Abstract
We developed a measure of pericyte/endothelial interaction, the desmin ensheathment ratio (DER), using the intermediate filament desmin as an indicator of pericyte ensheathment and have examined the DER in normal retinal vascular development and in the kitten retinopathy of prematurity (ROP) model. We also examined the role of mural cells in the pathogenesis of ROP. Postnatal day 1 to 45 kitten retinae were labeled for desmin, α-smooth muscle actin (SMA), and isolectin-B4. Newborn kittens exposed to hyperoxia and then returned to room air for 0 to 40 days (dRA) were similarly labeled. The ratio of desmin to lectin labeling on confocal images yielded the DER. Ultrastructural studies showed that mural cells were present on even the most primitive vessels. During normal development, immature vascular beds had DERs of 0.3 to 0.6 whereas mature beds, which predominated by postnatal day 28, had DERs greater than 0.9. Immature pericytes and smooth muscle cells did not prevent hyperoxia-induced vessel regression. During the vasoproliferative stage of ROP, the DERs of intra- and preretinal vessels ranged between 0.2 and 0.5. In the recovery stage, the DER increased in parallel with regression of pathology, reaching 0.9 at 34 dRA. Stabilization of the DER by the fifth postnatal week was temporally coincident with the development of resistance to hyperoxia-induced vessel regression previously reported in the kitten. These observations lead us to suggest that a DER of 0.9 represents a vascular stability threshold and that a low DER observed during ROP raises the possibility that mural cell abnormalities play a key role in the pathogenesis of ROP.
Vessel stability has important implications for many disease processes including sight-threatening diseases of the retina, tumor biology, and diabetic nephropathy. The capillaries of mature vascular beds are considered stable when vascular cell proliferation and vessel regression are negligible and their endothelial cells do not require vascular endothelial growth factor for their survival and are ensheathed by mature mural cells.1–3 In contrast, the capillaries of angiogenic plexuses are considered unstable and are characterized by significant endothelial proliferation, vessel regression in response to vascular endothelial growth factor withdrawal, and ensheathment by immature mural cells. The forming retinal vasculature is an unstable vascular bed and this instability underlies the initiating event in the pathogenesis of retinopathy of prematurity (ROP), the significant vaso-obliteration that occurs when the premature infant is exposed to therapeutic hyperoxia.
Mural cells are thought to play a role in vessel stabilization. In vitro, close contact between mural cells and endothelial cells inhibited endothelial cell proliferation and migration.4,5 More recently impaired mural cell recruitment has been associated with aberrant angiogenesis and vessel regression.6–9 Using retinal digest preparations it was noted that new vessel formation in retina ceased only when pericytes became discernable and neovascularization observed in diabetic retinopathy only occurred when pericytes were lost, leading the authors to suggest that pericytes may inhibit vessel formation.10–12
The retinal digest preparation permitted a quantitative measure of the ratio between the numbers of endothelial cells and pericytes. In diabetic retinopathy, one of the earliest indicators of disease is pericyte dropout. This led to the idea that the absolute ratio of these two cell types is critical to normal retinal vascular function. However, the accuracy of the endothelial cell to pericyte (E/P) ratio is limited by the difficulty in distinguishing these cell types because of their often ambiguous nuclear morphology13,14 and the possibility that the nuclei of perivascular glia may also be included.15
These limitations have led us to develop a new measure of pericyte/endothelial interaction using the intermediate filament desmin as an indicator of pericyte ensheathment. Desmin is expressed by mature and immature pericytes16–18 and a subpopulation of smooth muscle cells (SMCs) on mature and developing arteries, arterioles, venules, and veins.19,20 In this study, we introduce the desmin ensheathment ratio (DER), which is the relative occurrence of desmin to lectin labeling as a measure of vessel stability. Changes in the DER were determined during postnatal maturation of the cat retinal vasculature to test whether the DER correlated with attainment of vessel stability.
To further validate the applicability of the DER as a measure of vascular stability, we applied it to a neovascularizing disease of the retina: ROP. In the kitten model of ROP, kittens are exposed to hyperoxia to produce vaso-obliteration. When the kittens are returned to room air, the absence of retinal vasculature produces massive tissue hypoxia resulting in aberrant neovascularization both within the retina and in the vitreous chamber (the vasoproliferative phase of kitten ROP).21,22 This neovasculature later recovers with substantial vessel regression and the re-establishment of the blood-retinal barrier coinciding with close ensheathment of the vessels by astrocytes.23 DER was determined during the vasoproliferative and recovery phases of the kitten model of ROP as examples of vascular beds undergoing active angiogenesis and remodeling, respectively.
Mural cell abnormalities have been implicated in the pathogenesis of diabetic retinopathy and atherosclerosis. We postulated that mural cells might also play a role in the pathogenesis of ROP. During normal formation of the retinal vasculature under the influence of physiological hypoxia,24,25 the newly formed vessels are closely ensheathed by astrocytes26 and pericytes.27–29 Our earlier studies have demonstrated a significant role played by astrocytes in the pathogenesis of ROP,23 but no previous studies of mural cell changes have been reported in ROP.
In the retina, extensive SMA expression by mural cells has been shown to be associated with vessel stability.30,31 The authors concluded that there was a window of vascular plasticity characterized by the lack of ensheathment by SMA+ pericytes. However more recent reports have shown SMA+ pericytes are also evident on unstable vessels in tumors32,33 indicating that SMA expression alone is not an ideal indicator of vessel stability. Our recent results suggest that mural cells differentiate from a common precursor through a number of immature phenotypes to give rise to pericytes and SMCs in the developing rat retina. Immature mural cells were observed on angiogenic and regressing vessels, leading us to conclude that vessel stability is not conferred by the mere presence of immature mural cells but requires ensheathment by mature mural cells.3
We examined the mural cells during various stages of ROP to further our understanding of the role of mural cells in the pathogenesis of ROP. Mural cells also control blood flow. Clinically, this has implications for ROP with plus-disease in which retinal vessels are dilated and tortuous and are associated with a worse prognosis.34
Materials and Methods
Animals
To induce experimental hyperoxia, posnatal day (P) 1 kittens were placed with a lactating mother in a hyperoxic chamber (70 to 80% oxygen in air) for 4 days35 and then returned to room air for 0, 3, 7, 10, 14, 23, 27, 34, or 40 days (dRA) until sacrifice. Littermate controls were raised in room air from birth for 1, 3, 6, 17, 28, 32, or 45 postnatal days until sacrifice.
Tissue Preparation
Animals were anesthetized with an intramuscular injection of ketamine hydrochloride (33 mg/kg) and xylazine (1 mg/kg), perfused transcardially with 0.1 mol/L phosphate-buffered saline (PBS), pH 7.4 and 4% paraformaldehyde in 0.1 mol/L phosphate buffer, pH 7.4. Retinal whole mounts were prepared as described previously.36 Retinae for immunohistochemistry were immersion-fixed in 4% paraformaldehyde in phosphate buffer for 30 to 60 minutes at 4°C, permeabilized with 1% (v/v) Triton X-100 in PBS for 30 minutes, and then incubated for 30 minutes at room temperature with 1% bovine serum albumin in PBS.
Immunochemistry
Dual labeling was used to co-visualize mural cells and the vasculature. Retinae were incubated overnight at 4°C with primary antibody, washed with 0.1% Triton X-100 in PBS, incubated for 4 hours at room temperature with the appropriate secondary antibody and washed again. Retinae were then incubated with biotinylated Griffonia simplicifolia lectin followed by labeled streptavidin. All antibodies were diluted with 1% bovine serum albumin in PBS, and 0.1% Triton X-100 in 0.1 mol/L PBS was used for all washes. Washed retinal whole mounts were mounted ganglion cell layer up in glycerol: PBS (2:1, v/v) or Prolong Anti Fade (Molecular Probes, Eugene, OR).
Antibodies
To identify both mature and immature mural cells, antibodies against desmin and SMA were used. For desmin immunohistochemistry, we used mouse IgG1 monoclonal antibody (clone D33; DAKO, Carpinteria, CA) diluted 1 in 75. For SMA, we used a mouse IgG2a monoclonal (clone 1A4, Sigma-Aldrich, St. Louis, MO) diluted 1 in 75. To detect desmin labeling we used Texas Red-conjugated (rabbit) anti-mouse IgG1 secondary antibody (Southern Biotechnology Associates, Birmingham, AL) diluted 1 in 60. To detect SMA labeling we used a Texas Red (rabbit)-conjugated anti-mouse Ig secondary antibody (Amersham-Biosciences, Piscataway, NJ) diluted 1 in 50. Labeling with antibodies against desmin and S100 (an astrocyte-specific marker) confirmed that desmin+ cells are a distinct population to astrocytes (data not shown). The endothelium was labeled using biotinylated Griffonia simplicifolia lectin followed by streptavidin conjugated with fluorescein isothiocyanate (Amersham).36,37
Determination of DER
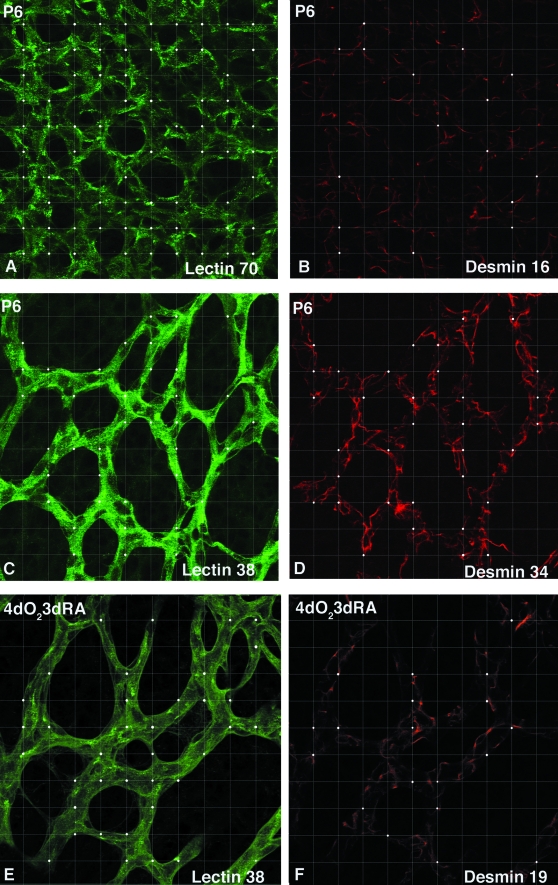
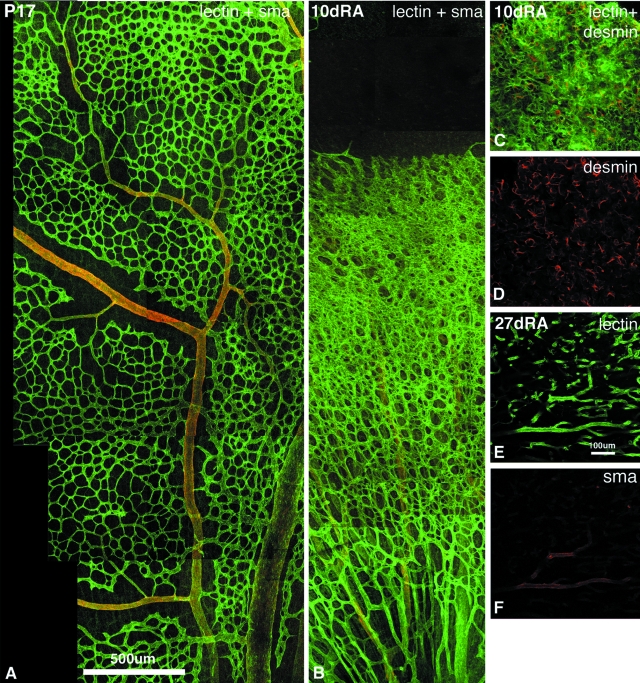
Fluorescently labeled retinal whole mounts were examined by confocal microscopy with a Leica argon-krypton laser mounted on a Leica Axiophot epifluorescence photomicroscope. Fluorescein isothiocyanate and Texas Red fluorescence were excited sequentially at 488 and 588 nm, respectively. The retina was divided arbitrarily into 12 sectors, akin to the 12 hours of a clock. Images were taken in 10 of the 12 sectors. In control cat retinae, regions of mature, remodeled vasculature were selected in the central retina, where the capillary plexus displayed an open capillary mesh with low capillary density and small capillary caliber (Figure 1A; Figure 2G, bottom box). Regions of immature vascular beds with high capillary density and large vessel caliber were captured, just proximal to the leading edge (Figure 1B; Figure 2G, top box). In ROP retinae, regions immediately proximal to the leading edge were selected for analysis. For each field of view selected for analysis, a desmin/G. simplicifolia lectin pair of images was generated. To preserve objectivity, areas captured were selected using the lectin (fluorescein isothiocyanate) channel only, with a ×40 objective. Further, the sequence of analysis was randomized. Each confocal image was overlaid with a 10 × 10 equally spaced grid using Adobe Photoshop V5.0. Figure 3 shows representative fields of view during normal development and ROP. The actual grid has been superimposed onto each image and the actual intersections with lectin and desmin present are shown with a white dot. Although the micrographs show a high resolution, the actual resolution obtained on screen was even higher as a 23-inch Apple studio display with a screen resolution of 1920 × 1200 pixels was used and each half of a field of view filled one entire screen during the actual counting process. The occurrence of desmin labeling relative to lectin labeling at the 100 intersection points yielded the DER. The DER as a function of postnatal age and recovery period in room air were analyzed and plotted using SigmaPlot.
Figure 1-4262.
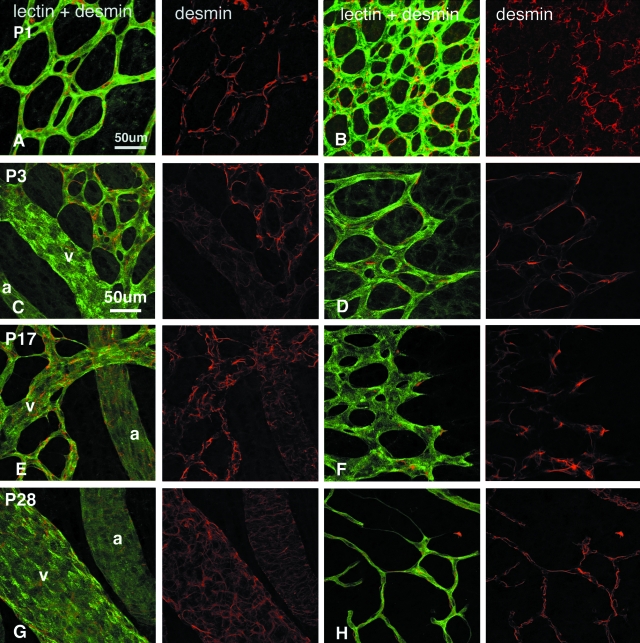
A–H: Retinal vessels double labeled with lectin (green) and antibodies against desmin (red) showing an increase in desmin ensheathment of inner retinal vessels as a function of maturation. Kittens were aged P1 (A, B), P3 (C, D), P17 (E, F), and P28 (G, H). A, C, E, and G: Central radial vessels. B, D, and F: Immature capillaries at the leading edge of vessel formation; and H: a remodeling, more mature, vascular plexus at the periphery. Scale bars, 50 μm.
Figure 2-4262.
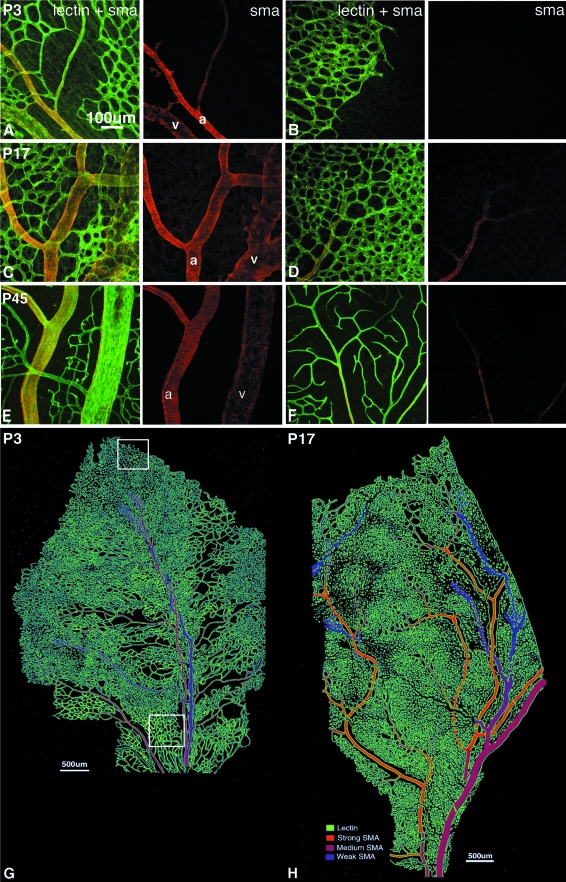
A–F: Retinal vessels double labeled with lectin (green) and antibodies against SMA (red) showing maturation of SMCs on inner vascular plexus during normal development. The intensity, extent, and organization of the SMA filaments increased with maturation. Kittens were aged P3 (A, B), P17 (C, D), and P45 (E, F). A, C, and E: Central radial vessels. B: Immature capillaries at the leading edge of vessel formation. D and F: Peripheral plexuses. G and H: Schematic representation of SMA distribution in the retinal vascular plexus at P3 (G) and P17 (H) in the kitten. Typical locations for image capture are shown for actively angiogenic (white box in peripheral retina) and remodeled, mature (white box in central retina) plexuses. Scale bars: 100 μm (A); 500 μm (G, H).
Figure 3-4262.
Determination of the DER. The figure shows representative fields of view during normal development and ROP. The actual grid with 100 intersection points has been superimposed onto each image and the actual intersections with lectin and desmin present are shown with a white dot. The numbers on each field of view indicate the intersection points with positive label. A and B: An area just proximal to the leading edge of vessel formation in a P6 kitten retina representative of an immature vasculature in which the DER = 0.23. C and D: A central area in a P6 kitten retina representative of a remodeled, mature vasculature where the DER = 0.89. E and F: An area just proximal to the leading edge of the intraretinal neovasculature in a kitten subjected to 4 days of hyperoxia from birth followed by return to room air for 3 days (4dO23dRA) in which the DER = 0.5.
Electron Microscopy
Retinae from control kittens aged P1, 3, 6, 32, and 45 and from ROP kittens at 0, 23, 34, and 40 dRA were examined with transmission electron microscopy. One preretinal membrane obtained during the recovery phase of ROP at 23 dRA was also examined. A sector including the optic nerve head was fixed by immersion in 4% paraformaldehyde at room temperature for 24 hours then transferred to 2% paraformaldehyde at 4°C for storage and transport. Paraformaldehyde-fixed retinas were postfixed in 2.5% glutaraldehyde in 0.1 mol/L cacodylate buffer, pH 7.2. Tissue blocks were treated with in 1% osmium tetroxide, dehydrated in ethanol, and embedded in Spurr’s resin.
Results
Pericyte and SMC Differentiation During Normal Development
Mural cells encompass a continuum of phenotypes from SMCs to pericytes that are characterized by a combination of cell-specific markers, morphology, and location on the vascular tree. By definition, pericytes were found on capillaries, whereas SMCs were found on arteries, arterioles, venules, and veins. In the kitten, pericytes were desmin +/SMA− whereas SMCs were desmin+/−/SMA+.
Figure 1, A to H, shows the changes in desmin ensheathment of retinal vessels and vessel morphology in the central retina (Figure 1; A, C, E, G) and at the leading edge of vessel formation (Figure 1; B, D, F, H). Desmin+ pericytes were found from birth throughout the vascular plexus, including newly formed vessels at the leading edge of vessel formation as well as on major vessels. Their processes ranged from bipolar through to stellate in appearance.
SMA+ SMCs were evident on radial arteries and veins, arterioles, and venules at P3 (Figure 2; A, B, G). At this stage arteries were narrower and more strongly labeled with SMA than were veins, and SMA staining was amorphous, lacking filamentous structure. With maturation, SMA labeling became more widespread, evident on secondary and tertiary arterioles (Figure 2H). From P17 to P45, SMA labeling increased in intensity and revealed organized circumferentially oriented filaments on arteries and distinct stellate processes on veins (Figure 2; C to F) indicative of arterial and venous SMC maturation. SMC differentiation began on the radial arteries and veins, spread to the arterioles and venules, and matured with a proximal-distal topography.
DER During Normal Development
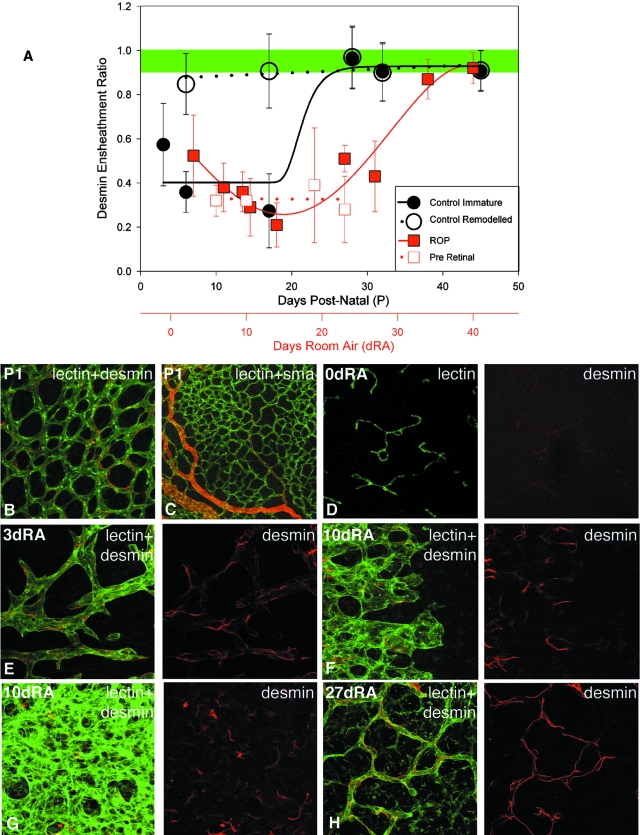
Our determination of the DER based on the relative occurrence of desmin and G. simplicifolia lectin labeling was examined on both immature actively angiogenic and mature, remodeled vascular beds during retinal development in the kitten. In the immature vascular beds at the leading edge of vessel formation, the DER was between 0.3 and 0.6 until P17, before increasing between P17 and P28, reaching 0.97 at P28, and remained greater than 0.9 thereafter (Figure 4A, filled circles). In contrast, in the central regions of the retina where the vascular bed had already undergone substantial remodeling, the DER was 0.85 at P6, reached 0.97 at P28, and remained greater than 0.9 until the last measurement at P45 (Figure 4A, open circles). By the end of the fourth postnatal week the peripheral DER reached that of the central vasculature. The convergence of central and peripheral DER at P28 was consistent with the morphological appearance of vascular maturity at this age.
Figure 4-4262.
A: Plots showing changes in DER as a function of age during normal development, in the peripheral retina, regions of active angiogenesis (filled black circles, r2 = 0.89, sigmoid Chapman 4 parameter fit) and in central retina where the vascular plexus had undergone substantial selection and remodeling (open black circles). Plots also show changes in the DER as a function of days in room air (dRA) after exposure to 70 to 80% for 4 days at birth (filled red squares, r2 = 0.93, fourth order linear regression). DER of preretinal vascular aggregates as a function of days in room air remained less than 0.5 throughout the period of observation (open red squares). Shaded band is above vascular stability threshold. B: Retinal vessels double labeled with lectin (green) and antibodies against desmin (red) showing extensive desmin ensheathment of vessels at P1, before introduction of kitten to hyperoxia. C: Retinal vessels double labeled with lectin (green) and antibodies against SMA (red) at P1 before introduction of kitten to hyperoxia. D–H: Retinal vessels double labeled with lectin (green) and antibodies against desmin (red). D: Weakly labeled desmin+ vascular remnants after 4 days hyperoxia (0 dRA). E, F, and H: The leading edge of vessel formation at 3, 10, and 27 dRA, respectively. G: A dense, central capillary plexus at 10 dRA. Scale bar, 50 μm.
Immature Pericytes and SMCs Do Not Prevent Hyperoxia-Induced Vessel Obliteration
Before kittens were introduced into hyperoxia, desmin+ filaments were already present on virtually all vessels including capillaries at the leading edge of vessel formation and immature SMA+ SMCs were evident on radial arteries and veins (Figure 4, B and C). Despite this extensive ensheathment by mural cells, exposure to 70 to 80% oxygen for 4 days resulted in obliteration of the retinal vasculature including radial vessels. Thus, immature pericytes and SMCs did not prevent hyperoxia-induced vaso-obliteration.
Vascular and Mural Cell Changes During the Hyperoxic Phase of Kitten ROP
After 4 days of exposure to hyperoxia from P1, extensive vascular obliteration occurred, and lectin-labeled vascular remnants were scattered in the central retina (Figure 4D). Weakly labeled desmin processes persisted on some vascular remnants, but SMA was not detected (Figure 4D).
Vascular and Mural Cell Changes During the Vasoproliferative and Recovery Phases of Kitten ROP
On return to room air, a circular multilayered neovascular front formed, centered on the optic disk. Formation of the neovasculature took place in predominantly two layers: a dense superficial capillary layer and a deeper radial plexus with major vessels apparent after 3 dRA. During the early vasoproliferative phase the endothelial cells were characterized by an abnormal rounded morphology (Figure 4, E and F). Desmin+ pericytes were present throughout the neovascular plexus; however, their occurrence was decreased relative to the abnormally dense endothelium seen in the vasoproliferative phase of ROP (Figure 4; E to G). During the recovery phase between 27 dRA and 34 dRA vascular density decreased, the endothelial cells acquired a more elongated morphology and endothelial and mural cells adopted their normal relationship as seen in remodeled vasculature during normal development (Figure 4H).
Large numbers of radial vessels were seen in the deep layers of the retinal neovasculature. Despite the aberrant nature of the neovasculature, SMA immunoreactivity was similar to that observed during normal development, revealing a distinction in labeling between arteries and veins (Figure 5, A–B). As the leading edge of neovascularization approached the edge of the retina at 10 dRA, a lag of ∼300 to 400 μm between the neovascularization front and the appearance of SMA immunoreactivity was observed on radial vessels. The magnitude of this lag was similar to that observed during normal development (Figure 5B and Figure 2H). During the recovery phase, the proximal-distal gradient of SMC maturation associated with normal selection of major vessels was preserved, as was the increase in intensity, extent, and filamentous organization of SMA.
Figure 5-4262.
A and B: Photographic montages of retinal vascular segments double labeled with lectin (green) and antibodies against SMA (red) showing maturation of SMCs during normal development (A) and delayed vascular remodeling during the vasoproliferative phase of ROP (B). Radial vessels ensheathed by SMA+ SMCs were evident deeper to the dense neovascularization in B. C and D: Pericyte ensheathment of preretinal vascular membrane at 10 dRA. E and F: SMC ensheathment of preretinal vascular membrane at 27 dRA. Scale bars: 500 μm (A); 100 μm (E).
DER During Vasoproliferative and Recovery Phases of Kitten ROP
During the vasoproliferative, angiogenic phase, the intraretinal neovasculature had a DER of 0.52 at 3 dRA. The DER reached a minimum of 0.21 at 14 dRA, rose during the recovery phase to 0.87 at 34 dRA, and remained greater than 0.9 thereafter (Figure 4A, red squares). Thus, a DER greater than 0.9 coincided with regression of pathology in the kitten model of ROP.
Changes in the Vasculature, Mural Cells, and DER in Preretinal Membranes
Preretinal membranes consisting of capillary-sized vessels were present in the vitreous humor at 10, 14, 23, and 27 dRA. Desmin+ pericytes were associated with these vascular membranes (Figure 5, C and D). The endothelial cells were ensheathed by desmin filaments, although the density of desmin coverage relative to the density of vascular endothelial cells was markedly reduced, as measured by DER, which varied between 0.2 and 0.4 (Figure 4A, open red squares). SMA ensheathment was only evident on a small number of the slightly larger caliber vessels within preretinal membranes (Figure 5, E and F).
Mural Cell Ensheathment on Unstable Vessels: Ultrastructural Evidence from Normal Development and ROP
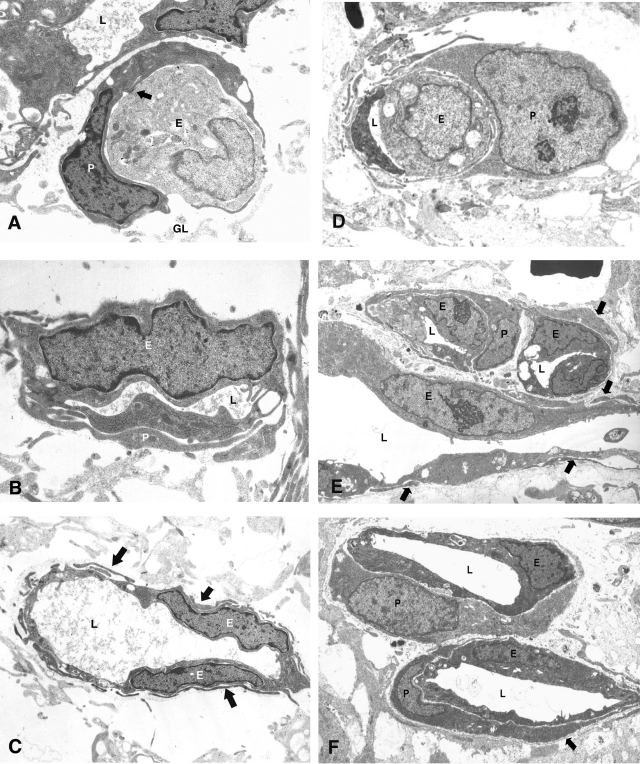
Ultrastructural analyses provided morphological evidence of pericytes on all vessels including the most primitive capillaries both in the normal developing vasculature and in the neovasculature of kitten ROP (Figure 6). Newly formed vessels in the region 40 to 200 μm central to the most anterior extension of the vasculature were examined. During development pericytes (Figure 6; A to C) were seen on the most primitive vessels including those undergoing lumen formation (Figure 6A). Similarly, in the hypoxia-induced neovasculature of the ROP kittens, pericytes were seen on primitive capillaries with slit-like lumens (Figure 6D) as well as more established vessels (Figure 6, E and F). Primitive capillaries during the vasoproliferative phase of kitten ROP with narrow lumen and plump endothelial cells were ensheathed by immature pericytes characterized by their close apposition to the endothelium, lack of basement membrane, and a cytoplasm rich in polysomes but without significant rough endoplasmic reticulum. Established capillary profiles were characterized by endothelial cells with euchromatic nuclei and a cytoplasm rich in cell organelles. Ensheathing pericytes had similar nuclear and cytoplasmic features and covered a significant proportion of the abluminal side of the endothelium.
Figure 6-4262.
Pericyte ensheathment during normal development in a P0 kitten retina (A–C) and during the vasoproliferative phase of the kitten model of ROP at 23 dRA (D–F). A: EM of developing capillaries proximal to the most anterior extension of the retinal vasculature in a P0 kitten. A pale endothelial cell (E), that on serial section was shown to be engaged in lumen formation, is ensheathed by a more darkly stained pericyte (P). There are extensive areas of close apposition and possible adhesion (arrow) between the two cell types and the vessel is enmeshed in a flocculent basement membrane that separates the vascular cells from the pale-stained processes of the surrounding glia (GL); L marks lumen of adjacent vessel. B: EM of primitive capillary adjacent to that depicted in A showing an endothelial cell (E) with narrow lumen (L) and closely applied pericyte processes (P). C: Small vessel with established lumen (L) from same region but upstream of those in A and B is surrounded by numerous pericyte processes (arrows). D: Electron micrograph showing a primitive capillary with a narrow lumen (L) and plump endothelial cell. The vessel is primarily ensheathed by an immature pericyte, so defined by its close apposition to the endothelium, lack of basement membrane, and cytoplasm rich in polysomes but without significant rough endoplasmic reticulum. E: Electron micrograph showing two transverse and one longitudinal capillary profiles (lumena, L) within a neovascular complex. The plump endothelial cells (E) have euchromatic nuclei with prominent nucleoli and a cytoplasm rich in cell organelles. Ensheathing pericytes have similar nuclear and cytoplasmic features and cover a significant proportion of the abluminal side of the endothelium. Longitudinal section reveals that many pericyte processes (arrows) are extremely attenuated. F: Electron micrograph showing two well-established capillary profiles within a neovascular complex. The plump endothelial cells (E) have euchromatic nuclei and cytoplasm rich in cell organelles. Ensheathing pericytes have similar nuclear and cytoplasmic features and cover a significant proportion of the abluminal side of the endothelium. A scanty basement membrane is present (arrow). All micrographs were taken from a region 40 to 200 μm central to the most anterior extension of neovascular tissue.
This analysis provided ultrastructural evidence that even the most immature vascular segments are ensheathed by a pericyte, thus confirming our results above and our earlier conclusion3 that the mere presence of mural cells is not an indicator of vessel stability.
Discussion
The limitations of earlier measures of vessel stability led us to explore an alternative. Using the combined advantages offered by the retinal whole mount preparation and the feline model of ROP, our studies aimed to develop a new measure of pericyte-endothelial interaction using the intermediate filament desmin as an indicator of pericyte ensheathment. We measured the DER during normal retinal vascular development and during the various stages of ROP in the kitten.
A DER of 0.9 Is Indicative of Vascular Stability
We have introduced a new measure of pericyte/endothelial interaction using the frequency of desmin ensheathment of lectin-labeled vasculature. During normal development of the feline retina vascular beds deemed immature by qualitative criteria had a low DER. The DER increased with vessel maturation and reached a plateau of 0.9 in plexuses with mature morphology. A DER of 0.9 was observed across the entire retina at P28. Stable levels of DER emerged between P21 to P28 and took place with disk to peripheral topography of maturation. In ROP, the preretinal vascular membranes and the intraretinal neovasculature observed within the first 4 weeks of return to room air were characterized by a DER between 0.2 and 0.5, which, during the recovery phase increased in parallel with regression of pathology to reach 0.9 at 34 dRA. We hypothesize that a DER of 0.9 and greater is indicative of vascular stability.
Stabilization of the DER by the fifth postnatal week was temporally coincident with the development of resistance to hyperoxia-induced vessel regression previously reported in the kitten; obliteration was severe when hyperoxia was initiated at P1 to P14, mild at P15 to P21, and doubtful or absent when started at P22 or later.38 Our theory predicts that hyperoxia at P17 should selectively obliterate vessels in the peripheral retina with DERs less than 0.4 and spare remodeled central vessels with DERs greater than 0.9 (Figure 4). The observations of Ashton and colleagues38 who noted that hyperoxia exposure at P15 to P21 in the kitten resulted in partial vaso-obliteration, only in the peripheral retina, fit exactly with our predictions from the DER values during normal development.
We have shown that by P28 the DER in both the central and peripheral vasculature is greater than 0.9 (Figure 4). Our hypothesis predicts that at P28 and older, the kitten retinal vasculature will not be susceptible to hyperoxia-induced vessel regression. The early work of Ashton and colleagues38 showed that kittens aged P29, P50, and P58 were not susceptible to hyperoxia-induced vaso-obliteration, which is in accord with our prediction. These observations lead us to suggest that a DER of 0.9 represents a vascular stability threshold and that the DER could serve as an indicator of vessel stability in disease states, such as diabetic retinopathy. Further experimentation is required to provide compelling evidence for this claim in other disease models.
A higher DER value may reflect a decrease in capillary endothelial density or it may reflect an increase in desmin filament coverage of the endothelium. A decrease in endothelial density is supported by our morphological examination in which immature plexuses are characterized by the presence of plump, rounded, vascular endothelial cells that mature into more narrow and linear networks. Desmin is a member of the family of intermediate filament proteins with the widely accepted function of providing structural rigidity to the cell’s cytoskeleton and acting as an anchor for the cell’s contractile machinery. We posit that an absolute increase in desmin coverage also contributes to the rise in DER with vessel maturation, although further studies are needed to confirm this.
Other evidence for a potential role of desmin in vessel stabilization comes from studies using the desmin-deficient mice.39In mice deficient of desmin, both passive and active stress in arterioles was lower than in wild-type animals.39 Further, Osborn and colleagues19 discussed the possibility that desmin expression may coincide with the turning on of a specially regulated contractility program. Although these insights into desmin function have come from studies of arterial SMCs, it is possible that desmin may play a similar role in pericytes.
Comparisons with Earlier Indices of Mural Cell Ensheathment: E/P Ratio, Mural Cell Coverage Index
The accuracy of the E/P ratio derived from retinal digests depended on the clear morphological distinction between endothelial and pericyte nuclei, as seen in the human,11 dog,40 and cat.41 In the mouse, nuclear distinctions were ambiguous in at least 25% of the nuclei counted, which led the authors to conclude that the calculation of E/P ratio in this species had poor reproducibility.14 Thus, in the mouse any pathological changes would require large perturbations to be detected because of the lower sensitivity of this measure.
This may explain why our DER results for normal development in the cat differ from the E/P ratio results of Wu and colleagues,42 for the postnatal development of the mouse retina in which they found no changes in this ratio from P7 to P21. Another possible explanation for this difference could be that the trypsin digest method might also include somas of astrocytes, perivascular microglia, as well as pericytes, whereas the DER is a measure of desmin ensheathment by pericytes. The observed differences might also be explained by species differences, because formation of the retinal vasculature involves both vasculogenesis and angiogenesis in the human3 and kitten retina,37 whereas in the mouse, evidence for vasculogenesis in the mouse retina is currently lacking.
Abramsson and colleagues33 developed a mural cell coverage index by quantifying vessel profiles associated with SMA+ cells and showed that mural cell coverage index varied between tumor types but is constant throughout a range of vascular densities for a given tumor type. The authors concluded that this index is influenced by mural cell density, spreading, and shape and therefore provides limited information. However, our present observations and our earlier report3 have shown that SMA is expressed transiently by a population of mural precursors and is again expressed by earliest appearing SMCs as well as some pericytes found on mature capillaries. Thus, an index using SMA would be unable to reliably distinguish between immature unstable vessels and mature stable vessels.
Immature SMCs Do Not Stabilize Vessels
We have shown in this study that the mural cells of undifferentiated vessels and immature SMCs do not prevent hyperoxia-induced vessel regression and thus do not stabilize vessels. Our earlier observation3 that vessel stability in the developing rat retina was coincident with the expression of the regulatory proteins, caldesmon and calponin in arteriolar SMCs, led us to suggest that SMC maturation, as indicated by the expression of these two regulatory proteins and the resulting capacity to fine tune autoregulatory responses to changes in metabolic demand, may play an important role in vessel stabilization. Recent studies have shown that transforming growth factor-β1-expressing pericytes and SMCs are specifically found on vessels resistant to oxygen-induced regression and have been suggested to be another possible indicator of vessel stability in the mouse during retinal development.43
Taken together, observations from the present study and from previous work support the notion that vessel stabilization results from a complex interplay of maturation processes such as the expression of proteins that contribute to the regulation of blood flow, as well as the expression of other markers indicative of pericyte and SMC maturation.
Role of Mural Cells in the Pathogenesis of ROP
Earlier reports have demonstrated the key role played by astrocytes23 and vascular endothelial growth factor22 in the pathogenesis of ROP, but little has been known of the role of pericytes and SMCs in this disease. Given the end stage of ROP, plus-disease has a poor prognosis in the human infant and because this stage is characterized by tortuosity and vasodilatation, which is thought to result from the shunting of blood flow at the ridge, it suggested to us that SMCs and pericytes play a key role in this disease because of their functions, known and postulated, in regulating retinal blood flow.
This study has shown that mural cells play a role in virtually every stage in the pathogenesis of ROP. Our observations show that mural cell immaturity as reflected by a low DER determine the susceptibility of a vascular bed to hyperoxia-induced vaso-obliteration, the initiating event in ROP. During the vasoproliferative phase, the retina undergoes an intense phase of hypoxia-induced neovascularization. The neovasculature shows an extremely high vascular density and although all vessels at the leading edge of neovascularization, as well as preretinal vascular membranes, are ensheathed by desmin+ pericytes, the DER varied between 0.2 and 0.5. These data support a low DER being associated with significant pathology because preretinal vessels are never observed except in significant sight-threatening disease. The low DER appears to be associated with endothelial abnormality and could be related to the inability of immature mural cells to constrain endothelial cell proliferation. The DER increased in parallel with regression of pathology, reaching 0.9 at 34 dRA. Although we have shown that changes in DER correlated with disease regression, it is unclear whether this is a causative.
Conclusions
The DER is an indicator of the extent of coverage of the endothelium by an intermediate filament protein, desmin. Using this novel measure, we have shown that during normal development of the feline retina, vessel maturation was associated with an increase in the DER, which reached a plateau of 0.9 in remodeled vascular plexuses. Stabilization of the DER across the entire retina by the fifth postnatal week was temporally coincident with the development of resistance to hyperoxia-induced vessel regression previously reported in the kitten. A reduced DER characterized the neovasculature of the vasoproliferative phase of ROP, whereas a stable DER of 0.9 emerged during the recovery phase. These observations led us to suggest that a DER of 0.9 represents a vascular stability threshold. What has to date been a qualitative and morphological assessment of vascular maturation appears to be embodied quantitatively in the DER. Further studies are required to validate the robustness of this measure and to determine whether the DER could serve as an indicator of pericyte changes in other disease states.
Acknowledgments
We thank Andrew Thompson and Clive Jeffrey for assistance with digital imaging.
Footnotes
Address reprint requests to Tailoi Chan-Ling Ph.D., Department of Anatomy and Histology (F13), University of Sydney, Sydney, NSW 2006, Australia. E-mail: tailoi@anatomy.usyd.edu.au.
Supported by the National Health and Medical Research Council of Australia (grant 153789 to T.C.L.) and the Ronald Geoffrey Arnott Foundation.
References
- Alon T, Hemo I, Itin A, Pe’er J, Stone J, Keshet E. Vascular endothelial growth factor acts as a survival factor for newly formed retinal vessels and has implications for retinopathy of prematurity. Nat Med. 1995;1:1024–1028. doi: 10.1038/nm1095-1024. [DOI] [PubMed] [Google Scholar]
- Benjamin LE, Keshet E. Conditional switching of vascular endothelial growth factor (VEGF) expression in tumors: induction of endothelial cell shedding and regression of hemangioblastoma-like vessels by VEGF withdrawal. Proc Natl Acad Sci USA. 1997;94:8761–8766. doi: 10.1073/pnas.94.16.8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes S, Chan-Ling T. Characterization of smooth muscle cell and pericyte differentiation in the rat retina in vivo. Invest Ophthalmol Vis Sci. 2004;45:2795–2806. doi: 10.1167/iovs.03-1312. [DOI] [PubMed] [Google Scholar]
- Orlidge A, D’Amore P. Inhibition of capillary endothelial cell growth by pericytes and smooth muscle cells. J Cell Biol. 1987;105:1455–1462. doi: 10.1083/jcb.105.3.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y, Rifkin DB. Inhibition of endothelial cell movement by pericytes and smooth muscle cells: activation of a latent transforming growth factor-beta 1-like molecule by plasmin during co-culture. J Cell Biol. 1989;109:309–315. doi: 10.1083/jcb.109.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S, Sato TN, Yancopoulos GD. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 1996;87:1171–1180. doi: 10.1016/s0092-8674(00)81813-9. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, Declercq C, Pawling J, Moons L, Collen D, Risau W, Nagy A. Abnormal blood vessel development and lethality in embryo is lacking a single VEGF allele. Nature. 1996;380:435–439. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, Compton D, McClain J, Aldrich TH, Papadopoulos N, Daly TJ, Davis S, Sato TN, Yancopoulos GD. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997;277:55–60. doi: 10.1126/science.277.5322.55. [DOI] [PubMed] [Google Scholar]
- Enge M, Bjarnegard M, Gerhardt H, Gustafsson E, Kalen M, Asker N, Hammes HP, Shani M, Fassler R, Betsholtz C. Endothelium-specific platelet-derived growth factor-B ablation mimics diabetic retinopathy. EMBO J. 2002;21:4307–4316. doi: 10.1093/emboj/cdf418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogan DG, Toussaint D, Kuwabara T. Retinal vascular patterns IV Diabetic retinopathy. Arch Ophthalmol. 1961;66:366–377. doi: 10.1001/archopht.1961.00960010368014. [DOI] [PubMed] [Google Scholar]
- Kuwabara T, Cogan DG. Retinal vascular patterns (VI) Mural cells of the retinal capillaries. Arch Ophthalmol. 1963;69:472–502. doi: 10.1001/archopht.1963.00960040498013. [DOI] [PubMed] [Google Scholar]
- Hammes HP, Lin J, Renner O, Shani M, Lundqvist A, Betsholtz C, Brownlee M, Deutsch U. Pericytes and the pathogenesis of diabetic retinopathy. Diabetes. 2002;51:3107–3112. doi: 10.2337/diabetes.51.10.3107. [DOI] [PubMed] [Google Scholar]
- Glatt HJ, Henkind P. Aging changes in the retinal capillary bed of the rat. Microvasc Res. 1979;18:1–17. doi: 10.1016/0026-2862(79)90014-1. [DOI] [PubMed] [Google Scholar]
- Cuthbertson RA, Mandel TE. Anatomy of the mouse retina Endothelial cell-pericyte ratio and capillary distribution. Invest Ophthalmol Vis Sci. 1986;27:1659–1664. [PubMed] [Google Scholar]
- Kuwabara T, Cogan DG. Studies of retinal vascular patterns Part I Normal architecture. Arch Ophthalmol. 1960;64:904–911. doi: 10.1001/archopht.1960.01840010906012. [DOI] [PubMed] [Google Scholar]
- Verhoeven D, Buyssens N. Desmin-positive stellate cells associated with angiogenesis in a tumour and non-tumour system. Virchows Arch B Cell Pathol. 1988;54:263–272. doi: 10.1007/BF02899222. [DOI] [PubMed] [Google Scholar]
- Nehls V, Denzer K, Drenckhahn D. Pericyte involvement in capillary sprouting during angiogenesis in situ. Cell Tissue Res. 1992;270:469–474. doi: 10.1007/BF00645048. [DOI] [PubMed] [Google Scholar]
- Hellstrom M, Kalen M, Lindahl P, Abramsson A, Betsholtz C. Role of PDGF-B and PDGFR-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development. 1999;126:3047–3055. doi: 10.1242/dev.126.14.3047. [DOI] [PubMed] [Google Scholar]
- Osborn M, Caselitz J, Weber K. Heterogeneity of intermediate filament expression in vascular smooth muscle: a gradient in desmin positive cells from the rat aortic arch to the level of the arteria iliaca communis. Differentiation. 1981;20:196–202. doi: 10.1111/j.1432-0436.1981.tb01176.x. [DOI] [PubMed] [Google Scholar]
- Kacem K, Seylaz J, Aubineau P. Differential processes of vascular smooth muscle cell differentiation within elastic and muscular arteries of rats and rabbits: an immunofluorescence study of desmin and vimentin distribution. Histochem J. 1996;28:53–61. doi: 10.1007/BF02331427. [DOI] [PubMed] [Google Scholar]
- Chan-Ling T, Tout S, Hollander H, Stone J. Vascular changes and their mechanisms in the feline model of retinopathy of prematurity. Invest Ophthalmol Vis Sci. 1992;33:2128–2147. [PubMed] [Google Scholar]
- Stone J, Chan-Ling T, Pe’er J, Itin A, Gnessin H, Keshet E. Roles of vascular endothelial growth factor and astrocyte degeneration in the genesis of retinopathy of prematurity. Invest Ophthalmol Vis Sci. 1996;37:290–299. [PubMed] [Google Scholar]
- Chan-Ling T, Stone J. Degeneration of astrocytes in feline retinopathy of prematurity causes failure of the blood-retinal barrier. Invest Ophthalmol Vis Sci. 1992;33:2148–2159. [PubMed] [Google Scholar]
- Chan-Ling T, Gock B, Stone J. The effect of oxygen on vasoformative cell division Evidence that “physiological hypoxia” is the stimulus for normal retinal vasculogenesis. Invest Ophthalmol Vis Sci. 1995;36:1201–1214. [PubMed] [Google Scholar]
- Stone J, Itin A, Alon T, Pe’er J, Gnessin H, Chan-Ling T, Keshet E. Development of retinal vasculature is mediated by hypoxia-induced vascular endothelial growth factor (VEGF) expression by neuroglia. J Neurosci. 1995;15:4738–4747. doi: 10.1523/JNEUROSCI.15-07-04738.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling TL, Stone J. The development of astrocytes in the cat retina: evidence of migration from the optic nerve. Brain Res Dev Brain Res. 1988;44:73–85. doi: 10.1016/0165-3806(88)90119-8. [DOI] [PubMed] [Google Scholar]
- Penfold PL, Provis JM, Madigan MC, van Driel D, Billson FA. Angiogenesis in normal human retinal development: the involvement of astrocytes and macrophages. Graefe’s Arch Clin Exp Ophthalmol. 1990;228:255–263. doi: 10.1007/BF00920031. [DOI] [PubMed] [Google Scholar]
- Ozerdem U, Grako KA, Dahlin-Huppe K, Monosov E, Stallcup WB. NG2 proteoglycan is expressed exclusively by mural cells during vascular morphogenesis. Dev Dyn. 2001;222:218–227. doi: 10.1002/dvdy.1200. [DOI] [PubMed] [Google Scholar]
- Fruttiger M. Development of the mouse retinal vasculature: angiogenesis versus vasculogenesis. Invest Ophthalmol Vis Sci. 2002;43:522–527. [PubMed] [Google Scholar]
- Benjamin LE, Hemo I, Keshet E. A plasticity window for blood vessel remodelling is defined by pericyte coverage of the preformed endothelial network and is regulated by PDGF-B and VEGF. Development. 1998;125:1591–1598. doi: 10.1242/dev.125.9.1591. [DOI] [PubMed] [Google Scholar]
- Benjamin LE, Golijanin D, Itin A, Pode D, Keshet E. Selective ablation of immature blood vessels in established human tumors follows vascular endothelial growth factor withdrawal. J Clin Invest. 1999;103:159–165. doi: 10.1172/JCI5028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morikawa S, Baluk P, Kaidoh T, Haskell A, Jain RK, McDonald DM. Abnormalities in pericytes on blood vessels and endothelial sprouts in tumors. Am J Pathol. 2002;160:985–1000. doi: 10.1016/S0002-9440(10)64920-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abramsson A, Berlin O, Papayan H, Paulin D, Shani M, Betsholtz C. Analysis of mural cell recruitment to tumor vessels. Circulation. 2002;105:112–117. doi: 10.1161/hc0102.101437. [DOI] [PubMed] [Google Scholar]
- Anonymous Supplemental therapeutic oxygen for prethreshold retinopathy of prematurity (STOP-ROP), a randomized, controlled trial I: primary outcomes. Pediatrics. 2000;105:295–310. doi: 10.1542/peds.105.2.295. [DOI] [PubMed] [Google Scholar]
- Chan-Ling T, Gock B, Stone J. Supplemental oxygen therapy Basis for noninvasive treatment of retinopathy of prematurity. Invest Ophthalmol Vis Sci. 1995;36:1215–1229. [PubMed] [Google Scholar]
- Chan-Ling T. Glial, vascular, and neuronal cytogenesis in whole-mounted cat retina. Microsc Res Tech. 1997;36:1–16. doi: 10.1002/(SICI)1097-0029(19970101)36:1<1::AID-JEMT1>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Chan-Ling T, Halasz P, Stone J. Development of retinal vasculature in the cat: processes and mechanisms. Curr Eye Res. 1990;9:459–478. doi: 10.3109/02713689008999612. [DOI] [PubMed] [Google Scholar]
- Ashton N, Ward B, Serpell G. Effect of oxygen on developing retinal vessels with particular reference to the problem of retrolental fibroplasia. Br J Ophthalmol. 1954;38:397–432. doi: 10.1136/bjo.38.7.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wede OK, Lofgren M, Li Z, Paulin D, Arner A. Mechanical function of intermediate filaments in arteries of different size examined using desmin deficient mice. J Physiol. 2002;540:941–949. doi: 10.1113/jphysiol.2001.014910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutlu F, Leopold IH. Structure of the retinal vascular system. Am J Ophthalmol. 1964;58:261–270. doi: 10.1016/0002-9394(64)91575-2. [DOI] [PubMed] [Google Scholar]
- Mutlu F, Leopold IH. Structure of the retinal vascular system of cat and rabbit. Am J Ophthalmol. 1964;57:804–814. doi: 10.1016/0002-9394(64)92229-9. [DOI] [PubMed] [Google Scholar]
- Wu Z, Wang S, Sorenson CM, Sheibani N. Hypoxia-mediated loss of astrocytes promotes the pathogenesis of oxygen-induced ischemic retinopathy. Invest Ophthalmol Vis Sci. 2003;244(Suppl):S2095. [Google Scholar]
- Shih SC, Ju M, Liu N, Mo JR, Ney JJ, Smith LE. Transforming growth factor beta1 induction of vascular endothelial growth factor receptor 1: mechanism of pericyte-induced vascular survival in vivo. Proc Natl Acad Sci USA. 2003;100:15859–15864. doi: 10.1073/pnas.2136855100. [DOI] [PMC free article] [PubMed] [Google Scholar]