Abstract
Variability in valacyclovir bioavailability and the potential for cephalexin-valacyclovir interaction were evaluated. The intraindividual acyclovir area under the concentration-time curve (AUC) varied minimally, whereas interindividual differences were substantial. Coadministration of the human peptide transporter 1 (hPEPT1) substrates valacyclovir and cephalexin minimally reduced the acyclovir AUC. These results suggest a stable valacyclovir absorption phenotype, significant interindividual variability, and minimal interaction between these hPEPT1 substrates.
Human peptide transporter 1 (hPEPT1) is associated with the absorption of peptidomimetic drugs such as valacyclovir and cephalexin (2, 3, 10, 11). Animal and in vitro models have documented a 50% reduction in the hPEPT1 uptake of valacyclovir in the presence of equimolar cephalexin (4, 6-8). While valacyclovir is a substrate of hPEPT2 and organic anion transporter 1 (OAT1), uptake by hPEPT1 is thought to be the primary mechanism of valacyclovir absorption (1, 4-8). Since 99% of the absorbed valacyclovir is hydrolyzed to acyclovir within 3 h of oral administration (6-7), valacyclovir reaches hPEPT2 and OAT1 in the form of acyclovir, which is a substrate of neither hPEPT2 nor OAT1 (12). Considering its rapid metabolism to acyclovir, the oral bioavailability of valacyclovir is a reflection of the acyclovir area under the concentration-time curve (AUC).
The intra- and interindividual variabilities of the oral bioavailability of valacyclovir have not been well studied. We evaluated the variability of valacyclovir absorption, as measured by the acyclovir AUC, and the impact of cephalexin on the acyclovir AUC.
This study was conducted at the University of California at San Francisco (UCSF) General Clinical Research Center (GCRC). All study subjects gave informed consent. The protocol was approved by the Committee on Human Research.
Volunteers were excluded if they had (i) diabetes, cardiovascular disease, or renal or hepatic disease; (ii) a recent history of drug abuse, alcoholism, or nicotine dependence; (iii) a history of intolerance to acyclovir or its analogues, cephalosporins, or penicillins; (iv) participated in other studies during the preceding month; or (v) taken any medication or dietary supplement other than oral contraceptives, vitamins, or minerals within the preceding 2 weeks; and (vi) female volunteers were excluded if they were pregnant, lactating, or sexually active without using adequate contraceptive measures. All female volunteers were required to provide urine for a urine dipstick pregnancy test.
With a random-number generator, subjects were randomly assigned to a single oral dose of (i) 500 mg of valacyclovir at both visits 1 and 2 (control group; n = 6) (ii), 500 mg of valacyclovir at visit 1 and 500 mg of valacyclovir plus 500 mg of cephalexin at visit 2 (treatment group A; n = 5), or (iii) 500 mg of valacyclovir plus 500 mg of cephalexin at visit 1 and 500 mg of valacyclovir at visit 2 (treatment group B; n = 5).
Subjects were admitted to the GCRC for two admissions, separated by at least 7 days. During each admission, subjects were allowed to continue their existing medication regimen. Subjects were required to abstain from alcohol-, caffeine-, or xanthine-containing products within 24 h prior to and during each admission, fast the night before each admission, and abstain from fluid intake within 2 h prior to and after the administration of each valacyclovir dose.
Blood samples were collected in 5-ml sodium heparinized tubes before dosing and then at 0.5, 1, 2, 4, 8, and 12 h following the administration of each valacyclovir dose. Plasma was separated via centrifugation at 1,300 × g for 5 min and then frozen in 2 aliquots at −20°C. After overnight storage at −20°C, plasma samples were stored at −80°C until assayed.
Concentrations of acyclovir in plasma were determined by a validated liquid chromatography-tandem mass spectrometry method developed at the UCSF Drug Study Unit (E. T. Lin, unpublished data). The standard curve was linear over a concentration range of 50 to 6,000 ng/ml (r2, ≥0.996). The lower limit of quantitation was 50 ng/ml, and the coefficients of variation were less than 15%. The assay was reproducible and accurate (coefficient of variation, ≤10%) and without interference from the matrix and anticoagulant. Although the samples were analyzed within 1 month of collection, acyclovir was stable in frozen human plasma at −80°C for at least 3 months. Acyclovir was stable in plasma for up to five freeze-and-thaw cycles.
The above-mentioned liquid chromatography-tandem mass spectrometry method was modified to monitor the conversion of valacyclovir to acyclovir.
WinNonLin 3.1 was used to perform noncompartmental pharmacokinetic analysis. Acyclovir concentration-versus-time curves were integrated and extrapolated by the linear/log trapezoidal rule and the 1/concentration weighted method to yield the AUC from zero to infinity (AUC0→∞). λz was calculated by selecting the terminal portion of the curve and subsequently used to extrapolate the AUC from the last measurable concentration to infinity (AUClast→∞).
Eleven previously identified single-nucleotide polymorphisms (SNPs) in and surrounding the hPEPT1-encoding gene were selected for sequencing on the basis of their allelic variation frequency (>1%) or phenotype (P. Anderle and W. Sadee, unpublished data).
Genotyping of hPEPT1 was performed as previously described (9). Briefly, primers for exons and adjoining intronic regions (ca. 50 bp) for hPPEP1 (National Center for Biotechnology Information reference sequence, NM_005073) were designed by using the Virtual Genome center website at http://alces.med.umn.edu/VGC.html and ordered from Operon (Alameda, Calif.). A collection of 247 ethnically identified genomic DNA samples was obtained from the Coriell Institute of Medicine and used to screen for hPEPT1 variants. PCR was performed with TaqGold on the GeneAmp 9700 thermocycler from PE. Samples were pooled 3 deep so that 96 samples were ready for high-performance liquid chromatography (dHPLC). dHPLC was performed on Varian HPLC machines with Varian Helix columns. The sequence for each amplicon was submitted to the dHPLC melt program at Stanford University (http://insertion.stanford.edu/melt.html). This information was used as the basis for analysis. Typically, the highest recommended temperature was run along with at least one other temperature, depending on the complexity of the melt profile. The results were scored manually. If a well was scored positive, the three PCRs corresponding to that well were cleaned with 2 U of SAP and ExoI (from USB), sequenced with ABI BigDye v2, cleaned with 96-well gel filtration blocks from Edge BioSystems, and run on an ABI 3700 DNA analyzer.
Parametric, paired, two-tailed t tests were used to determine the statistical significance (P = 0.05) of intra- and interindividual variability, the period effect, and the effect of concomitant treatment with cephalexin on acyclovir AUC0→∞.
Sixteen healthy volunteers (nine females, seven males) with a mean age of 27 years (range, 22 to 39 years) enrolled in the study. Their mean height and weight were 167 cm (range, 152 to 182 cm) and 62 kg (range, 45 to 77 kg), respectively. All subjects were calculated to be within their ideal body weight range.
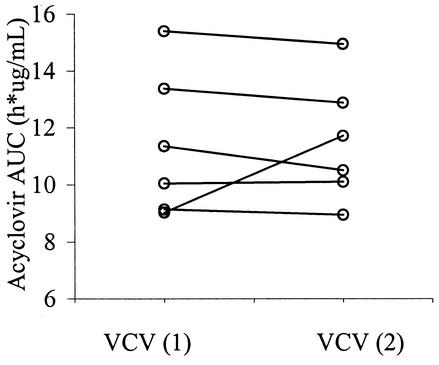
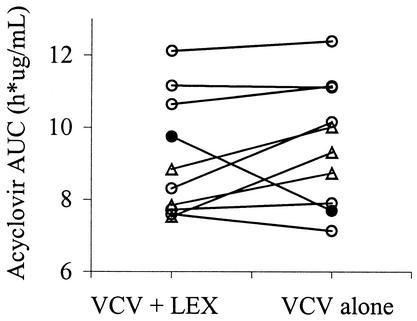
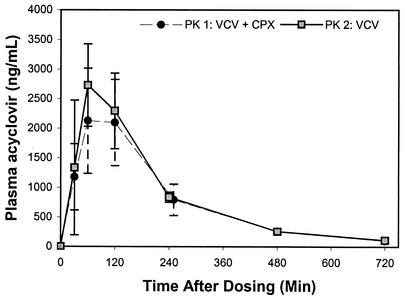
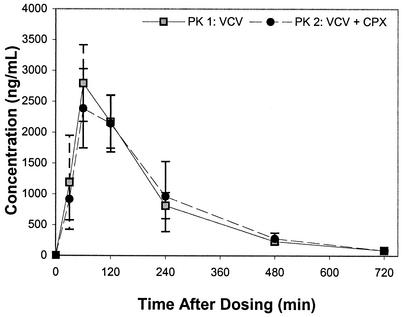
The acyclovir AUC0→∞ values for the control and treatment groups are shown in Fig. 1 and 2. While considerable interindividual variability was observed, no significant intraindividual variability in the AUC was demonstrated (P = 0.82) between study periods. Coadministration of cephalexin reduced the acyclovir AUC0→∞ by 7.1% or from 9.8 ± 1.7 to 9.1 ± 1.8 h · μg/ml (P = 0.034). However, this AUC reduction was only observable after exclusion of an outlier who had an increased acyclovir AUC with concomitant cephalexin. In 2 of 10 subjects, oral bioavailability was reduced by 20% with concomitant administration of cephalexin. Figures 3 and 4 display acyclovir concentrations in plasma over time in patients receiving valacyclovir with or without cephalexin. While a reduction in the peak concentration of acyclovir in plasma and the AUC was observed with concomitant administration of cephalexin, no change in the time to the peak concentration of acyclovir in plasma or the terminal half-life took place.
FIG. 1.
Acyclovir AUC0→∞ in the absence of cephalexin. VCV (1) is valacyclovir during PK visit 1, and VCV (2) is valacyclovir during PK visit 2. The interindividual standard deviations for VCV (1) and VCV (2) were 2.5 and 2.2, respectively. The intraindividual standard deviation was 0.56 or about four times less than the interindividual variability. There was no difference in the acyclovir AUC0→∞ between the periods (P = 0.82).
FIG. 2.
Acyclovir AUC0→∞ in the presence and absence of cephalexin. VCV + LEX is concomitant administration of valacyclovir and cephalexin, and VCV alone is single treatment with only valacyclovir. The 7.1% reduction in oral bioavailability was determined without the outlier (•). Concomitant cephalexin reduced the acyclovir AUC0→α from 9.8 ± 1.7 to 9.1 ± 1.8 h · μg/ml (P = 0.034). The AUC0→α was reduced by 20% in subjects represented by the symbol ▵.
FIG. 3.
Mean plasma concentration-versus-time profiles of acyclovir in treatment group A (n = 5; 500 mg of valacyclovir [VCV] plus 500 mg of cephalexin [CPX] at PK 1 and 500 mg of VCV at PK 2). Standard deviations are shown as error bars.
FIG. 4.
Mean plasma concentration-versus-time profiles of acyclovir in treatment group B (n = 5; 500 mg of valacyclovir [VCV] at PK 1 and 500 mg of VCV plus 500 mg of cephalexin [CPX] at PK 2). Standard deviations are shown as error bars.
Genotypic analysis did not reveal any contributions of the selected SNPs to the observed responses. In a separate study, none of the nonsynonymous SNPs assayed here had any effect on hPEPT1 transport activity for cephalexin (Anderle and Sadee, unpublished).
The experimental procedures and medications were generally well tolerated. Most subjects reported nonpainful erythema at the site of venous access.
While considerable interindividual variability was observed, the intraindividual oral bioavailability of valacyclovir was remarkably constant. As hPEPT1 is assumed to play a major role in valacyclovir absorption, the stability of hPEPT1 activity, as indicated by minimal intraindividual variability at two observation periods, is consistent with results from animal studies (9, 10). Interindividual variability, while significantly greater than intraindividual variability, did not exceed a twofold range, thereby, supporting the utility of hPEPT1 as a drug target. While a more substantial reduction in valacyclovir absorption (measured by the acyclovir AUC) was associated with concom-itant administration of cephalexin in some patients, the reduction was less than the 50% decrease suggested by isolated hPEPT1 models (5-7). Furthermore, one patient actually experienced an increased acyclovir AUC with simultaneous administration of cephalexin. One explanation for the discrepancy between studies is that the isolated hPEPT1 models used much higher concentrations and differing ratios of the respective agents. The reduction in bioavailability may be more significant with increased valacyclovir doses, longer duration of therapy, and concomitant administration with a more potent hPEPT1 competitive substrate, such as cefadroxil (11). It is possible that additional subjects with increased genetic polymorphism would produce more substantial differences in bioavailability. The results also suggest that isolated models of hPEPT1 do not account for all of the factors affecting the pharmacokinetics of valacyclovir. It is also possible that cephalexin and valacyclovir are absorbed via other transporters and less dependent on hPEPT1-mediated transport than previously suggested.
Acknowledgments
These studies were carried out in part at the GCRC, Moffitt Hospital, University of California at San Francisco, with funds provided by the National Center for Research Resources, U.S. Public Health Service (5 M01 RR-00079). This project also was supported by an unrestricted grant from Glaxo SmithKline, Philadelphia, Pa. In addition, National Institutes of Health General Medical Sciences grant GM31690 provided partial support for this work.
We thank R. Shih and the Drug Study Unit team for bioanalytical support. We also thank the GCRC for clinical and statistical support.
REFERENCES
- 1.Balimane, P. V., I. Tamai, A. Guo, T. Nakanishi, H. Kitada, F. H. Leibach, A. Tsuji, and P. J. Sinko. 1998. Direct evidence for peptide transporter PEPT1-mediated uptake of a nonpeptide prodrug, VACY. Biochem. Biophys. Res. Commun. 250:246-251. [DOI] [PubMed] [Google Scholar]
- 2.Chu, X., G. P. Sanchez-Castano, K. Higaki, D. Oh, C. Hsu, and G. L. Amidon. 2001. Correlation between epithelial cell permeability of CPX and expression of intestinal oligopeptide transporter. J. Pharmacol. Exp. Ther. 299:275-282. [PubMed] [Google Scholar]
- 3.Dantzig, A. H., and L. Bergin. 1990. Uptake of the cephalosporin, CPX, by a dipeptide transport carrier in the human intestinal cell line, Caco-2. Biochim. Biophys. Acta 1027:211-217. [DOI] [PubMed] [Google Scholar]
- 4.de Vrueh, R. L., P. L. Smith, and C. P. Lee. 1998. Transport of l-valine-ACY via the oligopeptide transporter in the human intestinal cell line, Caco-2. J. Pharmacol. Exp. Ther. 286:1166-1170. [PubMed] [Google Scholar]
- 5.Ganapathy, M. E., W. Huang, H. Wang, V. Ganapathy, and F. H. Leibach. 1998. VACY: a substrate for the intestinal and renal peptide transporters PEPT1 and PEPT2. Biochem. Biophys. Res. Commun. 246:470-475. [DOI] [PubMed] [Google Scholar]
- 6.Guo, A., P. Hu, P. V. Balimane, F. H. Leibach, and P. J. Sinko. 1999. Interactions of a nonpeptide drug, VACY, with a human intestinal peptide transporter (hPEPT1) expressed in a mammalian cell line. J. Pharmacol. Exp. Ther. 289:448-454. [PubMed] [Google Scholar]
- 7.Han, H. K., R. L. de Vrueh, K. Rhie, K. Y. Covitz, P. L. Smith, C. Lee, D. M. Oh, W. Sadee, and G. L. Amidon. 1998. 5′ amino acid esters of antiviral nucleosides, ACY, and AZT are absorbed by the intestinal PEPT1 peptide transporter. Pharm. Res. 15:1154-1159. [DOI] [PubMed] [Google Scholar]
- 8.Han, H. K., D. M. Oh, and G. L. Amidon. 1998. Cellular uptake mechanism of amino acid ester prodrugs in Caco-2/hPEPT1 cells overexpressing a human peptide transporter. Pharm. Res. 15:1382-1386. [DOI] [PubMed] [Google Scholar]
- 9.Leabman, M. K., C. C. Huang, M. Kawamoto, S. J. Johns, D. Stryke, T. E. Ferrin, J. DeYoung, T. Taylor, A. G. Clark, Herskowitz, I., and K. M. Giacomini. 2002. Polymorphisms in a human kidney xenobiotic transporter, OCT2, exhibit altered function. Pharmacogenetics 12:395-405. [DOI] [PubMed] [Google Scholar]
- 10.Lee, V. H. L. 2000. Membrane transporters. Eur. J. Pharm. Sci. 11:S41-S50. [DOI] [PubMed] [Google Scholar]
- 11.Tamai, I., N. Tomizawa, T. Takeuchi, K. Nakayama, H. Higashida, and A. Tsuji. 1995. Functional expression of transporter for beta-lactam antibiotics and dipeptides in Xenopus laevis oocytes injected with messenger RNA from human, rat and rabbit small intestines. J. Pharmacol. Exp. Ther. 273:26-31. [PubMed] [Google Scholar]
- 12.Wada, S., M. Tsuda, T. Sekine, S. H. Cha, M. Kimura, Y. Kanai, and H. Endow. 2000. Rat multispecific organic anion transporter 1 (OAT1) transports zidovudine, ACY, and other antiviral nucleoside analogs. J. Pharmacol. Exp. Ther. 294:844-849. [PubMed] [Google Scholar]