Abstract
Controversy persists pertaining to the role of CCR4 ligands, namely CCL17 (or thymus and activation regulated chemokine; TARC) and CCL22 (or macrophage-derived chemokine; MDC), in Th2-type cytokine-dominated responses in the lung. Accordingly, the present study addressed the relative role of each of these CC chemokines during an evolving pulmonary granulomatous response elicited by the intrapulmonary embolization of live Schistosoma mansoni eggs into S. mansoni-sensitized mice. CCL22 protein expression peaked at day 4, but CCL17 levels were not increased significantly at any time after egg challenge. CCR4 transcript and protein expression were highest at day 8 after egg embolization and CCR4 protein was prominently expressed in macrophages surrounding S. mansoni eggs. Systemic immunoneutralization of CCL22 from the time of egg injection into S. mansoni-sensitized mice for 8 days significantly decreased CCR4 protein expression, the eosinophil content, the overall size of the egg granuloma, and its hydroxyproline content. Whole lung levels of interferon-γ were also significantly increased at day 8 in anti-CCL22-treated mice. The systemic immunoneutralization of CCL17 had a lesser effect on all of the granuloma parameters listed above, but this antibody treatment significantly decreased granuloma hydroxyproline content to a greater extent than the anti-CCL22 antibody treatment. In addition, the immunoneutralization of CCL17 significantly increased whole lung levels of interleukin (IL)-4, IL-5, IL-13, transforming growth factor-β, IL-12, and tumor necrosis factor-α at day 8 after egg infusion. Thus, these studies demonstrate a major role for CCL22 and a lesser role for CCL17 during an evolving S. mansoni egg granuloma in the lung.
Understanding the biology of chemotactic cytokines or chemokines and their receptors continues to challenge a number of researchers working in diverse fields such as inflammation, immune regulation, angiogenesis, virology, and lymphoid development.1–3 Consistent with their first recognized role, chemokines direct cell movement and positioning in every organ and tissue examined to date, and it is this property that has garnered considerable interest among investigators pursuing therapies for a myriad of inflammatory diseases that play a significant role in the recruitment of leukocytes during an inflammatory response.4,5 This task is daunting considering that more than 40 human chemokines have been discovered and these factors bind to at least 17 receptors, many in a redundant and promiscuous manner.6
The recognition that certain chemokines direct the recruitment of both Th2 cells and other effector cells of allergic inflammation led to the pursuit of therapeutic strategies that relied on the targeting of these chemokines.7 Unfortunately, it has become clear that chemokines and chemokine receptors detected in diseased clinical tissue do not necessarily reflect an actual role for these mediators and their receptors in the disease process. For example, increased CCR48–11 and its corresponding ligands CCL17 (TARC)12–16 and CCL22 (MDC)14,15,17 have been detected in various clinical samples from asthmatic and allergic patients. However, the relative role for these chemokines and its receptor in experimental forms of these diseases remains controversial because some groups report a role for CCL1718 and CCL2219 but a lack20,21 or partial22,23 role for CCR4 during Th2-type cytokine-driven allergic airway responses in mice.
Although originally described as thymus-specific chemokines,24 both CCL17 and CCL22 are expressed in a number of organs including lung, liver, and skin. CCL17 expression has been associated with normal human bronchial epithelial cells,8,12 fibroblasts,25 and smooth muscle cells,26 and this chemokine is regulated by both Th1- and Th2-type cytokines. CCL22 is highly expressed constitutively in mononuclear cells including macrophages, B cells, and monocyte-derived dendritic cells,27,28 but not in other immune cells such as natural killer cells, monocytes, and CD4 lymphocytes until appropriately stimulated.28 Nonimmune cells such as those of epithelial, endothelial, or fibroblastic origin do not constitutively nor are they induced to express CCL22.27 Both ligands bind CCR4,28,29 a chemokine receptor shown to be expressed on various T-cell subsets including Th2 cells,11,28,30,31 natural killer cells,32 dendritic cells, and macrophages.33
The pulmonary granulomatous response is a highly effective albeit potentially tissue-damaging response to the presence of poorly digestible elements in several organ systems including the lung.34 Chemokines have emerged as major players in the orchestration of the inflammatory leukocytes that comprise the granuloma35 but they also participate directly in the activation of structural cells, which provide critical components such as extracellular matrix to the granuloma.36 To examine more thoroughly the relative contribution of CCL17 and CCL22 to heavily-skewed pulmonary Th2-type cytokine response, we used a model initiated by the sensitization of mice to Schistosoma mansoni egg antigen followed by an intravenous bolus of live S. mansoni eggs.37 Previous studies have demonstrated that this granulomatous response is mediated exclusively by Th2 cells,38,39 and is modulated by interleukin (IL)-10,40 IL-12,41 IL-13,42 and CCL2.43,44 In the present study, we examined the relative expression of CCR4 and it ligands after the intravenous introduction of live S. mansoni eggs into S. mansoni-sensitized mice. Using specific immunoneutralization approaches, the relative roles of CCL17 and CCL22 were also examined after pulmonary embolization into mice. Together, these data show a major role for CCL22 over CCL17 in the initiation and maintenance of the Th2 granulomatous response elicited by S. mansoni eggs in the sensitized lung.
Materials and Methods
S. mansoni Egg-Induced Pulmonary Granuloma Model
Female, CBA/J mice, 6 to 8 weeks of age (Jackson Laboratories, Bar Harbor, ME) were immunized intraperitoneally with 3000 live, mature S. mansoni eggs as described in detail previously.37 Live S. mansoni eggs were purified from Swiss-Webster mice heavily infected with S. mansoni, which were kindly provided by Dr. Fred Lewis (Biomedical Research Laboratory, Rockville, MD). Fourteen days later, secondary, synchronous pulmonary granulomas were subsequently induced in S. mansoni egg-sensitized mice by an intravenous infusion of 3000 live S. mansoni eggs. Groups of 10 mice were examined immediately before egg infusion (ie, T = 0) and similarly sized groups of mice were examined at days 4 and 8 after S. mansoni egg embolization.
Isolation and Culture of Primary Pulmonary Fibroblast Lines
Whole lungs from five S. mansoni-sensitized mice at day 8 after S. mansoni egg infusion were prepared as previously described in detail.36,45 Briefly, each mouse was euthanized by a sodium pentobarbital overdose, and whole lung samples were dissected free from the thoracic cavity. The whole lung samples were then finely dispersed on sterile steel mesh and the dispersed cells were then placed into 150-cm2 cell culture flasks (Corning Inc., Corning, NY) containing Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 15% fetal bovine serum (DMEM-15). Normal fibroblasts were obtained from five noninflamed, SPF mice according to the same protocol. The dispersed lung cells were maintained in DMEM-15 at 37°C in a 5% CO2 incubator and were serially passaged a total of five times to yield pure populations of lung fibroblasts as previously described.46 Our previous characterization of these primary fibroblast cell lines is described in detail elsewhere.36,45 Each well in a six-well tissue culture plate was seeded with ∼2.5 × 105 fibroblasts. Twenty-four hours later, the DMEM growth medium was replaced with RPMI containing 10% fetal bovine serum (RPMI 10) containing IL-4, interferon (IFN)-γ, or IL-13 at 10 ng/ml. All cytokines were obtained from R&D Systems (Minneapolis, MN). Twenty-four hours later, cell-free supernatants were removed from each well for the measurement of CCL17 levels by enzyme-linked immunosorbent assay (ELISA) (see below).
Alveolar Macrophage Isolation and Culture
Alveolar macrophages were isolated from bronchoalveolar lavage samples taken from S. mansoni-sensitized mice at day 8 after S. mansoni egg infusion. Bronchoalveolar lavages were obtained through the multiple intratracheal introduction of 1 ml of phosphate-buffered saline containing 50 mmol/L ethylenediaminetetraacetic acid. Preparations of alveolar macrophages were typically greater than 95% pure, and these cells were suspended in RPMI 1640 containing 10% fetal bovine serum (RPMI 10) at 1.0 × 105 cells/well of a six-well tissue culture plate. Individual wells were then exposed to RPMI 10 alone for 24 hours. Cell-free supernatants were subsequently removed from each well for the measurement of CCL17 and CCL22 levels by ELISA (see below).
Anti-CCL17 and Anti-CCL22 Therapy During Pulmonary Granulomatous Responses to Embolized S. mansoni Eggs
Anti-CCL17 and anti-CCL22 neutralizing antibodies have both been previously used in in vivo studies directed at understanding their roles in experimental Th2-cytokine-mediated allergic airway disease.18,19 Both antibodies were tested for cross-reactivity and neither polyclonal antibody cross-reacted with a panel of CC and CXC chemokine ligands (CCL1-CCL28 and CXCL1-CXCL8). In the present study, groups of 15 mice received intraperitoneal injections of 20 μg/dose of either anti-CCL17 or anti-CCL22 antibodies (goat anti-mouse antibodies, R&D Systems) dissolved in 500 μl of sterile normal saline starting immediately after the S. mansoni egg infusion and every other day thereafter until days 4 or 8. Control groups received a 20 μg/dose of normal goat IgG (R&D Systems) dissolved in 500 μl of sterile normal saline via the intraperitoneal route throughout the same time course. Mice were then examined at days 4 or 8 after the induction of the pulmonary granulomatous response. At both times after egg embolization, whole lung samples from each mouse were subjected to molecular, ELISA, histological, morphometric, and immunohistochemical analysis (see below). The effects of anti-CCL17 and anti-CCL22 antibodies on the S. mansoni egg granulomatous response were examined in three separate experiments.
Real-Time TaqMan Polymerase Chain Reaction (PCR) Analysis
Total RNA was isolated from right lung lobes from groups of five mice at days 0 (ie, immediately before eggs), 4, and 8 after the S. mansoni egg infusion. A total of 0.5 μg of total RNA was reverse-transcribed to yield cDNA, and CCL17, CCL22, and CCR4 gene expression were analyzed by real-time quantitative reverse transcriptase-PCR procedure using an ABI PRISM 7700 sequence detection system (Applied Biosystems, Foster City, CA). GAPDH was analyzed as an internal control. All primers and probes were purchased from Applied Biosystems. Gene expression was normalized to GAPDH before the fold change in gene expression was calculated. The fold increases in CCL17, CCL22, and CCR4 gene expressions were calculated via the comparison of gene expression of this chemokine receptor in naïve mice. CCR4 levels in naïve mice were assigned a value of 1.
Lung Histological, Morphometric, and Immunohistochemical Analysis
Whole lungs from untreated, anti-CCL17 antibody, anti-CCL22 antibody, and control IgG groups of mice (n = 5/group/time point/experiment) at days 4 or 8 after the S. mansoni egg challenge were fully inflated with 10% formalin, dissected, and placed in fresh formalin for an additional 24 hours. Routine histological techniques were used to paraffin-embed the entire lung, and 5-μm sections of whole lung were stained with hematoxylin and eosin (H&E) or with Masson trichrome. Inflammatory infiltrates and structural alterations were examined around individual (ie, containing a single egg) pulmonary granulomas using light microscopy at a magnification of ×200. Morphometric analysis of egg granuloma size was performed as previously described in detail.37 A minimum of 15 granulomas per lung section was analyzed for granuloma size. Once the average granuloma size in each mouse was determined, these measurements were then used for the group mean analysis. Routine immunohistochemical techniques were used to stain whole lung samples for murine CCR4 [goat anti-mouse CCR4 antibody (N-terminal); Capralogics, Hardwick, MA] and F4/80 (rat anti-mouse monoclonal antibody; Abcam Inc., Cambridge, MA) at day 8 after IgG, anti-CCL17, or anti-CCL22 antibody treatment in egg-challenged mice. Control goat IgG was applied to whole lung sections as a negative control.
ELISA Analysis
Murine chemokine (CCL1, CXCL5, CXCL10, CXCL13, CCL17, and CCL22) and cytokine [IL-4, IL-5, IL-13, transforming growth factor (TGF)-β, IFN-γ, IL-12, and tumor necrosis factor (TNF)-α] levels were measured in 50-μl samples from cell-free supernatants either from whole lung homogenates and/or from tissue culture plates using a standardized sandwich ELISA technique. Each ELISA was screened to ensure antibody specificity and recombinant murine cytokines, and chemokines were used to generate the standard curves from which the concentrations present in the samples were derived. The limit of ELISA detection for each cytokine was consistently greater than 50 pg/ml. The cytokine levels in each sample were normalized to total protein levels measured using the Bradford assay.
Hydroxyproline Assay
Left lobe samples from anti-CCL17, anti-CCL22, and control IgG groups of mice (n = 5/group/time point/experiment) at days 4 and 8 after the S. mansoni egg challenge were measured for hydroxyproline using a previously described assay.47 Briefly, a 500-μl sample of lung homogenate prepared for ELISA analysis (see above) was added to 1 ml of 6 N HCl for 8 hours at 120°C. To a 5-μl sample of the digested lung, 5 μl of citrate/acetate buffer (5% citric acid, 7.2% sodium acetate, 3.4% sodium hydroxide, and 1.2% glacial acetic acid, pH 6.0) and 100 μl of chloramine-T solution (282 mg chloramine-T, 2 ml of n-propanol, 2 ml of distilled water, and 16 ml of citrate/acetate buffer) were added. The resulting samples was then incubated at room temperature for 20 minutes before 100 μl of Ehrlich’s solution (Aldrich, Milwaukee, WI), 9.3 ml of n-propanol, and 3.9 ml of 70% perchloric acid were added (Aldrich). These samples were incubated for 15 minutes at 65°C and cooled samples were read at 550 nm in a Beckman DU 640 spectrophotometer. Hydroxyproline concentrations were calculated from a hydroxyproline standard curve (0 to 100 μg of hydroxyproline/ml).
Statistical Analysis
All results are expressed as mean ± SEM (SE). A one-way analysis of variance and a Dunnett’s multiple comparisons test were used to reveal statistical differences between the IgG control, anti-CCL17 antibody, and anti-CCL22 antibody groups before and at days 4 and 8 after the S. mansoni egg challenge; P < 0.05 was considered statistically significant.
Results
Temporal Changes in CCL17 and CCL22 Transcript and Protein Expression During S. mansoni Egg-Induced Pulmonary Granuloma Formation
Quantitative TaqMan analysis for CCL17 and CCL22 transcripts during the course of S. mansoni egg challenge in S. mansoni-sensitized mice is shown in Figure 1. Transcript levels for both CC chemokines were significantly increased in a time-dependent manner, and the highest expression for both was observed at day 8 after egg infusion (Figure 1). These data showed that the introduction of S. mansoni eggs into S. mansoni-sensitized mice resulted in a progressive and significant increase in transcript levels for both CCL17 and CCL22.
Figure 1-4247.
Whole lung CCL17 and CCL22 transcript levels before and at days 4 and 8 after S. mansoni egg infusion into S. mansoni-sensitized mice. RNA was isolated from whole lung samples as described in the Materials and Methods section, and TaqMan PCR was used to quantify chemokine transcript levels. Data are mean ± SEM of n = 5 per group. *, P ≤ 0.05 compared with chemokine transcript levels measured in whole lung samples removed from S. mansoni-sensitized mice immediately before egg injection (ie, day 0).
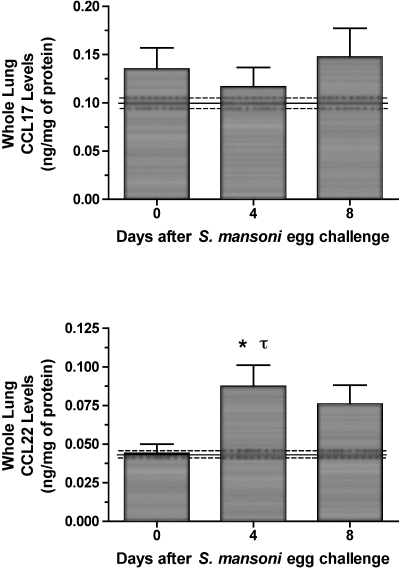
Immunoreactive CCL17 and CCL22 in whole lung samples removed before egg infusion (ie, day 0) and at days 4 and 8 after S. mansoni egg infusion are shown in Figure 2. Although higher levels of CCL17 than CCL22 were found in all whole lung samples analyzed, whole lung CCL17 levels were not significantly elevated above baseline levels (ie, those detected in naïve mouse lung; indicated by dashed lines in Figure 2) during the 8-day course of the S. mansoni egg challenge. In contrast, whole lung CCL22 was significantly elevated at day 4 after egg infusion. Together, these data indicate that CCL22 levels are altered during the pulmonary granulomatous response to embolized S. mansoni eggs.
Figure 2-4247.
Whole lung CCL17 and CCL22 protein levels before and at days 4 and 8 after S. mansoni egg infusion into S. mansoni-sensitized mice. Whole lung samples were processed as described in the Materials and Methods section, and ELISA was used to determine specific chemokine levels in cell-free supernatants. Data are mean ± SEM of n = 5 per group. *, P ≤ 0.05 compared with chemokine levels detected in whole lung samples removed from S. mansoni-sensitized mice immediately before egg injection (ie, day 0). τ, P ≤ 0.05 compared with chemokine levels detected in naïve mice (mean ± SEM denoted by solid and dashed lines, respectively).
Cellular Sources of CCL17 and CCL22 Include Alveolar Macrophages and Pulmonary Fibroblasts
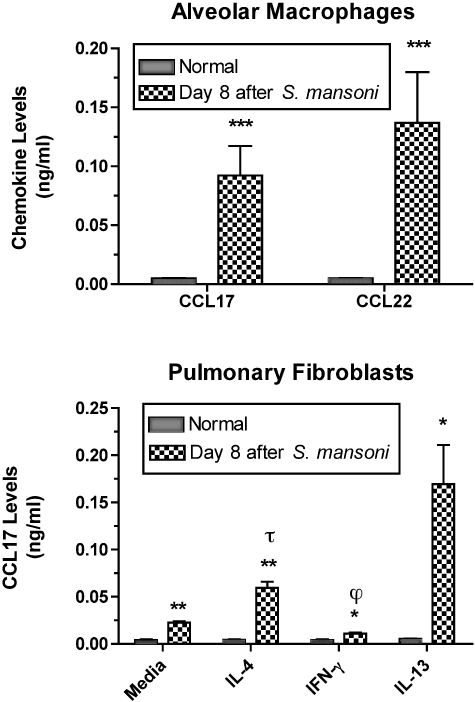
The cellular sources of CCL17 and CCL22 have been extensively studied and it is generally accepted that immune cells generate CCL17 and CCL22 whereas structural cells generate CCL17 but not CCL22.28,33 In the present study, we examined the synthetic capacity of alveolar macrophages and pulmonary fibroblasts derived from naïve and S. mansoni-sensitized mice at day 8 after egg infusion. As shown in the top panel of Figure 3, alveolar macrophages from naïve mice did not constitutively generate CCL17 or CCL22 after 24 hours in culture. Conversely, the same number of alveolar macrophages from S. mansoni-challenged mice generated both CCL17 and CCL22.
Figure 3-4247.
Spontaneous CCL17 and CCL22 generation by isolated alveolar macrophages (top) from naïve (ie, normal) and S. mansoni-sensitized and -challenged mice. Spontaneous and cytokine-induced CCL17 generation by cultured pulmonary fibroblasts (bottom) from naïve and S. mansoni-sensitized and -challenged mice. ELISA was used to determine specific chemokine levels in cell-free supernatants removed at 24 hours after culture. Data are mean ± SEM of triplicate or quadruplicate samples. *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001 compared with chemokine levels detected in culture wells containing normal alveolar macrophages or pulmonary fibroblasts. τ, P ≤ 0.05 compared with chemokine levels detected in tissue culture wells containing media-treated S. mansoni pulmonary fibroblasts. ϕ, P ≤ 0.05 compared with chemokine levels detected in tissue culture wells containing IL-4- or IL-13-treated S. mansoni pulmonary fibroblasts.
In cultures of pulmonary fibroblasts, CCL17, but not CCL22, was detected constitutively and after cytokine treatment for 24 hours (Figure 3, bottom). Significantly greater levels of CCL17 were detected in cultures of S. mansoni pulmonary fibroblasts compared with cultures that contained the same number of normal fibroblasts. Although none of the cytokine treatments affected CCL17 synthesis by normal fibroblasts, IL-4 and IL-13 were strong inducers whereas IFN-γ strongly inhibited CCL17 synthesis by S. mansoni pulmonary fibroblasts (Figure 3). Thus, these data show that S. mansoni egg challenge in S. mansoni-sensitized mice dramatically alters the spontaneous and cytokine-induced synthetic capacity of alveolar macrophages and pulmonary fibroblasts to generate CCL17 and/or CCL22.
Temporal Changes in CCR4 Transcript Expression During S. mansoni Egg-Induced Granulomatous Responses
The primary G-protein-coupled CC chemokine receptor that binds CCL17 and CCL22 is CCR4,24,28,29 although CCL17 has been shown to exhibit binding affinity for CCR8.48 Using quantitative TaqMan PCR and immunohistochemical analyses, we examined whether CCR4 expression was altered during the 8-day course of the egg granulomatous response. As shown in Figure 4, CCR4 transcripts in whole lung samples removed at day 8 after egg infusion were significantly increased fourfold above transcript levels in naïve mouse lung. In addition, the fold-change in CCR4 transcript expression observed at day 8 after egg embolization was significantly greater than the fold change in CCR4 transcript expression observed in S. mansoni-sensitized mice before egg embolization (day 0 group; Figure 4).
Figure 4-4247.
Quantitative TaqMan PCR analysis of CCR4 transcript expression in whole lung sample removed before (ie, day 0) and at days 4 and 8 after S. mansoni egg infusion into S. mansoni-sensitized mice. The fold-increase in CCR4 transcript expression above transcript levels in naïve mouse lungs is shown. Data are mean ± SEM of n = 5 per group. *, P ≤ 0.05 compared with CCR4 transcript levels detected in whole lung samples removed from S. mansoni-sensitized mice immediately before egg injection (ie, day 0).
Immunoneutralization of CCL17 or CCL22 Markedly Decreased the Presence of CCR4-Positive Lung Cells in the Egg Granuloma
Previous studies in our laboratory suggested that the endogenous generation of CCL17 and CCL22 required the presence of CCR4,23 suggesting major sources of both chemokines included CCR4-expressing cells. Our in vitro studies described above suggested that alveolar macrophages and recruited monocytes isolated from the lungs of S. mansoni-sensitized mice at day 8 after egg embolization were an excellent source of both chemokines. Consistent with our hypothesis that these cells also expressed the receptor for both chemokines, abundant CCR4 protein was localized predominantly to alveolar macrophages and recruited monocytes situated around egg granulomas (Figure 5B). Confirmation that the CCR4-positive cells were of monocyte/macrophage origin was confirmed by double staining of these cells with a monoclonal antibody directed against the specific macrophage marker, F4/80, and the anti-CCR4 antibody (staining not shown). In the present study, we examined whether the immunoneutralization of either CCL17 or CCL22 affected the presence of CCR4-positive cells in the lung. As shown in Figure 5, compared with the control IgG group (Figure 5B), immunoneutralization of either CCL17 (Figure 5D) or CCL22 (Figure 5F) markedly diminished the presence of CCR4-positive mononuclear cells in interstitial as well as peri-oval areas in the lung. Additional immunohistochemical analysis using the anti-F4/80 monoclonal antibody demonstrated the absence of F4/80-positive monocytes/macrophages around embolized S. mansoni eggs in both antibody treatment groups (immunostaining not shown). Thus, CCL17 and CCL22 appeared to be responsible for the presence of CCR4-expressing cells in the lung during secondary S. mansoni egg granulomatous responses.
Figure 5-4247.
Immunohistochemical analysis of CCR4 protein expression in formalin-fixed and paraffin-embedded whole lung samples from S. mansoni-sensitized mice at day 8 after S. mansoni egg infusion. Control IgG antibody staining is shown in A, C, and E. Representative CCR4 expression is shown for the control group (B), anti-CCL17 antibody group (D), day 4 (F), and anti-CCL22 antibody group. Original magnifications, ×400.
Immunoneutralization of CCL22 Significantly Reduced the S. mansoni Egg Granuloma Size and Eosinophilia
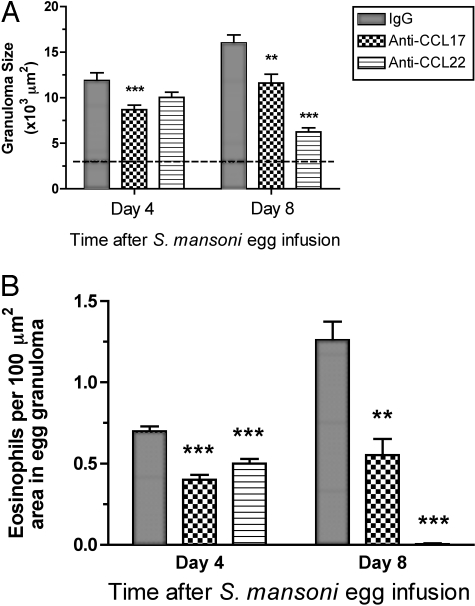
Given that immunoneutralization of CCL17 or CCL22 dramatically reduced the presence of CCR4-expressing cells around the egg granulomas, we next examined whether either antibody treatment altered the overall size of the S. mansoni egg granuloma. At day 4 after egg challenge, egg granulomas in the anti-CCL17 antibody-treated group, but not the anti-CCL22 antibody-treated group, were significantly smaller than egg granulomas measured in the IgG control group (IgG = 11.9 ± 0.8, anti-CCL17 = 8.7 ± 0.5, and anti-CCL22 = 10.0 ± 0.6; × 103 μm2) (Figure 6, top). At day 8 after egg challenge, egg granulomas were significantly smaller in both antibody-treated groups compared with the IgG control group (Figure 6, top). The greatest reduction in S. mansoni egg granuloma size was observed in the group of mice that received the anti-CCL22 antibody treatment until day 8 after egg challenge (IgG = 16.0 ± 0.9, anti-CCL17 = 11.6 ± 1.0, and anti-CCL22 = 6.2 ± 0.4; × 103 μm2).
Figure 6-4247.
Effects of anti-CCL17 and anti-CCL22 antibodies on the size (top) and eosinophil cellularity (bottom) of secondary granulomas at days 4 and 8 after egg infusion into S. mansoni-sensitized mice. Mice received IgG, anti-CCL17 antibody, or anti-CCL22 antibody by intraperitoneal injection beginning immediately after intravenous S. mansoni egg infusion and every other day thereafter. Data are mean ± SEM of n = 5 per group. **, P ≤ 0.01; ***, P ≤ 0.001 compared with granuloma size or eosinophil counts in egg granulomas in S. mansoni-sensitized mice infused with S. mansoni eggs and treated with IgG.
S. mansoni egg granulomas are composed of a number of immune and nonimmune cells including eosinophils, macrophages, lymphocytes, neutrophils, mast cells, and fibroblasts.49 Given the strong Th2-driven response invoked by the S. mansoni egg, eosinophils typically comprise between 50 to 70% of the cells present in the circumoval granulomas.50 The effects of antibody treatment on eosinophil counts in egg granulomas at days 4 and 8 after S. mansoni egg infusion are shown in Figure 6 (bottom). Eosinophils counts were significantly lower in granulomas analyzed from anti-CCL17 antibody-treated mice compared with granulomas analyzed in the control IgG group at day 8 after egg challenge (day 4, 0.47 ± 0.03 versus 0.75 ± 0.03 eosinophils per 100-μm2 area in the egg granuloma; day 8, 0.55 ± 0.10 versus 1.26 ± 0.11 eosinophils per 100-μm2 area in the egg granuloma). In the anti-CCL22 antibody treatment group, eosinophils counts were significantly lower at day 4 after egg embolization compared with the IgG control group (0.51 ± 0.03 versus 0.75 ± 0.03 eosinophils per 100-μm2 area in the egg granuloma), and these cells were completely absent in granulomas in the anti-CCL22 antibody-treated group at day 8.
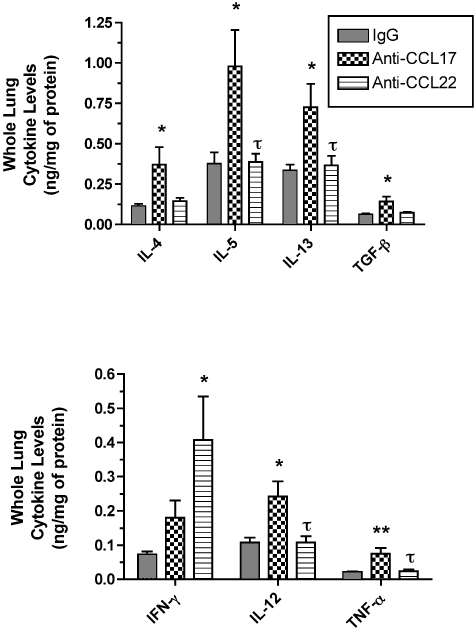
Anti-CCL17 Antibody Treatment Increased the Whole Lung Protein Levels of Th1 and Th2 Cytokines in the Lung Whereas Anti-CCL22 Antibody Treatment Selectively Increased Whole Lung IFN-γ
At day 8 after S. mansoni egg infusion, whole lung samples were removed from each group of mice and ELISA analysis was performed. As shown in Figure 7 (top), the treatment of S. mansoni egg-challenged mice with anti-CCL17 antibody significantly increased the whole lung levels of Th2-associated cytokines including IL-4, IL-5, IL-13, and TGF-β greater than levels detected in whole lung samples from the control IgG group and in the anti-CCL22 antibody group (for IL-5 and IL-13). The anti-CCL17 antibody treatment also significantly increased whole lung levels of IL-12 and TNF-α greater than those whole lung levels detected in the control IgG and anti-CCL22 antibody groups (Figure 7, bottom). However, the anti-CCL17 antibody treatment did not significantly alter whole lung levels of IFN-γ, instead the anti-CCL22 antibody treatment resulted in significant elevations in this Th1-type cytokine in whole lung samples compared with the control IgG group (Figure 7, bottom). Thus, the immunoneutralization of CCL17 generally increased whole lung Th2- and Th1-type cytokines whereas the immunoneutralization of CCL22 appeared to selectively increase whole lung IFN-γ in S. mansoni-sensitized mice challenged with S. mansoni eggs.
Figure 7-4247.
Whole lung IL-4, IL-5, IL-13, TGF-β, IFN-γ, IL-12, and TNF-α at day 8 after S. mansoni egg infusion into S. mansoni-sensitized mice that received control IgG, anti-CCL17 antibody, or anti-CCL22 antibody. Whole lung samples were processed as described in the Materials and Methods section, and ELISA was used to determine specific cytokine levels in cell-free supernatants. Data are mean ± SEM of n = 5 per group. *, P ≤ 0.05 compared with chemokine levels detected in whole lung samples removed from the IgG control group at day 8 after S. mansoni egg embolizaton. τ, P ≤ 0.05 compared with cytokine levels detected in anti-CCL17 antibody-treated mice.
Anti-CCL17 Antibody Treatment Increased the Whole Lung Protein Levels of CC and CXC Chemokines in the Lung
Chemokines are prominently expressed and involved in S. mansoni egg antigen-driven pulmonary granulomatous responses.35,51 In the present study, we examined a large panel of CC and CXC chemokine ligands at day 8 after egg challenge in the control IgG and antibody-treated groups. We observed that anti-CCL17 antibody treatment significantly increased whole lung levels of CCL3, CXCL5, CXCL10, and CXCL13 protein compared with the control and anti-CCL22 antibody treatment groups (Figure 8). Thus, the immunoneutralization of CCL17 significantly increased the levels of CC and CXC chemokine ligands in the egg-challenged lung.
Figure 8-4247.
Whole lung CCL1, CXCL5, CXCL10, and CXCL13 at day 8 after S. mansoni egg infusion into S. mansoni-sensitized mice that received control IgG, anti-CCL17 antibody, or anti-CCL22 antibody. Whole lung samples were processed as described in the Materials and Methods section, and ELISA was used to determine specific chemokine levels in cell-free supernatants. Data are mean ± SEM of n = 5 per group. *, P ≤ 0.05; **, P ≤ 0.01 compared with chemokine levels detected in whole lung samples removed from the IgG control group at day 8 after S. mansoni egg embolizaton. τ, P ≤ 0.05 compared with chemokine levels detected in anti-CCL17 antibody-treated mice.
Hydroxyproline Levels in Lung Homogenates from Anti-CCL17 and Anti-CCL22 Antibody-Treated Mice
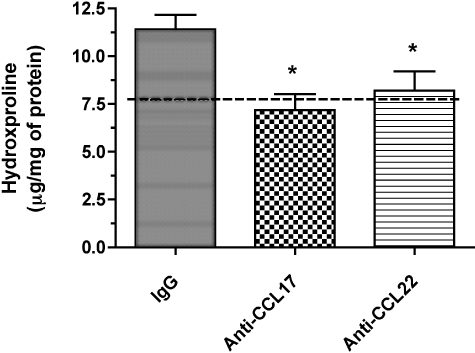
Peri-oval and lung fibrosis are prominent features of granulomatous response to S. mansoni eggs.50,52 This response is driven by Th252–54 and regulated by Th155 cytokines. Biochemical analysis of hydroxyproline levels in whole lung samples is routinely used to assess the degree of peri-oval and lung fibrosis evoked by S. mansoni eggs. The impact of anti-CCL17 and anti-CCL22 antibody treatments on the pulmonary fibrotic response elicited during the egg granulomatous response is shown in Figure 9. The highest whole lung hydroxyproline levels were measured in the control IgG and the lowest levels of whole lung hydroxyproline were measured in the anti-CCL17 antibody group (Figure 9). Statistically significant differences were observed between the antibody treatment groups and the control IgG group at the day 8 time point after egg embolization (Figure 9). Thus, CCL17 and CCL22 contribute to the fibrotic response elicited by S. mansoni egg embolization into S. mansoni-sensitized mice.
Figure 9-4247.
Whole lung hydroxyproline at day 8 after S. mansoni egg infusion into S. mansoni-sensitized mice that received control IgG, anti-CCL17 antibody, or anti-CCL22 antibody (bottom). Whole lung samples were processed as described in the Materials and Methods section. Data are mean ± SEM of n = 5 per group. Dashed line at bottom indicates mean hydroxyproline in S. mansoni-sensitized mouse lungs immediately before egg infusion (7.9 ± 1.5 μg/mg protein) *, P ≤ 0.05 compared with hydroxyproline levels detected in whole lung samples removed from the IgG-treated group at day 8 after S. mansoni egg embolization.
Discussion
Although CCL17, CCL22, and CCR4 have all been shown to be associated with clinical diseases associated with a strong Th2 response such as allergy and asthma, the relative role of these CC chemokines and CCR4 in experimental Th2 diseases remains controversial. Consequently, debate persists as to whether CCR4 and/or its ligands should be targeted in these diseases.56 In the present study, we addressed the relative roles of CCL17 and CCL22 in a heavily skewed Th2-type granuloma response elicited by the intravenous infusion of S. mansoni eggs into S. mansoni-sensitized mice. Examination of this secondary granuloma model has revealed the critical role for Th2 cytokines38,52,57 and CCL237 in the development of these aggressive granulomas. First, we observed that CCL22 and CCR4 levels were markedly increased during the course of this pulmonary granulomatous response. Next, we observed that the immunoneutralization of CCL22, more so than CCL17, resulted in changes in several parameters associated with the formation of egg granulomas in S. mansoni-sensitized mice. These parameters included the presence of CCR4, granuloma size, eosinophil content of the granulomas, whole lung cytokine levels, and the fibrotic response around the egg granuloma. Together, these data show that CCL22 and CCL17, to a lesser extent, are integrally involved in the Th2-driven granuloma response elicited by S. mansoni eggs.
Cellular sources of CCL17 and CCL22 have been extensively examined and it is widely accepted that both chemokines are generated by immune cells27,58,59 whereas nonimmune cells generate CCL17 but not CCL22.28 The induction of CCL17 and CCL22 in immune cells has been shown to be dependent on the presence of Th2 cytokines such as IL-4 and IL-13, but it has been shown that IFN-γ is also a potent stimulus for CCL17 synthesis by bronchial epithelial cells.12,60 This effect of IFN-γ may be restricted to bronchial epithelial cells because airway smooth muscle cells26 and fibroblasts25 do not appear to increase CCL17 synthesis in response to this Th1 cytokine. In addition, we observed that IFN-γ significantly decreased CCL17 synthesis by murine pulmonary fibroblasts grown from day 8 S. mansoni egg-challenged lungs. However, the ability of IFN-γ to regulate CCL17 production lends support to its immunomodulatory role recently elucidated by Katakura and colleagues.61 They recently showed that CCL17, collaboratively with IL-10, is a potent inhibitor of the generation and activation of classically activated macrophages (ie, those macrophages critical to antimicrobial innate immunity). The selective generation of CCL17 by structural cells such as fibroblasts may provide a protective mechanism to prevent overt activation of macrophages, and possibly other immune cells, in its environment. In the present study, we observed that CCL22 but not CCL17 levels were increased during the course of the egg granulomatous response, and this finding is consistent with those of Qui and colleagues34 who showed a marked increase in CCL22 protein levels during schistosomal antigen-elicited pulmonary granuloma formation. However, these investigators did not examine changes in CCL17 protein in their study. The selective increase in CCL22 in the S. mansoni egg granuloma model may reflect the fact that CCL22-generating immune cells were recruited to the lung, or that the resident immune cells were induced to generate this chemokine. Both scenarios are possible, but our in vitro data showed that the spontaneous release of both CCL17 and CCL22 was significantly increased by lung macrophages isolated from day 8 S. mansoni egg-challenged lungs versus the same number of macrophages from naïve lungs.
Numerous investigations have documented that CCR4 is expressed on T cells; not only Th28,28,62 but also other T-cell subsets.30,31,63–65 Although considerable attention has given to T-cell expression of this chemokine receptor, more recent studies have elucidated CCR4 expression on a wide array of immune and nonimmune cells.66,67 Using immunohistochemistry, we observed in the present study that resident alveolar macrophages in the naïve lung stained intensely for CCR4 but other resident cells including bronchial epithelium and smooth muscle were negative. The resident lung phagocytic macrophage is a key cell in the formation and maintenance of the egg granulomatous response34 so it is perhaps not surprising that this cell is equipped to immediately respond to CCL17 and CCL22 in the context of lung inflammatory events.
Numerous studies have shown the therapeutic effect of immunoneutralizing either CCL17 or CCL22 during allergic or Th2-cytokine-driven lung inflammation in mice. Specifically, it has been shown that targeting either CCL1718 or CCL2219 with specific antibodies attenuated ovalbumin-induced airway eosinophilia and diminished the degree of airway hyperresponsiveness. The immunoneutralization of CCL17 also decreased Th2 cytokine levels.18 Later studies indicated that CCL22 was responsible for progressive increase in CCR4-positive cells in more chronic allergic airway responses to ovalbumin.22 The relative role of CCL17 and CCL22 in these responses has been questioned by other data showing that targeting CCR4 either by gene deletion20 or specific antibody21 fails to prevent the development of allergic airway responses although CCR4 does appear to be involved in the maintenance of chronic allergic responses to Aspergillus fumigatus.23 The present study confirmed that CCL22 and CCL17, in part, are critical for the development of aggressive Th2 granulomatous responses in the lung. However, our studies also point to immunomodulatory effects of CCL17 that may explain the lack of anti-inflammatory effect observed in acute allergic airway models in which CCR4 was targeted. In our hands, the immunoneutralization of CCL17 clearly augmented a number of Th2 cytokines that can participate in Th2 responses but at least in the context of the S. mansoni egg granulomatous response, this increase was balanced by a concomitant increase in IL-12, which is a potent down-regulator of the granulomatous response.41,55 In addition, anti-CCL17 antibody treatment significantly increased whole lung levels of certain CC and CXC chemokine ligands such as CCL1, CXCL5, CXCL10, and CXCL13. Specifically, the profound increases in whole lung CXCL10 and CXCL13 levels after anti-CCL17 antibody treatment are interesting and require further investigation. CXCL10 is a potent Th1-associated chemokine68 whereas CXCL13 is the major chemoattractant for B cells, which can induce the apoptosis of CD4(+) T cells during schistosomal granuloma responses.69 In contrast, the immunoneutralization of CCL22, which had the most dramatic effect on all granulomatous parameters examined selectively increased IFN-γ. Thus, although CCL17 and CCL22 have demonstrable regulatory effects on the secondary granulomatous response to S. mansoni eggs, it is plausible that the mechanism through which this regulation is achieved differs markedly between the two CCR4 ligands.
CCL17 appeared to have a greater effect than CCL22 on the presence of extracellular matrix within the granulomatous lung. Although it is not know whether either chemokine directly activates pulmonary fibroblasts to generate collagen, both chemokines have been shown to regulate the movement of eosinophils into the lung.18,19 Human70 and mouse71 eosinophils express CCR4 particularly during an allergic inflammatory response, and these cells have been implicated in experimental and clinical fibrotic responses in the lung.72 In the present study, we observed that the immunoneutralization of either CCL17 or CCL22 significantly reduced the eosinophilia associated with the egg granuloma. The relative contribution of recruited eosinophils to the fibrotic response in granulomas requires further investigation but it is possible that the absence of these cells in the egg granuloma modulated the fibrotic response evoked in this model.
Thus, the present study demonstrates the relative roles for CCL17 and CCL22 during the development of secondary S. mansoni egg granulomas in S. mansoni-sensitized mice. CCL22 appeared to have a more important role than CCL17 in the granulomatous response studied herein, presumably because this chemokine did not appear to have the same immunomodulatory properties as CCL17. In addition, it has recently been reported that CCL22 was a potent and rapid inducer of CCR4 internalization, whereas CCL17 did not induce the same effect.73 The authors of this study speculate that CCL17’s effects may be limited to the endothelial surface where its interaction with CCR4 may be important in vascular recognition whereas CCL22 may exert its major effects within the tissue microenvironment where its interaction with CCR4 guides the localization of various immune cells.73 Regardless of their exact roles, both chemokines appeared to be important in the egg granulomatous response and thus both chemokines may be important targets in pathological granulomatous responses that exhibit a type-2 cytokine phenotype in the lung.
Footnotes
Address reprint requests to Cory M. Hogaboam, Ph.D., Associate Professor, Department of Pathology, University of Michigan Medical School, 1301 Catherine Rd., Ann Arbor MI 48109-0602. E-mail: hogaboam@med.umich.edu.
Supported, in part, by the National Institutes of Health.
References
- Rossi D, Zlotnik A. The biology of chemokines and their receptors. Annu Rev Immunol. 2000;18:217–242. doi: 10.1146/annurev.immunol.18.1.217. [DOI] [PubMed] [Google Scholar]
- Sallusto F, Mackay CR, Lanzavecchia A. The role of chemokine receptors in primary, effector, and memory immune responses. Annu Rev Immunol. 2000;18:593–620. doi: 10.1146/annurev.immunol.18.1.593. [DOI] [PubMed] [Google Scholar]
- Mackay CR. Chemokines: immunology’s high impact factors. Nat Immunol. 2001;2:95–101. doi: 10.1038/84298. [DOI] [PubMed] [Google Scholar]
- Power CA, Proudfoot AE. The chemokine system: novel broad-spectrum therapeutic targets. Curr Opin Pharmacol. 2001;1:417–424. doi: 10.1016/s1471-4892(01)00072-8. [DOI] [PubMed] [Google Scholar]
- Mackay CR. New avenues for anti-inflammatory therapy. Nat Med. 2002;8:117–118. doi: 10.1038/nm0202-117. [DOI] [PubMed] [Google Scholar]
- Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12:121–127. doi: 10.1016/s1074-7613(00)80165-x. [DOI] [PubMed] [Google Scholar]
- Romagnani S. Cytokines and chemoattractants in allergic inflammation. Mol Immunol. 2002;38:881–885. doi: 10.1016/s0161-5890(02)00013-5. [DOI] [PubMed] [Google Scholar]
- Panina-Bordignon P, Papi A, Mariani M, Di Lucia P, Casoni G, Bellettato C, Buonsanti C, Miotto D, Mapp C, Villa A, Arrigoni G, Fabbri LM, Sinigaglia F. The C-C chemokine receptors CCR4 and CCR8 identify airway T cells of allergen-challenged atopic asthmatics. J Clin Invest. 2001;107:1357–1364. doi: 10.1172/JCI12655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurashima K, Fujimura M, Myou S, Kasahara K, Tachibana H, Amemiya N, Ishiura Y, Onai N, Matsushima K, Nakao S. Effects of oral steroids on blood CXCR3+ and CCR4+ T cells in patients with bronchial asthma. Am J Respir Crit Care Med. 2001;164:754–758. doi: 10.1164/ajrccm.164.5.2008132. [DOI] [PubMed] [Google Scholar]
- Banwell ME, Robinson DS, Lloyd CM. Adenoid-derived TH2 cells reactive to allergen and recall antigen express CC chemokine receptor 4. J Allergy Clin Immunol. 2003;112:1155–1161. doi: 10.1016/j.jaci.2003.08.029. [DOI] [PubMed] [Google Scholar]
- Nouri-Aria KT, Wilson D, Francis JN, Jopling LA, Jacobson MR, Hodge MR, Andrew DP, Till SJ, Varga EM, Williams TJ, Pease JE, Lloyd CM, Sabroe I, Durham SR. CCR4 in human allergen-induced late responses in the skin and lung. Eur J Immunol. 2002;32:1933–1938. doi: 10.1002/1521-4141(200207)32:7<1933::AID-IMMU1933>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Berin MC, Eckmann L, Broide DH, Kagnoff MF. Regulated production of the T helper 2-type T-cell chemoattractant TARC by human bronchial epithelial cells in vitro and in human lung xenografts. Am J Respir Cell Mol Biol. 2001;24:382–389. doi: 10.1165/ajrcmb.24.4.4360. [DOI] [PubMed] [Google Scholar]
- Sekiya T, Yamada H, Yamaguchi M, Yamamoto K, Ishii A, Yoshie O, Sano Y, Morita A, Matsushima K, Hirai K. Increased levels of a TH2-type CC chemokine thymus and activation-regulated chemokine (TARC) in serum and induced sputum of asthmatics. Allergy. 2002;57:173–177. doi: 10.1034/j.1398-9995.2002.5720256.x. [DOI] [PubMed] [Google Scholar]
- Hirata H, Arima M, Cheng G, Honda K, Fukushima F, Yoshida N, Eda F, Fukuda T. Production of TARC and MDC by naive T cells in asthmatic patients. J Clin Immunol. 2003;23:34–45. doi: 10.1023/a:1021948214742. [DOI] [PubMed] [Google Scholar]
- Bochner BS, Hudson SA, Xiao HQ, Liu MC. Release of both CCR4-active and CXCR3-active chemokines during human allergic pulmonary late-phase reactions. J Allergy Clin Immunol. 2003;112:930–934. doi: 10.1016/j.jaci.2003.08.012. [DOI] [PubMed] [Google Scholar]
- Nomura T, Terada N, Kim WJ, Nakano K, Fukuda Y, Wakita A, Numata T, Konno A. Interleukin-13 induces thymus and activation-regulated chemokine (CCL17) in human peripheral blood mononuclear cells. Cytokine. 2002;20:49–55. doi: 10.1006/cyto.2002.1979. [DOI] [PubMed] [Google Scholar]
- Lezcano-Meza D, Negrete-Garcia MC, Dante-Escobedo M, Teran LM. The monocyte-derived chemokine is released in the bronchoalveolar lavage fluid of steady-state asthmatics. Allergy. 2003;58:1125–1130. doi: 10.1034/j.1398-9995.2003.00273.x. [DOI] [PubMed] [Google Scholar]
- Kawasaki S, Takizawa H, Yoneyama H, Nakayama T, Fujisawa R, Izumizaki M, Imai T, Yoshie O, Homma I, Yamamoto K, Matsushima K. Intervention of thymus and activation-regulated chemokine attenuates the development of allergic airway inflammation and hyperresponsiveness in mice. J Immunol. 2001;166:2055–2062. doi: 10.4049/jimmunol.166.3.2055. [DOI] [PubMed] [Google Scholar]
- Gonzalo JA, Pan Y, Lloyd CM, Jia GQ, Yu G, Dussault B, Powers CA, Proudfoot AE, Coyle AJ, Gearing D, Gutierrez-Ramos JC. Mouse monocyte-derived chemokine is involved in airway hyperreactivity and lung inflammation. J Immunol. 1999;163:403–411. [PubMed] [Google Scholar]
- Chvatchko Y, Hoogewerf AJ, Meyer A, Alouani S, Juillard P, Buser R, Conquet F, Proudfoot AE, Wells TN, Power CA. A key role for CC chemokine receptor 4 in lipopolysaccharide-induced endotoxic shock. J Exp Med. 2000;191:1755–1764. doi: 10.1084/jem.191.10.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conroy DM, Jopling LA, Lloyd CM, Hodge MR, Andrew DP, Williams TJ, Pease JE, Sabroe I. CCR4 blockade does not inhibit allergic airways inflammation. J Leukoc Biol. 2003;74:558–563. doi: 10.1189/jlb.0103030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd CM, Delaney T, Nguyen T, Tian J, Martinez AC, Coyle AJ, Gutierrez-Ramos JC. CC chemokine receptor (CCR)3/eotaxin is followed by CCR4/monocyte-derived chemokine in mediating pulmonary T helper lymphocyte type 2 recruitment after serial antigen challenge in vivo. J Exp Med. 2000;191:265–274. doi: 10.1084/jem.191.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuh JM, Power CA, Proudfoot AE, Kunkel SL, Lukacs NW, Hogaboam CM. Airway hyperresponsiveness, but not airway remodeling, is attenuated during chronic pulmonary allergic responses to Aspergillus in CCR4−/− mice. FASEB J. 2002;16:1313–1315. doi: 10.1096/fj.02-0193fje. [DOI] [PubMed] [Google Scholar]
- Imai T, Chantry D, Raport CJ, Wood CL, Nishimura M, Godiska R, Yoshie O, Gray PW. Macrophage-derived chemokine is a functional ligand for the CC chemokine receptor 4. J Biol Chem. 1998;273:1764–1768. doi: 10.1074/jbc.273.3.1764. [DOI] [PubMed] [Google Scholar]
- Fukuda K, Fujitsu Y, Seki K, Kumagai N, Nishida T. Differential expression of thymus- and activation-regulated chemokine (CCL17) and macrophage-derived chemokine (CCL22) by human fibroblasts from cornea, skin, and lung. J Allergy Clin Immunol. 2003;111:520–526. doi: 10.1067/mai.2003.59. [DOI] [PubMed] [Google Scholar]
- Faffe DS, Whitehead T, Moore PE, Baraldo S, Flynt L, Bourgeois K, Panettieri RA, Shore SA. IL-13 and IL-4 promote TARC release in human airway smooth muscle cells: role of IL-4 receptor genotype. Am J Physiol. 2003;285:L907–L914. doi: 10.1152/ajplung.00120.2003. [DOI] [PubMed] [Google Scholar]
- Godiska R, Chantry D, Raport CJ, Sozzani S, Allavena P, Leviten D, Mantovani A, Gray PW. Human macrophage-derived chemokine (MDC), a novel chemoattractant for monocytes, monocyte-derived dendritic cells, and natural killer cells. J Exp Med. 1997;185:1595–1604. doi: 10.1084/jem.185.9.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrew DP, Chang MS, McNinch J, Wathen ST, Rihanek M, Tseng J, Spellberg JP, Elias CG., III STCP-1 (MDC) CC chemokine acts specifically on chronically activated Th2 lymphocytes and is produced by monocytes on stimulation with Th2 cytokines IL-4 and IL-13. J Immunol. 1998;161:5027–5038. [PubMed] [Google Scholar]
- Imai T, Baba M, Nishimura M, Kakizaki M, Takagi S, Yoshie O. The T cell directed CC chemokine TARC is a highly specific ligand for CC chemokine receptor 4. J Biol Chem. 1997;272:15036–15042. doi: 10.1074/jbc.272.23.15036. [DOI] [PubMed] [Google Scholar]
- Campbell JJ, Brightling CE, Symon FA, Qin S, Murphy KE, Hodge M, Andrew DP, Wu L, Butcher EC, Wardlaw AJ. Expression of chemokine receptors by lung T cells from normal and asthmatic subjects. J Immunol. 2001;166:2842–2848. doi: 10.4049/jimmunol.166.4.2842. [DOI] [PubMed] [Google Scholar]
- Chantry D, Romagnani P, Raport CJ, Wood CL, Epp A, Romagnani S, Gray PW. Macrophage-derived chemokine is localized to thymic medullary epithelial cells and is a chemoattractant for CD3(+), CD4(+), CD8(low) thymocytes. Blood. 1999;94:1890–1898. [PubMed] [Google Scholar]
- Inngjerdingen M, Damaj B, Maghazachi AA. Human NK cells express CC chemokine receptors 4 and 8 and respond to thymus and activation-regulated chemokine, macrophage-derived chemokine, and I-309. J Immunol. 2000;164:4048–4054. doi: 10.4049/jimmunol.164.8.4048. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Gray PA, Van Damme J, Sozzani S. Macrophage-derived chemokine (MDC) J Leukoc Biol. 2000;68:400–404. [PubMed] [Google Scholar]
- Qiu B, Frait KA, Reich F, Komuniecki E, Chensue SW. Chemokine expression dynamics in mycobacterial (type-1) and schistosomal (type-2) antigen-elicited pulmonary granuloma formation. Am J Pathol. 2001;158:1503–1515. doi: 10.1016/S0002-9440(10)64101-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu BC, Freeman CM, Stolberg VR, Komuniecki E, Lincoln PM, Kunkel SL, Chensue SW. Cytokine-chemokine networks in experimental mycobacterial and schistosomal pulmonary granuloma formation. Am J Respir Cell Mol Biol. 2003;29:106–116. doi: 10.1165/rcmb.2002-0241OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogaboam CM, Bone-Larson CL, Lipinski S, Lukacs NW, Chensue SW, Strieter RM, Kunkel SL. Differential monocyte chemoattractant protein-1 and chemokine receptor 2 expression by murine lung fibroblasts derived from Th1- and Th2-type pulmonary granuloma models. J Immunol. 1999;163:2193–2201. [PubMed] [Google Scholar]
- Matsukawa A, Lukacs NW, Standiford TJ, Chensue SW, Kunkel SL. Adenoviral-mediated overexpression of monocyte chemoattractant protein-1 differentially alters the development of Th1 and Th2 type responses in vivo. J Immunol. 2000;164:1699–1704. doi: 10.4049/jimmunol.164.4.1699. [DOI] [PubMed] [Google Scholar]
- Chensue SW, Warmington KS, Hershey SD, Terebuh PD, Othman M, Kunkel SL. Evolving T cell responses in murine schistosomiasis Th2 cells mediate secondary granulomatous hypersensitivity and are regulated by CD8+ T cells in vivo. J Immunol. 1993;151:1391–1400. [PubMed] [Google Scholar]
- Townsend MJ, Fallon PG, Matthews DJ, Jolin HE, McKenzie AN. T1/ST2-deficient mice demonstrate the importance of T1/ST2 in developing primary T helper cell type 2 responses. J Exp Med. 2000;191:1069–1076. doi: 10.1084/jem.191.6.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores-Villanueva PO, Zheng XX, Strom TB, Stadecker MJ. Recombinant IL-10 and IL-10/Fc treatment down-regulate egg antigen-specific delayed hypersensitivity reactions and egg granuloma formation in schistosomiasis. J Immunol. 1996;156:3315–3320. [PubMed] [Google Scholar]
- Wynn TA, Eltoum I, Oswald IP, Cheever AW, Sher A. Endogenous interleukin 12 (IL-12) regulates granuloma formation induced by eggs of Schistosoma mansoni and exogenous IL-12 both inhibits and prophylactically immunizes against egg pathology. J Exp Med. 1994;179:1551–1561. doi: 10.1084/jem.179.5.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu JS, Frait KA, Reich F, Zhu Z, Elias JA, Chensue SW. IL-13 transgene state impairs mycobacterial (type-1) and schistosomal (type-2) antigen-elicited responses. Cell Immunol. 2001;213:114–121. doi: 10.1006/cimm.2001.1870. [DOI] [PubMed] [Google Scholar]
- Chensue SW, Warmington KS, Lukacs NW, Lincoln PM, Burdick MD, Strieter RM, Kunkel SL. Monocyte chemotactic protein expression during schistosome egg granuloma formation Sequence of production, localization, contribution, and regulation. Am J Pathol. 1995;146:130–138. [PMC free article] [PubMed] [Google Scholar]
- Lu B, Rutledge BJ, Gu L, Fiorillo J, Lukacs NW, Kunkel SL, North R, Gerard C, Rollins BJ. Abnormalities in monocyte recruitment and cytokine expression in monocyte chemoattractant protein 1-deficient mice. J Exp Med. 1998;187:601–608. doi: 10.1084/jem.187.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogaboam CM, Gallinat CS, Bone-Larson C, Chensue SW, Lukacs NW, Strieter RM, Kunkel SL. Collagen deposition in a non-fibrotic lung granuloma model after nitric oxide inhibition. Am J Pathol. 1998;153:1861–1872. doi: 10.1016/S0002-9440(10)65700-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RE, Hogaboam CM, Strieter RM, Lukacs NW, Kunkel SL. Cell-to-cell and cell-to-matrix interactions mediate chemokine expression: an important component of the inflammatory lesion. J Leukoc Biol. 1997;62:612–619. doi: 10.1002/jlb.62.5.612. [DOI] [PubMed] [Google Scholar]
- Jakubzick C, Choi ES, Joshi BH, Keane MP, Kunkel SL, Puri RK, Hogaboam CM. Therapeutic attenuation of pulmonary fibrosis via targeting of IL-4- and IL-13-responsive cells. J Immunol. 2003;171:2684–2693. doi: 10.4049/jimmunol.171.5.2684. [DOI] [PubMed] [Google Scholar]
- Bernardini G, Hedrick J, Sozzani S, Luini W, Spinetti G, Weiss M, Menon S, Zlotnik A, Mantovani A, Santoni A, Napolitano M. Identification of the CC chemokines TARC and macrophage inflammatory protein-1 beta as novel functional ligands for the CCR8 receptor. Eur J Immunol. 1998;28:582–588. doi: 10.1002/(SICI)1521-4141(199802)28:02<582::AID-IMMU582>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Metwali A, Elliott D, Blum AM, Li J, Sandor M, Lynch R, Noben-Trauth N, Weinstock JV. The granulomatous response in murine Schistosomiasis mansoni does not switch to Th1 in IL-4-deficient C57BL/6 mice. J Immunol. 1996;157:4546–4553. [PubMed] [Google Scholar]
- Jakubzick C, Kunkel SL, Joshi BH, Puri RK, Hogaboam CM. Interleukin-13 fusion cytotoxin arrests Schistosoma mansoni egg-induced pulmonary granuloma formation in mice. Am J Pathol. 2002;161:1283–1297. doi: 10.1016/S0002-9440(10)64405-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu BC, Chensue SW. Chemokine responses in schistosomal antigen-elicited granuloma formation. Parasite Immunol. 2002;24:285–294. doi: 10.1046/j.1365-3024.2002.00466.x. [DOI] [PubMed] [Google Scholar]
- Cheever AW, Williams ME, Wynn TA, Finkelman FD, Seder RA, Cox TM, Hieny S, Caspar P, Sher A. Anti-IL-4 treatment of Schistosoma mansoni-infected mice inhibits development of T cells and non-B, non-T cells expressing Th2 cytokines while decreasing egg-induced hepatic fibrosis. J Immunol. 1994;153:753–759. [PubMed] [Google Scholar]
- Wahl SM, Frazier-Jessen M, Jin WW, Kopp JB, Sher A, Cheever AW. Cytokine regulation of schistosome-induced granuloma and fibrosis. Kidney Int. 1997;51:1370–1375. doi: 10.1038/ki.1997.187. [DOI] [PubMed] [Google Scholar]
- Fallon PG, Richardson EJ, McKenzie GJ, McKenzie AN. Schistosome infection of transgenic mice defines distinct and contrasting pathogenic roles for IL-4 and IL-13: IL-13 is a profibrotic agent. J Immunol. 2000;164:2585–2591. doi: 10.4049/jimmunol.164.5.2585. [DOI] [PubMed] [Google Scholar]
- Wynn TA, Cheever AW, Jankovic D, Poindexter RW, Caspar P, Lewis FA, Sher A. An IL-12-based vaccination method for preventing fibrosis induced by schistosome infection. Nature. 1995;376:594–596. doi: 10.1038/376594a0. [DOI] [PubMed] [Google Scholar]
- Lloyd CM, Rankin SM. Chemokines in allergic airway disease. Curr Opin Pharmacol. 2003;3:443–448. doi: 10.1016/s1471-4892(03)00069-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan MH, Whitfield JR, Boros DL, Grusby MJ. Th2 cells are required for the Schistosoma mansoni egg-induced granulomatous response. J Immunol. 1998;160:1850–1856. [PubMed] [Google Scholar]
- Rodenburg RJ, Brinkhuis RF, Peek R, Westphal JR, Van Den Hoogen FH, van Venrooij WJ, van de Putte LB. Expression of macrophage-derived chemokine (MDC) mRNA in macrophages is enhanced by interleukin-1beta, tumor necrosis factor alpha, and lipopolysaccharide. J Leukoc Biol. 1998;63:606–611. doi: 10.1002/jlb.63.5.606. [DOI] [PubMed] [Google Scholar]
- Bonecchi R, Sozzani S, Stine JT, Luini W, D’Amico G, Allavena P, Chantry D, Mantovani A. Divergent effects of interleukin-4 and interferon-gamma on macrophage-derived chemokine production: an amplification circuit of polarized T helper 2 responses. Blood. 1998;92:2668–2671. [PubMed] [Google Scholar]
- Sekiya T, Miyamasu M, Imanishi M, Yamada H, Nakajima T, Yamaguchi M, Fujisawa T, Pawankar R, Sano Y, Ohta K, Ishii A, Morita Y, Yamamoto K, Matsushima K, Yoshie O, Hirai K. Inducible expression of a Th2-type CC chemokine thymus- and activation-regulated chemokine by human bronchial epithelial cells. J Immunol. 2000;165:2205–2213. doi: 10.4049/jimmunol.165.4.2205. [DOI] [PubMed] [Google Scholar]
- Katakura T, Miyazaki M, Kobayashi M, Herndon DN, Suzuki F. CCL17 and IL-10 as effectors that enable alternatively activated macrophages to inhibit the generation of classically activated macrophages. J Immunol. 2004;172:1407–1413. doi: 10.4049/jimmunol.172.3.1407. [DOI] [PubMed] [Google Scholar]
- Imai T, Nagira M, Takagi S, Kakizaki M, Nishimura M, Wang J, Gray PW, Matsushima K, Yoshie O. Selective recruitment of CCR4-bearing Th2 cells toward antigen-presenting cells by the CC chemokines thymus and activation-regulated chemokine and macrophage-derived chemokine. Int Immunol. 1999;11:81–88. doi: 10.1093/intimm/11.1.81. [DOI] [PubMed] [Google Scholar]
- Kim CH, Rott L, Kunkel EJ, Genovese MC, Andrew DP, Wu L, Butcher EC. Rules of chemokine receptor association with T cell polarization in vivo. J Clin Invest. 2001;108:1331–1339. doi: 10.1172/JCI13543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrew DP, Ruffing N, Kim CH, Miao W, Heath H, Li Y, Murphy K, Campbell JJ, Butcher EC, Wu L. C-C chemokine receptor 4 expression defines a major subset of circulating nonintestinal memory T cells of both Th1 and Th2 potential. J Immunol. 2001;166:103–111. doi: 10.4049/jimmunol.166.1.103. [DOI] [PubMed] [Google Scholar]
- Chiu BC, Shang XZ, Stolberg VR, Komuniecki E, Chensue SW. Population analysis of CD4+ T cell chemokine receptor transcript expression during in vivo type-1 (mycobacterial) and type-2 (schistosomal) immune responses. J Leukoc Biol. 2002;72:363–372. [PubMed] [Google Scholar]
- Chantry D, Burgess LE. Chemokines in allergy. Curr Drug Targets Inflamm Allergy. 2002;1:109–116. doi: 10.2174/1568010023344995. [DOI] [PubMed] [Google Scholar]
- Maghazachi AA. G protein-coupled receptors in natural killer cells. J Leukoc Biol. 2003;74:16–24. doi: 10.1189/jlb.0103019. [DOI] [PubMed] [Google Scholar]
- Luster AD, Ravetch JV. Biochemical characterization of a gamma interferon-inducible cytokine (IP-10) J Exp Med. 1987;166:1084–1097. doi: 10.1084/jem.166.4.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundy SK, Lerman SP, Boros DL. Soluble egg antigen-stimulated T helper lymphocyte apoptosis and evidence for cell death mediated by FasL(+) T and B cells during murine Schistosoma mansoni infection. Infect Immun. 2001;69:271–280. doi: 10.1128/IAI.69.1.271-280.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu LY, Jarjour NN, Busse WW, Kelly EA. Chemokine receptor expression on human eosinophils from peripheral blood and bronchoalveolar lavage fluid after segmental antigen challenge. J Allergy Clin Immunol. 2003;112:556–562. doi: 10.1016/s0091-6749(03)01798-6. [DOI] [PubMed] [Google Scholar]
- Pinho V, Oliveira SH, Souza DG, Vasconcelos D, Alessandri AL, Lukacs NW, Teixeira MM. The role of CCL22 (MDC) for the recruitment of eosinophils during allergic pleurisy in mice. J Leukoc Biol. 2003;73:356–362. doi: 10.1189/jlb.0502243. [DOI] [PubMed] [Google Scholar]
- Gharaee-Kermani M, Phan SH. The role of eosinophils in pulmonary fibrosis. Int J Mol Med. 1998;1:43–53. [PubMed] [Google Scholar]
- Mariani M, Lang R, Binda E, Panina-Bordignon P, D’Ambrosio D. Dominance of CCL22 over CCL17 in induction of chemokine receptor CCR4 desensitization and internalization on human Th2 cells. Eur J Immunol. 2004;34:231–240. doi: 10.1002/eji.200324429. [DOI] [PubMed] [Google Scholar]