Abstract
Podocytes are highly specialized and terminally differentiated glomerular cells that play a vital role in renal physiology, including the prevention of proteinuria. Cyclin-dependent kinase 5 (CDK5) has been shown to influence several cellular processes in other terminally differentiated cells, in particular neurons. In this study, we examined the role of CDK5 in podocyte differentiation, proliferation, and morphology. In conditionally immortalized mouse podocytes in culture, CDK5 increased in association with podocyte differentiation. During mouse glomerulogenesis in vivo, CDK5 expression was predominantly detected in podocytes from the capillary loop stage to maturation and persisted in the podocytes of adult glomeruli. In contrast, CDK5 was markedly decreased in the proliferating and dedifferentiated podocytes of mice with anti-glomerular basement membrane nephritis and in human immunodeficiency virus transgenic mice. p35, the activator of CDK5, was also detected in podocytes and the p35/CDK5 complex was active. Cell fractionation studies showed that active p35/CDK5 was mainly localized to the plasma membrane. Specific inhibition of CDK5 in differentiated cultured podocytes, either pharmacologically or with siRNA, induced shape changes, with cellular elongation and loss of process formation compared to the characteristic arborized phenotype. These data suggest a role for CDK5 as a regulator of podocyte differentiation, proliferation, and morphology.
Podocytes, also called visceral glomerular epithelial cells, are terminally differentiated cells overlying the outer aspect of the glomerular basement membrane of renal glomeruli. Podocytes have several key functions, including the prevention of proteinuria, synthesis of basement membrane components, regulation of glomerular filtration, and counteraction of the intraglomerular hydrostatic pressure.1 Injury to podocytes is associated with proteinuria and progressive glomerulosclerosis.2
Podocytes derive from epithelial cells originating in the metanephric mesenchyme, which develop into postmitotic terminally differentiated cells,3 and therefore have similarities to neurons.4 During glomerulogenesis, podocytes proliferate until the S-shape body stage, and exit the cell cycle at the capillary loop stage.5,6 Podocytes then acquire their fully differentiated phenotype, a process that in the mouse is not complete until 1 week after birth. Mature podocytes tightly regulate and maintain their quiescent and differentiated phenotype, and therefore the majority of diseases involving podocytes are not associated with proliferation and an increase in cellularity. Indeed, studies have shown that the inability to proliferate contributes to glomerular scarring.7 In contrast, podocytes may dedifferentiate, and proliferate, in human immunodeficiency virus (HIV)-associated nephropathy, which is characterized by a rapid decline in renal function, emphasizing the importance of podocyte quiescence for glomerular function.8 These studies show that the state of podocyte differentiation is closely linked with its proliferative potential. Studies have also shown that podocyte morphology is critical for normal function.9,10 After injury, flattening and effacement of podocytes leads to loss of normal function.
Proliferation and differentiation are controlled by specific cell cycle regulatory proteins.11 Cyclins bind to and activate their partner cyclin-dependent kinases (CDK). Cyclin-CDK complexes are inhibited by CDK inhibitors. In contrast to the classical, cell cycle-associated CDKs, CDK5 accumulates in postmitotic cells.12 CDK5 was originally isolated on the basis of its close primary sequence homology to the human Cdc2 serine threonine kinase.13 The brain has the highest levels of CDK5 activity, because of the expression of its regulatory partner, p35.14 Although CDK5 activity does not promote cell cycle progression,13 it has a crucial role in the direction of neuronal migration in the developing central nervous system.12 Dysregulation of CDK5 activity has been closely linked to specific neurodegenerative diseases, including Alzheimer’s disease and amyotrophic lateral sclerosis.15,16 Although CDK5 function was originally thought to be restricted to the nervous system, several groups have recently demonstrated that CDK5 is also active in nonneuronal tissue, including muscle, lens, and hematopoietic cells.12 In these cells, CDK5 activity is critical for development and differentiation.
In the current study we measured the expression and role of active CDK5/p35 in podocytes in vitro and in vivo. Our results show that CDK5 increases in podocytes during differentiation in culture, and in developing fetal kidneys. CDK5 declines in experimental diseases associated with podocyte proliferation. The CDK5 activator p35 is also expressed in podocytes, and the active complex is principally localized to the plasma membrane. Finally, we demonstrate that a novel role for CDK5 is to maintain podocyte morphology.
Materials and Methods
Primary Antibodies
Mouse monoclonal anti-CDK5 antibody (Ab-4; NeoMarkers, Fremont, CA) was used for immunohistochemical (1:50) and immunofluorescent (1:20) studies and Western blot analysis (1:500). Rabbit polyclonal anti-CDK5 antibody (C-8, 1:50; Santa Cruz Biotechnology, Santa Cruz, CA) was used for the histone H1 kinase assay. Rabbit polyclonal anti-p35 antibody (C-19; Santa Cruz Biotechnology) was used for immunohistochemical (1:500) and immunofluorescent (1:100) studies and histone H1 kinase assay (1:50). Mouse monoclonal anti-actin (Chemicon, Temecula, CA) was used for immunofluorescent studies (1:200) and Western blot analysis (1:4000). Mouse monoclonal anti-cyclin B1 antibody (GNS1, 1:200; Santa Cruz Biotechnology), rabbit polyclonal anti-cyclin A antibody (C-19, 1:1000; Santa Cruz Biotechnology), and monoclonal anti-GAPDH antibody (6C5, 1:8000, Abcam, Cambridge, UK) were used for Western blotting.
Conditionally Immortalized Mouse Podocytes in Culture
To determine the expression and potential role of CDK5 in podocytes in vitro, we used a conditionally immortalized mouse podocyte cell line that was isolated from the kidneys of H-2Kb-tsA58 transgenic mice as previously described.17 In this cell line, a temperature-sensitive SV40 large T-cell antigen (tsA58 Tag) is controlled by a γ-interferon inducible H-2Kb promoter. To induce proliferation (and therefore dedifferentiation), cells were grown on collagen I (BD Biosciences, Bedford, MA)-coated plastic plates (Primaria tissue culture dish, 100 × 20 mm; Becton Dickinson Labware, Franklin Lakes, NJ), at 33°C in RPMI 1640 culture medium (Life Technologies, Inc., Gaithersburg, MD) supplemented with 10% fetal calf serum (Summit Biotechnology, Ft. Collins, CO), 2 mmol/L glutamine (Sigma, St. Louis, MO), 10 mmol/L HEPES (Sigma), 1 mmol/L sodium pyruvate (Irvine Scientific, Santa Ana, CA), and 100 U/ml penicillin and 100 μg/ml streptomycin (Irvine Scientific), to which recombinant mouse γ-interferon 10 U/ml (Coulter, Hialeah, FL) was added (growth permissive conditions). To induce quiescence and the differentiated phenotype, cells were grown at 37°C in the same medium with no γ-interferon (growth restrictive conditions). All experiments were performed at least three times. Unless specified otherwise, all studies were performed on day 3 in cells growing under permissive (proliferating/dedifferentiated) conditions, and on days 10 to 14 for cells grown under restrictive (quiescent/differentiated) conditions. To inhibit CDK5 activity, selective and potent CDK5 inhibitors, roscovitine18 (50 μmol/L; Calbiochem Novabiochem, San Diego, CA) or alsterpaullone19 (5 μmol/L; Alexis Biochemicals, San Diego, CA) were used.
siRNA Synthesis and Transfection
Double-stranded CDK5 small interfering RNA (siRNA) was synthesized by in vitro transcription using the Silencer siRNA construction kit (Ambion, Austin, TX) according to the manufacturer’s instructions. All of the molecular biology reagents described below were supplied with this kit. The CDK5 target mRNA sequence was 5′ AATGGCCTGCCATGACCAAGC 3′. Sense and anti-sense siRNA oligonucleotide templates with a 3′ 8-nucleotide sequence complimentary to the T7 RNA polymerase promoter were obtained from Invitrogen (Carlsbad, CA) (sense: 5′ AAGCTTGGTCATGGCAGGCCACCTGTCTC 3′; anti-sense: 5′ AATGGCCTGCCATGACCCCGCCCTGTCTC 3′). Briefly, the oligonucleotide templates were hybridized to a T7 promoter primer, and the 3′ ends extended using Klenow. The sense and anti-sense siRNA templates were transcribed by T7 RNA polymerase and the resulting RNA transcripts hybridized to create dsRNA, consisting of 5′ terminal single-stranded leader sequences, a 19-nucleotide target-specific dsRNA, and 3′ terminal UUs. The leader sequences were removed by digestion with a single-strand-specific ribonuclease, and the DNA template digested by deoxyribonuclease. The resulting siRNA was purified by glass fiber filter binding and elution, and the concentration determined by measuring the absorbance at 260 nm. A scrambled siRNA sequence was included with the kit and used as a negative control to exclude nonspecific effects on gene expression.
CDK5-specific and control siRNA were spiked with random 20-mer fluorescein isothiocyanate-conjugated oligonucleotides (TriLink BioTech, San Diego, CA) and transfected into 50% confluent conditionally immortalized mouse podocytes at day 3 of growth restriction using the n-fect transfection reagent, according to the manufacturer’s instructions (Neuromics, Northfield, MN). After 48 hours, transfection was confirmed by uptake of fluorescein isothiocyanate-conjugated oligonucleotides, cell morphology analyzed by light microscopy before fixation, and protein harvested for Western blotting.
Embryonal and Adult Rodent Kidneys
To determine the temporal expression of CDK5 during podocyte development by immunostaining (see below), embryonal kidneys were harvested from gravid C57BL6 mice (Simonsen, Gilroy, CA) at embryonic (E) days 15 and 18 as previously described.20 Kidneys were also harvested from normal adult C57BL6 mice and Wistar rats (2 to 4 months old; Simonsen). To examine the role of CDK5 in renal development, kidneys were studied from CDK5−/− and CDK5+/+ mice at day E18. The generation of these mice has been reported previously.21 Kidneys were fixed in 10% buffered formalin for immunohistochemistry (see below).
Experimental Models of Glomerular Disease
To examine the expression of CDK5 in podocytes after injury, we studied three models of glomerular disease. 1) Anti-glomerular basement membrane (GBM) glomerulonephritis, characterized by podocyte injury, was induced in p21−/− and p21+/+ mice as previously described.22 In brief, a sheep anti-rabbit GBM antibody was injected intraperitoneally (0.5 ml/20 g body weight) on 2 consecutive days, and animals were studied on days 5 and 14 (n = 4 and n = 6). 2) HIV-1 transgenic mice (a gift of Dr. Jeffrey Kopp), characterized by HIV-associated nephropathy and podocyte injury, were created by introducing proviral HIV genome, lacking the gag and pol genes, into FVB/N mice, as previously described.23 Kidneys were examined at 10 weeks after birth (n = 4). 3) The Habu snake venom model of mesangial cell injury was induced in C57BL6/129SV mice by injection of Habu snake venom (4 mg/kg; Sigma) through the tail vein (n = 3).24 This disease model was used as a control, because podocytes are not the primary target of injury.
Immunofluorescence and Immunohistochemical Analysis
Cell Culture
To determine the expression of specific proteins by immunostaining, cultured podocytes were grown on eight-well Permanox chamber slides (Nalge Nunc, Naperville, IL) and fixed in methanol and acetone (1:1) at −20°C for 30 minutes. Cells were incubated overnight at 4°C with primary antibodies for CDK5, p35, or actin diluted in 1% bovine serum albumin in phosphate-buffered saline (PBS). A secondary biotinylated anti-mouse IgG or anti-rabbit IgG (Vector, Burlingame, CA) was incubated at room temperature for 30 minutes. Immunofluorescent staining was detected by streptavidin-Alexa Fluor 594 (Molecular Probes, Eugene, OR) for CDK5 and actin, and by streptavidin-fluorescein (Amersham Pharmacia Biotech, Piscataway, NJ) for p35.
Histology
Indirect immunoperoxidase immunostaining was performed on formalin-fixed paraffin-embedded kidney specimens from embryonal, normal, and diseased mice as previously described.20 Briefly, 4-μm cut tissue sections were deparaffinized in Histo-Clear (National Diagnostics, Atlanta, GA) and rehydrated in graded ethanol. Antigen retrieval was performed by boiling tissues for 10 minutes in 10 mmol/L citric acid, and endogenous peroxidases were blocked with 3% hydrogen peroxide. Endogenous biotin was blocked using an Avidin/Biotin blocking kit (Vector). Sections were incubated overnight with the primary antibody diluted in 1% bovine serum albumin in PBS. The sections were washed repeatedly in PBS before incubation with the appropriate biotinylated secondary antibody (Vector), diluted in 1% bovine serum albumin in PBS, for 1 hour at room temperature. The ABC-Elite reagent (Vector) was used for signal amplification and 3,3′-diaminobenzidine (Sigma) with nickel enhancement was used as chromogen. Slides were counterstained with methyl green or hematoxylin and eosin, dehydrated, and coverslipped.
Protein Extraction and Western Blot Analysis
Protein was extracted from cultured mouse podocytes20 and from glomeruli isolated from normal adult rats25 using TG buffer (1% Triton, 10% glycerol, 20 mmol/L HEPES, 100 mmol/L NaCl) with protease inhibitors (Boehringer Mannheim, Indianapolis, IN), or buffer A (20 mmol/L Tris-HCl, pH 7.2, 2 mmol/L MgCl2, 0.5% Nonidet P-40, 150 mmol/L NaCl, 1 mmol/L dithiothreitol) with protease inhibitors. Rats were used instead of mice to yield a highly purified preparation of glomeruli with minimal tubular contamination, a frequent problem when isolating mouse glomeruli. Protein concentration was determined by the BCA protein assay (Pierce, Rockford, IL) according to the manufacturer’s directions.
To determine the subcellular localization of the active p35/CDK5 complex, proteins from the cytoplasmic and crude membrane fractions were isolated using a modification of a published method.26,27 Cells were harvested by trypsin digestion, rinsed with cold PBS, and placed on ice for 15 minutes in hypotonic buffer (10 mmol/L HEPES, pH 7.9, 10 mmol/L KCl, 0.1 mmol/L EDTA, 0.1 mmol/L EGTA, 1 mmol/L dithiothreitol, 0.5 mmol/L phenylmethyl sulfonyl fluoride) and protease inhibitors. After homogenization with a Dounce-type homogenizer 30 times, the lysate was centrifuged for 15 minutes at 4000 × g to discard nuclei and intact cells. To extract the cytoplasmic fraction, the supernatant was collected after a 60-minute centrifugation at 100,000 × g. The pellet was resolved in buffer A with protease inhibitors (as above) on ice, and was gently vortexed for 15 minutes. After centrifugation for 15 minutes at 15,000 × g, the supernatant was collected, representing the crude plasma membrane fraction.
For Western blot analysis, 5 to 40 μg of protein extracts were separated under reduced conditions on 8 or 15% sodium dodecyl sulfate-polyacrylamide gels and transferred to polyvinyl difluoride membrane (Immobilon-P; Millipore, Bedford, MA) as we have reported previously.20 Membranes were incubated with primary antibodies overnight at 4°C, washed, and incubated with an alkaline phosphatase-conjugated anti-mouse IgG or anti-rabbit IgG antibody (Promega, Madison, WI) at room temperature for 1 hour. After further washing, detection of bound antibody was performed using the chromagen 5-bromo-4-chloro-3-indolyl phosphate/nitro blue tetrazolium (Sigma).
Co-Immunoprecipitation and Histone H1 Kinase Assay
To determine CDK5 activity, co-immunoprecipitation followed by a histone H1 kinase assay was performed as previously described.26,28 Briefly, 100 μg of total or fractionated protein (membrane and cytoplasm) extracted from cultured cells or isolated glomeruli was immunoprecipitated with primary antibodies for p35 or CDK5 in buffer A for 1 hour at 4°C, followed by incubation with protein A-Sepharose beads (RepliGen Corporation, Cambridge, MA) for 1 hour at 4°C. As a negative control, normal rabbit IgG was substituted for the specific antibody.
To perform the histone H1 kinase assay, the beads were washed four times with buffer A, then once in kinase reaction buffer (50 mmol/L HEPES, pH 7.0, 10 mmol/L MgCl2, 1 mmol/L dithiothreitol). The kinase reaction was performed at 37°C for 30 minutes in a kinase reaction buffer containing 5 μmol/L ATP, 2 μCi [32P] ATP (NEN Life Science Products, Boston, MA) and 0.6 μg histone H1 (Boehringer Mannheim). After stopping the reaction with 2× sodium dodecyl sulfate sample buffer, samples were resolved on a 15% sodium dodecyl sulfate-polyacrylamide gel under reduced condition. Finally, the gel was exposed to autoradiographic film (Amersham Pharmacia Biotech) to visualize the phosphorylated histone H1.
Polymerase Chain Reaction (PCR)
The expression of p35 was also confirmed by reverse transcriptase (RT)-PCR. Total RNA was isolated from 70% confluent differentiated heat-sensitive mouse podocytes using the Tri-Reagent (Sigma) according to the manufacturer’s instruction. To prevent contamination by genomic DNA, extracted RNA was treated with DNAase (RQ1 RNase-Free DNase Promega) at 37°C for 30 minutes. cDNA was synthesized using the oligo(dT) protocol contained in the Superscript First-Strand Synthesis System for RT-PCR (Life Technologies, Inc.). cDNA was amplified in a semiquantitative manner using primer sets specific for the mouse p35 gene (forward primer: 5′ CATGCTCTGCAGGGATGTTA 3′, reverse primer: 5′ GAAATAGTGTGGGTCGGCAT 3′). The PCR reaction was performed as follows: 94°C for 3 minutes, followed by 35 cycles of 94°C for 1 minute, 56°C for 1 minute, and 72°C for 1 minute. PCR products were resolved on a 2% agarose gel.
Results
CDK5 Is Increased in Protein Level During Differentiation of Cultured Podocytes
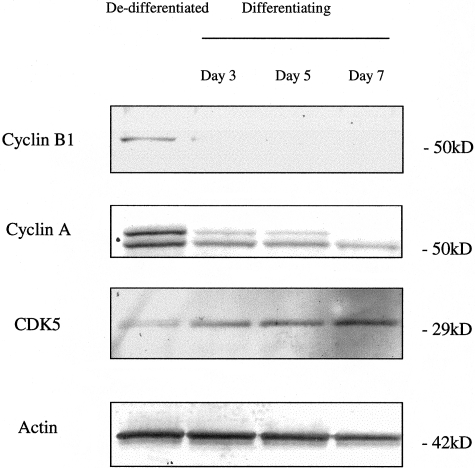
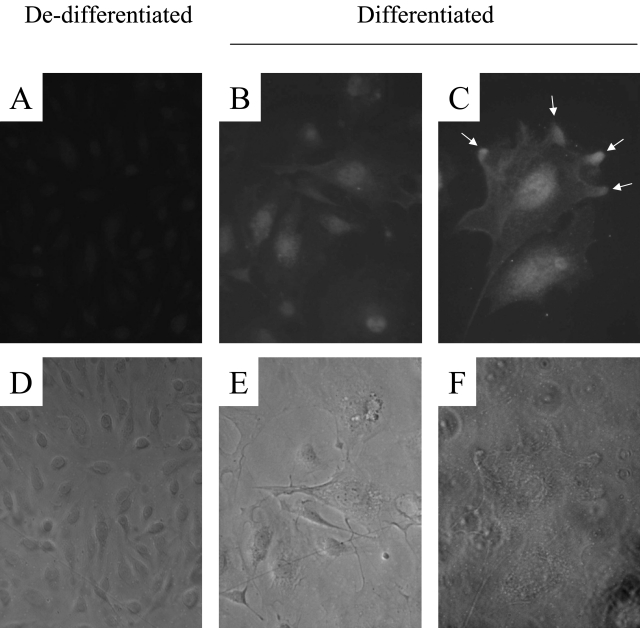
We began by using heat-sensitive mouse podocytes in culture. When grown under growth permissive conditions, these cells dedifferentiate and proliferate; when grown under restrictive conditions, they exit the cell cycle, differentiate, and acquire a quiescent phenotype similar to podocytes in vivo.17,20 As shown in Figure 1, cyclins B1 and A are increased in proliferating podocytes, grown under growth permissive conditions. In contrast, the protein levels for CDK5 were low in proliferating podocytes. However, there was an increase in CDK5 protein levels in differentiating podocytes when cultured under growth restrictive conditions (Figure 1). Actin was used to confirm equivalence of protein loading. CDK5 expression was also demonstrated in cultured podocytes by immunostaining. Consistent with the Western blot results, immunostaining for CDK5 was weak in proliferating and dedifferentiated podocytes (Figure 2). However, there was an increase in CDK5 staining in differentiated and quiescent podocytes, with CDK5 being detected in both the nucleus and cytoplasm, and abundant in cellular processes (Figure 2).
Figure 1-4249.
Differential expression of cyclin-CDKs during podocyte differentiation. The protein expression of cyclin-CDKs was measured by Western blot analysis. The switch from a dedifferentiated and proliferating phenotype to a differentiated and quiescent one was accompanied by a decrease in cyclins A and B. In contrast, CDK5 levels increased. Actin staining confirms equivalent protein loading.
Figure 2-4249.
CDK5 immunostaining in cultured mouse podocytes. A: Faint staining for CDK5 was seen in dedifferentiated podocytes. B and C: Both nuclear and cytoplasmic CDK5 immunostaining increased significantly in differentiated podocytes, with enhancement at the tips of cellular processes. D–F: Corresponding phase contrast images. Original magnifications: ×200 (A, B, D, E); ×400 (C and F).
CDK5 Is Expressed During Glomerulogenesis and in Adult Glomeruli
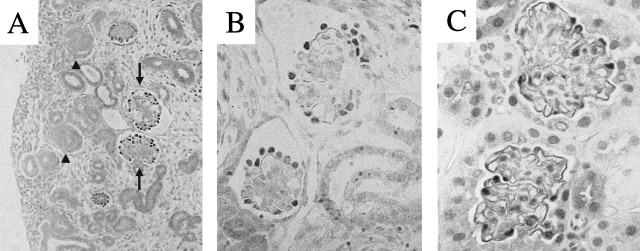
To test the hypothesis that CDK5 is also expressed in differentiating podocytes in vivo, we examined kidneys from normal embryonic mice. Our results showed faint staining of CDK5 in most kidney cells including the metanephric mesenchyme and tubular structures. Weak staining for CDK5 was also detected in early glomerular structures including the vesicle and comma and S-shaped bodies. In contrast, there was a marked increase in CDK5 staining at the capillary loop stage, in a distribution consistent with podocytes (Figure 3, A and B). Immunostaining for CDK5 was intense in podocytes of adult mice (Figure 3C). The specificity of the CDK5 antibody was confirmed by negative CDK5 staining in embryonal kidneys of CDK5−/− mice (data not shown).
Figure 3-4249.
CDK5 immunostaining in mouse kidneys. A: CDK5 staining was faint in immature glomeruli (examples indicated by arrowheads) during glomerulogenesis in embryonic kidneys. In contrast, CDK5 staining was detected in mature glomeruli (examples indicated by arrows) in embryonic kidneys (E18). B and C: CDK5 staining was abundant in podocytes in differentiated glomeruli of embryonic kidneys (E18) (B) and adult glomeruli (C). Original magnifications: ×200 (A); ×630 (B and C).
CDK5 Decreases in Proliferating Podocytes in Experimental Glomerular Disease
We next determined if CDK5 protein levels were altered after podocyte injury. Accordingly, we examined two models of experimental podocyte injury (anti-GBM nephritis and HIV nephropathy) and one model of glomerular disease (Habu nephritis) in which mesangial and endothelial cells, but not podocytes, are injured.
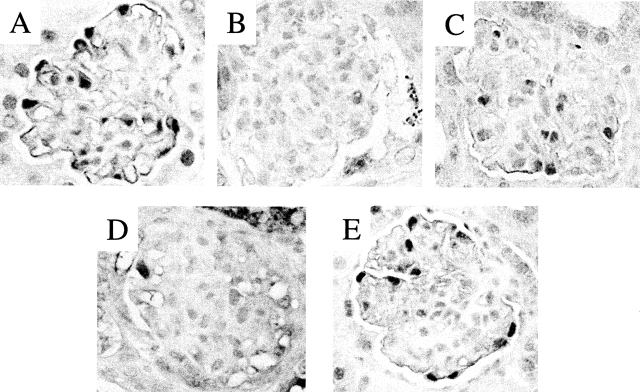
We have previously reported that p21−/− mice exhibit marked podocyte proliferation after the induction of glomerulonephritis with an anti-GBM antibody, and therefore used these mice to examine the expression of CDK5 in podocyte proliferation in vivo.22 Our results showed that CDK5 staining was barely detected in areas of podocyte proliferation in p21−/− nephritic mice (Figure 4B). In nephritic p21+/+ mice, podocyte proliferation is less marked, and although CDK5 immunostaining decreased this was to a lesser extent compared to nephritic p21−/− mice (Figure 4C).
Figure 4-4249.
CDK5 immunostaining in experimental renal disease. A: CDK5 is expressed in podocytes in normal mouse glomeruli. B: Immunostaining for CDK5 decreases in podocytes in the anti-GBM mouse model in p21CIP1−/− mice, which is characterized by podocyte dedifferentiation and proliferation. C: CDK5 immunostaining decreased to a lesser extent in the anti-GBM mouse model in wild-type mice, which is typically associated with a less severe course than in the p21CIP1−/− mice. D: CDK5 immunostaining decreased in podocytes in HIV transgenic mice, a second model characterized by podocyte dedifferentiation and proliferation. E: CDK5 immunostaining in podocytes was not altered in Habu nephritis, which is characterized by mesangial cell injury. Original magnifications, ×630.
Previous studies have shown that HIV-1 transgenic mice develop focal segmental glomerulosclerosis, accompanied by nephrotic syndrome and rapid progression to end-stage renal failure.23 However, before the sclerotic changes, podocytes enter the cell cycle, similarly to human HIV-associated nephropathy.29 In contrast to the intense CDK5 staining of podocytes in normal mice, CDK5 staining was barely detected in the podocytes of HIV transgenic mice (Figure 4D). Finally, we also determined CDK5 expression in the Habu nephritis model in mice, which is characterized by mesangial cell proliferation (and is not associated with podocyte injury).30Figure 4E shows that podocyte immunostaining for CDK5 was not altered in mice with Habu nephritis. Although the number of CDK5-positive cells was reduced in the area of mesangial expansion, this may be attributed to a reduction in podocyte density consequent to the enlargement of the mesangial area, and the intensity of staining for individual podocytes was unchanged from unmanipulated animals. Taken together, these results show that CDK5 is expressed in normal podocytes, and that levels decrease when podocytes re-enter the cell cycle.
p35 Is Expressed in Glomeruli and Cultured Podocytes and Activates CDK5
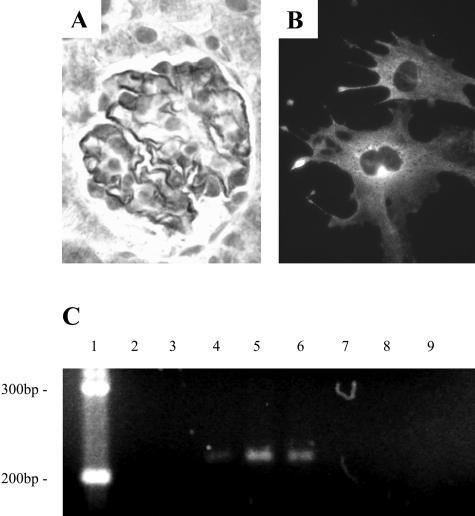
CDK5 is activated by p35 in neurons.14 To determine whether p35 was also expressed in podocytes, we performed immunostaining on adult mouse kidneys and cultured podocytes. In the normal mouse glomeruli, p35 staining was detected in podocytes (Figure 5A). The specificity of p35 antibody was confirmed using a blocking peptide, and by replacing it with normal rabbit IgG (data not shown). Figure 5B shows that p35 staining was detected in differentiated and quiescent podocytes in vitro, in a cytoplasmic distribution, and was abundant in cellular processes. To confirm p35 transcription in vivo in glomeruli and in vitro in podocytes, we measured p35 mRNA by RT-PCR. Figure 5C shows that p35 mRNA was present in both isolated rat glomeruli, and in differentiated and quiescent podocytes.
Figure 5-4249.
p35 is expressed by podocytes. A: p35 immunostaining was abundant in podocytes in normal mouse glomeruli. B: p35 immunostaining was present in differentiated mouse podocytes in culture. C: p35 mRNA was detected in podocytes by RT-PCR. Lane 1, DNA ladder; lane 2, H2O; lane 3, H2O, DNase, reverse transcriptase; lanes 4 and 7, normal rat brain; lanes 5 and 8, normal rat sieved glomeruli; lanes 6 and 9, differentiated cultured mouse podocytes. Lanes 4–6: With reverse transcriptase; lanes 7–9, without reverse transcriptase. Original magnifications, ×630 (A and B).
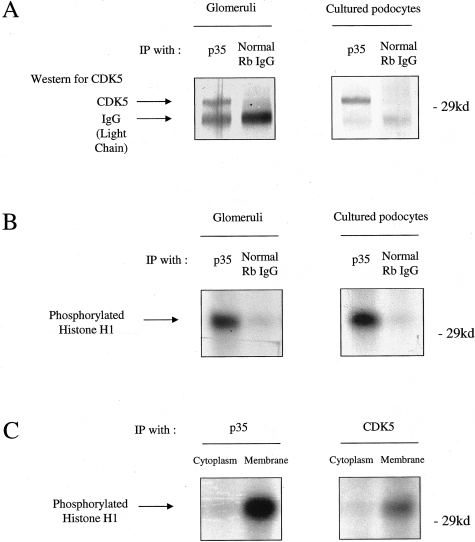
We also examined the p35/CDK5 kinase activity in isolated rat glomeruli and cultured podocytes by histone H1 kinase assay. As shown in Figure 6, A and B, CDK5 was co-immunoprecipitated with p35, and the complex was active. Our results also showed that the active p35/CDK5 complex was predominantly in the membrane-rich protein fraction compared to the cytoplasmic fraction (Figure 6C).
Figure 6-4249.
A: CDK5 complexes with p35 in podocytes. Protein from isolated glomeruli and cultured mouse podocytes was immunoprecipitated with an antibody to p35, and a Western blot performed for CDK5. No CDK5 bound in a control immunoprecipitation using rabbit IgG. B: CDK5 is active in podocytes. CDK5 activity was measured by the histone H1 kinase assay after immunoprecipitation with an anti-p35 antibody. CDK5 was active in protein derived from glomeruli and cultured differentiated podocytes. C: Subcellular fractionation demonstrates localization of active CDK5/p35 to the plasma membrane. Cytoplasmic and crude membrane fractions were isolated from cultured differentiated podocytes, immunoprecipitated with an anti-p35 antibody, and CDK5 activity measured by the histone H1 kinase assay. p35/CDK5 activity was principally localized to the membrane fraction.
CDK5 Contributes to Podocyte Cytoskeletal Integrity and Morphology
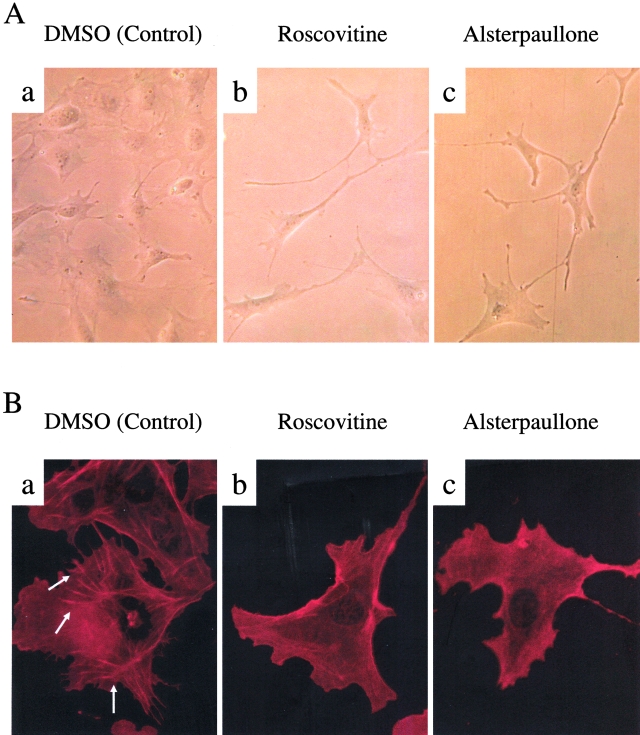
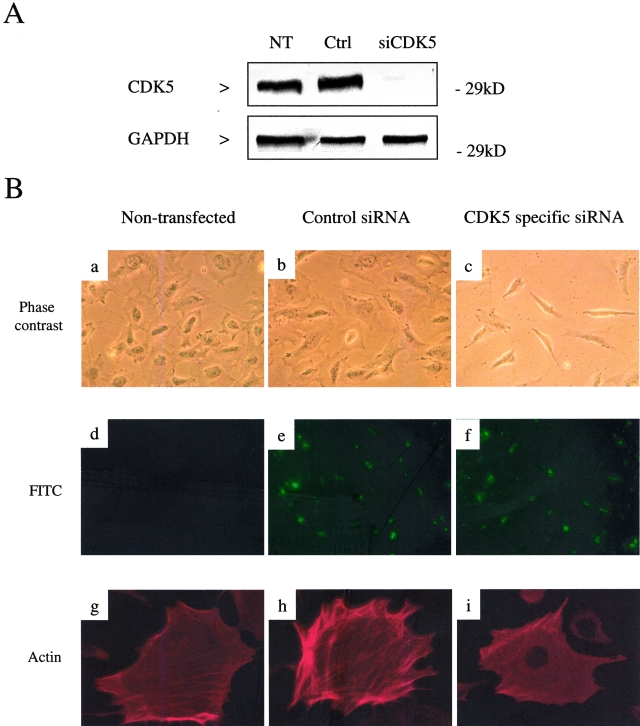
Finally, we examined the functional role of CDK5 in podocytes. Although the kidneys and podocytes of CDK5−/− mice appear morphologically normal during embryogenesis21 (and our own observations), the role of CDK5 in podocytes after birth is not known, as CDK5−/− mice undergo perinatal lethality because of brain abnormalities. Accordingly, to test the hypothesis that CDK5 has a critical function in mature podocytes, we inhibited CDK5 in podocytes in culture. Our results showed that specifically inhibiting CDK5 in differentiated cultured podocytes with roscovitine or alsterpaullone induced morphological changes with cellular elongation and loss of processes compared to the characteristic arborized phenotype (Figure 7A). Furthermore, these morphological changes were associated with actin rearrangement. In untreated podocytes, actin is distributed as long stress fiber-like bundles.17 Inhibiting CDK5 activity with either roscovitine or alsterpaullone was associated with loss of these actin bundles in the cytoplasm before cells become elongated (Figure 7B). To confirm the specificity of these results for CDK5, we reduced CDK5 protein levels using siRNA (Figure 8A). Cells transfected with CDK5-specific siRNA were smaller and more elongated than controls, with a loss of process formation (Figure 8B). Similarly to pharmacological inhibition of CDK5 activity, there was marked actin rearrangement with loss of the typical transverse stress fibers (Figure 8B).
Figure 7-4249.
A: Inhibiting CDK5 causes changes in podocyte morphology. a: Phase microscopy shows the normal cell morphology of differentiated podocytes in vitro, with multiple arborizing cell processes. When CDK5 is inhibited with either roscovitine (b) or alsterpaullone (c), podocytes lose their normal shape and become markedly elongated. B: Inhibiting CDK5 causes actin rearrangement. a: Immunofluorescent staining for actin shows that actin is normally arranged as long stress fiber-like bundles (examples indicated by arrows) in control differentiated podocytes. Inhibiting CDK5 with either roscovitine (b) or alsterpaullone (c) causes significant loss of these actin bundles. Original magnifications: ×200 (A); ×400 (B).
Figure 8-4249.
Specific inhibition of CDK5 with siRNA recapitulates the podocyte shape changes resulting from pharmacological inhibition. Differentiating mouse podocytes at day 3 of growth restriction were transfected with control or CDK5-specific siRNA. After 48 hours, protein was harvested, and cells examined by phase contrast microscopy and fixed for indirect immunofluorescence. A: Western blot of protein harvested from nontransfected (NT), control (Ctrl), and CDK5-specific siRNA (siCDK5)-transfected podocytes, confirming knock down of CDK5 protein. GAPDH staining confirms equivalent protein loading. B, a–c: Phase contrast microscopy of podocytes 48 hours after transfection with CDK5 siRNA demonstrates marked shape changes compared to non- and control-transfected cells, with loss of cytoplasmic spreading and reduced process formation. d–f: Corresponding fluorescent microscopy confirms transfection efficiency by detection of uptake of fluorescein isothiocyanate-labeled oligonucleotides. g–i: Actin immunostaining demonstrates the presence of stress fibers in both the non- and control-transfected cells, which is lost in the cells transfected with CDK5 siRNA. Original magnifications: ×200 (a–f); ×400 (g–i).
Discussion
The podocyte, or visceral glomerular epithelial cell, shares many similarities with neurons: both are specialized, terminally differentiated cells, with a complex cytoarchitecture and extensive, highly organized cell processes. Podocytes do not typically re-enter the cell cycle after injury, although under unusual circumstances podocytes will dedifferentiate and proliferate. Studies have shown that the inability to proliferate and replace those lost by detachment and apoptosis contributes to the development of glomerulosclerosis.2 Conversely, the poor outcome of conditions associated with aberrant podocyte proliferation underscores the importance of the maintenance of the mature differentiated phenotype for glomerular function. Therefore the regulation of podocyte proliferation and differentiation are critical in our understanding of glomerular disease.
The first finding in the current study was that de novo expression of CDK5 occurs in differentiating embryonal podocytes, and this persists in mature adult glomeruli. Similarly, in cultured podocytes, CDK5 expression increased during the switch from a nondifferentiated and proliferating phenotype, to a differentiated and quiescent one. In the kidney, CDK5 itself does not seem to be essential for morphological differentiation of podocytes before birth, as we have found that CDK5−/− mice have normal kidney development up to embryonal day 18 (data not shown). However the precise physiological role of CDK5 in podocytes after birth and in the adult remains undefined, because of the lethality of CDK5 deletion.21 Recently, a requirement for CDK5 has been reported in the differentiation of myoblasts31 and lens epithelial cells,32 the maturation of human hematopoietic cells to the monocytic lineage,33 and in the apoptosis associated with tissue remodeling during development.34 We present evidence that podocytes may now be added to the list of cells in which active CDK5 is constitutively expressed.
The second finding in the current study was that CDK5 is complexed with p35, and that p35-CDK5 complexes are active in both isolated glomeruli and cultured podocytes. Although CDK5 is present in many cell types, CDK5 activity was initially thought to be restricted to the nervous system, as expression of its activator protein, p35, is limited to neuronal cells.12 The immunohistochemical and immunofluorescent results in our study indicate the presence of CDK5 in the both the nucleus and cytoplasm of podocytes in vivo and in vitro. In contrast, staining for p35 was primarily restricted to the cytoplasm. The subcellular fractionation study showed that active p35/CDK5 localized predominantly to the plasma membrane in podocytes, suggesting that CDK5 elsewhere in the cell is either unbound, or complexed with other binding partners. We found that in both the nuclear and cytoplasmic fractions, CDK5 binds to cyclin D3, however this complex has no phosphorylating activity in the histone kinase assay (data not shown), suggesting an additional role for CDK5.
Little is known about the expression of CDK5 in inflammatory disease. It has previously been shown that podocytes dedifferentiate, and re-engage the cell cycle, in experimental HIV-associated nephropathy35 and in experimental anti-GBM nephritis.22 The third finding in this study was that in these disease models, podocyte dedifferentiation and proliferation is coincident with a marked decrease in CDK5 immunostaining. In contrast, CDK5 immunostaining was unaltered in an experimental model of mesangial cell injury (Habu nephritis). The correlation between reduced CDK5 expression and dedifferentiation in vivo, which is similar to the in vitro results, strongly implicates a role for CDK5 in maintaining the mature podocyte phenotype, and thus functional integrity. To further explore the role of CDK5 in podocytes, we inhibited CDK5 activity in cultured podocytes, both pharmacologically, and specifically with siRNA. Our results showed that inhibiting CDK5 caused marked changes in podocyte shape, with a concomitant dramatic reorganization of the actin cytoskeleton. These results indicate that CDK5 is involved in maintaining podocyte morphology, which is critical for its highly specialized function.
In summary, in this study we show that CDK5 is expressed in differentiated and quiescent podocytes in vitro and in vivo, and levels decrease during dedifferentiation and proliferation in podocytes in vitro and in experimental glomerular disease. In podocytes, CDK5 is activated by p35. Our results showed that a novel function for CDK5 is maintaining podocyte cytoskeletal structure, a prerequisite for the differentiated phenotype. Taken together, this study demonstrates that CDK5 is a regulator of podocyte differentiation, proliferation, and morphology.
Footnotes
Address reprint requests to Stuart J. Shankland M.D., Division of Nephrology, University of Washington School of Medicine, Box 356521, Seattle, WA 98195. E-mail: stuartjs@u.washington.edu.
Supported by the Public Health Service (grants DK34198, DK52121, DK51096, DK56799) and the George M. O’Brien Kidney Center (grant DK47659).
S.V.G. and K.H. contributed equally to this study.
S.J.S. is an Established Investigator of the American Heart Association.
References
- Pavenstadt H, Kriz W, Kretzler M. Cell biology of the glomerular podocyte. Physiol Rev. 2003;83:253–307. doi: 10.1152/physrev.00020.2002. [DOI] [PubMed] [Google Scholar]
- Kriz W. Podocyte is the major culprit accounting for the progression of chronic renal disease. Microsc Res Tech. 2002;57:189–195. doi: 10.1002/jemt.10072. [DOI] [PubMed] [Google Scholar]
- Kreidberg JA. Podocyte development and glomerulogenesis. J Am Soc Nephrol. 2003;14:806–814. doi: 10.1097/01.asn.0000054887.42550.14. [DOI] [PubMed] [Google Scholar]
- Kobayashi N. Mechanism of the process formation; podocytes vs neurons. Microsc Res Tech. 2002;57:217–223. doi: 10.1002/jemt.10077. [DOI] [PubMed] [Google Scholar]
- Combs HL, Shankland SJ, Setzer SV, Hudkins KL, Alpers CE. Expression of the cyclin kinase inhibitor p27kip1 in developing and mature human kidney. Kidney Int. 1998;53:892–896. doi: 10.1111/j.1523-1755.1998.00842.x. [DOI] [PubMed] [Google Scholar]
- Nagata M, Shibata S, Shigeta M, Yu-Ming S, Watanabe T. Cyclin-dependent kinase inhibitors: p27kip1 and p57kip2 expression during human podocyte differentiation. Nephrol Dial Transplant. 1999;14:48–51. doi: 10.1093/ndt/14.suppl_1.48. [DOI] [PubMed] [Google Scholar]
- Mundel P, Shankland SJ. Podocyte biology and response to injury. J Am Soc Nephrol. 2002;13:3005–3015. doi: 10.1097/01.asn.0000039661.06947.fd. [DOI] [PubMed] [Google Scholar]
- Barisoni L, Kriz W, Mundel P, D’Agati V. The dysregulated podocyte phenotype: a novel concept in the pathogenesis of collapsing idiopathic focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol. 1999;10:51–61. doi: 10.1681/ASN.V10151. [DOI] [PubMed] [Google Scholar]
- Shirato I. Podocyte process effacement in vivo. Microsc Res Tech. 2002;57:241–246. doi: 10.1002/jemt.10082. [DOI] [PubMed] [Google Scholar]
- Kriz W, Kretzler M, Provoost AP, Shirato I. Stability and leakiness: opposing challenges to the glomerulus. Kidney Int. 1996;49:1570–1574. doi: 10.1038/ki.1996.227. [DOI] [PubMed] [Google Scholar]
- Griffin SV, Pichler R, Wada T, Vaughan M, Durvasula R, Shankland SJ. The role of cell cycle proteins in glomerular disease. Semin Nephrol. 2003;23:569–582. doi: 10.1053/s0270-9295(03)00133-5. [DOI] [PubMed] [Google Scholar]
- Dhavan R, Tsai LH. A decade of cdk5. Nat Rev Mol Cell Biol. 2001;2:749–759. doi: 10.1038/35096019. [DOI] [PubMed] [Google Scholar]
- Tsai LH, Takahashi T, Caviness VS, Jr, Harlow E. Activity and expression pattern of cyclin-dependent kinase 5 in the embryonic mouse nervous system. Development. 1993;119:1029–1040. doi: 10.1242/dev.119.4.1029. [DOI] [PubMed] [Google Scholar]
- Lew J, Huang QQ, Qi Z, Winkfein RJ, Aebersold R, Hunt T, Wang JH. A brain-specific activator of cyclin-dependent kinase 5. Nature. 1994;371:423–426. doi: 10.1038/371423a0. [DOI] [PubMed] [Google Scholar]
- Patrick GN, Zukerberg L, Nikolic M, de la Monte S, Dikkes P, Tsai LH. Conversion of p35 to p25 deregulates Cdk5 activity and promotes neurodegeneration. Nature. 1999;402:615–622. doi: 10.1038/45159. [DOI] [PubMed] [Google Scholar]
- Nguyen MD, Lariviere RC, Julien JP. Deregulation of Cdk5 in a mouse model of ALS: toxicity alleviated by perikaryal neurofilament inclusions. Neuron. 2001;30:135–147. doi: 10.1016/s0896-6273(01)00268-9. [DOI] [PubMed] [Google Scholar]
- Mundel P, Reiser J, Zuniga Mejia Borja A, Pavenstadt H, Davidson GR, Kriz W, Zeller R. Rearrangements of the cytoskeleton and cell contacts induce process formation during differentiation of conditionally immortalized mouse podocyte lines. Exp Cell Res. 1997;236:248–258. doi: 10.1006/excr.1997.3739. [DOI] [PubMed] [Google Scholar]
- Meijer L, Borgne A, Mulner O, Chong JP, Blow JJ, Inagaki N, Inagaki M, Delcros JG, Moulinoux JP. Biochemical and cellular effects of roscovitine, a potent and selective inhibitor of the cyclin dependent kinases cdc2, cdk2 and cdk5. Eur J Biochem. 243:527–536. doi: 10.1111/j.1432-1033.1997.t01-2-00527.x. [DOI] [PubMed] [Google Scholar]
- Leost M, Schultz C, Link A, Wu YZ, Biernat J, Mandelkow EM, Bibb JA, Snyder GL, Greengard P, Zaharevitz DW, Gussio R, Senderowicz AM, Sausville EA, Kunick C, Meijer L. Paullones are potent inhibitors of glycogen synthase kinase-3beta and cyclin-dependent kinase 5/p25. Eur J Biochem. 2000;267:5983–5994. doi: 10.1046/j.1432-1327.2000.01673.x. [DOI] [PubMed] [Google Scholar]
- Hiromura K, Haseley LA, Zhang P, Monkawa T, Durvasula R, Petermann AT, Alpers CE, Mundel P, Shankland SJ. Podocyte expression of the CDK-inhibitor p57 during development and disease. Kidney Int. 2001;60:2235–2246. doi: 10.1046/j.1523-1755.2001.00057.x. [DOI] [PubMed] [Google Scholar]
- Ohshima T, Ward JM, Huh CG, Longenecker G, Veeranna Pant HC, Brady RO, Martin LJ, Kulkarni AB. Targeted disruption of the cyclin-dependent kinase 5 gene results in abnormal corticogenesis, neuronal pathology and perinatal death. Proc Natl Acad Sci USA. 1996;93:11173–11178. doi: 10.1073/pnas.93.20.11173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YG, Alpers CE, Brugarolas J, Johnson RJ, Couser WG, Shankland SJ. The cyclin kinase inhibitor p21CIP1/WAF1 limits glomerular epithelial cell proliferation in experimental glomerulonephritis. Kidney Int. 1999;55:2349–2361. doi: 10.1046/j.1523-1755.1999.00504.x. [DOI] [PubMed] [Google Scholar]
- Kopp JB, Klotman ME, Adler SH, Bruggeman LA, Dickie P, Marinos NJ, Eckhaus M, Bryant JL, Notkins AL, Klotman PE. Progressive glomerulosclerosis and enhanced renal accumulation of basement membrane components in mice transgenic for human immunodeficiency virus type 1 genes. Proc Natl Acad Sci USA. 1992;89:1577–1581. doi: 10.1073/pnas.89.5.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao N, Hiraiwa N, Yoshiki A, Ike F, Kusakabe M. Tenascin-C promotes healing of Habu-snake venom-induced glomerulonephritis: studies in knockout congenic mice and in culture. Am J Pathol. 1998;152:1237–1245. [PMC free article] [PubMed] [Google Scholar]
- Shankland SJ, Hugo C, Coats SR, Nangaku M, Pichler RH, Gordon KL, Pippin J, Roberts JM, Couser WG, Johnson RJ. Changes in cell-cycle protein expression during experimental mesangial proliferative glomerulonephritis. Kidney Int. 1996;50:1230–1239. doi: 10.1038/ki.1996.432. [DOI] [PubMed] [Google Scholar]
- Nikolic M, Tsai LH. Activity and regulation of p35/Cdk5 kinase complex. Methods Enzymol. 2000;325:200–213. doi: 10.1016/s0076-6879(00)25444-x. [DOI] [PubMed] [Google Scholar]
- Nikolic M, Chou MM, Lu W, Mayer BJ, Tsai LH. The p35/Cdk5 kinase is a neuron-specific Rac effector that inhibits Pak1 activity. Nature. 1998;395:194–198. doi: 10.1038/26034. [DOI] [PubMed] [Google Scholar]
- Hiromura K, Pippin JW, Fero ML, Roberts JM, Shankland SJ. Modulation of apoptosis by the cyclin-dependent kinase inhibitor p27(Kip1) J Clin Invest. 1999;103:597–604. doi: 10.1172/JCI5461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barisoni L, Mokrzycki M, Sablay L, Nagata M, Yamase H, Mundel P. Podocyte cell cycle regulation and proliferation in collapsing glomerulopathies. Kidney Int. 2000;58:137–143. doi: 10.1046/j.1523-1755.2000.00149.x. [DOI] [PubMed] [Google Scholar]
- Bradfield JW, Cattell V, Smith J. The mesangial cell in glomerulonephritis II Mesangial proliferation caused by Habu snake venom in the rat. Lab Invest. 1977;36:487–492. [PubMed] [Google Scholar]
- Lazaro JB, Kitzmann M, Poul MA, Vandromme M, Lamb NJ, Fernandez A. Cyclin dependent kinase 5, cdk5, is a positive regulator of myogenesis in mouse C2 cells. J Cell Sci. 1997;110:1251–1260. doi: 10.1242/jcs.110.10.1251. [DOI] [PubMed] [Google Scholar]
- Gao CY, Zakeri Z, Zhu Y, He H, Zelenka PS. Expression of Cdk5, p35, and Cdk5-associated kinase activity in the developing rat lens. Dev Genet. 1997;20:267–275. doi: 10.1002/(SICI)1520-6408(1997)20:3<267::AID-DVG9>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Chen F, Studzinski GP. Expression of the neuronal cyclin-dependent kinase 5 activator p35Nck5a in human monocytic cells is associated with differentiation. Blood. 2001;97:3763–3767. doi: 10.1182/blood.v97.12.3763. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Ahuja HS, Zakeri ZF, Wolgemuth DJ. Cyclin-dependent kinase 5 is associated with apoptotic cell death during development and tissue remodeling. Dev Biol. 1997;183:222–233. doi: 10.1006/dbio.1996.8494. [DOI] [PubMed] [Google Scholar]
- Barisoni L, Bruggeman LA, Mundel P, D’Agati VD, Klotman PE. HIV-1 induces renal epithelial dedifferentiation in a transgenic model of HIV-associated nephropathy. Kidney Int. 2000;58:173–181. doi: 10.1046/j.1523-1755.2000.00152.x. [DOI] [PubMed] [Google Scholar]