Abstract
Ischemia-reperfusion (I/R) is an important cause of acute renal failure (ARF). The complement system appears to be essentially involved in I/R injury. However, via which pathway the complement system is activated and in particular whether the mannose-binding lectin (MBL)-pathway is activated is unclear. This tempted us to study the activation and regulation of the MBL-pathway in the course of experimental renal I/R injury and in clinical post-transplant ARF. Mice subjected to renal I/R displayed evident renal MBL-depositions, depending on the duration of warm ischemia, in the early reperfusion phase. Renal deposition of C3, C6 and C9 was observed in the later reperfusion phase. The deposition of MBL-A and -C completely co-localized with the late complement factor C6, showing that MBL is involved in complement activation in the course of renal I/R injury. Moreover, the degree of early MBL-deposition correlated with complement activation, neutrophil-influx, and organ-failure observed in the later reperfusion phase. In serum of mice subjected to renal I/R MBL-A, levels increased in contrast to MBL-C levels, which dropped evidently. In line, liver mRNA levels for MBL-A increased, whereas MBL-C levels decreased. Renal MBL mRNA levels rapidly dropped in the course of renal I/R. Finally, in human biopsies, MBL-depositions were observed early after transplantation of ischemically injured kidneys. In line with our experimental data, in ischemically injured grafts displaying post-transplant organ-failure extensive MBL depositions were observed in peritubular capillaries and tubular epithelial cells. In conclusion, in experimental renal I/R injury and clinical post-transplant ARF the MBL-pathway is activated, followed by activation of the complement system. These data indicate that the MBL-pathway is involved in ischemia-induced complement activation.
Ischemia-reperfusion (I/R) is an important cause of acute renal failure, associated with a mortality rate of up to 50%.1,2 Post-transplant renal failure is a common and threatening complication after renal transplantation, in particular when organs of marginal donors, such as non-heart-beating (NHB) donors, are used.3 Effective treatment for I/R injury is currently not available and hemodialysis is, though symptomatic, the only treatment available. The pathophysiology of renal I/R injury is complicated. Recent studies have shown that the complement system plays a crucial role in pathogenesis of renal injury. Zhou et al4 demonstrated that complement-deficient mice are protected against renal I/R injury. We and others showed that renal I/R injury can be abrogated by treatment with complement inhibitors such as anti-C5 antibodies and C5a receptor antagonists.5–7 Renal deposition of complement has been well described for the complement factors C3, C6, and C9.4,7 However, via which pathway the complement system is activated in the course of renal I/R is not clear. Park et al8 demonstrated that renal I/R does not induce IgG or IgM deposition. Moreover, RAG-1 −/− mice subjected to I/R showed renal complement deposition, indicating that renal I/R is not mediated via the classical pathway. Recently, Thurman et al9 showed that mice lacking a functional alternative complement pathway (factor B −/− mice) are partially protected against renal ischemic injury. Whether the alternative pathway is the initiating pathway of ischemia-induced complement activation or an enhancing pathway for other complement-activating pathways remains unclear.
Next to the classical and alternative pathway, the mannose-binding lectin (MBL)-pathway forms a third activation route of the complement system. Interestingly, whereas in rodents two forms of MBL are present (MBL-A and -C), in humans only one MBL form exists. The MBL-pathway is initiated by binding of MBL to cell surface carbohydrates. Subsequently, two serine proteases, MBL-associated serine protease-1 and -2 (MASP-1 and -2), are activated, cleaving C2 and C4 to form the classical pathway C3 convertase.10 In vitro work shows that complement activation after endothelial oxidative stress is mediated by the MBL-pathway, by showing that C3-deposition after oxidative stress is attenuated by inhibition of the MBL-pathway.11 Activation of MBL in this in vitro model is reported to be mediated by cytokeratin-1 which is up-regulated and expressed on the cell surface in hypoxic endothelial cells.12
In vivo, cardiac I/R in rats induces MBL-deposition.11 Moreover, inhibition of the MBL-pathway reduces postischemic myocardial reperfusion injury in rats. Anti-MBL treatment reduced infarct size and inflammation (neutrophil-infiltration and pro-inflammatory gene expression).13 These data indicate that the MBL-pathway plays an important role in complement activation in the course of I/R injury. This tempted us to study the activation and regulation of the MBL-pathway in the course of experimental renal I/R injury and clinical post-transplant ARF.
Materials and Methods
Antibodies and Reagents
Monoclonal antibodies recognizing mouse MBL-A and -C were kindly provided by Dr. Jensenius (Aarhus University Hospital, Aarhus, Denmark). Mouse-anti-human MBL (clone 3E7) was kindly provided by Hbt (Uden, the Netherlands). Rat anti-mouse C1q was a gift from Dr. E. Kremmer (GSF-Forschungszentrum, München, Germany), rabbit anti-mouse C6 was kindly provided by Dr. N.R. Cooper (Scripps Research Institute, La Jolla, CA), rabbit anti-rat C9 by Dr. B.P. Morgan (University of Wales College of Medicine, Cardiff, UK). Goat anti-mouse C3 was purchased from Cappel (ICN Biomedicals, Aurora, OH). Secondary antibodies, peroxidase conjugated rabbit anti-goat, goat anti-rabbit and goat anti-rat IgG as well as Texas-red-labeled goat anti-rat IgG and FITC-labeled goat anti-rabbit IgG were purchased from Jackson (West Grove, PA). All other reagents were purchased from Sigma (St. Louis, MO).
Experimental Renal Ischemia-Reperfusion Model
Male Swiss mice weighing 20 to 25 g were obtained from Charles River Breeding Laboratories (Heidelberg, Germany). Animals were housed individually in standard laboratory cages and were allowed free access to food and water throughout the experiments. The studies were carried out under a protocol approved by the Institutional Animal Care Committee of the University of Maastricht. At the start of the experiments, mice were anesthetized with sodium pentobarbital (100 mg/kg i.p.). Body temperature was maintained at 39°C by a heating pad until animals recovered from anesthesia. Under aseptic conditions, a 1.0-cm long midline abdominal incision was made and ischemia was induced by applying a non-traumatic vascular clamp to the left renal pedicle for 15, 30, or 45 minutes. Subsequently, the wound was covered with cotton soaked in sterile phosphate-buffered saline (PBS). After removal of the clamp the left kidney was inspected for restoration of blood flow and the contralateral kidney was removed and stored for analysis. The wound was closed in two layers and 0.25% bupivacaine was applied topically for postoperative pain management. The animals were sacrificed at indicated time points after reperfusion (n = 6 per group). At the time of sacrifice, blood was collected and the left kidney and liver were harvested for analysis.
Human Renal Biopsy Material
As part of our clinical transplantation protocol, pre-transplant needle biopsies are routinely taken from all donor kidneys before start of cold machine-preservation (pre-transplant biopsy). Another biopsy is obtained after approximately 30 to 60 minutes of reperfusion during the transplantation procedure (post-transplant biopsy).
The biopsies evaluated in the present study were chosen based on post-transplant organ function. We studied heart-beating (HB) donor kidneys which are not subjected to evident warm ischemia and functioned immediately after transplantation (n = 2). Also non-heart-beating (NHB) kidneys were used, these organs suffer per definition from evident warm ischemia and often display post-transplant organ-failure.14,15 Pre- and post-transplant biopsies of ischemically injured NHB kidneys displaying a delayed graft function (n = 3) or primary non-function (n = 3) were analyzed.
For the studied kidneys there were no differences in the duration of cold storage (28 ± 3 hours) and no acute rejections were observed. All biopsies were immediately embedded in Tissue-Tek (EMS; Washington, PA) and snap-frozen in isopentane at −80°C. Biopsies are stored at −80°C until further processing.
Renal Histology
Cryostat sections (5 μm) of frozen murine kidneys were fixed with acetone and stained for complement factors C3 or C6 as reported previously,16 and for C1q, MBL-A and MBL-C. For MBL-A and -C as well as C1q staining, slides were immersed in PBS for 5 minutes and subsequently in 5% normal goat serum in PBS to block aspecific antibody binding. Slides were incubated for 1 hour at room temperature with the anti-MBL-A, -MBL-C or -C1q primary antibody in PBS with 0.1% bovine serum albumin (BSA). After three washes in PBS for 5 minutes each, slides were incubated for 30 minutes with the peroxidase-labeled secondary antibody diluted in the same buffer. After three washes in PBS, staining was visualized by 3-amino-9-ethylcarbazole (AEC) followed by hematoxylin counterstain. Finally, the slides were cover-slipped, and viewed with a light microscope. No significant staining was detected in slides incubated with rat IgG instead of the primary antibody indicating the absence of significant background staining.
Murine cryostat sections (5 μm) were also double-stained for MBL-A or MBL-C and complement factor C6. Briefly, slides were dried, fixed in acetone for 10 minutes, and air-dried. Slides were immersed in PBS for 5 minutes and subsequently in 5% normal goat serum in PBS to block aspecific antibody binding. Slides were incubated for 1 hour at room temperature with anti-MBL-A or -C in combination with anti-C6 primary antibody in PBS with 0.1% BSA. After three washes in PBS for 5 minutes each, slides were incubated for 30 minutes with the appropriate FITC-labeled and Texas-red-labeled secondary antibody diluted in the same buffer. After three washes in PBS, the slides were mounted using glycerol-PBS with 1,4-diazabicyclo(2,2,2)octane (DABCO) and 4,6-diamidino(2)phenylindole (DAPI), and viewed with an immunofluorescence microscope. No significant staining was detected in slides incubated with control rabbit serum and/or rat IgG instead of the primary antibodies indicating the absence of significant background staining or cross-reactivity.
Human cryostat sections (2 μm) were stained for MBL and F-actin. Briefly, slides were immersed in PBS for 5 minutes and subsequently in 5% normal goat serum in PBS to block aspecific antibody binding. Slides were incubated with anti-MBL primary antibody in PBS with 0.1% BSA for 2 hours at room temperature. After three washes in PBS for 5 minutes each, slides were incubated for 30 minutes with the appropriate FITC-labeled secondary antibody diluted in the same buffer with the addition of Texas Red-phalloidin that specifically binds to F-actin. After three washes in PBS, the slides were mounted using glycerol-PBS with 1,4-diazabicyclo(2,2,2)octane (DABCO) and 4,6-diamidino(2)phenylindole (DAPI), and viewed with an immunofluorescence microscope.
MBL-A and -C ELISA
Sandwich ELISAs were used to measure serum MBL-A and -C concentrations in mice subjected to renal I/R. These ELISAs were kindly provided by Hbt (Uden, the Netherlands).
Measurement of Renal and Hepatic MBL-A and -C mRNA Levels by Reverse Transcriptase-PCR
For measurement of renal and hepatic MBL-A and -C mRNA levels in the course of experimental renal I/R injury, total RNA was extracted from kidneys and livers using the SV Total RNA isolation system (Promega, Madison, WI) and treated with RQ1 RNase-Free DNase (Promega). Total RNA was reverse-transcribed using oligo (dT) primer and Moloney murine leukemia virus reverse transcriptase. cDNA samples were standardized based on the content of β-actin cDNA as housekeeping gene. β-actin cDNA was evaluated by performance of a β-actin PCR on multiple dilutions of each cDNA sample. The amount of amplified product was estimated by densitometry of ethidium bromide-stained 1.2% agarose gels using a CCD camera and Imagemaster VDS software (Pharmacia, Uppsala, Sweden). Primers used for the amplification of murine β-actin were: 5′-TAA AAC GCA GCT CAG TAA CAG TCG G-3′ (sense primer) and 5′-TGC AAT CCT GTG GCA TCC ATG AAA C-3′ (antisense primer). To determine renal and hepatic MBL-A and -C mRNA expression, PCR reactions with specific primers were performed using appropriate dilutions of the cDNA. The sequences of these primers were as follows: MBL-A, 5′-CCA AAG GGG AGA AGG GAG AAC-3′ (sense primer) and 5′-GCC TCG TCC GTG ATG CCT AG-3′ (antisense primer); MBL-C, 5′-GAC GTG ACG GTG CCA AGG G-3′ (sense primer) and 5′-CTT TCT GGA TGG CCG AGT TTT C-3′ (antisense primer). The following PCR conditions were used: MBL-A and -C, 95°C for 30 seconds, 56°C for 45 seconds, 72°C for 30 seconds; MBL-A, 35 cycles for kidney and 25 cycles for liver samples; and MBL-C, 40 cycles for kidney and 30 cycles for liver samples.
PCR reactions were performed in a total volume of 25 μl in PCR buffer (Perkin Elmer, Boston, MA), in the presence of 0.2 mmol/L dNTP (Pharmacia), 1.0 μmol/L of each primer, 0.3 mmol/L MgCl2, and 0.5 U Taq polymerase (Perkin Elmer). After separation on 1.2% agarose gel, the amplified product was visualized using a CCD camera and Imagemaster VDS software (Pharmacia).
Renal Myeloperoxidase Assay
To quantify the extent of renal neutrophil accumulation, renal MPO content was determined.17 In brief, tissue samples were homogenized in 0.5% hexadecyltrimethylammonium bromide in 50 mmol/L PBS, pH = 6.0, 0.17 g tissue/ml. After heat incubation (2 hours, 60°C) and three freeze-thaw cycles, myeloperoxide (MPO) content was measured in triplicate by incubating supernatants with tetramethylbenzidine (TMB) substrate in a 96-well sample plate (Costar, Cambridge, MA) followed by measurement of optical density (OD) at 450 nm. MPO activity was calculated per mg renal tissue by comparing OD of samples with a standard titration curve of horseradish peroxidase. Data were standardized with respect to wet/dry ratios of the assayed renal tissue and are presented relative to the amount of MPO present in the contralateral kidney.
Renal Function
Blood urea nitrogen (BUN) was measured in serum obtained 24 hours after reperfusion using a Urea 25 kit (ABX Diagnostics, Eindhoven, Holland) in a Cobas Fara autoanalyzer (Roche, Basel, Switzerland).
Statistical Analysis
Data are expressed as medians with inter-quartile ranges or means with SEM and statistical analysis was performed by Mann-Whitney U-test. P < 0.05 was taken to denote statistical significance.
Results
MBL-Pathway Is Activated in the Course of Experimental Renal Ischemia-Reperfusion Injury
To investigate activation of the MBL-pathway in the course of renal I/R, we first examined renal deposition of MBL-A and -C by immunohistochemistry. In line with a previous report,18 in murine renal control tissue both MBL-A and -C are localized to glomeruli with a mesangial expression pattern, whereas the vasculature and tubular epithelium is negative (Figure 1A and F, respectively). Renal warm ischemia of 15, 30, or 45 minutes without reperfusion did not induce any changes in MBL-A or -C expression as compared to healthy control tissue (data not shown). Warm ischemia of 15 minutes followed by reperfusion did not induce activation of the MBL-pathway (Figure 1, B and G). Longer renal ischemia of 30 minutes and, in particular, 45 minutes induced a rapid deposition of MBL-A and MBL-C to tubular and interstitial cells and in peritubular capillaries. These depositions were already detectable after 1 hour of reperfusion for MBL-C (data not shown) and after 2 hours of reperfusion for both MBL-A (Figure 1, C and D) and MBL-C (Figure 1, H and I). MBL-A and -C depositions were most pronounced after 24 hours of reperfusion when tubular casts also stained positive for MBL (Figure 1, E and J). These data show that warm ischemia followed by reperfusion induces the activation of the MBL-pathway depending on the duration of warm ischemia.
Figure 1.
Ischemia followed by reperfusion leads to renal deposition of MBL-A and MBL-C. Positive staining for MBL-A and -C in kidneys from healthy mice is only observed in glomeruli (A and F, respectively, right top corner). Renal ischemia for 15 minutes followed by 2 hours of reperfusion did not induce MBL-deposition (B and G). After 30 to 45 minutes of renal ischemia, MBL-A depositions were already evident after 2 hours of reperfusion, mainly localized to tubular epithelium in the medullar region (C and D, respectively). MBL-C depositions were already evident after 1 to 2 hours of reperfusion after 30 minutes and most pronounced after 45 minutes of renal ischemia (H and I, respectively) and localized to peritubular capillaries (H, right top corner) and interstitium (I, right top corner). At the late phase (24 hours of reperfusion) evident depositions of MBL-A were observed in the cortico-medullary region (E) and the medulla (E, right top corner). Most pronounced, depositions of MBL-C were observed in the vasculature (J, right bottom corner) and on tubular epithelial cells in the cortico-medullary region (J) and the medulla (J, right bottom corner). Magnification, ×200 (×600 for the insets in A, C, F, H, I and bottom corner, J.
Next, we examined whether, in addition to the MBL-pathway, the classical pathway is also activated in the course of renal I/R injury. As reported by others, we did not observe renal C1q deposition (Table 1). Furthermore, we examined the kinetics of renal deposition of the complement factors C3 (common pathway of the complement cascade), C6 and C9 (both part of the membrane attack complex (MAC)). Ischemia-induced renal deposition of C3 started after 2 hours reperfusion, deposition of C6 and C9, was first observed after 12 and 24 hours of reperfusion, respectively (Table 1).
Table 1.
Immunohistochemical Analysis of Renal Complement/Deposition after Ischemia-Reperfusion*
| Ischemia (min) | Reperfusion (hours) | Activation pathway
|
Common pathway
|
MAC
|
|||
|---|---|---|---|---|---|---|---|
| MBL-A | MBL-C | C1q | C3 | C6 | C9 | ||
| − | − | − | − | − | − | − | − |
| 45 | − | − | − | − | − | − | − |
| 45 | 1 | − | + | − | − | − | − |
| 45 | 2 | + | ++ | − | ++ | − | − |
| 45 | 12 | + | ++ | − | ++ | + | − |
| 45 | 24 | ++ | ++ | − | ++ | ++ | ++ |
| − | 24 | − | − | − | − | − | − |
, Renal deposition of various complement-factors was scored arbitrarily as negative (−), positive (+), or intensively positive (++) after immunohistochemical staining of kidneys from various experimental groups (n = 4 per group) subjected to renal ischemia reperfusion.
Taken together, these data show that the MBL-pathway of complement activation, in contrast to the classical pathway, is activated in the course of renal I/R. Ischemia-induced MBL-activation occurs at an early phase, before C3 deposition occurs, indicating that the MBL-pathway is an initiating pathway of complement activation induced by renal I/R.
Ischemia-Induced MBL-Deposition Co-Localizes with Renal Complement Deposition
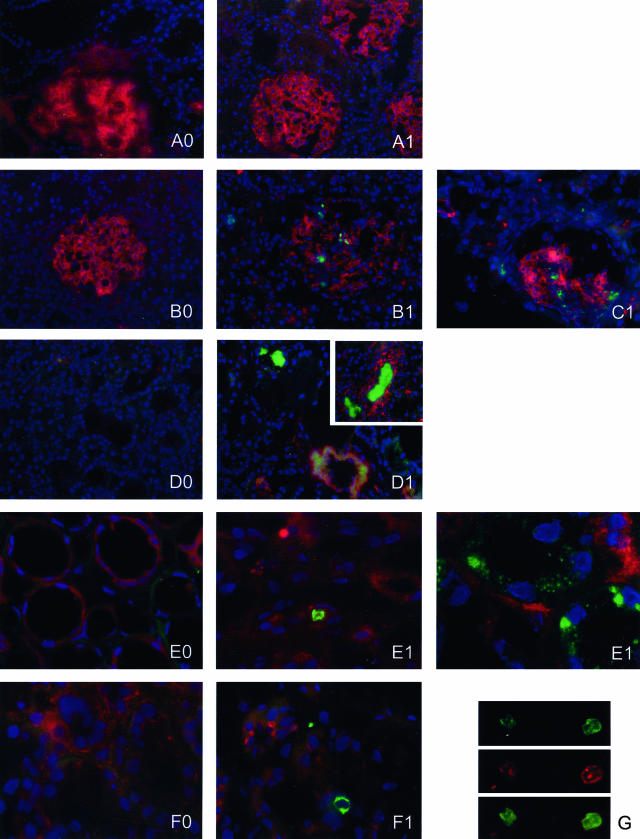
An important question is whether activation of the MBL-pathway is involved in complement activation in the course of renal I/R. Therefore we determined whether MBL-deposition co-localizes with the deposition of complement factor C6 which is part of the MAC and has been shown to be essentially involved in renal I/R injury.4,7 For the first time, we demonstrate that MBL-deposition almost completely co-localizes with renal C6 deposition (Figures 2 and 3). First, it is shown that in healthy kidneys both MBL-A and MBL-C are present in glomeruli without C6 deposition (Figure 2, A and D). Next, in the early reperfusion phase (1-hour reperfusion), after 45 minutes renal ischemia MBL-C, in contrast to MBL-A, is deposited to tubular epithelial cells, whereas no C6 deposition is observed (Figure 2, B and E). In the late reperfusion phase (24 hours of reperfusion), both MBL-A and -C are deposited and appear to be located at the same sites as C6 deposition (Figure 2, C and F, respectively). In Figure 3, the co-localization of both MBL-A and -C with C6 after 24 hours of reperfusion is further examined. MBL-A (left panel) and C6 (middle panel) displayed complete co-localization (right panel). Also MBL-C (left panel) and C6 (middle panel) almost completely co-localized (right panel). However, some tubuli appear to stain positive for C6 without evident MBL-C staining (Figure 3, bottom panel). These data indicate that activation of the MBL-pathway is involved in the initiation of complement activation and MAC formation induced by renal ischemia.
Figure 2.
Ischemia-induced MBL-deposition precedes C6-deposition. Kidneys of healthy animals (A and D) showed only glomerular MBL-A and MBL-C staining (in red) and no deposition of C6 (in green). Renal ischemia for 45 minutes followed by 1 hour of reperfusion (B and E) induced evident tubular epithelial deposition of MBL-C (E) but no MBL-A deposition (B). However, no C6-deposition is observed in the early reperfusion phase (B and E). At the late reperfusion phase (24 hours of reperfusion) both MBL-A and MBL-C depositions are observed (C and F, respectively). Interestingly, after 24 hours of reperfusion evident C6-staining is present which appears to co-localize to both MBL-A and -C (C and F). Red staining, MBL-A (A–C) and MBL-C (D–F) (Texas-red); green staining, C6 (FITC); blue staining, nuclei (DAPI). Magnification, ×200 and ×600 for right top corners.
Figure 3.
Ischemia-induced MBL-deposition co-localizes with C6-deposition. Kidneys subjected to 45 minutes ischemia followed by 24 hours reperfusion showed intense MBL-A and MBL-C depositions (left). At the same time, evident C6 deposition was observed (middle panel). The overlay pictures show that both MBL-A and MBL-C co-localize with C6 deposition (right). Interestingly, whereas C6 completely co-localizes with MBL-A, deposition of C6 does also occur without evident MBL-C deposition. Red staining, MBL-A and MBL-C (Texas-red); green staining, C6 (FITC); blue staining, nuclei (DAPI). Magnification, ×200 and ×600 for the bottom.
Renal Ischemia-Reperfusion Induces Differential Changes in Serum MBL-Levels
Next, serum levels of MBL-A and -C were measured to investigate the effects of renal I/R on circulating MBL levels. In normal mouse serum (Swiss mice) MBL-A and MBL-C levels were 11.2 and 49.5 μg/ml, respectively, which is comparable to reported MBL-levels in other mouse strains.19 Interestingly, the serum levels of MBL-A increased gradually during reperfusion, leading to a significant twofold increase 24 hours after reperfusion (Figure 4A). This is in line with data from Liu et al19 showing that MBL-A is an acute phase protein combined with data from our laboratory that renal I/R induces an acute phase response.20 In contrast, serum MBL-C levels decreased after renal I/R leading to a significant halving after 24 hours of reperfusion (Figure 4B). Thus, serum levels of MBL-A and -C are differentially regulated in the course of renal I/R injury.
Figure 4.
Serum MBL-A and -C levels in the course of renal I/R. Serum MBL-levels were determined after 45 minutes of ischemia followed by various reperfusion times using a sandwich ELISA. As compared to control mice, serum MBL-A levels increased twofold after 24 hours of reperfusion (A). In contrast, serum MBL-C levels dropped gradually during reperfusion resulting in a 50% reduction after 24 hours of reperfusion (B). Statistical significance as compared to control-treated animals was denoted at P < 0.05 (*). Data are expressed as median serum concentration of MBL (μg/ml) with interquartile ranges.
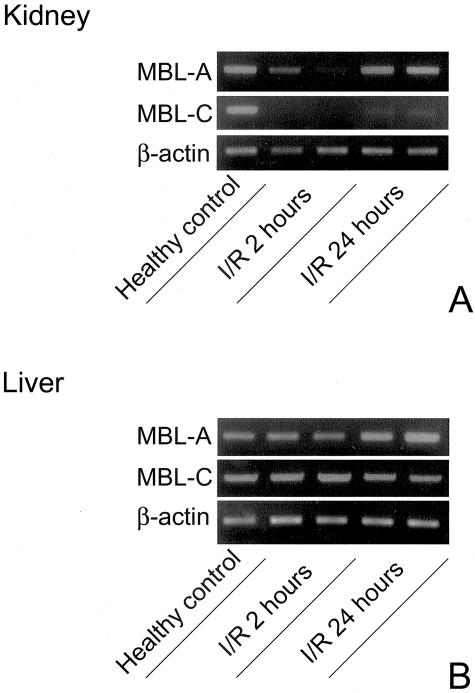
Hepatic and Renal MBL-A and -C mRNA Levels in the Course of Renal Ischemia-Reperfusion
To get more insight in the regulation of MBL-A and -C in the course of renal I/R injury we determined mRNA expression levels of both MBL-forms in kidney and liver tissue after renal I/R. First, both MBL-A and -C are expressed in healthy control kidneys as determined by RT-PCR (Figure 5A). Renal I/R induced an evident decrease of both renal MBL-A and -C mRNA levels at 2 hours reperfusion, which partially recovered for MBL-A at 24 hours of reperfusion (Figure 5A). Next, in normal liver tissue, MBL-A as well as MBL-C are expressed (Figure 5B). In the early reperfusion phase no changes in expression levels were observed, however, at 24 hours reperfusion MBL-A mRNA expression increased, in contrast to MBL-C levels, which decreased slightly (Figure 5B). The ischemia-induced changes in liver mRNA levels correlated well with serum MBL levels (Figure 4).
Figure 5.
Renal ischemia-reperfusion (I/R) differentially regulates renal and hepatic MBL-A and -C mRNA levels. Renal I/R induced a rapid down-regulation of renal MBL-A and -C mRNA levels as compared to healthy control tissue (A). Renal ischemia led to an evident up-regulation of hepatic MBL-A mRNA levels at 24 hours of reperfusion in contrast to MBL-C expression levels (B). Shown are representative samples (n = 4 per group) calibrated against equal amounts of β-actin mRNA.
Renal Ischemia-Induced MBL-Depositions Correlate with Postischemic Neutrophil-Influx and Organ Failure
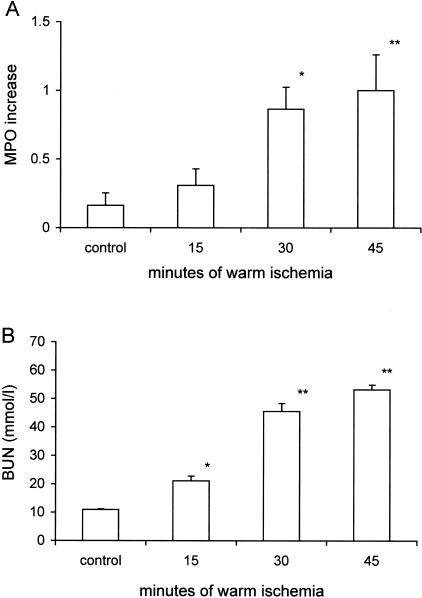
Activation of the complement system plays an important role in ischemia-induced inflammation, characterized by the infiltration of neutrophils and organ failure.4,6,7 We therefore questioned whether the early ischemia-induced deposition of MBL would correlate with inflammation and organ failure in the course of renal I/R injury. As reported, renal I/R induced no deposition of MBL after 15 minutes of ischemia, moderate MBL-depositions after 30 minutes of ischemia, and evident MBL-depositions after 45 minutes of ischemia followed by 1 to 2 hours of reperfusion (Figure 1). Interestingly, the early deposition of MBL correlated with the ischemia-induced influx of neutrophils 24 hours after reperfusion. Whereas 15 minutes of ischemia did not result in the renal infiltration of neutrophils, 30 and 45 minutes of warm ischemia resulted in an increasing amount of infiltrated neutrophils (Figure 6A). Moreover, the early deposition of MBL correlated with organ failure observed 24 hours after reperfusion. Whereas 15 minutes of ischemia only induced a marginal increase in BUN-levels, prolonged ischemia of 30 and 45 minutes resulted in strongly elevated BUN-levels reflecting severe organ failure (Figure 6B). Thus, the early deposition of MBL correlates with the development of I/R injury characterized by renal inflammation and organ failure. These data also suggest that the deposition of MBL, which results in complement activation, may be potentially involved in the development inflammation and organ failure in the course of renal I/R injury.
Figure 6.
Neutrophil-influx (A) and renal function loss (B) induced by renal warm ischemia. A: Renal neutrophil-influx was assessed quantitatively by determination of MPO increase in mice subjected to various periods of warm ischemia followed by 24 hours of reperfusion. Values are presented relative to the amount of MPO present in the contralateral kidney harvested immediately after reperfusion and normalized with respect to the MPO increase at 24 hours in mice subjected to 45 minutes of ischemia. Statistical significance as compared to control animals was denoted at P < 0.05 (*) and P < 0.01 (**). The presented data are means ± SEM. B: Renal function after 15, 30, and 45 minutes of ischemia and 24 hours of reperfusion as reflected by blood urea nitrogen (BUN) content. Statistical significance as compared to control animals was denoted at P < 0.05 (*) and P < 0.01 (**). The presented data are means ± SEM.
Post-Transplant MBL-Deposition in the Human Kidney
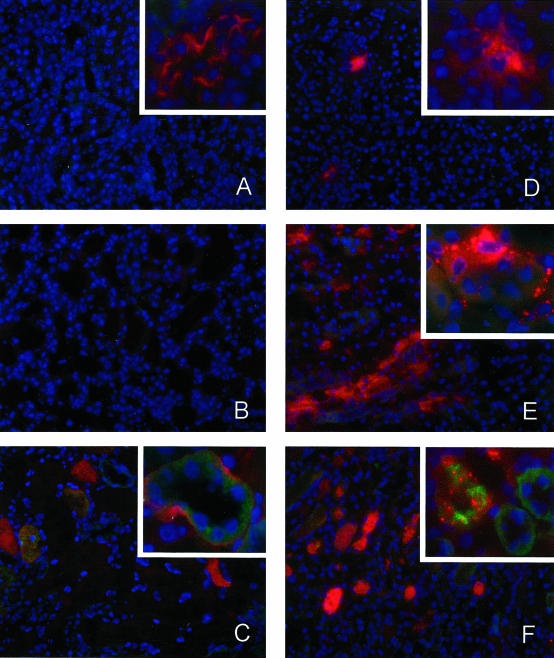
Finally, we determined whether the MBL-pathway is activated in human I/R injury by staining human pre- and post-transplant kidney biopsies for MBL. In normal human kidney no MBL could be detected as described by others.21 In line with our experimental data all pre-transplant biopsies were negative for MBL (Figure 7, A0 to F0). Interestingly, post-transplant biopsies of transplanted NHB donor kidneys, which per definition suffer from warm ischemia before organ procurement, stained positive for MBL (Figure 7, B1 to F1). In particular, glomerular staining was observed in such transplanted NHB grafts (Figure 7, B1 and C1). In contrast, transplanted HB kidneys (which do not suffer from evident warm ischemia) were negative for MBL (Figure 7A1). Strikingly, in transplanted NHB kidneys which did not function after transplantation (primary non-function), interlobular arteries and peritubular capillary walls, as well as tubular epithelial cells and casts, stained positive (Figure 7, D1 to F1), in contrast to delayed functioning grafts (Figure 7, B1 and C1). Double-staining of MBL with von Willebrand Factor (staining endothelial cells) shows that MBL is deposited on peritubular capillaries (Figure 7G).
Figure 7.
Renal deposition of MBL in early post-transplant biopsies of transplanted human kidneys. In pre-transplant renal biopsies no MBL could be detected (A0–F0). After transplantation of kidneys derived from heart-beating (HB) donors no MBL deposition is present (A1). In contrast, transplantation of ischemically injured non-heart-beating (NHB) donor kidneys induced rapid glomerular MBL deposition (B1 and C1). Moreover, in NHB grafts that displayed primary non-function extensive vascular and tubular MBL-depositions are observed (D1, interlobular artery and tubular casts; E1 peritubular capillary and tubular epithelium; F1 and G, peritubular capillary). A double-staining for MBL (green) and von Willebrand Factor (red) shows MBL deposition in peritubular capillaries (G). Red staining, filamentous actin (Texas-red), except for G, von Willebrand Factor (Texas-Red); green staining, MBL (FITC); blue staining, nuclei (DAPI). Magnification, ×200 for A–D and ×600 for E–G. Each letter represents a kidney (pre-transplant biopsy marked with 0, post-transplant biopsy marked with 1).
Our data show, for the first time, that the MBL-pathway of complement activation is initiated in the early reperfusion phase after renal transplantation of ischemically injured organs. Moreover, in this limited number of studied kidneys the intensity and localization of MBL-deposition appear to correlate with I/R injury and graft outcome after transplantation of ischemically injured NHB donor organs, which is in line with our experimental data.
Discussion
In the present study we show that experimental renal I/R induces significant changes in circulating MBL-levels after reperfusion. Interestingly, whereas levels of MBL-A increased twofold, MBL-C levels showed an almost 50% reduction. These data are in line with a study by Liu et al,19 showing that the induction of an acute phase response by casein as well as LPS injection in mice induced a twofold increase of serum MBL-A levels, whereas MBL-C levels were unchanged but tended to decrease after LPS injection. Interestingly, we previously showed that renal I/R induces an intense acute phase response comparable to the reaction observed after intraperitoneal LPS administration.20 These data indicate that in the course of renal I/R MBL-A behaves as an acute phase protein. On the other hand, serum MBL-C levels decreased significantly during renal I/R. MBL-A as an acute phase protein is mainly produced in the liver, although basal renal and pulmonary expression have been described.18,22,23 Basal MBL-C expression is described in the liver, kidney, thymus, and small intestine.23 In line, in the present study we show basal expression of MBL-A and -C in kidney and liver. In the postischemic kidney both MBL-A and -C expression rapidly decreased. This was in contrast to hepatic expression levels, which were regulated differentially. Whereas hepatic MBL-A levels increased after renal ischemia, as has been shown previously for other acute phase proteins,20 MBL-C levels decreased slightly. Hepatic levels MBL-A and -C levels correlated well with serum levels in the early as well as late reperfusion phase, indicating that the liver is the main site of MBL-production in the course of renal I/R injury.24
In humans, MBL deficiency is one of the most common immunodeficiencies, arising primarily from three single point mutations in codons 52, 54, and 57 of exon 1 in the MBL gene.25 These mutations prevent the assembly of fully functional multimeric protein, resulting in low serum levels of MBL. MBL deficiency predisposes to a broad range of serious bacterial, viral, and fungal infections as well as autoimmune disease.25,26 On the other hand, a recent report indicates that MBL-deficiency prevents complement activation and subsequent systemic inflammation in patients undergoing thoraco-abdominal aortic aneurysm repair, suggesting that MBL deficiency might be beneficial in conditions of pathophysiologic complement activation, such as ischemic injury.27
In renal disease, glomerular MBL-deposition is observed in glomerulonephritis, among others, primary and secondary IgA nephropathy, lupus nephropathy, membranous nephropathy, membranoproliferative glomerulonephritis, and anti-GBM nephritis.21,28–30 Recent work shows that the MBL-pathway is not activated in renal transplantation, however, in that study, only living donor recipients suffering acute humoral rejection were studied.31 In acute humoral rejection the classical pathway of complement activation is regarded to be the main activation route of the complement system.31,32 In the present study we show for the first time that the MBL-pathway is activated after transplantation of ischemically injured NHB donor kidneys in contrast to HB donor kidneys. This indicates that activation of the MBL-pathway is initiated on the basis of ischemic injury followed by reperfusion. In particular kidneys that did not function after transplantation (primary non-function) showed widespread MBL-depositions, located on glomeruli, large vessels, peritubular capillaries, tubular epithelial cells, and tubular casts, suggesting that the MBL-pathway might be essentially involved in post-transplant I/R injury. These clinical observations are supported by our experimental data, which show that the deposition of MBL is dependent on the duration of warm ischemia and correlates with postischemic inflammation and organ-failure.
Involvement of the complement system in experimental renal I/R injury was already reported in 1985 by Stein et al33 showing complement deposition in a rat model for renal I/R injury. Only recently, an active role in the pathophysiology of I/R injury was addressed to the complement system by Zhou and colleagues,4 showing that complement-deficient mice are protected against I/R injury. The complement system can be activated via three pathways: the classical pathway (activated by antigen-antibody interaction), the MBL-pathway (activated by mannose present on microbial surfaces), and the alternative pathway (activated by microbial products). All three pathways converge at the level of C3 activation, forming a C5-convertase, which activates C5. Activation of C5 results in the formation of C5a and C5b, the latter one being the first step in the formation of MAC. Both the formation of MAC as well as the generation of the anaphylatoxin C5a have been shown to mediate renal I/R injury.4–7 However, via which pathway(s) complement activation is initiated in the course of renal I/R injury is still unclear. Complement activation in the kidney has been attributed to the alternative pathway mainly by exclusion of classical complement activation. Park et al8 demonstrated that renal I/R does not induce IgG or IgM deposition. In concordance we did not observe C1q deposition in our experimental model. RAG-1 −/− mice subjected to I/R still showed renal complement deposition, indicating that renal I/R is not mediated via the classical pathway.8 Indeed, C4-deficient mice, in contrast to C3-deficient mice, were not protected against renal I/R injury.4 Moreover, Thurman et al9 showed recently that mice lacking a functional alternative complement pathway (factor B −/− mice) are partially protected against renal ischemic injury, showing that the alternative pathway plays an important role in the pathophysiology of renal I/R injury. In an experimental mouse model for renal I/R injury we show for the first time that renal I/R induces the activation of the MBL-pathway. In the healthy mouse kidney only glomerular mesangial cells stained positive as reported by others.18 After renal I/R both MBL-A and -C deposited to injured tubular epithelial cells. The site of renal MBL-deposition observed in the present study was similar to ischemia-induced C3, C6, and C9 deposition, as reported previously.7 Therefore, co-localization studies were performed to demonstrate that MBL-deposition precedes and co-localizes with complement activation. Our data show that MBL-deposition precedes C3 and C6 deposition and moreover that C6 deposition completely co-localizes with MBL-deposition. These data are in line with recent in vitro studies showing that endothelial hypoxia followed by re-oxygenation induces MBL-deposition which co-localizes with C3 deposition.11,34 In vivo, MBL-A deposition has been observed during the early phase of myocardial I/R in a rat model. Moreover, inhibition of MBL-A protected against myocardial I/R injury, attenuating C3 deposition, reducing infarct size and neutrophil infiltration.13 In combination with our experimental and clinical data showing that the deposition of MBL correlates with postischemic organ-failure, these studies indicate that the MBL-pathway of complement activation plays an active role in I/R injury. However, additional intervention studies are needed to unravel the precise role of this pathway in renal I/R injury.
How the MBL-pathway is activated in the course of I/R injury remains unclear. Recent in vitro work indicates that cytokeratin 1 is involved in activation of the MBL-pathway.12 The membrane expression of cytokeratin 1 is up-regulated after endothelial hypoxic stress and inhibition of cytokeratin-1 prevented endothelial complement deposition (MBL and C3).12 Whether cytokeratin 1 plays a role in complement activation in renal I/R injury in vivo remains to be established.
Taken together, in the present study we show for the first time that the MBL-pathway is activated in the course of experimental as well as clinical renal I/R injury. Moreover, the MBL-pathway precedes and co-localizes with complement deposition in the course of I/R. These data indicate that the MBL-pathway of complement activation is essentially involved in renal I/R injury.
Footnotes
Address reprint requests to Dr. W.A. Buurman, Department of General Surgery, Maastricht University, P.O. Box 616, Universiteitssingel 50, 6200 MD Maastricht, the Netherlands. E-mail address: W.Buurman@ah.unimaas.nl.
Supported by the Dutch Kidney Foundation, Grant C99.1840 (to W.A.B.) and by AGIKO Stipendium of The Netherlands Organization of Scientific Research (to B.d.V.).
References
- Levy EM, Viscoli CM, Horwitz RI. The effect of acute renal failure on mortality: a cohort analysis. JAMA. 1996;275:1489–1494. [PubMed] [Google Scholar]
- Thadhani R, Pascual M, Bonventre JV. Acute renal failure. N Engl J Med. 1996;334:1448–1460. doi: 10.1056/NEJM199605303342207. [DOI] [PubMed] [Google Scholar]
- Nicholson ML, Metcalfe MS, White SA, Waller JR, Doughman TM, Horsburgh T, Feehally J, Carr SJ, Veitch PS. A comparison of the results of renal transplantation from non-heart-beating, conventional cadaveric, and living donors. Kidney Int. 2000;58:2585–2591. doi: 10.1046/j.1523-1755.2000.00445.x. [DOI] [PubMed] [Google Scholar]
- Zhou W, Farrar CA, Abe K, Pratt JR, Marsh JE, Wang Y, Stahl GL, Sacks SH. Predominant role for C5b-9 in renal ischemia/reperfusion injury. J Clin Invest. 2000;105:1363–1371. doi: 10.1172/JCI8621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arumugam TV, Shiels IA, Strachan AJ, Abbenante G, Fairlie DP, Taylor SM. A small molecule C5a receptor antagonist protects kidneys from ischemia/reperfusion injury in rats. Kidney Int. 2003;63:134–142. doi: 10.1046/j.1523-1755.2003.00737.x. [DOI] [PubMed] [Google Scholar]
- de Vries B, Kohl J, Leclercq WK, Wolfs TG, van Bijnen AA, Heeringa P, Buurman WA. Complement factor C5a mediates renal ischemia-reperfusion injury independent from neutrophils. J Immunol. 2003;170:3883–3889. doi: 10.4049/jimmunol.170.7.3883. [DOI] [PubMed] [Google Scholar]
- de Vries B, Matthijsen RA, Wolfs TG, Van Bijnen AA, Heeringa P, Buurman WA. Inhibition of complement factor C5 protects against renal ischemia-reperfusion injury: inhibition of late apoptosis and inflammation. Transplantation. 2003;75:375–382. doi: 10.1097/01.TP.0000044455.05584.2A. [DOI] [PubMed] [Google Scholar]
- Park P, Haas M, Cunningham PN, Bao L, Alexander JJ, Quigg RJ. Injury in renal ischemia-reperfusion is independent from immunoglobulins and T lymphocytes. Am J Physiol. 2002;282:F352–F357. doi: 10.1152/ajprenal.00160.2001. [DOI] [PubMed] [Google Scholar]
- Thurman JM, Ljubanovic D, Edelstein CL, Gilkeson GS, Holers VM. Lack of a functional alternative complement pathway ameliorates ischemic acute renal failure in mice. J Immunol. 2003;170:1517–1523. doi: 10.4049/jimmunol.170.3.1517. [DOI] [PubMed] [Google Scholar]
- Thiel S, Vorup-Jensen T, Stover CM, Schwaeble W, Laursen SB, Poulsen K, Willis AC, Eggleton P, Hansen S, Holmskov U, Reid KB, Jensenius JC. A second serine protease associated with mannan-binding lectin that activates complement. Nature. 1997;386:506–510. doi: 10.1038/386506a0. [DOI] [PubMed] [Google Scholar]
- Collard CD, Vakeva A, Morrissey MA, Agah A, Rollins SA, Reenstra WR, Buras JA, Meri S, Stahl GL. Complement activation after oxidative stress: role of the lectin complement pathway. Am J Pathol. 2000;156:1549–1556. doi: 10.1016/S0002-9440(10)65026-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collard CD, Montalto MC, Reenstra WR, Buras JA, Stahl GL. Endothelial oxidative stress activates the lectin complement pathway: role of cytokeratin 1. Am J Pathol. 2001;159:1045–1054. doi: 10.1016/S0002-9440(10)61779-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan JE, Montalto MC, Stahl GL. Inhibition of mannose-binding lectin reduces postischemic myocardial reperfusion Injury. Circulation. 2001;104:1413–1418. doi: 10.1161/hc3601.095578. [DOI] [PubMed] [Google Scholar]
- Wijnen RM, Booster MH, Stubenitsky BM, de Boer J, Heineman E, Kootstra G. Outcome of transplantation of non-heart-beating donor kidneys. Lancet. 1995;345:1067–1070. [PubMed] [Google Scholar]
- Weber M, Dindo D, Demartines N, Ambuhl PM, Clavien PA. Kidney transplantation from donors without a heartbeat. N Engl J Med. 2002;347:248–255. doi: 10.1056/NEJMoa020274. [DOI] [PubMed] [Google Scholar]
- de Vries B, Matthijsen RA, van Bijnen AA, Wolfs TG, Buurman WA. Lysophosphatidic acid prevents renal ischemia-reperfusion injury by inhibition of apoptosis and complement activation. Am J Pathol. 2003;163:47–56. doi: 10.1016/S0002-9440(10)63629-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laight DW, Lad N, Woodward B, Waterfall JF. Assessment of myeloperoxidase activity in renal tissue after ischemia/reperfusion. Eur J Pharmacol. 1994;292:81–88. doi: 10.1016/0926-6917(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Wagner S, Lynch NJ, Walter W, Schwaeble WJ, Loos M. Differential expression of the murine mannose-binding lectins A and C in lymphoid and nonlymphoid organs and tissues. J Immunol. 2003;170:1462–1465. doi: 10.4049/jimmunol.170.3.1462. [DOI] [PubMed] [Google Scholar]
- Liu H, Jensen L, Hansen S, Petersen SV, Takahashi K, Ezekowitz AB, Hansen FD, Jensenius JC, Thiel S. Characterization and quantification of mouse mannan-binding lectins (MBL-A and MBL-C) and study of acute phase responses. Scand J Immunol. 2001;53:489–497. doi: 10.1046/j.1365-3083.2001.00908.x. [DOI] [PubMed] [Google Scholar]
- Daemen MA, Heemskerk VH, van’t Veer C, Denecker G, Wolfs TG, Vandenabeele P, Buurman WA. Functional protection by acute phase proteins alpha(1)-acid glycoprotein and alpha(1)-antitrypsin against ischemia/reperfusion injury by preventing apoptosis and inflammation. Circulation. 2000;102:1420–1426. doi: 10.1161/01.cir.102.12.1420. [DOI] [PubMed] [Google Scholar]
- Endo M, Ohi H, Ohsawa I, Fujita T, Matsushita M. Glomerular deposition of mannose-binding lectin (MBL) indicates a novel mechanism of complement activation in IgA nephropathy. Nephrol Dial Transplant. 1998;13:1984–1990. doi: 10.1093/ndt/13.8.1984. [DOI] [PubMed] [Google Scholar]
- Sastry R, Wang JS, Brown DC, Ezekowitz RA, Tauber AI, Sastry KN. Characterization of murine mannose-binding protein genes Mbl1 and Mbl2 reveals features common to other collectin genes. Mamm Genome. 1995;6:103–110. doi: 10.1007/BF00303252. [DOI] [PubMed] [Google Scholar]
- Uemura K, Saka M, Nakagawa T, Kawasaki N, Thiel S, Jensenius JC, Kawasaki T. L-MBP is expressed in epithelial cells of mouse small intestine. J Immunol. 2002;169:6945–6950. doi: 10.4049/jimmunol.169.12.6945. [DOI] [PubMed] [Google Scholar]
- Sastry K, Zahedi K, Lelias JM, Whitehead AS, Ezekowitz RA. Molecular characterization of the mouse mannose-binding proteins: the mannose-binding protein A but not C is an acute phase reactant. J Immunol. 1991;147:692–697. [PubMed] [Google Scholar]
- Eisen DP, Minchinton RM. Impact of mannose-binding lectin on susceptibility to infectious diseases. Clin Infect Dis. 2003;37:1496–1505. doi: 10.1086/379324. [DOI] [PubMed] [Google Scholar]
- Turner MW. The role of mannose-binding lectin in health and disease. Mol Immunol. 2003;40:423–429. doi: 10.1016/s0161-5890(03)00155-x. [DOI] [PubMed] [Google Scholar]
- Fiane AE, Videm V, Lingaas PS, Heggelund L, Nielsen EW, Geiran OR, Fung M, Mollnes TE. Mechanism of complement activation and its role in the inflammatory response after thoracoabdominal aortic aneurysm repair. Circulation. 2003;108:849–856. doi: 10.1161/01.CIR.0000084550.16565.01. [DOI] [PubMed] [Google Scholar]
- Matsuda M, Shikata K, Wada J, Sugimoto H, Shikata Y, Kawasaki T, Makino H. Deposition of mannan binding protein and mannan binding protein-mediated complement activation in the glomeruli of patients with IgA nephropathy. Nephron. 1998;80:408–413. doi: 10.1159/000045212. [DOI] [PubMed] [Google Scholar]
- Lhotta K, Wurzner R, Konig P. Glomerular deposition of mannose-binding lectin in human glomerulonephritis. Nephrol Dial Transplant. 1999;14:881–886. doi: 10.1093/ndt/14.4.881. [DOI] [PubMed] [Google Scholar]
- Endo M, Ohi H, Ohsawa I, Fujita T, Matsushita M. Complement activation through the lectin pathway in patients with Henoch-Schonlein purpura nephritis. Am J Kidney Dis. 2000;35:401–407. doi: 10.1016/s0272-6386(00)70192-2. [DOI] [PubMed] [Google Scholar]
- Sund S, Hovig T, Reisaeter AV, Scott H, Bentdal O, Mollnes TE. Complement activation in early protocol kidney graft biopsies after living-donor transplantation. Transplantation. 2003;75:1204–1213. doi: 10.1097/01.TP.0000062835.30165.2C. [DOI] [PubMed] [Google Scholar]
- Mauiyyedi S, Colvin RB. Humoral rejection in kidney transplantation: new concepts in diagnosis and treatment. Curr Opin Nephrol Hypertens. 2002;11:609–618. doi: 10.1097/00041552-200211000-00007. [DOI] [PubMed] [Google Scholar]
- Stein JH, Osgood RW, Barnes JL, Reineck HJ, Pinckard RN, McManus LM. The role of complement in the pathogenesis of postischemic acute renal failure. Miner Electrolyte Metab. 1985;11:256–261. [PubMed] [Google Scholar]
- Collard CD, Lekowski R, Jordan JE, Agah A, Stahl GL. Complement activation following oxidative stress. Mol Immunol. 1999;36:941–948. doi: 10.1016/s0161-5890(99)00116-9. [DOI] [PubMed] [Google Scholar]