Abstract
Interleukin-1β (IL-1) expression is associated with a spectrum of neuroinflammatory processes related to chronic neurodegenerative diseases. The single-bolus microinjection of IL-1 into the central nervous system (CNS) parenchyma gives rise to delayed and localized neutrophil recruitment, transient blood-brain barrier (BBB) breakdown, but no overt damage to CNS integrity. However, acute microinjections of IL-1 do not mimic the chronic IL-1 expression, which is a feature of many CNS diseases. To investigate the response of the CNS to chronic IL-1 expression, we injected a recombinant adenovirus expressing IL-1 into the striatum. At the peak of IL-1 expression (days 8 and 14 post-injection), there was a marked recruitment of neutrophils, vasodilatation, and breakdown of the BBB. Microglia and astrocyte activation was evident during the first 14 days post-injection. At days 8 and 14, extensive demyelination was observed but the number of neurons was not affected by any treatment. Finally, at 30 days, signs of inflammation were no longer present, there was evidence of tissue reorganization, the BBB was intact, and the process of remyelination was noticeable. In summary, our data show that chronic expression of IL-1, in contrast to its acute delivery, can reversibly damage CNS integrity and implicates this cytokine or downstream components as major mediators of demyelination in chronic inflammatory and demyelinating diseases.
Inflammation is a key component of the defense mechanism against infection in the periphery and in the central nervous system (CNS). Inflammation in the CNS has different features than in the periphery. For example, differential induction of cytokines may be involved in the atypical pattern of leukocyte recruitment induced in the brain.1 Importantly, a dysregulated inflammatory response has been associated with many chronic CNS diseases, the prototype of which is multiple sclerosis (MS). However, in comparison with the periphery, many basic facts about inflammation in the CNS and its consequences are still unresolved.
One useful approach to shed light on the distinct characteristics of the CNS inflammatory response is to study the response of brain tissue to individual components of inflammation, such as pro-inflammatory cytokines. In the CNS, resident cells express cytokines and their receptors2 and these cytokines can in turn affect the viability of neurons and oligodendrocytes, among other effects on CNS function.3,4 In particular, interleukin-1β (IL-1) has been shown to play a pivotal role in the exacerbation of acute neurodegeneration caused by ischemia, head trauma, or stroke and has been implicated in the pathology of MS, Alzheimer’s disease, and other chronic diseases of the CNS.2,5–9 In MS, the IL-1 levels in the CSF correlates with disease activity and certain IL-1 genotypes or the balance between IL-1 and IL-1ra are associated with disease severity, susceptibility, and/or progression.4,10,11 Moreover, blocking IL-1 action reduces neuronal loss and inflammation induced by experimental brain insults9,12,13 and IL-1 has been shown to be cytotoxic for oligodendrocytes both in vitro and in vivo.14,15 On the other hand, although most of the evidence points to a detrimental role of IL-1 in MS, it has been shown to promote repair of cuprizone-derived demyelination via IGF-1.16
It has been shown that a single-bolus injection of IL-1 into the CNS parenchyma gives rise to delayed and localized neutrophil (PMN) recruitment, but no overt damage to CNS integrity.17,18 However, no study has examined the consequences of chronic expression of IL-1 in the brain parenchyma, which would be more relevant to the pattern of expression observed in chronic inflammatory diseases of the CNS. In this study, we were particularly keen to determine whether the atypical recruitment profile, which is observed after a single-bolus injection of IL-1, is conserved during extended IL-1 expression and whether extended expression would result in CNS damage. To achieve this, we used a recombinant replication-deficient adenovirus vector to overexpress human IL-1 for a transient, but prolonged period in the rat brain. We then analyzed the inflammation, gliosis, and any evidence of tissue damage that might be caused by the chronic expression of IL-1.
Materials and Methods
Vectors
Adenoviral vectors were generated as already described.19 Briefly, for construction of AdhIL-1β, human IL-1β cDNA (in pGEM3Z vector, a gift from British Biotech Pharmaceuticals Ltd., Oxford, United Kingdom) was cloned into a shuttle vector with a human cytomegalovirus promoter and cotransfected on 293 cells with a plasmid containing E1- to E3-deleted type 5 adenoviral genome. The correct recombination was verified with restriction digestions of the purified viral DNA obtained by HIRT, and further confirmed by Southern blot. Transgene expression was observed by Western blot with an anti-human IL-1-specific antibody.20 The adenoviral vectors were purified by plaque-formation under agar. Stocks were obtained from large-scale preparations in HEK293 cells by double cesium chloride gradients, and were quantified by plaque assay (final titers: Adβgal = 1.5 × 1010 pfu/μl, AdIL-1 = 1 × 1010 pfu/μl). Stocks had less than 1 ng/ml of endotoxin, assayed with E-TOXATE reagents (Sigma, St. Louis, MO). Viral stocks were free of autoreplicative particles as assessed by PCR and transduction of non-transcomplementary cells (HeLa, ATCC).21 The adenoviral vector expressing β-galactosidase (Adβgal) was gently provided by Dr. J. Mallet (Hospital Pitie Salpetriere, Paris, France).22
Animals and Injections
Adult male Wistar rats (200 g to 280 g) (Anmat, Buenos Aires), housed in groups of two animals, under controlled temperature (22°C ± 2°C), artificially lit under a 12-hour cycle period and with water and food ad libitum.
For central injections, the animals were anesthetized with ketamine chlorhydrate (80 mg/kg) and xylazine (8 mg/kg). The adenovirus were administered with a 50-μm tipped finely drawn glass capillary, stereotactically directed to the center of the right striatum (bregma, +1 mm; lateral, −3 mm; ventral, −4.5 mm).23 Striatal injections of 1 μl of adenoviral vectors or vehicle were infused over a 5-minute period and the pipette was kept in place for additional 2 minutes before removal. All surgical procedures took place in the morning to avoid effects of circadian variations in cytokine expression. Human IL-1β and β-galactosidase expressing adenoviral vectors were diluted in sterile PBS (pH 7.4) and administered at a dose of 107 pfu/rat. The animals were killed at 2, 8, 14, 21, or 30 days post-surgery.
All animal procedures were performed according to the rules and standards of the German animal protection law and the regulations for the use of laboratory animals of the National Institutes of Health, USA.
Histology
The animals were deeply anesthetized, then perfused transcardially with heparinized saline followed by cold 4% paraformaldehyde in 0.1 mol/L phosphate buffer (PB) (pH 7.2). After dissecting the brains, they were placed in the same fixative overnight at 4°C. Tissues were then cryoprotected by immersion in 30% sucrose, frozen in isopentane, and serially sectioned in a cryostat (14 μm and 40 μm, alternating). The 14-μm sections were mounted on gelatin-coated slides and the 40-μm sections were used for free-floating immunohistochemistry. Serial sections (14 μm) were stained with Luxol fast blue/cresyl violet stain to determine demyelination in the nervous tissue. Serial free-floating sections were used to detect β-galactosidase activity, using X-gal (5-bromo-4-chloro-3-indoyl-β-D-galactopyranoside) as substrate.22
To identify neurons we used immunohistochemistry to detect the neuronal marker NeuN in unfixed tissue rats were deeply anesthetized and perfused transcardially with heparinized saline. The brain was then dissected and quickly frozen in isopentane. Serial sections (10 μm) were cut in a cryostat, mounted on gelatin-subbing slides and kept at −20°C.
Identification of Leukocytes
Polymorphonuclear neutrophil (PMN) cells were identified by their nuclear morphology appearance in 40-μm thick cresyl violet-stained sections and confirmed by immunohistochemistry with MBS II antibody.24 Leukocytes were classified as: “marginated”, those cells that appeared to be adherent to the luminal side of the endothelium; “cuffed”, those that appeared on the abluminal side of the vessels; and “recruited”, those cells that had crossed the vascular endothelium and the basement membrane and were located in the parenchyma.24 Cells of the mononuclear phagocyte lineage, microglia and macrophages, were identified using the lectin Griffonia simplicifolia (GSA-1B4)25 and the antibody ED1 which stains recently recruited monocytes/macrophages and activated but not quiescent microglia.
Immunohistochemistry
Free-floating sections were incubated in blocking buffer (1% donkey serum, 0.1% Triton in 0.1 mol/L PB) for 45 minutes, rinsed in 0.1% Triton in 0.1 mol/L PB and incubated overnight with primary antibodies diluted in blocking solution. The antibodies used were: anti-human IL-1β (that recognizes both human and rat IL-1β) (1:100; Peprotech, Mexico), ED1 (1:200; Serotec, Raleigh, NC), MBS II (1:100; specific for neutrophils,24 ED2 (1:200; Serotec), anti-GFAP (1:700; Dako, Carpinteria, CA), and anti-NeuN (1:100; Chemicon, Temecula, CA). We used the biotinylated lectin Griffonia simplicifolia (GSA-1B4, 1:50; Vector Laboratories, Burlingame, CA), that specifically binds to the terminal α-D-galactose residues in the plasma membrane of transforming microglial cells.25 After three 5-minute washes with 0.1 mol/L PB, the sections were incubated with either indocarbocyanine Cy3 (Cy3) conjugated donkey anti-mouse antibody (1:250; Jackson ImmunoResearch Laboratories Inc., West Grove, PA), cyanine Cy2 (Cy2) conjugated donkey anti-rabbit antibody (1:250; Jackson), or Cy2 conjugated streptavidin (1:250; Jackson) for 2 hours, rinsed in 0.1 mol/L PB, and mounted in Mowiol (Calbiochem, San Diego, CA). Digital images were collected in a Zeiss LSM 510 laser scanning confocal microscope equipped with a krypton-argon laser.
Quantification
For the quantification of neutrophils, cell types were identified by their morphology on cresyl violet staining under ×40 magnification. Every sixth 40-μm thick serial section was counted and the area of the striatum was measured using the Image Pro image analysis system (Media Cybernetics, Silver Spring, MD). Graphs show the number of cells per mm3. For the quantification of activated macrophages, we measured the ED1-positive area in every sixth 40-μm serial section using Image Pro image analysis system. For the quantification of the total number of neurons in the striatum, NeuN-positive cells were counted in every tenth 10-μm thick serial section under ×20 magnification. In addition, the areas in which we counted the cells were measured with the Image Pro image analysis software and the striatal volume was calculated based on these measurements and the distance among areas. Nuclear staining for NeuN was counted as positive only for those nuclei showing an intact morphology. False-positive cells (mostly composed of PMN cells) were discarded based on cellular morphology. All measurements were performed throughout the striatum. Graphs show the total number of neurons in the whole striatum.
Electron Microscopy
Anesthetized animals were intracardially perfused with a modified saline solution (0.8% NaCl, 0.8% sucrose, 0.4% glucose) followed by a fixative made of 2% glutaraldehyde, 2% paraformaldehyde in 0.1 mol/L cacodylate buffer (pH 7.2). The 1-mm3 samples of striatum were then immersed in the same fixative at 4°C overnight. The tissue was stored in 0.1 mol/L cacodylate buffer (CB) with 0.2 mol/L sucrose. After 5-minute washes in CB, they were post-fixed in OsO4 in CB for 1 hour, washed in CB, dehydrated in ethanol series, cleared in acetone, and embedded in Araldite. Semi-thin and ultra-thin sections were cut on a Sorvall-Porter-Blum ultramicrotome and stained with toluidine blue and double-stained with uranyl acetate and lead citrate, respectively. Ultra-thin sections were examined in either a JEOL 1200EX2 or Zeiss EM10-C electron microscopes. The electronic photomicrograhs were obtained by using standard photographic and darkroom procedures.
Assessment of Blood-Brain Barrier Permeability
Thirty minutes before perfusion the animals were injected intravenously with 104 U/kg of type II horseradish peroxidase (HRP, Sigma, St. Louis, MO). Then, the animals were perfused as previously described with Karnovsky′s fixative and their brains processed as previously described. HRP was detected in free-floating sections by a modified Hanker-Yates method.26
Staining for Degenerating Neurons
Fourteen-μm sections mounted on gelatin slides were stained with Fluoro-Jade B (Histo-Chem, Jefferson, AR) according to the technique previously described.27 As a positive control, the substantia nigra of a rat brain, which had been injected intrastrially with 6OH-dopamine, was used.
ELISA Method
The animals were perfused as previously described with heparinized saline to clear the blood from the brain samples. The brains were quickly removed, sliced into 3-mm sections and both the injected and uninjected striatum were punched out, snap-frozen in liquid nitrogen, and stored at −80°C. The tissue was homogenized on ice in 400 μl of Tris-HCl buffer (pH 7.3) containing protease inhibitors (10 μg/ml aproteinin, 5 μg/ml peptastin, 5 μg/ml leupeptin, 1 mmol/L PMSF, Sigma). Homogenates were centrifuged at 10000 × g at 4°C for 10 minutes and the supernatants were then ultracentrifuged at 40000 rpm for 2 hours. The supernatants were stored at −80°C until used. Bradford protein assays were performed to determine total protein concentration in each sample. A commercially available human IL-1β (hIL-1β) ELISA kit (R&D, Minneapolis, MN) was used according to the manufacturer’s instructions to quantify hIL-1β with high sensitivity (2 pg/ml). To estimate protein recovery, hIL-1β standard was added exogenously to brain homogenates and the ELISA was performed as described before. A recovery of 85 to 90% of the added hIL-1β standard was found.
Statistical Analysis
Statistical differences among treatments were determined using one-way analysis of variance test, followed by Newman-Keuls Multiple Comparison test. Alternatively, Student’s t-tests were used.
Results
Chronic IL-1β Expression in the Striatum
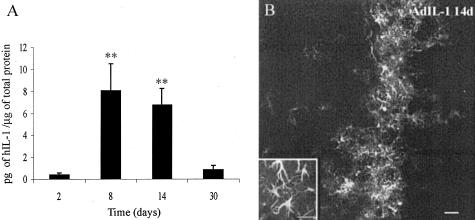
We achieved chronic expression of hIL-1β in the rat striatum with the administration of a low dose (107 pfu) of a replication-deficient, recombinant adenoviral vector (AdIL-1). We chose this dose because it was the maximal dose of control vector (Adβgal) that failed to cause an inflammatory response assessed by the presence or abscence of inflammatory infiltrate in Nissl-stained histological sections. In AdIL-1β injected animals, hIL-1β expression started 2 days after administration, peaked between 8 and 14 days and was still detected by ELISA 30 days post-inoculation (Figure 1A). hIL-1β expression was not detected in control animals, reflecting the specificity of the ELISA kit. The calculated mean amounts of total hIL-1β in the whole striatum (2.5 ng, 41.1 ng, 40.6 ng, and 2.1 ng, at 2, 8, 14, and 30 days, respectively) are within the range that produces an inflammatory response when injected in the periphery in rodents and humans.28 At 2 and 8 days post-AdIL-1 injection, ramified IL-1β-positive cells were restricted to the injection site and surrounding vessels (data not shown). At 14 days, most of the IL-1β-positive cells were located close to the injection region within the coronal sections through the injection site (Figure 1B). However, scattered IL-1β-positive cells were widespread throughout the antero-posterior axis of the striatum. No IL-1β staining was observed outside the striatum. Thirty days post-injection, expression of IL-1β was not detected by immunofluorescence, probably due to a lack of sensitivity. After the inoculation of control vector (Adβgal), β-galactosidase activity was detected in the injected striatum only by X-gal staining at all the analyzed time points (data not shown).
Figure 1.
Chronic IL-1β expression in the striatum after AdIL-1 inoculation. A: Time course of the expression of IL-1β after AdIL-1 injection as quantified by ELISA. Levels of IL-1β are shown as mean pg hIL-1β per μg of total protein ± SE. **, P < 0.01 when compared to 2 days, analysis of variance (P = 0.0015) followed by Neuman-Keuls tests. B: Immunofluorescence against IL-1β 14 days post-AdIL-1 injection. Inset: Detail of ramified cells expressing IL-1β. Bar, 50 μm.
Chronic IL-1β Expression in the Striatum Results in a Massive Recruitment of Polymorphonuclear Neutrophils
Little or no recruitment of any type of leukocyte or vasodilatation was observed in the uninjected hemispheres or in animals injected with similar doses of Adβgal or saline solution at any of the time points studied (Figure 2A and data not shown).
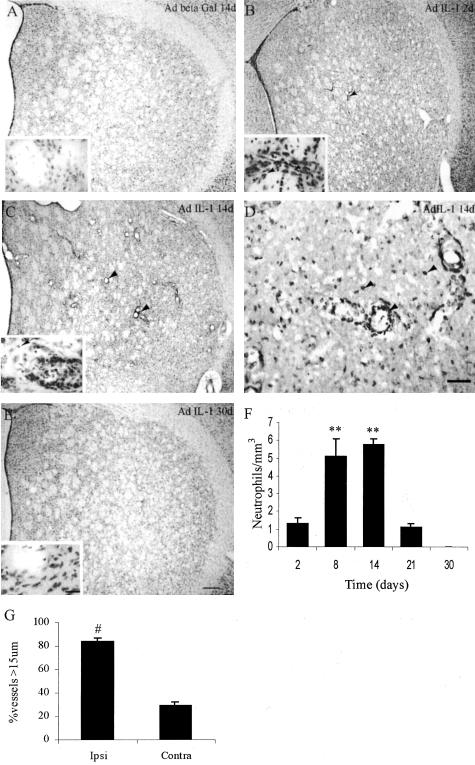
Figure 2.
Chronic expression of IL-1 in the striatum induces recruitment of inflammatory cells and vasodilatation. A: Cresyl violet staining of a representative striatal section 14 days after ipsilateral Adβgal injection. B: Cresyl violet staining of a representative ipsilateral striatal section 2 days after the injection of AdIL-1β. Note the inflammatory infiltrate with PMN or monocyte morphology mostly located in the lumen of blood vessels (arrow). Inset: Note the infiltrate in the lumen of the vessels (*) and also some in the brain parenchyma (arrow). C: Cresyl violet staining of a representative ipsilateral striatal section 14 days after chronic expression of IL-1. Massive amounts of neutrophils are observed throughout the striatum. Vasodilatation is also evident (arrowhead). Inset: the infiltrate is located in the lumen of blood vessels (*) and the parenchyma (arrow). D: Neutrophil-specific immunohistochemical analysis of ipsilateral striatal sections 14 days after AdIL-1 administration. Arrowhead: neutrophils. E: Cresyl violet staining of a representative ipsilateral striatal section 30 days after the ipsilateral injection of AdIL-1. Inset shows the nervous tissue without any sign of inflammatory response or vasodilatation. F: Quantification of the neutrophil influx in the striatum at different time points ipsilateral to the AdIL-1β administration. Number of neutrophils/mm3 is the number of PMN/mm3 along the entire striatum × 100. **, P < 0.01 when compared to 2 days, analysis of variance (P = 0.0014) followed by Newman-Keuls tests. G: Quantification of vasodilatation 14 days post-AdIL-1 administration as determined by counting the number of vessels with a diameter higher than 15 μm in the ipsilateral striatum. #, P < 0.05, Student′s t-test. Bar, 100 μm (A, B, C, and E). Bar, 50 μm (D). Bar insets, 10 μm.
Two days after AdIL-1 injection, marginated leukocytes filled the vessels located near the injection site. This leukocyte population was composed predominantly of monocytes/macrophages and only scarce PMN were observed (Figure 2, B and F). No cuffed leukocytes were observed at this time point (Figure 2B). However, at 8 days, cuffed and recruited neutrophils to the brain parenchyma were found to be widespread throughout the whole striatum (Figure 2F and data not shown). At 14 days, a very large number of neutrophils were observed throughout the striatum (Figure 2, C, D, and F) and the blood vessels were vasodilated and filled with marginated PMN and few monocytes/macrophages (Figure 2, C, D, and G). However, there was no inflammatory response in the meninges of either hemisphere at this time point (see supplementary data 1). The inflammatory response in the parenchyma was totally resolved by 30 days after surgery, when no signs of vasodilatation or inflammatory infiltrate were visualized (Figure 2E).
Macrophages/Microglial and Astroglial Reaction to the Chronic Expression of IL-1β
Control animals injected either with Adβgal or PBS exhibited minor microglial/macrophage activation 2 and 8 days after injection, as seen by GSA and ED1 immunostaining (Figure 3, A, D, E, and data not shown). Microglial activation was evident close to the injection site, reflecting the non-specific response of the tissue to the surgery (Figure 3, A, E, and data not shown). The contribution of peripheral monocytes/macrophages to the total number of activated microglial cells was not possible to determine based on morphology or immunostaining. No GSA-positive (GSA+) or ED-1-positive (ED-1+) microglia was observed in control animals at 14, 21, or 30 days post-injection (Figure 3, D, H, and data not shown). No GFAP-positive (GFAP+) astrocytes were detected in control animals at any time point studied (Figure 3, I, L, and data not shown).
Figure 3.
Glial reaction to the chronic expression of IL-1. A–C: ED1 immunoreactivity in the ipsilateral striatum after the injection of Adβgal or AdIL-1. A: Representative striata shown 2 days after Adβgal injection. B: Representative striata shown 2 days after AdIL-1 injection. C: Representative striata shown 14 days after AdIL-1β injection. D: Quantification of the ED1-positive areas (mm2) in the whole ipsilateral AdIL-1β- and Adβgal-treated striata. E–H: GSA-1B4 immunofluorescence in the striatum. E: Representative striata shown 2 days after Adβgal injection, cells with macrophage morphology (arrowhead) were detected. F: Representative striata shown 2 days after AdIL-1β injection. G: Representative striata shown 14 days after AdIL-1β injection. H: Representative striata shown 14 days after Adβgal injection. I–L: GFAP immunofluorescence in the striatum. I: Two days after Adβgal injection. J: Representative striata shown 2 days after AdIL-1β injection. K: Representative striata shown 14 days after AdIL-1 injection. L: Representative striata shown 14 days after Adβgal injection. Bars, 20 μm (A and B); 10 μm (C); 50 μm (E–L).
Two days after the injection of AdIL-1, activated microglia defined as GSA+ or ED-1+ cells, with either thick and stout processes or round-shaped morphology were detected (Figure 3, B, D, and F). The ED-1+ cells were more restricted to the needle tract (Figure 3B). An extensive and widespread astrogliosis, defined as GFAP+ ramified cells, was observed in the striatum (Figure 3J).
At 8 and 14 days, immunoreactive cells for ED1 and with morphological signs of phagocytic activity (round shape and vacuolated cytoplasm) were observed in the whole striatum (Figure 3C). The area covered by ED1+ cells reached a maximum at these stages (Figure 3, C and D, and data not shown). The GSA+ cells located nearest to the injection site exhibited a typical appearance of phagocytic cells, ie, round shape with vacuolated cytoplasm, while the GSA+ cells distant to the injection site exhibited the typical ramified morphology of microglial cells (Figure 3G). Finally, the whole striatum was filled with GFAP+ cells at this stage, reflecting an intense astrogliosis after the inflammatory insult (Figure 3K). At 30 days, the inflammatory response had resolved and there were no signs of GSA+, ED-1+, or GFAP+ cells in the striatum of the animals injected with AdIL-1 (Figure 3D).
Loss of Integrity of the Blood-Brain Barrier (BBB) Caused by Chronic IL-1β Expression
To evaluate whether neutrophil recruitment was accompanied by BBB damage, we examined the permeability of the BBB by the intravenous injection of HRP in treated animals, followed by the HRP detection as described in the methods.
No breakdown of the BBB was observed in the animals injected with Adβgal or in the contralateral striatum of AdIL-1β injected rats at any time point studied (Figure 4A).
Figure 4.
Assessment of the BBB integrity using the Hanker-Yates method. A: Representative striata 14 days after ipsilateral Adβgal administration. No Hanker-Yates reaction is observed. B: Representative striata 2 days after ipsilateral AdIL-1β administration. No BBB breakdown is observed, but some phagocytic cells and neutrophils are labeled with the HRP reaction. C: Representative striata 8 days after ipsilateral AdIL-1β administration. Evident BBB breakdown is observed. D: Representative striata 14 days after ipsilateral AdIL-1β administration. BBB damage is evident, visualized as an extensive region where HRP reaction can be observed as a dark precipitate. E: Representative striata 21 days after ipsilateral AdIL-1β administration. The recovery of the BBB damage is evident. F: Representative striata 30 days after ipsilateral AdIL-1β administration. The BBB integrity is totally restored. G: Quantitation of the peroxidase-positive areas after ipsilateral AdIL-1β administration. Bar, 100 μm.
At 2 days, no marked breakdown of the BBB was observed in animals injected with AdIL-1 (Figure 4, B and G). However damage to the BBB was evident at 8 days, and was conspicuous at 14 days, as demonstrated by extravasation of HRP from vessels in the parenchyma of the striatum (Figure 4, C, D, and G). A large number of HRP-positive neutrophils and phagocytic cells were also evident in the brain parenchyma with this technique (data not shown). The integrity of the BBB was still not restored by 21 days after AdIL-1 injection, but it was totally restored by 30 days post-injection (Figure 4, E, F, and G).
Chronic IL-1β Expression Causes Reversible Demyelination in the Striatum
The effects of the chronic expression of IL-1 on the integrity of myelin were also studied. No evidence of myelin damage or demyelination was observed in Adβgal-injected animals or in the uninjected striatum of AdIL-1 injected rats by Luxol staining, in semi-thin sections and at the ultrastructural level at any time point studied (Figure 5, A, D, and L).
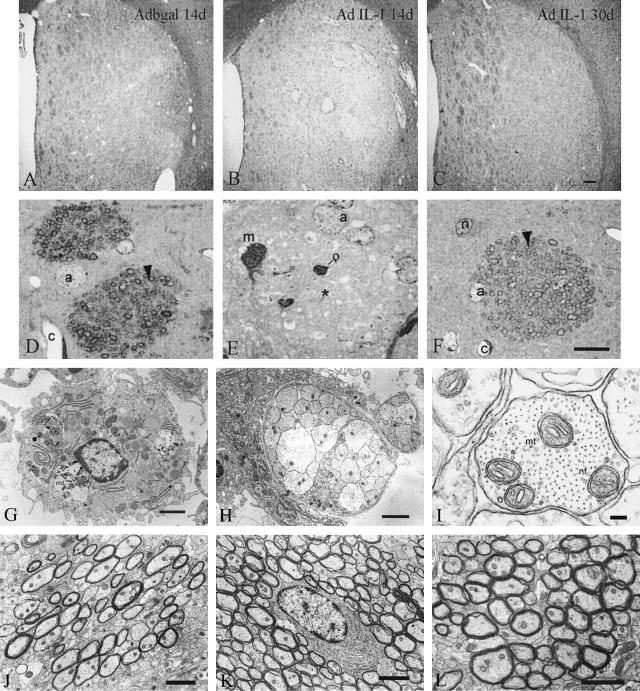
Figure 5.
Analysis of the effects of the chronic expression of IL-1β on demyelination of the nerve bundles in the striatum by Luxol/cresyl violet stain (A–C), semi-thin sections (D–F), or electron microscopy (G and L). A: No demyelination can be observed in the control animals 14 days post-Adβgal injection. B: Fourteen days after AdIL-1β injection severe demyelination is visualized. C: Thirty days after AdIL-1β injection remyelination is observed. D: Semi-thin section showing myelinated axons (arrowhead) in animals treated as in A. E: Semi-thin section showing the breakdown of the myelin sheaths (*) in the nerve bundles 14 days after AdIL-1 injection. F: Semi-thin section showing a nerve bundle with myelinated axons (arrowhead) at 30 days. G–I: Representative photomicrographs of transversal sections of the striatum at the injection site 21 days postadenoviral administration. G: A macrophage containing myelin debris (md) and numerous lipid vacuoles (L) can be seen within the lesion. Bar, 2 μm. H: A bundle of non-myelinated axons can be observed within the inflammatory region. Bar, 2 μm. I: Demyelinated axons have a normal appearance with microtubules (mt), neurofilaments (nf) and mitochondria in its axoplasm. Bar, 100 nm. J: Representative electronmicrograph at the edge of lesioned area at 21 days post-lesion showing that a large portion of the axons are remyelinated, characterized by a thin myelin sheath surrounding each axon (*). Bar, 2 μm. K: At 30 days post-lesion almost all of the axons show signs of remyelination, here surrounding an oligodendrocyte (O). Bar, 2 μm. L: Representative figure of normal myelinated axons of the contralateral hemisphere in rats operated in the right striatum at any time point studied (picture taken at 21 days post-injection). Bar, 2 μm. m, microgial cell; a, astrocyte; o, oligodendrocyte; c, capillary; n, neuron.
At 2 days, no myelin damage was seen in the striatum of AdIL-1-injected animals (data not shown). At 8 days post-injection, an evident loss of the regular distribution of the nerve bundles across the striatum was observed but only in those regions where the inflammatory infiltrate penetrated into the parenchyma (data not shown). However, no demyelination was evident at this time point.
In contrast, overt disorganization and widespread demyelination of the nervous tissue were observed under Luxol fast blue/cresyl violet staining at 14 days post-AdIL-1 injection (Figure 5B). Demyelination was also observed in semi-thin sections (Figure 5E). Furthermore, we observed several signs of demyelination at the ultrastructural level between 14 and 21 days (Figure 5, G, H, and data not shown). Macrophage/microglia with myelin debris and lipid vacuoles could be observed at the lesion site (Figure 5G). A representative bundle of demyelinated axons at the lesion site is shown in Figure 5H. Neurofilaments and microtubules of the vast majority of axons were found intact, suggesting a preservation of axonal integrity (Figure 5I). In addition, a minority of the axons showed signs of damage (supplementary data 2). These signs of demyelination were not present at 30 days or at the contralateral injected side as seen by Luxol fast blue staining, semi-thin sections or at the ultrastructural level (Figure 5, C, F, K, and L). Thinner myelin sheaths were observed around axons at 21 days, compared with the contralateral side or the striatum at 30 days, suggesting the presence of an ongoing recovery process after demyelination (Figure 5, J, K, and L). The oligodendrocytes had no evident ultrastructural alterations at any time point studied (Figure 5K).
IL-1-Mediated No Neuronal Cell Loss
To evaluate the effects of the chronic expression of IL-1 in the striatum on neuronal integrity, NeuN-positive (NeuN+) cells were counted throughout the striatum as described in Materials and Methods. A similar number of intact, NeuN+ nuclei was found in the ipsilateral striatum of animals treated with AdIL-1 at 14 and 30 days, or control vectors at 14 days post-treatment (Figure 6, A to C). In addition, no changes in the total number of NeuN+ cells were observed among hemispheres (data not shown). Furthermore, neurons presented no alterations at the ultrastructural level at any time point studied (Figure 6D). Moreover, no positive staining with Fluoro-Jade B was observed in this tissue, indicating the lack of degenerating neurons in the injected striatum (data not shown).
Figure 6.
Analysis of the chronic expression of IL-1 on neuronal viability. A: NeuN expression in the striatum after 14 days of chronic expression of IL-1. Notice a non-specific label within the vessels filled with infiltrate (arrowhead). B: NeuN immunoreactivity 30 days post-AdIL-1 injection. C: Quantification of the total number of NeuN-positive neurons in the ipsilateral striatum. No significant differences were observed between groups. D: Electron photomicrograph of a representative striatal neuron 14 days after injection of AdIL-1 showing no ultrastructural alterations. However, in the same field, some axons exhibit a normal appearance (1) and other display splits in its myelin sheath (2). (n) nucleus, (d) dendrite. Bars, 50 μm (A–B); 500 nm (D).
Discussion
In these experiments we have investigated the impact of chronic IL-1 expression on the integrity of the brain parenchyma and its constituent cell types. We have shown that the chronic expression of IL-1 leads to the recruitment of PMN to the brain parenchyma and induces the activation of microglia and astrocytes as revealed by GSA+, ED1+, and GFAP+ cells, BBB breakdown, reversible demyelination, but no overt neurodegeneration. These changes in the brain parenchyma were reversible, as they had been largely resolved by day 30 post-injection.
Although it has been stated that the adenoviral vectors cause inflammation both in the peripheral tissues and also in the brain,29,30 we only detected modest and restricted microglial activation at early time points in both control adenoviral vectors and vehicle-injected brains. This is likely due to the low pfu that we used for this study and to the use of finely polished capillaries for the infusion that minimize the trauma associated with the surgery.31 Under our conditions, we achieved long transgene expression (for 30 days) with no chronic inflammatory response due to the vector itself.
Cellular and Temporal Characteristics of the Striatal-Restricted Inflammatory Infiltrate after Chronic IL-1 Expression in the Brain
One of the main features of the study is the magnitude and the specificity of the recruitment of PMN into the striatum. The earliest event detected at 2 days post-AdIL-1 injection was the presence of mainly macrophages/monocytes in the lumen of blood vessels or close to enter the parenchyma and also some marginated PMN. At 8 and 14 days, the striatum was virtually full of cuffed and marginated PMN and most of the brain parenchyma was filled with PMNs. No other type of leukocytes, except few monocytes/macrophages in or around the vessels, was observed. At 30 days, no PMN or other leukocyte was detected in any brain compartment although IL-1 was still detectable at levels similar to those measured 2 days after surgery. Considering that neutrophils live 24 to 48 hours after leaving the blood,32 our data suggest a constant influx of neutrophils for at least 21 days. As the amount of hIL-1β detected at 30 days (2.5 ng) was sufficient for the recruitment of PMN at 2 days, we suggest some degree of tachyphylaxis in the brain 1 month after chronic IL-1 expression (see Figures 1A and 2F).
In the periphery, neutrophils are the earliest inflammatory cells to infiltrate the damaged tissue, playing an important role in phagocytosis and in releasing chemokines and cytokines that, in turn, amplify the inflammatory response.32 These cells are generally followed by monocytes/macrophages during an inflammatory reaction in the periphery. On the contrary, the contribution of macrophages/monocytes to the inflammatory infiltrate was only noticeable at early (2 days post-IL-1 injection) but not late time points. In previous studies in which the same adenoviral vector AdIL-1 was introduced into the peritoneum33 or into the lung,19 neutrophils dominated the inflammatory response, but the mononuclear cells were also present. Similar numbers of neutrophils and monocytes were present in the lung 14 days after injection and this paralleled increases in TNF-α and IL-6.19 Thus, in peripheral tissue, prolonged IL-1 expression induces TNF-α expression, resulting in mixed neutrophil and mononuclear cell recruitment to the site of injury. This has led to the concept of cross-talk in peripheral cytokine signaling pathways. In the brain parenchyma, however, we observe restricted patterns of leukocyte recruitment following the focal injection of pro-inflammatory agents into the brain and after adenoviral-mediated prolonged expression of IL-1 (this study) and TNF-α.34 We have previously found that a single-bolus injection of IL-1 induces de novo synthesis of additional IL-1, but not TNF-α, and that TNF-α induces neither itself nor IL-1, and suggested that the signaling pathways that are present in the periphery are modified in the brain.1 It is now clear even that prolonged expression does not eventually give rise to mixed infiltrate, suggesting that the cytokine profile remains restricted. The specificity of IL-1β in recruiting PMN could be related to the potential chemokine and cytokine network induced. Therefore, it would be relevant to study whether crucial chemokines for neutrophil infiltration, such as MIP-2 or IL-8, are induced in our model of chronic IL-1 expression. These differences in the recruitment profile and the tachyphylaxis observed in the CNS add to the distinct features of the inflammatory response in the CNS in comparison with the periphery.
A single-bolus injection of a similar amount of IL-1 as those achieved at days 8 and 14 after AdIL-1 administration into the striatum has been previously shown to recruit PMN.24 However, the infiltrate described for acute IL-1 expression in the adult rat differs from the chronic expression described in the present study. After acute IL-1, the infiltrate was mostly located in the meninges and the distribution of the infiltrate in the nervous parenchyma was circumscribed only to few millimeters to each site of the injection site, while no vessel permeability can be observed.24 The cellular recruitment observed after chronic IL-1 expression was specific for the ipsilateral striatum; eg, the contralateral hemisphere, the ipsilateral cortex, and the meninges were free of any infiltrate. It will be interesting to unravel the different signals that mediate or restrict the involvement of the meninges in the inflammatory reaction during IL-1 synthesis in the parenchyma.
BBB breakdown followed the neutrophil recruitment as expected since we previously showed that the breakdown of the BBB is not necessary for neutrophil recruitment but is a consequence of their recruitment.35,36
Macrophages/Microglial and Astroglial Reaction
One of the expected events associated with IL-1 expression was the presence of GSA+, ED1+, and GFAP+ cells in the striatum. In animals injected with control adenoviral vectors or PBS GSA+ and ED-1+ cells were seen only at the earliest time point studied and this could be localized to the site of the injection track. On the other hand, GSA+ and ED-1+ cells were detected during 21 days after adenoviral-mediated IL-1 expression. However, at the latest time point studied (30 days), GSA+ and ED-1+ cells were no longer observed, although the amount of IL-1 present was similar to the one detected at 2 days, when those cell populations were active (Figures 1 and 3).
GFAP+ cells were not observed in animals injected with control adenoviral vectors or in the contralateral hemispheres of AdIL-1-administered animals. On the contrary, GFAP+ cells were detected throughout the ipsilateral striatum at 8 and 14 days after AdIL-1 inoculation. As in the case of GSA+ and ED-1+ cells, no GFAP+ cell could be visualized at 30 days post-treatment. These results suggest that, as in the case of the IL-1-mediated effect on PMN recruitment, astrocyte and microglia activation appears to become refractory to the chronic expression of IL-1.
Neurodegeneration
IL-1 has been shown to be associated with neurodegeneration and neuroprotection in a variety of in vitro and in vivo models.2,37,38 IL-1 can mediate neuronal death or more typically, exacerbate on-going neuronal demise.2,5 Using three different approaches, we were not able to detect differences in the total number of neurons (NeuN+ cells) in the striatum or signs of active neurodegeneration. Similar number of neurons was counted throughout the striatum of animals injected with AdIL-1 or Adβgal at the peak of transgene expression (14 days). This number was the expected one for an undamaged striatum39 and was comparable to the number of neurons present in rat striata 30 days after AdIL-1 administration. This last observation rules out a non-specific effect of the adenoviral vector that could have reduced neuronal numbers non-specifically in the previous groups (AdIL-1- and Adβgal-injected animals 14 days post-treatment). Thus, despite pronounced and sustained PMN recruitment and the activation of microglial and astroglial cells, neuronal viability was not affected by the expression of IL-1 for 1 month. This result suggests that at levels sufficient to trigger a florid inflammatory response, IL-1 is not neurotoxic per se during the time frame studied and that large numbers of PMNs or their products are not injurious to neurons unless the tissue is already compromised, as in an ischemic lesion.
IL-1 and Demyelination
The chronic expression of IL-1 induced extensive striatal demyelination at the peak of the inflammatory process and IL-1 expression. An endogenous remyelinating process that led to the recovery of the affected white matter fascicles in the striatum by 30 days followed this demyelination, as shown by two different analyses and confirmed at the ultrastructural level. Interestingly, the ultrastructural characteristics that we have described in the present model resemble the ones observed in a model of demyelination using Theiler’s virus.40
IL-1 per se has been shown to be cytotoxic to adult oligodendrocytes in vitro,14,15 while the treatment of rats subjected to experimental autoimmune encephalomyelitis (EAE) with IL-1ra, an endogenous antagonist of IL-1, resulted in delayed and milder disease.41,42 Moreover, certain IL-1 polymorphisms and a high ratio of IL-1 over IL-1ra production have been associated with increased susceptibility to relapse-onset multiple sclerosis in families.4,11 On the other hand, in a cuprizone model of demyelination, IL-1 was not associated with demyelination but with the remyelinating process, as the lack of IL-1 delayed the differentiation of oligodendrocyte progenitor cells to mature oligodendrocytes and the consequent remyelination.16 In our study, the chronic expression of IL-1 resulted in significant demyelination but did not inhibit the remyelination process despite its continued presence. It is clear that although IL-1 above a certain threshold may cause tissue damage, the CNS has the capacity to repair in the continuing presence of this inflammatory cytokine. Whether another increase in IL-1, such as that which may occur during reactivation of the inflammation in an MS plaque, would lead to the same sequence of events or more prolonged demyelination, remains to be investigated.
In parallel with the action of IL-1 per se, the influx of immune cells in the CNS has been historically associated with demyelination and remyelination. In our model, IL-1 expression is mostly associated with the influx of PMNs and it does not include a T-cell-mediated mechanism. Clinical severity in an EAE model has been associated with the degree of infiltration of neutrophils and macrophages in the brain,43 and studies on CCR2 knockout mice concluded that the presence of neutrophils in the CNS led to demyelination.44 On the other hand, some authors propose that oligodendrocytes are rather resistant to the presence of inflammatory cells in the spinal cord, suggesting that activated macrophages and microglia are able to distinguish intact oligodendrocytes and myelin and destroy only the degenerating myelin.45 In accord with that observation, activated macrophages and microglia were observed over a large part of the striatum in our study, but the demyelination was largely confined to the center of the lesion. Only further experiments will allow for better dissecting the role of IL-1 per se or the inflammatory infiltrate and associated mediators on the demyelination observed. Whatever the individual contribution of IL-1 or the IL-1-mediated infiltrate on the demyelination observed, this study shows demyelination could be an IL-1-triggered, T-cell-independent phenomenon.
In conclusion, our studies have shown that prolonged expression of IL-1 in the brain parenchyma of the adult will lead to a selective recruitment of PMN, in contrast to that observed in other organs. The prolonged recruitment of PMNs is associated with breakdown of the BBB and loss of the myelin sheaths from axons. However, after 30 days, despite the continuing presence of readily detectable levels of IL-1 protein, the inflammatory response resolves, the BBB is restored and remyelination ensues, suggesting some degree of tachyphylaxis in the brain. These results contrast with those in the periphery where persistent IL-1 expression leads to PMN and also monocyte recruitment and more long-lasting fibrosis. Whether repeated rounds of challenge will overcome the natural repair processes of the CNS remains to be investigated. The expression of IL-1 by adenoviral vectors provides an excellent model of the demyelination/remyelination process and will serve to investigate the mechanisms of action of IL-1 in chronic inflammatory CNS diseases.
Supplementary Material
Acknowledgments
We thank Isabel López de Farías for excellent technical assistance, Dr. H. Aldana Marcos for his help with the electron microscopy, and Dr. Rodolfo Tarelli for his help with the preparation of the figures.
Footnotes
Address reprint requests to Fernando Pitossi, Leloir Institute, Av. Patricias Argentinas 435, (1405) Buenos Aires, Argentina. E-mail: fpitossi@leloir.org.ar.
Supported by the Wellcome Trust grant number 059911/Z/99/Z.
References
- Blond D, Campbell SJ, Butchart AG, Perry VH, Anthony DC. Differential induction of interleukin-1beta and tumour necrosis factor-alpha may account for specific patterns of leukocyte recruitment in the brain. Brain Res. 2002;958:89–99. doi: 10.1016/s0006-8993(02)03473-x. [DOI] [PubMed] [Google Scholar]
- Allan SM, Rothwell NJ. Cytokines and acute neurodegeneration. Nat Rev Neurosci. 2001;2:734–744. doi: 10.1038/35094583. [DOI] [PubMed] [Google Scholar]
- Rothwell N. Interleukin-1 and neuronal injury: mechanisms, modification, and therapeutic potential. Brain Behav Immun. 2003;17:152–157. doi: 10.1016/s0889-1591(02)00098-3. [DOI] [PubMed] [Google Scholar]
- Mann CL, Davies MB, Stevenson VL, Leary SM, Boggild MD, Ko Ko C, Jones PW, Fryer AA, Strange RC, Thompson AJ, Hawkins CP. Interleukin 1 genotypes in multiple sclerosis and relationship to disease severity. J Neuroimmunol. 2002;129:197–204. doi: 10.1016/s0165-5728(02)00181-9. [DOI] [PubMed] [Google Scholar]
- Perry VH, Newman TA, Cunningham C. The impact of systemic infection on the progression of neurodegenerative disease. Nat Rev Neurosci. 2003;4:103–112. doi: 10.1038/nrn1032. [DOI] [PubMed] [Google Scholar]
- Griffin WS, Mrak RE. Interleukin-1 in the genesis and progression of and risk for development of neuronal degeneration in Alzheimer’s disease. J Leukoc Biol. 2002;72:233–238. [PMC free article] [PubMed] [Google Scholar]
- Huitinga I, van der Cammen M, Salm L, Erkut Z, van Dam A, Tilders F, Swaab D. IL-1beta immunoreactive neurons in the human hypothalamus: reduced numbers in multiple sclerosis. J Neuroimmunol. 2000;107:8–20. doi: 10.1016/s0165-5728(00)00248-4. [DOI] [PubMed] [Google Scholar]
- Pearson VL, Rothwell NJ, Toulmond S. Excitotoxic brain damage in the rat induces interleukin-1beta protein in microglia and astrocytes: correlation with the progression of cell death. Glia. 1999;25:311–323. [PubMed] [Google Scholar]
- Boutin H, LeFeuvre RA, Horai R, Asano M, Iwakura Y, Rothwell NJ. Role of IL-1alpha and IL-1beta in ischemic brain damage. J Neurosci. 2001;21:5528–5534. doi: 10.1523/JNEUROSCI.21-15-05528.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser SL, Doolittle TH, Lincoln R, Brown RH, Dinarello CA. Cytokine accumulations in CSF of multiple sclerosis patients: frequent detection of interleukin-1 and tumor necrosis factor but not interleukin-6. Neurology. 1990;40:1735–1739. doi: 10.1212/wnl.40.11.1735. [DOI] [PubMed] [Google Scholar]
- de Jong BA, Huizinga TW, Bollen EL, Uitdehaag BM, Bosma GP, van Buchem MA, Remarque EJ, Burgmans AC, Kalkers NF, Polman CH, Westendorp RG. Production of IL-1beta and IL-1Ra as risk factors for susceptibility and progression of relapse-onset multiple sclerosis. J Neuroimmunol. 2002;126:172–179. doi: 10.1016/s0165-5728(02)00056-5. [DOI] [PubMed] [Google Scholar]
- Loddick SA, Wong ML, Bongiorno PB, Gold PW, Licinio J, Rothwell NJ. Endogenous interleukin-1 receptor antagonist is neuroprotective. Biochem Biophys Res Commun. 1997;234:211–215. doi: 10.1006/bbrc.1997.6436. [DOI] [PubMed] [Google Scholar]
- Grundy RI, Rothwell NJ, Allan SM. Site-specific actions of interleukin-1 on excitotoxic cell death in the rat striatum. Brain Res. 2002;926:142–148. doi: 10.1016/s0006-8993(01)03325-x. [DOI] [PubMed] [Google Scholar]
- Merrill JE, Scolding NJ. Mechanisms of damage to myelin and oligodendrocytes and their relevance to disease. Neuropathol Appl Neurobiol. 1999;25:435–458. doi: 10.1046/j.1365-2990.1999.00200.x. [DOI] [PubMed] [Google Scholar]
- Brogi A, Strazza M, Melli M, Costantino-Ceccarini E. Induction of intracellular ceramide by interleukin-1 beta in oligodendrocytes. J Cell Biochem. 1997;66:532–541. doi: 10.1002/(sici)1097-4644(19970915)66:4<532::aid-jcb12>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Mason JL, Suzuki K, Chaplin DD, Matsushima GK. Interleukin-1beta promotes repair of the CNS. J Neurosci. 2001;21:7046–7052. doi: 10.1523/JNEUROSCI.21-18-07046.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson PB, Perry VH, Gordon S. Intracerebral injection of proinflammatory cytokines or leukocyte chemotaxins induces minimal myelomonocytic cell recruitment to the parenchyma of the central nervous system. J Exp Med. 1992;176:255–259. doi: 10.1084/jem.176.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry VH, Bell MD, Brown HC, Matyszak MK. Inflammation in the nervous system. Curr Opin Neurobiol. 1995;5:636–641. doi: 10.1016/0959-4388(95)80069-7. [DOI] [PubMed] [Google Scholar]
- Kolb M, Margetts PJ, Anthony DC, Pitossi F, Gauldie J. Transient expression of IL-1beta induces acute lung injury and chronic repair leading to pulmonary fibrosis. J Clin Invest. 2001;107:1529–1536. doi: 10.1172/JCI12568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher S, Winston SE, Fuller SA, Hurrell J. Immunoblotting and immunodetection. Ausubel FK, Brent R, Kingston R, Moore D, Seidman G, Smith J, Struhl K, editors. Boston, MA: John Wiley and Sons, Inc.; Current Protocols in Molecular Biology. 1995:pp 10.8.7–10.8.24. [Google Scholar]
- Lochmüller H, Jani A, Huard J, Prescott S, Simoneau M, Massie B, Karpati G, Acsadi G. Emergence of early region 1-containing replication-competent adenovirus in stocks of replication-defective adenovirus recombinants (DE1 + DE3) during multiple passages in 293 cells. Hum Gene Ther. 1994;5:1485–1491. doi: 10.1089/hum.1994.5.12-1485. [DOI] [PubMed] [Google Scholar]
- Le Gal La Salle G, Roberts JJ, Berrard S, Ridoux V, Stratford-Perricaudet LD, Perricaudet M, Mallet J. An adenovirus vector for gene transfer into neurons and glia in the brain. Science. 1993;259:988–990. doi: 10.1126/science.8382374. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. Orlando: Academic Press; The Rat Brain in Stereotaxic Coordinates. 1986 [Google Scholar]
- Anthony DC, Bolton SJ, Fearn S, Perry VH. Age-related effects of interleukin-1 beta on polymorphonuclear neutrophil-dependent increases in blood-brain barrier permeability in rats. Brain. 1997;120:435–444. doi: 10.1093/brain/120.3.435. [DOI] [PubMed] [Google Scholar]
- Kaur C, Ling EA. Study of the transformation of amoeboid microglial cells into microglia labelled with the isolectin Griffonia simplicifolia in postnatal rats. Acta Anat (Basel) 1991;142:118–125. doi: 10.1159/000147175. [DOI] [PubMed] [Google Scholar]
- Perry VH, Linden R. Evidence for dendritic competion in the developing retina. Nature. 1982;297:683–685. doi: 10.1038/297683a0. [DOI] [PubMed] [Google Scholar]
- Schmued LC, Hopkins KJ. Fluoro-Jade B: a high affinity fluorescent marker for the localization of neuronal degeneration. Brain Res. 2000;874:123–130. doi: 10.1016/s0006-8993(00)02513-0. [DOI] [PubMed] [Google Scholar]
- Dinarello CA. Interleukin-1. Cytokine Growth Factor Rev. 1997;8:253–265. doi: 10.1016/s1359-6101(97)00023-3. [DOI] [PubMed] [Google Scholar]
- Thomas CE, Abordo-Adesida E, Maleniak TC, Stone D, Gerdes CA, Lowenstein PR. Gene transfer into rat brain using adenoviral vectors. Crawley JN, Gerfen C, McKay R, Rogawski M, Sibley DR, Skolnick P, editors. Boston, MA: John Wiley & Sons, Inc.; Current Protocols in Neuroscience. 2000:pp 4.24.21–24.24.37. doi: 10.1002/0471142301.ns0424s13. [DOI] [PubMed] [Google Scholar]
- Stone D, Xiong W, Williams JC, David A, Lowenstein PR, Castro MG. Adenovirus expression of IL-1 and NF-kappaB inhibitors does not inhibit acute adenoviral-induced brain inflammation, but delays immune system-mediated elimination of transgene expression. Mol Ther. 2003;8:400–411. doi: 10.1016/s1525-0016(03)00178-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmoto Y, Wood MJ, Charlton HM, Kajiwara K, Perry VH, Wood KJ. Variation in the immune response to adenoviral vectors in the brain: influence of mouse strain, environmental conditions, and priming. Gene Ther. 1999;6:471–481. doi: 10.1038/sj.gt.3300851. [DOI] [PubMed] [Google Scholar]
- Simon HU. Neutrophil apoptosis pathways and their modifications in inflammation. Immunol Rev. 2003;193:101–110. doi: 10.1034/j.1600-065x.2003.00038.x. [DOI] [PubMed] [Google Scholar]
- Margetts PJ, Kolb M, Yu L, Hoff CM, Holmes CJ, Anthony DC, Gauldie J. Inflammatory cytokines, angiogenesis, and fibrosis in the rat peritoneum. Am J Pathol. 2002;160:2285–2294. doi: 10.1016/S0002-9440(10)61176-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibson NR, Blamire AM, Perry VH, Gauldie J, Styles P, Anthony DC. TNF-alpha reduces cerebral blood volume and disrupts tissue homeostasis via an endothelin- and TNFR2-dependent pathway. Brain. 2002;125:2446–2459. doi: 10.1093/brain/awf256. [DOI] [PubMed] [Google Scholar]
- Anthony DC, Ferguson B, Matyzak MK, Miller KM, Esiri MM, Perry VH. Differential matrix metalloproteinase expression in cases of multiple sclerosis and stroke. Neuropathol Appl Neurobiol. 1997;23:406–415. [PubMed] [Google Scholar]
- Schnell L, Fearn S, Schwab ME, Perry VH, Anthony DC. Cytokine-induced acute inflammation in the brain and spinal cord. J Neuropathol Exp Neurol. 1999;58:245–254. doi: 10.1097/00005072-199903000-00004. [DOI] [PubMed] [Google Scholar]
- Carlson NG, Wieggel WA, Chen J, Bacchi A, Rogers SW, Gahring LC. Inflammatory cytokines IL-1 alpha, IL-1 beta, IL-6, and TNF-alpha impart neuroprotection to an excitotoxin through distinct pathways. J Immunol. 1999;163:3963–3968. [PubMed] [Google Scholar]
- Patel HC, Boutin H, Allan SM. Interleukin-1 in the brain: mechanisms of action in acute neurodegeneration. Ann NY Acad Sci. 2003;992:39–47. doi: 10.1111/j.1749-6632.2003.tb03136.x. [DOI] [PubMed] [Google Scholar]
- Hardman CD, Henderson JM, Finkelstein DI, Horne MK, Paxinos G, Halliday GM. Comparison of the basal ganglia in rats, marmosets, macaques, baboons, and humans: volume and neuronal number for the output, internal relay, and striatal modulating nuclei. J Comp Neurol. 2002;445:238–255. doi: 10.1002/cne.10165. [DOI] [PubMed] [Google Scholar]
- Sathornsumetee S, McGavern DB, Ure DR, Rodriguez M. Quantitative ultrastructural analysis of a single spinal cord demyelinated lesion predicts total lesion load, axonal loss, and neurological dysfunction in a murine model of multiple sclerosis. Am J Pathol. 2000;157:1365–1376. doi: 10.1016/S0002-9440(10)64650-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badovinac V, Mostarica-Stojkovic M, Dinarello CA, Stosic-Grujicic S. Interleukin-1 receptor antagonist suppresses experimental autoimmune encephalomyelitis (EAE) in rats by influencing the activation and proliferation of encephalitogenic cells. J Neuroimmunol. 1998;85:87–95. doi: 10.1016/s0165-5728(98)00020-4. [DOI] [PubMed] [Google Scholar]
- Martin D, Near SL. Protective effect of the interleukin-1 receptor antagonist (IL-1ra) on experimental allergic encephalomyelitis in rats. J Neuroimmunol. 1995;61:241–245. doi: 10.1016/0165-5728(95)00108-e. [DOI] [PubMed] [Google Scholar]
- Reiseter BS, Miller GT, Happ MP, Kasaian MT. Treatment of murine experimental autoimmune encephalomyelitis with a myelin basic protein peptide analog alters the cellular composition of leukocytes infiltrating the cerebrospinal fluid. J Neuroimmunol. 1998;91:156–170. doi: 10.1016/s0165-5728(98)00171-4. [DOI] [PubMed] [Google Scholar]
- Gaupp S, Pitt D, Kuziel WA, Cannella B, Raine CS. Experimental autoimmune encephalomyelitis (EAE) in CCR2(−/−) mice: susceptibility in multiple strains. Am J Pathol. 2003;162:139–150. doi: 10.1016/S0002-9440(10)63805-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frei E, Klusman I, Schnell L, Schwab ME. Reactions of oligodendrocytes to spinal cord injury: cell survival and myelin repair. Exp Neurol. 2000;163:373–380. doi: 10.1006/exnr.2000.7379. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.