Abstract
Chronic Bartonella quintana bacteremia is known to occur in homeless people exposed to lice. We present here the results of an open randomized trial performed to evaluate the efficacy of doxycycline in combination with gentamicin in the eradication of B. quintana bacteremia. From 1 January 2001 to 1 April 2002, homeless people with blood cultures positive for B. quintana were randomized to receive either no treatment (untreated controls) or a combination of gentamicin (3 mg/kg of body weight/day intravenously for 14 days) and doxycycline (200 mg/day orally for 28 days). Patients were evaluated from the results of blood cultures performed between day 28 (the end of treatment) and day 90 postinclusion. Intention-to-treat analysis of 20 included patients showed eradication of bacteremia in 7 out of 9 treated patients versus 2 out of 11 untreated controls (P = 0.01). In the per-protocol analysis, eradication was obtained for 7 out of 7 treated patients versus 2 out of 9 untreated controls (P = 0.003). This study demonstrates the efficiency of the combination of doxycycline and gentamicin in eradicating B. quintana bacteremia.
Bartonella quintana infection of humans was first described during World War I as being responsible for trench fever, a fever occurring every five days and associated with headaches, shin pain, and dizziness (13, 18). Recent reports have indicated a reemergence of B. quintana infections among the homeless population in cities in both Europe and the United States (2, 9, 24, 25). In 1994 the first cases of B. quintana endocarditis in homeless people were observed (5, 23), and in 1995 urban trench fever was reported in France and in the United States (24, 25). Seroepidemiological studies were then performed, and significant seroprevalence against B. quintana was reported in France (1, 2) and in the United States (3, 10). In a study performed in 1997 in emergency departments of the university hospital in Marseilles, France (2), it was demonstrated that 14% of the homeless people tested had blood cultures positive for B. quintana.
This bacterium is known to be responsible for endocarditis in homeless people (5, 7, 15, 22, 23). Although the relationship between bacteremia and endocarditis has not yet been demonstrated, it is likely that the treatment of bacteremia may be of importance to prevent the occurrence of endocarditis. Recently we retrospectively reviewed data for bacteremic patients and found that only those who were treated with a combination of doxycycline and gentamicin were cured, while those treated with β-lactams or doxycycline alone were not (6). In order to confirm those preliminary retrospective results, we decided to conduct a randomized clinical trial to evaluate the efficacy of this antibiotic association.
MATERIALS AND METHODS
The study was conducted in the Infectious Diseases Unit of the university hospital (CHU-Nord) in Marseilles, France, from 1 January 2001 to 1 April 2002. The trial was performed in accordance with the Declaration of Helsinki and its Hong Kong Amendment. The trial protocol was reviewed and approval was given by an institutional review board (CCPPRB no. 00/62). Written informed consent was obtained from all patients. It was hypothesized that 70% of bacteremic subjects are chronically infected (2). Considering an objective of reduction of chronic bacteremia from 70 to 2% after treatment and assuming a type I error of 0.05 and a type II error of 0.20, it was determined necessary to include 18 patients in the study (9 in each group).
Patients were enrolled if they were more than 18 years old and had a blood culture positive for B. quintana. Exclusion criteria included pregnancy, underlying cardiac diseases, such as the presence of a valvulopathy or vegetation seen on the transesophageal echocardiography, previous vascular disease (aneurysm or vascular graft), immunosuppression as defined by positive human immunodeficiency virus serology, cancer, diabetes, or dialyzed renal failure. Patients were randomized to receive either no treatment or a combination of 3 mg of gentamicin/kg of body weight as a single intravenous daily dose for 14 days and 200 mg of doxycycline as a single oral daily dose for 28 days. Group assignment was performed blindly by one of the authors (P. Brouqui or C. Foucault) by random sampling from previously prepared blocks of envelops containing four treatments and four untreated controls. Randomization by block was performed in order to avoid recruitment bias. Untreated controls were monitored after the end of the trial, and patients with persistent positive blood cultures were treated with a combination of doxycycline and gentamicin, as proposed in the trial.
Patients were recruited from emergency departments of the CHU-Nord in Marseilles, France, or from Marseilles city shelters during an investigation performed to evaluate the health status of homeless people (P. Brouqui, unpublished data). Patients of the treatment group were hospitalized for the first 14 days for intravenous treatment with gentamicin and oral treatment with doxycycline. For the remaining 14 days oral treatment with doxycycline was performed either in the hospital or in medical facilities of the city shelters of Marseilles. Serum creatinin level and audiograms were monitored twice a week during treatment with gentamicin. Untreated controls were monitored either in the hospital or in medical facilities of the city shelters. Blood cultures for B. quintana were performed once a week for patients of both groups. Patients were evaluated on the basis of the results of blood cultures performed between day 28 (the end of treatment) and day 90 postinclusion.
Methods used to isolate B. quintana from blood have been described elsewhere (11). Briefly, aerobic blood culture bottles (Bactec plus; Becton Dickinson Instrument Systems, Sparks, Md.) were inoculated with whole blood and, even if no bacterial growth was detected, after 7 days 1 ml of the broth was plated onto sheep blood agar plates and incubated at 37°C at 5% CO2. Plates were examined weekly for 3 months. In addition, 1 ml of whole blood (collected in VACUTAINER tubes [Becton Dickinson Vacutainer System, Rutherford, N.J.]) was plated directly onto sheep blood agar plates which were incubated and monitored as described above. When Bartonella-like colonies were observed, confirmation was achieved by a PCR-based method (20, 21). Samples of heparinized blood were also inoculated into the ECV 304 human endothelial cell line by using the centrifugation-shell vial technique (11). Detection of B. quintana was carried out by immunofluorescence with rabbit polyclonal antibodies raised in our laboratory after 15, 30, and 45 days of incubation. If immunofluorescence was positive, identification was performed by using the PCR-based method outlined above.
Statistical analysis was performed both for the intention-to-treat and for the per-protocol patient populations. For the intention-to-treat analysis, loss of patients due to their dropping out of the study was considered treatment failure. Data were recorded by using a standardized questionnaire, were captured by using Excel software (Microsoft), and were analyzed by using Epi Info 6 software (at http://www.cdc.gov/epiinfo/). Frequencies were compared by the use of Fisher's exact test. Student's test was used to compare quantitative data. A difference of P < 0.05 was considered significant.
RESULTS
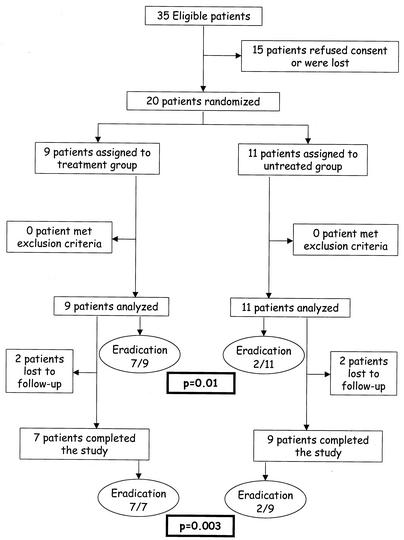
Between 1 January 2001 and 1 April 2002 a total of 35 homeless people had positive blood cultures for B. quintana and were eligible for the study. Fifteen refused consent or were discharged before the results of blood cultures were obtained and were not subsequently reached; 20 were randomized. Nine were assigned to the treatment group and 11 were assigned to the untreated group (Fig. 1). The subjects of both groups were not statistically different regarding age, sex, alcoholism, immune status, white blood cell count, mean corpuscular volume, and serologic status (Table 1). The delousing procedure was identical for the two groups (including a complete change of clothes). Four patients (two in the treatment group and two untreated controls) were dropped out of the study. These patients were considered treatment failures in the intention-to-treat analysis, and data for these patients were not included in the per-protocol analysis. Of the two patients in the treatment group who left the study, one was discharged from the hospital on day 9 postinclusion and did not complete intravenous treatment with gentamicin and oral treatment with doxycycline and the other one refused blood culture during follow up from day 21 postinclusion. Both of the two patients in the untreated group who left the study did not come to follow-up consultations, and blood cultures were not performed on them from day 21 postinclusion.
FIG. 1.
Trial profile.
TABLE 1.
Composition of the study populationa
| Group | Age (avg ± SD) | Sex ratio (male/ female) | No. consuming >70 ml of ethanol/day | Mean corpuscular vol (I SD) | No. with significant antibody titerb |
|---|---|---|---|---|---|
| Untreated | 52.9 ± 9.8 | 8/3 | 6 | 93.1 ± 7.8 | 7 |
| Treatment | 54.9 ± 9.5 | 7/2 | 2 | 90.6 ± 13 | 2 |
The P values were not significant for any of the study data shown here. No patient from either group was positive for immunosuppression, and no patient from either group had a white blood cell count of <3,000/mm3.
A significant antibody titer is defined as having a ratio of immunoglobulin G antibody titer to B. quintana of more than 1:100.
Among the 20 patients whose data were used for the intention-to-treat analysis, eradication of B. quintana bacteremia was obtained for 7 out of 9 in the treatment group and for 2 out of 11 in the untreated control group (P = 0.01). Among the 16 patients whose data were used in the per-protocol analysis, eradication was obtained for all 7 patients of the treatment group and for 2 out of 9 in the untreated control group (P = 0.003) (Fig. 1). Side effects were recorded for one patient in the treatment group. He presented with two different side effects. The first was a transient increase of serum creatinin level during treatment which did not necessitate renal dialysis and which was completely regressive. He also presented with a photosensitization with first-degree skin burns of the face after sun exposure which was regressive without any aesthetic result. No statistical difference regarding clinical outcome was recorded for the two groups during follow up. None of the patients of either group developed endocarditis. The mean time of resolution of bacteremia was 26 ± 3.4 days following the initiation of doxycycline plus gentamicin administration in the treatment group and 17.5 ± 4.9 days following enrollment in the trial in the untreated control group.
DISCUSSION
B. quintana is susceptible to many antibiotics (12), but aminoglycosides are the only effective bactericidal agents and should constitute the basis of the treatment of Bartonella chronic infections, except bacillary angiomatosis and peliosis (6, 16, 19). Preliminary clinical data have suggested the efficiency of the combination of gentamicin and doxycycline in treating B. quintana bacteremia, while β-lactams and doxycycline alone were not effective (6). Many of the reported cases of Bartonella endocarditis have been cured by empirical therapies for blood-culture-negative endocarditis that include β-lactams and aminoglycosides antibiotics. We retrospectively demonstrated that those receiving aminoglycosides were more likely to fully recover than those who did not, and when aminoglycosides were given for at least 14 days they were more likely to survive than those with a shorter duration of therapy (16). Brucella spp. are phylogenetically closely related to Bartonella spp. and share comparable microbiological, pathophysiological, and clinical findings (19). Among antimicrobial agents, only aminoglycosides (and to a lesser extent, doxycycline) are bactericidal on Brucella spp. (19), suggesting that the present knowledge of the treatment of the Brucella spp. infections (8) may help to define the optimum treatment of Bartonella-related infections. Altogether these data lead us to propose the combination of doxycycline and gentamicin for the treatment of chronic B. quintana bacteremia. The present trial demonstrates the efficiency of this combination. The mean time of resolution of bacteremia was different between the treatment group and the untreated controls, and this could be due to the chronic aspect of B . quintana infection, as the duration of the bacteremia at the time of recruitment of each patient was unknown and as some of the patients may be cured spontaneously.
To avoid confounding factors we have compared data that could influence the spontaneous outcome of bacteremia. The two groups were not different regarding age, sex, alcoholism, immune status, the presence of leukopenia, the mean corpuscular volume, and the presence of significant levels of antibodies to B. quintana. The treatment of lice was also identical between the two groups. Patients of the treatment group were all hospitalized and received good nutrition, while some of the untreated controls were monitored in city shelters. One could hypothesize that nutrition may affect the outcome of B. quintana bacteremia. Nevertheless, it has been demonstrated that malnutrition in homeless people is more frequently due to loss of appetite linked to chronic alcoholism than to the lack of food (P. Brouqui, unpublished). No statistical difference was detected between treatment group and untreated control groups regarding alcoholism.
Only 20 of 35 eligible homeless people were randomized in the trial. This is due to the particularity of the population studied here. The homeless, mainly young, are not seekers of medical care and often refuse hospitalization (4, 14). Primary isolation of B. quintana may require up to 45 days of incubation (13). Most homeless people involved in the trial were recruited during an investigation performed in city shelters to evaluate the health status of homeless people. Considering the duration of incubation of blood cultures, some homeless had left the shelters by the time positive results were obtained and were not reached despite intensive searches in emergency rooms of the university hospital and in city shelters (2).
The second major limit of this study is the high proportion of homeless who left the study. Up to 20% of randomized bacteremic homeless did not complete the study. Adherence to the follow-up portion of the study is difficult to obtain from homeless people and is often poor. In addition, access to health care may be limited by mental illness, transport problems, self neglect, and fear of institutionalization (4, 14).
Eradication of lice is difficult to achieve (17). The alternative for control of B. quintana is the eradication of its human reservoir (6). Because many bacteremic patients exhibit antibodies to B. quintana, we speculate that they are immune and may not be reinfected. Consequently, systematic treatment would decrease the prevalence of B. quintana infections, although the impact of such treatments remains to be evaluated.
B. quintana endocarditis is known to occur in homeless people (5, 7, 15, 22, 23). Death by unknown causes has been reported for one of our bacteremic homeless patients (6), and although this could not be definitely attributed to endocarditis, this possibility cannot be ruled out. Furthermore, the severity of B. quintana endocarditis has been well established, with deaths having been reported despite antibiotic therapy and valvular surgery (7). The efficiency of the combination of gentamicin and doxycycline in eradicating B. quintana bacteremia may efficiently prevent the occurrence of B. quintana endocarditis. Considering the poor adherence of the homeless population to medical care and treatment, a 1-month treatment including 14 days of intravenous treatment is difficult to manage. Moreover, side effects have been observed during the trial, and therefore the treatment should be carefully managed. Better adherence to treatment should be obtained with shorter duration, and this remains to be evaluated in the future. Although further studies are needed, combination of gentamicin and doxycycline should be recommended for treatment of chronic B. quintana bacteremia.
Acknowledgments
This work was supported by the “programme hospitalier de recherche clinique 2000: Protocole SDF 2000-TA,” Assistance Publique—Hôpitaux de Marseille.
REFERENCES
- 1.Brouqui, P., P. Houpikian, H. T. Dupont, P. Toubiana, Y. Obadia, V. Lafay, and D. Raoult. 1996. Survey of the seroprevalence of Bartonella quintana in homeless people. Clin. Infect. Dis. 23:756-759. [DOI] [PubMed] [Google Scholar]
- 2.Brouqui, P., B. Lascola, V. Roux, and D. Raoult. 1999. Chronic Bartonella quintana bacteremia in homeless patients. N. Engl. J. Med. 340:184-189. [DOI] [PubMed] [Google Scholar]
- 3.Comer, J. A., C. Flynn, R. L. Regnery, D. Vlahov, and J. E. Childs. 1996. Antibodies to Bartonella species in inner-city intravenous drug users in Baltimore, Md. Arch. Intern. Med. 156:2491-2495. [PubMed] [Google Scholar]
- 4.Crane, M., and A. M. Warnes. 2001. Primary health care services for single homeless people: defects and opportunities. Fam. Pract. 18:272-276. [DOI] [PubMed] [Google Scholar]
- 5.Drancourt, M., J. L. Mainardi, P. Brouqui, F. Vandenesch, A. Carta, F. Lehnert, J. Etienne, F. Goldstein, J. Acar, and D. Raoult. 1995. Bartonella (Rochalimaea) quintana endocarditis in three homeless men. N. Engl. J. Med. 332:419-423. [DOI] [PubMed] [Google Scholar]
- 6.Foucault, C., K. Barrau, P. Brouqui, and D. Raoult. 2002. Bartonella quintana bacteremia among homeless people. Clin. Infect. Dis. 35:684-689. [DOI] [PubMed] [Google Scholar]
- 7.Fournier, P. E., H. Lelievre, S. J. Eykyn, J. L. Mainardi, T. J. Marrie, F. Bruneel, C. Roure, J. Nash, D. Clave, E. James, C. Benoit-Lemercier, L. Deforges, H. Tissot-Dupont, and D. Raoult. 2001. Epidemiologic and clinical characteristics of Bartonella quintana and Bartonella henselae endocarditis: a study of 48 patients. Medicine (Baltimore) 80:245-251. [DOI] [PubMed] [Google Scholar]
- 8.Hall, W. H. 1990. Modern chemotherapy for brucellosis in humans. Rev. Infect. Dis. 12:1060-1099. [DOI] [PubMed] [Google Scholar]
- 9.Jackson, L. A., and D. H. Spach. 1996. Emergence of Bartonella quintana infection among homeless persons. Emerg. Infect. Dis. 2:141-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jackson, L. A., D. H. Spach, D. A. Kippen, N. K. Sugg, R. L. Regnery, M. H. Sayers, and W. E. Stamm. 1996. Seroprevalence to Bartonella quintana among patients at a community clinic in downtown Seattle. J. Infect. Dis. 173:1023-1026. [DOI] [PubMed] [Google Scholar]
- 11.La Scola, B., and D. Raoult. 1999. Culture of Bartonella quintana and Bartonella henselae from human samples: a 5-year experience (1993 to 1998). J. Clin. Microbiol. 37:1899-1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maurin, M., S. Gasquet, C. Ducco, and D. Raoult. 1995. MICs of 28 antibiotic compounds for 14 Bartonella (formerly Rochalimaea) isolates. Antimicrob. Agents Chemother. 39:2387-2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maurin, M., and D. Raoult. 1996. Bartonella (Rochalimaea) quintana infections. Clin. Microbiol. Rev. 9:273-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raoult, D., C. Foucault, and P. Brouqui. 2001. Infections in the homeless. Lancet Infect. Dis. 1:77-84. [DOI] [PubMed] [Google Scholar]
- 15.Raoult, D., P. E. Fournier, M. Drancourt, T. J. Marrie, J. Etienne, J. Cosserat, P. Cacoub, Y. Poinsignon, P. Leclercq, and A. M. Sefton. 1996. Diagnosis of 22 new cases of Bartonella endocarditis. Ann. Intern. Med. 125:646-652. [DOI] [PubMed] [Google Scholar]
- 16.Raoult, D., P. E. Fournier, F. Vandenesch, J. L. Mainardi, S. J. Eykyn, J. Nash, E. James, C. Benoit-Lemercier, and T. J. Marrie. 2003. Outcome and treatment of Bartonella endocarditis. Arch. Intern. Med. 163:226-230. [DOI] [PubMed]
- 17.Raoult, D., J. B. Ndihokubwayo, H. Tissot-Dupont, V. Roux, B. Faugere, R. Abegbinni, and R. J. Birtles. 1998. Outbreak of epidemic typhus associated with trench fever in Burundi. Lancet 352:353-358. [DOI] [PubMed] [Google Scholar]
- 18.Raoult, D., and V. Roux. 1999. The body louse as a vector of reemerging human diseases. Clin. Infect. Dis. 29:888-911. [DOI] [PubMed] [Google Scholar]
- 19.Rolain, J. M., M. Maurin, and D. Raoult. 2000. Bactericidal effect of antibiotics on Bartonella and Brucella spp.: clinical implications. J. Antimicrob. Chemother. 46:811-814. [DOI] [PubMed] [Google Scholar]
- 20.Roux, V., and D. Raoult. 1995. Inter- and intraspecies identification of Bartonella (Rochalimaea) species. J. Clin. Microbiol. 33:1573-1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roux, V., and D. Raoult. 1995. The 16S-23S rRNA intergenic spacer region of Bartonella (Rochalimaea) species is longer than usually described in other bacteria. Gene 156:107-111. [DOI] [PubMed] [Google Scholar]
- 22.Spach, D. H., K. P. Callis, D. S. Paauw, Y. B. Houze, F. D. Schoenknecht, D. F. Welch, H. Rosen, and D. J. Brenner. 1993. Endocarditis caused by Rochalimaea quintana in a patient infected with human immunodeficiency virus. J. Clin. Microbiol. 31:692-694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spach, D. H., A. S. Kanter, N. A. Daniels, D. J. Nowowiejski, A. M. Larson, R. A. Schmidt, B. Swaminathan, and D. J. Brenner. 1995. Bartonella (Rochalimaea) species as a cause of apparent “culture-negative” endocarditis. Clin. Infect. Dis. 20:1044-1047. [DOI] [PubMed] [Google Scholar]
- 24.Spach, D. H., A. S. Kanter, M. J. Dougherty, A. M. Larson, M. B. Coyle, D. J. Brenner, B. Swaminathan, G. M. Matar, D. F. Welch, and R. K. Root. 1995. Bartonella (Rochalimaea) quintana bacteremia in inner-city patients with chronic alcoholism. N. Engl. J. Med. 332:424-428. [DOI] [PubMed] [Google Scholar]
- 25.Stein, A., and D. Raoult. 1995. Return of trench fever. Lancet 345:450-451. [DOI] [PubMed] [Google Scholar]