Abstract
Two distinct clinical phenotypes of experimental autoimmune encephalomyelitis are observed in BALB interferon-γ knockout mice immunized with encephalitogenic peptides of myelin basic protein. Conventional disease, characterized by ascending weakness and paralysis, occurs with greater frequency after immunizing with a peptide comprising residues 59 to 76. Axial-rotatory disease, characterized by uncontrolled axial rotation, occurs with greater frequency in mice immunized with a peptide corresponding to exon 2 of the full length 21.5-kd protein. The two clinical phenotypes are histologically distinguishable. Conventional disease is characterized by inflammation and demyelination primarily in spinal cord, whereas axial-rotatory disease involves inflammation and demyelination of lateral medullary areas of brain. Both types have infiltrates in which neutrophils are a predominating component. By isolating T cells and transferring disease to naïve recipients, we show here that the type of disease is determined entirely by the inducing T cell. Furthermore, studies using CXCR2 knockout recipients, unable to recruit neutrophils to inflammatory sites, show that although neutrophils are critical for some of these T cells to effect disease, there are also interferon-γ-deficient T cells that induce disease in the absence of both interferon-γ and neutrophils. These results highlight the multiplicity of T-cell-initiated effector pathways available for inflammation and demyelination.
BALB/c mice are among those inbred strains resistant to developing experimental autoimmune encephalomyelitis (EAE) when immunized with myelin basic protein (MBP) in adjuvant. Although their T cells do not readily expand in vivo, BALB/c T cells with specificity for a number of class II MHC-associated MBP epitopes can be expanded in vitro. These cloned BALB/c T-cell lines can be highly encephalitogenic when adoptively transferred into naïve BALB/c recipients.1–3 We have previously reported that residues 59 to 76, 89 to 101, and 151 to 168 of MBP constitute epitopes for BALB/c-derived encephalitogenic T-cell clones.1–5 MBP exon 2 peptide, a 26-mer present in the 21.5- and 17.2-kd isoforms of MBP, is now shown to constitute a fourth and important epitope for BALB/c-derived T-cell clones.
Krakowski and Owens6 reported that BALB/c mice with a targeted disruption of the interferon (IFN)-γ gene (BALB-γ-knockout or GKO), are highly susceptible to developing EAE when immunized with bovine MBP. These findings were initially surprising, because IFN-γ, a known proinflammatory cytokine, has been shown to play an important role in promoting the pathology of EAE as well as multiple sclerosis.7,8 mRNA for IFN-γ is readily detectable in the central nervous system (CNS) of mice during peak severity of EAE, produced by infiltrating CD4+ T cells; when disease remits, levels of IFN-γ drop back down to undetectable levels.9 IFN-γ-secreting T cells have been shown to appear in the CNS just before onset of clinical EAE, and are present in significant numbers in cerebrospinal fluid of multiple sclerosis patients.7 IFN-γ-dependent up-regulation of MHC class II expression in the CNS is characteristic of EAE.10,11 Thus the pathological role played by IFN-γ in the effector stages of disease appears indisputable. However, in both the afferent and effector phases of immune response generation, IFN-γ may also play a role in limiting the peripheral expansion of TH1 cells, thereby regulating both the earliest stages of disease and its final clinical manifestation.12–14 The regulatory role played by IFN-γ has been demonstrated both by using monoclonal anti-IFN-γ antibodies to modify the active induction of EAE, and by immunizing mice with targeted mutations in either IFN-γ or the IFN-γ receptor with myelin components.15–17 IFN-γ may also play a role in limiting survival of nonlymphoid inflammatory cells such as microglia and neutrophils.18–23 IFN-γ-activated microglia up-regulate Fas expression and develop susceptibility to FasL-mediated apoptosis. Neutrophil clearance via apoptosis appears to be IFN-γ-dependent via an interleukin (IL)-6/sIL-6R pathway.23
Pathological mechanisms used by effector T cells from GKO mice to induce inflammatory disease in the absence of IFN-γ differ from the more conventional TH1 proinflammatory pathway involving primarily macrophage activation. Tran and colleagues,17 for example, described a neutrophil dominated pathological pathway in GKO mice immunized with bovine MBP, leading to rapidly progressive EAE.
To further study the differences in EAE induction and presentation in the presence or absence of IFN-γ, we immunized BALB-GKO mice with defined MBP peptides that were previously found to comprise epitopes for encephalitogenic BALB/c-derived T cells. We observed that the majority (>75%) of BALB-GKO mice immunized with MBP exon 2 peptide develop axial-rotatory EAE, presenting with a pronounced lean to one side and/or with continuous and unrelenting axial-rotation. This atypical EAE has been described in a small number of previous reports of disease induced in a variety of different models.24–27 In contrast to exon 2-immunized GKO mice, the majority of GKO mice immunized with MBP 59-76 develop conventional EAE, with ascending paralysis, as do GKO mice immunized with MBP 89-101. Interestingly, we find that cloned MBP-specific T cells from BALB-GKO mice with axial-rotatory EAE consistently and reliably transfer only axial-rotatory EAE to all BALB and GKO recipients. In this report, axial-rotatory EAE is compared clinically and histologically with the conventional EAE presentation of ascending paralysis, and T cells that induce axial-rotatory EAE are compared with T cells from wild-type BALB/c mice for characteristics that may be disease related. Because neutrophils play an important role in inflammatory disease in GKO mice, we also examined the nature of disease induced in CXCR2 knockout recipients of these T cells, in which neutrophils are deficient in a key chemokine receptor, CXCR2.
Results of this study highlight the diversity of cellular pathways available for immunological reactivity and, in this case, tissue damage. Although most often involving other cell types, the T cell is the primary determinant of the effector pathway used. Clinical presentation is determined by lesion site which, in turn, is determined by one or more properties of the inducing T cell. These appear to include both TCR specificity for antigen as well as cytokine expression.
Materials and Methods
Mice
Homozygous IFN-γ knockout BALB/c mice (BALB/c-Ifngtm1Ts), referred to as BALB-GKO mice, were purchased from Jackson Laboratories, Bar Harbor, ME, and maintained by brother-sister mating. BALB/c mice were purchased from Harlan (Indianapolis, IN). Females between the ages of 4 to 12 weeks were used in most experiments. Homozygous CXCR2 knockout mice (on BALB/c background) were purchased from Jackson Laboratories and bred after independent confirmation of genotype. CXCR2 is the receptor for neutrophil chemoattracting CXC cytokines such as MIP-2 and KC, thus these mice exhibit a defect in neutrophil recruitment.28 Tail DNA was analyzed by polymerase chain reaction to confirm CXCR2 KO genotype, using a sense primer (AACAGTTATGCTGTGGTTGTA) and an anti-sense primer (CAAACGGGATGTATTGTTACC). The mice were maintained by brother-sister matings for at least 10 generations. The derivation of BALB-shiverer mice has been previously described.5 C57BL/6J and B6-Ifngr (IFN-γ receptor) knockout mice were purchased from Jackson Laboratories. Mice were maintained in accord with guidelines of the Committee on Care and Use of Animals of Harvard Medical School and those prepared by the Animal Committee on Care and Use of Laboratory Animals of the National Research Council (Department of Health and Human Services Publication NIH 85-23; 1985).
Peptides
MBP peptides 59 to 76 (HTRTTHYGSLPQKSQHGR) and 89 to 101 (HFFKNIVTRRTPP), and exon 2 (VPWLKQSRSPLPSHARSRPGLCHMYK) and myelin oligodendrocyte glycoprotein peptide 35 to 55 (MEVGWYRSPFSRVVHLYRNGK) were synthesized by Dr. Chuck Dahl in the Biopolymers Facility, Dept. of Biological Chemistry, Harvard Medical School, Boston, MA.
Disease Induction and Evaluation
Active induction of disease was accomplished by two subcutaneous injections, 1 week apart, of 200 μg of peptide emulsified in complete Freund’s adjuvant (CFA). Disease was passively transferred by intravenous injection of 107 cells into 300 R irradiated recipients. No pertussis was used in either protocol. Assignment of disease to either conventional or axial-rotatory categories was straightforward based on clinical presentation. In conventional EAE, classical ascending paralysis is evident, beginning with tail limpness (score 1), ataxia and hind leg weakness (score 2), paralysis of hind legs (score 3), and finally quadriplegia (score 4) and death (score 5). Invariably, significant loss of body mass accompanies conventional disease. In contrast, axial-rotatory disease presents with mild (score 1) or marked tilting of head (score 2) and body (score 3), followed, often within hours, by involuntary and continuous axial rotation (score 4) and death (score 5), usually within 2 to 3 days from first disease onset. CNS tissue was harvested and fixed in situ in Bouin’s (Sigma-Aldrich, St. Louis, MO) solution for 1 week before dissection. Sections were cut from paraffin-embedded blocks and stained with hematoxylin and eosin (H&E) to assess inflammatory infiltrates or Luxol Fast Blue to assess demyelination.
Isolation and in Vitro Expansion and Cloning of CNS-Infiltrating Cells
Mononuclear cells in the CNS were isolated by a modification of the procedure of Havenith and colleagues.29 Briefly, suspensions of mechanically disrupted brain and spinal cord were filtered through 70-μm nylon mesh filters to remove debris, washed, then spun in a discontinuous Percoll (Amersham BioSciences, Uppsala, Sweden) gradient consisting of 70%, 35%, and 0% Percoll for 45 minutes at 1200 × g. Cells at the 70%/35% interface were harvested for use. Antigen-specific T-cell lines were initiated by addition of irradiated (3000 R) syngeneic spleen cells and peptide at 10 μg/ml to isolated mononuclear cells from diseased mice, followed by addition of a source of IL-2 (Research Diagnostics Inc., Flanders, NJ) after 24 to 48 hours. Cells were maintained and cloned as previously described.2,30
Derivation of T-Cell Clones from EAE-Resistant BALB/c Mice
Clones 8-4.G6 and BC.D9 are CD4+ TH1 cells from IFN-γ-secreting BALB/c mice, which are EAE-resistant. Derivation of MBP-recognizing T cells is accomplished by in vivo subcutaneous priming of mice with MBP, followed by in vitro restimulation of draining lymph node lymphocytes, as previously described.2,30 Clone 6-G.10 is similarly derived, but from a BALB-shiverer mouse. Cells found to be antigen-specific are expanded in culture and tested in vivo for encephalitogenicity by transfer into lightly irradiated BALB/c or BALB-GKO recipients.
In Vitro Assay of Antigen-Specific Proliferation
Triplicate wells of a flat-bottom 96-well plate (Becton-Dickinson Labware, Franklin Lakes, NJ), containing irradiated spleen as a source of antigen-presenting cells (APC) (5 × 105/well), T cells (5 × 104/well), in the presence or absence of peptide at indicated concentrations, were set up as previously described.2 Cultures were pulsed with 1 μCi of [3H]-methylthymidine (New England Nuclear, Boston, MA) at ∼24 hours, and harvested 18 to 24 hours later using a Wallac harvester (Turku, Finland) and scintillation counter. Mean cpm and SD of triplicate wells are shown.
Flow Cytometric Analysis
Isolated mononuclear cells or in vitro grown cells were washed twice in fluorescence-activated cell sorting buffer (phosphate-buffered saline containing 0.1% bovine serum albumin (Sigma-Aldrich) and 0.1% sodium azide (Sigma-Aldrich)) at 4°C. Fluorescence-activated cell sorting buffer was used for all antibody dilutions and cell washes. Potential Fc receptor binding was blocked by incubation for 15 minutes at 4°C with 0.5 μg of Fc Block (BD BioSciences, La Jolla, CA). One μg of prediluted fluorescein isothiocyanate- or phycoerythrin-conjugated antibody was added to pelleted cells in a volume of 50 μl. After 35 minutes of incubation at 4°C, cells were washed twice before analysis. Monoclonal antibodies used include phycoerythrin-conjugated anti-T-cell receptor (H57-597), anti-Vβ2 (B20.6), anti-Vβ7 (TR-310), anti-Vβ8.1,8.2,8.3 (F23.1), anti-neutrophil antibody (anti-Gr-1), anti-Mac-1 (M1/70), anti-MHC class II (2G9; binds to both I-Ad and I-Ed), and appropriate phycoerythrin- or fluorescein isothiocyanate-conjugated isotype controls (all from BD BioSciences). Two-step staining using hybridoma supernatants binding to Vβ8.1,8.2 (KJ-16) and anti-Vβ8.2 alone (F23.2) were used to further phenotype Vβ8+ cells. Neutrophils were identified as cells staining with anti-Gr-1 antibody and higher intensity Mac-1 staining.
Supernatant Preparation
Supernatants were collected at 20 hours from triplicate cultures set up in 96-well plates, with 105 T cells and 5 × 105 irradiated splenic feeder per well, either without or with 10 μg/ml peptide.
Enzyme-Linked Immunosorbent Assay of Cytokines
Capture enzyme-linked immunosorbent assay quantitation using paired antibodies to IFN-γ, IL-12, tumor necrosis factor (TNF)-α, IL-2, IL-5, IL-6, and GM-CSF was performed according to manufacturer’s instructions (BD BioSciences). An antibody pair to mouse G-CSF was purchased from R&D Systems (Minneapolis, MN). Standard recombinant cytokines were purchased from Research Diagnostics Inc. (Flanders, NJ).
Preparation of RNA
CNS tissue was homogenized in Trizol reagent (Life Technologies, Inc., Grand Island, NY) by trituration alternatively through 20-gauge then 23-gauge needles. RNA extraction was performed according to the manufacturer’s protocol. RNA concentration was determined by UV spectroscopy.
RNase Protection Assay (RPA)
RPA was performed using the RiboQuant multiprobe RPA system (BD BioSciences), according to the manufacturer’s protocol and as described previously.31 A custom set of templates was used, consisting of housekeeping genes GAPDH and L32, chemokines MCP-1, MIP-1α, MIP-2, KC, RANTES, and TNF-α. Gels were digitally scanned and normalized based on densitometric values of the housekeeping gene L32.
Results
Immunization of GKO Mice with MBP Exon 2 Peptide Induces Predominantly Axial-Rotatory EAE
Although BALB/c mice do not develop EAE after immunization with MBP, T cells with specificity for MBP can be isolated from lymph nodes of wild-type BALB/c mice after in vivo priming and multiple rounds of in vitro restimulation of cells.1–3 Previous studies from thislaboratory have established that T cells cloned from MBP-immunized BALB/c mice respond to at least four different epitopes including residues 59 to 76,2 89 to 101,5 151 to 168,1,3 and exon 2 peptide (unpublished observations), thereby establishing these sequences as potentially encephalitogenic in H-2d mice. The exon 2 encoded 26-mer is present only in the 21.5- and 17-kd isoforms of MBP (comprising <10% of adult isoforms) and absent from the more common 18.5- and 14-kd isoforms.32,33
In contrast to BALB/c mice, BALB-GKO mice develop clinical signs of EAE after immunization with native MBP.6,17 To examine responsiveness of BALB-GKO mice to various MBP epitopes, BALB-GKO mice were immunized with peptides of either the exon 2 sequence (26 residues in length), 59-76, or 89-101. Results in Table 1 show that MBP exon 2 and 59-76 peptides are strongly encephalitogenic in BALB/c-GKO mice; peptide 89-101 is less efficient. Of 22 mice immunized with the exon 2 peptide of MBP, all developed disease. The majority (77%) of BALB-GKO mice immunized with exon 2 peptide develop an unusual and striking form of EAE, in which vigorous axial rotation and spasticity, rather than the classical ascending paralysis, occurs. In its earliest stages, mice with axial-rotatory EAE present with a definite lean to one side, but rapidly (often within 24 hours) progress to the stage of axial rotation. Although axial-rotatory EAE was also observed in some 59-76-immunized BALB-GKO mice (4 of 13), the majority of these mice (69%) presented with the more conventional EAE phenotype of ascending paralysis.
Table 1.
Correlation of Clinical Disease Presentation with Peptide Immunization
| MBP peptide | No. of mice with ascending paralysis | Days of onset | No. of mice with axial-rotatory presentation | Days of onset |
|---|---|---|---|---|
| Exon 2 | 5/22 | 26, 45, 50, 75, 109 | 17/22 | 21, 21, 22, 23, 24, 24, 28, 30, 32, 32, 35, 35, 36, 38, 41, 42, 61 |
| 59–76 | 9/13 | 21, 24, 25, 30, 31, 33, 36, 39, 42 | 4/13 | 25, 28, 30, 33 |
| 89–101 | 2/6 | 62, 63 | 0/6 |
BALB-GKO mice were immunized on days 0 and 7 with 200 μg of the indicated peptides in CFA at multiple subcutaneous sites. No pertussis was administered.
Neutrophils Comprise a Significant Fraction of Inflammatory Infiltrates in All BALB-GKO Mice but Axial-Rotatory EAE Is Histologically Distinguishable from Conventional EAE by the Site of Inflammation
Histopathological examination of CNS tissues from peptide immunized GKO mice with EAE clearly distinguishes the two clinical manifestations of the disease based on sites of inflammatory infiltrates. Regardless of whether mice were immunized with exon 2 peptide or 59-76, mice with axial-rotatory disease presentation have severe inflammatory and demyelinating lesions in lateral medullary areas of the brainstem (Figure 1, A and E). The entry zone of the vestibulocochlear nerve root seems to be the earliest affected area. Extensive parenchymal areas of the lateral medulla are densely infiltrated by lymphocytes, neutrophils, and macrophages. Although focal spinal cord lesions are occasionally observed (Figure 1, B and F) they are not nearly as numerous or as severe as those present in mice with conventional EAE. In contrast, GKO mice with conventional EAE, whether immunized with 59-76 or exon 2 peptide, have no evidence of lesions in the lateral medulla (Figure 1, C and G), but have severe and numerous foci throughout their spinal cords, with extensive invasion into the parenchyma (Figure 1, D and H). MBP 89-101-immunized BALB-GKO mice that develop EAE have a similar histological presentation—severe and extensive inflammation in spinal cord parenchyma, with no apparent infiltrate in brain (not shown).
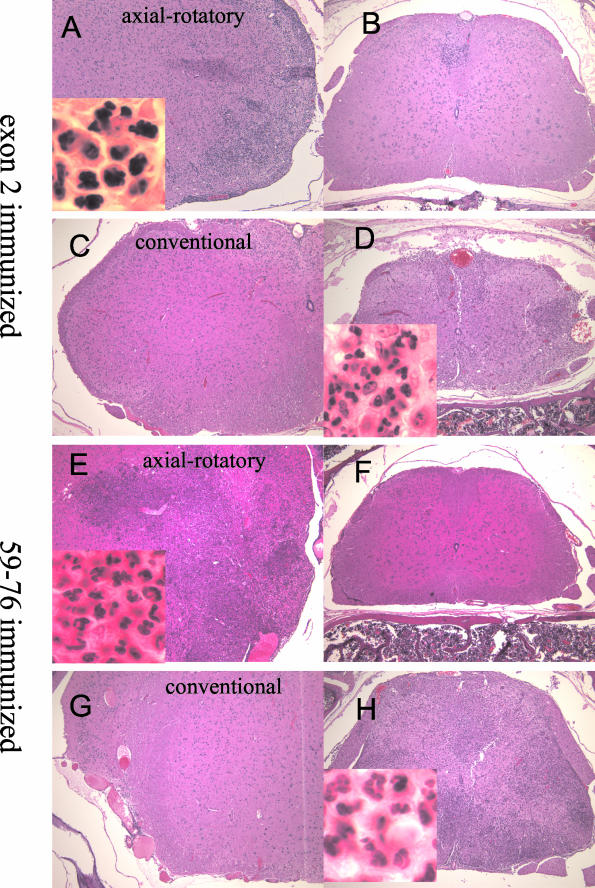
Figure 1.
Comparison of histopathology of actively induced axial-rotatory and conventional EAE in BALB-GKO mice. H&E-stained sections from mice immunized with either MBP exon 2 peptide (A–D) or MBP 59-76 peptide (E–H). A, C, E, G: Lateral medullary regions of brain. B, D, F, H: Spinal cord. A and B are from an exon 2-immunized mouse with axial-rotatory disease. C and D are from an exon 2-immunized mouse with conventional ascending paralysis. E and F are from a peptide 59-76-immunized mouse with axial-rotatory disease. G and H are from a peptide 59-76-immunized mouse with conventional ascending disease. Original magnifications: ×100; ×1000 (insets).
All GKO mice with actively induced disease appear to have mixed mononuclear and neutrophilic infiltrates, or predominantly neutrophilic infiltrates, whether clinical disease is conventional or axial-rotatory in nature (Figure 1, insets). The cellular composition of infiltrates was further quantitated by isolating CNS-infiltrating cells and analyzing their surface marker expression by flow cytometry. These results are summarized in Table 2.
Table 2.
Cellular Composition of Inflammatory Cells Isolated from CNS of Immunized GKO Mice
| Immunizing peptide | Day after immunization | Disease presentation | Percent of cells identified as:
|
Mac† | ||
|---|---|---|---|---|---|---|
| CD4 | CD8 | PMN* | ||||
| 59–76 | 24 | Conventional | 11 | <2 | 28 | 50 |
| 59–76 | 30 | Axial-rotatory | 12 | <2 | 44 | 36 |
| Exon 2 | 24 | Axial-rotatory | 9 | <2 | 44 | 39 |
| Exon 2 | 24 | Axial-rotatory | 8 | <2 | 31 | 51 |
Mononuclear infiltrating and glial cells were isolated from brain and spinal cord of diseased mice and stained with fluorescent-labeled antibodies as described in Materials and Methods. Results shown are from individual representative mice.
Polymorphonuclear leukocytes or neutrophils are Gr-1+ and Mac-1high cells. Total numbers of cells isolated from CNS of each mouse ranged from 0.4 to 1.2 × 106.
Macrophages are defined as Mac-1+ cells that are Gr-1−.
These data confirm that after immunization of BALB-GKO mice with either of the MBP peptides, neutrophils (Gr-1+Mac-1+) comprise a significant fraction of the infiltrating cells isolated from the CNS (28 to 44%), even at late time points after immunization. Thirty-six to fifty-one percent of the cells are Mac-1+ and Gr-1−; these probably are comprised of resident microglia and infiltrating macrophages. T cells comprise only 8 to 12% of the mononuclear population. These percentages do not differ significantly between GKO mice with conventional, as compared with axial-rotatory, EAE, nor do they differ between mice immunized with exon 2 compared with 59-76 peptide. Thus, the absence of IFN-γ qualitatively influences infiltrate composition.
Exon 2-Specific T-Cell Lines and Clones from GKO Mice with Axial-Rotatory EAE Adoptively Transfer Axial-Rotatory EAE
To determine the relative roles played by T cells compared with other infiltrating cells in inducing GKO axial-rotatory EAE, three independent exon 2-recognizing T-cell lines were established from three exon 2-immunized GKO mice with axial-rotatory EAE. When injected in vivo into either naïve wild-type BALB or BALB-GKO recipients (without pertussis), activated cells (107/recipient) from each of these lines (referred to as line 1, line 2, and line 3) transferred axial-rotatory EAE into 100% of recipients. No differences, either in kinetics or disease severity, were observed between wild-type and GKO recipients. Histopathological examination of H&E-stained CNS tissues from recipients of line 1 showed inflammatory infiltrates consisting of macrophages, lymphocytes, neutrophils, and eosinophils severelyaffecting lateral medullary regions of brain, although mild inflammation was sometimes present in spinal cord. Recipients of line 2 also had severe and extensive inflammation in lateral medullary regions, with very little or no spinal cord involvement; infiltrating cells included neutrophils, macrophages, and lymphocytes. Finally, line 3 recipients had both lateral medullary and often cerebellar white matter severely infiltrated with neutrophils, lymphocytes, and macrophages; spinal cord involvement was rare; when present, it was usually focal and relatively mild.
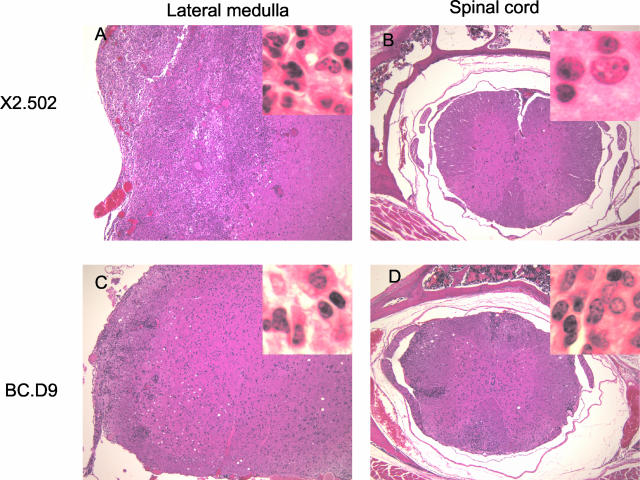
Seven T-cell clones derived from line 1 (referred to with the prefix X2) and one clone from line 3 (3a.56) were expanded and injected in vivo into GKO or BALB recipients. Each of these eight clones induced the atypical disease characterized by leaning and/or axial rotation in 100% of recipients. All recipient mice with this clinical phenotype had histologically severe disease in lateral medullary regions of brain. Line 1 and some of its clones also induce varying levels of inflammation in the spinal cord (lesions, when present in cord, were few and relatively small). Lateral medullary and spinal cord sections from a representative clone, X2.502, are shown in Figure 2, A and B. Line 3 induces inflammatory lesions in both lateral medulla and cerebellum, although clone 3a.56, derived from line 3, induces severe disease only in lateral medulla. Very few recipients of line 3 had any detectable spinal cord lesions or inflammation. Thus the common histopathological features of axial-rotatory disease are severe and extensive lateral medullary inflammation, and absence of, or relatively mild, spinal cord inflammation.
Figure 2.
Sections of lateral medulla and spinal cord from BALB-GKO recipients of GKO-derived clones X2.502 (A and B) and IFN-γ-secreting clone BC.D9 (C and D). A and C show lateral medulla. B and D show corresponding spinal cord sections. All sections are H&E stained. Original magnifications: ×100; ×1000 (insets).
Two IFN-γ-secreting exon 2-specific clones (derived from BALB/c and BALB/c-shiverer mice) were also tested for disease induction. Both of these clones were isolated from long-term cultures of in vitro restimulated MBP-primed lymph node T cells. Both clones induced mild-to-moderate disease in either GKO or BALB/c recipients; neither clone induced axial-rotatory presentation. 6-G.10 (BALB-shiverer-derived) induces inflammation mostly restricted to meningeal and perivascular areas of spinal cord, some in lateral medulla, and areas in the peripheral nervous system (PNS) (not shown). BALB/c-derived clone BC.D9 induces inflammation that is heterogeneous in location, with some inflammation in lateral medulla as well as spinal cord (Figure 2, C and D) and often significant peripheral nerve involvement (not shown). Infiltrates in lateral medullary regions of brain are significantly less extensive than those induced by GKO-derived T cells (Figure 2, compare A and C).
These adoptive transfer experiments highlight the fact that the disease phenotype, as defined by both the clinical and the histological presentation of EAE, are remarkably consistent properties of the T cells transferring disease. Results obtained by immunizing with exon 2 and studying cloned exon 2-specific T cells suggest, furthermore, an association between exon 2 peptide recognition and lateral medulla-associated inflammation in mice of BALB/c origin. The presence or absence of IFN-γ may influence the extent of lateral medullary involvement.
Characterization of T-Cell Clones Inducing Axial-Rotatory EAE
BALB-GKO-derived exon 2-specific T cells inducing axial-rotatory EAE were compared with two conventional EAE-inducing exon 2-specific T-cell lines for their intraexon 2 specificity and T-cell receptors, as well as cytokine and chemokine profiles. In this study of exon 2-specific clones and lines, all are MHC class II-restricted CD4+ cells. They react in vitro with the full-length exon 2 26-mer, with optimal proliferative responses induced by 1 μg/ml of peptide. Clone 6-G.10 (BALB-shiverer-derived) also responds optimally to 1 μg/ml of exon 2 peptide. BALB/c-derived clone BC.D9 responds optimally to far lower exon 2 concentrations, in the range of 0.01 to 0.001 μg/ml. Because exon 2 is 26 amino acids long, it is likely that multiple intrapeptide epitopes exist. Three overlapping peptides comprising the 26 amino acids of exon 2 were synthesized, comprising residues 1 to 15, 6 to 20, and 11 to 26. Figure 3 shows the proliferative responses of line 3 and clone 3a.56, two representative line 1-derived clones (X2.51 and X2.502), and two IFN-γ-secreting clones (BC.D9 and 6-G.10). Neither line 3 nor the line 3-derived clone 3a.56 reacts with any of the three overlapping exon 2 peptides tested, but they do recognize the full-length 26-mer. Antibody to I-Ad blocks proliferation of line 3 and clone 3a.56 to exon 2 (data not shown). Seven of seven line 1-derived clones (represented by X2.51 and X2.502) react with peptide 11 to 26 (and 14 to 26, data not shown here). Proliferation is blocked by anti-I-Ed antibody (data not shown). The sequences of both α and β TCR chains of all line 1-derived clones are identical (data not shown). IFN-γ-secreting clone BC.D9 reacts with an epitope in peptide 6 to 20 (restricted by I-Ad), whereas clone 6-G.10 reacts with an epitope in 11 to 26 (restricted by I-Ed). Use of a series of alanine substituted peptides, however, has shown that this 11 to 26 epitope is different from that recognized by GKO-derived line 1 clones (data not shown). TCR sequencing also confirms different α and β chains for 6-G.10 and the line 1 clones. Thus at least two different peptide/MHC/TCR complexes are represented among the exon 2-specific axial-rotatory disease-inducing T cells from GKO mice, and two others by the two IFN-γ-secreting clones. Table 3 summarizes data on epitope specificity, MHC restriction, and TCR Vβ usage of clones and lines used in this report.
Figure 3.
Analysis of intraexon 2 peptide epitopes recognized by exon 2-specific T cells. Proliferative responses of line 3 and clone 3a.56 (from line 3), clones X2.51 and X2.502 (both from line 1), and two IFN-γ-secreting clones 6-G.10 and BC.D9, to the full length exon 2 peptide (residues 1 to 26), or peptides of exon 2 residues 1 to 15, 6 to 20, or 11 to 26. Results show mean cpm of 3H-thymidine incorporated in triplicate wells. Results shown are for 10 μg/ml of each peptide.
Table 3.
Intra-Exon 2 Specificity, MHC Restriction, and TCR Vβ Usage of Exon 2-Specific T Cells
| T cell line or clone (origin) | Exon 2 epitope | MHC restriction‡ | TCR Vβ |
|---|---|---|---|
| line 3 (GKO) | aa 1–26* | I-Ad | Vβ2 (>90%) |
| I-Ed | Vβ7 (<5%) | ||
| 3a.56 (GKO) | aa 1–26* | I-Ad | Vβ2 |
| X2.502 (GKO) | aa 14–26 | I-Ed | Vβ8.3 |
| X2.51 (GKO) | aa 14–26 | I-Ed | Vβ8.3 |
| BC.D9 (BALB) | aa 6–20 | I-Ad | Vβ8.2 |
| 6-G.10 (BALB−shi/shi) | aa 14–26 | I-Ed | Vβ1† |
Amino acids 1–26 comprise the entire exon 2 peptide; these cells do not react with peptides comprised of amino acids 1–15, 1–13, 6–20, 11–26, or 14–26.
Identified by RT-PCR; all others identified by flow cytometry.
Determined by blocking antigen-specific proliferation with antibodies to either I-Ad (34-5-3) or I-Ed (14-4-4).
Cytokines secreted after antigen stimulation were analyzed by enzyme-linked immunosorbent assay. Table 4 compares levels of IL-2, IL-5, IL-12, TNF-α, IFN-γ, GM-CSF, and IL-6 in supernatants of three IFN-γ-secreting T-cell clones with three GKO clones and GKO line 3, 20 hours after in vitro stimulation with peptides and irradiated spleen cells. Lack of IFN-γ in supernatants of GKO-derived clones confirms their origins; in contrast, three BALB/c and BALB-shiverer clones secrete between 122 to 163 ng/ml of IFN-γ. IL-12 p70 levels were significantly decreased (to <10%) in the supernatants of antigen-stimulated GKO-derived T-cell/APC cultures as compared with cultures of IFN-γ-secreting T cells (mean of 36.5 versus 2.5 ng/ml). Levels of IL-2, IL-5, TNF-α, IL-6, and GM-CSF, however, did not differ significantly between the two groups of cells, although certain clone-to-clone variation was evident within each of the groups. No G-CSF was detected in any of the T-cell-APC culture supernatants.
Table 4.
Cytokines Secreted by Antigen-Activated GKO- Versus Non-GKO-Derived T Cells
| T cell (origin) | MBP specificity | Secreted cytokines (ng/ml):
|
||||||
|---|---|---|---|---|---|---|---|---|
| IFN-γ | IL-12 | IL-5 | TNF-α | IL-2 | GM-CSF | IL-6 | ||
| 8-4.G6 (BALB) | 59–76 | 163.5 | 45.5 | .26 | 8.5 | <.02 | 5.3 | 0.870 |
| 6-G.10 (BALB-shi) | Exon 2 | 122.8 | 17.5 | <.02 | 6.9 | 1.99 | 32.6 | 0.666 |
| BC.D9 (BALB) | Exon 2 | 150.6 | 46.1 | 20.88 | 14.4 | 4.57 | 40.9 | 0.334 |
| X2.51 (GKO) | Exon 2 | <.02 | 3.0 | .29 | 4.3 | 1.75 | 5.3 | 0.453 |
| X2.502 (GKO) | Exon 2 | <.02 | 2.1 | .48 | 6.4 | 2.41 | 27.1 | 0.674 |
| Line 3 (GKO) | Exon 2 | <.02 | 4.8 | .99 | 9.0 | 0.55 | 34.0 | 0.542 |
| 3a.56 (GKO) | Exon 2 | <.02 | 0.2 | <.02 | 9.2 | <.02 | 17.3 | 0.185 |
Levels of indicated cytokines in supernatants harvested 20 hours after activation of T cells with splenic feeder cells and 10 μg/ml indicated peptide antigen. No cytokines were detectable in the absence of peptide addition. Levels were quantitated by ELISA as described.
The Importance of Neutrophils in Disease Depends on the Initiating T Cell
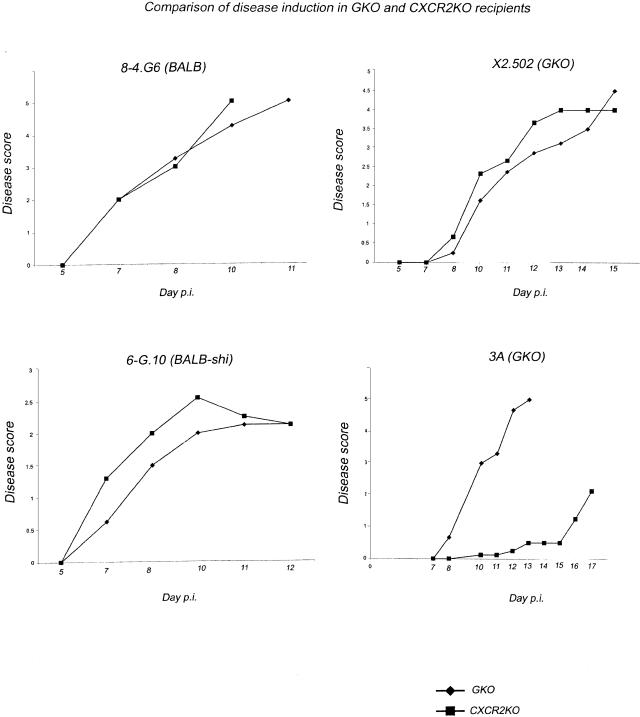
To establish whether or not neutrophils are, in fact, essential to disease induction and progression in the absence of IFN-γ secretion, a comparison of CXCR2 knockout and (CXCR2 expressing) GKO mice as recipients for GKO-derived or control (IFN-γ-secreting) T-cell clones, was made. CXCR2 is the neutrophil receptor for the neutrophil attracting CXC chemokines MIP-2 and KC, murine homologs of human IL-8. Data in Figure 4 show results of experiments in which two IFN-γ-secreting T cells (8-4.G6 and 6-G.10) and two GKO-derived T cells (X2.502 and line 3) were activated and then injected in parallel into either GKO or CXCR2 knockout recipients. Both IFN-γ-secreting T-cell clones, 8-4.G6 and 6-G.10, induce essentially identical disease with similar kinetics in either GKO or CXCR2 knockout recipients (Figure 4, left). Surprisingly, GKO clone X2.502, derived from line 1, was also able to induce EAE with essentially the same kinetics and severity whether recipients expressed CXCR2 or not. In contrast, although, the GKO-derived line 3 appears critically dependent on neutrophil recruitment to effect clinical disease. Thus although GKO recipients of line 3 have all died of disease by day 13, CXCR2 knockout recipients have extremely mild clinical disease, if any, occurring with significantly delayed kinetics. These results underscore the findings of heterogeneity and redundancy in immunopathological pathways available to T cells.
Figure 4.
A representative experiment showing clinical outcomes after injecting two IFN-γ-secreting T-cell lines (8-4.G6 and 6-G.10) and two GKO lines (X2.502 and line 3) into either BALB-GKO or BALB-CXCR2 KO recipients. Each T-cell line was activated in vitro, then injected in parallel into recipient groups of five mice each. Clinical scores were assigned as described in Materials and Methods. Both IFN-γ-secreting clones, shown at left, induced essentially identical disease with similar kinetics in either GKO or CXCR2 KO recipient mice. GKO clone X2.502 (derived from line 1), was also able to induce EAE with essentially the same kinetics and severity whether or not recipients expressed CXCR2. In contrast, GKO-derived line 3 is critically dependent on neutrophil recruitment to effect clinical disease.
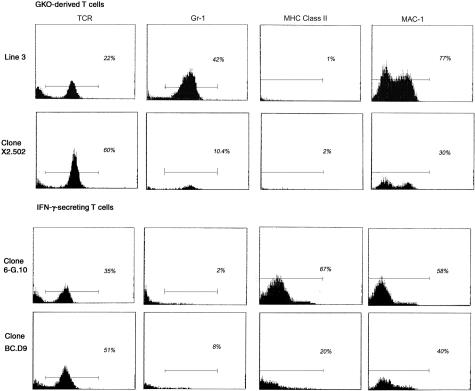
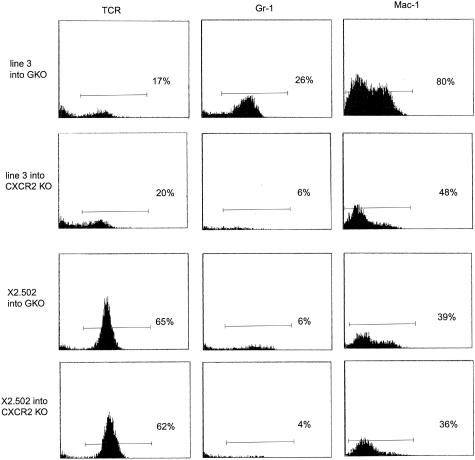
Flow cytometric profiles of mononuclear CNS infiltrates isolated 10 days after injection of two IFN-γ-secreting T cells and two GKO T cells into GKO recipients were compared. A representative experiment is shown in Figure 5. The total numbers of recovered cells did not differ significantly between the four groups. The data show the presence of Gr-1+ neutrophils in CNS of recipients of GKO T-cell lines 3 and X2.502, whereas no significant peak of Gr-1+ cells was observed in CNS of recipients of IFN-γ-secreting T cells BC.D9 and 6-G.10. However, there is an apparent quantitative difference between line 3 and X2.502 T cells in their levels of neutrophil recruitment to inflammatory sites. Line 3 infiltrates routinely contain >40% neutrophils and only ∼20% T cells (all of which have the predominant TCR Vβ of line 3, Vβ 2; data not shown), whereas infiltrates from X2.502 recipients have ∼10% neutrophils with a much higher percentage, ∼60%, T cells (all of which are Vβ8.3+, the TCR of the injected clone; data not shown). The remaining cells in both cases are Mac-1+ cells and are likely either resident or infiltrating macrophages, although they are not activated, as judged by the absence of MHC class II up-regulation. (Two-color immunofluorescent staining, not shown, confirms that the higher intensity Mac-1+ peak is also Gr-1+, constituting the neutrophil population; the lower intensity Mac-1+ peak is Gr-1−, and therefore probably includes resident microglia and infiltrating macrophages.) These results are entirely consistent with the clinical data presented in Figure 4, showing that neutrophil recruitment is essential for optimal disease induction by line 3 T cells but not by X2.502 T cells. For IFN-γ-secreting clones 6-G.10 and BC.D9, T cells constitute 35% and 50% of isolated mononuclear cells, respectively; the remaining cells are Mac-1+ macrophages and microglia. (All T cells isolated from BC.D9 recipients are Vβ8.2+; data not shown.) Although MHC class II is absent from Mac-1+ cells in recipients of GKO T cells, levels of MHC class II are high in CNS of recipients of these two IFN-γ-secreting T cells, reflecting the activation of Mac-1+ cells.
Figure 5.
Flow cytometric profiles of mononuclear cells isolated from CNS of diseased GKO recipient mice 10 days after T-cell injections. T cells injected were line 3 or X2.502, from GKO mice (top) or IFN-γ-secreting clones 6-G.10 and BC.D9 (bottom). Mononuclear cells were isolated on Percoll gradients, as described in Materials and Methods. Monoclonal antibodies used were H57-597, to detect TCR+ cells, anti-Gr-1 (RB6-8C5) to detect neutrophils, anti-MHC class II I-Ad/I-Ed (2G9) and anti-Mac-1 (CD11b) antibody M1/70, which binds to macrophages (lower intensity peak) and neutrophils (higher intensity peak). Analysis was done on three to five mice per group; representative examples from individual mice are shown.
To determine whether or not the absence of CXCR2 in recipients has any differential effect on the homing and trafficking patterns of the two different GKO T cells, X2.502 and line 3, mononuclear cells were isolated from the CNS of recipient GKO or CXCR2 KO mice 12 days after injection of these two clones, and examined by flow cytometry (Figure 6). The data show that the percentage of mononuclear cells isolated from the CNS that is TCR+ is virtually identical for a given clone whether it is injected into a GKO or a CXCR2 KO recipient. For line 3, for example, 17% and 20% of the mononuclear cells recovered from GKO and CXCR2 KO recipients, respectively, were T cells. These numbers were 65% and 62%, respectively, for GKO and CXCR2KO recipients of X2.502. Thus there is no deleterious effect on T-cell homing to the CNS in the absence of CXCR2. As shown earlier, infiltrates from GKO recipients of line 3 consist of a significantly higher percentage of neutrophils and lower percentage of T cells, whereas X2.502 infiltrates consist of a higher percentage of T cells, with far fewer neutrophils. As expected, there is no significant accumulation of neutrophils in the CNS of CXCR2 KO recipient mice. When present (in GKO recipients, as in Figure 5), the neutrophil population is apparent not only as the Gr-1+ peak, but also as the Mac-1high peak; this second peak is absent or diminished in CXCR2 KO recipients.
Figure 6.
Comparative quantitation of mononuclear cell composition from CNS of GKO versus CXCR2 KO recipients of line 3 (top rows) and clone X2.502 (bottom rows). Cells from diseased recipients were isolated by Percoll gradient 12 days after injection. Monoclonal antibodies were used to detect TCR+ cells (H57-597), neutrophils (anti-Gr-1), or Mac-1+ cells (anti-CD11b). Profiles shown are from individual representative mice; three to five mice per group were injected and analyzed.
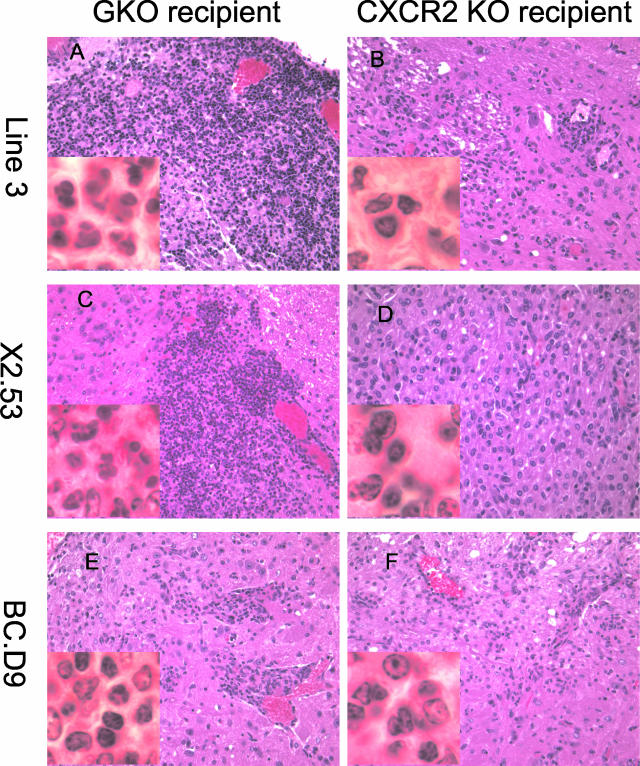
Localization of inflammation to lateral medulla by exon 2-recognizing GKO T cells is not dependent on the presence of neutrophils but is, rather, an independent property of the T cells. This is apparent from histopathological examination of CNS tissue from CXCR2 KO recipients of clones. Thus even in the case of line 3 T cells, which are dependent on neutrophil recruitment to effect severe clinical disease, milder infiltrates consisting of lymphocytes and macrophages can be found in lateral medulla of CXCR2 KO recipients (Figure 7, A and B). Although the localization is unchanged, the importance of neutrophil recruitment for line 3 is reflected in the differential degree of parenchymal invasion when GKO (Figure 7A) versus CXCR2KO mice (Figure 7B) are used as recipients. In the latter instance, the relatively mild infiltrates consist of lymphocytes and macrophages. Figure 7, C and D, show histopathology of clone X2.53, a sister clone to X2.502, injected into either GKO (Figure 7C) or CXCR2KO (Figure 7D) recipients. Like X2.502, this clone induces equivalent disease and histopathological changes even in the absence of neutrophil recruitment. Lymphocytes, neutrophils, and macrophages are evident in lateral medullary areas of the brain in the GKO recipient (Figure 7C). In the CXCR2 KO recipient, lymphocytes and macrophages (and an occasional eosinophil) are present in the same region (Figure 7D). The IFN-γ-secreting clone BC.D9, like other IFN-γ-secreting clones shown earlier, also induces equivalent disease and inflammation whether injected into GKO (Figure 7E) or CXCR2 KO recipients (Figure 7F). Macrophages and lymphocytes are primarily observed in both cases, although an occasional neutrophil may be present.
Figure 7.
H&E-stained photographs of lateral medulla from either GKO (A, C, E) or CXCR2 KO (B, D, F) recipients of T cells. A and B: Day 14 after injection of line 3 into GKO (A) or CXCR2 KO (B) recipients. C and D: Day 11 after injection of clone X2.53 into GKO (C) or CXCR2 KO (D) recipients. E and F: Day 17 after injection of clone BC.D9 into GKO (E) or CXCR2 KO (F) recipients. Original magnifications: ×400; ×1000 (insets).
Differential Chemokine Expression Correlates with Presence or Absence of IFN-γ
To study how IFN-γ differentially affected chemokine expression and thus cellular composition of infiltrates in this system, chemokine synthesis in affected areas of the CNS was studied by RPA analysis of RNA purified from tissue lysates. Comparison of RPA results for a panel of chemokines is shown in Figure 8. Among the set of chemokines looked at, RANTES is the dominantly expressed chemokine in CNS of recipients of wild-type, IFN-γ-producing clones, as shown in Figure 8A for clone 6-G.10. The level of RANTES expression is greatly reduced when IFN-γ is absent, for example in CNS of recipients of GKO T cells such as line 3 (Figure 8A). Differences in chemokine expression between brain and spinal cord tissue for line 3 reflect the histological profile of lesions reported previously. Levels of MIP-2 RNA, one of the murine IL-8 homologs, and a ligand for CXCR2, are generally higher in recipients of GKO-derived clones than wild type, although it is not highly expressed (Figure 8A). Tran and colleagues17 reported a correlation between MIP-2 expression and neutrophilia in GKO mice. Although the increase in MIP-2 expression may contribute to neutrophilia, an alternative or additional interpretation may be that the neutrophils themselves contribute to the level of MIP-2.34 That neutrophils may be the source of the MIP-2 is suggested by results in Figure 8B, which shows decreased MIP-2 in CXCR2KO recipients of X2.502, as compared with MIP-2 in CXCR2-expressing GKO recipients of this clone.
Figure 8.
Comparison of RPA results for a panel of chemokines is shown. A: mRNA expressed in brain (B, lanes 1 and 3) or spinal cord (SC, lanes 2 and 4) of GKO recipients of either 6-G.10 (IFN-γ-producing, lanes 1 and 2) or line 3 (non-IFN-γ-producing, lanes 3 and 4) T cells. B: Decreased MIP-2 in a CXCR2KO recipient of X2.502 (lane 2), as compared with MIP-2 in a CXCR2-expressing (GKO) recipient of this clone (lane 1). C: RPA of brain tissue from recipients of a C57BL/6-GRKO (B6-IFN-γR knockout)-derived myelin oligodendrocyte glycoprotein-specific clone, clone G. The activated clone was transferred in parallel into two wild-type C57BL/6 recipients (lanes 1 and 2) or two B6-GRKO recipients (lanes 3 and 4).
The effect of IFN-γ signaling on chemokine expression is further confirmed by results shown in Figure 8C, in which a single C57BL/6-GRKO (IFN-γR knockout)-derived myelin oligodendrocyte glycoprotein-specific clone (clone G) is transferred either into wild-type C57BL/6 recipients or B6-GRKO recipients. This clone synthesizes IFN-γ, but in the absence of the IFN-γ receptor, no signaling of the APC is transduced. Once again, this RPA confirms that in the presence of the IFN-γ pathway, RANTES levels are significantly higher and MIP-2 levels lower, than in the absence of IFN-γ signaling. In Figure 8C, the normalized values are as follows: in the two C57BL/6 recipients, RANTES is 0.77 and 0.81, compared with 0.20 and 0.23 in the two IFN-γR knockout recipients. MIP-2 levels in the C57BL/6 recipients are 0.08 and 0.12, compared with 0.30 and 0.33 in the B6-IFNγR knockout recipients of the same T cells. In these mice, histopathological examination showed the presence of activated macrophages (and some neutrophils) in cerebellum and mid-brain of wild-type C57BL/6 recipients of clone G; in B6-GRKO recipients, the cerebellum was densely infiltrated with neutrophils and macrophages.
Discussion
In recent years, it has become increasingly evident that both EAE and multiple sclerosis comprise inflammatory, demyelinating CNS diseases of heterogeneous clinical and pathological phenotype.24,25,35,36 Here we demonstrate two distinct EAE phenotypes induced in a single mouse strain and begin to investigate the cellular basis for its heterogeneous manifestations. Unlike EAE-resistant wild-type BALB/c mice, BALB-GKO mice are reported to present with the conventional clinical signs of EAE when immunized with native MBP.6,17 Here, intra-MBP peptides were used to induce disease in BALB-GKO mice, and two distinct phenotypes were observed. The majority of mice immunized with MBP peptide 59-76 develop the conventional signs of EAE with ascending paralysis, whereas the majority of mice immunized with MBP exon 2 peptide develop an axial-rotatory EAE, characterized by head tilting, leaning to one side, and rapid involuntary rolling or axial rotation. Each of these clinical phenotypes has a uniquely associated histological profile. Spinal cord lesions predominate in the conventional disease, whereas brainstem, especially lateral medulla, is the specific target of extensive infiltrates in axial-rotatory EAE. In fact, characteristically little inflammation is noted in spinal cords of mice with axial-rotatory EAE.
Atypical, or axial-rotatory EAE has been described in a small number of previous reports.24–27,37,38 Greer and colleagues,38 Muller and colleagues,24 and Sobel25 noted the occurrence of this atypical EAE phenotype in some C3H/HeJ mice immunized with PLP peptides 190-209 and 215-232. Muller and colleagues24 describe the pathology of axial-rotatory EAE in great detail in PLP peptide 190-209-immunized C3H/HeJ mice, and C3H/HeJ recipients of 190-209 in vitro-stimulated T cells. Wensky and colleagues26 describe an atypical or nonclassical clinical presentation of EAE induced in recipients of TH2 cells producing high levels of IL-5, derived from B10.PL mice transgenically expressing a TCR for MBP Ac1–11; the description of spinning and rolling is very similar to axial-rotatory EAE described by Greer and colleagues,38 Muller and colleagues,24 and Sobel,25 and observed here. Finally, Huseby and colleagues27 describe a similar clinical presentation of EAE induced in C3H recipients of class I-restricted MBP 79-87-recognizing CD8+ T cells from C3H/HeJ mice.
Studies presented here are in agreement with the correlation between histological profile and clinical presentation reported in detail by Muller and colleagues,24 and Sobel25 in that mice with conventional ascending paralysis tend to have more numerous and severe lesions in spinal cord, whereas mice with axial-rotatory EAE have significant lesions in the brainstem and/or cerebellar regions, and limited spinal cord disease. The consistent transfer of particular disease phenotypes with defined T-cell lines described in this report supports the notion of a given T-cell clone having distinct preference for a particular region or regions of the CNS. In our previous studies of cloned BALB/c T cells with specificity for other epitopes of MBP, we found that MBP 59-76-specific T cells only transferred disease characterized by ascending paralysis, and that MBP 151-168-recognizing T cells induced inflammation and demyelination in nerves of the peripheral nervous system.2–4 The studies presented here reinforce the finding of a correlation between T-cell specificity and a distinct pattern of inflammation site, in turn leading to a distinctive clinical presentation.
Seven exons, alternatively spliced, contribute to the five isoforms of MBP found in the adult murine CNS.39–43 Of the five isoforms, all include epitope 59-76, encoded in exon 3, whereas only two, of molecular weights 21.5 and 17.3 kd, contain exon 2. Together, these two exon 2-containing isoforms comprise ≤10% of the total MBP.32 In earlier studies, we had found both 59-76- and exon 2 peptide-specific T-cell clones among (wild-type) BALB T-cell lines primed in vivo and restimulated multiple times in vitro with native MBP, although 59-76-specific T cells predominated by a large margin. In studies presented here, BALB-GKO mice were immunized with the 26-mer comprising exon 2 to increase the frequency of exon 2-recognizing T cells. Among actively immunized BALB-GKO mice, MBP exon 2-immunized mice present with axial-rotatory EAE with higher frequency than 59-76-immunized mice. Although exon 2 in BALB-GKO mice is not unique in inducing axial-rotatory EAE, it is one more example of a strain/peptide combination that results in axial-rotatory EAE with reproducibly significant frequency. Two earlier studies reported that MBP exon 2 peptide induces relatively mild conventional EAE in SJL and in B10.RIII mice.44,45 These reports, together with results presented here, suggest the conclusion that, rather than being an absolute property of a given peptide antigen, properties of the T-cell population induced by a given peptide determine disease site.
Immune responses to MBP exon 2-encoded epitopes are of particular interest in studies of myelin antigens recognized by T cells of multiple sclerosis patients. Synthesis of exon 2-containing isoforms increases early in ontogeny, and in remyelination, which occurs in both acute and chronic multiple sclerosis and EAE lesions.40,46–50 Thus new expression of exon 2 in remyelinating areas of the CNS could possibly contribute to a second wave of immune reactivity to myelin in susceptible multiple sclerosis patients.51–53
There are a number of possible reasons for the localization of inflammation to a specific site, the lateral medulla. If site is determined by the T-cell receptor specificity for antigen, then it could be because of either particular T cells specifically recognizing preferentially expressed MBP isoforms or epitopes in the lateral medulla or, alternatively, to an absence or paucity of those isoforms or epitopes elsewhere, eg, in spinal cord. Possibly locally distinct processing or posttranslational modifications result in locally unique epitope presentation. The fact that the majority of exon 2-immunized BALB-GKO mice develop axial-rotatory EAE (as a result of lateral medullary infiltrates), and all GKO-derived exon 2-recognizing clones home to the lateral medulla, suggests that epitopes encoded in exon 2 or MBP isoforms containing exon 2, and recognized by this group of T cells, may be expressed in particular patterns in the CNS. The absence of IFN-γ after immunization of BALB-GKO mice might contribute to the expansion and survival of such T cells that would not have expanded in the presence of IFN-γ. These cells presumably constitute a subset of those T cells that are responsible for the increased susceptibility and/or increased severity of EAE induced in mice in the absence of either IFN-γ or the IFN-γ receptor.4,6,12,14–17,54
Alternatively, although not mutually exclusive, the absence of IFN-γ may influence the apparent site specificity by contributing to a quantitatively greater inflammatory infiltrate. Because infiltrates induced by GKO-derived T cells cover extensive areas of the lateral medulla, clinical presentation is distinctly axial-rotatory. In the case of the two IFN-γ-secreting clones BC.D9 and 6-G.10, infiltrates are also present in the lateral medulla, but are far less extensive. Additionally, other parts of the CNS, such as spinal cord, are also affected by these two clones. Thus clinical presentation is no longer distinctly axial-rotatory. Consistent with the extensive infiltrates observed, the absence of IFN-γ or IFN-γ signaling is associated with higher rates of expansion and/or decreased apoptosis of T lymphocytes12,55 as well as neutrophils23 and microglia.18,20 Thus the usual pathways for achieving homeostasis after EAE-associated inflammation are unavailable in the absence of IFN-γ.56–58
The absence of the classical IFN-γ-mediated TH1 proinflammatory pathway allows an unhindered view of some of the other immunological pathways that may contribute to EAE pathogenesis. Two of these are illustrated by two of the GKO-derived T-cell lines described here. The first (typified by line 3) is primarily a neutrophil-dominated pathway, such as that described by Tran and colleagues,17 in which extensive parenchymal infiltrates consist mostly of neutrophils, with relatively few lymphocytes present. In CNS tissue from disease occurring in the absence of IFN-γ, an increase in mRNA for the neutrophil chemoattractant, MIP-2, is observed. The increase in MIP-2 expression may be responsible for the neutrophilia, and/or may be a consequence of it. Neutrophils both respond to, and synthesize, MIP-2.28,34 Furthermore, IFN-γ has been shown to down-regulate IL-8 production by human neutrophils.59,60 Tissue damage and demyelination are most likely because of superoxide radicals and reactive oxygen intermediates produced by neutrophils.
A second pathological pathway is typified by clone X2.502. Although a small percentage of neutrophils are present in infiltrates induced by X2.502, flow cytometric analysis of infiltrating cells shows a large percentage of T cells. In CXCR2 knockout recipients of this clone, disease severity and kinetics are similar to that of GKO recipients. Macrophages, although present, are not activated in the classical sense, as judged by lack of MHC class II expression. The mechanism for tissue damage and demyelination used under these circumstances has not yet been determined. One possibility may be that a large number of T cells produce TNF-α which, in turn, may either directly affect CNS cells adversely, or influence Mac-1+ cells to express a limited set of inflammation-related genes. Gaupp and colleagues,61 studying EAE in CCR2 knockout mice, conclude that in the absence of the classical pathway of EAE mediation, there are other pathways, in this case neutrophil-mediated, that can compensate. Results presented here, using T cells deficient in IFN-γ production in mice deficient in neutrophil recruitment, highlight even further the multiplicity of available inflammatory pathways.
In summary, factors that contribute to the rise of a particular set of T cells in a particular strain immunized with a specific peptide sequence are still incompletely understood, but tendencies are consistently observed. Both genetics and epigenetics influence immune response development, contributing to the determination of the final set of effector T cells.25 Results presented here demonstrate, however, that once a given T cell is clonally expanded, its effector function, as defined by the specific CNS site and the cellular properties of the inflammatory lesions it elicits, appear to be primarily determined by properties of that T cell. MBP exon 2 peptide tends to induce axial-rotatory EAE in BALB-GKO mice, and T cells isolated from CNS of mice with axial-rotatory EAE always transfer axial-rotatory EAE to naïve recipients. Axial-rotatory EAE is characterized by massive infiltrates of inflammatory cells in lateral medullary areas of brainstem; spinal cord lesions are relatively mild in comparison. In the absence of IFN-γ, at least two differing pathological pathways are observed, depending on the inducing T cell. Some T cells may effect a neutrophil-dominated set of events whereas others are able to mediate disease without dependence on either neutrophils or classically activated macrophages. The pathological pathway used appears to be an invariant property of the encephalitogenic T cell.
Footnotes
Address reprint requests to Sara Abromson-Leeman, Ph.D., Department of Pathology, Harvard Medical School, New Research Building, 77 Louis Pasteur Ave., Boston, MA 02115. E-mail: sara@abromson-leeman@hms.harvard.edu.
Supported in part by the National Multiple Sclerosis Society (grants RG3310A3 to S.A.-L. and RG2989B3 to M.E.D.).
References
- Abromson-Leeman S, Hayashi M, Martin C, Sobel R, al-Sabbagh A, Weiner H, Dorf ME. T cell responses to myelin basic protein in experimental autoimmune encephalomyelitis-resistant BALB/c mice. J Neuroimmunol. 1993;45:89–101. doi: 10.1016/0165-5728(93)90168-x. [DOI] [PubMed] [Google Scholar]
- Abromson-Leeman S, Alexander J, Bronson R, Carroll J, Southwood S, Dorf M. Experimental autoimmune encephalomyelitis-resistant mice have highly encephalitogenic myelin basic protein (MBP)-specific T cell clones that recognize a MBP peptide with high affinity for MHC class II. J Immunol. 1995;154:388–398. [PubMed] [Google Scholar]
- Abromson-Leeman S, Bronson R, Dorf ME. Experimental autoimmune peripheral neuritis induced in BALB/c mice by myelin basic protein-specific T cell clones. J Exp Med. 1995;182:587–592. doi: 10.1084/jem.182.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshizawa I, Bronson R, Ben-Nun A, Richert JR, Dorf ME, Abromson-Leeman S. Differential recognition of MBP epitopes in BALB/c mice determines the site of inflammatory disease induction. J Neuroimmunol. 1998;89:73–82. doi: 10.1016/s0165-5728(98)00101-5. [DOI] [PubMed] [Google Scholar]
- Yoshizawa I, Bronson R, Dorf ME, Abromson-Leeman S. T-cell responses to myelin basic protein in normal and MBP-deficient mice. J Neuroimmunol. 1998;84:131–138. doi: 10.1016/s0165-5728(97)00205-1. [DOI] [PubMed] [Google Scholar]
- Krakowski M, Owens T. Interferon-gamma confers resistance to experimental allergic encephalomyelitis. Eur J Immunol. 1996;26:1641–1646. doi: 10.1002/eji.1830260735. [DOI] [PubMed] [Google Scholar]
- Olsson T. Cytokines in neuroinflammatory disease: role of myelin autoreactive T cell production of interferon-gamma. J Neuroimmunol. 1992;40:211–218. doi: 10.1016/0165-5728(92)90135-8. [DOI] [PubMed] [Google Scholar]
- Brosnan CF, Cannella B, Battistini L, Raine CS. Cytokine localization in multiple sclerosis lesions: correlation with adhesion molecule expression and reactive nitrogen species. Neurology. 1995;45:S16–S21. doi: 10.1212/wnl.45.6_suppl_6.s16. [DOI] [PubMed] [Google Scholar]
- Renno T, Lin JY, Piccirillo C, Antel J, Owens T. Cytokine production by cells in cerebrospinal fluid during experimental allergic encephalomyelitis in SJL/J mice. J Neuroimmunol. 1994;49:1–7. doi: 10.1016/0165-5728(94)90174-0. [DOI] [PubMed] [Google Scholar]
- McCombe PA, de Jersey J, Pender MP. Inflammatory cells, microglia and MHC class II antigen-positive cells in the spinal cord of Lewis rats with acute and chronic relapsing experimental autoimmune encephalomyelitis. J Neuroimmunol. 1994;51:153–167. doi: 10.1016/0165-5728(94)90077-9. [DOI] [PubMed] [Google Scholar]
- McCombe PA, Nickson I, Pender MP. Cytokine expression by inflammatory cells obtained from the spinal cords of Lewis rats with experimental autoimmune encephalomyelitis induced by inoculation with myelin basic protein and adjuvants. J Neuroimmunol. 1998;88:30–38. doi: 10.1016/s0165-5728(98)00068-x. [DOI] [PubMed] [Google Scholar]
- Chu CQ, Wittmer S, Dalton DK. Failure to suppress the expansion of the activated CD4 T cell population in interferon gamma-deficient mice leads to exacerbation of experimental autoimmune encephalomyelitis. J Exp Med. 2000;192:123–128. doi: 10.1084/jem.192.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willenborg DO, Fordham S, Bernard CC, Cowden WB, Ramshaw IA. IFN-gamma plays a critical down-regulatory role in the induction and effector phase of myelin oligodendrocyte glycoprotein-induced autoimmune encephalomyelitis. J Immunol. 1996;157:3223–3227. [PubMed] [Google Scholar]
- Willenborg DO, Fordham SA, Staykova MA, Ramshaw IA, Cowden WB. IFN-gamma is critical to the control of murine autoimmune encephalomyelitis and regulates both in the periphery and in the target tissue: a possible role for nitric oxide. J Immunol. 1999;163:5278–5286. [PubMed] [Google Scholar]
- Duong TT, Finkelman FD, Singh B, Strejan GH. Effect of anti-interferon-gamma monoclonal antibody treatment on the development of experimental allergic encephalomyelitis in resistant mouse strains. J Neuroimmunol. 1994;53:101–107. doi: 10.1016/0165-5728(94)90069-8. [DOI] [PubMed] [Google Scholar]
- Lublin FD, Knobler RL, Kalman B, Goldhaber M, Marini J, Perrault M, D’Imperio C, Joseph J, Alkan SS, Korngold R. Monoclonal anti-gamma interferon antibodies enhance experimental allergic encephalomyelitis. Autoimmunity. 1993;16:267–274. doi: 10.3109/08916939309014645. [DOI] [PubMed] [Google Scholar]
- Tran EH, Prince EN, Owens T. IFN-gamma shapes immune invasion of the central nervous system via regulation of chemokines. J Immunol. 2000;164:2759–2768. doi: 10.4049/jimmunol.164.5.2759. [DOI] [PubMed] [Google Scholar]
- Spanaus KS, Schlapbach R, Fontana A. TNF-alpha and IFN-gamma render microglia sensitive to Fas ligand-induced apoptosis by induction of Fas expression and down-regulation of Bcl-2 and Bcl-xL. Eur J Immunol. 1998;28:4398–4408. doi: 10.1002/(SICI)1521-4141(199812)28:12<4398::AID-IMMU4398>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- White CA, McCombe PA, Pender MP. Microglia are more susceptible than macrophages to apoptosis in the central nervous system in experimental autoimmune encephalomyelitis through a mechanism not involving Fas (CD95). Int Immunol. 1998;10:935–941. doi: 10.1093/intimm/10.7.935. [DOI] [PubMed] [Google Scholar]
- Badie B, Schartner J, Vorpahl J, Preston K. Interferon-gamma induces apoptosis and augments the expression of Fas and Fas ligand by microglia in vitro. Exp Neurol. 2000;162:290–296. doi: 10.1006/exnr.1999.7345. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Zhou T, Choi C, Wang Z, Benveniste EN. Differential regulation and function of Fas expression on glial cells. J Immunol. 2000;164:1277–1285. doi: 10.4049/jimmunol.164.3.1277. [DOI] [PubMed] [Google Scholar]
- Kohji T, Matsumoto Y. Coexpression of Fas/FasL and Bax on brain and infiltrating T cells in the central nervous system is closely associated with apoptotic cell death during autoimmune encephalomyelitis. J Neuroimmunol. 2000;106:165–171. doi: 10.1016/s0165-5728(00)00238-1. [DOI] [PubMed] [Google Scholar]
- McLoughlin RM, Witowski J, Robson RL, Wilkinson TS, Hurst SM, Williams AS, Williams JD, Rose-John S, Jones SA, Topley N. Interplay between IFN-gamma and IL-6 signaling governs neutrophil trafficking and apoptosis during acute inflammation. J Clin Invest. 2003;112:598–607. doi: 10.1172/JCI17129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller DM, Pender MP, Greer JM. A neuropathological analysis of experimental autoimmune encephalomyelitis with predominant brain stem and cerebellar involvement and differences between active and passive induction. Acta Neuropathol (Berl) 2000;100:174–182. doi: 10.1007/s004019900163. [DOI] [PubMed] [Google Scholar]
- Sobel RA. Genetic and epigenetic influence on EAE phenotypes induced with different encephalitogenic peptides. J Neuroimmunol. 2000;108:45–52. doi: 10.1016/s0165-5728(99)00270-2. [DOI] [PubMed] [Google Scholar]
- Wensky A, Marcondes MC, Lafaille JJ. The role of IFN-gamma in the production of Th2 subpopulations: implications for variable Th2-mediated pathologies in autoimmunity. J Immunol. 2001;167:3074–3081. doi: 10.4049/jimmunol.167.6.3074. [DOI] [PubMed] [Google Scholar]
- Huseby ES, Liggitt D, Brabb T, Schnabel B, Ohlen C, Goverman J. A pathogenic role for myelin-specific CD8(+) T cells in a model for multiple sclerosis. J Exp Med. 2001;194:669–676. doi: 10.1084/jem.194.5.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Cacalano G, Camerato T, Toy K, Moore MW, Wood WI. Chemokine binding and activities mediated by the mouse IL-8 receptor. J Immunol. 1995;155:2158–2164. [PubMed] [Google Scholar]
- Havenith CE, Askew D, Walker WS. Mouse resident microglia: isolation and characterization of immunoregulatory properties with naive CD4+ and CD8+ T-cells. Glia. 1998;22:348–359. [PubMed] [Google Scholar]
- Abromson-Leeman S, Maverakis E, Bronson R, Dorf ME. CD40-mediated activation of T cells accelerates, but is not required for, encephalitogenic potential of myelin basic protein-recognizing T cells in a model of progressive experimental autoimmune encephalomyelitis. Eur J Immunol. 2001;31:527–538. doi: 10.1002/1521-4141(200102)31:2<527::aid-immu527>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Fischer FR, Santambrogio L, Luo Y, Berman MA, Hancock WW, Dorf ME. Modulation of experimental autoimmune encephalomyelitis: effect of altered peptide ligand on chemokine and chemokine receptor expression. J Neuroimmunol. 2000;110:195–208. doi: 10.1016/s0165-5728(00)00351-9. [DOI] [PubMed] [Google Scholar]
- Barbarese E, Carson JH, Braun PE. Accumulation of the four myelin basic proteins in mouse brain during development. J Neurochem. 1978;31:779–782. doi: 10.1111/j.1471-4159.1978.tb00110.x. [DOI] [PubMed] [Google Scholar]
- Newman S, Kitamura K, Campagnoni AT. Identification of a cDNA coding for a fifth form of myelin basic protein in mouse. Proc Natl Acad Sci USA. 1987;84:886–890. doi: 10.1073/pnas.84.3.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scapini P, Lapinet-Vera JA, Gasperini S, Calzetti F, Bazzoni F, Cassatella MA. The neutrophil as a cellular source of chemokines. Immunol Rev. 2000;177:195–203. doi: 10.1034/j.1600-065x.2000.17706.x. [DOI] [PubMed] [Google Scholar]
- Lucchinetti CF, Brueck W, Rodriguez M, Lassmann H. Multiple sclerosis: lessons from neuropathology. Semin Neurol. 1998;18:337–349. doi: 10.1055/s-2008-1040885. [DOI] [PubMed] [Google Scholar]
- Lucchinetti C, Bruck W, Parisi J, Scheithauer B, Rodriguez M, Lassmann H. Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann Neurol. 2000;47:707–717. doi: 10.1002/1531-8249(200006)47:6<707::aid-ana3>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Endoh M, Tabira T, Kunishita T, Sakai K, Yamamura T, Taketomi T. DM-20, a proteolipid apoprotein, is an encephalitogen of acute and relapsing autoimmune encephalomyelitis in mice. J Immunol. 1986;137:3832–3835. [PubMed] [Google Scholar]
- Greer JM, Sobel RA, Sette A, Southwood S, Lees MB, Kuchroo VK. Immunogenic and encephalitogenic epitope clusters of myelin proteolipid protein. J Immunol. 1996;156:371–379. [PubMed] [Google Scholar]
- Takahashi N, Roach A, Teplow DB, Prusiner SB, Hood L. Cloning and characterization of the myelin basic protein gene from mouse: one gene can encode both 14 kd and 18.5 kd MBPs by alternate use of exons. Cell. 1985;42:139–148. doi: 10.1016/s0092-8674(85)80109-4. [DOI] [PubMed] [Google Scholar]
- Campagnoni AT, Sorg B, Roth HJ, Kronquist K, Newman SL, Kitamura K, Campagnoni C, Crandall B. Expression of myelin protein genes in the developing brain. J Physiol (Paris) 1987;82:229–238. [PubMed] [Google Scholar]
- Campagnoni AT. Molecular biology of myelin proteins from the central nervous system. J Neurochem. 1988;51:1–14. doi: 10.1111/j.1471-4159.1988.tb04827.x. [DOI] [PubMed] [Google Scholar]
- Campagnoni AT, Macklin WB. Cellular and molecular aspects of myelin protein gene expression. Mol Neurobiol. 1988;2:41–89. doi: 10.1007/BF02935632. [DOI] [PubMed] [Google Scholar]
- Staugaitis SM, Smith PR, Colman DR. Expression of myelin basic protein isoforms in nonglial cells. J Cell Biol. 1990;110:1719–1727. doi: 10.1083/jcb.110.5.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz RB, Zhao ML. Encephalitogenicity of myelin basic protein exon-2 peptide in mice. J Neuroimmunol. 1994;51:1–6. doi: 10.1016/0165-5728(94)90122-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal BM, Raine CS, McFarlin DE, Voskuhl RR, McFarland HF. Experimental allergic encephalomyelitis induced by the peptide encoded by exon 2 of the MBP gene, a peptide implicated in remyelination. J Neuroimmunol. 1994;51:7–19. doi: 10.1016/0165-5728(94)90123-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prineas JW, Barnard RO, Kwon EE, Sharer LR, Cho ES. Multiple sclerosis: remyelination of nascent lesions. Ann Neurol. 1993;33:137–151. doi: 10.1002/ana.410330203. [DOI] [PubMed] [Google Scholar]
- Raine CS, Mokhtarian F, McFarlin DE. Adoptively transferred chronic relapsing experimental autoimmune encephalomyelitis in the mouse. Neuropathologic analysis. Lab Invest. 1984;51:534–546. [PubMed] [Google Scholar]
- Jordan CA, Friedrich VL, Jr, de Ferra F, Weismiller DG, Holmes KV, Dubois-Dalcq M. Differential exon expression in myelin basic protein transcripts during central nervous system (CNS) remyelination. Cell Mol Neurobiol. 1990;10:3–18. doi: 10.1007/BF00733631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capello E, Voskuhl RR, McFarland HF, Raine CS. Multiple sclerosis: re-expression of a developmental gene in chronic lesions correlates with remyelination. Ann Neurol. 1997;41:797–805. doi: 10.1002/ana.410410616. [DOI] [PubMed] [Google Scholar]
- Nagasato K, Farris RW, II, Dubois-Dalcq M, Voskuhl RR. Exon 2 containing myelin basic protein (MBP) transcripts are expressed in lesions of experimental allergic encephalomyelitis (EAE). J Neuroimmunol. 1997;72:21–25. doi: 10.1016/S0165-5728(96)00137-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voskuhl RR, McFarlin DE, Tranquill LR, Deibler G, Stone R, Maloni H, McFarland HF. A novel candidate autoantigen in a multiplex family with multiple sclerosis: prevalence of T-lymphocytes specific for an MBP epitope unique to myelination. J Neuroimmunol. 1993;46:137–144. doi: 10.1016/0165-5728(93)90243-R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voskuhl RR, McFarlin DE, Stone R, McFarland HF. T-lymphocyte recognition of a portion of myelin basic protein encoded by an exon expressed during myelination. J Neuroimmunol. 1993;42:187–191. doi: 10.1016/0165-5728(93)90009-N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voskuhl RR, Robinson ED, Segal BM, Tranquill L, Camphausen K, Albert PS, Richert JR, McFarland HF. HLA restriction and TCR usage of T lymphocytes specific for a novel candidate autoantigen, X2 MBP, in multiple sclerosis. J Immunol. 1994;153:4834–4844. [PubMed] [Google Scholar]
- Ferber IA, Brocke S, Taylor-Edwards C, Ridgway W, Dinisco C, Steinman L, Dalton D, Fathman CG. Mice with a disrupted IFN-gamma gene are susceptible to the induction of experimental autoimmune encephalomyelitis (EAE). J Immunol. 1996;156:5–7. [PubMed] [Google Scholar]
- Refaeli Y, Van Parijs L, Alexander SI, Abbas AK. Interferon gamma is required for activation-induced death of T lymphocytes. J Exp Med. 2002;196:999–1005. doi: 10.1084/jem.20020666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabi Z, McCombe PA, Pender MP. Antigen-specific down-regulation of myelin basic protein-reactive T cells during spontaneous recovery from experimental autoimmune encephalomyelitis: further evidence of apoptotic deletion of autoreactive T cells in the central nervous system. Int Immunol. 1995;7:967–973. doi: 10.1093/intimm/7.6.967. [DOI] [PubMed] [Google Scholar]
- Pender MP, McCombe PA, Yoong G, Nguyen KB. Apoptosis of alpha beta T lymphocytes in the nervous system in experimental autoimmune encephalomyelitis: its possible implications for recovery and acquired tolerance. J Autoimmun. 1992;5:401–410. doi: 10.1016/0896-8411(92)90001-7. [DOI] [PubMed] [Google Scholar]
- Pender MP, Rist MJ. Apoptosis of inflammatory cells in immune control of the nervous system: role of glia. Glia. 2001;36:137–144. doi: 10.1002/glia.1103. [DOI] [PubMed] [Google Scholar]
- Kasama T, Strieter RM, Lukacs NW, Lincoln PM, Burdick MD, Kunkel SL. Interferon gamma modulates the expression of neutrophil-derived chemokines. J Invest Med. 1995;43:58–67. [PubMed] [Google Scholar]
- Ellis TN, Beaman BL. Interferon-gamma activation of polymorphonuclear neutrophil function. Immunology. 2004;112:2–12. doi: 10.1111/j.1365-2567.2004.01849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaupp S, Pitt D, Kuziel WA, Cannella B, Raine CS. Experimental autoimmune encephalomyelitis (EAE) in CCR2(−/−) mice: susceptibility in multiple strains. Am J Pathol. 2003;162:139–150. doi: 10.1016/S0002-9440(10)63805-9. [DOI] [PMC free article] [PubMed] [Google Scholar]