Abstract
Resistance to drugs can result from changes in drug transport, and this resistance can sometimes be overcome by a second drug that modifies the transport mechanisms of the cell. This strategy has been exploited to partly reverse resistance to chloroquine in Plasmodium falciparum. Studies with human tumor cells have shown that probenecid can reverse resistance to the antifolate methotrexate, but the potential for reversal of antifolate resistance has not been studied in P. falciparum. In the present study we tested the ability of probenecid to reverse antifolate resistance in P. falciparum in vitro. Probenecid, at concentrations that had no effect on parasite viability alone (50 μM), was shown to increase the sensitivity of a highly resistant parasite isolate to the antifolates pyrimethamine, sulfadoxine, chlorcycloguanil, and dapsone by seven-, five-, three-, and threefold, respectively. The equivalent effects against an antifolate-sensitive isolate were activity enhancements of approximately 3-, 6-, 1.2-, and 19-fold, respectively. Probenecid decreased the level of uptake of radiolabeled folic acid, suggesting a transport-based mechanism linked to folate salvage. When probenecid was tested with chloroquine, it chemosensitized the resistant isolate to chloroquine (i.e., enhanced the activity of chloroquine). This enhancement of activity was associated with increased levels of chloroquine accumulation. In conclusion, we have shown that probenecid can chemosensitize malaria parasites to antifolate compounds via a mechanism linked to reduced folate uptake. Notably, this effect is observed in both folate-sensitive and -resistant parasites. In contrast to the activities of antifolate compounds, the effect of probenecid on chloroquine sensitivity was selective for chloroquine-resistant parasites (patent P407595GB [W. P. Thompson & Co., Liverpool, United Kingdom] has been filed to protect this intellectual property).
Malaria remains one of the biggest killer diseases in the world, with almost 2 million people dying each year, the majority of them children in sub-Saharan Africa (44). Chloroquine has for decades been the mainstream treatment for uncomplicated malaria. However, the spread of chloroquine resistance (16, 36) has prompted the introduction of pyrimethamine-sulfadoxine as the first-line treatment of uncomplicated falciparum malaria in many countries, including Kenya, Tanzania, and Uganda, where the level of chloroquine resistance is already very high. Other African countries where chloroquine resistance is increasing are also considering changing to pyrimethamine-sulfadoxine. Unfortunately, resistance to pyrimethamine-sulfadoxine is already spreading at an alarming rate in East Africa (14, 17, 20, 30, 33, 38). In Kenya, for instance, when the rate of resistance to chloroquine (parasitological failure on days 7 to 14 after treatment) in the 1980s was more than 30% in most areas where malaria is endemic (18, 19, 43), parasites in the same areas were fully susceptible to pyrimethamine-sulfadoxine (15, 29). However, at present, the rate of resistance to pyrimethamine-sulfadoxine is greater than 30% (17, 20, 33, 38) in these areas in Kenya. This development is of great concern, since no alternative affordable drugs are available at present to replace pyrimethamine-sulfadoxine.
The mechanism of Plasmodium falciparum resistance to antifolate drugs is now well understood and is primarily due to alterations of the dihydrofolate reductase (DHFR) and dihydropteroate (DHPS) genotypes (23). At present, the only way to overcome resistance to antifolate is to develop better and more potent antifolate agents against these variant enzymes. Unfortunately, this is an expensive and time-consuming process. Consequently, new antifolate drugs may not become available in time to avert the present antifolate resistance crisis.
Similar problems of resistance have been associated with the use of the anticancer agent methotrexate. This drug is a potent inhibitor of human DHFR and is used in the treatment of diverse malignancies (3). Several potential resistance mechanisms have been identified or proposed. These include target site modification (mutations and altered expression levels), deficient uptake via the endogenous folate carrier, decreased polyglutamation, and enhanced drug efflux via an organic acid transporter (2, 4, 11). Importantly, some forms of methotrexate resistance can be reversed by probenecid through inhibition of a drug efflux mechanism (11, 25). Probenecid also inhibits folate salvage, and this can directly influence the activities of antifolates if they use this transporter for intracellular access, in addition to any indirect effects of reducing the intracellular folate pool (27). Folate salvage has been observed in P. falciparum, but it has not been studied extensively (40). In the present investigation, we have tested the hypothesis that antifolate susceptibility in P. falciparum may be influenced by a probenecid-sensitive transport process. These studies are a further step toward defining folate salvage in this parasite. Using a multidrug-resistant parasite isolate and an antifolate- and chloroquine-sensitive parasite isolate, we have measured the effects of probenecid on the DHPS inhibitors sulfadoxine and dapsone, the DHFR inhibitors pyrimethamine and chlorcycloguanil, and the nonantifolate 4-aminoquinoline chloroquine, as a control.
MATERIALS AND METHODS
The test drugs probenecid, pyrimethamine, dapsone, and chloroquine were purchased from Sigma Chemical Co., Dorset, United Kingdom. Sulfadoxine was purchased from Hoffmann-La Roche, Basel, Switzerland; and chlorcycloguanil was a gift from AstraZeneca, London, United Kingdom. Radiolabeled chloroquine was purchased from Moravek, Brea, Calif., while folic acid was purchased from Amersham, Littel Chalfont, United Kingdom. All other reagents were purchased from Sigma Chemical Co.
Three reference P. falciparum laboratory isolates were used in these studies: M24 (fully sensitive to antifolates and chloroquine), HB3 (fully sensitive to chloroquine), and V1/S (a multidrug-resistant isolate). The last isolate has four mutations in its DHFR (at codons 108, 51, 59, and 164) and is highly resistant to anti-DHFR drugs. The V1/S strain is also highly resistant to chloroquine but is moderately resistant to sulfadoxine and dapsone, which target DHPS. P. falciparum isolates were cultured by standard techniques (35). The culture medium, RPMI 1640 (GIBCO BRL, Paisley, United Kingdom) containing para-aminobenzoic acid (PABA) and folic acid at physiological concentrations (0.5 and 10 μg/liter, respectively), was supplemented with 10% (vol/vol) normal human serum, 25 mM bicarbonate, and 25 mM HEPES buffer. Antimalarial activity was measured in the presence of various concentrations of probenecid by the radioisotopic incorporation technique (28). The results were expressed as the drug concentration required for 50% inhibition of [3H]hypoxanthine incorporation into parasite nucleic acid (IC50) by nonlinear regression analysis of the dose-response curve. The mean ± standard deviation IC50s were determined by using a third log dilution series across concentration ranges of approximately 2 log orders on either side of the reported IC50s.
The uptake of 3H-radiolabeled folic acid (25 Ci/mmol) by strain V1/S was measured over a period of 90 min in the absence or presence of probenecid (200 μM). Because of the presence of folate derivatives in standard RPMI 1640 medium, we carried out the uptake experiment in Ringer's buffer by a previously reported protocol (6). The levels of accumulation of 3H-radiolabeled (50.4 Ci/mmol) chloroquine by V1/S and by sensitive isolate HB3 were determined in the presence of various concentrations of probenecid. The concentration of radiolabeled drug was fixed at 5 nM. The assay was conducted over 1 h at 37°C under conditions previously described in detail (6). The results were expressed as the cellular accumulation ratio, which is the ratio of the concentration of labeled chloroquine in the infected cells to the concentration of radiolabeled chloroquine in a similar volume of medium.
Statistical analysis was performed by using the EPI INFO statistical package (version 6). The mean values for the parameters obtained with the three different isolates of P. falciparum in the presence and absence of probenecid were compared by the Mann-Whitney or the Wilcoxon two-sample test, and a P value <0.05 was taken to be statistically significant.
RESULTS
Our first goal was to determine whether probenecid could increase the sensitivity of P. falciparum to various antifolate drugs. We assessed the impacts of various concentrations of probenecid on the in vitro activities of the antifolate drugs pyrimethamine, sulfadoxine, chlorcycloguanil, and dapsone against multidrug-resistant isolate V1/S and fully sensitive isolate M24, which were used as reference strains. The results are summarized in Table 1. Probenecid alone was found to be a very weak antimalarial agent, with a mean IC50 of >1,500 μM for the M24 and V1/S parasites. In preliminary experiments, we had established that at a concentration of 50 μM, probenecid had no antimalarial effect in vitro. We had also established that probenecid had no measurable antimalarial activity at concentrations below 150 μM even over a 2-day incubation period. In the presence of 50 μM probenecid, the IC50 of pyrimethamine for VI/S (representing the most antifolate-resistant isolate described to date) was reduced from 4,000 nM to 611 nM, a sevenfold increase in sensitivity (Table 1). The latter IC50 is within the range of in vivo pyrimethamine and sulfadoxine concentrations effective against P. falciparum parasites with mutations at codons 108 and 51 or codons 108 and 59 of DHFR (41). In the presence of 100 μM probenecid, there is a further 13-fold decrease in the IC50. The sensitivities of M24 to pyrimethamine and sulfadoxine increased almost three- and sixfold, respectively, and a similar concentration of probenecid increased the sensitivity of V1/S to sulfadoxine approximately fivefold (Table 1). The activities of chlorcycloguanil against the M24 and V1/S isolates were also potentiated two- and threefold, respectively, in the presence of 50 μM probenecid. Finally, the sensitivity of V1/S to dapsone was increased 3-fold and the sensitivity of M24 to dapsone was increased 19-fold in the presence of 50 μM probenecid. The differences in the activities of pyrimethamine, chlorcycloguanil, sulfadoxine, and dapsone in the presence versus absence of 50 μM probenecid were statistically significant (P < 0.05) for the V1/S isolate, the multidrug-resistant isolate. For M24 (fully sensitive), statistically significant differences in activities in the presence of 50 μM probenecid were found only with pyrimethamine and dapsone. Importantly, there was an increase in sensitivity with increased concentrations of probenecid (results not shown).
TABLE 1.
In vitro effect of probenecid on the antimalarial activities of chloroquine, pyrimethamine, sulfadoxine, dapsone, and chlorcycloguanila
| Antimalarial agent | Probenecid concn (μM) | M24 |
V1S |
V1/S sensitivity to drug in vivod | ||
|---|---|---|---|---|---|---|
| IC50b | Chemosensitization ratioc | IC50b | Chemosensitization ratio | |||
| PM | 0 | 1.42 ± 0.52 | 2.7e | 4,196 ± 864 | 6.9e | Highly resistant to PM and SDX |
| 50 | 0.52 ± 0.36 | 611 ± 433 | Fully sensitive to PM and SDS | |||
| CCG | 0 | 0.31 ± 0.14 | 1.2 | 69.81 ± 7.43 | 3.1e | Resistant to CPG and DDS |
| 50 | 0.25 ± 0.26 | 22.25 ± 10.39 | Fully sensitive to CPG and DDS | |||
| SDX | 0 | 215 ± 150 | 5.9 | 2,334 ± 532 | 5.0e | |
| 50 | 36.50 ± 26.08 | 467 ± 391 | ||||
| DDS | 0 | 33.53 ± 12.30 | 18.9e | 53.36 ± 19.69 | 2.9e | |
| 50 | 1.77 ± 2.70 | 18.32 ± 13.81 | ||||
| CQ | 0 | 24.19 ± 7.09 | 0.9 | 202 ± 25.88 | 4.7e | Highly resistant to CQ |
| 50 | 27.29 ± 10.44 | 43.42 ± 2.86 | Fully sensitive to CQ | |||
PM, pyrimethamine; CCG, chlorcycloguanil; SDX, sulfadoxine; DDS, dapsone; CQ, chlorcycloguanil.
The IC50s are in nanomolar for pyrimethamine, chlorcycloguanil, and chloroquine and in micromolar for sulfadoxine and dapsone.
The chemosensitization ratio is the IC50 without probenecid/IC50 with probenecid.
Predictive clinical outcome for infection with a V1/S-like parasite on the basis of the IC50 of chloroquine (34) and pyrimethamine (41) for the parasite in the presence of probenecid.
Differences are statistically significant (P < 0.05 with EPI INFO [version 6]).
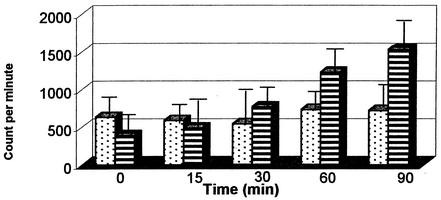
On the basis of the results of studies with tumor cells, we tested directly whether probenecid affected folate uptake in the parasites. We used 200 μM probenecid and measured the level of drug accumulation over 90 min. Figure 1 demonstrates that probenecid inhibited the accumulation of radiolabeled folic acid in the V1/S parasite compared with that in the control cultures. In these preliminary experiments, we did not establish the specific form of the folate or its contribution to the folate pool after parasite uptake.
FIG. 1.
Uptake of radiolabeled folic acid (y axis, in counts per minute) by erythrocytes infected with isolate V1/S (highly antifolate resistant) in the presence (░⃞) and absence (▤) of 200 μM probenecid.
We tested the effect of probenecid on the sensitivities of two chloroquine-sensitive isolates, M24 and HB3. In the absence of probenecid, the chloroquine IC50 for M24 and HB3 were 24 and 27 nM, respectively. These values remained similar after the addition of 50 μM probenecid (27 and 26 nM for M24 and HB3, respectively). However, 50 μM probenecid increased the sensitivity of chloroquine-resistant isolate V1/S, with the IC50 decreasing from approximately 200 to 43 nM (an almost fivefold increase in activity) (Table 1; Fig. 2A and B), which is generally accepted as a level of full sensitivity (34). This difference in the IC50 for V1/S was statistically significant (P < 0.05). An increase in the probenecid concentration to 100 μM resulted in a further decrease in the chloroquine IC50 to 16 nM. Previous studies have clearly implicated reduced levels of drug accumulation as a feature of chloroquine resistance. Treatment with verapamil, for example, partially reverses this trend. Consequently, we tested the ability of probenecid to enhance chloroquine uptake. Probenecid was shown to increase the steady-state accumulation of chloroquine by 100% selectively in the V1/S isolate (Fig. 2C). Probenecid had no effect on the chloroquine uptake by HB3 (Fig. 2C).
FIG. 2.
Effects of chloroquine on the growth rate (measured as counts per minute of incorporated hypoxanthine [hypoxan]) of HB3, a sensitive isolate (solid line), and V1/S, a multidrug-resistant isolate (dashed line), in the absence (A) and presence (B) of 50 μM probenecid. The arrows indicate the cutoff point for sensitive and resistant isolates (100 nM for chloroquine). (C) Uptake of radiolabeled chloroquine, expressed as the cellular accumulation ratio, by V1/S (░⃞) and HB3 (▤) in the presence of various concentrations of probenecid. The concentration of radiolabeled chloroquine was fixed at 5 nM.
DISCUSSION
The results of this study indicate that probenecid, an inhibitor of organic anion transporters and multiresistance-associated protein (4, 5, 22, 31), can chemosensitize P. falciparum cells to antifolate agents (i.e., enhance the activities of these compounds against the parasite). This process is not associated with sensitivity status or with the parasite DHFR and DHPS genotypes. This is the first report on the potentiation or chemosensitization of antifolate drug activity in microorganisms in general and in P. falciparum in particular.
The ability of probenecid to increase the sensitivities of cancer cells to antifolates has been reported previously. It has been shown that in these cells probenecid can (i) reverse methotrexate resistance in vitro (11) and (ii) increase the activities of other antifolates (27). Further molecular analyses have clearly demonstrated that the ability of probenecid to reverse methotrexate resistance is associated with the inhibition of multiresistance-associated proteins. These proteins are overexpressed in methotrexate-resistant cells, and this overexpression is correlated with an increase in the level of methotrexate efflux. As a result, probenecid treatment leads to an increase in the steady-state concentration of methotrexate in the cell. As an organic anion, methotrexate could be expected to be a multiresistance-associated protein substrate (4, 11, 32, 37); however, this chemical classification does not extend to the drugs that we have used in this study. Therefore, it is unlikely that these drugs are targets of multiresistance-associated proteins. Probenecid can enhance antifolate activity in drug-sensitive cancer cells via inhibition of endogenous folate transport as well (27). This may be an alternative explanation for our findings.
Two types of folate transport systems have been reported in mammalian cells. The first is the reduced folate carrier (RFC), also known as the micromolar folate transporter. RFC has a very low affinity for folic acid and a higher affinity for reduced folate derivatives. This receptor also mediates the transport of methotrexate (13, 26). The second receptor, the folate binding protein or folate receptor, is specific for oxidized folate derivatives such as folic acid (1, 24). Studies have demonstrated that both the oxidized and reduced folate (folic and folinic acid, respectively) can cross the membranes of the red blood cells parasitized with P. falciparum, because they can both negate the effects of antifolate drugs (12, 42). Methotrexate, an analog of folic acid, uses the RFC receptor to gain intracellular access and has been shown to inhibit P. falciparum at IC50s similar to those that inhibit human cells (nanomolar range) (10, 39). Taken together, these data suggest the existence of functional folate salvage in P. falciparum. This leads us to hypothesize that the sensitization of antifolate by probenecid may be explained, at least partly, by the decrease in folate uptake by the parasite, analogous to observations with cancer cells (27). Our data showing the decrease in the level of folate uptake into parasites in the presence of probenecid provide further support for this argument. This observation has clinical relevance, because it indicates that the activities of antimalarial antifolate agents could be potentiated in vivo with appropriate anion organic inhibitors such as probenecid, a strategy that has been demonstrated in antitumor therapy (27).
The molecular basis for our observations requires further elucidation. It could be argued that our data support the presence of red blood cell and parasite folate transporters; however, the new permeability pathway could provide the initial route of entry for anions such as folates (8, 21). Clearly, there must exist parasite-specific transporters that enable folate movement into the parasite. Furthermore, although all the evidence points to an effect at the level of folate salvage, parasites also salvage PABA for folate synthesis (46). PABA is also an inorganic anion, and it is possible that probenecid could exert its effect by inhibiting PABA accumulation, thereby starving the de novo folate pathway of an essential precursor. Our data do not rule out this possibility.
In addition to the effects on the activities of antifolate drugs, we have demonstrated that probenecid sensitizes chloroquine-resistant isolates by increasing the level of chloroquine accumulation, a hallmark feature of the chloroquine-resistant phenotype. These data suggest an additional effect of probenecid (directly or indirectly) on pfcrt, the gene most closely associated with chloroquine resistance (9, 45).
Our results demonstrate that the anion transporter inhibitor probenecid chemosensitizes both drug-sensitive and drug-resistant isolates to the actions of antifolate drugs. In addition, probenecid selectively chemosensitizes chloroquine-resistant parasites to chloroquine. Probenecid is an old drug that has been used for the treatment of several clinical conditions, including in combination with penicillin to decrease the renal elimination of penicillin (7). It is inexpensive and has a long history of use without serious toxicity (http://www.medscape.com). The highest single dose of probenecid that can be administered to humans is 3 g, and a dose of 2 g yields peak concentrations ranging between 500 and 700 μM (http:www.medscape.com). This range is far beyond the 50 μM required to produce chemosensitization in vitro. We have yet to establish whether these promising findings in vitro can be extrapolated to the in vivo setting with therapeutically acceptable doses of probenecid.
Overall, our data suggest a potential solution to the burgeoning problem of antifolate resistance based on the reversal of resistance through inhibition of folate salvage. These data also provide the platform for more detailed investigations of the molecular and biochemical aspects of the mode of action of probenecid.
Acknowledgments
We thank the director of the Kenya Medical Research Institute for permission to publish the data presented here. We also thank Carol Sibley of the University of Washington for reading and commenting on the manuscript.
This work was supported by the Wellcome Trust of Great Britain (grant 056769). A.N. and E.M. are grateful to the Wellcome Trust for personal support. P.W., S.W., and P.B. are grateful to the Wellcome Trust for institutional support.
REFERENCES
- 1.Antony, A. C. 1996. Folate receptors. Annu. Rev. Nutr. 16:501-521. [DOI] [PubMed] [Google Scholar]
- 2.Banerjee, D., P. Mayer-Kuckuk, G. Capiaux, T. Budak-Alpdogan, R. Gorlick, and J. R. Bertino. 2002. Novel aspects of resistance to drugs targeted to dihydrofolate reductase and thymidylate synthase. Biochim. Biophys. Acta 1587:164-173. [DOI] [PubMed] [Google Scholar]
- 3.Bertino, J. R. 1993. Karnofsky Memorial Lecture. Ode to methotrexate. J. Clin. Oncol. 11:5-14. [DOI] [PubMed] [Google Scholar]
- 4.Borst, P., R. Evers, M. Kool, and J. Wijnholds. 2000. A family of drug transporters: the multidrug resistance-associated proteins. J. Natl. Cancer Inst. 92:1295-1302. [DOI] [PubMed] [Google Scholar]
- 5.Borst, P., R. Evers, M. Kool, and J. Wijnholds. 1999. The multidrug resistance protein family. Biochim. Biophys. Acta 1461:347-357. [DOI] [PubMed] [Google Scholar]
- 6.Bray, P. G., O. Janneh, K. J. Raynes, M. Mungthin, H. Ginsburg, and S. A. Ward. 1999. Cellular uptake of chloroquine is dependent on binding to ferriprotoporphyrin IX and is independent of NHE activity in Plasmodium falciparum. J. Cell Biol. 145:363-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cunningham, R. F., Z. H. Israili, and P. G. Dayton. 1981. Clinical pharmacokinetics of probenecid. Clin. Pharmacokinet. 6:135-151. [DOI] [PubMed] [Google Scholar]
- 8.Desai, S. A., S. M. Bezrukov, and J. Zimmerberg. 2000. A voltage-dependent channel involved in nutrient uptake by red blood cells infected with the malaria parasite. Nature 406:1001-1005. [DOI] [PubMed] [Google Scholar]
- 9.Djimde, A., O. K. Doumbo, J. F. Cortese, K. Kayentao, S. Doumbo, Y. Diourte, A. Dicko, X. Z. Su, T. Nomura, D. A. Fidock, T. E. Wellems, C. V. Plowe, and D. Coulibaly. 2001. A molecular marker for chloroquine-resistant falciparum malaria. N. Engl. J. Med. 344:257-263. [DOI] [PubMed] [Google Scholar]
- 10.Fidock, D. A., and T. E. Wellems. 1997. Transformation with human dihydrofolate reductase renders malaria parasites insensitive to WR99210 but does not affect the intrinsic activity of proguanil. Proc. Natl. Acad. Sci. USA 94:10931-10936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hooijberg, J. H., H. J. Broxterman, M. Kool, Y. G. Assaraf, G. J. Peters, P. Noordhuis, R. J. Scheper, P. Borst, H. M. Pinedo, and G. Jansen. 1999. Antifolate resistance mediated by the multidrug resistance proteins MRP1 and MRP2. Cancer Res. 59:2532-2535. [PubMed] [Google Scholar]
- 12.Kinyanjui, S. M., E. K. Mberu, P. A. Winstanley, D. P. Jacobus, and W. M. Watkins. 1999. The antimalarial triazine WR99210 and the prodrug PS-15: folate reversal of in vitro activity against Plasmodium falciparum and a non-antifolate mode of action of the prodrug. Am. J. Trop. Med. Hyg. 60:943-947. [DOI] [PubMed] [Google Scholar]
- 13.Matherly, L. H. 2001. Molecular and cellular biology of the human reduced folate carrier. Prog. Nucleic Acids Res. Mol. Biol. 67:131-162. [DOI] [PubMed] [Google Scholar]
- 14.Mutabingwa, T., A. Nzila, E. Mberu, E. Nduati, P. Winstanley, E. Hills, and W. Watkins. 2001. Chlorproguanil-dapsone for treatment of drug-resistant falciparum malaria in Tanzania. Lancet 358:1218-1223. [DOI] [PubMed] [Google Scholar]
- 15.Nguyen-Dinh, P., H. C. Spencer, S. Chemangey-Masaba, and F. C. Churchill. 1982. Susceptibility of Plasmodium falciparum to pyrimethamine and sulfadoxine/pyrimethamine in Kisumu, Kenya. Lancet i:823-825. [DOI] [PubMed]
- 16.Nuwaha, F. 2001. The challenge of chloroquine-resistant malaria in sub-Saharan Africa. Health Policy Plan. 16:1-12. [DOI] [PubMed] [Google Scholar]
- 17.Nzila, A. M., E. K. Mberu, J. Sulo, H. Dayo, P. A. Winstanley, C. H. Sibley, and W. M. Watkins. 2000. Towards an understanding of the mechanism of pyrimethamine-sulfadoxine resistance in Plasmodium falciparum: genotyping of dihydrofolate reductase and dihydropteroate synthase of Kenyan parasites. Antimicrob. Agents Chemother. 44:991-996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okelo, G. B. 1984. A review of chloroquine resistance by Plasmodium falciparum in Kenya. East Afr. Med. J. 61:150-153. [PubMed] [Google Scholar]
- 19.Oloo, A. J. 1991. Sensitivity of falciparum malaria to chloroquine and amodiaquine in four districts of western Kenya (1985-1987). East Afr. Med. J. 68:606-610. [PubMed] [Google Scholar]
- 20.Omar, S. A., I. S. Adagu, D. W. Gump, N. P. Ndaru, and D. C. Warhurst. 2001. Plasmodium falciparum in Kenya: high prevalence of drug-resistance-associated polymorphisms in hospital admissions with severe malaria in an epidemic area. Ann. Trop. Med. Parasitol. 95:661-669. [DOI] [PubMed] [Google Scholar]
- 21.Saliba, K. J., and K. Kirk. 2001. Nutrient acquisition by intracellular apicomplexan parasites: staying in for dinner. Int. J. Parasitol. 31:1321-1330. [DOI] [PubMed] [Google Scholar]
- 22.Sekine, T., S. H. Cha, and H. Endou. 2000. The multispecific organic anion transporter (OAT) family. Pflugers Arch. 440:337-350. [DOI] [PubMed] [Google Scholar]
- 23.Sibley, C. H., J. E. Hyde, P. F. Sims, C. V. Plowe, J. G. Kublin, E. K. Mberu, A. F. Cowman, P. A. Winstanley, W. M. Watkins, and A. M. Nzila. 2001. Pyrimethamine-sulfadoxine resistance in Plasmodium falciparum: what next? Trends Parasitol. 17:582-588. [DOI] [PubMed] [Google Scholar]
- 24.Sierra, E. E., and I. D. Goldman. 1999. Recent advances in the understanding of the mechanism of membrane transport of folates and antifolates. Semin. Oncol. 26:11-23. [PubMed] [Google Scholar]
- 25.Sirotnak, F. M., D. M. Moccio, and C. W. Young. 1981. Increased accumulation of methotrexate by murine tumor cells in vitro in the presence of probenecid which is mediated by a preferential inhibition of efflux. Cancer Res. 41:966-970. [PubMed] [Google Scholar]
- 26.Sirotnak, F. M., and B. Tolner. 1999. Carrier-mediated membrane transport of folates in mammalian cells. Annu. Rev. Nutr. 19:91-122. [DOI] [PubMed] [Google Scholar]
- 27.Sirotnak, F. M., H. G. Wendel, W. G. Bornmann, W. P. Tong, V. A. Miller, H. I. Scher, and M. G. Kris. 2000. Co-administration of probenecid, an inhibitor of a cMOAT/MRP-like plasma membrane ATPase, greatly enhanced the efficacy of a new 10-deazaaminopterin against human solid tumors in vivo. Clin. Cancer Res. 6:3705-3712. [PubMed] [Google Scholar]
- 28.Sixsmith, D. G., W. M. Watkins, J. D. Chulay, and H. C. Spencer. 1984. In vitro antimalarial activity of tetrahydrofolate dehydrogenase inhibitors. Am. J. Trop. Med. Hyg. 33:772-776. [DOI] [PubMed] [Google Scholar]
- 29.Spencer, H. C., W. W. Watkins, D. G. Sixsmith, and D. K. Koech. 1986. Response of Plasmodium falciparum to dihydrofolate reductase inhibitors in Malindi, Kenya. Trans. R. Soc. Trop. Med. Hyg. 80:201-203. [DOI] [PubMed] [Google Scholar]
- 30.Staedke, S. G., M. R. Kamya, G. Dorsey, A. Gasasira, G. Ndeezi, E. D. Charlebois, and P. J. Rosenthal. 2001. Amodiaquine, sulfadoxine/pyrimethamine, and combination therapy for treatment of uncomplicated falciparum malaria in Kampala, Uganda: a randomised trial. Lancet 358:368-374. [DOI] [PubMed] [Google Scholar]
- 31.Sweet, D. H., K. T. Bush, and S. K. Nigam. 2001. The organic anion transporter family: from physiology to ontogeny and the clinic. Am. J. Physiol. Renal Physiol. 281:F197-F205. [DOI] [PubMed] [Google Scholar]
- 32.Takeda, M., S. Khamdang, S. Narikawa, H. Kimura, M. Hosoyamada, S. H. Cha, T. Sekine, and H. Endou. 2002. Characterization of methotrexate transport and its drug interactions with human organic anion transporters. J. Pharmacol. Exp. Ther. 302:666-671. [DOI] [PubMed] [Google Scholar]
- 33.Terlouw, D. J., J. M. Courval, M. S. Kolczak, O. S. Rosenberg, A. J. Oloo, P. A. Kager, A. A. Lal, B. L. Nahlen, and F. O. ter Kuile. 2003. Treatment history and treatment dose are important determinants of sulfadoxine-pyrimethamine efficacy in children with uncomplicated malaria in Western Kenya. J. Infect. Dis. 187:467-476. [DOI] [PubMed] [Google Scholar]
- 34.Thomas, S. M., O. Ndir, T. Dieng, S. Mboup, D. Wypij, J. H. Maguire, and D. F. Wirth. 2002. In vitro chloroquine susceptibility and PCR analysis of pfcrt and pfmdr1 polymorphisms in Plasmodium falciparum isolates from Senegal. Am. J. Trop. Med. Hyg. 66:474-480. [DOI] [PubMed] [Google Scholar]
- 35.Trager, W., and J. B. Jensen. 1976. Human malaria parasites in continuous culture. Science 193:673-675. [DOI] [PubMed] [Google Scholar]
- 36.Trape, J. F. 2001. The public health impact of chloroquine resistance in Africa. Am. J. Trop. Med. Hyg. 64:12-17. [DOI] [PubMed] [Google Scholar]
- 37.Uwai, Y., H. Saito, and K. Inui. 2000. Interaction between methotrexate and nonsteroidal anti-inflammatory drugs in organic anion transporter. Eur. J. Pharmacol. 409:31-36. [DOI] [PubMed] [Google Scholar]
- 38.Van Dillen, J., M. Custers, A. Wensink, B. Wouters, T. van Voorthuizen, W. Voorn, B. Khan, L. Muller, and C. Nevill. 1999. A comparison of amodiaquine and sulfadoxine-pyrimethamine as first-line treatment of falciparum malaria in Kenya. Trans. R. Soc. Trop. Med. Hyg. 93:185-188. [DOI] [PubMed] [Google Scholar]
- 39.Walter, R. D., B. Bergmann, M. Kansy, M. Wiese, and J. K. Seydel. 1991. Pyrimethamine-resistant Plasmodium falciparum lack cross-resistance to methotrexate and 2,4-diamino-5-(substituted benzyl) pyrimidines. Parasitol. Res. 77:346-350. [DOI] [PubMed] [Google Scholar]
- 40.Wang, P., R. K. Brobey, T. Horii, P. F. Sims, and J. E. Hyde. 1999. Utilization of exogenous folate in the human malaria parasite Plasmodium falciparum and its critical role in antifolate drug synergy. Mol. Microbiol. 32:1254-1262. [DOI] [PubMed] [Google Scholar]
- 41.Watkins, W. M., E. K. Mberu, P. A. Winstanley, and C. V. Plowe. 1997. The efficacy of antifolate antimalarial combination in Africa: a predictive model based on pharmacodynamic and pharmacokinetic analyses. Parasitol. Today 13:459-464. [DOI] [PubMed] [Google Scholar]
- 42.Watkins, W. M., D. G. Sixsmith, J. D. Chulay, and H. C. Spencer. 1985. Antagonism of sulfadoxine and pyrimethamine antimalarial activity in vitro by p-aminobenzoic acid, p-aminobenzoylglutamic acid and folic acid. Mol. Biochem. Parasitol. 14:55-61. [DOI] [PubMed] [Google Scholar]
- 43.Watkins, W. M., D. G. Sixsmith, H. C. Spencer, D. A. Boriga, D. M. Kariuki, T. Kipingor, and D. K. Koech. 1984. Effectiveness of amodiaquine as treatment for chloroquine-resistant Plasmodium falciparum infections in Kenya. Lancet i:357-359. [DOI] [PubMed]
- 44.World Health Organization. 1997. World malaria situation in 1994. Wkly. Epidemiol. Rec. 72:269-276.9293226 [Google Scholar]
- 45.Zhang, H., E. M. Howard, and P. D. Roepe. 2002. Analysis of the antimalarial drug resistance protein Pfcrt expressed in yeast. J. Biol. Chem. 277:49767-49775. [DOI] [PubMed] [Google Scholar]
- 46.Zhang, Y., S. Merali, and S. R. Meshnick. 1992. p-Aminobenzoic acid transport by normal and Plasmodium falciparum-infected erythrocytes. Mol. Biochem. Parasitol. 52:185-194. [DOI] [PubMed] [Google Scholar]