Abstract
Spinal muscular atrophy (SMA) is characterized by degeneration of lower motor neurons caused by mutations of the survival motor neuron 1 gene (SMN1). SMN is involved in various processes including the formation of the spliceosome, pre-mRNA splicing and transcription. To know whether SMN has an essential role in all mammalian cell types or an as yet unknown specific function in the neuromuscular system, deletion of murine Smn exon 7, the most frequent mutation found among SMA patients, has been restricted to liver. Homozygous mutation results in severe impairment of liver development associated with iron overload and lack of regeneration leading to dramatic liver atrophy and late embryonic lethality of mutant mice. These data strongly suggest an ubiquitous and essential role of full-length SMN protein in various mammalian cell types. In SMA patients, the residual amount of SMN allows normal function of various organs except motor neurons. However, data from mouse and human suggest that other tissues might be involved in severe form of SMA or during prolonged disease course which reinforce the need of therapeutic approaches targeted to all tissues. In addition, liver function of patients should be carefully investigated and followed up before and during therapeutic trials.
Spinal muscular atrophy (SMA) is a recessive autosomal disorder with an incidence of 1 in 6000.1,2 SMA is characterized by degeneration of lower motor neurons leading to muscle paralysis and atrophy. Homozygous deletion or conversion events of the Survival Motor Neuron (SMN1) gene exon 7 are the most frequent mutations found in SMA patients.3 SMN1 is duplicated with a highly homologous copy called SMN2 and both genes are transcribed.3 The SMN2 gene remains present in all patients but the low amount of SMN protein encoded by full-length SMN2 transcripts is not able to compensate for the SMN1 gene defects (4 for review). SMA has been ascribed to a dose effect of SMN (4 for review). SMN is an ubiquitously expressed protein that has been involved in various processes including cytoplasmic assembly of snRNP into the spliceosomal complex, pre-mRNA splicing, transcription, and metabolism of ribosomal RNA (5 for review). The molecular pathway linking marked deficiency of SMN to SMA phenotype remains unknown. Selective involvement of the neuromuscular system in SMA suggests that cells of different types do not require the same amount of SMN for survival unless SMN may have an as yet unknown function specific to neurons or skeletal muscle. To answer these questions, mouse models of SMA have been generated. Targeted disruption of the Smn gene, which is not duplicated in mouse, resulted in an embryonic lethal phenotype caused by the failure of blastocyst implantation of Smn−/− embryos.6 The early developmental defect prevented the use of these embryos to analyze the role of SMN in the development of the neuromuscular system. A mouse line carrying two loxP sequences flanking Smn exon 7 (SmnF7) has been established through homologous recombination.7 Cre-mediated deletion of Smn exon 7, the most frequent mutation found in SMA patients, has been directed to either neurons or skeletal muscle by using transgenic mice expressing the Cre recombinase in neurons or skeletal muscle, respectively (“neuronal” or “muscular” mutant).7–10 Both mutants develop severe degenerative process suggesting the involvement of both tissues in SMA. To know whether SMN has an essential role in all mammalian cell types or a specific function in the neuromuscular system, deletion of Smn exon 7 has been directed to liver, a tissue not affected in human SMA.
Materials and Methods
Mice
Mice carrying Smn exon 7 flanked by two loxP sites (SmnF7) were generated through homologous recombination in embryonic stem cells.7 Transgenic line expressing the Cre recombinase transgene driven by both the mouse albumin regulatory elements and the α-fetoprotein enhancers was used for hepatocyte-specific expression of the Cre recombinase (Alfp-Cre).11 (Alfp-Cre, SmnF7/F7) mutant mice were generated by crossing homozygous (SmnF7/F7) mice to those carrying the Cre recombinase transgene (Alfp-Cre) and the (SmnF7/+) genotype. Mice were maintained on C57BL/6J genetic background. (SmnF7/+) or (SmnF7/F7) mice from litter were used as controls. Animals were genotyped by PCR amplification of DNA extracted from tail biopsies.7,12 Ex7sou1 and GS8 primers were used to amplify the wild-type (Smn+, 430 bp), the SmnF7 allele (630 bp) or both. The transmission of the Alfp-Cre transgene was confirmed by PCR amplification of DNA using primers Cre1 and Cre2.12 All animal procedures were performed in accordance with institutional guidelines (agreement numbers A91–228-2 and 3429). Mice were maintained in a pathogen-free animal house.
Analysis of Cre Recombinase Transgene Activity
To characterize the pattern of Cre recombinase activity, Rosa26 reporter mice were used (R26R).13 β-galactosidase expression was assayed on tissues of adult or whole embryos carrying both R26R locus and Alfp-Cre transgene. Double transgenic mice were identified using PCR amplification analysis of DNA for Cre and R26R transgenes as previously described.13 Noon of the day of the plug was considered as 0.5 days p.c. Embryos were dissected in 0.1 mol/L phosphate buffer (PB) then fixed in 0.2% glutaraldehyde, 5 mmol/L EGTA, 2 mmol/L MgCl2 in 1X PB for 10 minutes at 4°C. Fixed embryos were washed three times in 2 mmol/L MgCl2, 1% Nonidet P-40 (NP-40) in 1X PB and incubated for 3 to 4 hours at 37°C in 1 mg/ml X-Gal, 5 mmol/L potassium ferrocyanide, 5 mmol/L potassium ferricyanide in the washing solution as described above. After staining, embryos were rinsed twice in the washing solution. Tissues from adult mice were frozen in isopentane cooled on liquid nitrogen. Frozen sections of 10 μm were fixed in 2% formaldehyde and 0.2% glutaraldehyde in 1X PB for 10 minutes at 4°C. Fixed tissues were washed twice in 1X PB, and incubated overnight at 37°C in the staining solution described above without NP-40. After staining, tissues were washed twice in 1X PB and counterstained with safranine.
To test the efficiency of Smn exon 7 excision by the Cre recombinase, Alfp-Cre transgenic mice were crossed to mice carrying the SmnF7 allele and double transgenic mice were selected. Genomic DNA was extracted from a variety of adult tissues. The wild-type and SmnF7 alleles were simultaneously amplified by PCR using one set of primers (ex7sou1-GS8).7 The efficiency of excision was estimated by comparing the intensity of the band amplified from the SmnF7 allele with that from the wild-type allele which differs only by the absence of the loxP site. Detection of the SmnΔ7 allele was performed by PCR amplification using primers pHR5 and GS8. 7
Morphological and Immunohistochemical Analyses
Liver, spinal cord, or skeletal muscle samples of adult mice or whole embryos were fixed in fresh 10% formalin for at least 24 hours at 4°C. At least three mutant or control mice or embryos were analyzed. Samples were processed for routine light histochemistry. Fixation was followed by dehydration using a series of graded concentrations of alcohol and toluene and embedding in paraffin. Five-μm sections were de-waxed, washed and stained with hematoxylin eosin safran (HES), periodic acid-Schiff (PAS), Perls staining, and Masson method. Sections were observed under light microscope. The slides were examined by three different observers in blind manner.
For immunolabeling experiments, liver samples were frozen in Tissue-Tek (Sakura) on dry ice. For α-fetoprotein (AFP) immunostaining and TdT-mediated x-dUTP nick end labeling (TUNEL) analyses, 5-μm frozen sections were prepared from liver of control and mutant embryos. For AFP immunostaining, sections were fixed in cold acetone for 10 minutes and incubated in blocking solution containing 3% donkey serum in PBS for 30 minutes, then with the anti-AFP antibody (1:40, Santa Cruz Biotechnology Inc., Santa Cruz, CA) overnight at +4°C. After washing in PBS, sections were incubated with Cy3-conjugated anti-goat antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA) for 1 hour at room temperature. For CD68 labeling, sections were fixed with formalin, blocked, then incubated with anti-CD68 (1:200, clone PG-M1, DAKO, Denmark). The TUNEL assay was performed using the In Situ Cell Death Detection kit (Fluorescein, Roche Applied Science, Penzberg, Germany). For Ki67 immunostaining, 7-μm frozen sections were prepared and immediately fixed in Zamboni’s fixative (2% paraformaldehyde and 15% picric acid in PB) for 10 minutes.14 Sections were washed in Tris-buffered saline (TBS), pH 7.6 and incubated in blocking solution containing 3% donkey serum in TBS for 10 minutes, and then with the anti-Ki67 antiserum (1:200, Novocastra Laboratories Ltd.) for 1 hour at room temperature. After washing in TBS, sections were incubated with FITC-conjugated anti-rabbit antibodies (1:1000, Jackson ImmunoResearch Laboratories) for 45 minutes at room temperature. Sections were then mounted with Vectashield (Vector Laboratories) and observed under Zeiss Axiophot fluorescence microscope.
Transcript Analysis
Total RNA was extracted from freshly isolated tissues using the Trizol procedure (Gibco-BRL). First-strand cDNA synthesis was performed by using either random or oligo(dT) primers. PCR amplification analysis of single-strand cDNA was performed using primers flanking Smn exon 7 (ex5sou2, 5′- TGC TGG ATG CCC CCG TTC CCT TCA- 3′ and ex8sou, 5′- GGC ACG CTC TGC TGC TGA CTT AG - 3′) and revealed Smn transcripts containing (350 bp) or lacking exon 7 (290 bp). Transcript analysis of genes encoding transferrin receptor 1 (TFR1), transferrin receptor 2 (TFR2), HFE, hepcidin 1 and 2 (HAMP1 and 2) was performed using the following primers. TFR1: TFR1-Ex2F, 5′-GTAGATGGAGATAACAGTCATGTG-3′ and TFR1-Ex5R, 5′- AGTTACCATTT GACTGCACTATG-3′; TFR2: TFR2-Ex1F, 5′-CACAAGCATGGAGCAACGTTGG-3′ and TFR2-Ex6R, 5′-CTTTTAGGTCCTGTAGGTCCTC-3′; HFE: HFE-Ex2, 5′-GGACCATCAT GGGCAACTATAAC-3′ and HFE-Ex6, 5′-TGTTAAGACATAGCCACCCATGG-3′, HAMP1: HEPC1-F, 5′-CCTATCTCCATCAACAGATGAGAC-3′ and HEPC1-R, 5′-GATGTGGCTCT AGGCTATGTTTTG-3′; HAMP2: HEPC2-F, 5′-CCTATCTCCAGCAACAGATGAGAC-3′ and HEPC2-R, 5′-TTCTTCACAACAGATACCACAGGA-3′. As internal control for RT-PCR amplification analysis, aldolase A cDNA was co-amplified using primers aldo 1 (5′ - TAA GAA GGA TGG AGC CGA CTT TG -3′) and aldo 600 (5′- GCG AGG CTG TTG GCC AGG GCG CG-3′). RT-PCR products were separated by agarose gel electrophoresis and labeled with ethidium bromide.
Serum Level of Liver Proteins
Serum enzymatic activities of transaminases and γ-glutamyl transferase (γ-GT) from control (SmnF7/F7) or (Alfp-Cre, SmnF7/+) mice were evaluated according to standard procedures. Blood was collected by retro-orbital puncture of anesthetized mice and the serum was stored at −80°C before measurement. Serum activities of transaminases, γ-GT and dosage of α-fetoprotein and albumin were determined from blood samples of SMA patients according to standard procedures and after informed consent of families or patients.
Immunoblotting Experiments
Frozen liver of (SmnF7/+), (SmnF7/F7), (Alfp-Cre, SmnF7/+) or (Alfp-Cre, SmnF7/F7) mice were rapidly frozen and crushed in liquid nitrogen using a mortar and pestle. Pulverized tissue samples were transferred into 5 volumes of a buffer containing 25 mmol/L sodium phosphate (pH 7.2), 5 mmol/L ethylene diamine tetraacetic acid (EDTA) and 1% sodium dodecyl sulfate (SDS) supplemented with protease inhibitor cocktail (Sigma, St. Louis, MO), and boiled for 5 minutes. Protein sample (25 to 50 μg) was mixed with an equal volume of 2X Laemmli buffer, electrophoresed, and then transferred. For SMN and actin immunodetection, anti-SMN and anti-actin monoclonal antibodies were used (1:5000, Transduction Laboratories, Lexington, CA; 1:10000, Chemicon International Inc., Temecula, CA, respectively). After washes in PBS, 0.05% Tween 20, membranes were incubated with anti-mouse IgG conjugated to horseradish peroxydase (1:5000) and the immune complexes were revealed using chemiluminescent detection reagents (Pierce, Rockford, IL). For AFP and albumin immunodetections, goat anti-AFP and anti-albumin antibodies were used (1:500, Santa Cruz Biotechnology; 1:15000, Bethyl Lab. Inc., Montgomery, TX) and revealed by anti-goat IgG conjugated to horseradish peroxidase (1:500).
Results
Liver-Specific Activity of Cre Recombinase in Alfp Transgenic Line
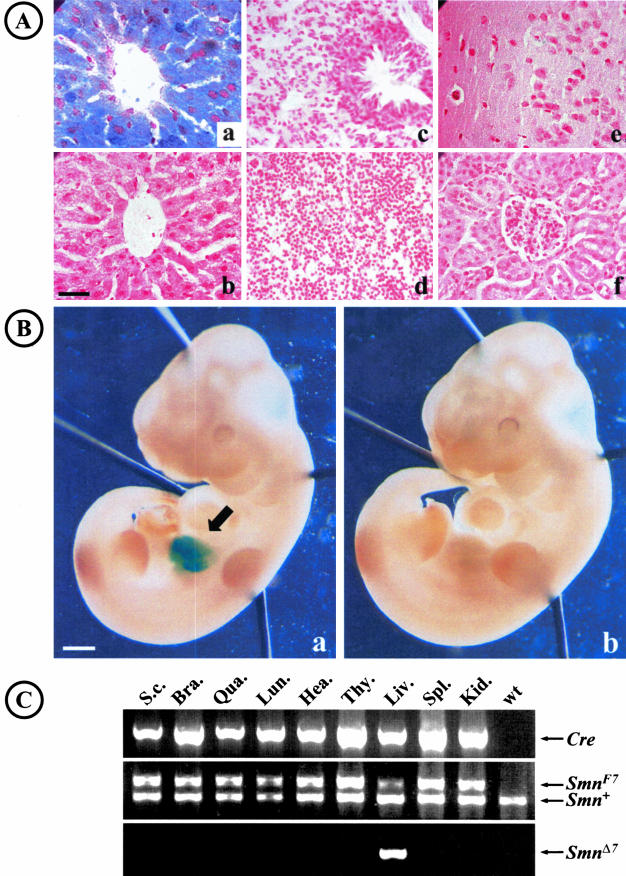
To characterize the Cre recombinase expression pattern of Alfp-Cre transgene, (Alfp-Cre) transgenic mice were crossed to either Rosa26 reporter mice (R26R) or mice harboring SmnF7 allele. In adult transgenic mice carrying both Alfp-Cre and R26R transgenes, liver stained uniformly blue with standard β-galactosidase incubation times, indicating a specific and strong Cre recombinase activity in liver (Figure 1A). No β-galactosidase activity was detected in other tissues including lung, kidney, brain, heart, or spleen (Figure 1A). These results were further supported by PCR amplification analysis of genomic DNA extracted from various tissues of adult mice carrying both Alfp-Cre transgene and SmnF7/+ genotype. Cre-mediated deletion of Smn exon 7 was observed in liver but not in other tissues, indicating that deletion of Smn exon 7 was restricted to liver (Figure 1C).
Figure 1.
Liver-specific activity of Alfp-Cre transgene. A: In adult transgenic mouse carrying both Alfp-Cre and R26R loci, β-galactosidase activity was observed in liver (a) but not in lung (c), spleen (d), brain (e), or kidney (f). No β-galactosidase activity was observed in transgenic mouse carrying R26R locus only (b). B: During development, β-galactosidase activity was observed in liver (arrow) of 12 days p.c. embryos carrying both Alfp-Cre and R26R transgenes (a). No β-galactosidase activity was detected in transgenic mouse carrying R26R locus only (b). C: PCR amplification analysis of genomic DNA extracted from various tissues of adult mouse carrying both Alfp-Cre and SmnF7/+ loci. PCR amplification products of Cre recombinase (top), Smn exon 7 locus (middle), Smn deleted allele (SmnΔ7, bottom) are shown. Deletion of Smn exon 7 was observed in liver only (liv). S.c., spinal cord; Bra, brain; Qua, quadriceps; Lun, lung; Hea, heart; Thy, thymus; Liv, liver; Spl, spleen; Kid, kidney; wt, DNA from wild-type mouse. Scale bar: 30 μm (A); 1 mm (B).
To characterize Cre recombinase activity during development, embryos that inherit both Alfp-Cre and R26R transgenes were selected. β-galactosidase activity was observed in liver from 10 days postcoitum (d.p.c.) corresponding to activation of the endogenous albumin gene (Figure 1B and data not shown). In other tissues, no β-galactosidase activity was observed at that stage or in older embryos (Figure 1B and data not shown). These findings led us to use the Alfp-Cre transgenic line for the targeting of Smn in liver but not in the other tissues.
Homozygous Deletion of Smn Exon 7 Directed to Liver Leads to Late Embryonic Lethality
To generate mutant mice carrying homozygous deletion of Smn exon 7 directed to liver (Alfp-Cre, SmnF7/F7) in a 1:4 ratio, mice homozygous for the SmnF7 allele (SmnF7/F7) were crossed to mice carrying both the Alfp-Cre transgene and (SmnF7/+) genotype. These crosses generated no living mutant mice carrying (Alfp-Cre, SmnF7/F7) genotype of 201 newborns analyzed (Table 1 and Figure 2A). To determine the timing of the lethality, embryos were analyzed during gestation. Until 16 days p.c., embryos showed the expected ratio for the various genotypes, then progressive lack of embryos carrying the (Alfp-Cre, SmnF7/F7) genotype was observed (Figure 2A). These data indicate that homozygous deletion of Smn exon 7 directed to liver leads to late embryonic lethality from 18 days p.c. to birth.
Table 1.
Late Embryonic Lethality of Liver Smn Mutant Mice
| Genotype of living embryos
|
Number | p (χ2) | ||||
|---|---|---|---|---|---|---|
| Smn+/F7 | SmnF7/F7 | Alfp-Cre, Smn+/F7 | Alfp-Cre, SmnF7/F7 | |||
| 16 d.p.c. | 30% | 21% | 23% | 26% | 61 | 0.9245 |
| 18 d.p.c. | 35% | 29% | 22% | 14% | 59 | 0.3353 |
| Neonates | 34% | 30% | 36% | 0% | 201 | <0.0001 |
Figure 2.
Macroscopic characteristics of Smn mutant mice. A: Percentage of living embryos carrying the various genotypes at 16, (16) 18 days p.c. (18) and at birth (NN). Note the progressive lack of embryos carrying the (Alfp-Cre, SmnF7/F7) genotype from 18 days p.c. to birth. B: Weight of the liver from mice carrying the various genotypes at 16 (16) and 18 days p.c. (18) Note the dramatic reduction of liver weight of (Alfp-Cre, SmnF7/F7) mice. Asterisks indicate statistical significant difference between (Alfp-Cre, SmnF7/F7) mutant mice and those carrying the other genotypes. At the top of bars are indicated the number of animals analyzed in each subgroup. C: Macroscopic aspect of liver in (Alfp-Cre, SmnF7/F7) mutant mouse (a) and control mouse (SmnF7/+, b). Scale bar: 2 mm
To determine the effect of Cre-mediated deletion of Smn exon 7 on Smn transcripts, RT-PCR amplification analysis of RNA extracted from liver of 18 days p.c. (Alfp-Cre, SmnF7/F7) mutant or (SmnF7/+or SmnF7/F7) control embryos was performed (Figure 3A). Using primers flanking Smn exon 7, RT-PCR analysis of Smn in liver of mutant embryos revealed a marked reduction of the full-length transcript (Figure 3A). Surprisingly, SmnΔ7 transcripts were detected in liver of heterozygous but not homozygous mutant mice suggesting that cells carrying homozygous Cre-mediated deletion of Smn exon 7 do not survive (Figure 3A). Immunoblotting of proteins extracted from liver of 18 days p.c. mutant (Alfp-Cre, SmnF7/F7) or control embryos (SmnF7/+ or SmnF7/F7) was performed using a monoclonal antibody directed against the N-terminus of the SMN protein. Dramatic reduction of SMN was observed in liver of mutant embryos, which correlated with the marked decrease of full-length Smn transcripts (Figure 3B). The residual amount of the SMN protein likely resulted from non-deleted liver or hematopoeitic cells.
Figure 3.
Molecular analysis of liver from Smn mutant mice. A: RT-PCR analysis of Smn transcripts extracted from control (SmnF7/+ or SmnF7/F7), mutant embryos (Alfp-Cre, SmnF7/F7, left) or heterozygous adult mice (Alfp-Cre, SmnF7/+, right). Note the marked decrease of full-length Smn transcripts associated with lack of SmnΔ7 transcripts in (Alfp-Cre, SmnF7/F7) liver when compared with control and aldolase expression (left panel). In heterozygous mutant mice (Alfp-Cre, SmnF7/+), SmnΔ7 transcripts are detectable (right). SmnΔ7/+ mouse was used as positive control on the right. B: Immunoblotting experiments of SMN in control and mutant mice. Note the marked reduction of SMN in homozygous (left) or half dose in heterozygous mutant mice (right) when compared to control and actin expression. (C and D) Immunoblotting experiments of AFP (C) and albumin (D) in control and mutant mice. Note the marked reduction of AFP and albumin expression in mutant embryos (Alfp-Cre, SmnF7/F7, left) when compared to control of the same age and actin expression (SmnF7/+ or SmnF7/F7). No increased amount of AFP was observed in heterozygous adult mutant mice (C, right). A mouse wild-type fetus was used as positive control on the right.
Lack of Smn Exon 7 Leads to Severe Defect of Liver Development
Macroscopic examination of (Alfp-Cre, SmnF7/F7) mutant embryos revealed a dramatic reduction of liver mass (Figure 2C). From 16 to 21 days p.c., weight of liver was markedly diminished in mutant embryos (Alfp-Cre, SmnF7/F7; 10 mg ± 1.3 SE, 86% reduction at 18 days p.c., P < 0.0001) when compared with controls of the same age (SmnF7/+ or SmnF7/F7; 73 mg ± 2.1 SE, Figure 2B). Hematoxylin and eosin staining of liver sections of 18 days p.c. mutant and control embryos was performed. At 18 days p.c., the morphology of liver from mutant mice revealed markedly reduced number of hepatocytes and bile ducts, liver tissue being mainly composed of hematopoeitic cells (Figure 4). No liver fibrosis was observed as determined by Masson method (data not shown). The morphology of the remaining hepatocytes was similar to that of control embryos and no nuclear fragmentation was observed. TUNEL staining did not reveal any increased number of apoptotic cells in mutant when compared to control embryos (Figure 4 and data not shown). Marked reduction in number of hepatocytes and bile ducts strongly suggested that homozygous mutation of Smn resulted in dramatic impairment of liver development.
Figure 4.
Histological and immunolabelling experiments of mutant embryos (Alfp-Cre, SmnF7/F7). a and b: Hematoxylin eosin safran staining reveals a dramatic reduction of hepatocyte number in mutant (b) when compared to control embryos of the same age (a, arrow). Liver tissue is mainly composed of hematopoeitic cells in mutant embryos (arrowhead). c and d: AFP immunolabeling on liver tissue sections. Note the dramatic reduction of cells labeled with AFP antibody in mutant (d) when compared to control embryos (c, arrow). e and f: TUNEL staining in control (e) and mutant embryos (f). No increased number of apoptotic cells is detectable in mutant embryo. g and h: Ki67 immunolabeling on liver tissue sections of control (g) and mutant embryos (h). Number of dividing cells is similar in control (g) or mutant embryos (h, arrow). Scale bar: 30 μm (a and b, c to h).
To determine whether this defect was associated with liver-specific dysfunction, expression of albumin and α-fetoprotein was evaluated. Dramatic reduction of albumin and α-fetoprotein expression was observed in (Alfp-Cre, SmnF7/F7) mutant embryos when compared to that of (SmnF7/+) control embryos and actin expression (Figure 3). Consistently, immunolabeling experiments performed on frozen liver sections revealed very few hepatocytes labeled by α-fetoprotein antibody in mutant embryos as compared to control embryos. These results indicate further a marked reduction of functional hepatocyte number (Figure 4). Defect of liver functions was also suspected by the absence of blood clot after umbilical cord section of dissected mutant but not of control embryos. These data suggest a defect in hemostasis, which could be responsible for neonatal lethality during delivery.
To ascertain whether liver regeneration occurred in mutant embryos, immunohistochemical experiments were performed on frozen liver sections using antibody directed against Ki67, an antigen specific to cell cycle division. The density of Ki67-positive cells was similar in both control and mutant embryos suggesting that liver regeneration did not occur in mutant embryos (Figure 4). Altogether, these data strongly suggest that Cre-mediated deletion of Smn exon 7 occurred in hepatoblasts leading to marked defect of liver development associated with lack of liver regeneration. The presence of rare hepatocytes likely results from cells that do not express the Cre recombinase in agreement with the low amount of SMN in mutant embryos.
Mutant Embryos Display Massive Iron Overload Restricted to Liver
Surprisingly, Perls staining showed massive iron overload in liver of 16 days p.c. mutant embryos when compared to control embryos of the same age (Figure 5). Examination of other tissues including brain, intestine, kidney, pancreas, bone, or heart from mutant embryos did not reveal any accumulation of iron indicating that it was restricted to liver (data available on request). To determine whether iron accumulated in hepatocytes or reticulo-endothelial cells, serial sections of liver were labeled with Perls and anti-CD68, a marker specific to reticulo-endothelial cells. Iron overload was observed in both types of cells. The same labeling was applied to lung and revealed high proportion of reticulo-endothelial cells without any iron accumulation. These results indicate that iron overload occurred in hepatocytes and reticulo-endothelial cells of liver (data available on request).
Figure 5.
Massive iron overload in Smn mutant embryos. Perls staining was performed on liver sections of 16 days p.c. mutant (B) and control embryos (A). Note the marked deposition of iron in mutant liver (arrow). Scale bar: 40 μm.
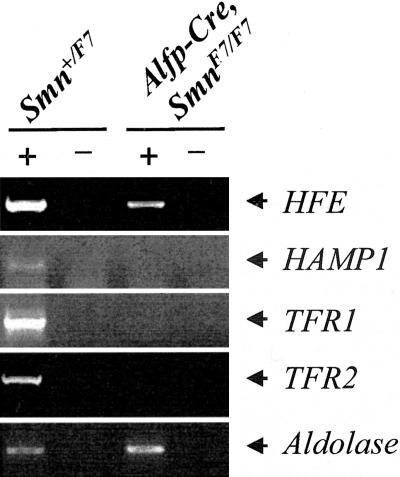
Previous studies have shown that SMN played an essential role in RNA metabolism (5 for review). We were wondering whether the lack of SMN directed to liver might result in defect of splicing or processing of transcripts encoding proteins involved in iron homeostasis. Genes including transferrin receptor 2 (TFR2), HFE, hepcidin 1 and 2 (HAMP 1 and 2), or transferrin receptor (TFR1) might be regarded as candidates based on the presence of alternative spliced products of TFR2 and HFE transcripts, predominant liver expression of HFE, TFR2, or hepcidin, or the presence of iron responsive element (IRE) in untranslated region of TFR1 transcripts.15–19 To avoid any bias in RNA analysis, cDNA synthesis was carried out using either random or oligo (dT) primers. Semi-quantitative RT-PCR analysis of transcripts was performed from liver of 16 days p.c. mutant (Alfp-Cre, SmnF7/F7) and control embryos of the same age. No hepcidin 2 transcripts were detectable in either mutant or control liver indicating that hepcidin 2 was not expressed during liver development. Dramatic reduction of HFE and lack of HAMP1, TFR1, or TFR2 transcripts were observed in mutant liver embryos when compared to control and aldolase expression (Figure 6). No abnormal spliced products were detected in mutant liver when compared to control. Similar results were obtained using either random or oligo(dT) primers for cDNA synthesis.
Figure 6.
Transcript analysis of genes involved in iron homeostasis. RT-PCR analysis of transcripts encoding HFE, TFR1, TFR2, and HAMP1 was performed in control and mutant embryos. Aldolase expression was used as internal control. RT-PCR amplification was performed with (+) or without (−) reverse transcriptase. Lack of TFR1, TFR2, and HAMP1 transcripts or marked reduction HFE transcripts were observed in mutant embryos when compared to control and aldolase expression.
Finally, the same Smn mutation had been previously directed to other tissues including neurons (neuronal model)7,9 or skeletal muscle (muscular model).8,10 To determine whether lack of SMN was responsible for iron accumulation in other tissues mutated for Smn, Perls staining was performed in skeletal muscle and spinal cord of 25-day-old muscular and neuronal models, respectively. No iron accumulation was observed in these tissues. These data indicate that SMN defect did not trigger ubiquitous protein involved in iron homeostasis (data available on request).
Lack of Dominant Negative Effect of Heterozygous Deletion of Smn Exon 7 in Vivo
To determine whether heterozygous deletion of Smn exon 7 might have any deleterious effect on liver, (Alfp-Cre, SmnF7/+) mice were analyzed. Macroscopic examination of liver from (Alfp-Cre, SmnF7/+) embryos did not reveal any abnormalities (Figure 2B) and ratio of liver to total body weight of heterozygous or control adult mice did not differ significantly (mean of 3.7% in SmnF7/F7 and 3.9% in Alfp-Cre, SmnF7/+, Student’s t-test, P = 0.34). In addition, histological analysis of liver from (Alfp-Cre, SmnF7/+) adult mice did not show any change when compared to control mice (SmnF7/F7, data not shown). No iron accumulation was detected in liver of adult heterozygous mice (data not shown). RT-PCR analysis of RNA extracted from liver of heterozygous mice (Alfp-Cre, SmnF7/+) revealed the presence of truncated transcripts lacking exon 7 and immunoblotting experiments showed as expected half dose of SMN in liver of (Alfp-Cre, SmnF7/+) adult mice when compared to control mice and actin expression (Figure 3).
Serum activities of enzymes expressed in liver including transaminases and γ-glutamyl transferase (γ-GT) did not reveal any change in either young (1- to 2-month-old, n = 11) or older heterozygous mice (7- to 13-month-old, n = 7) when compared with controls of the same age (SmnF7/F7, n = 9, Table 2). Finally, immunoblotting experiments revealed no increase of α-fetoprotein protein amount in (Alfp-Cre, SmnF7/+) mice with respect to that of (SmnF7/F7) control of the same age and actin expression (Figure 3C). These data indicate that heterozygous deletion of Smn exon 7 is sufficient for normal liver function and did not provide any evidence for liver necrosis nor for regeneration as determined by liver enzyme or α-fetoprotein analyses, respectively.
Table 2.
Liver Function of Control, Heterozygous Mutant Mice, and Human SMA Patients
| Genotype | Young mice (1–2 months)
|
Old mice (7–13 months)
|
SMA patients
|
||
|---|---|---|---|---|---|
| SmnF7/F7 | Alfp-Cre, Smn+/F7 | SmnF7/F7 | Alfp-Cre, Smn+/F7 | SMN1Δ7/Δ7 | |
| Number | 9 | 11 | 9 | 7 | 4 |
| SGOT (U/L) | 146 ± 30 | 161 ± 28 | 111 ± 7 | 120 ± 9 | 28 ± 4 [20–120] |
| SGPT (U/L) | 46 ± 7 | 53 ± 9 | 42 ± 3 | 45 ± 1 | 23 ± 10 [10–50] |
| γ-GT (U/L) | 2 ± 2 | 0 | 0 | 0 | 15 ± 2 [10–80] |
| Proteins (g/L) | 57 ± 4 | 58 ± 4 | 60 ± 4 | 66 ± 2 | nd |
| Albumin (g/L) | nd | nd | nd | nd | 40 ± 2 [35–42] |
| AFP (ng/mL) | nd | nd | nd | nd | 1.1 ± 0.4 [0–10] |
Results are given as mean with ± S.E. Normal values in humans are given in square parenthesis. nd, not done.
To determine whether liver dysfunction might occur in SMA patients, enzymatic activities of γ-GT, transaminases and dosage of α-fetoprotein and albumin were evaluated in serum of four patients affected with SMA type II, the intermediate form of SMA. In these patients, onset of symptoms occurred within the first year of life.20 They were able to sit unaided but not to walk. Patients carried homozygous deletion of SMN1 exon 7 (data not shown). Serum analysis was performed at a mean time of 13.3 years after the onset of symptoms (11 to 17 years). No change was observed indicating that the residual amount of SMN in liver of SMA patients is sufficient for normal liver function during natural SMA disease course (Table 2).
Discussion
Using the Aflp-Cre transgenic line, deletion of Smn exon 7 has been directed to liver from 10 days postcoitum. Homozygous mutation leads to late embryonic lethality of mutant mice. Mutant phenotype was characterized by dramatic liver atrophy associated with severe liver dysfunction, iron overload, and lack of regeneration. Liver-specific deletion of Smn indicates that defect of liver function was responsible for death. Molecular analysis of liver from (Alfp-Cre, SmnF7/F7) mutant embryos revealed dramatic reduction of SMN protein. Residual amount of SMN found in liver of mutant embryos likely comes from hepatocytes or hematopoeitic cells which do not express Cre recombinase. Marked rarefaction of hepatocytes expressing α-fetoprotein associated with the absence of SmnΔ7 transcripts strongly suggest that hepatocytes carrying homozygous Cre-mediated deletion of Smn exon 7 do not survive. Negative TUNEL staining excludes apoptosis as the pathogenic mechanism involved in cell loss. Surprisingly, no liver regeneration was observed from the remaining hepatocytes or hepatoblasts as determined by both the marked reduction of α-fetoprotein and the absence of increased number of dividing cells in liver of mutant embryos. It can be hypothesized that hepatocyte progenitors entering into cell cycle division lead to the generation of new hepatocytes in which Cre recombinase activity will induce deletion of Smn and cell loss.
These results demonstrate that Smn transcripts lacking exon 7 (SmnΔ7), which represent the predominant transcript isoform encoded by the SMN2 gene in human,3 are not able to compensate for the lack of full-length Smn transcripts and lead to loss of function. Loss of function rather than dominant negative effect of SmnΔ7 was further supported by the analysis of heterozygous mutant mice. Heterozygous deletion of Smn exon 7 in liver, which results in the expression of both full-length and truncated transcripts, did not result in any changes in either morphology or function of liver. In these mice, the presence of half dose of SMN protein strongly suggests an instability of SmnΔ7 protein as previously suspected.8 These results indicate that half dose of SMN is sufficient for normal liver function and exclude the hypothesis of a dominant negative effect of SmnΔ7 on wild-type allele in vivo. These data are consistent with previous in vivo data in either mice8 or human SMA carriers.
The most striking feature found in mutant embryos was massive iron deposition in the liver. Examination of other organs did not provide any evidence for iron accumulation in other tissues. Moreover, examination of tissues in which homozygous deletion of Smn exon 7 had been previously targeted, namely skeletal muscle and neurons, did not reveal iron overload.7–9 Therefore, genes encoding proteins predominantly expressed in liver and playing an essential role in iron homeostasis might be regarded as targets of SMN defect, a protein involved in RNA processing in cell systems (5 for review). Marked reduction or lack of transcripts encoding TFR2, TFR1, HFE, and HAMP1 was observed in mutant liver embryos, which could be ascribed to marked reduction of functional hepatocytes in mutant embryos. Several spontaneous or induced mutations have been reported in murine genes encoding proteins involved in iron metabolism. Interestingly, iron accumulation in liver was observed from 1-month-old mutant mice but not in embryos lacking hepcidin or HFE21,22 excluding both genes as candidates to account for liver iron deposition in Smn mutant embryos. In mice carrying homozygous mutation of Tfr2 or β2-microglobulin genes, liver iron accumulation was observed from 1 to 8 months of age but no data are available in liver of mutant embryos.23,24 However, these genes have been regarded as candidates in human neonatal hemochromatosis (OMIM 231100), a rare syndrome in which congenital cirrhosis or fulminant hepatitis in early infancy is associated with marked iron deposition in the liver and extrahepatic tissues. Autosomal recessive inheritance has been suggested in familial forms. No pathogenic mutations in β2-microglobulin, HFE, and haem oxygenases 1 and 2 genes have been identified in multiplex families.25 In the same way, mutations of the TFR2 gene have been identified in juvenile but not in neonatal form of hemochromatosis.26 Our data and previous studies in various mouse models of hemochromatosis or in human inherited neonatal hemochromatosis did not provide any rational candidate gene to account for iron accumulation in liver embryos deficient for SMN. Evidence from human patients with neonatal hemochromatosis strongly suggests that iron accumulation might be secondary to disrupted hepatocellular development resulting from various non-inherited insults.27 In Smn mutant mice, severe impairment of liver development could be responsible for defect of various liver functions including iron regulation leading to liver iron overload. Alternatively, liver being the major site of iron storage, iron accumulation could result from marked loss of hepatocytes no longer able to ensure this function.
Importantly, this study indicates that the lack of full-length SMN protein in various mouse cell types including neurons, skeletal muscle fibers, or hepatocytes leads to severe cell dysfunction. However, in human SMA, the disease phenotype is restricted to degeneration of the neuromuscular system and no liver dysfunction had been reported so far. How to explain the phenotypic difference in response to SMN defect in liver between human and mouse? Murine Smn gene is present as a single copy while in humans, SMN1 is duplicated in a highly homologous copy SMN2 (4 for review). The residual amount of SMN produced by full-length transcripts of the SMN2 gene, which remains present in patients, is likely sufficient to ensure normal functions of various organs including liver but not motor neurons. Motor neuron is one of the largest cells in the body by both volume and surface area. Such large cellular unit requires molecular machinery able to produce and regulate molecules, including proteins and RNA, from the cell body to the neuromuscular junction through motor axons. Mild impairment in production or stability of molecules involved in these processes could have detrimental effect on axonal growth. This hypothesis is consistent with features found in mouse or Drosophila carrying mutation of Smn9,28 or in in vitro experiments.29,30 Identifying defective transcripts encoding proteins involved in or regulating structural specificities of motor neurons should contribute to elucidate SMA pathogenesis.
However, other neurons including sensory neurons are also very large in size. Recently, involvement of these neurons has been described in severe cases of SMA.31 Skeletal muscle has also been recently shown to be involved when lack of murine SMN has been directed to that tissue.8,10 Although motor neurons are the predominant cell type affected in the human disease, data from mouse and human suggest that other tissues might be involved during prolonged SMA disease course or in very severe form of SMA. Our results reinforce the need of therapeutic approaches targeted to all tissues in human SMA. In addition, liver function of patients should be carefully investigated and followed up before and during therapeutic trials.
Acknowledgments
We thank Michèle Hadchouel and Anne Weber for fruitful discussions and Benedicte Desforges for helpful technical assistance.
Footnotes
Address reprint requests to Judith Melki, Molecular Neurogenetics Laboratory, INSERM E-223, 2 rue Gaston Crémieux, CP5724, 91057 Evry, France. E-mail: j.melki@genopole.inserm.fr.
Supported by INSERM, the Association Française contre les Myopathies (AFM), Families of SMA (USA), and the Fondation Bettencourt Schueller.
References
- Pearn J. The gene frequency of acute Werdnig-Hoffmann disease (SMA type I): a total population survey in Northeast England. J Med Genet. 1973;10:260–265. doi: 10.1136/jmg.10.3.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearn J. Incidence, prevalence, and gene frequency studies of chronic childhood spinal muscular atrophy. J Med Genet. 1978;15:409–413. doi: 10.1136/jmg.15.6.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre S, Bürglen L, Reboullet S, Clermont O, Burlet P, Viollet L, Benichou B, Cruaud C, Millasseau P, Zeviani M, Le Paslier D, Frezal J, Cohen D, Weissenbach J, Munnich A, Melki J. Identification and characterization of a spinal muscular atrophy-determining gene. Cell. 1995;80:155–165. doi: 10.1016/0092-8674(95)90460-3. [DOI] [PubMed] [Google Scholar]
- Frugier T, Nicole S, Cifuentes-Diaz C, Melki J. The molecular bases of spinal muscular atrophy. Curr Opin Genet Dev. 2002;12:294–298. doi: 10.1016/s0959-437x(02)00301-5. [DOI] [PubMed] [Google Scholar]
- Paushkin S, Gubitz AK, Massenet S, Dreyfuss G. The SMN complex, an assemblyosome of ribonucleoproteins. Curr Opin Cell Biol. 2002;14:305–31. doi: 10.1016/s0955-0674(02)00332-0. [DOI] [PubMed] [Google Scholar]
- Schrank B, Gotz R, Gunnersen JM, Ure JM, Toyka KV, Smith AG, Sendtner M. Inactivation of the survival motor neuron gene, a candidate gene for human spinal muscular atrophy, leads to massive cell death in early mouse embryos. Proc Natl Acad Sci USA. 1997;94:9920–9925. doi: 10.1073/pnas.94.18.9920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frugier T, Tiziano FD, Cifuentes-Diaz C, Miniou P, Roblot N, Dierich A, Le Meur M, Melki J. Nuclear targeting defect of SMN lacking the C-terminus in a mouse model of spinal muscular atrophy. Hum Mol Genet. 2000;9:849–858. doi: 10.1093/hmg/9.5.849. [DOI] [PubMed] [Google Scholar]
- Cifuentes-Diaz C, Frugier T, Tiziano FD, Lacene E, Roblot N, Joshi V, Moreau MH, Melki J. Deletion of murine SMN exon 7 directed to skeletal muscle leads to severe muscular dystrophy. J Cell Biol. 2001;152:1107–1114. doi: 10.1083/jcb.152.5.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cifuentes-Diaz C, Nicole S, Velasco ME, Borra-Cebrian C, Panozzo C, Frugier T, Millet G, Roblot N, Joshi V, Melki J. Neurofilament accumulation at the motor endplate and lack of axonal sprouting in a spinal muscular atrophy mouse model. Hum Mol Genet. 2002;11:1439–1447. doi: 10.1093/hmg/11.12.1439. [DOI] [PubMed] [Google Scholar]
- Nicole S, Desforges B, Millet G, Lesbordes J, Cifuentes-Diaz C, Vertes D, Cao ML, De Backer F, Languille L, Roblot N, Joshi V, Gillis JM, Melki J. Intact satellite cells lead to remarkable protection against Smn gene defect in differentiated skeletal muscle. J Cell Biol. 2003;161:571–582. doi: 10.1083/jcb.200210117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellendonk C, Opherk C, Anlag K, Schutz G, Tronche F. Hepatocyte-specific expression of Cre recombinase. Genesis. 2000;26:151–153. doi: 10.1002/(sici)1526-968x(200002)26:2<151::aid-gene17>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Miniou P, Tiziano D, Frugier T, Roblot N, Le Meur M, Melki J. Gene targeting restricted to mouse striated muscle lineage. Nucleic Acids Res. 1999;27:e27. doi: 10.1093/nar/27.19.e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Stefanini M, De Martino C, Zamboni L. Fixation of ejaculated spermatozoa for electron microscopy. Nature. 1967;216:173–174. doi: 10.1038/216173a0. [DOI] [PubMed] [Google Scholar]
- Theil EC. Iron regulatory elements (IREs): a family of mRNA non-coding sequences. Biochem J. 1994;304:1–11. doi: 10.1042/bj3040001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawabata H, Germain RS, Ikezoe T, Tong X, Green EM, Gombart AF, Koeffler HP. Regulation of expression of murine transferrin receptor 2. Blood. 2001;98:1949–1954. doi: 10.1182/blood.v98.6.1949. [DOI] [PubMed] [Google Scholar]
- Jeffrey GP, Basclain K, Hajek J, Chakrabarti S, Adams PC. Alternate splicing produces a soluble form of the hereditary hemochromatosis protein HFE. Blood Cells Mol Dis. 1999;25:61–67. doi: 10.1006/bcmd.1999.0227. [DOI] [PubMed] [Google Scholar]
- Thenie A, Orhant M, Gicquel I, Fergelot P, Le Gall JY, David V, Mosser J. The HFE gene undergoes alternate splicing processes. Blood Cells Mol Dis. 2000;26:155–162. doi: 10.1006/bcmd.2000.0291. [DOI] [PubMed] [Google Scholar]
- Nicolas G, Bennoun M, Devaux I, Beaumont C, Grandchamp B, Kahn A, Vaulont S. Lack of hepcidin gene expression and severe tissue iron overload in upstream stimulatory factor 2 (USF2) knockout mice. Proc Natl Acad Sci USA. 2001;98:8780–8785. doi: 10.1073/pnas.151179498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munsat TL. Workshop report: International SMA Collaboration. Neuromuscul Disord. 1991;1:81. [Google Scholar]
- Nicolas G, Viatte L, Lou DQ, Bennoun M, Beaumont C, Kahn A, Andrews NC, Vaulont S. Constitutive hepcidin expression prevents iron overload in a mouse model of hemochromatosis. Nat Genet. 2003;34:97–101. doi: 10.1038/ng1150. [DOI] [PubMed] [Google Scholar]
- Zhou XY, Tomatsu S, Fleming RE, Parkkila S, Waheed A, Jiang J, Fei Y, Brunt EM, Ruddy DA, Prass CE, Schatzman RC, O’Neill R, Britton RS, Bacon BR, Sly WS. HFE gene knockout produces mouse model of hereditary hemochromatosis. Proc Natl Acad Sci USA. 1998;95:2492–2497. doi: 10.1073/pnas.95.5.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming RE, Ahmann JR, Migas MC, Waheed A, Koeffler HP, Kawabata H, Britton RS, Bacon BR, Sly WS. Targeted mutagenesis of the murine transferrin receptor-2 gene produces hemochromatosis. Proc Natl Acad Sci USA. 2002;99:10653–10658. doi: 10.1073/pnas.162360699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenberg BE, Voland JR. Beta2 knockout mice develop parenchymal iron overload: a putative role for class I genes of the major histocompatibility complex in iron metabolism. Proc Natl Acad Sci USA. 1996;93:1529–1534. doi: 10.1073/pnas.93.4.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly AL, Lunt PW, Rodrigues F, Berry PJ, Flynn DM, McKiernan PJ, Kelly DA, Mieli-Vergani G, Cox TM. Classification and genetic features of neonatal haemochromatosis: a study of 27 affected pedigrees and molecular analysis of genes implicated in iron metabolism. J Med Genet. 2001;38:599–610. doi: 10.1136/jmg.38.9.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camaschella C, Roetto A, Cali A, De Gobbi M, Garozzo G, Carella M, Majorano N, Totaro A, Gasparini P. The gene TFR2 is mutated in a new type of haemochromatosis mapping to 7q22. Nat Genet. 2000;25:14–15. doi: 10.1038/75534. [DOI] [PubMed] [Google Scholar]
- Knisely AS, Mieli-Vergani G, Whitington PF. Neonatal hemochromatosis. Gastroenterol Clin North Am. 2003;32:877–89. doi: 10.1016/s0889-8553(03)00050-5. [DOI] [PubMed] [Google Scholar]
- Chan YB, Miguel-Aliaga I, Franks C, Thomas N, Trulzsch B, Sattelle DB, Davies KE, van den Heuvel M. Neuromuscular defects in a Drosophila survival motor neuron gene mutant. Hum Mol Genet. 2003;12:1367–76. doi: 10.1093/hmg/ddg157. [DOI] [PubMed] [Google Scholar]
- Zhang HL, Pan F, Hong D, Shenoy SM, Singer RH, Bassell GJ. Active transport of the survival motor neuron protein and the role of exon-7 in cytoplasmic localization. J Neurosci. 2003;23:6627–6637. doi: 10.1523/JNEUROSCI.23-16-06627.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossoll W, Jablonka S, Andreassi C, Kroning AK, Karle K, Monani UR, Sendtner M. Smn, the spinal muscular atrophy-determining gene product, modulates axon growth and localization of beta-actin mRNA in growth cones of motoneurons. J Cell Biol. 2003;163:801–812. doi: 10.1083/jcb.200304128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudnik-Schoneborn S, Goebel HH, Schlote W, Molaian S, Omran H, Ketelsen U, Korinthenberg R, Wenzel D, Lauffer H, Kreiss-Nachtsheim M, Wirth B, Zerres K. Classical infantile spinal muscular atrophy with SMN deficiency causes sensory neuronopathy. Neurology. 2003;60:983–987. doi: 10.1212/01.wnl.0000052788.39340.45. [DOI] [PubMed] [Google Scholar]