Abstract
Epidemiological studies suggest links between cholesterol metabolism and Alzheimer’s disease (AD), with hypercholesterolemia associated with increased AD risk, and use of cholesterol-lowering drugs associated with decreased risk. Animal models using cholesterol-modifying dietary or pharmacological interventions demonstrate similar findings. Proposed mechanisms include effects of cholesterol on the metabolism of amyloid-β (Aβ), the protein that deposits in AD brain. To investigate the effect of genetic alterations in plasma cholesterol on Aβ pathology, we crossed the PDAPP transgenic mouse model of AD-like cerebral amyloidosis to apolipoprotein AI-null mice that have markedly reduced plasma cholesterol levels due to a virtual absence of high density lipoproteins, the primary lipoprotein in mice. Interestingly and in contrast to models using non-physiological high fat diets or cholesterol-lowering drugs to modify plasma cholesterol, we observed no differences in Aβ pathology in PDAPP mice of the various apoAI genotypes despite robust differences in plasma cholesterol levels between the groups. Absence of apoAI also resulted in reductions in brain but not cerebrospinal fluid cholesterol, but had no effect on brain apolipoprotein E levels. These and other data suggest that it is perhaps the level of brain apolipoprotein E, not cholesterol per se, that plays a primary role in brain Aβ metabolism.
Recent evidence suggests a link between cholesterol metabolism and the pathogenesis of Alzheimer’s disease (AD). Epidemiological studies report positive associations between hypercholesterolemia (high plasma cholesterol levels) and risk for AD,1–4 although findings are inconsistent.5 Potentially consistent with such a link is the observation that the ε4 allele of apolipoprotein E (apoE), the isoform associated with elevated levels of plasma cholesterol,6 is also the strongest genetic risk factor for late-onset AD.7 ApoE4 also influences the age of clinical disease onset in families exhibiting an AD-causing gene mutation8 and in AD associated with Down syndrome.9 Finally, retrospective epidemiological studies demonstrate associations between use of HMG-Co-A reductase inhibitors (the cholesterol-lowering drugs known as statins) and reduced AD prevalence10 and dementia risk.11
Experimental studies suggest a potential mechanism by which cholesterol influences AD may be via effects on the metabolism of amyloid-β (Aβ), the protein that accumulates and deposits in the AD brain. Cholesterol is found in dense core plaques in AD and transgenic mouse models of AD-like cerebral amyloidosis.12 In addition, a portion of Aβ in plasma and cerebrospinal fluid (CSF) is associated with cholesterol-containing lipoproteins13–16 and thus may be influenced by processes governing lipoprotein metabolism. Cholesterol can regulate amyloid precursor protein (APP) processing and Aβ generation in vitro,17–19 and alterations in Aβ deposition have been observed in animal models of hyper- and hypocholesterolemia induced by high fat diets20–23 or treatment with cholesterol-lowering drugs,19,24 respectively. Finally, data from recent clinical trials demonstrate decreases in serum Aβ25 and APP metabolites in CSF26 after statin treatment, although other studies report minimal effects.27,28
While these data are suggestive, several issues must be resolved. With the exception of one study,23 experimental high fat diets can be considered non-physiological because of other pathological consequences, including vascular inflammation and blood-brain barrier disruption.29 In addition, potential effects of cholesterol-lowering drugs on AD risk differ for the various compounds despite equivalent cholesterol-lowering capabilities.10,11 The statins also have pleiotropic effects (including anti-inflammatory, vascular, and antioxidant effects)30 apart from their ability to lower cholesterol, thus raising the question of mechanism of action. Therefore, to circumvent the limitations and caveats of previous studies, we used a direct genetic approach to investigate whether life-long, non-dietary, non-pharmacological differences in plasma cholesterol levels influence the development of Aβ-related pathology in a well-characterized transgenic mouse model of AD-like cerebral amyloidosis. Genetic variations in plasma cholesterol levels in APPV717F (PDAPP) transgenic mice were achieved by modifying apoAI gene dose through breedings to apoAI−/− mice, known to exhibit marked deficiencies in plasma cholesterol level.31,32 We observed significant reductions in plasma cholesterol in PDAPP+/−, apoAI−/− mice, but no differences in brain Aβ pathology. Absence of apoAI also resulted in significant reductions in cholesterol measured in brain but had no effect on brain apolipoprotein E (apoE) levels. These data suggest that it is perhaps the level of brain apoE, and not cholesterol per se, that may be playing a primary role in brain Aβ metabolism.
Materials and Methods
Animals and Tissue Preparation
Transgenic mice expressing APPV717F (PDAPP;33 were bred with mice lacking the gene for apolipoprotein AI (apoAI−/−)31 (Jackson Labs, Bar Harbor, ME) to ultimately generate PDAPP+/− mice expressing two (apoAI+/+), one (apoAI+/−), or no (apoAI−/−) copies of the endogenous mouse apoAI gene within the same litter. PDAPP animals were on a mixed (50% C57BL/6/DBA, 50% Swiss Webster) background,34 and apoAI−/− mice were on a C57BL/6 background. Animals were screened for the presence of the APPV717F transgene35 and apoAI genes (Jackson Labs) by PCR from tail DNA. ApoAI genotype was further confirmed by semi-quantitative Western blotting of plasma (see below). Animals were sacrificed at 6, 9, 12, or 15 months of age. Mice were anesthetized with sodium pentobarbital, and CSF was collected from the cisterna magna as described,36 and blood (for plasma) was obtained via cardiac puncture. Following transcardial perfusion with 0.1 mol/L phosphate-buffered saline (PBS) (pH 7.4), brains were divided into left and right hemispheres. The right hemisphere was immersion-fixed in paraformaldehyde (4% in 0.1 mol/L phosphate buffer, pH 7.4) overnight and cryoprotected for 24 hours in 30% sucrose in PBS at 4°C for subsequent histological analysis. The left hemisphere was regionally dissected and frozen in dry ice for subsequent biochemical analysis.
Histological Analysis
Tissue sections were cut at 50 μm in the coronal plane on a freezing sliding microtome from the genu of the corpus callosum through the caudal extent of the hippocampus. For analysis of Aβ-immunoreactive (IR) deposits, sections were immunostained with a pan anti-Aβ antibody (Biosource; Camarillo, CA) as described.37 Thioflavine-S (Thio-S) staining was used to identify amyloid (ie, fibrillar Aβ), as described.35 Quantitative analysis of Aβ and amyloid deposition in the hippocampus was performed, defined as the percent hippocampal area covered by Aβ-IR and Thio-S-positivity, respectively, in three tissue sections, 300 μm apart starting 900 μm caudal to the beginning of the hippocampus in coronal section. The percentage of hippocampal area covered by Aβ-IR or Thio-S-positivity (% Αβ or amyloid load, respectively) was determined in an unbiased fashion using the Cavalieri point counting method38,39 with the assistance of a stereology system (MicroBrightField, Inc.; Colchester, VT). Statistical comparisons were made with analysis of variance followed by Tukey post-hoc tests using GraphPad Prism software (version 4.0) for Windows (San Diego, CA). In addition, sections from a subset of animals of each genotype displaying amyloid deposition at 15 months of age were stained with the de Olmos silver stain40 to identify neuritic dystrophy associated with amyloid plaques. Power calculations indicate that we can detect a 30 to 40% difference in the amount of Aβ deposition between groups (at 15 months of age) using 10 to 15 animals per group.
Biochemical Analysis
Soluble and insoluble fractions of brain tissue were prepared for Aβ analysis as described.41 Half of the hippocampus from each animal was Dounce homogenized in carbonate buffer (100 mmol/L Na2CO3, 50 mmol/L NaCl, pH 11.5) containing protease inhibitors (20 μg/ml aprotinin, 10 μg/ml leupeptin) and centrifuged at 14,000 rpm for 20 minutes at 4°C. The supernatant (soluble fraction) was transferred to another tube, kept on ice, and immediately analyzed (see below). The pellet was then homogenized in 5 mol/L guanidine buffer (5 mol/L guanidine-HCl in 50 mmol/L Tris-HCl, pH 8.0) and rotated for 3.5 hours at room temperature (RT). Following centrifugation at 14,000 rpm for 20 minutes at 4°C, the supernatant (insoluble fraction) was transferred to another tube and stored at −70°C until analyzed. Levels of human Aβ40 and Aβ42 in the soluble and insoluble brain fractions and CSF and plasma were quantified by sensitive ELISA, as described.41 Statistical comparisons were made with analysis of variance followed by Tukey post-hoc tests or Pearson’s correlation. Power analyses indicate that we would be able to detect a 20% difference in tissue Aβ levels between groups before Aβ deposition (≤9 months) and a 60 to 70% difference between groups with deposition (eg, 15 months) using 10 to 15 animals per group. Thus, non-statistical differences in Aβ levels are interpreted as indicating differences less than 20% for young animals and 60% for older animals.
Western Blot
SDS-PAGE and Western blotting were performed as described.42 Blots of mouse plasma were incubated with rabbit anti-mouse apoAI antibodies (Biodesign International; Saco, ME), followed by HRP-conjugated goat anti-rabbit antibodies (BioRad; Hercules, CA). Signal was detected by chemiluminescence (SuperSignal West Pico Chemiluminescence Substrate, Pierce; Rockford, IL) and quantified by Kodak Image Station (Rochester, NY).
Gel Filtration Chromatography
Samples of plasma (250 μl) from PDAPP+/− , apoAI+/+ and PDAPP+/−, apoAI−/− mice (12 months old, n = 2 each, fasted and non-fasted) were fractionated under non-denaturing conditions over tandem Superose-6 HR 10/30 columns (Amersham Biosciences; Piscataway, NJ) using a BioLogic Workstation (BioRad) as described.43 Adjacent fractions were pooled and assayed for total cholesterol as described below.
Cholesterol Assay
Plasma from all animals and cortical brain lysates (homogenized in PBS containing protease inhibitors) from a subset of 9- to 12-month-old animals before Aβ deposition were assayed for total cholesterol (Amplex Red Cholesterol Assay Kit, Molecular Probes; Eugene, OR) as previously described44 and normalized to tissue wet weight. Small tissue volumes prevented us from analyzing both Aβ and cholesterol in the same hippocampal region, so another region known to exhibit Aβ deposition (parietal cortex) was chosen for cholesterol measures. Tissue homogenates included both soluble and insoluble (eg, membrane) fractions. Statistical comparisons between groups were made as described above.
Mouse apoE ELISA
Plasma from 15-month-old animals and brain tissue from 9-month-old animals before Aβ deposition were assayed for endogenous mouse apoE expression by an ELISA developed in our lab. Briefly, brain tissue (parietal cortex) was sonicated for 3 seconds on ice in apoE ELISA lysis buffer (PBS containing 0.05% Tween and protease inhibitors) before centrifugation at 14,000 rpm for 15 minutes at 4°C. The supernatant was transferred to another tube and stored at −70°C until analyzed. For the apoE ELISA procedure, microtiter plates were coated overnight with a monoclonal mouse anti-apoE antibody that recognizes mouse apoE (WUE445) at a concentration of 4.5 μg/ml in carbonate-coating buffer (35 mmol/L NaHCO3, 16 mmol/L Na2CO3, 0.02% Na azide, pH 9.6), and then blocked with 1% dry milk in PBS for 2 hours at RT. ApoE standards (Swiss Webster mouse plasma estimated to contain 50 μg/ml apoE) and samples of plasma or brain lysate from PDAPP+/−, apoAI mice were diluted in apoE ELISA sample buffer (PBS containing 0.025% Tween, 0.1% bovine serum albumin (BSA) and protease inhibitors), loaded onto blocked ELISA plates, and incubated for 4 hours at RT. Plates were then incubated overnight at 4°C in biotinylated goat anti-apoE antibodies (125 μg/ml; Calbiochem; San Diego, CA) in PBS containing 1% BSA and 0.1% Na azide, followed by a 2-hour incubation in Strep-Poly HRP (Pierce) at RT and color development in Slow TMB for ELISA (Sigma; St. Louis, MO). Plates were read at 650 nm and quantified via FL600 Fluorescence Reader (Bio-Tek; Winooski, VT). Plates were rinsed 5 to 8 times with PBS between each step, and all incubations were carried out with rotation. This assay is sensitive down to 1.5 ng apoE/ml. ApoE levels in brain lysates were normalized to total protein levels, as measured by bicinchoninic acid (BCA) assay (Pierce). Statistical comparisons between groups were made as described above. Power analyses indicate an ability to detect differences of ≥60% between groups given the relatively small number of animals (n = 5) in each group.
Results
Total Cholesterol Levels in Plasma and Brain of PDAPP+/− Mice Are Significantly Reduced in the Absence of apoAI
The goal of the present study was to create a mouse model that develops AD-like pathology (ie, cerebral amyloidosis) and has variable levels of plasma cholesterol without the use of non-physiological dietary or pharmacological interventions. Consistent with previous studies of apoAI−/− mice,31,32 PDAPP+/− mice lacking the endogenous mouse apoAI gene exhibited significant reductions (mean, 77%) in plasma cholesterol levels (Figure 1A) at all ages analyzed. Levels within groups did not differ as a function of age (data not shown). Isolation of plasma lipoproteins from PDAPP+/−, apoAI+/+ and PDAPP+/−, apoAI−/− mice via size exclusion chromatography confirmed that this reduction was due to a marked decrease in plasma high density lipoprotein (HDL), the primary plasma lipoprotein in mice (Figure 1B), although decreases were also observed in very low density lipoprotein (VLDL) and low density lipoprotein (LDL). Fasting did not alter this pattern (data not shown). Thus we were successful in creating an animal model of AD-like cerebral amyloidosis that markedly differed in its level of plasma cholesterol (predominantly HDL).
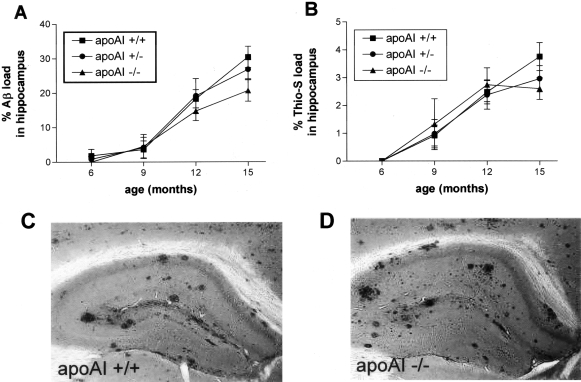
Figure 1-4255.
Effects of apoAI gene dose on plasma and brain lipid profiles in PDAPP+/− mice. A: Mean plasma total cholesterol levels significantly differ as a function of apoAI genotype in a gene dose-dependent manner (apoAI+/−, 33% decrease compared to apoAI+/+; apoAI−/−, 77% decrease). B: Representative fractionation profile of plasma from 12-month-old PDAPP+/−, apoAI+/+ and PDAPP+/−, apoAI−/− mice via gel filtration chromatography demonstrates a virtual absence of plasma HDL (the primary plasma lipoprotein in mice), as well as decreases in plasma VLDL and LDL in apoAI−/− mice. Total plasma cholesterol level in apoAI+/+ =4827 μg/ml. Total plasma cholesterol level in apoAI−/− = 318 μg/ml. C: Absence of apoAI (apoAI−/−) also results in a significant decrease (43%) in mean total cholesterol levels measured in the brain (parietal cortex) of PDAPP+/− mice (9 to 12 months of age). D: Levels of brain total cholesterol are positively correlated (Pearson correlation) with levels of plasma total cholesterol in PDAPP+/−, apoAI mice. HDL, high density lipoproteins; LDL, low density lipoproteins; VLDL, very low density lipoproteins.
Interestingly, we also observed significant reductions in cortical brain cholesterol levels in PDAPP+/−, apoAI−/− mice compared to PDAPP+/−, apoAI+/+ mice (Figure 1C), although the magnitude of the difference was not as dramatic as was seen in plasma. There was ∼40% less total brain cholesterol measured in PDAPP+/−, apoAI−/− mice compared to PDAPP+/−, apoAI+/+ mice, although there was overlap between the groups. Brain cholesterol levels were significantly correlated with plasma cholesterol levels (Figure 1D). However, we observed no differences in the level of cholesterol in the CSF of apoAI−/− mice compared to C57BL/6 controls (17.7 ± 1.26 μg/ml in C57BL/6; 17.02 ± 0.49 μg/ml in apoAI−/−, P > 0.05, mean ± SEM), nor significant differences between CSF apoE level in these animals (618 ± 304 ng/ml apoE in C57BL/6; 863 ± 240 ng/ml in apoAI−/−, P > 0.05, mean ± SEM). To the extent that CSF reflects the composition of brain extracellular fluid, these data suggest that the reduction in brain total cholesterol we observed in PDAPP/apoAI−/− mice is due to changes in lipid pools other than lipoproteins in brain extracellular fluid. This could represent changes in brain cellular pools or could conceivably be related to residual plasma cholesterol associated with brain vasculature that is possibly not removed with standard systemic perfusion methods. Together these data suggest that apoAI, a protein produced predominantly by cells of the periphery (liver and intestine) and not the CNS (except perhaps by brain endothelial cells46,47), in some way influences cholesterol levels measured in the CNS, either through direct effects of apoAI on the brain or perhaps through interactions between cholesterol and/or lipoproteins in the plasma and the brain.
Reduction in Plasma Cholesterol Level Has No Effect on Age-Dependent Increases in Soluble or Insoluble Aβ40 and Aβ42 in the Hippocampus of PDAPP+/− Mice
Results from cell culture experiments17–19 and in vivo models of pharmacological or dietary induced hypo- or hypercholesterolemia, respectively,19–24 suggest a role for cholesterol in APP processing and Aβ generation. To directly test whether non-dietary and non-pharmacological variations in plasma cholesterol levels influence brain Aβ levels, PDAPP+/−, apoAI+/+ (mean plasma cholesterol ± SEM = 3931 μg/ml ± 180), PDAPP+/−, apoAI+/− (mean plasma cholesterol ± SEM = 2631 μg/ml ± 166), and PDAPP+/−, apoAI−/−(mean plasma cholesterol ± SEM = 896 μg/ml ± 68) mice were sacrificed at various ages, and the hippocampus was assayed for human Aβ40 and Aβ42 in the carbonate-soluble and carbonate-insoluble (guanidine-soluble) fractions. Consistent with previous reports of total Aβ (soluble plus insoluble),48,49 levels of soluble and insoluble Aβ40 and Aβ42 in the hippocampus of PDAPP mice increased with age (Figure 2). These increases were all statistically significant (P < 0.001) except for soluble Aβ40 (P = 0.07). Levels of insoluble Aβ42 increased between 500- to 1000-fold from 6 to 15 months of age in all genotype groups. However, despite significant reductions in plasma and brain cholesterol levels (by 77% and 43%, respectively) with the absence of apoAI, the amount of soluble and insoluble Aβ40 and Aβ42 in the hippocampus and the time course of its increase did not differ between the genotype groups (Figure 2), nor was there a significant genotype by age interaction. Although levels of insoluble Aβ40 and Aβ42 in PDAPP+/−, apoAI−/− mice were lower than the apoAI-expressing groups at 12 months of age (Figure 2, C and D), this difference was not observed in younger animals (6 to 9 months old) and values were not statistically different between the genotypes at older ages (15 months old). Consistent with the above findings, we observed no correlations between the level of brain cholesterol and any of the hippocampal Aβ levels (data not shown). In addition, levels of Aβ40 and Aβ42 in the CSF and plasma did not differ between the genotype groups (data not shown). These data demonstrate that life-long, non-dietary and non-pharmacological variations (up to fourfold) in the level of plasma cholesterol do not significantly influence steady-state Aβ levels in the CNS or plasma of PDAPP mice.
Figure 2-4255.
Levels of soluble and insoluble Aβ40 and Aβ42 in the hippocampus of PDAPP+/−, apoAI mice with age. Levels of soluble (A) Aβ40 and (B) Aβ42 and insoluble (C) Aβ40 and (D) Aβ42 increase with age in PDAPP, apoAI mice, but do not differ significantly as a function of apoAI genotype. Values correspond to means ± SEM, 6 months, n = 2 to 3 animals per group; 9 months, n = 9 to 12 animals per group; 12 months, n = 9 to 14 animals per group; 15 months, n = 9 to 10 animals per group.
The Amount, Pattern, and Age of Onset of Plaque Deposition in PDAPP+/− Mice Does Not Differ as a Function of Plasma Cholesterol Levels Due to apoAI Genotype
Since cholesterol accumulates in senile plaques in AD brain and APP transgenic mice,12 binds to Aβ50 and promotes Aβ fibril formation,51 we next investigated whether the reductions in plasma cholesterol observed in PDAPP+/−, apoAI−/− mice had any effect on the deposition of Aβ as diffuse or amyloid plaques. PDAPP+/− mice of the different apoAI genotypes were sacrificed at 6, 9, 12, and 15 months of age, and the amount of Aβ and amyloid deposition in the hippocampus was quantified by unbiased, stereologic methods. In agreement with previous reports,48,49 Aβ deposition in PDAPP+/− mice (wild-type for the apoAI gene) increased with age (Figure 3A). The amount and age of onset of Aβ deposition, however, did not differ significantly between the apoAI genotype groups (Figure 3A), nor did the pattern of plaque distribution within the hippocampus (Figure 3, C and D). There were also no significant differences between the numbers of amyloid plaques, as defined by staining with Thioflavine-S (Figure 3B), nor in the amount of neuritic dystrophy associated with amyloid plaques, as assessed by the de Olmos silver stain (data not shown). Consistent with these findings, we observed no correlation between plasma (R2 = 0.006, P = 0.64) or brain (R2 = 0.045, P = 0.24) cholesterol levels and hippocampal Aβ deposition. Thus, dramatic reductions in plasma cholesterol secondary to the absence of apoAI does not appear to influence Aβ levels or deposition in this mouse model.
Figure 3-4255.
Aβ deposition in the hippocampus of PDAPP mice as a function of apoAI genotype. A: Aβ load (defined as the percentage of hippocampal area in three tissue sections covered by Aβ immunoreactivity) and (B) amyloid load (defined as the percentage of hippocampal area in three tissue sections covered by Thioflavine-S positivity) increases with age in PDAPP+/−, apoAI mice, but do not differ significantly as a function of apoAI genotype. Aβ plaques are observed in similar patterns throughout the hippocampus and overlying cortex in both (C) PDAPP+/−, apoAI+/+ and (D) PDAPP+/−, apoAI−/− mice (12 months of age). Values correspond to means ± SEM, 6 months, n = 2 to 3 animals per group; 9 months, n = 9 to 12 animals per group; 12 months, n = 9 to 14 animals per group; 15 months, n = 9 to 10 animals per group.
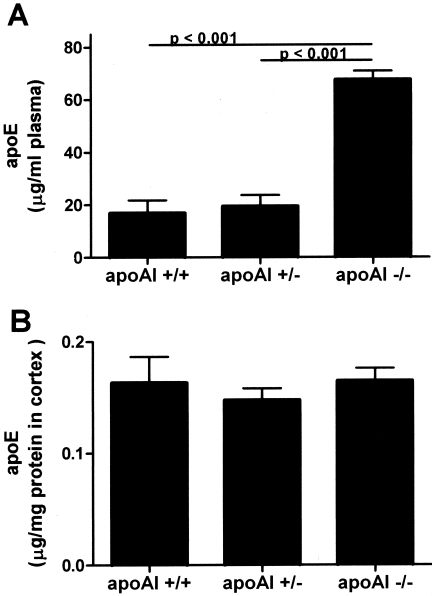
ApoE Expression Is Increased in the Plasma But Not the Brain of PDAPP+/− Mice Lacking apoAI
Given the finding of a lack of effect of plasma cholesterol on Aβ-related pathology in this animal model, we quantified the expression of another apolipoprotein, apoE, in the brain and plasma of PDAPP mice of the different apoAI genotypes before Aβ deposition. ApoE is normally expressed in both the brain and the periphery, but its levels are regulated independently in these two compartments.16,52 Furthermore, apoE is known to exert profound effects on Aβ fibrillogenesis,53,54 and Aβ metabolism in human AD55 and mouse models of AD-like cerebral amyloidosis in a dose-dependent fashion.35,37,49,56–58 ApoE levels in plasma (15 months old) and homogenates of parietal cortex (9 months old without Aβ deposition) from animals of each genotype were quantified by a sensitive ELISA. We observed a marked increase in apoE levels in the plasma of PDAPP+/− mice lacking apoAI, consistent with previous studies of apoAI−/− mice59,60(Figure 4A). Interestingly, however, there was no significant difference in apoE levels in the brain between any of the apoAI genotype groups (Figure 4B). Our combined observations of equivalent Aβ pathology in animals with equal expression of brain apoE but reduced levels of cholesterol are consistent with the hypothesis that it is perhaps the level of apoE, and not cholesterol per se, that influences Aβ metabolism in this mouse model.
Figure 4-4255.
ApoE protein levels in the plasma and brain of PDAPP+/− mice as a function of apoAI genotype. A: ApoE level in the plasma of PDAPP+/−, apoAI−/− mice is significantly greater than that of PDAPP+/−, apoAI+/+ and PDAPP+/−, apoAI+/− mice (all 9 months old without Aβ deposition). B: ApoE levels in the brain of PDAPP+/−, apoAI mice do not differ as a function of apoAI genotype. Values correspond to means ± SEM, n = 5 to 6 animals per group.
Discussion
Results of the present study demonstrate that absence of apoAI, the major plasma HDL-associated apolipoprotein, leads to marked (mean, 77%) reductions in plasma cholesterol levels in PDAPP+/− mice. Hence, we were able to use this genetic model to directly test whether non-dietary, non-pharmacological variations in plasma cholesterol level influence brain Aβ levels and deposition, a hypothesis proposed to explain the reported link between high plasma cholesterol and increased risk for AD.61,62 Interestingly and in contrast to animal models in which non-physiological high fat diets or pharmacological means are used to modify plasma cholesterol levels, we observed no differences in the age-related development, pattern or extent of Aβ-related pathology in PDAPP mice of the various apoAI genotypes despite up to fourfold differences in normal plasma cholesterol levels between the groups. The absence of apoAI also resulted in reduced levels of cholesterol measured in the brain (mean, 43% reduction) but not CSF, but had no effect on CNS apoE levels. Together, these data are consistent with the idea that it is the level of brain apoE, not plasma cholesterol per se, which influences Aβ metabolism and its deposition in the brain.
Low HDL cholesterol is a known risk factor for coronary artery disease,63,64 perhaps by impairing reverse cholesterol transport capability. ApoAI is the major apolipoprotein associated with HDL, and apoAI deficiency in humans leads to a phenotype of low plasma HDL levels and premature atherosclerosis.65–67 ApoAI knockout mice also exhibit a marked reduction in plasma HDL levels31,32 that is reflected in levels of total plasma cholesterol since HDL is the primary plasma lipoprotein in mice. Interestingly, apoAI−/− mice do not develop atherosclerosis,60 although they have been reported to exhibit diminished HDL cholesteryl ester flux and tissue uptake of HDL cholesteryl esters.32 However, HMG-CoA reductase activity (important for cholesterol biosynthesis) and LDL receptor levels are normal in apoAI−/− mice (except in steroidogenic tissues), as are cholesterol and cholesteryl ester stores in a variety of tissues examined.32 Cholesterol levels in the brain with apoAI deficiency have not been examined. We observed reduced levels of cholesterol in brain but not in CSF in mice lacking apoAI. Although our methods at the time did not permit assessment of the different pools of cholesterol in brain (ie, free versus esterified cholesterol), more recent experiments using tissue from various mouse strains (including apoAI-null mice) has demonstrated that brain contains predominantly (>95%) free (non-esterified) cholesterol (S. Wahrle, unpublished observations). Therefore, it is free cholesterol that is most likely decreased in PDAPP/apoAI−/− mice.
The observation of reduced levels of brain cholesterol in PDAPP/apoAI−/− mice may indicate a direct or indirect effect of apoAI on brain cholesterol metabolism or alternatively may reflect plasma HDL cholesterol associated with brain vasculature that is possibly not removed by systemic perfusion. ApoAI is synthesized primarily by cells of the liver and intestine68,69 but is found in brain homogenates,70 perhaps a product of brain endothelial cells,46,47 and in CSF,16,71,72 as a presumed filtrate of plasma. Thus, to the extent that apoAI can enter brain parenchyma from the plasma and CSF, apoAI could conceivably interact directly with neural tissue elements and modify local cholesterol metabolism. The cellular (eg, neurons or glia) or subcellular (eg, myelin, lipid rafts, interstitial fluid) origins of the observed brain cholesterol deficit in PDAPP, apoAI−/− mice remain to be determined. In general, the cellular and molecular mechanisms governing cholesterol metabolism in the CNS are poorly understood and are likely complex. Indeed, the overlap in brain cholesterol levels observed between the apoAI genotype groups suggests that molecules in addition to apoAI are involved in brain cholesterol metabolism. The fact that CSF cholesterol did not differ between wild-type and apoAI−/− mice suggests that brain extracellular lipoprotein metabolism is not affected by apoAI deficiency. As mentioned above, while our methods of quantifying cholesterol in tissue are very sensitive and reproducible, the possible contribution of residual plasma HDL cholesterol that remains associated with brain vasculature after systemic perfusion has not been defined. Thus, the changes in total brain cholesterol in PDAPP/apoAI−/− mice may not be due to changes in neuronal or glial cholesterol but may possibly reflect vascular cholesterol of a plasma origin. This issue will need to be addressed in future studies.
Reduced brain cholesterol levels in the absence of apoAI may alternatively indicate indirect actions of apoAI on the brain, secondary to reductions in plasma HDL and total cholesterol levels. Although regulation of brain cholesterol metabolism has long been considered to be independent of that in plasma, we have recently reported a strong positive correlation between the level of CSF lipoproteins (known to be HDL-like) and plasma HDL, but not LDL, in cognitively normal elderly individuals.16 Furthermore, a positive correlation was observed between the level of apoAI, but not apoE, in CSF and plasma, suggesting a possible role of plasma apoAI/HDL in modulating CNS lipoprotein metabolism.16 Interestingly, decreased HDL and plasma apoAI concentrations have been reported to correlate highly with the severity of dementia in AD.73 Whether other diseases that lead to reduced plasma HDL levels (eg, apoAI mutations or Tangier’s disease) affect CNS cholesterol levels or influence AD risk has not been reported.
The absence of an Aβ phenotype in PDAPP, apoAI−/− mice was somewhat surprising given data supporting a role for cholesterol in influencing AD risk and Aβ metabolism. However, a closer inspection of the published data point to a possible reason for this discrepancy and, perhaps more importantly, allows for an alternative interpretation of the published data that is consistent with the present results. In human and animal studies, hyper- and hypocholesterolemia induced by high fat diets and use of the cholesterol-lowering drugs known as statins, respectively, are also associated with alterations in brain apoE levels. High fat diets not only increase the level of cholesterol, but also apoE, in the brain,20,21,74 and statins decrease them both.75,76 Thus, it is not possible to distinguish putative effects of cholesterol from those of apoE on brain Aβ metabolism in these studies. Indeed, it is conceivable that effects of high fat diets and statin treatment previously attributed to cholesterol are actually due to altered levels of brain apoE. Consistent with this idea are studies showing that cholesterol effects on APP processing appear to require the presence of apoE,21 and lovastatin treatment influences brain cholesterol levels in wild-type mice but has no effect in apoE−/− mice.77 Our present finding of no alterations in Aβ-related measures in PDAPP, apoAI−/− mice in the presence of reduced plasma and brain cholesterol levels but equivalent levels of brain apoE would thus be consistent with this proposed primary role of apoE, rather than cholesterol, in brain Aβ metabolism in vivo. ApoE is known to exert profound effects on Aβ fibrillogenesis in vitro53,54 and on Aβ deposition in human AD.55 Murine and human apoE have also been shown to have marked dose-dependent effects on Aβ fibrillogenesis, clearance, and toxicity in vivo in mouse models of AD-like cerebral amyloidosis.35,37,49,56–58 It is particularly noteworthy that apoE−/− mice have extremely high levels of plasma cholesterol associated with VLDL and normal levels of brain cholesterol,44 yet mouse models of amyloidosis lacking apoE display significant reductions in Aβ deposition, especially deposits that are true amyloid (ie, Thioflavine-S positive).35,37 This dissociation strongly argues that the main effect of apoE on Aβ metabolism is not obviously linked with total brain or plasma cholesterol but is much more likely due to its direct effect as an Aβ chaperone.
Together, our findings suggest that the reported link between plasma cholesterol metabolism and AD pathogenesis may be due to mechanisms other than, or in addition to, direct effects of cholesterol on Aβ metabolism, and further strengthen the idea that regulating the level of brain apoE may be an important therapeutic approach for AD treatment. Studies aimed at directly modifying apoE level in the brain (independent of cholesterol) in mouse models, for example through gene transfer approaches, are currently in progress to test this hypothesis.
Acknowledgments
We thank Drs. John Cirrito, Eugene Johnson, and Chengjie Xiong for helpful comments, and Hong Jiang for technical assistance.
Footnotes
Address reprint requests to Anne M. Fagan, Ph.D., Department of Neurology and Center for the Study of Nervous System Injury, Washington University School of Medicine, 660 S. Euclid Ave., Box 8111, St. Louis, MO 63110. E-mail: fagana@neuro.wustl.edu.
Supported by grants IIRG-01–2751 from the Alzheimer’s Association (A.M.F.), a MetLife Award (D.M.H.), and National Institutes of Health grants 1K01-AG00861–01 (A.F.M.), and AG13956, AG05681, and AG11355 (D.M.H.).
References
- Jarvik J, Wijsman E, Kukull W, Schellenberg G, Yu C, Larson E. Interactions of apolipoprotein E genotype, total cholesterol level, age, and sex in prediction of Alzheimer’s disease: a case-control study. Neurology. 1995;45:1092–1096. doi: 10.1212/wnl.45.6.1092. [DOI] [PubMed] [Google Scholar]
- Kalmijn S, Launer L, Ott A, Witteman J, Hofman A, Breteler M. Dietary fat intake and the risk of incident dementia in the Rotterdam Study. Ann Neurol. 1997;42:776–782. doi: 10.1002/ana.410420514. [DOI] [PubMed] [Google Scholar]
- Kuo Y, Emmerling M, Bisgaier C, Essenburg A, Lampert H, Drumm D, Roher A. Elevated low-density lipoprotein in Alzheimer’s disease correlates with brain Aβ1–42 levels. Biochem Biophys Res Commun. 1998;252:711–715. doi: 10.1006/bbrc.1998.9652. [DOI] [PubMed] [Google Scholar]
- Notkola I, Sulkava R, Pekkanen J, Erkinjuntti T, Ehnholm C, Kivinen P, Tuomilehto J, Nissinen A. Serum cholesterol, apolipoprotein E epsilon 4 allele, and Alzheimer’s disease. Neuroepidemiol. 1998;17:14–20. doi: 10.1159/000026149. [DOI] [PubMed] [Google Scholar]
- Romas S, Tang M, Berglund L, Mayeux R. APOE genotype, plasma lipids, lipoproteins, and AD in community elderly. Neurology. 1999;53:517–521. doi: 10.1212/wnl.53.3.517. [DOI] [PubMed] [Google Scholar]
- Davignon J, Gregg RE, Sing CF. Apolipoprotein E polymorphism and atherosclerosis. Arteriosclerosis. 1988;8:1–21. doi: 10.1161/01.atv.8.1.1. [DOI] [PubMed] [Google Scholar]
- Strittmatter WJ, Saunders AM, Schmechel D, Pericak-Vance M, Enghild J, Salvesen GS, Roses AD. Apolipoprotein E: high avidity binding to β-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc Natl Acad Sci USA. 1993;90:1977–1981. doi: 10.1073/pnas.90.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastor P, Roe C, Villegas A, Bedoya G, Chakraverty S, Garcia G, Tirado V, Norton J, Rios S, Martinez M, Kosik K, Lopera F, Goate A. Apolipoprotein E ε4 modifies Alzheimer’s disease onset in an E280A PS1 kindred. Ann Neurol. 2003;54:163–169. doi: 10.1002/ana.10636. [DOI] [PubMed] [Google Scholar]
- Deb S, Braganza J, Norton N, Williams H, Kehoe P, Williams J, Owen M. APOE epsilon 4 influences the manifestation of Alzheimer’s disease in adults with Down’s syndrome. Br J Psychiatry. 2000;177:469–470. doi: 10.1192/bjp.176.5.468. [DOI] [PubMed] [Google Scholar]
- Wolozin B, Kellman W, Ruosseau P, Celesia G, Siegel G. Decreased prevalence of Alzheimer disease associated with 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors. Arch Neurol. 2000;57:1439–1443. doi: 10.1001/archneur.57.10.1439. [DOI] [PubMed] [Google Scholar]
- Jick H, Zornberg G, Jick S, Seshadri S, Drachman D. Statins and the risk of dementia. Lancet. 2000;356:1627–1631. doi: 10.1016/s0140-6736(00)03155-x. [DOI] [PubMed] [Google Scholar]
- Mori T, Paris D, Town T, Rojiani A, Sparks D, Delledonne A, Crawford F, Abdullah L, Humphrey J, Dickson D, Mullan M. Cholesterol accumulates in senile plaques of Alzheimer disease patients and in transgenic APPsw mice. J Neuropathol Exp Neurol. 2001;60:778–785. doi: 10.1093/jnen/60.8.778. [DOI] [PubMed] [Google Scholar]
- Koudinov A, Matsubara E, Frangione B, Ghiso J. The soluble form of Alzheimer’s amyloid beta protein is complexed to high density lipoprotein 3 and very high density lipoprotein in normal human plasma. Biochem Biophys Res Commun. 1994;205:1164–1171. doi: 10.1006/bbrc.1994.2788. [DOI] [PubMed] [Google Scholar]
- Koudinov AR, Koudinova NV, Kumar A, Beavis RC, Ghiso J. Biochemical characterization of Alzheimer’s soluble beta protein in human cerebrospinal fluid: association with high density lipoprotein. Biochem Biophys Res Commun. 1996;223:592–597. doi: 10.1006/bbrc.1996.0940. [DOI] [PubMed] [Google Scholar]
- Matsubara E, Ghiso J, Frangione B, Amari M, Tomidokoro Y, Ikeda Y, Harigaya Y, Okamoto K, Shoji M. Lipoprotein-free amyloidogenic peptides in plasma are elevated in patients with sporadic Alzheimer’s disease and Down’s syndrome. Ann Neurol. 1999;45:537–541. [PubMed] [Google Scholar]
- Fagan A, Younkin L, Morris J, Fryer J, Cole T, Younkin S, Holtzman D. Differences in the Aβ40/Aβ42 ratio associated with cerebrospinal fluid lipoproteins as a function of apolipoprotein E genotype. Ann Neurol. 2000;48:201–210. [PubMed] [Google Scholar]
- Simons M, Keller P, De Strooper B, Beyreuther K, Dotti C. Cholesterol depletion inhibits the generation of β-amyloid in hippocampal neurons. Proc Natl Acad Sci USA. 1998;95:6460–6464. doi: 10.1073/pnas.95.11.6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frears E, Stephens D, Walters C, Davies H, Austen B. The role of cholesterol in the biosynthesis of β-amyloid. NeuroReport. 1999;10:1699–1705. doi: 10.1097/00001756-199906030-00014. [DOI] [PubMed] [Google Scholar]
- Fassbender K, Simons M, Bergmann C, Stroick M, Lutjohann D, Keller P, Runz H, Kuhl S, Bertsch T, Von Bergmann K, Hennerici M, Beyreuther K, Hartmann T. Simvastatin strongly reduces levels of Alzheimer’s disease β-amyloid peptides Aβ42 and Aβ40 in vitro and in vivo. Proc Natl Acad Sci USA. 2001;98:5856–5861. doi: 10.1073/pnas.081620098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparks D, Liu H, Gross D, Scheff S. Increased density of cortical apolipoprotein E immunoreactive neurons in rabbit brain after dietary administration of cholesterol. Neurosci Lett. 1995;187:142–144. doi: 10.1016/0304-3940(95)11357-6. [DOI] [PubMed] [Google Scholar]
- Howland D, Trusko S, Savage M, Reaume A, Lang D, Hirsch JD, Maeda N, Siman R, Greenberg B, Scott R, Flood D. Modulation of secreted β-amyloid precursor protein and amyloid β-peptide in brain by cholesterol. J Biol Chem. 1998;273:16576–16582. doi: 10.1074/jbc.273.26.16576. [DOI] [PubMed] [Google Scholar]
- Refolo L, Pappolla M, Malester B, LaFrancois J, Bryant-Thomas T, Wang R, Tint G, Sambamurti K, Duff K. Hypercholesterolemia accelerates the Alzheimer’s amyloid pathology in a transgenic mouse model. Neurobiol Dis. 2000;7:321–331. doi: 10.1006/nbdi.2000.0304. [DOI] [PubMed] [Google Scholar]
- Levin-Allerhand J, Lominska C, Smith J. Increased amyloid-β levels in APPswe transgenic mice treated chronically with a physiological high-fat high-cholesterol diet. J Nutr Health Aging. 2002;6:315–319. [PubMed] [Google Scholar]
- Refolo L, Pappolla M, LaFrancois J, Malester B, Schmidt S, Thomas-Bryant T, Tint G, Wang R, Mercken M, Petanceska S, Duff K. A cholesterol-lowering drug reduces β-amyloid pathology in a transgenic mouse model of Alzheimer’s disease. Neurobiol Dis. 2001;8:890–899. doi: 10.1006/nbdi.2001.0422. [DOI] [PubMed] [Google Scholar]
- Buxbaum J, Cullen E, Friedhoff L. Pharmacological concentrations of the HMG-CoA reductase inhibitor lovastatin decrease the formation of the Alzheimer β-amyloid peptide in vitro and in patients. Front Biosci. 2002;7:a50–a59. doi: 10.2741/A739. [DOI] [PubMed] [Google Scholar]
- Sjogren M, Gustafsson K, Syversen S, Olsson A, Edman A, Davidsson P, Wallin A, Blennow K. Treatment with simvastatin in patients with Alzheimer’s disease lowers both α- and β-cleaved amyloid precursor protein. Dement Geriatr Cognit Disord. 2003;16:25–30. doi: 10.1159/000069989. [DOI] [PubMed] [Google Scholar]
- Fassbender K, Stroick M, Bertsch T, Ragoschke A, Kuehl S, Walter S, Walter J, Brechtel K, Muehlhauser F, von Bergmann K, Lutjohann D. Effects of statins on human cerebral cholesterol metabolism and secretion of Alzheimer amyloid peptide. Neurology. 2002;59:1257–1258. doi: 10.1212/wnl.59.8.1257. [DOI] [PubMed] [Google Scholar]
- Simons M, Schwarzler F, Lutjohann D, von Bergmann K, Beyreuther K, Dichgans J, Wormstall H, Hartmann T, Schulz J. Treatment with simvastatin in normocholesterolemic patients with Alzheimer’s disease: a 26-week randomized, placebo-controlled, double-blind trial. Ann Neurol. 2002;52:346–350. doi: 10.1002/ana.10292. [DOI] [PubMed] [Google Scholar]
- Sparks D, Kuo Y, Roher A, Martin T, Lukas R. Alterations of Alzheimer’s disease in the cholesterol-fed rabbit, including vascular inflammation: preliminary observations. Ann NY Acad Sci. 2000;903:335–344. doi: 10.1111/j.1749-6632.2000.tb06384.x. [DOI] [PubMed] [Google Scholar]
- Vaughan C, Murphy M, Buckley B. Statins do more than just lower cholesterol. Lancet. 1996;348:1079–1082. doi: 10.1016/S0140-6736(96)05190-2. [DOI] [PubMed] [Google Scholar]
- Williamson R, Lee D, Hagaman J, Maeda N. Marked reduction of high density lipoprotein cholesterol in mice genetically modified to lack apolipoprotein A-I. Proc Natl Acad Sci USA. 1992;89:7134–7138. doi: 10.1073/pnas.89.15.7134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plump A, Azrolan N, Odaka H, Wu L, Jiang X, Tall A, Eisenberg S, Breslow J. ApoA-I knockout mice: characterization of HDL metabolism in homozygotes and identification of a post-RNA mechanism of apoA-I up-regulation in heterozygotes. J Lipid Res. 1997;38:1033–1047. [PubMed] [Google Scholar]
- Games D, Adams D, Alessandrini R, Barbour R, Berthelette P, Blackwell C, Carr T, Clemens J, Donaldson T, Gillespie F, Guido T, Hagopian S, Johnson-Wood K, Khan K, Lee M, Leibowitz P, Lieberburg I, Little S, Masliah E, McConlogue L, Montoya-Zavala, Mucke L, Paganini L, Penniman E, Power M, Schenk D, Seubert P, Snyder B, Soriano F, Tan H, Vitale J, Wadsworth S, Wolozin B, Zhao J. Alzheimer-type neuropathology in transgenic mice overexpressing V717F β-amyloid precursor protein. Nature. 1995;373:523–527. doi: 10.1038/373523a0. [DOI] [PubMed] [Google Scholar]
- Holtzman DM, Bales KR, Wu S, Bhat P, Parsadanian M, Fagan AM, Chang LK, Sun Y, Paul SM. Expression of human apolipoprotein E reduces amyloid-β deposition in a mouse model of Alzheimer’s disease. J Clin Invest. 1999;103:R15–R21. doi: 10.1172/JCI6179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bales KR, Verina T, Dodel RC, Du Y, Altstiel L, Bender M, Hyslop P, Johnstone EM, Little SP, Cummins DJ, Piccardo P, Ghetti B, Paul SM. Lack of apolipoprotein E dramatically reduces amyloid β-peptide deposition. Nat Genet. 1997;17:263–264. doi: 10.1038/ng1197-263. [DOI] [PubMed] [Google Scholar]
- DeMattos RB, Bales KR, Parsadanian M, O’Dell MA, Foss EM, Paul SM, Holtzman DM. Plaque-associated disruption of CSF and plasma amyloid-β (Aβ) equilibrium in a mouse model of Alzheimer’s disease. J Neurochem. 2002;81:229–236. doi: 10.1046/j.1471-4159.2002.00889.x. [DOI] [PubMed] [Google Scholar]
- Holtzman DM, Fagan AM, Mackey B, Tenkova T, Sartorius L, Paul SM, Bales K, Hsiao-Ashe K, Irizarry MC, Hyman BT. ApoE is required for neuritic and cerebrovascular plaque formation in the APPsw mouse model of Alzheimer’s disease. Ann Neurol. 2000;47:739–747. [PubMed] [Google Scholar]
- Cavalieri B. Unione Tipografico,; Torino: Geometria Degli Indivisibili. 1966 [Google Scholar]
- West MJ. New stereological methods for counting neurons. Neurobiol Aging. 1993;14:275–285. doi: 10.1016/0197-4580(93)90112-o. [DOI] [PubMed] [Google Scholar]
- Wozniak D, Brosnan-Watters G, Nardi A, McEwen M, Corso T, Olney J, Fix A. MK-801 neurotoxicity in male mice: histologic effects and chronic impairment in spatial learning. Brain Res. 1996;707:165–179. doi: 10.1016/0006-8993(95)01230-3. [DOI] [PubMed] [Google Scholar]
- DeMattos R, O’Dell M, Parsadanian M, Taylor W, Harmony JK, Bales R, Paul S, Aronow B, Holtzman D. Clusterin promotes amyloid plaque formation and is critical for neuritic toxicity in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci USA. 2002;99:10843–10848. doi: 10.1073/pnas.162228299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Wu S, Bu G, Onifade MK, Patel SN, LaDu MJ, Fagan AM, Holtzman DM. GFAP-apoE transgenic mice: astrocyte-specific expression and differing biological effects of astrocyte-secreted apoE3 and apoE4 lipoproteins. J Neurosci. 1998;18:3261–3272. doi: 10.1523/JNEUROSCI.18-09-03261.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagan AM, Holtzman DM, Munson G, Mathur T, Schneider D, Chang LK, Getz GS, Reardon CA, Lukens J, Shah JA, LaDu MJ. Unique lipoproteins secreted by primary astrocytes from wild-type, apoE (−/−), and human apoE transgenic mice. J Biol Chem. 1999;274:30001–30007. doi: 10.1074/jbc.274.42.30001. [DOI] [PubMed] [Google Scholar]
- Han X, Cheng H, Fryer J, Fagan A, Holtzman D. Novel role of apolipoprotein E in the central nervous system: modulation of sulfatide content. J Biol Chem. 2003;278:8043–8051. doi: 10.1074/jbc.M212340200. [DOI] [PubMed] [Google Scholar]
- Krul ES, Tang J. Secretion of apolipoprotein E by an astrocytoma cell line. J Neurosci Res. 1992;32:227–238. doi: 10.1002/jnr.490320212. [DOI] [PubMed] [Google Scholar]
- Weiler-Guttler H, Sommerfeldt M, Papandrikopoulou A, Mischek U, Bonitz D, Frey A, Grupe M, Scheerer J, Gassen H. Synthesis of apolioprotein A-I in pig brain microvascular endothelial cells. J Neurochem. 1990;54:444–450. doi: 10.1111/j.1471-4159.1990.tb01892.x. [DOI] [PubMed] [Google Scholar]
- Mockel B, Zinke H, Flach R, Weib B, Weiler-Guttler H, Gassen H. Expression of apolipoprotein A-I in porcine brain endothelium in vitro. J Neurochem. 1994;62:788–798. doi: 10.1046/j.1471-4159.1994.62020788.x. [DOI] [PubMed] [Google Scholar]
- Johnson-Wood K, Lee M, Motter R, Hu K, Gordon G, Barbour R, Khan K, Gordon M, Tan H, Games D, Lieberburg I, Schenk D, Seubert P, McConlogue L. Amyloid precursor protein processing and Aβ42 deposition in a transgenic mouse model of Alzheimer’s disease. Proc Natl Acad Sci USA. 1997;94:1550–1555. doi: 10.1073/pnas.94.4.1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagan AM, Watson M, Parsadanian M, Bales KR, Paul SM, Holtzman DM. Human and murine apoE markedly influence Aβ metabolism before and after plaque formation in a mouse model of Alzheimer’s disease. Neurobiol Dis. 2002;9:305–318. doi: 10.1006/nbdi.2002.0483. [DOI] [PubMed] [Google Scholar]
- Avdulov NA, Chochina SV, Igbavboa U, Warden CS, Vassiliev AV, Wood WG. Lipid binding to amyloid beta-peptide aggregates: preferential binding of cholesterol as compared with phosphatidylcholine and fatty acids. J Neurochem. 1997;69:1746–1752. doi: 10.1046/j.1471-4159.1997.69041746.x. [DOI] [PubMed] [Google Scholar]
- Kakio A, Nishimoto S-I, Yanagisawa K, Kozutsumi Y, Matsuzaki K. Cholesterol-dependent formation of GM1 ganglioside-bound amyloid β-protein, an endogenous seed for Alzheimer amyloid. J Biol Chem. 2001;276:24985–24990. doi: 10.1074/jbc.M100252200. [DOI] [PubMed] [Google Scholar]
- Linton MF, Gish R, Hubl ST, Butler E, Esquivel C, Bry WI, Boyles JK, Wardell MR, Young SG. Phenotypes of apolipoprotein B and apolipoprotein E after liver transplantation. J Clin Invest. 1991;88:270–281. doi: 10.1172/JCI115288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Yee A, Brewer HB, Das S, Potter H. Amyloid-associated proteins alpha-1-antichymotrypsin and apolipoprotein E promote assembly of Alzheimer beta-protein into filaments. Nature. 1994;372:92–94. doi: 10.1038/372092a0. [DOI] [PubMed] [Google Scholar]
- Castano EM, Prelli F, Wisniewski T, Golabek A, Kumar RA, Soto C, Frangione B. Fibrillogenesis in Alzheimer’s disease of amyloid beta peptides and apolipoprotein E. Biochem J. 1995;306:599–604. doi: 10.1042/bj3060599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmechel DE, Saunders AM, Strittmattter WJ, Crain BJ, Hulette CM, Joo SH, Pericak-Vance MA, Goldgaber D, Roses AD. Increased amyloid β-peptide deposition in cerebral cortex as a consequence of apolipoprotein genotype in late-onset Alzheimer disease. Proc Natl Acad Sci USA. 1993;90:9649–9653. doi: 10.1073/pnas.90.20.9649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bales KR, Verina T, Cummins DJ, Du Y, Dodel RC, Saura J, Fishman CE, DeLong CA, Piccardo P, Petegnief V, Ghetti B, Paul SM. Apolipoprotein E is essential for amyloid deposition in the APPV717F transgenic mouse model of Alzheimer’s disease. Proc Natl Acad Sci USA. 1999;96:15233–15238. doi: 10.1073/pnas.96.26.15233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzman DM, Bales KR, Tenkova T, Fagan AM, Parsadanian M, Sartorius LJ, Mackey B, Olney J, McKeel D, Wozniak D, Paul SM. Apolipoprotein E isoform-dependent amyloid deposition and neuritic degeneration in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci USA. 2000;97:2892–2897. doi: 10.1073/pnas.050004797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter DB, Dunn E, McKinley DD, Stratman NC, Boyle TP, Kuiper SL, Oostveen JA, Weaver RJ, Boller JA, Gurney ME. Human apolipoprotein E4 accelerates β-amyloid deposition in APPsw transgenic mouse brain. Ann Neurol. 2001;50:468–475. doi: 10.1002/ana.1134. [DOI] [PubMed] [Google Scholar]
- Huang Y, Zhu Y, Langer C, Raabe M, Wu S, Wiesenhutter B, Seedorf U, Maeda N, Assmann G, von Eckardstein A. Effects of genotype and diet on cholesterol efflux into plasma and lipoproteins of normal, apolipoprotein A-I-, and apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 1997;17:2010–2019. doi: 10.1161/01.atv.17.10.2010. [DOI] [PubMed] [Google Scholar]
- Li H, Reddick R, Maeda N. Lack of apoA-I is not associated with increased susceptibility to atherosclerosis in mice. Arterioscler Thromb. 1993;1993:1814–1821. doi: 10.1161/01.atv.13.12.1814. [DOI] [PubMed] [Google Scholar]
- Hartmann T. Cholesterol, Aβ, and Alzheimer’s disease. Trends Neurosci. 2001;24:S45–S48. doi: 10.1016/s0166-2236(00)01990-1. [DOI] [PubMed] [Google Scholar]
- Puglielli L, Tanzi R, Kovacs D. Alzheimer’s disease: the cholesterol connection. Nature Neurosci. 2003;6:345–351. doi: 10.1038/nn0403-345. [DOI] [PubMed] [Google Scholar]
- Gordon T, Castelli W, Hjortland M, Kannel W, Dawber T. High density lipoprotein as a protective factor against coronary heart disease: The Framingham Study. Am J Med. 1977;62:707–714. doi: 10.1016/0002-9343(77)90874-9. [DOI] [PubMed] [Google Scholar]
- Reichl D, Miller N. Pathophysiology of reverse cholesterol transport: insights from inherited disorders of lipoprotein metabolism. Arteriosclerosis. 1989;9:785–797. doi: 10.1161/01.atv.9.6.785. [DOI] [PubMed] [Google Scholar]
- Norum R, Lakier J, Goldstein S, Angel A, Goldberg R, Block W, Noffze D, Dolphin P, Edelglass J, Bogorad D, Alaupovic P. Familial deficiency of apolipoproteins A-I and C-III and precocious coronary-artery disease. N Engl J Med. 1982;306:1513–1519. doi: 10.1056/NEJM198206243062503. [DOI] [PubMed] [Google Scholar]
- Schaefer E, Heaton W, Wetzel M, Brewer HJ. Plasma apolipoprotein A-I absence associated with a marked reduction of high density lipoproteins and premature coronary artery disease. Arteriosclerosis. 1982;2:16–26. doi: 10.1161/01.atv.2.1.16. [DOI] [PubMed] [Google Scholar]
- Karathanasis S, Zannis V, Breslow J. A DNA insertion in the apolipoprotein A-I gene of patients with premature atherosclerosis. Nature. 1983;305:823–825. doi: 10.1038/305823a0. [DOI] [PubMed] [Google Scholar]
- Blue M, Ostapchuk P, Gordon J, Williams D. Synthesis of apolipoprotein AI by peripheral tissues of the rooster: a possible mechanism of cellular cholesterol efflux. J Biol Chem. 1982;257:11151–11159. [PubMed] [Google Scholar]
- Banerjee D, Mukherjee T, Redman C. Biosynthesis of high density lipoprotein by chicken liver: intracellular transport and proteolytic processing of nascent apolipoprotein A-I. J Cell Biol. 1985;101:1219–1226. doi: 10.1083/jcb.101.4.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harr SD, Uint L, Hollister R, Hyman BT, Mendez AJ. Brain expression of apolipoproteins E, J, and A-I in Alzheimer’s disease. J Neurochem. 1996;66:2429–2435. doi: 10.1046/j.1471-4159.1996.66062429.x. [DOI] [PubMed] [Google Scholar]
- Roheim PS, Carey M, Forte T, Vega GL. Apolipoproteins in human cerebrospinal fluid. Proc Natl Acad Sci USA. 1979;76:4646–4649. doi: 10.1073/pnas.76.9.4646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitas RE, Boyles JK, Lee SH, Hui D, Weisgraber KH. Lipoproteins and their receptors in the central nervous system. J Biol Chem. 1987;262:14352–14360. [PubMed] [Google Scholar]
- Merched A, Xia Y, Visvikis S, Serot J, Siest G. Decreased high-density lipoprotein cholesterol and serum apolipoprotein AI concentrations are highly correlated with the severity of Alzheimer’s disease. Neurobiol Aging. 2000;21:27–30. doi: 10.1016/s0197-4580(99)00103-7. [DOI] [PubMed] [Google Scholar]
- Wu C, Liao P, Lin C, Kuo C, Chen S, Chen H, Kuo Y. Brain region-dependent increases in beta-amyloid and apolipoprotein E levels in hypercholesterolemic rabbits. J Neural Transm. 2003;110:641–649. doi: 10.1007/s00702-002-0809-1. [DOI] [PubMed] [Google Scholar]
- Naidu A, Xu Q, Catalano R, Cordell B. Secretion of apolipoprotein E by brain glia requires protein prenylation and is suppressed by statins. Brain Res. 2002;958:100–111. doi: 10.1016/s0006-8993(02)03480-7. [DOI] [PubMed] [Google Scholar]
- Petanceska S, Papolla M, Refolo L. Modulation of Alzheimer’s amyloidosis by statins: mechanisms of action. Curr Med Chem Immunol Endosc Metab Agents. 2003;3:233–243. [Google Scholar]
- Eckert G, Kirsch C, Mueller W. Differential effects of lovastatin treatment on brain cholesterol levels in normal and apoE-deficient mice. NeuroReport. 2001;12:883–887. doi: 10.1097/00001756-200104170-00003. [DOI] [PubMed] [Google Scholar]