Abstract
Calcineurin is an important signaling molecule in the kidney and may be involved in a variety of processes. The phosphatase subunit of calcineurin (CnA) has three isoforms, α, β, and γ. In this study, we investigated the effect of loss of calcineurin A-α (CnA-α) or calcineurin A-β (CnA-β) on the development and function of the kidney. Total calcineurin expression and activity was significantly reduced in whole kidney homogenates from both CnA-α −/− and CnA-β −/− mice. Kidneys of CnA-β −/− mice appear normal and the mice develop with no phenotypic abnormalities. In contrast, kidneys of CnA-α −/− animals fail to fully develop. In particular, postnatal maturation of the nephrogenic zone (NZ) is defective. Within the NZ, glomeruli also fail to mature and lack mesangial cells. In addition to alterations in development, there is an absence of proliferation and an increase of cell death in the NZ with loss of CnA-α. Finally, increased collagen deposition is observed and serum creatinine levels are significantly increased in CnA-α −/− animals compared to wild-type littermates, indicating that kidney function is impaired. In summary, absence of CnA-α but not CnA-β leads to a defect in normal maturation of the NZ and glomeruli, alterations in the cell cycle, and impaired kidney function.
Calcineurin is a calcium-dependent, serine/threonine phosphatase that functions as a signaling intermediate in a variety of cell signaling pathways. Initially characterized as a component of the activated T cell receptor (TCR) complex and as a target of therapeutically successful immune-suppressing drugs,1 calcineurin has since been identified as a downstream signaling element in a variety of signal transduction systems. Factors including angiotensin II,2–4 IGF-I,5–8 and TGFβ9 have all been shown to signal through calcineurin. In addition, calcineurin has been shown to be an important intracellular phosphatase in the regulation of cytoskeletal integrity in neurons.10,11 Dephosphorylation of the cytoskeletal component tau by calcineurin maintains neuron integrity. Build-up of hyperphosphorylated tau, one feature of neurofibrillary plaques that are characteristic of Alzheimer’s disease, is due at least in part to decreased activity of calcineurin.12 Calcineurin has also been implicated in a number of other organ systems including the heart, where it participates in hypertrophic responses,13–15 and the kidney where inhibitors of calcineurin result in renal dysfunction, matrix accumulation, and fibrosis.16,17
Calcineurin is made up of a catalytic subunit called A and a regulatory subunit, designated B. The A subunit contains the phosphatase domain which is activated only when the B subunit is bound to both calmodulin and calcium. There are three known isoforms of the A subunit, α, β, and γ. Calcineurin A-α (CnA-α) and -β (CnA-β) are reported to be widely expressed while the γ isoform is limited to the testes and, to a lesser extent, the brain.1 Despite the broad tissue distribution of CnA-α and CnA-β, there appears to be some specificity of action among the isoforms. For example, under hypertrophic conditions, CnA-β appears to be specifically up-regulated in the heart15 while CnA-α appears to be the predominant isoform up-regulated in the diabetic kidney.18
Further evidence of tissue-specific action of calcineurin A isoforms is seen in transgenic mice lacking each isoform. CnA-α knockout mice were created and develop brain lesions consistent with a build-up of hyperphosphorylated tau19 and show memory impairment.20 Interestingly, the immune systems of the mice are essentially normal and T cell responses to agonists are only partially impaired under culture conditions.21,22 In contrast, mice lacking CnA-β develop normally but fail to produce mature T cells.23 These mice also show an impaired cardiac hypertrophic response.24 Mice lacking calcineurin B or mice lacking both CnA-α and CnA-β die in utero,13,25,26 suggesting that there is some degree of compensation between the two isoforms.
Use of calcineurin inhibitors is therapeutically limited because of nephrotoxicity. Moreover, use of calcineurin inhibitors during pregnancy is becoming increasingly common. As a result, there is a need for greater understanding of the role of calcineurin in kidney function and development. Genetic knockout of calcineurin A isoforms provides unique model systems to study the role of calcineurin in normal kidney development and function. Therefore, the purpose of this study was to examine the effect of the loss of calcineurin isoforms on the development and function of the kidney.
Materials and Methods
Materials
Total CnA antibody used in Western blotting and anti-platelet endothelial cell adhesion molecule (PECAM) antibody were from Chemicon (Temecula, CA), synaptopodin was from Research Diagnostics International (Flanders, NJ), p27 antibodies were purchased from Santa Cruz (Santa Cruz, CA), and α-smooth muscle actin antibody was purchased from Sigma (St. Louis, MO). Proliferating cell nuclear antigen (PCNA) antibody was obtained from Dako (Carpinteria, CA). Antibodies used for loading controls included actin (from Sigma) and Erk1/2 (Santa Cruz).
Transgenic Mice
Calcineurin A-α Transgenic Mice
Mice were created by J. Seidman (Howard Hughes Medical Institute, Harvard Medical School, Boston, MA) as previously described21 and kindly provided to our laboratory. Animals were maintained and bred in the animal facility at the Audie Murphy Veterans Hospital, San Antonio, in accordance with Institutional Animal Care and Use Committee standards. Genotypes of offspring from heterozygous breeding pairs were determined by PCR reaction using the following primers: 5′ - GGC AGG AGA GTA AAT TCT TGC, 3′ - GTG GAA TTC TCT GGA GAC AAA CCA CC, and neo - TCT TGA TTC CCA CTT TGT GGT TCT A.
The majority of CnA-α −/− mice have a shortened lifespan (unpublished observation also confirmed by J. Seidman). Therefore, most of the studies described were conducted at 18 days of age, a time point when postnatal maturation of the kidney is complete and when majority of CnA-α −/− mice were viable. CnA-α −/− mice were created on a mixed genetic background. Therefore all experiments were carried out with wild-type and, where indicated, heterozygous littermates.
Calcineurin A-β Transgenic Mice
These mice were created as previously described24 and kindly provided to our laboratory by J. Molkentin (Children’s Hospital Medical Center, Cincinnati, OH). Animals were maintained and bred in the animal facility at the Audie Murphy Veterans Hospital, San Antonio, in accordance with Institutional Animal Care and Use Committee standards. Genotypes of offspring from heterozygous breeding pairs were determined by PCR reaction using the following primers: 5′ −TCA GTA AGG TGT CTG CAT TC, 3′ −TTA GGC TAT ATT AAG CAA TCT G, and neo −GAA GGA GCC AAG CTG CTA TT.
Creatinine Determinations
Urine was collected by bladder massage and blood was collected at the time of sacrifice by cardiac puncture. Serum and urine creatinine levels were determined by the Jaffe method, according to the manufacturer’s instructions (Sigma).
Western Blot
Whole kidneys were homogenized in TNESV lysis buffer (50 mmol/L Tris-HCl (pH 7.4), 2 mmol/L ethylenediamine tetraacetic acid (EDTA), 1% NP-40, 100 mmol/L NaCl, 100 mmol/L Na orthovanadate, 100 μg/ml leupeptin, 20 μg/ml aprotonin, and 10−7 M phenylmethylsulfonyl [PMSF]). Twenty-five μg of protein was analyzed by 10% SDS-PAGE. Following transfer of the proteins to nitrocellulose, the membrane was incubated in 5% milk-TBST (20 mmol/L Tris-HCl, pH 7.6, 137 mmol/L NaCl, 0.1% Tween 20) and then immunoblotted with 1:500 dilution of primary antibodies. Horse-radish peroxidase (HRP)-conjugated donkey-anti-rabbit secondary antibodies were added at 1:2000 and proteins were visualized by enhanced chemiluminescence (Pierce, Rockford, IL).
Calcineurin Phosphatase Assay
Calcineurin phosphatase activity was determined following a protocol published by Fruman et al.27 Briefly, the calcineurin substrate, RII, was phosphorylated in vitro with 250 U recombinant PKA, 50 mmol/L adenosine triphosphate (ATP), 50μCi [γ32-P]ATP, 0.15 mmol/L RII, and 500 μl of 2X reaction buffer (40 mmol/L MOPS, 4 mmol/L MgCl2, 0.1 mmol/L CaCl2, 0.4 mmol/L EDTA, 0.8 mmol/L ethylene glycol-bis(2-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA), 0.5 mmol/L dithiothreitol (DTT), and 0.1 mg/ml bovine serum albumin [BSA]). Lysates were prepared by resuspending homogenized kidneys in a hypotonic lysis buffer (50 mmol/L Tris (pH 7.5), 1 mmol/L EDTA, 1 mmol/L EGTA, 0.5 mmol/L DTT, 50 μg/ml PMSF, 10 μg/ml leupeptin, and 10 μg/ml aprotinin) followed by three cycles of freeze-thawing in liquid nitrogen and a 30°C water bath. Calcineurin activity in each sample was determined by incubating equal parts lysate, 3X reaction buffer (40 mmol/L Tris (pH 7.5), 0.1 mol/L NaCl, 6 mmol/L MgCl2, 0.1 mmol/L CaCl2, 0.5 mmol/L DTT, 500 nmol/L okadaic acid, and 0.1 mg/ml BSA), and labeled RII peptide at 30°C for 10 minutes. The reaction was stopped by addition of 0.1 mol/L KPO4 in 5% trichloro acetic acid (TCA). Control reactions were simultaneously performed using a reaction buffer in which EGTA was substituted for CaCl2. To determine the amount of phosphate released by calcineurin in each sample, reactions were then added to PolyPrep columns (BioRad, Hercules, CA) containing AG-50X Dowex ion exchange resin (BioRad) prepared as described.27 Finally, 5 ml scintillation fluid was added to the flow-through from each column and the released phosphate was measured in a scintillation counter. Final calcineurin activity was calculated by subtracting the calcium-independent activity in EGTA buffer from each reaction and expressed per μg protein.
Histology
Kidneys were immediately immersed in formalin or quickly frozen in liquid nitrogen for further analyses. Routine histology was performed in hematoxylin and eosin-stained, 4-μm-thick sections of kidneys from each genotype. In addition, kidneys from three to five animals of each genotype were analyzed using TdT-mediated d-UTP nick-end labeling (TUNEL) assay to identify cells undergoing cell death, and stained with trichrome to identify extracellular matrix deposition.
Immunohistochemistry
Four-μmol/L-thick paraffin-embedded tissue sections were prepared by de-waxing followed by quenching of endogenous peroxidases by incubation in 0.3% H2O2 in methanol for 30 minutes. Antigens were unmasked by incubation in 10 mmol/L citrate buffer at 80°C for 10 minutes. Sections were then rehydrated in PBS-0.1% BSA for 15 minutes before addition of appropriate blocking serum for 15 minutes. Sections were incubated with primary antibodies (dilutions of 1:100 to 1:250) overnight in a humidified chamber at 4°C. On the following day, sections were washed three times in PBS-0.1% BSA and then incubated with biotinylated secondary antibodies (dilutions of 1:100) for 1 hour at room temperature. Bound antibody was identified by immunoperoxidase ABC staining following the manufacturer’s instructions (Vector Laboratories, Burlingame, CA). In addition, sections were counterstained with hemotoxylin. Finally, coverslips were mounted with Permount (Sigma) and sections were viewed by brightfield microscopy.
Immunofluorescence
Six-μmol/L-thick frozen sections were mounted on glass slides and then fixed in acetone. Sections were rehydrated in PBS-0.1% BSA before blocking with the appropriate IgG. Primary antibodies were added at concentrations between 10 and 20 μg/ml for 1 hour at room temperature. After incubation with primary antibodies, sections were washed three times for 5 minutes each time in PBS-0.1% BSA. Fluorescence-conjugated secondary antibodies were added at dilutions of 1:100 for 45 minutes at room temperature followed by washing in PBS-0.1% BSA. Sections were mounted with Crystal Mount (Dako) and allowed to dry before viewing with fluorescence microscopy. Cell types within the glomerulus were identified by detecting proteins characteristic of each cell type. Synaptopodin was used to detect mature podocytes, PECAM to detect endothelial cells, and α-smooth muscle actin was used to identify mesangial cells.
Plastic Embedding and Electron Microscopy
Cortices from kidneys of animals of each genotype were dissected, finely chopped, and then embedded in plastic. Ultra-thin sections were cut and stained with toludine blue. Sections were examined by electron microscopy.
Morphometric Analyses
Kidney blocks were oriented to optimally cut sections through the central axis of the kidney to allow full measurement of all zones of the kidney including the tip of the papilla. Regions of the kidneys were determined by anatomical boundaries as follows: the cortex is defined as the outer portion of the kidney containing glomeruli (cortical and juxtamedullary), proximal, and distal tubules beginning from the capsule to the transition point of S2 to S3 segments of proximal tubules. The outer stripe of the outer medulla (OSOM) includes S3 proximal tubules and thick ascending loop of Henle to the boundary where S3 transitions to thin descending loops of Henle. The inner stripe of the outer medulla (ISOM) includes thin and thick loops of Henle flanked by S3/thin limb and thin limb/thick limb of Henle boundaries. The inner medulla is defined as the region from the inner stripe of the outer medulla boundary to the tip of the papilla. Collecting ducts (cortical and medullary) are located in all zones of the kidney.
Results
Defects in Nephrogenic Zone Development with Loss of CnA-α But Not CnA-β
The phenotypes of mice which lack the α isoform of calcineurin A α (CnA-α)21 and mice lacking CnA-β24 have been reported. However, the effects of CnA-α or CnA-β knockout on the development and function of the kidney have not been described.
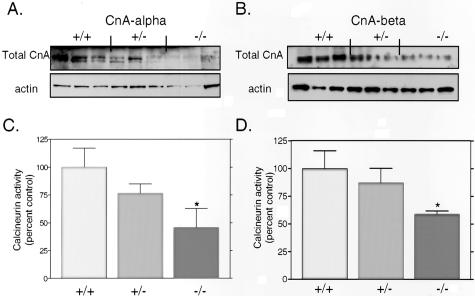
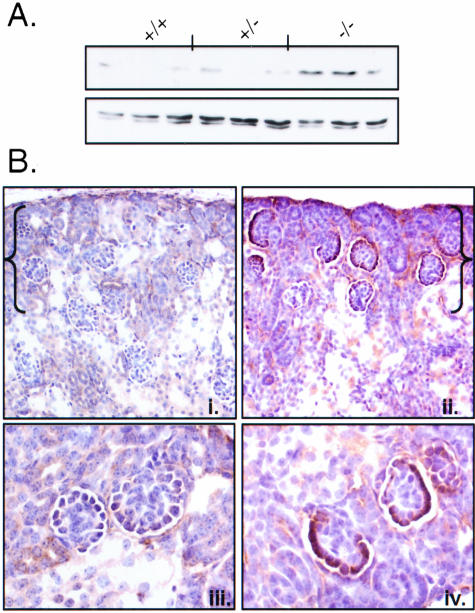
Using total protein lysates from kidneys of wild-type, heterozygous, and null animals, we examined expression of total CnA in CnA-α and CnA-β transgenic mice. Figure 1A shows that expression of total calcineurin is decreased in both CnA-α heterozygous and homozygous animals. Similarly, expression of total calcineurin was reduced in whole kidney homogenates of CnA-β −/− mice compared to wild-type and heterozygous littermates (Figure 1B). We next examined calcineurin activity in whole kidney homogenates from each transgenic line and found a roughly 60% decrease in activity from CnA-α −/− kidneys (Figure 1C, P < 0.05) and an approximate 40% decrease in activity from CnA-β −/− kidneys (Figure 1D, P < 0.05).
Figure 1.
Calcineurin A expression and activity in knockout animals. A: Expression of total calcineurin A was examined in protein lysates from homogenized kidneys of CnA-α wild-type, heterozygous, and homozygous mice by immunoblotting with an antibody specific for the phosphatase domain. Levels of actin were also determined to confirm equal loading of samples. B: Expression of total calcineurin A was examined in protein lysates from homogenized kidneys of CnA-β wild-type, heterozygous, and homozygous mice by immunoblotting with an antibody specific for the phosphatase domain. Levels of actin were also determined to confirm equal loading of samples. C: Calcineurin activity was determined in whole kidney lysates of CnA-α wild-type, heterozygous, and homozygous animals using an in vitro assay previously described (see Materials and Methods). Data shown are the mean levels of calcineurin activity in five animals of each genotype ± SEM. *, P < 0.05 compared to wild-type. D: Calcineurin activity was determined in whole kidney lysates of CnA-β wild-type, heterozygous, and homozygous animals using an in vitro assay previously described (see Materials and Methods). Data shown are the mean levels of calcineurin activity in five animals of each genotype ± SEM. *, P < 0.05 compared to wild-type.
Body mass and individual organ weights from CnA-α and CnA-β wild-type, heterozygous, and knockout animals were measured at 18 days of age and the results are summarized in Table 1. CnA-β −/− animals are not different from wild-type whereas CnA-α −/− mice are significantly smaller. Further, the average weights of brain, heart, liver, and kidney of CnA-α−/− mice are all significantly reduced compared to wild-type animals. Heart, liver, and kidney weights also show a trend toward a decrease in heterozygous animals. To determine whether the reduction in organ weights was consistent with overall reduction in body mass of CnA-α transgenic animals, organ weights were normalized by body weight, and the results are presented in Table 2. Heart size is proportional to body size, but both liver and kidney weights are significantly reduced independent of body weight. Moreover, heterozygous animals demonstrate a reduction in kidney mass that is significantly different from wild-type.
Table 1.
Growth and Organ Weights of Transgenic Animals at 18 Days Postnatal
| n | Body weight (g) | Femur (cm) | Brain (mg) | Heart (mg) | Liver (mg) | Kidney (mg) | |
|---|---|---|---|---|---|---|---|
| CnAα | |||||||
| +/+ | 11 | 9.56 ± .2 | 1.6 ± .04 | 385 ± 6.7 | 60.7 ± 4.7 | 392 ± 16 | 145 ± 5.3 |
| +/− | 11 | 9.62 ± .2 | 1.6 ± .03 | 400 ± 8.7 | 57.6 ± 3.0 | 360 ± 14 | 130 ± 5.4 |
| −/− | 9 | 5.69 ± .4* | 1.5 ± .04 | 357 ± 8.4** | 33.0 ± 2.0* | 169 ± 12* | 70.6 ± 2.7* |
| CnAβ | |||||||
| +/+ | 8 | 9.60 ± .8 | 1.74 ± .05 | 425 ± 11 | 59.1 ± 5.8 | 383 ± 37 | 139 ± 11 |
| +/− | 8 | 9.62 ± .9 | 1.76 ± .04 | n/a | 52.3 ± 3.7 | 368 ± 32 | 136 ± 8.2 |
| −/− | 8 | 9.06 ± .8 | 1.75 ± .05 | 402 ± 11 | 53.5 ± 5.0 | 386 ± 43 | 140 ± 9.3 |
Data shown are the mean ± SEM.
, p < 0.01 compared to +/+;
, p < 0.05 compared to +/+.
Table 2.
CnA-α Organ Weights Normalized by Body Weight
| n | Heart (% bw) | Liver (% bw) | Kidney (% bw) | |
|---|---|---|---|---|
| +/+ | 11 | .610 ± .04 | 4.01 ± .10 | 1.52 ± .04 |
| +/− | 11 | .587 ± .03 | 3.81 ± .15 | 1.38 ± .04* |
| −/− | 9 | .646 ± .04 | 3.28 ± .09** | 1.27 ± .05** |
Data shown are the mean ± SEM.
, p < 0.05 compared to +/+;
, p < 0.01 compared to +/+.
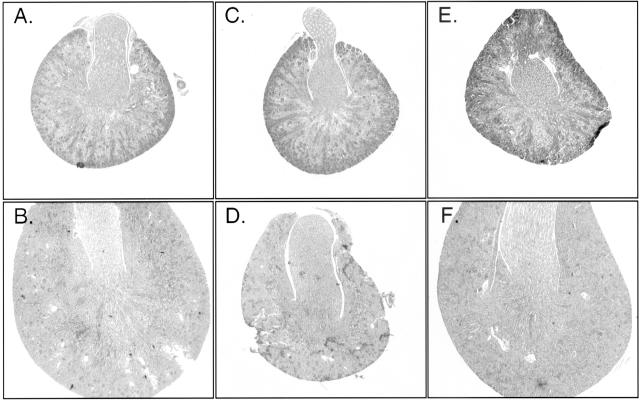
Examination of organ sizes indicated that kidney growth may be particularly impaired in CnA-α −/− mice. Therefore, kidney sections from 4- and 18-day-old wild-type and knockout animals were examined. Figure 2 shows that in wild-type animals, postnatal kidney maturation progresses from day 4 (Figure 2A), which still retain many developmental features, to fully mature by 18 days postnatal (Figure 2B). Kidneys increase in size, develop mature medullary rays, and develop significant cortical mass. At 4 days postnatal, kidneys from CnA-α null animals are relatively normal and comparable to wild-type animals (Figure 2C). However, by 18 days postnatal, there is a noticeable delay of maturation evident as a lack of overall mass, poorly defined medullary rays, and decreased cortical mass (Figure 2D) compared to wild-type animals. Kidneys from CnA-β null mice appear to develop normally postnatally (Figure 2, E and F).
Figure 2.
Effect of CnA-α −/− on postnatal kidney maturation. Cross-sections of kidneys from 4 day and 18 day postnatal wild-type and CnA-α and CnA-β homozygous animals were stained with hemotoxylin and eosin. Original magnification, ×40. All pictures are to scale. A: Four-day-old wild-type animal, B: Eighteeen-day-old wild-type animal, C: 4-day-old CnA-α −/− animal, D: Eighteen-day-old CnA-α −/− animal, E: Four-day-old CnA-β −/− animal, F: Eighteeen-day-old CnA-β −/− animal.
To determine what regions of the kidney are most affected by loss of CnA-α, the cross-sectional axes of kidney sections from wild-type, heterozygous, and knockout animals were examined and the lengths of each of the four major regions were determined and are presented in Table 3. There were no significant differences in the inner medulla, inner stripe of the outer medulla (ISOM), or cortex of heterozygous animals compared to the wild-type animals. Interestingly, the outer stripe of the outer medulla (OSOM) of heterozygous animals was also significantly decreased. When the regions of the CnA-α −/− kidneys were examined, all four regions were found to be affected by loss of calcineurin. However, the inner medulla and ISOM were only mildly altered (18% decrease and 16% decrease, respectively) while the OSOM and cortical regions were more markedly decreased (55% and 47%, respectively).
Table 3.
CnA-α Mice: Cross-Sectional Axis of Kidney Regions (mm)
| n | Inner medulla | ISOM | OSOM | Cortex | |
|---|---|---|---|---|---|
| +/+ | 9 | 1.63 ± .10 | 0.68 ± .04 | 0.63 ± .04 | 0.93 ± .04 |
| +/− | 8 | 1.53 ± .15 | 0.83 ± .08 | 0.47 ± .05* | 0.93 ± .06 |
| −/− | 8 | 1.33 ± .09* | 0.57 ± .09 | 0.29 ± .03** | 0.49 ± .04** |
Data shown are mean ± SEM.
, p < 0.05 compared to +/+;
, p < 0.001 compared to +/+.
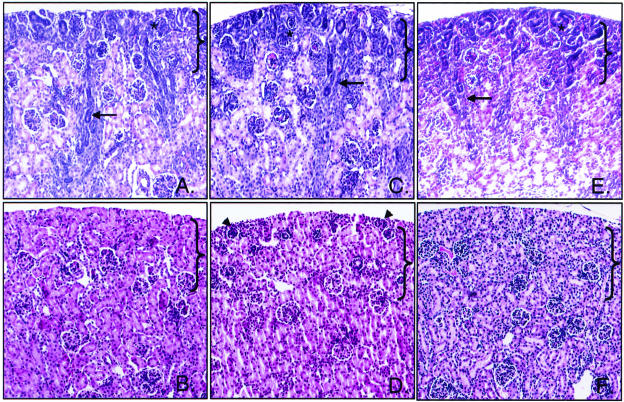
Closer examination of cortical sections from wild-type and knockout animals at 4 days postnatal and 18 days postnatal shown in Figure 3 confirms this observation. At 4 days postnatal, wild-type, CnA-α −/−, and CnA-β −/− cortical sections have a characteristic range of maturation from immature late-S phase glomeruli (identified with an *) to fully mature, juxtamedullary glomeruli. Medullary rays are also visible in all genotypes at this stage (indicated by arrows). By 18 days postnatal, however, the maturation process evident in wild-type and CnA-β −/− animals is absent in CnA-α knockout animals. Poorly developed, surface glomeruli persist (arrowhead), and there is a significant lack of maturation of tubules within the NZ.
Figure 3.
Effect of CnA-α −/− on postnatal development of the nephrogenic zone (NZ). Cortical sections of kidneys from 4-day-old and 18-day-old animals were stained with hemotoxylin and eosin and examined by light microscopy. Original magnification was ×400. Brackets designate the nephrogenic zone (NZ). A: Four-day-old wild-type animal. B: Eighteen-day-old wild-type animal. C: Four-day-old CnA-α −/− animal. D: Eighteen-day-old CnA-α −/− animal. E: Four-day-old CnA-β −/− animal. F: Eighteen-day-old CnA-β −/− animal. Arrows identify developing cortical rays, asterisks denote late S-phase glomeruli, and arrowheads identify poorly developed, surface glomeruli.
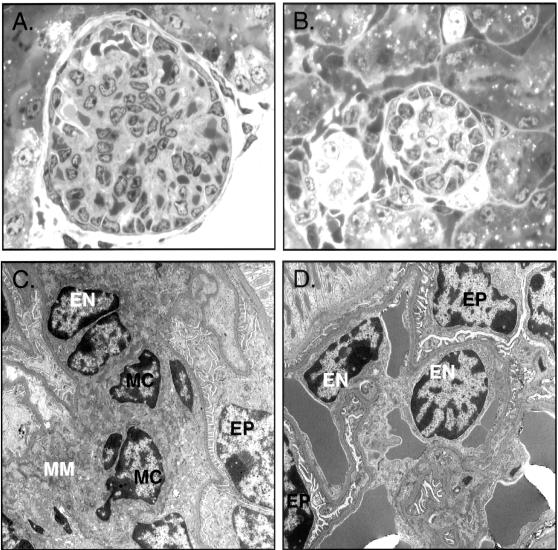
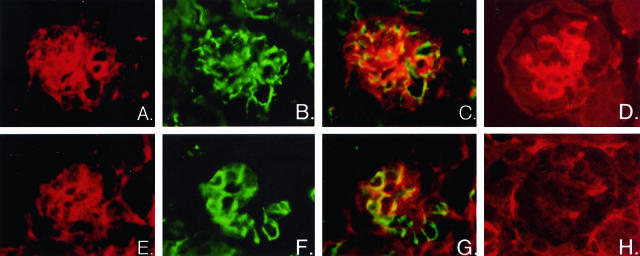
While there were no differences in total number of immature and mature glomeruli (data not shown), there were alterations in the development of glomeruli within the NZ of CnA-α −/− animals. Figure 4 shows that not only are NZ glomeruli from CnA-α knockout animals smaller, but there appears to be a defect in cellular development (Figure 4, A and B). Ultrastructural evaluation by electron microscopy revealed a marked attenuation of mesangial cells in NZ glomeruli of CnA-α −/− animals (Figure 4, C and D). Furthermore, no mesangial matrix was observed by electron microscopy or by staining with PAS (data not shown). To further investigate the cell types present in wild-type and knockout NZ glomeruli, mesangial, epithelial, and endothelial cells were examined by identification of specific markers for each cell type. Figure 5 shows that while mesangial cells are readily detected by anti-α-smooth muscle actin antibodies in wild-type kidneys (Figure 5A), there was again a marked attenuation of cells within glomeruli of CnA-α −/− animals the mesangial cell marker (Figure 5E). Also consistent with results obtained by electron microscopic evaluation of glomeruli, both endothelial and epithelial cells were detected by expression of characteristic proteins PECAM and synaptopodin, respectively (wild-type, Figure 5, B, C, and D (merged image) and CnA-α −/−, Figure 5, F, G, and H (merged image)).
Figure 4.
Loss of calcineurin A-α results in marked reduction in mesangial cells in juxtamedullary glomeruli. Ultra-thin cortical sections from 18-day-old wild-type and −/− animals were stained with toluidine blue and examined by light microscopy and by high-power electron microscopy. A: Glomerulus from a wild-type animal. Original magnification, ×600. B: Glomerulus from a CnA-α −/− animal. Original magnification, ×600. C: Glomerulus from a wild-type animal. Epithelial cells (EP), endothelial cells (EN), mesangial cells (MC), and mesangial matrix (MM) are labeled. Original magnification, ×5600. D: A representative cortical glomerulus from a CnA-α −/− animal. Epithelial cells (EP) and endothelial cells (EN) are labeled. Original magnification, ×5600.
Figure 5.
Expression of cell-specific markers demonstrates lack of mesangial cells in CnA-α −/− juxtamedullary glomeruli. Expression of cell markers was determined in cortical sections from 18- day-old wild-type (A–D) and CnA-α −/− animals (E–H) by immunofluorescence using specific antibodies to synaptopodin to identify podocytes (A and E) and PECAM to detect endothelial cells (B and F) and their areas of co-expression (merged images C and G). α-smooth muscle actin was used to identify mesangial cells in juxtamedullary glomeruli of newborn animals (D and H). Data shown are representative of at least three animals.
Alterations in the Cell Cycle within the NZ of CnA-α −/− Kidneys
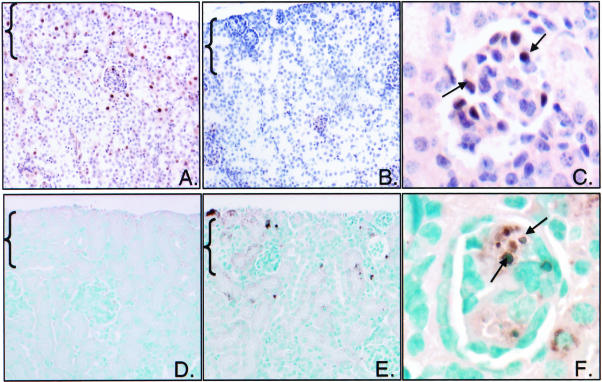
The lack of maturation in the NZ of CnA-α knockout mice was further examined. First, proliferation within the NZ was investigated by identifying cells that stain positive for PCNA (Figure 6). In wild-type animals, PCNA staining is strongest within the NZ and occurs progressively less often in the inner, more mature areas of the cortex (Figure 6A). In contrast, there is very little evidence of PCNA-positive cells within the NZ of CnA-α knockout animals (Figure 6B). However, proliferating cells could be identified in some mature glomeruli (Figure 6C) indicating that there is a defect in proliferation specific to the NZ and not a generalized proliferative failure in CnA-α −/− animals. PCNA staining in the NZ was not different in CnA-β wild-type and knockout animals (data not shown).
Figure 6.
Decreased proliferation and increased cell death in the NZ of homozygous animals. Proliferating cells were identified in cortical sections of 18-day-old wild-type and CnA-α −/− animals by immunohistochemical staining with anti-PCNA antibody (A–C). PCNA expression was detected with DAB chromagen and sections were counterstained with hemotoxylin. The nephrogenic zone (NZ) is indicated with brackets. A: PCNA expression in an 18-day-old wild-type animal. B: PCNA expression in an 18-day-old −/− animal. C: PCNA expression in a representative mature glomeruli of an 18-day-old −/− animal. Arrows indicate cells positive for PCNA expression. Data shown are representative of at least three animals of each genotype. Cortical sections from wild-type and CnA-α −/− animals were examined for evidence of cell death using TUNEL method (see Materials and Methods). TUNEL-positive cells were detected using the DAB chromogen and sections were counter-stained with methyl green. The nephrogenic zone (NZ) is indicated with brackets. D: TUNEL stain of 18-day-old wild-type cortical section. E: TUNEL stain of an 18-day-old −/− cortical section. F: TUNEL stain of a representative glomeruli from an 18-day-old −/− animal. Arrows indicate a glomeruli positive for cell death. Data shown are representative of at least three animals of each genotype.
Next, cell death was examined in the cortex of wild-type and CnA-α knockout animals by TUNEL assay. In wild-type cortical sections, there is very little evidence of cell death (Figure 6D). Loss of CnA-α, however, results in a significant increase in cell death, particularly within the NZ (Figure 6E) and juxtamedullary glomeruli (Figure 6F). Further examination of individual glomeruli revealed that there is a 3.5-fold increase in the number of glomeruli that show signs of cell death from 2.1% in wild-type animals to 7.4% in knockout animals (P < 0.05). There was no difference in rates of cell death in the NZ with loss of CnA-β (data not shown).
Decreased proliferation and increased cell death in the NZ indicate an alteration in the cell cycle due to loss of CnA-α. The cyclin-dependent kinase inhibitor p27 has been implicated in control of proliferation and cell survival in the NZ.28–30 Therefore, we examined expression of p27 in CnA-α +/+ and −/− kidneys. Figure 7A shows that expression of p27 is increased in CnA-α knockout kidneys compared to kidneys from wild-type and heterozygous animals. The proliferative defects in CnA-α mice are concentrated in the NZ. As such, we examined the regions of CnA-α kidneys to determine where p27 is overexpressed by immunohistochemistry (Figure 7B). Figure 7, Bi and Biii, show normal expression of p27 in a 4 day old mouse kidney. Compared to wild-type, CnA-α knockout results in a dramatic overexpression of p27 in the NZ (Figure 7Bii) and specifically in the epithelium of NZ glomeruli (Figure 7Biv). Loss of CnA-β has no effect on p27 expression in the NZ (data not shown).
Figure 7.
Loss of CnA-α results in increased p27 expression in the NZ. A: p27 protein expression was examined in whole kidney homogenates by direct immunoblotting. Each lane represents one animal. Actin was also detected as a control for equal loading. B: p27 expression was determined by immunohistochemistry in wild-type and CnA-α −/− mice using a specific antibody. Protein expression was detected with DAB chromagen and sections were counter-stained with hemotoxylin. The nephrogenic zone (NZ) is indicated in brackets. (i) Four-day-old wild-type animal showing normal p27 expression, (ii) Four-day-old CnA-α −/− animal showing increased p27 expression, (iii) higher magnification of wild-type cortical section showing normal p27 expression in glomeruli, and (iv) higher magnification of CnA-α −/− cortical section showing increased p27 expression in glomeruli. Results are representative of at least three animals of each genotype.
Kidney Function Is Impaired
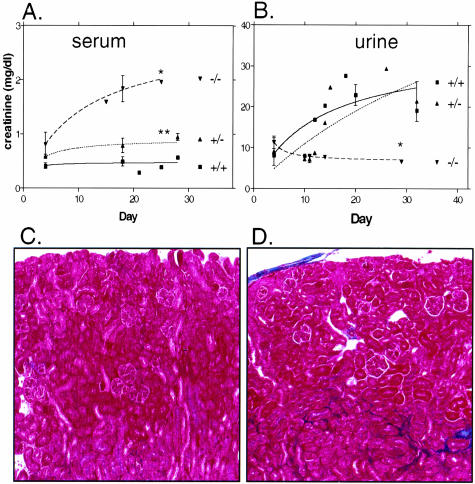
Finally, we investigated the effect of loss of CnA-α and CnA-β on kidney function. First, we examined creatinine excretion as determined by levels of creatinine in both serum and urine from wild-type, heterozygous, and knockout animals. Figure 8A shows that wild-type animals maintain very low serum levels of creatinine throughout the first 4 weeks postnatal. Urine creatinine excretion increases steadily to reach a stable level between 2 and 3 weeks of age, consistent with final postnatal maturation of the kidney (Figure 8B). Serum and urine creatinine levels of CnA-β −/− mice were not different from wild-type (data not shown). Interestingly, CnA-α heterozygous animals show a slight increase in serum creatinine levels significantly different from wild-type animals. However, urine creatinine secretion in heterozygous animals is not different from wild-type mice. Finally, CnA-α −/− mice show significant elevations of serum creatinine by 4 days of age that increase throughout the study period. In addition, knockout animals demonstrate a significant decrease in urine creatinine concentration compared to wild-type and heterozygous animals. Next, we examined collagen deposition by trichrome stain in wild-type and knockout kidney sections. Figure 8C shows that there is little evidence of collagen deposition in wild-type and CnA-β −/− animals but CnA-α −/− kidneys demonstrate an increased amount of collagen deposition (Figure 8D). Interestingly, the area of increased extracellular matarix (ECM) appears to be limited to the tubulointerstitium and does not include glomeruli. There was no difference in trichrome staining with loss of CnA-β (data not shown). Taken together, the results suggest a significant impairment of kidney function in CnA-α knockout animals.
Figure 8.
Evidence of impaired kidney function in CnA-α knockout mice. Serum creatinine (A) and urine creatinine (B) levels were determined in wild-type, heterozygous, and homozygous mice over time. Data shown are the mean of two to six measurements at multiple time points ± SEM. A curve was generated using non-linear regression. *, P < 0.001 and **, P < 0.01 compared to wild-type. Presence of ECM, particularly collagen, was detemined in cortical sections of 18-day-old wild-type (C), and CnA-α −/− (D) animals by trichrome staining (see Materials and Methods). Collagen deposition is evident as areas that stain blue. Results are representative of at least three animals of each genotype.
Discussion
In this study, we describe for the first time the effect of loss of calcineurin A isoforms on kidney function and development. First, we show that loss of CnA-α or CnA-β significantly reduces both total calcineurin A protein expression and activity in the kidney. Interestingly, CnA-α appears to make up a slightly greater proportion of both total calcineurin A protein levels and activity, supporting earlier observations that the α isoform may be the predominant isoform expressed in the kidney.31 However, despite expression of CnA-α and CnA-β in the kidney, it is clear that CnA-α plays a more critical role in the development of the kidney and in postnatal kidney function. In our laboratory, mice lacking CnA-α are born at a normal rate, but are smaller than littermates within days after birth, and have a shortened lifespan. CnA-α mice suffer from progressive kidney failure and most animals die between 21 and 28 days of age. On closer examination, loss of calcineurin is associated with significant alterations in kidney development. In this study, we report three interrelated findings that are specific to mice lacking CnA-α: postnatal defect in development of the NZ and superficial glomeruli, cell cycle alterations in the NZ, and impaired kidney function with loss of CnA-α. Each of these alterations represents a previously undescribed role for calcineurin in the kidney and suggests that there are specific functions of CnA-α isoform.
First, we report that loss of CnAα impairs development of the kidney, specifically within the NZ. While the kidneys of CnAα −/− mice are smaller due to an overall decrease in size of the animals, we found that kidney growth was further impaired as evidenced by a significantly decreased kidney/body weight ratio compared to wild-type littermates. Of the four main regions of the kidney, inner medulla, ISOM, OSOM, and cortex, the OSOM and the cortex are particularly affected. A dose response was also observed as heterozygous animals also showed a significant decrease in kidney size unrelated to body size (Table 2) and a small but significant decrease in the OSOM. Together, these data indicate that calcineurin is required for normal development and growth of the kidney, especially within regions most affected by postnatal growth. Interestingly, the defect in cortical development appears to be specific to the NZ. Nephrons within the juxta-medullary region appear to develop normally and are sufficient to support renal function several weeks postnatally. This suggests that distinct mechanisms govern the development of early versus late nephrogenesis. Interestingly, our finding that calcineurin is important for NZ development is mirrored in a recent study that specifically targeted inhibition of calcineurin activity to the gestational period coinciding with onset of nephrogenesis. Tendron et al32 describe that administration of cyclosporin A to pregnant rabbits during a 5-day period when embryonic nephrogenesis is initiated is sufficient to reduce nephron mass in the pups. Specifically, the pups showed a defect in NZ development. This experimental finding supports our conclusion from CnA-α −/− mice that calcineurin is required for normal kidney development.
Within the NZ, glomeruli were also affected by loss of CnA-α. Specifically, there is a deficiency of mesangial cells. Interestingly, mesangial cells appear to be present in juxta-medullary glomeruli, which compete development antenatally, and are only absent in glomeruli within the postnatally developing NZ. It will be interesting to determine whether the loss of mesangial cells is due to decreased proliferation and increased cell death as was observed in the NZ, which would suggest a direct role for calcineurin in mesangial cells, or due to a lack of recruitment of mesangial cells by endothelial and/or epithelial cells, suggesting a role for calcineurin in these cell types with an indirect effect on mesangial cells.
Concomitant with defects in development within the NZ, we report that loss of CnA-α results in alterations in the cell cycle. Further supporting a role for calcineurin in control of proliferation in the kidney is a recent study by Chang et al,33 showing that targeted disruption of the regulatory calcineurin B subunit (and, therefore targeted inhibition of both CnA-α and CnA-β) in the urinary tract resulted in a defect in proliferation. The knockout was targeted to metanephric mesenchymal cells and a loss of proliferation was observed in glomeruli as well as proximal and distal tubules. Interestingly, we find decreased proliferation and increased cell death specifically within the NZ of CnA-α −/− mice, suggesting that there may be a specific role for the α isoform in terminal nephrogenesis. The nature of the defect appears to be disruption of normal cell cycle as evidenced by increased levels of the cyclin-dependent kinase inhibitor p27, also specifically in the NZ. This is consistent with inhibition of proliferation in these structures and increased cell death. Interestingly, knockout of p27 in a transgenic mouse model results in organomegaly, including the kidney.34 Also, up-regulation of cux-1, a transcription factor implicated in transcriptional repression of p27 and expressed in the NZ of the developing kidney, also decreases p27. Transgenic mice over-expressing cux 1 have increased proliferation in the NZ29 and an increased number of mesangial cells in NZ glomeruli.30 Thus, it is possible that calcineurin is also important in a pathway that functions to regulate cell cycle events specifically in the developing kidney. Interestingly, over-expression of cux 1 also results in a loss of p27 in the epithelium of glomeruli and an over-proliferation of mesangial cells,30 an observation that parallels the increase of p27 in glomerular epithelial cells and loss of mesangial cells in CnA-α −/− mice. The two models suggest coordination between epithelial cell cycle regulation and survival/proliferation of mesangial cells.
Last, there is a defect in kidney function that may be a direct result of loss of calcineurin, but may also be due to the lack of development of the cortical nephrons. In the case of the latter, these data would suggest that juxtamedullary nephrons are not sufficient for postnatal kidney maturation or function as the animal grows. However, it is also possible that loss of calcineurin directly impairs renal function. For example, as early as 4 days postnatal, serum creatinine levels are slightly increased suggesting that normal filtration is not taking place before significant defects in cortical nephrogenesis are observed. While there appears to be increased ECM deposition, it is not possible to determine whether the up-regulation is due to a direct loss of calcineurin or due to an indirect effect of immature nephrons and/or decreased kidney function.
In interpreting the data from this model, several considerations should be noted. Kidney function appears to be impaired from birth and undoubtedly poor function may contribute to the structural/histological findings. In addition, the animals have several other defects. Notably, their livers are also significantly smaller. It is unknown what the defect in liver growth/maturation may contribute to the kidney phenotype. Data from this transgenic model may also have implications for the clinical use of calcineurin inhibitors. In particular, inhibition of calcineurin may impair the ability of some nephrons to recover from injury, especially if proliferation of the mesangium is required.
Calcineurin is a ubiquitous signaling protein that has been implicated in a variety of pathways in multiple organ systems. Specific roles for calcineurin A subunit isoforms may be a key mechanism for tissue-specific effects of calcineurin. In the kidney, calcineurin is involved in up-regulation of ECM16,17 as well as in the inhibition of ECM accumulation5,9 suggesting a cell and/or tissue-specific mechanism of action. Calcineurin A isoform expression/action may be one way calcineurin acts in a tissue-specific manner. For example, CnA-α, and not CnA-β or CnA-γ, is specifically up-regulated in diabetic glomeruli.18 Inhibition of calcineurin action in a rat model of diabetes improved glomerular hypertrophy and accumulation of TGFβ and ECM proteins,35 suggesting that CnA-α may be involved in these processes. Our data are consistent with a role for CnA-α, particularly in the mesangium and possibly endothelial cells. Also, we observed changes in ECM production in CnA-α −/− mice, however it is not clear whether increased ECM is due to a direct effect of CnA-α loss or the result of an indirect response to impaired nephron function. It is interesting to note that the increase in ECM was in the tubulointerstitium and not in glomeruli. This is consistent with previous findings that inhibition of calcineurin is associated with tissue-specific regulation of ECM proteins.35
In summary, loss of CnA-α results in a postnatal defect in development of NZ and in the development and/or survival of mesangial cells. These defects appear to be the result of cell cycle changes, in particular increased expression of the cyclin-dependent kinase inhibitor p27. In addition to, or as a result of, these developmental alterations, loss of CnA-α is associated with poor kidney function evidenced by increased ECM production and high serum creatinine values. Collectively, CnA-α knockout mice provide an interesting model of growth impairment specifically in NZ, calcineurin-mediated cell cycle alterations, and progressive, early renal failure.
Acknowledgments
We thank Jonathon Seidman and Jeffrey Molkentin for kindly providing CnA-α and CnA-β knockout mice, Greg Vanden-Huevel for helpful discussion, and Hanna Abboud for helpful discussion and review of the manuscript.
Footnotes
Address reprint requests to Jennifer L. Gooch, Ph.D., Medicine/Nephrology, University of Texas Health Science Center, San Antonio, 7703 Floyd Curl Drive, San Antonio, TX 78229-3900. E-mail: Gooch@uthscsa.edu.
Supported by the American Diabetes Association (J.L.G.), the Department of Veterans Affairs (J.L.G. and J.L.B.), and the National Institutes of Health George O’Brien Kidney Center (J.L.G. and J.L.B.).
References
- Rusnak F, Mertz P. Calcineurin: form and function. Physiol Rev. 2000;80:1483–1521. doi: 10.1152/physrev.2000.80.4.1483. [DOI] [PubMed] [Google Scholar]
- Suzuki E, Nishimatsu H, Satonaka H, Walsh K, Goto A, Omata M, Fujita T, Nagai R, Hirata Y. Angiotensin II induces myocyte enhancer factor 2- and calcineurin/nuclear factor of activated T cell-dependent transcriptional activation in vascular myocytes. Circ Res. 2002;90:1004–1011. doi: 10.1161/01.res.0000017629.70769.cc. [DOI] [PubMed] [Google Scholar]
- Murat A, Pellieux C, Brunner H-R, Pedrazzini T. Calcineurin blockade prevents cardiac mitogen-activated protein kinase activation and hypertrophy in renovascular hypertension. J Biol Chem. 2000;275:40867–40873. doi: 10.1074/jbc.M008071200. [DOI] [PubMed] [Google Scholar]
- Lea J, Kin S, Roberts B, Shuler M, Marrero M, Tumlin J. Angiotensin II stimulates calcineurin activity in proximal tubule epithelia through AT-1 receptor-mediated tyrosine phosphorylation of the PLC-γ1 isoform. J Am Soc Nephrol. 2002;13:1750–1756. doi: 10.1097/01.asn.0000022029.50356.2c. [DOI] [PubMed] [Google Scholar]
- Gooch JL, Tang Y, Ricono JM, Abboud HE. Insulin-like growth factor-I induces renal cell hypertrophy via a calcineurin-dependent mechanism. J Biol Chem. 2001;276:42492–42500. doi: 10.1074/jbc.M102994200. [DOI] [PubMed] [Google Scholar]
- Musaro A, McCullagh KJ, Naya FJ, Olsen EN, Rosenthal N. IGF-I induces skeletal myocyte hypertrophy in association with GATA-2 and NF-ATc1. Nature. 1999;400:581–585. doi: 10.1038/23060. [DOI] [PubMed] [Google Scholar]
- Pons S, Torres-Aleman I. Insulin-like growth factor-I stimulates dephosphorylation of IkB through the serine phosphatase calcineurin (protein phosphatase 2B). J Biol Chem. 2000;275:38620–38625. doi: 10.1074/jbc.M004531200. [DOI] [PubMed] [Google Scholar]
- Semsarian C, Wu M-J, Ju Y-K, Marciniec T, Yeoh T, Allen DG, Harvey RP, Graham RM. Skeletal muscle hypertrophy is mediated by a Ca2+-dependent calcineurin signalling pathway. Nature. 1999;400:576–580. doi: 10.1038/23054. [DOI] [PubMed] [Google Scholar]
- Gooch JL, Gorin Y, Zhang BX, Abboud HE. Involvement of calcineurin in TGFβ-mediated regulation of extracellular matrix accumulation. J Biol Chem. 2004;279:15561–15570. doi: 10.1074/jbc.M308759200. [DOI] [PubMed] [Google Scholar]
- Garver TA, Kincaid RL, Conn RA, Bellingsley ML. Reduction of calcineurin activity in brain by antisense oligonucleotides leads to persistent phosphorylation of tau protein at Thr181 and Thr231. Mol Pharmacol. 1999;55:632–641. [PubMed] [Google Scholar]
- Mandelkow E-M, Bjiernat J, Drewes G, Gustke N, Trinczek B, Mandelkow E. Tau domains, phosphorylation, and interactions with microtubules. Neurobiol Aging. 1995;16:355–363. doi: 10.1016/0197-4580(95)00025-a. [DOI] [PubMed] [Google Scholar]
- Ladner C, Czech J, Maurice J, Lorens S, Lee J. Reduction of calcineurin anzymatic activity in Alzheimer’s disease: correlation with neuropathologic changes. J Neuropathol Exp Neurol. 1996;55:924–931. doi: 10.1097/00005072-199608000-00008. [DOI] [PubMed] [Google Scholar]
- Molkentin J, Lu J, Antos C, Markham B, Richardson J, Robbins J, Grant S, Olsen E. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell. 1998;93:215–228. doi: 10.1016/s0092-8674(00)81573-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Windt J, Lim H, Taigen T, Wencker D, Condorelli G, Dorn Gn, Kitsis R, Molkentin J. Calcineruin-mediated hypertrophy protects cardiomyocytes from apoptosis in vitro and in vivo: an apoptosis-independent model of dilated heart failure. Circ Res. 2000;86:255–263. doi: 10.1161/01.res.86.3.255. [DOI] [PubMed] [Google Scholar]
- Taigen T, De Windt LJ, Lim HW, Molkenten JD. Targeted inhibition of calcineurin prevents agonist-induced cardiomyocyte hypertrophy. Proc Natl Acad Sci USA. 2000;97:1196–1201. doi: 10.1073/pnas.97.3.1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DW, Saunders HJ, Johnson FJ, Huq SO, Field MJ, Pollock CA. Cyclosporin exerts a direct fibrogenic effect on human tubulointerstitial cells: roles of insulin-like growth factor I, transforming growth factor β1, and platelet-derived growth factor. J Pharmacol Exp Ther. 1999;289:535–542. [PubMed] [Google Scholar]
- Wolf G, Killen PD, Neilson EG. Cyclosporin A stimulates transcription and procollagen secretion in tubulointersititial fibroblasts and proximal tubule cells. J Am Soc Nephol. 1990;6:918–922. doi: 10.1681/ASN.V16918. [DOI] [PubMed] [Google Scholar]
- Gooch JL, Pergola PE, Guler RL, Abboud HE, Barnes JL. Differential expression of calcineurin A isoforms in the diabetic kidney. J Am Soc Nephol. 2004;15:1421–1429. doi: 10.1097/01.asn.0000128076.91545.bb. [DOI] [PubMed] [Google Scholar]
- Kayyali U, Zhang W, Yee A, Seidman J, Potter H. Cytoskeletal changes in the brains of mice lacking calcineurin A α. J Neurochem. 1997;68:1668–1678. doi: 10.1046/j.1471-4159.1997.68041668.x. [DOI] [PubMed] [Google Scholar]
- Zhuo M, Zhang W, Son H, Mansuy I, Sobel R, Seidman J, Kandel E. A selective role of calcineurin A α in synaptic depotentiation in hippocampus. Proc Natl Acad Sci USA. 1999;96:4650–4655. doi: 10.1073/pnas.96.8.4650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Zimmer G, Chen J, Ladd D, Li E, Alt F, Wiederrecht G, Cryan J, O’Neill E, Seidman C, Abbas A, Seidman J. T cell responses in calcineurin A α-deficient mice. J Exp Med. 1996;183:413–420. doi: 10.1084/jem.183.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan VS, Wong C, Ohashi PS. Calcineurin Aα plays an exclusive role in TCR signaling in mature but not immature T cells. Eur J Immunol. 2002;32:1223–1229. doi: 10.1002/1521-4141(200205)32:5<1223::AID-IMMU1223>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Bueno OF, Brandt EB, Rothenberg ME, Molkentin JD. Defective T cell development and function in calcineurin A β-deficient mice. Proc Natl Acad Sci USA. 2002;99:9398–9403. doi: 10.1073/pnas.152665399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bueno OF, Wilkins BJ, Tymitz KM, Glascock BJ, Kimball TF, Lorenz JN, Molkentin JD. Impaired cardiac hypertrophic response in calcineurin A β-deficient mice. Proc Natl Acad Sci USA. 2002;99:4586–4591. doi: 10.1073/pnas.072647999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons SA, Wilkins BJ, Bueno OF, Molkentin JD. Altered skeletal muscle phenotypes in calcineurin Aα and Aβ gene-targeted mice. Mol Cell Biol. 2003;23:4331–4343. doi: 10.1128/MCB.23.12.4331-4343.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graef I, Chen F, Chen L, Kuo A, Crabtree G. Signals transduced by Ca(2+)/calcineurin and NFATc3/c4 pattern the developing vasculature. Cell. 2001;105:863–875. doi: 10.1016/s0092-8674(01)00396-8. [DOI] [PubMed] [Google Scholar]
- Fruman DA, Pai S-Y, Klee CN, Burakoff SJ, Bierer BE. Measurement of calcineurin phosphatase activity in cell extracts. Methods Enzymol. 1996;9:146–154. doi: 10.1006/meth.1996.0020. [DOI] [PubMed] [Google Scholar]
- Combs HL, Shankland SJ, Setzer SV, Hudkins KL, Alpers CE. Expression of the cyclin kinase inhibitor, p27kip1, in developing and mature human kidney. Kidney Int. 1998;53:892–896. doi: 10.1111/j.1523-1755.1998.00842.x. [DOI] [PubMed] [Google Scholar]
- Ledford AW, Brantley JG, Kemeny G, Foreman TL, Quaggin SE, Igarashi P, Oberhaus SM, Rodova M, Calvet JP, Vanden Heuvel GB. Deregulated expression of the homeobox gene Cux-1 in transgenic mice results in downregulation of p27(kip1) expression during nephrogenesis, glomerular abnormalities, and multiorgan hyperplasia. Dev Biol. 2002;245:157–171. doi: 10.1006/dbio.2002.0636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brantley JG, Sharma M, Alcalay NI, Heuvel GB. Cux-1 transgenic mice develop glomerulosclerosis and interstitial fibrosis. Kidney Int. 2003;63:1240–1248. doi: 10.1046/j.1523-1755.2003.00889.x. [DOI] [PubMed] [Google Scholar]
- Tumlin JA, Someren JT, Swanson CE, Lea JP. Expression of calcineurin activity and α-subunit isoforms in specific segments of the rat nephron. Am J Physiol. 1995;269:F558–F563. doi: 10.1152/ajprenal.1995.269.4.F558. [DOI] [PubMed] [Google Scholar]
- Tendron A, Decramer S, Justrabo E, Gouyon JB, Semama DS, Gilbert T. Cyclosporin A administration during pregnancy induces a permanent nephron deficit in young rabbits. J Am Soc Nephrol. 2003;14:3188–3196. doi: 10.1097/01.asn.0000095637.13193.89. [DOI] [PubMed] [Google Scholar]
- Chang CP, McDill BW, Neilson JR, Joist HE, Epstein JA, Crabtree GR, Chen F. Calcineurin is required in urinary tract mesenchyme for the development of the pyeloureteral peristaltic machinery. J Clin Invest. 2004;113:1051–1058. doi: 10.1172/JCI20049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama K, Ishida N, Shirane M, Inomata A, Inoue T, Shishido N, Horii I, Loh DY. Mice lacking p27(Kip1) display increased body size, multiple organ hyperplasia, retinal dysplasia, and pituitary tumors. Cell. 1996;85:707–720. doi: 10.1016/s0092-8674(00)81237-4. [DOI] [PubMed] [Google Scholar]
- Gooch JL, Barnes JL, Garcia S, Abboud HE. Calcineurin is activated in diabetes and is required for glomerular hypertrophy and ECM accumulation. Am J Physiol Renal Physiol. 2003;284:F144–F154. doi: 10.1152/ajprenal.00158.2002. [DOI] [PubMed] [Google Scholar]