HCMV isolates was generally superior to GCV and, in addition, were active against GCV-resistant mutants. Their toxicity in tissue culture cells appeared to be somewhat similar to that observed with GCV and CDV.
DISCUSSION
Of the current diseases caused by the herpesviruses, infections due to HSV or VZV have been treated somewhat successfully with ACV, valacyclovir, or famciclovir. Treatment of CMV infections with CDV or GCV, however, has not been optimal due to the toxicity and poor oral bioavailability of these drugs. There is a need, therefore, for long-term therapeutic options with new orally active drugs that do not share cross-resistance to GCV and CDV. Additionally, there is a need for effective therapy for EBV, HHV-6, and HHV-8 infections.
The mechanism of action of the benzimidazole nucleosides is complex. Not only do the ribonucleosides BDCRB and 1263W94 act by different mechanisms, but a third mechanism for action of deoxylyxosl benzimidazoles has also been reported (D. L. Evers, G. Komazin, D.-J. Shin, B. T. Emmer, L. B. Townsend, and J. C. Drach, submitted for publication). For BDCRB and related analogs, there is excellent genotypic evidence from drug-resistant viruses that these compounds target the products of HCMV genes UL56 and UL89. These two genes, which are involved in DNA maturation, are conserved open reading frames that are found throughout the herpesviruses (8). These essential genes have homologous sequences to open reading frames found in the UL15 and UL28 genes of HSV-1, respectively, which are important in the maturation of viral DNA (8, 18). Further studies have also indicated that the UL89 gene may be a putative CMV terminase because of its homology with the bacteriophage T4 terminase complex (8, 32). Although the role of UL89 in the packaging of DNA still remains to be determined, there is evidence that it interacts with UL56 (18, 28, 29). This gene product exhibits ATPase activity, which is a main function in the packaging of DNA in HCMV (3, 28). It has been suggested that it provides energy for the translocation of the DNA into the procapsids (15). Recently, it has been demonstrated that the target of 1263W94 in HCMV replication is the product of gene UL97 (2). Direct inhibition of the UL97 protein by 1263W94 inhibits the phosphorylation of viral DNA processivity factor pUL44, which may explain the inhibition of viral DNA synthesis (K. Biron, unpublished results).
Results from the current study indicate that the benzimidazole ribonucleosides had little if any effect on the replication of HSV, VZV, HHV-6, or HHV-8. All three of the compounds, however, did have good activity against the betaherpesvirus HCMV, including strains that were either sensitive or resistant to GCV. Biron et al. (2) and McSharry et al. (21) reported activity of 1263W94 against laboratory strains and clinical isolates that are resistant to GCV as well. In addition, 1263W94, which is an l-riboside, had activity against the gammaherpesvirus EBV, apparently due to an effect on the phosphorylation of viral proteins (11). The beta- and gammaherpesviruses have a protein kinase that is homologous to the alphavirus HSV protein UL13, and this enzyme has also been reported to be involved in viral replication and pathogenesis of EBV (11) and is a target of 1263W94. Although BDCRB is not active against EBV (Table 6) (35), 175X is (Table 6). This is surprising because initial mode-of-action studies suggested that 175X is more like the DNA processing inhibitors TCRB and BDCRB than 1263W94 (M. R. Underwood, J. C. Drach, and K. K. Biron, unpublished results).
Although these compounds are very active against HCMV replication in vitro, their lack of activity against the animal surrogate viruses has prevented the validation of their activity in animal model systems. Nonetheless, in a clinical study in which a small number of patients received 1263W94 in a dose escalation study, a significant reduction of HCMV titers in the semen of these patients was observed (20), thereby validating the activity of 1263W94 in humans. The pharmacokinetics and toxicity of 1263W94 has been studied in animal models (17) and in humans (20, 33). Both profiles were very favorable, leading to the initial clinical trial mentioned above and described previously (20). In both animal models and in humans, good oral bioavailability and lower toxicity were found for 1263W94 in comparison to the drugs currently approved for the treatment of HCMV infections. Although additional clinical studies will have to be performed before the potential of these compounds for use in treatment of CMV or EBV infections in humans can be realized, at least one of the compounds has the potential to become a drug for the treatment of CMV infections.
Acknowledgments
This work was supported by Public Health Service Contract NO1-AI-85347 and Grants 5-U19-A131718 and P01-AI46390 from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Md. Support to E.R.K. from Glaxo SmithKline, Research Triangle Park, N.C. is gratefully acknowledged.
We also thank James McSharry for his help with the flow cytometry studies.
REFERENCES
- 1.Beadle, J. R., C. B. Hartline, K. A. Aldern, N. Rodriguez, E. A. Harden, E. R. Kern, and K. Y. Hostetler. 2002. Alkoxyalkyl esters of cidofovir and cyclic cidofovir exhibit multiple-log enhancement of antiviral activity against cytomegalovirus and herpesvirus replication in vitro. Antimicrob. Agents Chemother. 46:2381-2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biron, K. K., R. J. Harvey, S. C. Chamberlain, S. S. Good, A. A. Smith III, M. G. Davis, C. L. Talarico, W. H. Miller, R. Ferris, R. E. Dornsife, S. C. Stanat, J. C. Drach, L. B. Townsend, and G. W. Koszalka. 2002. Potent and selective inhibition of human cytomegalovirus replication by 1263W94: a benzimidazole l-riboside with a unique mode of action. Antimicrob. Agents Chemother. 46:2365-2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bogner, E., K. Radsak, and M. F. Stinski. 1998. The gene product of human cytomegalovirus open reading frame UL56 binds the pac motif and has specific nuclease activity. J. Virol. 72:2259-2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chrisp, P., and S. P. Clissold. 1991. Foscarnet: a review of its antiviral activity, pharmacokinetic properties, and the therapeutic use in immunocompromised patients with cytomegalovirus retinitis. Drugs 41:104-129. [DOI] [PubMed] [Google Scholar]
- 5.Chulay, J., K. K. Biron, L. Wang, M. Underwood, S. Chamberlain, L. Frick, S. Good, M. Davis, R. Harvey, L. Townsend, J. Drach, and G. Koszalka. 1999. Development of novel benzimidazole riboside compounds for treatment of cytomegalovirus disease. Adv. Exper. Med. Biol. 458:129-134. [DOI] [PubMed] [Google Scholar]
- 6.Crumpacker, C. S. 1996. Ganciclovir. N. Engl. J. Med. 335:721-729. [DOI] [PubMed] [Google Scholar]
- 7.Curran, M., and S. Noble. 2001. Valganciclovir. Drugs 61:1145-1150. [DOI] [PubMed] [Google Scholar]
- 8.Davison, A. J. 1992. Channel catfish virus: a new type of herpesvirus. Virology 186:9-14. [DOI] [PubMed] [Google Scholar]
- 9.Erice, A. 1999. Resistance of human cytomegalovirus to antiviral drugs. Clin. Microbiol. Rev. 12:286-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Field, A. K., and K. K. Biron. 1991. “The end of innocence” revisited: resistance of herpesviruses to antiviral drugs. Clin. Microbiol. Rev. 7:1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gershburg, E., and J. S. Pagano. 2002. Phosphorylation of Epstein-Barr (EBV) DNA polymerase processivity factor EA-D by the EBV-encoded protein kinase and effects of the l-riboside benzimidazole 1263W94. J. Virol. 76:998-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Good, S. S., B. S. Owens, L. B. Townsend, and J. C. Drach. 1994. The disposition in rats and monkeys of 2-bromo-5,6-dichloro-1-(β-d-ribofuranosyl)-benzimidazole (BDCRB) and its 2,5,6-trichloro congener (TCRB). Antiviral Res. 23:(Suppl. I)103. [Google Scholar]
- 13.Gudmundsson, K. S., J. Tidwell, N. Lippa, G. W. Koszalka, N. van Draanen, R. G. Ptak, J. C. Drach, and L. B. Townsend. 2000. Synthesis and antiviral evaluation of halogenated β-d- and l-erythrofuranosylbenzimidazoles. J. Med. Chem. 43:2464-2472. [DOI] [PubMed] [Google Scholar]
- 14.Hitchcock, M. J. M., H. S. Jaffe, J. C. Martin, and R. J. Stagg. 1996. Cidofovir, a new agent with potent anti-herpesvirus activity. Antivir. Chem. Chemother. 7:115-127. [Google Scholar]
- 15.Hwang, J. S., and E. Bogner. 2002. ATPase activity of the terminase subunit pUL56 of human cytomegalovirus. J. Biol. Chem. 277:6943-6948. [DOI] [PubMed] [Google Scholar]
- 16.Koszalka, G. W., S. D. Chamberlain, R. J. Harvey, L. W. Frick, S. S. Good, M. L. Davis, A. Smith, J. C. Drach, L. B. Townsend, and K. K. Biron. 1996. Benzimidazoles for the treatment of human cytomegalovirus infections. Antivir. Res. 30:A43. [Google Scholar]
- 17.Koszalka, G. W., N. W. Johnson, S. S. Good, L. Boyd, S. C. Chamberlain, L. B. Townsend, J. C. Drach, and K. K. Biron. 2002. Preclinical and toxicology studies of 1263W94, a potent and selective inhibitor of human cytomegalovirus replication. Antimicrob. Agents Chemother. 46:2373-2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krosky, P. M., M. R. Underwood, S. R. Turk, K. W.-H. Feng, R. K. Jain, R. G. Ptak, A. C. Westerman, K. K. Biron, L. B. Townsend, and J. C. Drach. 1998. Resistance of human cytomegalovirus to benzimidazole ribonucleosides maps to two open reading frames: UL89 and UL56. J. Virol. 72:4721-4728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krosky, P. M., R. G. Ptak, M. R. Underwood, K. K. Biron, L. B. Townsend, and J. C. Drach. 2000. Differences in DNA packaging genes and sensitivity to benzimidazole ribonucleosides between human cytomegalovirus strains AD169 and Towne. Antivir. Chem. Chemother. 11:349-352. [DOI] [PubMed] [Google Scholar]
- 20.Lalezari, J. P., J. A. Aberg, L. H. Wang, M. B. Wire, R. Miner, W. Snowden, C. L. Talarico, S. Shaw, M. A. Jacobson, and W. L. Drew. 2002. Phase I dose escalation trial evaluating the pharmacokinetics, anti-human cytomegalovirus (HCMV) activity, and safety of 1263W94 in human immunodeficiency virus-infected men with asymptomatic HCMV shedding. Antimicrob. Agents Chemother. 46:2969-2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McSharry, J. J., A. McDonough, B. Olson, C. Talarico, M. Davis, and K. K. Biron. 2001. Inhibition of ganciclovir-susceptible and -resistant human cytomegalovirus clinical isolates by the benzimidazole l-riboside 1263W94. Clin. Diagn. Lab. Immunol. 8:1279-1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mocarski, E. S., Jr., and C. Tan Courcelle. 2001. Cytomegaloviruses and their replication, p. 2629-2673. In D. M. Knipe et al. (ed.), Fields virology, 4th ed. Lippincott, Williams & Wilkins, Philadelphia, Pa.
- 23.Nassiri, M. R., S. G. Emerson, R. V. Devivar, L. B. Townsend, J. C. Drach, and R. S. Taichman. 1996. Comparison of benzimidazole nucleosides and ganciclovir on the in vitro proliferation and colony formation of human bone marrow progenitor cells. Br. J. Haematol. 93:273-279. [DOI] [PubMed] [Google Scholar]
- 24.Perry, C. M., and J. A. Barman Balfour. 1999. Fomivirsen. Drugs 57:375-380. [DOI] [PubMed] [Google Scholar]
- 25.Prichard, M. N., and C. Shipman, Jr. 1990. A three-dimensional model to analyze drug-drug interactions. Antivir. Res. 14:181-206. [DOI] [PubMed] [Google Scholar]
- 26.Prichard, M. N., S. R. Turk, L. A. Coleman, S. L. Engelhardt, C. Shipman, Jr., and J. C. Drach. 1990. A microtiter virus yield reduction assay for the evaluation of antiviral compounds against human cytomegalovirus and herpes simplex virus. J. Virol. Methods 28:101-106. [DOI] [PubMed] [Google Scholar]
- 27.Rybak, R. J., C. B. Hartline, Y.-L. Qiu, J. Zemlicka, E. A. Harden, G. Marshall, J.-P. Sommadossi, and E. R. Kern. 2000. In vitro activities of methylenecyclopropane analogues of nucleosides and their phosphoralaninate prodrugs against cytomegalovirus and other herpesvirus infections. Antimicrob. Agents Chemother. 44:1506-1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scheffczik, H., C. G. W. Savva, A. Holzenburg, L. Kolesnikova, and E. Bogner. 2002. The terminase subunits pUL56 and pUL89 of human cytomegalovirus are DNA-metabolizing proteins with toroidal structure. Nucleic Acids Res. 30:1695-1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scholz, B., S. Rechter, J. C. Drach, L. B. Townsend, and E. Bogner. 2003. Identification of the ATP-binding site in the terminase subunit pUL56 of human cytomegalovirus. Nucleic Acids Res. 31:1426-1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Townsend, L. B., R. V. Devivar, S. R. Turk, M. R. Nassiri, and J. C. Drach. 1995. Design, synthesis, and antiviral activity of certain 2,5,6-trihalo-1-(β-d-ribofuranosyl)benzimidazoles. J. Med. Chem. 38:4098-4105. [DOI] [PubMed] [Google Scholar]
- 31.Townsend, L. B., K. S. Gudmundsson, S. M. Daluge, J. J. Chen, Z. Zhu, G. W. Koszalka, L. Boyd, S. D. Chamberlain, G. A. Freeman, K. K. Biron, and J. C. Drach. 1999. Studies designed to increase the stability and antiviral activity (HCMV) of the active benzimidazole nucleoside, TCRB. Nucleosides Nucleotides 18:509-519. [DOI] [PubMed] [Google Scholar]
- 32.Underwood, M. R., R. J. Harvey, S. C. Stanat, M. L. Hemphill, T. Miller, J. C. Drach, L. B. Townsend, and K. K. Biron. 1998. Inhibition of human cytomegalovirus DNA maturation by a benzimidazole ribonucleoside is mediated through the UL89 gene product. J. Virol. 72:717-725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang, L. H., R. W. Peck, Y. Yin, J. Allanson, R. Wiggs, and M. B. Wire. 2003. Phase I safety and pharmacokinetic trials of 1263W94, a novel oral anti-human cytomegalovirus agent, in healthy and human immunodeficiency virus-infected subjects. Antimicrob. Agents Chemother. 47:1334-1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Whitley, R. J. 2001. Herpes simplex viruses, p. 2461-2509. In D. M. Knipe et al. (ed.), Fields virology, 4th ed. Lippincott, Williams & Wilkins, Philadelphia, Pa..
- 35.Zacny, V. L., E. Gershburg, M. G. Davis, K. K. Biron, and J. S. Pagano. 1999. Inhibition of Epstein-Barr replication by benzimidazole l-riboside: novel antiviral mechanism of 5,6-dichloro-2-(isopropylamino)-1-β-l-ribofuranosyl-1-H benzimidazole. J. Virol. 73:7271-7277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zou, R., R. Ayres, J. C. Drach, and L. B. Townsend. 1996. Synthesis and antiviral evaluation of certain disubstituted benzimidazole ribonucleosides. J. Med. Chem. 39:3477-3482. [DOI] [PubMed] [Google Scholar]