Abstract
Levels of prostaglandin E2 (PGE2), a potent inhibitor of fibroblast function, are decreased in the lungs of patients with pulmonary fibrosis, which has been shown to be because of limited expression of cyclooxygenase-2 (COX-2). To further investigate the relative importance of COX-2 and PGE2 in the development of fibrosis we have used a selective COX-2 inhibitor and COX-2-deficient (−/− and +/−) mice in studies of bleomycin-induced lung fibrosis. We demonstrate in wild-type mice that bleomycin-induced lung PGE2 production is predominantly COX-2 mediated. Furthermore, COX-2+/− mice show limited induction of PGE2 and an enhanced fibrotic response with increased lung collagen content compared with wild-type mice after bleomycin injury (P < 0.001). In contrast, COX-2−/− mice show increased levels of lung PGE2, compared with wild-type mice after injury (P < 0.05), because of compensatory up-regulation of COX-1, which appears to be associated with macrophage/monocytes but not fibroblasts derived from these mice. COX-2−/− mice show an enhanced and persistent inflammatory response to bleomycin, however the fibrotic response to injury was unaltered compared with wild-type animals. These data provide further direct evidence for the importance of up-regulating COX-2 and PGE2 expression in protecting against the development of fibrosis after lung injury.
Idiopathic pulmonary fibrosis is a chronic and progressive interstitial lung disease characterized by fibroblast proliferation and excessive deposition of extracellular matrix proteins including collagens, which severely impairs lung function and is often fatal.1 Although the pathogenic processes involved in the initiation and perpetuation of fibrosis are not fully understood, dysregulation of both pro- and anti-fibrotic mediators that regulate fibroblast proliferation and collagen production after injury are thought to play an important role.2 The majority of studies have focused on the role of stimulatory growth factors and cytokines in the pathogenesis of both human disease and animal models of lung fibrosis.3,4 However, there is substantial evidence to suggest that inhibitory regulators of fibroblast function such as prostaglandin E2 (PGE2),5–7 or interferons,8,9 may play an important role, whereby defective suppression of proinflammatory and profibrotic cytokines may lead to unrestrained fibroblast proliferation and collagen production.
PGE2 is the major prostanoid product in the lung and of fibroblasts.10–12 PGE2 is a potent inhibitor of fibroblast proliferation,6,13 collagen synthesis,14,15 chemotaxis,16 fibroblast to myofibroblast differentiation,17 connective tissue growth factor (CTGF) expression,18 and has also been shown to promote degradation of newly synthesized collagen.19,20 Furthermore, autocrine synthesis of PGE2 inhibits transforming growth factor (TGF)-β1-induced fibroblast proliferation and collagen synthesis.5,6 Despite increased levels of a range of mediators known to induce its synthesis, levels of PGE2 in bronchoalveolar lavage fluid (BALF) from patients with pulmonary fibrosis have been shown to be 50% lower than in normal individuals.21 Macrophages obtained by bronchoalveolar lavage show a reduced capacity to produce PGE2.11 Moreover, fibroblasts derived from the lungs of patients with pulmonary fibrosis generally show both decreased basal synthesis of PGE2 and fail to induce its synthesis on stimulation with interleukin-1β, lipopolysaccharide, TGF-β1, or tumor necrosis factor-α,7,22,23 although this is controversial.24 The reduced synthesis of PGE2 in these cells results in a more fibrogenic phenotype, characterized by an increase in procollagen production, a loss of the anti-proliferative response to TGF-β1,7 and decreased production of hepatocyte growth factor.25
The prostanoids, including PGE2, are a class of lipid mediators generated via the cyclooxygenase (COX) pathway. Three COX isoforms have been described to date. COX-1 is constitutively expressed in nearly all tissues and is generally thought to be responsible for homeostatic functions. In contrast, COX-2 is normally undetectable in most tissues, but is readily induced in response to a variety of mediators including proinflammatory and profibrotic cytokines, hormones, and oncogenes,26–28 and has been associated with the pathogenesis of a number of diseases.29,30 However, in the lung there appears to be constitutive expression of COX-2 associated predominantly with epithelial cells and macrophages.31,32 A third isoform, COX-3 has recently been described.33 This protein is similar to COX-1 with the addition of an N-terminal extension because of the retention of intron-1 and the retained signal peptide, however no functional data have been described to date.
The failure to synthesize PGE2 in fibroblasts derived from patients with pulmonary fibrosis has been shown to be associated with a decreased capacity to up-regulate COX-2, at both the mRNA and protein levels.7,22 Together, these data suggest that a decreased capacity to up-regulate synthesis of PGE2 via dysregulation of COX-2 expression may play a key role in the pathogenesis of pulmonary fibrosis. Therefore to assess the relative importance of limited COX-2 and PGE2 synthesis in the development of lung fibrosis this study examines the fibrotic response to bleomycin-induced lung injury in homozygote (COX-2−/−) and heterozygote (COX-2+/−) COX-2 gene-deficient mice. We show that mice heterozygous for COX-2 demonstrate limited induction of PGE2 synthesis after injury, which is coupled with an enhanced fibrotic response compared with wild-type (WT) mice. However in COX-2−/− mice after bleomycin injury, levels of PGE2 were increased because of, at least in part, a compensatory up-regulation of COX-1. This was associated with an enhanced and persistent inflammatory response characterized by chronic lymphocytosis and neutrophilia but the fibrotic response was similar to that in WT mice. Together these data provide further compelling evidence of the protective roles played by COX-2 and PGE2 in limiting pulmonary fibrosis.
Materials and Methods
Animals
COX-2-deficient (COX-2+/− and COX-2−/−) and WT SV129/C57BL/6, F4 mice34 were bred at University College London from breeding pairs obtained from The Jackson Laboratory, Bar Harbor, ME. The animals were housed on a 12-hour light/12-hour dark cycle at 25°C. Food and water were available ad libitum. Litters were weaned at 4 weeks of age and the presence of the disrupted COX-2 gene in COX-2+/− and COX-2−/− mice was detected using polymerase chain reaction amplification of genomic DNA isolated from a tail snip. Three primers were used to amplify the specific region of DNA; a common downstream primer: 5′-CAC CAT AGA ATC CAG TCC GG-3′ (547); a specific upstream primer for the WT allele: 5′-ATC TCA GCA CTG CAT CCT GC-3′ (546); and a specific primer for COX-2-deficient alleles: 5′-CTT GGG TGG AGA GGC TAT TC-3′ (013). The polymerase chain reaction consisted of a 10-μl reaction mix containing 2 μl of DNA (100 ng/μl), 3 μl of distilled water, 5 μl of reaction mixture (final concentrations 50 mmol/L KCL, 10 mmol/L dNTPs, 1.5 mmol/L MgCl2, 0.2 μmol/L primer 546, 0.2 μmol/L primer 547, 0.04 μmol/L primer 013). TaqDNA polymerase (5 U/μl; Amersham Biosciences, Amersham, UK) was added to the reaction mixture to give a final concentration of 2 U/μl. The polymerase chain reaction conditions used were one cycle of initial denaturing (94°C, 3 minutes), followed by 34 cycles of denaturing, annealing, and extension (94°C, 1 minute; 62°C, 2 minutes; 72°C, 2 minutes), with one cycle of final extension (72°C, 5 minutes). The products were separated by electrophoresis using a 2% (w/v) agarose gel and the DNA bands were visualized by phosphorimaging. Animal genotype was determined by the band pattern; WT DNA produced a single band at 1.44 kb, COX-2−/− DNA a single band at 0.922 kb, and both bands were seen in COX-2+/− DNA.
Bleomycin Model of Lung Fibrosis
Equal numbers of male and female mice 6 to 10 weeks of age were used. Animals were anesthetized with halothane (3%) in a stream of oxygen (2 L/min). Mice received a single intratracheal instillation of either bleomycin sulfate (1 mg/kg body weight; Kyowa Hakko, Slough, UK) dissolved in 50 μl of 0.9% saline or 0.9% saline alone as a control. At least six animals per group were analyzed for biochemical and cytological assessment and at least three for histological assessment. Animals were terminally anesthetized using an intraperitoneal injection of 100 μl of pentobarbitone sodium (200 mg/ml; Sanofi Animal Health, Watford, UK) at 3, 7, 14, 28, or 84 days after bleomycin instillation. After laparotomy and exanguination, the trachea was cannulated with a 22-gauge venflon (Ohmeda BOC, Sweden) and the lungs lavaged with 5 ml of phosphate-buffered saline (PBS) in 10 0.5-ml aliquots. The BALF was kept on ice throughout the procedure and more than 90% of the instilled volume was consistently recovered. After lavage the lungs were subsequently removed, snap-frozen in liquid nitrogen, and stored at −80°C before analysis.
For histological analysis of lung fibrosis, animals were terminally anesthetized at 28 days after injury, as described above. After laparotomy and exanguination, the pulmonary vasculature was perfused with heparinized PBS via the vena cava followed by 4% paraformaldehyde. The lungs were inflated by intratracheal instillation of 4% paraformaldehyde fixative in PBS at a pressure of 20-cm H2O. The trachea was ligated and the heart and lungs removed en bloc, immersed in 4% paraformaldehyde overnight at 4°C, and processed to paraffin wax.
Pharmacological Inhibition of COX-2
To determine the relative importance of COX-2 and COX-2-derived PGE2 in response to bleomycin injury, mice were treated with the highly selective COX-2 inhibitor NS398 [N-(-2-cyclohexyloxy)-4-nitrophenyl methanesulphonamide; Cayman Chemicals/Alexis Corporation, Nottingham, UK]. NS398 was dissolved in dimethyl sulfoxide (Sigma, UK) at 25 mg/ml and stock solutions stored at −40°C. Immediately before administration, NS398 was thawed and diluted in PBS containing 1% Tween 80 to a concentration of 750 μg/ml and administered to mice within 1 hour of preparation. Each animal received 3 μg of NS398/kg body weight in a final volume of 30 μl. The inhibitor was administered by oral gavage every 12 hours as previously described.35 Control animals received 30 μl of vehicle alone. The first dose of NS398 was administered 1 hour before intratracheal instillation of bleomycin.
Cytological Analysis of BALF
Lavage samples were centrifuged (300 × g) at 4°C for 5 minutes to pellet the cells and the fluid removed, aliquoted, and stored at −80°C until eicosanoid levels were analyzed. The cell pellet was resuspended in 500 μl of Dulbecco’s modified Eagle’s medium (DMEM) (Gibco-BRL, Paisley, UK) containing 10% fetal bovine serum, (Imperial Laboratories, Andover, UK). Total lavage cell numbers were determined using a hemocytometer. Cytospins of BALF were made by centrifuging 0.5 to 1 × 106 cells/ml onto poly-l-lysine-coated microscope slides (Cytospin 3; Shandon, UK). The slides were allowed to air-dry and were then fixed in methanol and stained using DiffQuik (DADE AG, Switzerland). At least 500 cells per sample were differentiated, using conventional morphological criteria for macrophages/monocytes, lymphocytes, and polymorphonuclear leukocytes (PMNs).
Measurement of BALF Eicosanoid and TGF-β1 Levels
PGE2 levels were measured directly in BALF obtained 3 to 84 days after bleomycin instillation using a specific enzyme immunoassay according to manufacturer’s instructions (Amersham Biosciences). Previous extraction of lipids was required to measure leukotriene C4 (LTC4) levels. Briefly, lipids were methanol-extracted from BALF using C18 Sep-Pak light cartridges (Millipore, Watford, UK) using previously described methods.36 Samples were eluted using methyl formate and dried under a stream of nitrogen. LTC4 was resuspended in DMEM and measured using a specific immunoassay (Cayman Chemicals/Alexis Corporation) according to the manufacturer’s instructions. Total TGF-β1 in BALF was measured using a specific immunoassay according to the manufacturer’s instructions (R&D Systems Europe Ltd., Abingdon, UK).
Analysis of Lung Collagen Content
Total lung collagen was quantified by analysis of hydroxyproline, using previously described methods.5,37 Lungs obtained from animals 14 to 84 days after bleomycin instillation were crushed to a fine powder under liquid nitrogen using a pestle and mortar. Aliquots of 10- to 20-mg lung powder were hydrolyzed for 16 hours in 2 ml of 6 mol/L HCl at 110°C. Samples were then decolorized by the addition of ∼20 mg of charcoal (VWR Int., Poole, UK) and filtered (0.65-μm pore, Millipore) before chromatography. Hydroxyproline was isolated and quantified by reverse-phase high-pressure liquid chromatography of 7-chloro-4-nitrobenzo-oxa-1,3-diazole (NBD-Cl)-derivatized hydrosylates as described previously.5
Western Blotting
COX-1, COX-2, and cPLA2 protein expression was examined using Western analysis of lung tissue taken 14 days after instillation of bleomycin. Protein was extracted from powdered lung tissue by five freeze-thaw cycles in 200 μl of RIPA buffer [1% Triton X-100 (w/v), 0.1% sodium dodecyl sulfate (w/v), 158 mmol/L NaCl, 1 mmol/L ethylenediaminetetraacetic acid, 10 mmol/L Tris-HCl, 1 mmol/L NaVO3, 1 mmol/L 4-(2-aminoethyl)benzenesulfonyl fluoride HCl (AEBSF), 50 μg/ml leupeptin, pH 7.2]. Samples were then centrifuged (5 minutes, 10,000 × g, 4°C) and protein in the supernatant measured using a bicinchoninic acid assay (Pierce, Tattenhall, UK). Approximately 100 μg of protein was fractionated on sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels, transferred onto polyvinylidene difluoride membranes (Hybond-ECL, Amersham Biosciences) and incubated with blocking buffer [COX-1: 3% bovine serum albumin in TBST (50 mmol/L Tris, 150 mmol/L NaCl, 0.02% Tween 20, pH 7.4) for 3 hours; COX-2: 5% skimmed milk in PBS overnight at 4°C; cPLA2: 0.2%w/v I-Block dissolved in Tris-buffered saline (TBS) (20 mmol/L Tris, 137 mmol/L NaCl, pH 7.6) for 3 hours]. To examine COX-2 expression, membranes were incubated with a COX-2 polyclonal rabbit anti-mouse antibody (Cayman Chemicals/Alexis Corp.) at a 1:2000 dilution in 2% skimmed milk TBST for 2 hours at 4°C. Alternatively, to examine the expression of COX-1 or cPLA2, membranes were incubated overnight at room temperature with goat anti-rabbit polyclonal or rabbit anti-mouse monoclonal antibodies, respectively (both Santa Cruz, Calne, UK) diluted 1:1000 in TBST. All blots were then washed with TBST and incubated for 1 hour at room temperature with horseradish peroxidase-conjugated anti-rabbit IgG (COX-2 1: 2000 dilution, cPLA2 1:10000 in TBST) or anti-goat IgG (COX-1: 1:10,000 dilution in TBST). After further washing in TBST, immunoreactive bands were visualized by enhanced chemiluminescence (ECL, Amersham Biosciences) according to manufacturer’s instructions. Band intensities were calculated by scanning the films and quantifying band densities using Image Master software (Amersham Biosciences).
Histological Analysis
For histological analysis of lung fibrosis 3-μm sections were stained with modified Martius Scarlet Blue. The extent of fibrosis was scored in a blinded manner by three independent observers based on a previously described method.7,38 Each lung lobe was scored on a scale of 0 to 4 and a mean derived from the five lobe scores for each individual mouse.
To examine expression of TGF-β1, 4-μm sections of lung tissue were deparaffinized with xylene, rehydrated through graded ethanol, and washed in TBS (20 mmol/L Tris, 137 mmol/L NaCl, pH 7.6). After antigen retrieval treatment using proteinase K (VWR) at 10 μg/ml (10 minutes, room temperature), endogenous peroxidase was blocked using 3% H2O2 in methanol for 30 minutes. After a further wash in TBS, sections were treated with 1:6 goat serum in TBS containing avidin block (Vector Laboratories, Peterborough, UK), drained, and incubated overnight at 4°C in rabbit anti-TGF-β1 polyclonal antibody (0.5 μg/ml, Santa Cruz) in TBS containing 1% bovine serum albumin and biotin block (Vector Laboratories). The tissues were incubated for 1 hour at room temperature in a goat-biotinylated anti-rabbit IgG antiserum (1:200; DAKO Corp., Ely, UK) followed by streptavidin (1:200, DAKO Corp.) for 30 minutes. Visualization was performed using 3,3′-diaminobenzidine (Vector Laboratories) for 10 minutes; sections were counterstained using hematoxylin, dehydrated, cleared, and mounted.
Isolation of COX-2−/− and WT Fibroblasts and Measurement of PGE2 Synthesis
After anesthesia the mouse was exanguinated and lungs removed and cut into small pieces with a sterile scalpel in a Petri dish. Tissue was incubated in 1 mg/ml of type 2 bacterial collagenase (Worthington Biochemical Corp., Freehold, NJ) in DMEM at 37°C for 2 hours in a shaking water bath. The cell suspension was filtered through a 100-μm nylon filter (Falcon; BD Biosciences, Cowley, UK) and diluted at a ratio of 1:1 with fetal bovine serum (Autogen Bioclear, Calne, UK) and centrifuged (300 × g for 5 minutes) to pellet the cells. Cells were resuspended in DMEM containing 20% fetal bovine serum and recentrifuged. The final cell pellet was resuspended in DMEM containing 20% fetal bovine serum, 100 U/ml penicillin, 100 μg/ml streptomycin, and 2.5 μg/ml amphotericin and incubated in a humidified atmosphere of air containing 10% CO2 at 37°C. Cells were grown to confluence, and for experiments cells between passages 3 to 6 were trypsinized and plated at 105 cells/well in 12-well plates and grown to confluence. Once confluent, cells were incubated for a further 24 hours. The media was removed and replaced with 1 ml of preincubation media containing 0.4% (v/v) fetal bovine serum, 50 μg/ml ascorbic acid, and 0.2 mmol/L proline for 24 hours. After this time the preincubation media was replaced with 1 ml of fresh media with or without TGF-β1 (1 ng/ml) and NS398 (5 μg/ml) and incubated for a further 24 hours. The media was then removed and frozen at −80°C before being analyzed for PGE2 as described above.
Isolation of COX-2−/− and WT Macrophages/Monocytes and Measurement of PGE2 Synthesis
Peritoneal macrophages/monocytes were isolated using an established technique.39 Briefly, COX-2−/− and WT mice were injected intraperitoneally with 2 ml (4% w/v) of thioglycolate. After 5 days the animals were sacrificed by cervical dislocation and the peritoneum lavaged using 5 ml of sterile PBS. Lavage fluid was kept on ice at all times. The inflammatory cells were isolated by centrifuging the lavage fluid (300 × g) for 5 minutes at 4°C. The cells were resuspended in serum-free DMEM and seeded at 100,000 cells/well in 100 μl of DMEM into 96-well plates and incubated in a humidified atmosphere of air containing 10% CO2 at 37°C overnight to adhere. The cell layer was washed three times with DMEM to remove red blood cells and nonadherent inflammatory cells. Fresh media (150 μl) was then added to each well with or without a selective COX-1 inhibitor, piroxicam (2.5 ng/ml), or the nonselective COX inhibitor, indomethacin (1 μg/ml) and the plate incubated for 24 hours. After 24 hours the media was removed and assayed for PGE2 as previously described. Each group contained three to four mice and six wells were analyzed for each.
Statistical Analysis
Data are presented as mean ± SEM. Statistical analysis was performed using an unpaired Student’s t-test for single, or analysis of variance for multiple, group comparison. A P value of less than 0.05 was considered significant.
Results
PGE2 Synthesis after Bleomycin-Induced Lung Injury Is Predominantly via COX-2
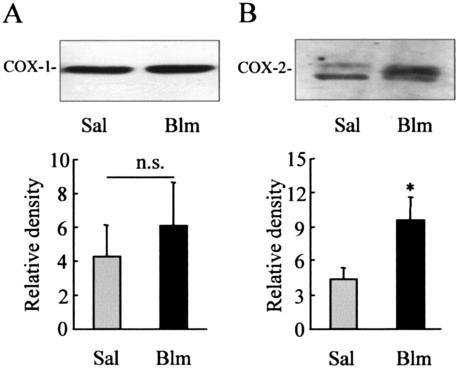
To examine the hypothesis that induction of PGE2 synthesis after intratracheal instillation of bleomycin is primarily because of an up-regulation of COX-2, Western analysis was performed on whole lung tissue at day 14 to examine COX protein expression. Both COX-1 and COX-2 protein were detectable in the lungs of WT mice after instillation of saline alone. Although expression of COX-1 did not significantly increase after instillation of bleomycin, expression of COX-2 increased 2.5-fold 14 days after injury (Figure 1).
Figure 1.
COX expression in WT mice. Bleomycin (Blm; 1 mg/kg body weight) or 0.9% saline (Sal) was administered intratracheally to WT mice. After 14 days the lungs were removed, and protein was extracted and run on sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels. Levels of COX-1 (A) and COX-2 (B) were detected using Western blotting with goat polyclonal COX-1 and rabbit polyclonal COX-2 antibodies. Band densities were quantified and represent the mean ± SEM for five to six animals per group. *, P < 0.05 compared with saline alone.
After this, WT mice were treated twice daily with the highly selective COX-2 inhibitor NS398 (3 mg/kg) after instillation of either bleomycin or vehicle. Control animals received saline and either NS398 or vehicle. PGE2 levels were quantified in lavage fluid obtained 7 and 14 days after instillation. Lavage PGE2 increased significantly after administration of 1 mg/kg of bleomycin at both days 7 and 14 compared with saline in vehicle-treated mice (Figure 2, P < 0.05). However, lavage PGE2 synthesis was almost completely inhibited by NS398 for 14 days after bleomycin instillation (Figure 2). There was no significant difference between BALF PGE2 levels in saline/vehicle-treated mice and those given saline/NS398 at any time point (Figure 2).
Figure 2.
Effect of pharmacological inhibition of COX-2 on PGE2 synthesis in WT mice. WT mice were pretreated with either the selective COX-2 inhibitor NS398 (3 mg/kg body weight) or vehicle by oral gavage for 1 hour before intratracheal administration of either 0.9% saline (gray bars) or bleomycin (1 mg/kg, black bars) and twice daily thereafter for either 7 or 14 days. The lungs were lavaged with PBS and BALF PGE2 was quantified using an EIA. Each value represents the mean ± SEM from six to eight animals per group. *, P < 0.05 compared with saline control. †, P < 0.05 compared with vehicle-treated bleomycin.
PGE2 Production after Bleomycin-Induced Lung Injury in WT and COX-2-Deficient Mice
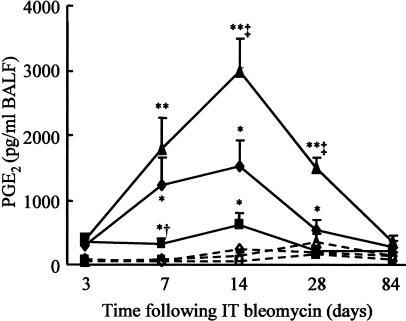
PGE2 levels were quantified in lavage fluid obtained from WT, COX-2+/−, and COX-2−/− mice at various time points up to 84 days after intratracheal instillation of saline or bleomycin. Control levels of BALF PGE2 were measurable, but low. There were no significant differences between different time points or genotypes of saline-instilled mice (Figure 3). Bleomycin administration in WT littermate mice showed a modest, but significant increase in BALF PGE2 3 days after injury, which increased up to 7 days, remaining constant until 14 days and then began to decline, although levels at 28 days were still significantly increased above saline control values (P < 0.05). In comparison, mice heterozygous for COX-2 showed a more limited induction of PGE2 synthesis after instillation of bleomycin. Levels were raised at day 3 and remained constant until day 14 however by day 28 PGE2 was not significantly increased compared with instillation of saline alone. At day 7 COX-2+/− BALF PGE2 levels were significantly lower than in WT mice (P < 0.05). In contrast, COX-2−/− mice showed a greater increase in PGE2 synthesis after bleomycin injury than WT mice. At 14 days after instillation of bleomycin, BALF PGE2 levels were elevated 20-fold compared with saline controls (P < 0.001) and were twofold greater than bleomycin-exposed WT animals (P < 0.05). Levels declined at 28 days but remained significantly higher than bleomycin-treated WT animals (P < 0.005). By 84 days levels had returned to control values in all genotypes. These data suggest a compensatory up-regulation of BALF PGE2 in homozygous COX-2-deficient mice.
Figure 3.
BALF PGE2 production in COX-2-deficient mice. Bleomycin (1 mg/kg body weight) or 0.9% saline was administered intratracheally to WT, COX-2+/−, and COX-2−/− mice. Lungs were lavaged with PBS at 3, 7, 14, 28, and 84 days after administration and BALF PGE2 was quantified using an EIA. Solid lines and filled symbols represent animals given bleomycin and dashed lines and open symbols represent animals given saline: ♦, WT; ▪, COX-2+/−; ▴, COX-2−/−. Each point represents the mean ± SEM for seven to nine animals. *, P < 0.05 compared with respective genotype saline; **, P < 0.01 compared with respective genotype saline; †, P < 0.05 compared with WT and COX-2−/− bleomycin at day 7; ‡, P < 0.05 compared with WT and COX-2+/− bleomycin at days 14 and 28.
Mice Heterozygous for COX-2 Exhibit an Enhanced Fibrotic Response to Bleomycin
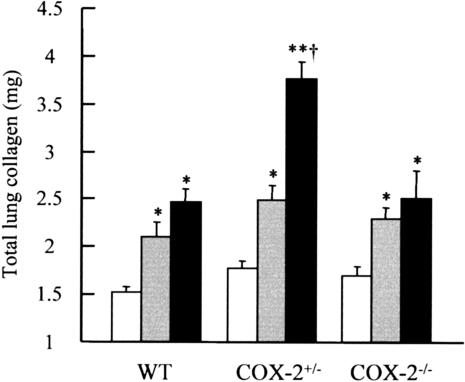
The fibrotic response to bleomycin was evaluated biochemically and histologically. Lung collagen content was determined at 14, 28, and 84 days after injury. Similar basal levels of collagen were seen in all genotypes after instillation of saline alone, and did not alter with time. Therefore data were combined across the time course for each genotype (Figure 4). After 14 days after instillation of bleomycin all three genotypes showed a significant increase in total lung collagen compared with animals given saline alone (P < 0.01 in all groups), but there was no difference between either COX-2−/− or COX-2+/− mice compared with WT animals. At day 28 after bleomycin there was a trend toward a further increase in lung collagen in WT and COX-2−/− mice compared with 14 day values but this was not statistically significant. However, 28 days after bleomycin injury total lung collagen in COX-2+/− mice was ∼50% greater than in WT and COX-2−/− mice (P < 0.001 in both cases). No further increase in lung collagen was observed at day 84 in any genotype, however the fibrotic response was maintained (WT: 2.44 ± 0.3 mg collagen/lung; COX-2+/−: 3.14 ± 0.3 mg collagen/lung; COX-2−/−: 2.56 ± 0.2 mg collagen/lung).
Figure 4.
Total lung collagen content in WT, COX-2+/−, and COX-2−/− mice. Animals were instilled intratracheally with either 0.9% saline (light gray bars) or bleomycin (1 mg/kg body weight) and lungs removed at either 14 (dark gray bars) or 28 (black bars) days after injury. Each value represents the mean ± SEM of 9 to 13 animals for bleomycin-treated groups and 20 to 25 animals for pooled control groups. *, P < 0.01; **, P < 0.001 compared with genotype saline; †, P < 0.001 compared with WT and COX-2−/− bleomycin.
Figure 5 shows Martius Scarlet Blue staining for collagen and other matrix proteins in representative sections obtained 28 days after instillation of either saline (Figure 5; A to C) or bleomycin (Figure 5; D to F). Intratracheal administration of saline did not affect the lung, with matrix staining limited to airways, blood vessels, and normal alveolar interstitium. There were no apparent differences between genotypes. In contrast, in support of the biochemical data for lung collagen content, Figure 5, D to F, shows that COX-2+/− mice develop a more severe fibrotic response to bleomycin injury than either WT or COX-2−/− mice. Both WT and COX-2−/− mice show a patchy loss of alveolar architecture and destruction of the interstitium, accompanied by increased matrix deposition in areas where alveolar architecture was disrupted. A widespread and increased inflammatory response was observed in COX-2−/− mice. Bleomycin injury in COX-2+/− mice resulted in widespread destruction of lung architecture, which appeared more extensive and severe than that seen in WT or COX-2−/− mice. Affected areas of the lung were associated with intense staining for matrix proteins and increased cellularity. Semiquantitative scoring showed no difference between the genotypes in lungs of animals after intratracheal saline. Mean scores were: WT saline, 0.56 ± 0.20 (n = 5); COX-2+/− saline, 0.18 ± 0.14 (n = 6); COX-2−/− saline, 0.64 ± 0.17 (n = 5). Mean scores for bleomycin-treated mice were all significantly greater than those of their respective genotype control: WT bleomycin, 1.99 ± 0.14 (n = 7, P < 0.001); COX-2+/− bleomycin, 2.20 ± 0.16 (n = 8, P < 0.001); COX-2−/− bleomycin, 1.97 ± 0.67 (n = 3, P < 0.05). The histological score for COX-2+/− mice treated with bleomycin tended to be higher than those for WT and COX-2−/− mice although the difference was not statistically significant.
Figure 5.
Effect of bleomycin on WT and COX-2-deficient mouse lung. Lung sections were stained with modified Martius Scarlet Blue 28 days after intratracheal instillation of either 0.9% saline (A–C) or 1 mg/kg bleomycin (D–F). Representative sections of WT (A, D), COX-2+/− (B, E), and COX-2−/− (C, F) mice are shown. In saline-instilled lungs normal architecture was preserved with no obvious differences between genotypes (A–C). After bleomycin WT (D) and COX-2−/− (F) lungs showed similar patchy fibrotic lesions within increased deposition of blue-staining extracellular matrix proteins. In COX-2+/− mice (E) the fibrotic lesions were more widespread with intense blue staining of the extracellular matrix. Scale bar, 5 μm.
Effect of COX-2 Genotype on Inflammatory Response to Bleomycin-Induced Lung Injury
Total bronchoalveolar lavage cell numbers from animals instilled with saline were not significantly different between genotypes or at any time point (Table 1). Three days after bleomycin instillation, BAL cell numbers tended to be lower than for animals instilled with saline but these differences were not statistically significant. Between 7 and 28 days after bleomycin BAL cell numbers tended to be increased by 30 to 200% although because of variability between animals these differences were not always significant (Table 1). There was no clear difference between genotypes, although the highest cell number was observed in COX-2−/− animals 28 days after bleomycin, with a value three times that of saline-treated animals. By 84 days after bleomycin total lavage cell numbers were returning toward basal levels.
Table 1.
Total BALF Cell Numbers (×10−3) in WT, COX-2+/−, and COX-2−/− Mice
| Genotype | Treatment | Time after instillation (days)
|
||||
|---|---|---|---|---|---|---|
| 3 | 7 | 14 | 28 | 84 | ||
| WT | Saline | 238.5 ± 34.0 | 346.0 ± 38.5 | 177.9 ± 27.7 | 207.7 ± 21.6 | 184.7 ± 18.1 |
| WT | Bleomycin | 195.0 ± 31.6 | 459.3 ± 59.2 | 430.4 ± 50.9* | 415.5 ± 46.5* | 245.7 ± 28.8 |
| COX-2+/− | Saline | 310.6 ± 35.2 | 277.4 ± 27.0 | 230.0 ± 40.9 | 258.9 ± 71.6 | 153.9 ± 12.0 |
| COX-2+/− | Bleomycin | 229.7 ± 43.2 | 407.7 ± 53.0* | 428.3 ± 40.1* | 520.5 ± 68.1* | 277.3 ± 18.0* |
| COX-2−/− | Saline | 254.5 ± 32.9 | 361.4 ± 63.7 | 207.3 ± 33.1 | 174.2 ± 31.6 | 205.5 ± 41.7 |
| COX-2−/− | Bleomycin | 231.9 ± 20.3 | 493.2 ± 52.5 | 319.9 ± 51.7 | 534.0 ± 84.4* | 243.4 ± 18.3 |
Animals were instilled intratracheally with either 0.9% saline or bleomycin (1 mg/kg body weight) and lungs were lavaged using PBS at 3, 7, 14, 28, and 84 days after injury. Each value represents the mean ± SEM of 6 to 10 animals.
P < 0.05 compared with relative genotype saline control.
BAL cell profiles were analyzed at 3, 7, 14, 28, and 84 days after injury. In each genotype, cell profiles did not alter significantly between time points in response to saline alone and were therefore combined. In WT animals macrophage numbers significantly increased from 7 days after bleomycin and remained significantly greater than saline alone at days 14 and 28 after injury (P < 0.001 at each time point, Figure 6A). The increase in macrophage numbers was delayed in COX-2+/− and COX-2−/− mice with increases observed only at 14 and 28 days after injury, however by 84 days after bleomycin macrophage numbers were no longer increased compared with saline alone in any genotype.
Figure 6.
BALF macrophage (A), PMN (B), and lymphocyte (C) numbers in WT (light gray bars), COX-2+/− (dark gray bars), and COX-2−/− (black bars) mice. Animals were instilled intratracheally with either 0.9% saline or bleomycin (1 mg/kg body weight) and lungs were lavaged with PBS at 3, 7, 14, 28, and 84 days after injury. Saline values were unaltered throughout the time course and therefore combined. Each value represents the mean ± SEM of 6 to 10 animals for bleomycin-treated groups and 34 to 40 animals for pooled control groups. *, P < 0.05; **, P < 0.001 compared with relative genotype saline control; †, P < 0.05 compared with WT Blm at day 84; ‡, P < 0.05 compared with WT and COX-2+/− Blm at day 84.
In contrast, numbers of PMNs increased significantly by day 3 after bleomycin in all three genotypes, and remained significantly elevated compared with instillation of saline alone up to day 14 (Figure 6B). In all three genotypes, PMN numbers peaked at day 7. After this time numbers decreased in both WT and COX-2+/− mice and by day 28 were no longer significantly increased after bleomycin in WT animals. However, after bleomycin PMN numbers remained significantly increased in COX-2−/− mice. At day 28 PMN numbers were significantly greater than both WT and COX-2+/− animals (Figure 6B, P < 0.05) and remained significantly greater than WT mice 84 days after injury, P < 0.05.
Lymphocyte numbers followed a similar pattern (Figure 6C). In all three genotypes there was a sharp rise in cell numbers peaking at day 7 (P < 0.001 for all genotypes) with a trend toward increased numbers of lymphocytes in COX-2−/− mice compared with both WT and COX-2+/− animals. Lymphocyte numbers 14 days after instillation of bleomycin remained significantly increased compared with saline alone in all three genotypes (P < 0.001 in all cases). By day 28 after bleomycin, lymphocyte numbers had returned to baseline in WT mice, but remained significantly increased in both groups of COX-2-deficient animals. At this time point, lymphocyte numbers were significantly greater in COX-2−/− mice compared with both WT and COX-2+/− mice (P < 0.05 in both cases). This difference was maintained at 84 days after administration of bleomycin when COX-2−/− mice still showed significantly elevated levels of BALF lymphocytes compared with WT and COX-2+/− animals, at similar levels to day 28 (P < 0.05 in both cases).
COX-2 Deficiency Does Not Affect BALF LTC4 Production
A possible explanation for both the enhanced fibrotic or inflammatory response to bleomycin-injury observed in COX-2+/− and COX-2−/− mice, respectively, could be increased production of leukotrienes (LTs). LTs are alternative metabolites of arachidonic acid derived from the 5-lipoxygenase (5-LO) pathway and are known to be both proinflammatory and profibrotic.40 In the absence of COX-2, or potentially after its reduction, arachidonic acid metabolism may be shunted through the 5-LO pathway to produce increased levels of LTs. Levels of cysteinyl-LTs, particularly LTC4, have been shown to be the predominant murine lung LT product in lavage fluid, lung homogenate, and alveolar macrophages.36,40,41 Therefore levels of BALF LTC4 were measured in WT, COX-2+/−, and COX-2−/− mice 7 days after instillation of either saline or bleomycin. LTC4 was measurable in all three genotypes but was not significantly increased after bleomycin (data not shown) suggesting that in this model, COX-2 deficiency does not lead to shunting through the LT pathway.
Expression of TGF-β1 in WT and COX-2+/− Mice
Our data suggests that the decreased capacity of COX-2+/− mice to up-regulate PGE2 synthesis in response to bleomycin injury is directly linked to the enhanced fibrotic response observed in these animals. However, indirect up-regulation of profibrotic mediators may also contribute to the enhanced fibrotic response. For this reason, we compared the expression of the potent profibrotic mediator TGF-β1 in the lungs of WT and COX-2+/− mice 28 days after instillation of bleomycin. Immunohistochemical staining for total TGF-β1 was localized predominantly to bronchial epithelial cells, macrophages, and the extracellular matrix (Figure 7). However, there was no apparent difference in the localization or staining intensity between WT and COX-2+/− mice. Similarly, there was no significant difference in total lavage TGF-β1 levels between WT and COX-2+/− mice (WT, 111 ± 69 pg/ml; COX-2+/−, 155 ± 41 pg/ml).
Figure 7.
Immunohistochemical staining of TGF-β1 in the lungs of WT (A) and COX-2+/− (B) mice 28 days after intratracheal instillation of bleomycin. Intense staining for TGF-β1 was localized to the bronchial epithelium and macrophages with more diffuse staining of the extracellular matrix. There were no apparent differences between WT and COX-2-deficient mice. Scale bar, 2 μm.
Mechanism of Compensatory PGE2 Synthesis in COX-2−/− Mice
To begin to elucidate the mechanism by which COX-2−/− mice increase BALF PGE2 synthesis after bleomycin, Western analyses for cPLA2 and COX-1 were performed on lung tissue from WT and COX-2−/− mice. cPLA2, the rate limiting enzyme in the release of arachidonic acid, was increased ∼50% in both WT and COX-2−/− bleomycin-exposed mice compared with their respective saline controls (Figure 8A). This suggests that increased substrate availability does not explain the increased synthesis of PGE2 seen in COX-2−/− mice. However, after bleomycin the expression of COX-1 protein increased 4.6-fold in COX-2−/− mice compared with only a 1.5-fold increase in WT animals (Figure 8B). There was no significant difference in baseline values of either cPLA2 or COX-1 between mice of either genotype. As shown in Figure 1, COX-2 expression increased 2.5-fold in WT mice after bleomycin injury, and as expected, COX-2 was absent in COX-2−/− mice (data not shown).
Figure 8.
cPLA2 and COX-1 expression in the lungs of WT and COX-2−/− mice. Bleomycin (Blm; 1 mg/kg body weight) or 0.9% saline (Sal) was administered intratracheally. After 14 days the lungs were removed and the protein extracted and run on sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels. Levels of cPLA2 (A) and COX-1 (B) were detected using Western blotting with mouse monoclonal cPLA2 and goat polyclonal COX-1 antibodies. Band densities were quantified and represent the mean ± SEM of the fold increase after bleomycin administration of five to six animals. **, P < 0.01 compared with WT.
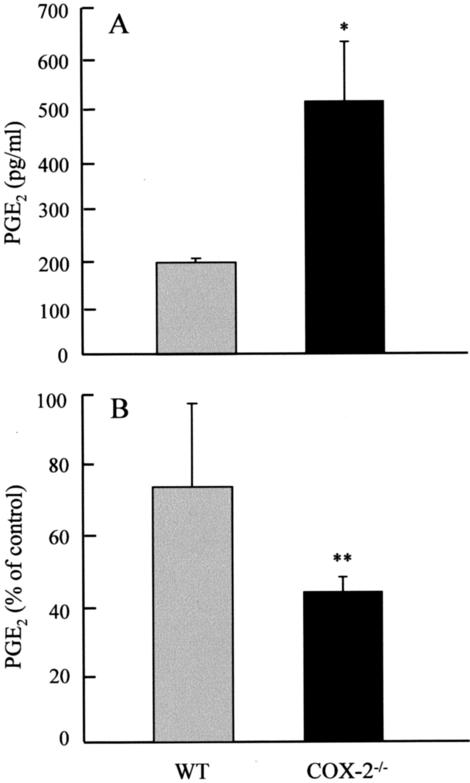
To further investigate the possible cellular source of PGE2 in COX-2−/− mice, pulmonary fibroblasts and peritoneal macrophages/monocytes were isolated from WT and COX-2−/− mice. PGE2 levels were measured in cell-conditioned media taken from both cell types. Figure 9 shows that WT fibroblasts produced PGE2 that was further stimulated by the addition of TGF-β1 for 24 hours (P < 0.05). Addition of the selective COX-2 inhibitor NS398 reduced basal PGE2 production by 80% and TGF-β1-stimulated PGE2 production by 90% (P < 0.001 in both cases). This suggests PGE2 production in these cells is mediated predominantly via COX-2. In comparison, COX-2−/− fibroblasts synthesized PGE2 at or below the limit of detection (20 pg/ml) indicating a minimal basal production of PGE2 via constitutive COX-1 only, which is not inducible by TGF-β1, and is unchanged in the presence of NS398. In contrast, Figure 10A shows PGE2 production in COX-2−/− macrophages/monocytes is ∼2.5-fold greater than WT cells, demonstrating a capability to generate high levels of prostanoids via an alternative COX isoform, even in the absence of COX-2. Figure 10B shows that piroxicam, a selective COX-1 inhibitor, tended to reduce WT macrophage/monocyte PGE2 production by ∼25% but this was not statistically significant. In contrast piroxicam inhibited COX-2−/− macrophage/monocyte production by ∼55% (P < 0.001). Indomethacin, a nonselective COX inhibitor, completely inhibited PGE2 production by both macrophage genotypes. Because piroxicam was used at a concentration 750 times lower than the reported IC50 for COX-2 in macrophages,42 these data suggest that PGE2 production by WT macrophages/monocytes under these conditions is mediated predominantly via COX-2, whereas in COX-2−/− macrophages/monocytes compensatory up-regulation of COX-1 plays a significant role in PGE2 production.
Figure 9.
PGE2 production by WT (gray bars) and COX-2−/− (black bars) mouse lung fibroblasts. Fibroblasts were cultured with either 1 ng/ml of TGF-β1 or the selective COX-2 inhibitor NS398 (5 μg/ml) for 24 hours. PGE2 was measured in cell-conditioned media. Each bar represents the mean ± SEM for six replicate cultures. *, P < 0.05 compared with WT control cells; †, P < 0.05 compared with COX-2−/− TGF-β1-treated cells; ‡, P < 0.001 compared with WT cells. Data are representative of three separate experiments.
Figure 10.
PGE2 production in WT and COX-2−/− macrophages/monocytes. Macrophages/monocytes were isolated 5 days after peritoneal injection with the inflammatory stimulant thioglycolate. A: Cells were cultured in serum-free conditions for 24 hours and PGE2 measured in the cell-conditioned media. B: The effect of piroxicam (2.5 ng/ml) on WT and COX-2−/− macrophage/monocyte PGE2 production. Each bar represents the mean ± SEM for at least six replicate wells of three to four separate animals per group. *, P < 0.05 compared with WT control cells; **, P < 0.001 compared with media control.
Discussion
Initial experiments using WT mice demonstrated that intratracheal instillation of bleomycin results in a marked increase in protein expression of COX-2, but only a minimal increase in COX-1. Subsequently, BAL fluid PGE2 levels were significantly elevated over saline alone for at least 28 days after bleomycin. Administration of a COX-2 inhibitor almost completely inhibited BALF PGE2 for at least 14 days demonstrating that PGE2 synthesis in WT mice after bleomycin administration is predominantly COX-2-derived.
Response of COX-2+/− Mice to Bleomycin-Induced Lung Injury
Heterozygous COX-2-deficient mice show reduced synthesis of PGE2 after bleomycin injury compared with WT mice, developing a more severe and extensive fibrosis histologically and biochemically. These data are consistent with data from patients with pulmonary fibrosis in which there is limited induction of COX-2, and levels of PGE2 in BALF are lower than in nonfibrotic controls.7,21,22 This suggests that in this respect COX-2+/− mice provide a good model of pulmonary fibrosis.
This enhanced fibrotic response in COX-2+/− mice appears to be directly linked to the reduction in PGE2 levels. LTC4, the predominant murine leukotriene was not increased in COX-2+/− mice suggesting that COX-2 deficiency did not induce shunting of arachidonic acid through the profibrotic leukotriene pathway. In addition, immunohistochemical staining and BALF levels of TGF-β1 were similar in COX-2+/− and WT mice suggesting that an indirect up-regulation of TGF-β1 expression was not responsible for the greater fibrotic response in COX-2+/− mice. Furthermore, the enhanced fibrotic response seen in COX-2+/− mice is not because of an increased inflammatory response to bleomycin injury because a similar inflammatory response is seen compared with WT mice. This supports recent suggestions that development of inflammation and fibrosis are not interdependent.43,44
COX-2-deficient mice will also have a reduced ability to produce other prostanoid products. These include prostacyclin, PGF2α, and thromboxane A2 that have previously been shown to be up-regulated after bleomycin injury.45,46 Thromboxane A2 and PGF2α are weak stimulants of fibroblast proliferation and collagen production, whereas prostacyclin is a weak inhibitor of proliferation and collagen synthesis. Reduced levels of these mediators after lung injury could potentially modulate the fibrotic response. However, these prostanoid mediators have relatively weak and opposing effects on fibroblast function, and are present in the lung at much lower concentrations than PGE2.11 They are therefore unlikely to play a major role in the outcome of these studies.
Reduced levels of PGE2 have previously been linked to a more profibrotic phenotype. Moore and colleagues36 reported that GM-CSF−/− mice show enhanced fibrosis after bleomycin that correlated with reduced levels of PGE2 in both alveolar macrophages and whole lung homogenates. In addition, this study showed that administration of the COX inhibitor indomethacin to WT animals enhanced the fibrotic response to bleomycin. Conversely, leukotriene-deficient mice are protected from bleomycin-induced lung fibrosis40 at least in part, because of increased levels of PGE2 produced via shunting through the COX pathway. Together these data suggests that limited induction of PGE2 is an important mechanism in the progression of fibrotic disease.
Response of COX-2−/− Mice to Bleomycin-Induced Lung Injury
In contrast to COX-2+/− mice, BALF PGE2 levels were increased in COX-2−/− mice after bleomycin injury compared with bleomycin-exposed WT and COX-2+/− animals and the fibrotic response was similar to that of WT mice. The data presented here suggests that increased levels of PGE2 in bleomycin-exposed COX-2−/− mice are at least partly because of compensatory up-regulation of COX-1 by macrophages.
Other studies investigating the effect of lung injury on COX-2−/− mice have not shown increased levels of PGE2. Studies by both Bonner and colleagues47 and Zeldin and colleagues48 showed reduced expression of PGE2 compared with WT animals after exposure to V2O5 and lipopolysaccharide, respectively, and Gavett and colleagues,49 reported unchanged levels of PGE2 synthesis in COX-2−/− mice using a model of ovalbumin-induced asthma. The reasons for these differences are uncertain, however instillation of bleomycin appears to cause a more severe injury than ovalbumin challenge, lipopolysaccharide, or V2O5. Indeed, BALF PGE2 levels in WT mice 28 days after bleomycin were higher than those measured at early time points after lipopolysaccharide and ovalbumin challenge, and are comparable with PGE2 levels 3 to 15 days after instillation of V2O5. Therefore previous studies may not have reached an injury threshold whereby compensatory mechanisms are induced in these animals.
Compensatory synthesis of PGE2 in the absence of COX-2 has been previously reported in vitro. Kirtikara and co-workers50 described increased basal and interleukin-1β-stimulated release of PGE2 in COX-2−/− lung fibroblasts compared with WT cells. We observed no basal increase in PGE2 production either in vivo after instillation of saline, or in vitro in COX-2−/− fibroblasts. The studies of Kirtikara and colleagues50 suggest the compensatory basal synthesis of PGE2 was because of increased levels of COX-1 and cPLA2. However, in COX-2−/− lung tissue we find no increase in either cPLA2 or COX-1 production basally, and increased COX-1 expression alone compared with WT mice after bleomycin injury. The differences may be because of the nature of the experiments involved, our experiments were conducted in vivo and using primary cells, whereas the data described by Kirtikara and colleagues50 were generated with transformed cells. To begin to determine the source of compensatory PGE2 synthesis we studied isolated fibroblasts and macrophages. Both cell types are known to generate substantial amounts PGE2 in the lung and are present in increased numbers in pulmonary fibrosis. Treatment with TGF-β1 significantly stimulated PGE2 production in WT fibroblasts, and the selective COX-2 inhibitor NS398 inhibited both basal and TGF-β1-induced PGE2 synthesis suggesting that WT fibroblasts synthesize PGE2 predominantly via COX-2. However, as described above, but contradictory to work described by Kirtikara and colleagues,50 COX-2−/− fibroblasts produced significantly less PGE2 basally than WT cells, showed no induction of PGE2 synthesis after stimulation by TGF-β1, and PGE2 synthesis was unaffected by NS398. This corresponds with data generated by Dinchuk and co-workers34 who showed that COX-2−/− primary embryonic fibroblasts produced at least 10-fold less PGE2 than WT cells after stimulation with serum.
In comparison with fibroblasts, stimulated COX-2-deficient peritoneal macrophages/monocytes showed significantly increased production of PGE2 compared with WT cells. PGE2 production by COX-2−/− macrophages was inhibited by piroxicam when used at a concentration that is highly selective for COX-1. In addition, a recent study has shown compensatory expression of COX-1 mRNA in peritoneal macrophages from COX-2-deficient mice.51 Furthermore, it has recently been shown that COX-2-deficient alveolar epithelial cells are incapable of synthesizing PGE2.52 Together these in vitro data suggest that the increased synthesis of PGE2 seen in COX-2−/− mice is likely to be via macrophage/monocytes, but not fibroblasts or type II epithelial cells. Furthermore, our data in whole lung tissue and in macrophages/monocytes suggests that this is because of a compensatory expression of COX-1 in these cells.
Because PGE2 levels were higher in COX-2−/− than WT mice exposed to bleomycin, it might have been expected that these mice would be protected from fibrosis. However, deposition of collagen was similar in COX-2−/− and WT mice assessed biochemically and histologically. The reason for this is unclear. It may be that the additional increase in PGE2 in COX-2−/− mice is insufficient to limit the fibrotic response although this seems an unlikely explanation given that PGE2 levels were twofold to threefold greater in COX-2−/− mice at 14 and 28 days after bleomycin. The response of COX-2−/− mice to lung injury is complex and the failure of the increased levels of PGE2 to limit fibrosis may, at least partly, relate to the persistent neutrophilia and lymphocytosis described in these animals.
The mechanism for the more severe and persistent lymphocytosis and neutrophilia seen in COX-2−/− mice is uncertain. Levels of BALF LTC4 were low therefore it is unlikely that increased production of proinflammatory LTs is responsible and this suggests the enhanced inflammatory response to be a result of the increased levels of proinflammatory PGE2. Therefore the enhanced inflammation may not be a direct consequence of an absence of COX-2 either promoting or limiting resolution of inflammation, but the compensatory expression of COX-1 resulting in high levels of PGE2. Despite this suggestion, previous studies suggest that COX-2 may have prostaglandin-independent effects that contribute to the inflammatory response. For example, Bonner and co-workers47 described increased lung inflammation after V2O5 instillation in the presence of low levels of PGE2 in COX-2−/− mice. Other studies demonstrating evidence of enhanced inflammation in COX-2−/− mice include: enhanced pulmonary eosinophilia and lymphocytosis after ovalbumin sensitization and challenge;49 persistent chronic inflammation in a carrageenan-induced paw injury;53 and enhanced colonic inflammation in dextran sodium sulfate-induced bowel injury.54 However, the inflammatory response of COX-2−/− mice to different models of injury has not been consistent. Two studies have demonstrated no difference at early time points after injury,34,48 and a further two studies have demonstrated reduced inflammation, in collagen-induced arthritis,55 and in a sepsis model.56 This inconsistency in the inflammatory response to injury may relate to the varying insults, the time point assessed, and the degree to which compensatory up-regulation of COX-1 and PGE2 occurs.
In summary, we have demonstrated that in WT animals, up-regulation of PGE2 synthesis in response to lung injury is mediated predominantly via induction of COX-2. Using mice deficient in one or more allele of COX-2 we have shown that reduced expression, rather than an absence of COX-2 in heterozygous mice, results in limited induction of PGE2 synthesis and subsequently an enhanced fibrotic response to bleomycin. This phenotype models that of patients with pulmonary fibrosis who show reduced PGE2 biosynthesis21 and a reduced capacity to up-regulate COX-2.7,22 In the absence of a functioning COX-2 gene, COX-2−/− mice are still capable of up-regulating PGE2 synthesis and exhibit a similar fibrotic response to that observed in WT mice. We have shown this to be mediated, at least in part, via up-regulation of COX-1. This response is cell-type-specific with macrophage/monocyte populations being the predominant source of PGE2 synthesis and we suggest this is via increased COX-1 in these cells. However, COX-2−/− mice showed differences in their inflammatory response with a severe and persistent lymphocytosis and neutrophilia up to 3 months after the instillation of bleomycin, which suggests a prostaglandin-independent role for COX-2 in the resolution of inflammation. Together, these data confirm the importance of up-regulating COX-2 and PGE2 in the protection against fibrosis after lung injury. In addition, they provide further support for the theory that fibrosis is not dependent on a sustained inflammatory response because, despite the development of persistent inflammation in COX-2−/− mice in response to bleomycin, these animals demonstrated similar levels of collagen deposition to WT mice, in which inflammation resolved within 28 days. Further studies to determine the molecular mechanisms of COX-2 insufficiency may identify novel, highly specific, therapeutic targets for the treatment of patients with idiopathic pulmonary fibrosis.
Footnotes
Address reprint requests to Dr. Robin J. McAnulty, Centre for Respiratory Research, University College London, Rayne Bldg., 5 University St., London WC1E 6JJ, UK. E-mail: r.mcanulty@ucl.ac.uk.
Supported by The Wellcome Trust (grant no. 071124), Aventis Pharmaceuticals Inc., the Arthritis Research Campaign (grant no. JO520), and the British Lung Foundation (grant nos. P01/04 and P03/12).
R.J.H. and R.G.J. contributed equally to this work.
References
- Gross TJ, Hunninghake GW. Idiopathic pulmonary fibrosis. N Engl J Med. 2001;345:517–525. doi: 10.1056/NEJMra003200. [DOI] [PubMed] [Google Scholar]
- McAnulty RJ, Laurent GJ. Collagen and its regulation in pulmonary fibrosis. Phan SH, Thrall RJ, editors. New York: Marcel Dekker,; 1995:pp 135–171. [Google Scholar]
- Coker RK, Laurent GJ. Pulmonary fibrosis: cytokines in the balance. Eur Respir J. 1998;11:1218–1221. doi: 10.1183/09031936.98.11061218. [DOI] [PubMed] [Google Scholar]
- Allen JT, Spiteri MA. Growth factors in idiopathic pulmonary fibrosis: relative roles. Respir Res. 2002;3:13–22. doi: 10.1186/rr162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAnulty RJ, Chambers RC, Laurent GJ. Regulation of fibroblast procollagen production: transforming growth factor-beta 1 induces prostaglandin E2 but not procollagen synthesis via a pertussis toxin-sensitive G-protein. Biochem J. 1995;307:63–68. doi: 10.1042/bj3070063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAnulty RJ, Hernandez-Rodriguez NA, Mutsaers SE, Coker RK, Laurent GJ. Indomethacin suppresses the anti-proliferative effects of transforming growth factor-beta isoforms on fibroblast cell cultures. Biochem J. 1997;321:639–643. doi: 10.1042/bj3210639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keerthisingam CB, Jenkins RG, Harrison NK, Hernandez-Rodriguez NA, Booth H, Laurent GJ, Hart SL, Foster ML, McAnulty RJ. Cyclooxygenase-2 deficiency results in a loss of the anti-proliferative response to transforming growth factor-beta in human fibrotic lung fibroblasts and promotes bleomycin-induced pulmonary fibrosis in mice. Am J Pathol. 2001;158:1411–1422. doi: 10.1016/s0002-9440(10)64092-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez SA, Freundlich B, Rosenbloom J. Selective inhibition of human diploid fibroblast collagen synthesis by interferons. J Clin Invest. 1984;74:1112–1116. doi: 10.1172/JCI111480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurujeyalakshmi G, Giri SN. Molecular mechanisms of antifibrotic effect of interferon gamma in bleomycin-mouse model of lung fibrosis: downregulation of TGF-beta and procollagen I and III gene expression. Exp Lung Res. 1995;21:791–808. doi: 10.3109/01902149509050842. [DOI] [PubMed] [Google Scholar]
- Korn JH. Fibroblast prostaglandin E2 synthesis. Persistence of an abnormal phenotype after short-term exposure to mononuclear cell products. J Clin Invest. 1983;71:1240–1246. doi: 10.1172/JCI110873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki T, Rennard SI, Crystal RG. Cyclooxygenase metabolites are compartmentalized in the human lower respiratory tract. J Appl Physiol. 1987;62:219–222. doi: 10.1152/jappl.1987.62.1.219. [DOI] [PubMed] [Google Scholar]
- Brock TG, McNish RW, Peters-Golden M. Arachidonic acid is preferentially metabolized by cyclooxygenase-2 to prostacyclin and prostaglandin E2. J Biol Chem. 1999;274:11660–11666. doi: 10.1074/jbc.274.17.11660. [DOI] [PubMed] [Google Scholar]
- Bitterman PB, Wewers MD, Rennard SI, Adelberg S, Crystal RG. Modulation of alveolar macrophage-driven fibroblast proliferation by alternative macrophage mediators. J Clin Invest. 1986;77:700–708. doi: 10.1172/JCI112364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RH, Polgar P. The effect and interaction of bradykinin and prostaglandins on protein and collagen production by lung fibroblasts. J Biol Chem. 1982;257:8630–8633. [PubMed] [Google Scholar]
- Saltzman LE, Moss J, Berg RA, Hom B, Crystal RG. Modulation of collagen production by fibroblasts. Effects of chronic exposure to agonists that increase intracellular cyclic AMP. Biochem J. 1982;204:25–30. doi: 10.1042/bj2040025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohyama T, Ertl RF, Valenti V, Spurzem J, Kawamoto M, Nakamura Y, Veys T, Allegra L, Romberger D, Rennard SI. Prostaglandin E2 inhibits fibroblast chemotaxis. Am J Physiol. 2001;281:L1257–L1263. doi: 10.1152/ajplung.2001.281.5.L1257. [DOI] [PubMed] [Google Scholar]
- Kolodsick JE, Peters-Golden M, Larios J, Toews GB, Thannickal VJ, Moore BB. PGE2 inhibits fibroblast to myofibroblast transition via EP2 signaling and cAMP elevation. Am J Respir Cell Mol Biol. 2003;29:537–544. doi: 10.1165/rcmb.2002-0243OC. [DOI] [PubMed] [Google Scholar]
- Yu J, Prado GN, Schreiber B, Polgar P, Polgar P, Taylor L. Role of prostaglandin E2 EP receptors and cAMP in the expression of connective tissue growth factor. Arch Biochem Biophys. 2002;404:302–308. doi: 10.1016/s0003-9861(02)00276-x. [DOI] [PubMed] [Google Scholar]
- Baum BJ, Moss J, Breul SD, Berg RA, Crystal RG. Effect of cyclic AMP on the intracellular degradation of newly synthesized collagen. J Biol Chem. 1980;255:2843–2847. [PubMed] [Google Scholar]
- Brilla CG, Zhou G, Rupp H, Maisch B, Weber KT. Role of angiotensin II and prostaglandin E2 in regulating cardiac fibroblast collagen turnover. Am J Cardiol. 1995;76:8D–13D. doi: 10.1016/s0002-9149(99)80485-8. [DOI] [PubMed] [Google Scholar]
- Borok Z, Gillissen A, Buhl R, Hoyt RF, Hubbard RC, Ozaki T, Rennard SI, Crystal RG. Augmentation of functional prostaglandin E levels on the respiratory epithelial surface by aerosol administration of prostaglandin E. Am Rev Respir Dis. 1991;144:1080–1084. doi: 10.1164/ajrccm/144.5.1080. [DOI] [PubMed] [Google Scholar]
- Wilborn J, Crofford LJ, Burdick MD, Kunkel SL, Strieter RM, Peters-Golden M. Cultured lung fibroblasts isolated from patients with idiopathic pulmonary fibrosis have a diminished capacity to synthesize prostaglandin E2 and to express cyclooxygenase-2. J Clin Invest. 1995;95:1861–1868. doi: 10.1172/JCI117866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vancheri C, Sortino MA, Tomaselli V, Mastruzzo C, Condorelli F, Bellistri G, Pistorio MP, Canonico PL, Crimi N. Different expression of TNF-alpha receptors and prostaglandin E2 production in normal and fibrotic lung fibroblasts: potential implications for the evolution of the inflammatory process. Am J Respir Cell Mol Biol. 2000;22:628–634. doi: 10.1165/ajrcmb.22.5.3948. [DOI] [PubMed] [Google Scholar]
- Cruz-Gervis R, Stecenko AA, Dworski R, Lane KB, Loyd JE, Pierson R, King G, Brigham KL. Altered prostanoid production by fibroblasts cultured from the lungs of human subjects with idiopathic pulmonary fibrosis. Respir Res. 2002;3:17–22. doi: 10.1186/rr166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchand-Adam S, Marchal J, Cohen M, Soler P, Gerard B, Castier Y, Leseche G, Valeyre D, Mal H, Aubier M, Dehoux M, Crestani B. Defect of hepatocyte growth factor secretion by fibroblasts in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2003;168:1156–1161. doi: 10.1164/rccm.200212-1514OC. [DOI] [PubMed] [Google Scholar]
- Smith WL, Dewitt DL. Prostaglandin endoperoxide H synthases-1 and -2. Adv Immunol. 1996;62:167–215. doi: 10.1016/s0065-2776(08)60430-7. [DOI] [PubMed] [Google Scholar]
- Smith WL, Garavito RM, DeWitt DL. Prostaglandin endoperoxide H synthases (cyclooxygenases)-1 and -2. J Biol Chem. 1996;271:33157–33160. doi: 10.1074/jbc.271.52.33157. [DOI] [PubMed] [Google Scholar]
- Herschman HR. Prostaglandin synthase 2. Biochim Biophys Acta. 1996;1299:125–140. doi: 10.1016/0005-2760(95)00194-8. [DOI] [PubMed] [Google Scholar]
- Hinz B, Brune K. Cyclooxygenase-2: 10 years later. J Pharmacol Exp Ther. 2002;300:367–375. doi: 10.1124/jpet.300.2.367. [DOI] [PubMed] [Google Scholar]
- Dubois RN, Abramson SB, Crofford L, Gupta RA, Simon LS, Van De Putte LB, Lipsky PE. Cyclooxygenase in biology and disease. FASEB J. 1998;12:1063–1073. [PubMed] [Google Scholar]
- O’Neill GP, Ford-Hutchinson AW. Expression of mRNA for cyclooxygenase-1 and cyclooxygenase-2 in human tissues. FEBS Lett. 1993;330:156–160. doi: 10.1016/0014-5793(93)80263-t. [DOI] [PubMed] [Google Scholar]
- Asano K, Lilly CM, Drazen JM. Prostaglandin G/H synthase-2 is the constitutive and dominant isoform in cultured human lung epithelial cells. Am J Physiol. 1996;271:L126–L131. doi: 10.1152/ajplung.1996.271.1.L126. [DOI] [PubMed] [Google Scholar]
- Chandrasekharan NV, Dai H, Roos KL, Evanson NK, Tomsik J, Elton TS, Simmons DL. COX-3, a cyclooxygenase-1 variant inhibited by acetaminophen and other analgesic/antipyretic drugs: cloning, structure, and expression. Proc Natl Acad Sci USA. 2002;99:13926–13931. doi: 10.1073/pnas.162468699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinchuk JE, Car BD, Focht RJ, Johnston JJ, Jaffee BD, Covington MB, Contel NR, Eng VM, Collins RJ, Czerniak PM, Gorry SA, Trzaskos JM. Renal abnormalities and an altered inflammatory response in mice lacking cyclooxygenase II. Nature. 1995;378:406–409. doi: 10.1038/378406a0. [DOI] [PubMed] [Google Scholar]
- Gilroy DW, Tomlinson A, Willoughby DA. Differential effects of inhibition of isoforms of cyclooxygenase (COX-1, COX-2) in chronic inflammation. Inflamm Res. 1998;47:79–85. doi: 10.1007/s000110050285. [DOI] [PubMed] [Google Scholar]
- Moore BB, Coffey MJ, Christensen P, Sitterding S, Ngan R, Wilke CA, McDonald R, Phare SM, Peters-Golden M, Paine R, III, Toews GB. GM-CSF regulates bleomycin-induced pulmonary fibrosis via a prostaglandin-dependent mechanism. J Immunol. 2000;165:4032–4039. doi: 10.4049/jimmunol.165.7.4032. [DOI] [PubMed] [Google Scholar]
- Mutsaers SE, Foster ML, Chambers RC, Laurent GJ, McAnulty RJ. Increased endothelin-1 and its localization during the development of bleomycin-induced pulmonary fibrosis in rats. Am J Respir Cell Mol Biol. 1998;18:611–619. doi: 10.1165/ajrcmb.18.5.2898. [DOI] [PubMed] [Google Scholar]
- Ashcroft T, Simpson JM, Timbrell V. Simple method of estimating severity of pulmonary fibrosis on a numerical scale. J Clin Pathol. 1988;41:467–470. doi: 10.1136/jcp.41.4.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handel-Fernandez ME, Lopez DM. Isolation of macrophages from tissues, fluids, and immune response sites. Paulnock DM, editor. Oxford: Oxford University Press,; 2000:pp 1–30. [Google Scholar]
- Peters-Golden M, Bailie M, Marshall T, Wilke C, Phan SH, Toews GB, Moore BB. Protection from pulmonary fibrosis in leukotriene-deficient mice. Am J Respir Crit Care Med. 2002;165:229–235. doi: 10.1164/ajrccm.165.2.2104050. [DOI] [PubMed] [Google Scholar]
- Rouzer CA, Scott WA, Hamill AL, Cohn ZA. Synthesis of leukotriene C and other arachidonic acid metabolites by mouse pulmonary macrophages. J Exp Med. 1982;155:720–733. doi: 10.1084/jem.155.3.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frolich JC. A classification of NSAIDs according to the relative inhibition of cyclooxygenase isoenzymes. Trends Pharmacol Sci. 1997;18:30–34. doi: 10.1016/s0165-6147(96)01017-6. [DOI] [PubMed] [Google Scholar]
- Selman M, Pardo A. Idiopathic pulmonary fibrosis: an epithelial/fibroblastic cross-talk disorder. Respir Res. 2002;3:3–10. doi: 10.1186/rr175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauldie J, Kolb M, Sime PJ. A new direction in the pathogenesis of idiopathic pulmonary fibrosis? Respir Res. 2002;3:1–3. doi: 10.1186/rr158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler DB, Giri SN. Changes in plasma concentrations of prostaglandins and plasma angiotensin-converting enzyme during bleomycin-induced lung fibrosis in hamsters. Am Rev Respir Dis. 1983;128:71–76. doi: 10.1164/arrd.1983.128.1.71. [DOI] [PubMed] [Google Scholar]
- Giri SN, Witt TC. Effects of intratracheal administration of bleomycin on prostaglandins and thromboxane-B2 and collagen levels of the lung in hamsters. Exp Lung Res. 1985;9:119–133. doi: 10.3109/01902148509061532. [DOI] [PubMed] [Google Scholar]
- Bonner JC, Rice AB, Ingram JL, Moomaw CR, Nyska A, Bradbury A, Sessoms AR, Chulada PC, Morgan DL, Zeldin DC, Langenbach R. Susceptibility of cyclooxygenase-2-deficient mice to pulmonary fibrogenesis. Am J Pathol. 2002;161:459–470. doi: 10.1016/S0002-9440(10)64202-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeldin DC, Wohlford-Lenane C, Chulada P, Bradbury JA, Scarborough PE, Roggli V, Langenbach R, Schwartz DA. Airway inflammation and responsiveness in prostaglandin H synthase-deficient mice exposed to bacterial lipopolysaccharide. Am J Respir Cell Mol Biol. 2001;25:457–465. doi: 10.1165/ajrcmb.25.4.4505. [DOI] [PubMed] [Google Scholar]
- Gavett SH, Madison SL, Chulada PC, Scarborough PE, Qu W, Boyle JE, Tiano HF, Lee CA, Langenbach R, Roggli VL, Zeldin DC. Allergic lung responses are increased in prostaglandin H synthase-deficient mice. J Clin Invest. 1999;104:721–732. doi: 10.1172/JCI6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirtikara K, Morham SG, Raghow R, Laulederkind SJ, Kanekura T, Goorha S, Ballou LR. Compensatory prostaglandin E2 biosynthesis in cyclooxygenase 1 or 2 null cells. J Exp Med. 1998;187:517–523. doi: 10.1084/jem.187.4.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Goorha S, Raghow R, Ballou LR. The tissue-specific, compensatory expression of cyclooxygenase-1 and -2 in transgenic mice. Prostaglandins Other Lipid Mediat. 2002;67:121–135. doi: 10.1016/s0090-6980(01)00177-0. [DOI] [PubMed] [Google Scholar]
- Lama V, Moore BB, Christensen P, Toews GB, Peters-Golden M. Prostaglandin E2 synthesis and suppression of fibroblast proliferation by alveolar epithelial cells is cyclooxygenase-2-dependent. Am J Respir Cell Mol Biol. 2002;27:752–758. doi: 10.1165/rcmb.4857. [DOI] [PubMed] [Google Scholar]
- Wallace JL, Bak A, McKnight W, Asfaha S, Sharkey KA, MacNaughton WK. Cyclooxygenase 1 contributes to inflammatory responses in rats and mice: implications for gastrointestinal toxicity. Gastroenterology. 1998;115:101–109. doi: 10.1016/s0016-5085(98)70370-1. [DOI] [PubMed] [Google Scholar]
- Morteau O, Morham SG, Sellon R, Dieleman LA, Langenbach R, Smithies O, Sartor RB. Impaired mucosal defence to acute colonic injury in mice lacking cyclooxygenase-1 or cyclooxygenase-2. J Clin Invest. 2000;105:469–478. doi: 10.1172/JCI6899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers LK, Kang AH, Postlethwaite AE, Rosloniec EF, Morham SG, Shlopov BV, Goorha S, Ballou LR. The genetic ablation of cyclooxygenase 2 prevents the development of autoimmune arthritis. Arthritis Rheum. 2000;43:2687–2693. doi: 10.1002/1529-0131(200012)43:12<2687::AID-ANR8>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Ejima K, Layne MD, Carvajal IM, Kritek PA, Baron RM, Chen YH, Vom Saal J, Levy BD, Yet SF, Perrella MA. Cyclooxygenase-2-deficient mice are resistant to endotoxin-induced inflammation and death. FASEB J. 2003;17:1325–1327. doi: 10.1096/fj.02-1078fje. [DOI] [PubMed] [Google Scholar]