Abstract
CD44, a polymorphic hyaluronate receptor, may participate in chronic inflammation. We hypothesized that CD44 variants contribute to the development of arterial diseases. CD44 levels vary in normal and diseased arterial tissues in the following order: unaffected arteries < fibrous plaques ≤ abdominal aortic aneurysm < atheromatous plaques; and correlate with macrophage content. Furthermore, plaque microvessels express CD44, and anti-CD44v3 or anti-CD44v6 treatment reduces endothelial cell proliferation but not apoptosis in vitro, suggesting functionality of these receptors. Endothelial cells express CD44H and CD44v6 after exposure to interleukin-1β and tumor necrosis factor-α. Macrophages, a major source of abundant CD44 in vitro, express not only CD44H but also variants CD44v4/5, CD44v6, and CD44v7/8, isoforms distinctively regulated by proinflammatory cytokines. Several proinflammatory cytokines induce shedding of CD44 from the surface of macrophages and endothelial cells. Soluble CD44 stimulates the expression and release of interleukin-1β from endothelial cells, suggesting a positive feedback loop of this cytokine. By demonstrating augmented expression of CD44 and variants within human atheroma and in abdominal aortic aneurysm as well as the vascular cell release of sCD44, a process regulated by proinflammatory cytokines, this study provides new insights on the functions of CD44 in arterial diseases.
CD44 is an adhesion molecule of the hyaluronate receptor family. Its standard form, CD44H, lacks variable spliced exons (v), and its apparent molecular weight of 80 kd exceeds the theoretical weight of 37 kd because of extensive posttranslational modifications.1 Additionally, variable exons in the extracellular domain of CD44H give rise to multiple variants distinct in molecular weight and function. Most CD44 variants bind ligands of the extracellular matrix. In addition to its major ligand, hyaluronan (HA), CD44 binds to other components of extracellular matrix such as osteopontin, collagen, fibronectin, and laminin.1 CD44 presumably maintains organ and tissue structure via cell-cell and cell-matrix interactions.1
CD44 contributes to many cellular functions, including adhesion of lymphocytes to inflamed endothelium,2,3 homing,4,5 migration,6,7 cell activation,8 and apoptosis.9–11 Elevated levels of CD44 induce shedding of the elastin-binding protein and lead to impaired production of elastic fibers in Costello syndrome patients,12 thus suggesting that increased levels of CD44 may participate in the formation of dysfunctional elastic fibers.
CD44 proteins may participate in inflammatory processes.13 CD44 contributes to host defenses by activating macrophages (MΦ) to induce Th1 cytokines while inhibiting Th2 cytokines.14 Chronic inflammation increases CD44H expression on T cells.3 Furthermore, the expression of CD44v3 (also known as CD44-HSPG or CD44-HS), CD44v4, CD44v5, CD44v6, CD44v7, and CD44v915 during cutaneous inflammation increases in infiltrating monocytes.
In addition to its role as a transmembrane receptor on many cell types, serum and lymph contain a soluble form of CD44 (sCD44). Proteolytic cleavage of its extracellular domain by enzymes such as membrane-type 1 matrix metalloproteinase,16 a chymotrypsin-like sheddase,17,18 serine proteases, and matrix metalloproteinases19 removes CD44 from the cell surface. Atherosclerotic lesions contain proteinases implicated in degradation of components of extracellular matrix such as collagen and elastin. Such lesions commonly exhibit an imbalance between matrix-degrading enzymes and their inhibitors. Shedding of CD44 plays a major role in the inhibition of CD44-HA-dependent cell-matrix interactions at the inflammatory site.20 The release of CD44 during inflammatory processes may indicate ongoing matrix remodeling because of enhanced proteolytic activity. Interestingly, the severity of arthritis, another inflammatory condition, correlates with shedding of cell surface CD44. The physiological inducers of CD44 release and the role of sCD44 remain incompletely defined.
In light of these many diverse functions of CD44 related to cell-cell and cell-matrix interactions, we hypothesized that CD44 variants contribute to the development of inflammatory arterial diseases. The present study investigated the expression of CD44H and several distinct variants (CD44v3, CD44v4/5, CD44v6, CD44v7/8, and CD44v10) in atheromata as well as in abdominal aortic aneurysm (AAA). We also studied the regulation by proinflammatory cytokines of CD44 expression and shedding in human endothelial cells (ECs) in vitro and MΦ in vitro.
Materials and Methods
Cell Culture
Human monocytes were isolated from buffy coats by density gradient centrifugation using Cappel LSM lymphocyte separation media according to the manufacturer’s instructions (ICN Biomedical Inc., Aurora, OH). Freshly plated monocytic cells in serum-free medium (SFM) were denoted “day 0” and incubated for 48 hours in either SFM alone or SFM supplemented with 10 ng/ml of interleukin (IL)-1β and tumor necrosis factor (TNF)-α, 1000 U/ml interferon (IFN)-γ (all from Endogen, Woburn, MA), or 10 μg/ml CD40L (Leinco Technologies Inc., St. Louis, MO). To obtain maximal cell activation, we used a cocktail containing IL-1β, TNF-α, and well-established cytokine concentrations.21–23 In addition, aliquots of monocyte preparation were differentiated for 10 (day 10) days in RPMI 1640 (Life Technologies, Inc., Gaithersburg, MD) supplemented with 100 U/ml penicillin/streptomycin, 2 mmol/l l-glutamine, 1.26 mg/ml amphotericin B, 20 mmol/l sodium pyruvate, 0.1% NaHCO3, 2% human serum, and 10% fetal bovine serum. Cells were growth-arrested in SFM for 24 hours and then incubated for 48 hours as described for day 0. Purity of monocyte preparations was ≥92% as determined by fluorescence-activated cell sorting analysis using fluorescein isothiocyanate-labeled anti-CD68 (Pharmingen, San Diego, CA).
Human ECs were isolated from saphenous veins and cultured in M-199 (Life Technologies, Inc.) supplemented with 100 U/ml penicillin/streptomycin, 1.26 mg/ml amphotericin B, 100 mg/ml heparin, 50 mg/ml endothelial cell growth factor, and 5% fetal calf serum. Cells were growth-arrested for 16 hours before cytokine incubations in M199 supplemented with 100 U/ml penicillin/streptomycin, 1.26 mg/ml amphotericin B, and 0.1% bovine serum albumin. Cytokines were diluted in SFM and used at concentrations described above. Immunostaining with mouse anti-CD31 (DAKO, Carpinteria, CA) characterized EC isolates. Culture media for all cell types, fetal bovine serum, and CD44-specific antibodies contained <40 pg endotoxin/ml as determined by the chromogenic Limulus amebocyte assay (QLC-1000; Bio-Whittaker, Walkersville, MD).
Proliferation and Apoptosis Assay
For the proliferation assay, ECs from three different donors were seeded in duplicate in 96-well plates in 100 μl of culture media as described above. Cells were incubated either with or without antibodies against CD44H, CD44v3, CD44v6 (R&D Systems, Abingdon, UK), and CD44v7/8 (Chemicon, Temecula, CA) at 100 μg/ml. After 24 hours, cell proliferation was quantified using a colorimetric assay according to the manufacturer’s instructions (XTT; Roche Diagnostics GmbH, Mannheim, Germany). Mouse IgG1, IgG2A, and IgG2B were used as negative controls for the respective CD44 antibodies. Optical density readings obtained from negative controls were subtracted from each individual reading of the corresponding CD44 antibody.
For the apoptosis assay, ECs from three different donors were seeded in eight-chamber slides and antibodies were added as described above for the proliferation assay. After 24 hours, slides were washed twice with phosphate-buffered saline (PBS), fixed with 1% paraformaldehyde for 20 minutes, and then washed three times with Tris-buffered saline containing 0.1% Triton X-100. Samples were subsequently immunostained for active caspase 3 as described.24
Tissue Samples
We obtained fresh surgical specimens of human carotid atheroma and nonatherosclerotic aorta (cardiac transplantation donors) as well as discarded aneurysmal tissue from AAA repair surgery according to protocols approved by the Human Investigation Review Committee at the Brigham and Women’s Hospital. All tissue samples were immediately divided into two macroscopically identical portions and used for both morphological and biochemical studies. All tissue samples used for Western blotting were morphologically characterized. Atherosclerotic plaques were dichotomized into fibrous (n = 4) and atheromatous (n = 5) subsets by morphological criteria as described previously.25 Briefly, this classification is based on fibrous cap thickness as well as smooth muscle cell (SMC) and MΦ content. Fibrous plaques (designated “fibrous cap atheroma” by Virmani and colleagues26) are defined as lesions with maximal fibrous cap thickness >0.8 mm (in the area of minimal thickness) and positive area for MΦ (CD68 staining) <10% and for SMC (α-actin) >10%. Atheromatous plaques (designated “thin fibrous cap atheroma” by Virmani and colleagues26) are characterized by a minimal fibrous cap thickness <0.3 mm and a positive area for MΦ >20% and for SMC <10%.
Immunohistochemistry
Serial cryostat tissue sections (6 μm) were fixed in acetone, air-dried, and stained by the avidin-biotin-peroxidase method. After blocking with 0.3% hydrogen peroxide and PBS supplemented with 4% species-appropriate normal serum, sections were processed according to the manufacturer’s recommendations (Universal DAKO LSAB kit, DAKO). Primary antibodies from R&D Systems (CD44H, CD44v3, CD44v4/5, CD44v6) and Chemicon (CD44v7/8, CD44v10) were all used at 10 μg/ml, except CD44v4/5 (100 μg/ml). The reaction was visualized with 3-amino-9-ethyl carbazole (DAKO). Sections were counterstained with Gill’s hematoxylin solution. Mouse IgG1 (M-9269; Sigma Immuno Chemicals, St. Louis, MO) diluted to the same IgG concentration as the primary antibodies was used as negative control.
Western Blot
Specimens of nonatherosclerotic arterial tissue (n = 6), fibrous (n = 6) and atheromatous (n = 5) atherosclerotic plaques, and AAA tissue (n = 7) were snap-frozen, homogenized under liquid nitrogen, lysed, and prepared as described previously.27 For cultured cells, supernatants were removed and cells were washed two times in PBS. Cells were lysed in buffer containing 0.15 mol/l NaCl, 10 mmol/l Tris, 5 mmol/l MgCl2, 2 mmol/l ethylenediaminetetraacetic acid, 1% Triton X-100 (pH 7.2) supplemented with proteinase inhibitors 0.1 mmol/l 4-(2-aminoethyl) benzenesulfonyl fluoride hydrochloride, 2 μg/ml aprotinin, 2 μg/ml leupeptin, and 1 μg/ml pepstatin A. Cells and matrix were scraped into Eppendorf tubes. Lysates were incubated on ice for 20 minutes then cleared by centrifugation for 5 minutes at 300 × g (4°C). Total protein was measured with the Micro BCA protein assay (Pierce, Rockford, IL). Total protein from tissue extracts (25 μg) and cell culture lysates (40 μg) was analyzed under nonreducing conditions on 8% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels and transferred to polyvinylidene fluoride membranes as described.25 All primary antibodies were used at 1 μg/ml, except CD44v10 (2 μg/ml). Peroxidase-conjugated goat anti-mouse IgG (Jackson ImmunoResearch Laboratories, West Grove, PA) was applied as the secondary antibody at a dilution of 1:10,000. Immunoreactive bands were digitized and analyzed densitometrically; the integrated optical density was calculated using the Gel Pro Analyzer software (Media Cybernetics Inc., Des Moines, IA).
Secretion of CD44
Conditioned media from ECs and MΦ was analyzed for the presence of sCD44 with a sCD44std module set (Bender MedSystems, Vienna, Austria). This enzyme-linked immunosorbent assay (ELISA) recognizes the framework portion of CD44 common to all isoforms and gives a measurement of total sCD44, including both standard CD44 and splice variants. Cells were cultured in SFM in the presence or absence of IL-1β, TNF-α, IFN-γ, or CD40L, as described above. Supernatants (four to seven donors) were analyzed undiluted. We ran each supernatant in duplicate.
Cytokine Expression by sCD44-Stimulated ECs
Supernatants from early monocytes in SFM were collected and concentrated with Centriprep-30 with a 30-kd molecular weight cutoff (Amicon, Beverly, MA).19 sCD44 concentration was determined by sCD44 ELISA, as described above. After growth arrest ECs (n = 3) were incubated with sCD44 (80 ng/ml) in the presence or absence of a 100-fold excess (8 μg/ml) of anti-CD44H antibody (R&D Systems). As a control, cells were incubated with either heat-inactivated (56°C, 30 minutes) sCD44-enriched supernatant or an IgG2A. Cytokine ELISA was performed for IL-8, IL-1β, IFN-γ, and TNF-α, as described for IL-1β.28 Samples from each donor were analyzed in duplicate.
Reverse Transcriptase-Polymerase Chain Reaction
Total RNA was isolated from cultured ECs using the RNeasy mini kit (Qiagen, Valencia, CA). One hundred ng of total RNA was reverse-transcribed into cDNA for 10 minutes at 20°C, 15 minutes at 42°C, and 5 minutes at 99°C. The reaction mixture was cooled to 4°C before amplification. Samples were denatured at 95°C for 2 minutes and then amplified with IL-1β primers29 for 36 cycles at 95°C for 1 minute, 60°C for 1 minute, and 72°C for 2 minutes. Polymerase chain reaction products (388 bp) were run on 1% agarose gels and stained with ethidium bromide.
Statistics
Results were analyzed with the Mann-Whitney test (Western blot on tissue extracts) and Student’s t-test (cell culture experiments). P ≤ 0.05 was regarded as statistically significant.
Results
Elevated Protein Levels of CD44 in Human Atheroma and AAA
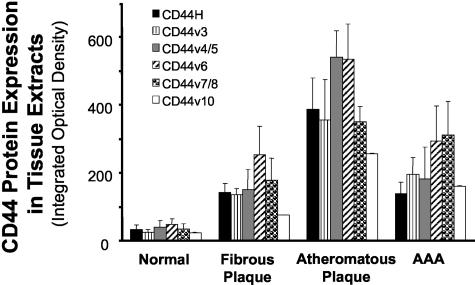
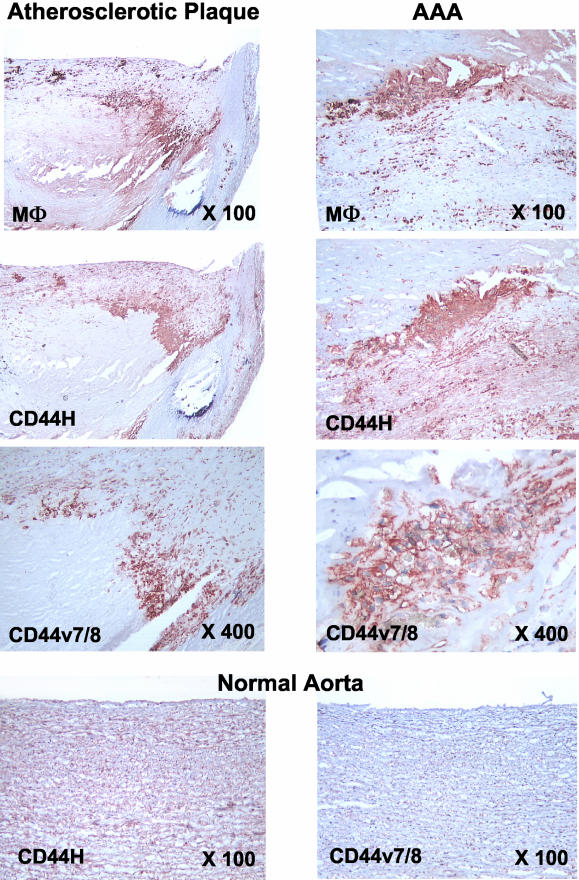
Normal arterial tissue expresses CD44H but almost no splice variants. Western blot analyses of extracts from nondiseased tissues show either weak or no detectable signal for CD44 or its variants (Figure 1); immunohistochemical stainings of corresponding tissue sections confirmed these findings (Figure 2). ECs, identified by CD31-staining, express only CD44H of the isoforms tested (data not shown). Fibrous plaques contain all CD44 splice variants sought compared to nondiseased arterial tissue (Figure 1). In diseased arterial tissue, CD44H co-localizes predominantly with MΦ in both atherosclerotic plaques and AAA, as determined by CD68 staining performed on serial sections (Figure 2) and to a lesser extent with SMC (data not shown). MΦ show immunodetectable levels of all tested CD44 variants [CD44v3, CD44v4/5, CD44v6, CD44v7/8 (Figure 2), and CD44v10], whereas SMCs yield only weak staining for CD44v7/8 similar to nondiseased tissues (data not shown). Levels of CD44H and its variants were highest in MΦ-rich atheromatous plaques, as determined by immunohistochemistry and Western blot analysis (Figures 1 and 2; Table 1). These lesions have 11.9-fold higher CD44H levels (P = 0.01, Table 1), and levels of all CD44 variants studied increased 10- to 15-fold compared to nondiseased tissue (Table 1). Interestingly, because of higher MΦ content, atheromatous plaques showed substantially increased levels (approximately twofold to fourfold) of CD44H and variants compared to either fibrous plaques or AAA tissue (Table 1), which contain less MΦ.
Figure 1.
CD44 expression is enhanced in tissue extracts from atheroma and AAA. Tissue extracts (25 μg total protein/lane) from nonatherosclerotic arteries (normal, n = 6), fibrous plaques (n = 6), and atheromatous plaques (n = 5), as well as AAA (n = 7) were analyzed by Western blotting for CD44H and variants expression. The integrated optical density of each band was densitometrically determined as described in Materials and Methods. Bars represent mean ± SEM.
Figure 2.
Increased CD44 expression in macrophage-rich areas in atheroma and AAA. Both CD44 and its splice variants are prominently expressed in macrophage-rich areas of atherosclerotic plaques (left) and AAA tissue (right). Representative stainings are shown. Staining of tissue samples from seven patients showed similar results. Normal, nondiseased, aorta (bottom, n = 5) is positive for CD44H but not CD44v7/8. Original magnifications are indicated.
Table 1.
Changes in CD44 Levels in Tissue Extracts
| CD44H | CD44v3 | CD44v4/5 | CD44v6 | CD44v7/8 | CD44v10 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fibrous plaque versus normal | +4.4 | P = 0.01 | +5.5 | P = 0.01 | +3.8 | P = 0.01 | +5.3 | P = 0.03 | +5.1 | P = 0.03 | +3.3 | P = 0.05 |
| Atheromatous plaque versus normal | +11.9 | P = 0.01 | +14.6 | P = 0.01 | +13.5 | P = 0.01 | +11.1 | P = 0.01 | +9.9 | P = 0.01 | +11.1 | P = 0.01 |
| Fibrous plaque versus atheromatous plaque | −2.7 | P = 0.05 | −2.6 | P = 0.05 | −3.6 | P = 0.02 | −2.1 | P = 0.05 | −2.0 | P = 0.05 | −3.4 | P = 0.03 |
| AAA versus normal | +4.3 | P = 0.03 | +7.9 | P = 0.02 | +4.5 | P = 0.01 | +6.1 | P = 0.02 | +8.8 | P = 0.004 | +6.9 | P = 0.02 |
| AAA versus fibrous plaque | P = NS | P = NS | P = NS | P = NS | P = NS | P = NS | ||||||
| AAA versus atheromatous plaque | −2.8 | P = 0.04 | −1.8 | P = 0.04 | −3.0 | P = 0.02 | P = NS | P = NS | P = NS | |||
Western Blot analysis of tissue extracts of nondiseased (normal, n = 6), fibrous (n = 6), and atheromatous (n = 5) plaque, and aortic aneurysm (AAA, n = 7) are shown as fold increase (+) or fold decrease (−). P values ≤0.05 were regarded as statistically significant. NS denotes no significant change.
In aneurysmal tissue, CD44H co-localizes not only with MΦ (Figure 2) but also with SMC and CD4+ T cells (data not shown). AAA also showed induced expression of CD44H and variants compared to nondiseased tissue. As in atheromatous plaques, all variants studied here (CD44v3, CD44v4/5, CD44v6, CD44v7/8, and CD44v10) co-localized with MΦ and showed weak staining for CD44v7/8 in SMC (data not shown). AAA showed lower levels of CD44 variants compared to atheromatous plaques: CD44H (2.8-fold lower levels, P = 0.04), CD44v3 (1.8-fold lower levels, P = 0.04), and CD44v4/5 (threefold reduction, P = 0.02), as determined by Western blot analysis (Figure 1, Table 1). To validate our observations, we used CD44 antibodies from different sources, and obtained similar results with monoclonal rat anti-CD44S (same specificity as mouse anti-CD44H), polyclonal rabbit anti-CD44v3, and monoclonal mouse anti-CD44v5 (all from Chemicon Int. Inc.) (data not shown).
In summary, these observations show elevated expression of CD44 in diseased tissues compared to unaffected arteries, varying in a rank order of normal < fibrous plaques ≤ AAA < atheromatous plaques and correlating with the inflammation level (MΦ content) in the tissue samples.
Macrophages Elaborate Abundant CD44 in Vitro
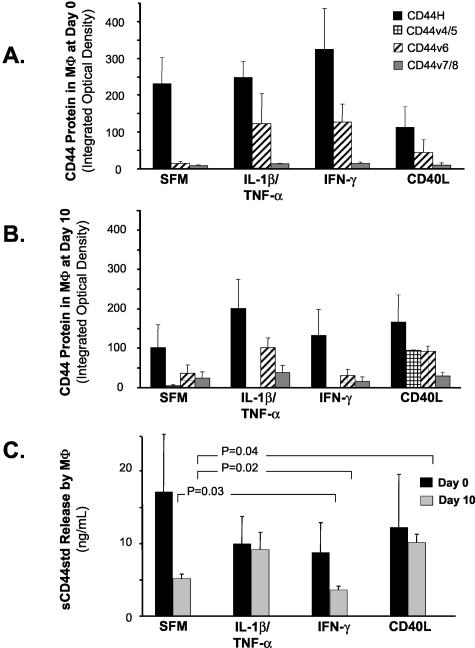
Because MΦ in lesions show exuberant expression of CD44H and many of its variants, we used human blood-derived mononuclear phagocytes to test the hypothesis that proinflammatory cytokines found in human atheroma augment CD44 levels in vitro. Freshly isolated monocytes (day 0) constitutively express CD44H, CD44v6, and CD44v7/8, and the proinflammatory cytokines (IL-1β, TNF-α, and CD40L) did not affect the protein levels of these CD44 variants (Figure 3A). Differentiated MΦ (cultured for 10 days in the presence of serum) also express CD44H constitutively (Figure 3B). Proinflammatory cytokines elevated these levels [IL-1β/TNF-α (P = 0.02) or CD40L (P = 0.05)] by twofold, whereas IL-1β/TNF-α stimulation augments CD44v6 and CD44v7/8 protein levels (2.7-fold and 1.6-fold, respectively, both P = 0.01) (Figure 3B).
Figure 3.
Cytokines stimulate CD44 expression and release in MΦ. MΦ express both CD44H and variants CD44v6 and CD44v7/8 in vitro. MΦ were cultured for 0 (day 0, A) and 10 (day 10, B) days in the presence of serum. Cells were then incubated with or without IL-1β/TNF-α, IFN-γ, or CD40L and cell lysates analyzed by Western blotting. Bars represent mean ± SEM from four different donors. C: Growth-arrested MΦ released CD44 on stimulation with the proinflammatory cytokine CD40L. Bars represent mean ± SEM.
To test the hypothesis that inflammatory mediators involved in the development of arterial diseases regulate CD44 release, we measured sCD44 in supernatants from cytokine-stimulated MΦ (Figure 3C). The cytokines in this study do not affect sCD44 secretion in freshly isolated MΦ (day 0). Unstimulated MΦ in SFM secrete 5.12 ± 0.66 ng/ml sCD44 at day 10, and IFN-γ reduces sCD44 expression by 28.8% (3.64 ± 0.46 ng/ml, P = 0.02) in these cells. Interestingly, CD40L strongly induces sCD44 secretion compared to IL-1β/TNF-α or IFN-γ, and fully differentiated MΦ at day 10 secrete 10.1 ± 1.20 ng/ml sCD44 in response to CD40L, an increase of 197.4% (P = 0.04).
Microvessels in AAA Express CD44 and Inflammatory Mediators Regulate CD44 Expression by ECs
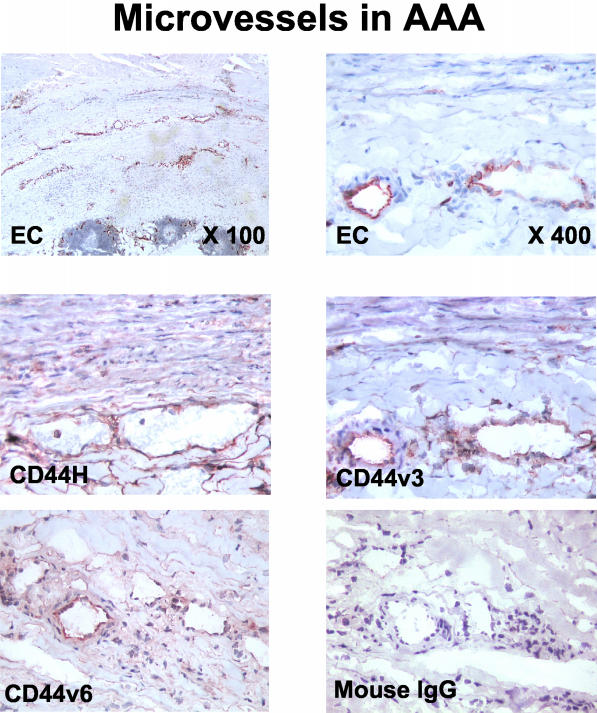
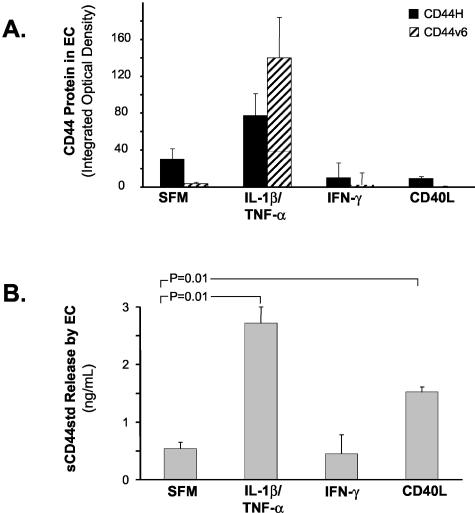
Microvascular ECs express CD44H, CD44v3, CD44v6 (Figure 4), and CD44v7/8 (data not shown) in aneurysmal tissue containing very prominent neovascularization. Both CD44H and CD44v6 have been implicated in regulating cell proliferation.28–31 We therefore studied the protein levels of these isoforms in response to inflammatory mediators in more detail in cultured ECs. Unstimulated ECs show low levels of CD44H, which increase with IL-1β stimulation (4.4-fold, P = 0.05). IL-1β and TNF-α significantly elevated CD44v6 levels 23-fold (P = 0.05) and 14-fold (P = 0.05), respectively. CD40L, the strongest inducer of CD44 expression in MΦ, did not affect the levels of CD44H or its variants in ECs (Figure 5A).
Figure 4.
CD44 variant expression in AAA microvessels. CD44H, CD44v3, and CD44v6 stainings co-localize with endothelium lining microvessels. Cell-type-specific staining for ECs with anti-CD31 (top) and negative control staining (mouse IgG, bottom right) is also shown. Staining of tissue samples from six patients showed similar results. Original magnifications, ×400.
Figure 5.
Cytokines stimulate CD44 expression and release in ECs. A: IL-1β and TNF-α enhance CD44 expression in ECs. ECs were incubated with or without IL-1β/TNF-α, IFN-γ, and CD40L. Cell lysates were analyzed for CD44 expression. Bars represent mean ± SEM from four different donors. B: Proinflammatory cytokines stimulate CD44 release from growth-arrested ECs. CD40L and IL-1β/TNF-α induce sCD44 release in ECs. Bars represent mean ± SEM.
ECs stimulated by cytokines release levels of sCD44 (0.54 ± 0.11 ng/ml) (Figure 5B) 10-fold lower than those observed in MΦ (Figure 3C). IL-1β, TNF-α, or CD40L induce considerable sCD44 secretion by ECs. Cells incubated in the presence of IL-1β (n = 7) secrete 1.37 ± 0.28 ng/ml sCD44, an overall increase of 254% (P = 0.02) compared to controls in SFM. TNF-α (n = 7) exerts a similar effect with 1.35 ± 0.32 ng/ml secreted sCD44 (249.9% increase, P = 0.03), and CD40L (n = 4) induced 1.52 ± 0.14 ng/ml sCD44 secretion (282.6% increase, P = 0.01) compared to control (Figure 5B).
CD44 Expression in ECs: A Potential Feedback Loop in Vitro
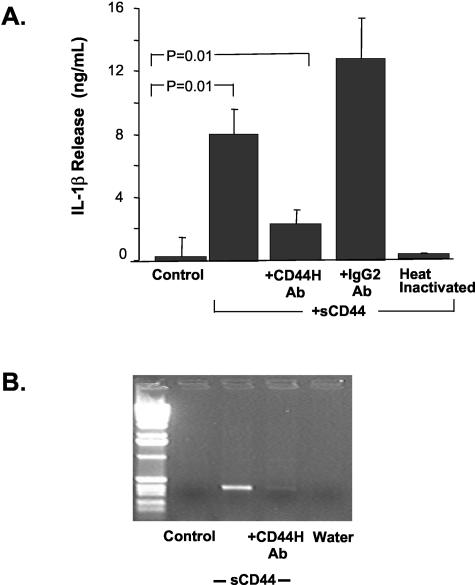
sCD44 induces IL-1β expression and release in ECs, whereas in SFM, ECs only release minor amounts of IL-1β (0.1 ± 1.3 ng/ml). In response to sCD44, these ECs secrete 8 ± 1.6 ng/ml of IL-1β (P = 0.01, Figure 6A). This expression falls to 2.3 ± 0.9 ng/ml when sCD44-enriched supernatants are preincubated with an anti-CD44 antibody (P = 0.01). A mouse IgG2 antibody (IgG control) did not reduce IL-1β expression in sCD44-stimulated ECs. Furthermore, heat-inactivated sCD44-enriched supernatants do not stimulate IL-1β expression in ECs, arguing against endotoxin contamination as an explanation for IL-1β expression. Reverse transcriptase-polymerase chain reaction analyses showed that sCD44 induces IL-1β mRNA expression in ECs (Figure 6B); treatment with an anti-CD44 antibody during cell culture almost completely negates this effect, suggesting that sCD44 enhances IL-1β expression in ECs through elevated IL-1β mRNA levels. Soluble CD44 affects neither the expression of IL-8 nor the expression of TNF-α or IFN-γ in ECs (data not shown). It is possible that the elevated IL-1β levels could then stimulate CD44 expression and shedding in ECs in an autocrine manner.
Figure 6.
Soluble CD44 stimulates IL-1β expression and release from ECs. Growth-arrested ECs from three different donors were stimulated with sCD44 (80 ng/ml) in the presence or absence of a CD44H antibody. Soluble CD44 induces IL-1β protein in ECs (A) as well as IL-1β mRNA (B). This effect is abolished by the addition of an anti-CD44H antibody or by heat-inactivation of the sCD44-containing medium. Anti-IgG2A (negative control) does not affect IL-1β protein levels.
CD44 Variants Inhibit EC Proliferation but Do Not Affect EC Apoptosis
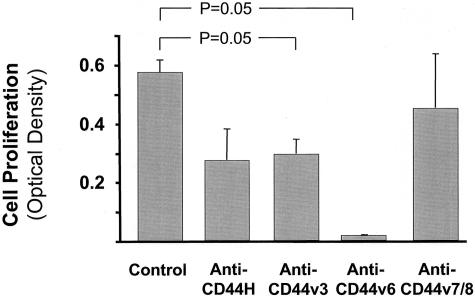
The co-localization of CD44H and its variants CD44v3, CD44v6, and CD44v7/8 to ECs lining microvessels in AAA suggests that CD44 isoforms participate in EC function. To address the possible role of CD44 variants in EC proliferation, we monitored proliferation of human saphenous vein ECs in the presence and absence of antibodies directed against CD44H, CD44v3, CD44v6, and CD44v7/8. Indeed, anti-CD44v3 reduces EC proliferation by 63% (P = 0.05) whereas anti-CD44v6 reduces proliferation almost 100% (P = 0.05) compared to controls without antibody. Addition of anti-CD44v7/8 does not effect EC cell proliferation (Figure 7). Addition of same amount of respective IgG did not affect EC proliferation. We used the same antibodies to study CD44-mediated apoptosis in ECs. The addition of neither CD44H, CD44v3, CD44v6, nor CD44v7/8 influenced EC apoptosis significantly (data not shown).
Figure 7.
CD44 inhibition diminishes the ability of human ECs to proliferate in vitro. ECs were plated as described in Materials and Methods and cultured in the presence or absence of antibodies against CD44H, CD44v3, CD44v6, and CD44v7/8. Cell proliferation was determined using a colorimetric (XTT based) assay. Bars represent mean ± SEM of three donors run in duplicate.
Discussion
Interactions between extracellular matrix constituents and vascular cells have received intense scrutiny. Although we have learned much about integrin signaling in this regard, glycosaminoglycan interaction has received less attention thus far. CD44, the primary receptor for HA may participate in inflammatory diseases via numerous pathways, including cell adhesion, migration or proliferation, and lymphocyte activation and homing. This study addressed the role of CD44H and splice variants in both human AAA and atheroma. We found the presence or absence of MΦ, a major source of CD44H and splice variants in atheroma and AAA tissue, may explain the differences in CD44 protein levels observed in these two inflammatory arterial diseases. Because all tissue samples used for Western blotting were morphologically characterized and divided into two groups according to MΦ and SMC content, we could establish a correlation between the MΦ content and the amount of CD44 protein in the different tissue groups (normal, fibrous, and atheromatous plaques, as well as AAA). Fibrous plaques, which contain fewer MΦ than lipid-rich lesions, exhibit CD44 expression similar to AAA, whereas atheromatous plaques, dominated by MΦ, show much higher levels of CD44.
Low levels of CD44 variants in nondiseased tissue agree with a survey that shows CD44H primarily restricted to epithelia.32 Although all major cell types in human atheroma and AAA express CD44, we show here in vitro that MΦ are the major source of CD44H and some of its variants. Although CD44 variant expression previously was limited to diseased tissue, MΦ activation itself induces expression of certain CD44 variants.15
Both injury and inflammation induce CD44 expression, as seen in restenosis after balloon angioplasty,33 injured carotid arteries in rats,34,35 the expanded intima in transplanted mouse arteries,36 and atherosclerotic tissue in humans and rabbits.37,38 Interestingly, enhanced CD44 expression may coincide with augmented cytokine expression in arterial tissues. Whereas normal arteries express few or no cytokines, atheromatous plaques contain IL-1β, TNF-α, and IFN-γ as well as biologically active IL-1β.25
A recent study shows reduction in aortic lesion size (50 to 70%) in CD44-deficient mice and increased levels of HA in atherosclerotic lesions of apolipoprotein E-deficient mice.39 Elevated levels of both CD44 and HA result in more CD44-HA interactions, thus promoting SMC motility in response to injury in vitro.40 Another study demonstrates that sCD44 releases spontaneously from bronchial epithelial cells and accumulates in the matrix.41 Although an increased release of sCD44 may indicate tissue injury, its interaction with other CD44-associated matrix components may benefit matrix deposition.2 The interaction of sCD44 with HA may compete with regular CD44-HA interactions. Thus, sCD44 inhibits melanoma tumor growth by preventing HA from binding cell surface CD44.19
In this study, proinflammatory cytokines induce sCD44 release from ECs. Because sCD44 can induce IL-1β expression in ECs, this released IL-1β, together with IL-1β expressed by other surrounding vascular cells, might act in an autocrine manner to stimulate CD44 expression in ECs and other cells. Interestingly, IL-1β localizes most prominently in the MΦ-rich shoulder area of the atherosclerotic plaque, and mature IL-1β is found primarily in extracts of atheromatous lesions with morphological characteristics of lesions prone to rupture (eg, with abundant MΦ and few SMCs).28,42 Furthermore, IL-1β activates CD44 by augmenting the HA-binding phenotype of CD44 through increased sulfation of CD44.43
We found CD44H, CD44v3, CD44v6, and CD44v7/8 expression in EC lining microvessels in AAA tissue. Although previous studies show the involvement of nonspliced CD44H in EC angiogenesis,30,44 we extend these findings by suggesting that EC angiogenesis may also involve alternatively spliced CD44 variants, a hypothesis supported by significantly reduced EC proliferation in the presence of anti-CD44v3 and anti-CD44v6 antibodies. Reduced heparin-binding growth factor immobilization, which is mediated through the v3 exon,45 may contribute to this effect. ECs in capillaries of tonsil tissue and umbilical vein express CD44v3, where it may have similar function,46 and it binds several growth factors, including vascular endothelial growth factor.47 CD44v3 is expressed in vessel endothelium, where it is involved in leukocyte extravasation.48 On the other hand, partial hepatectomy induces CD44v6, which promotes proliferation of residual hepatocytes.31 Inhibition of CD44v7/8 does not affect EC proliferation in this study, although CD44v7/8 has a role in trophoblast invasion and placental angiogenesis,49 suggesting that its effect is cell-type specific.
Several studies have investigated the role of CD44 in apoptosis, but results regarding CD44 as a pro- or anti-apoptotic mediator are inconclusive thus far. It is clear that the scenario is complicated by the occurrence of CD44 splice forms and the diverse signaling pathways that CD44 uses in different cells. Thus, CD44-mediated anti-apoptotic effects occur in a B-cell lymphoma cell line,50 tubular epithelial cells,51 and cancer cells.52,53 In contrast, CD44 is proapoptotic for T cells,54,55 leukocytes,56 and thymic lymphomas and T-cell hybridomas.57 Interestingly, in contrast to CD44H, CD44v7 supports survival of activated T cells by interfering with activation-induced cell death,58 suggesting differential roles for CD44 and its splice variants in cellular responses. Our study did not detect CD44-mediated apoptosis in human ECs from saphenous veins, leading to our conclusion that CD44 does not have proapoptotic properties in these cells. Our CD44H protein results on primary human vascular cells agree with the stimulatory effects exerted by IL-1β on CD44 mRNA in rat SMC36 and TNF-α in a human EC cell line.59 Together, IL-1β and TNF-α induce CD44-HA interaction in MΦ60 but also increase HA expression in ECs, thereby affecting CD44-HA primary adhesion.61
Several CD44 splice variants are enhanced in diseased arterial tissues, as determined by Western blot, and are highly expressed by MΦ and ECs in situ, although we did not detect these isoforms in vitro. This suggests that other factors, eg, cell-cell or cell-matrix interactions, may be important for the induction of these splice variants. Recently, Jones and colleagues47 showed that fully differentiated MΦ express CD44v3 after activation with IL-1 or LPS in vitro. Furthermore, the endothelium produces several CD44 isoforms in situ,46,48 and CD44 plays an essential role in EC injury.62
This study extends previous knowledge by showing augmented expression of CD44 and variants within human atheroma and in AAA. Furthermore, we demonstrate that proinflammatory cytokines trigger both CD44 expression and its release from the cell surface. A positive feedback loop found in ECs suggests that sCD44 may participate in the expression of IL-1β. These data provide new support for CD44 as a participant rather than as a bystander in arterial diseases.
Acknowledgments
We thank Eugenia Shvartz and Elissa Simon-Morrissey (Brigham and Women’s Hospital) for technical assistance and Karen Williams for editorial assistance.
Footnotes
Address reprint requests to Peter Libby, M.D., Brigham and Women’s Hospital, 77 Avenue Louis Pasteur, NRB-741, Boston, MA 02115. E-mail: plibby@rics.bwh.harvard.edu.
Supported by the National Heart, Lung, and Blood Institute (HL-34636 to P.L and HL-67249 to G.K.S.); the Donald W. Reynolds Foundation; Göteborg University (Göteborg, Sweden) (postdoctoral grant to A.K.); and the Swedish Heart Lung Foundation (to A.K.).
References
- Goodison S, Urquidi V, Tarin D. CD44 cell adhesion molecules. Mol Pathol. 1999;52:189–196. doi: 10.1136/mp.52.4.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesley J, Hyman R, Kincade PW. CD44 and its interaction with extracellular matrix. Adv Immunol. 1993;54:271–335. doi: 10.1016/s0065-2776(08)60537-4. [DOI] [PubMed] [Google Scholar]
- Siegelman MH, DeGrendele HC, Estess P. Activation and interaction of CD44 and hyaluronan in immunological systems. J Leukoc Biol. 1999;66:315–321. doi: 10.1002/jlb.66.2.315. [DOI] [PubMed] [Google Scholar]
- Christ O, Gunthert U, Haas R, Zoller M. Importance of CD44v7 isoforms for homing and seeding of hematopoietic progenitor cells. J Leukoc Biol. 2001;69:343–352. [PubMed] [Google Scholar]
- Asosingh K, Gunthert U, De Raeve H, Van Riet I, Van Camp B, Vanderkerken K. A unique pathway in the homing of murine multiple myeloma cells: CD44v10 mediates binding to bone marrow endothelium. Cancer Res. 2001;61:2862–2865. [PubMed] [Google Scholar]
- Weiss JM, Renkl AC, Sleeman J, Dittmar H, Termeer CC, Taxis S, Howells N, Schopf E, Ponta H, Herrlich P, Simon JC. CD44 variant isoforms are essential for the function of epidermal Langerhans cells and dendritic cells. Cell Adhes Commun. 1998;6:157–160. doi: 10.3109/15419069809004472. [DOI] [PubMed] [Google Scholar]
- Yoshinari C, Mizusawa N, Byers HR, Akasaka T. CD44 variant isoform CD44v10 expression of human melanoma cell lines is upregulated by hyaluronate and correlates with migration. Melanoma Res. 1999;9:223–231. doi: 10.1097/00008390-199906000-00003. [DOI] [PubMed] [Google Scholar]
- Rosel M, Foger N, Zoller M. Involvement of CD44 exon v10 in B-cell activation. Tissue Antigens. 1998;52:99–113. doi: 10.1111/j.1399-0039.1998.tb02273.x. [DOI] [PubMed] [Google Scholar]
- Chen D, McKallip RJ, Zeytun A, Do Y, Lombard C, Robertson JL, Mak TW, Nagarkatti PS, Nagarkatti M. CD44-deficient mice exhibit enhanced hepatitis after concanavalin A injection: evidence for involvement of CD44 in activation-induced cell death. J Immunol. 2001;166:5889–5897. doi: 10.4049/jimmunol.166.10.5889. [DOI] [PubMed] [Google Scholar]
- Wittig BM, Johansson B, Zoller M, Schwarzler C, Gunthert U. Abrogation of experimental colitis correlates with increased apoptosis in mice deficient for CD44 variant exon 7 (CD44v7). J Exp Med. 2000;191:2053–2064. doi: 10.1084/jem.191.12.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii K, Fujii Y, Hubscher S, Tanaka Y. Cd44 is the physiological trigger of fas up-regulation on rheumatoid synovial cells. J Immunol. 2001;167:1198–1203. doi: 10.4049/jimmunol.167.3.1198. [DOI] [PubMed] [Google Scholar]
- Hinek A, Smith AC, Cutiongco EM, Callahan JW, Gripp KW, Weksberg R. Decreased elastin deposition and high proliferation of fibroblasts from Costello syndrome are related to functional deficiency in the 67-kD elastin-binding protein. Am J Hum Genet. 2000;66:859–872. doi: 10.1086/302829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pure E, Cuff CA. A crucial role for CD44 in inflammation. Trends Mol Med. 2001;7:213–221. doi: 10.1016/s1471-4914(01)01963-3. [DOI] [PubMed] [Google Scholar]
- Weber GF, Ashkar S. Molecular mechanisms of tumor dissemination in primary and metastatic brain cancers. Brain Res Bull. 2000;53:421–424. doi: 10.1016/s0361-9230(00)00379-8. [DOI] [PubMed] [Google Scholar]
- Weiss JM, Renkl AC, Ahrens T, Moll J, Mai BH, Denfeld RW, Schopf E, Ponta H, Herrlich P, Simon JC. Activation-dependent modulation of hyaluronate-receptor expression and of hyaluronate-avidity by human monocytes. J Invest Dermatol. 1998;111:227–232. doi: 10.1046/j.1523-1747.1998.00286.x. [DOI] [PubMed] [Google Scholar]
- Kajita M, Itoh Y, Chiba T, Mori H, Okada A, Kinoh H, Seiki M. Membrane-type 1 matrix metalloproteinase cleaves CD44 and promotes cell migration. J Cell Biol. 2001;153:893–904. doi: 10.1083/jcb.153.5.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MC, Alpaugh ML, Nguyen M, Deato M, Dishakjian L, Barsky SH. Myoepithelial-specific CD44 shedding is mediated by a putative chymotrypsin-like sheddase. Biochem Biophys Res Commun. 2000;279:116–123. doi: 10.1006/bbrc.2000.3918. [DOI] [PubMed] [Google Scholar]
- Alpaugh ML, Lee MC, Nguyen M, Deato M, Dishakjian L, Barsky SH. Myoepithelial-specific CD44 shedding contributes to the anti-invasive and antiangiogenic phenotype of myoepithelial cells. Exp Cell Res. 2000;261:150–158. doi: 10.1006/excr.2000.5056. [DOI] [PubMed] [Google Scholar]
- Ahrens T, Sleeman JP, Schempp CM, Howells N, Hofmann M, Ponta H, Herrlich P, Simon JC. Soluble CD44 inhibits melanoma tumor growth by blocking cell surface CD44 binding to hyaluronic acid. Oncogene. 2001;20:3399–3408. doi: 10.1038/sj.onc.1204435. [DOI] [PubMed] [Google Scholar]
- Mikecz K, Dennis K, Shi M, Kim JH. Modulation of hyaluronan receptor (CD44) function in vivo in a murine model of rheumatoid arthritis. Arthritis Rheum. 1999;42:659–668. doi: 10.1002/1529-0131(199904)42:4<659::AID-ANR8>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Schonbeck U, Gerdes N, Varo N, Reynolds RS, Horton DB, Bavendiek U, Robbie L, Ganz P, Kinlay S, Libby P. Oxidized low-density lipoprotein augments and 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors limit CD40 and CD40L expression in human vascular cells. Circulation. 2002;106:2888–2893. doi: 10.1161/01.cir.0000043029.52803.7b. [DOI] [PubMed] [Google Scholar]
- Horton DB, Libby P, Schonbeck U. Ligation of CD40 on vascular smooth muscle cells mediates loss of interstitial collagen via matrix metalloproteinase activity. Ann NY Acad Sci. 2001;947:329–336. doi: 10.1111/j.1749-6632.2001.tb03957.x. [DOI] [PubMed] [Google Scholar]
- Gerdes N, Sukhova GK, Libby P, Reynolds RS, Young JL, Schonbeck U. Expression of interleukin (IL)-18 and functional IL-18 receptor on human vascular endothelial cells, smooth muscle cells, and macrophages: implications for atherogenesis. J Exp Med. 2002;195:245–257. doi: 10.1084/jem.20011022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Hirahashi J, Cullere X, Mayadas TN. Elucidation of molecular events leading to neutrophil apoptosis following phagocytosis: cross-talk between caspase 8, reactive oxygen species, and MAPK/ERK activation. J Biol Chem. 2003;278:28443–28454. doi: 10.1074/jbc.M210727200. [DOI] [PubMed] [Google Scholar]
- Sukhova GK, Schonbeck U, Rabkin E, Schoen FJ, Poole AR, Billinghurst RC, Libby P. Evidence for increased collagenolysis by interstitial collagenases-1 and -3 in vulnerable human atheromatous plaques. Circulation. 1999;99:2503–2509. doi: 10.1161/01.cir.99.19.2503. [DOI] [PubMed] [Google Scholar]
- Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2000;20:1262–1275. doi: 10.1161/01.atv.20.5.1262. [DOI] [PubMed] [Google Scholar]
- Galis Z, Sukhova G, Lark M, Libby P. Increased expression of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques. J Clin Invest. 1994;94:2493–2503. doi: 10.1172/JCI117619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JL, Sukhova GK, Foster D, Kisiel W, Libby P, Schonbeck U. The serpin proteinase inhibitor 9 is an endogenous inhibitor of interleukin 1beta-converting enzyme (caspase-1) activity in human vascular smooth muscle cells. J Exp Med. 2000;191:1535–1544. doi: 10.1084/jem.191.9.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A, Doyle M, Mark D. Quantitation of mRNA by the polymerase chain reaction. Proc Natl Acad Sci USA. 1989;86:9717–9721. doi: 10.1073/pnas.86.24.9717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savani RC, Cao G, Pooler PM, Zaman A, Zhou Z, DeLisser HM. Differential involvement of the hyaluronan (HA) receptors CD44 and receptor for HA-mediated motility in endothelial cell function and angiogenesis. J Biol Chem. 2001;276:36770–36778. doi: 10.1074/jbc.M102273200. [DOI] [PubMed] [Google Scholar]
- Della Fazia MA, Pettirossi V, Ayroldi E, Riccardi C, Magni MV, Servillo G. Differential expression of CD44 isoforms during liver regeneration in rats. J Hepatol. 2001;34:555–561. doi: 10.1016/s0168-8278(00)00065-9. [DOI] [PubMed] [Google Scholar]
- Fox SB, Fawcett J, Jackson DG, Collins I, Gatter KC, Harris AL, Gearing A, Simmons DL. Normal human tissues, in addition to some tumors, express multiple different CD44 isoforms. Cancer Res. 1994;54:4539–4546. [PubMed] [Google Scholar]
- Savani RC, Turley EA. The role of hyaluronan and its receptors in restenosis after balloon angioplasty: development of a potential therapy. Int J Tissue React. 1995;17:141–151. [PubMed] [Google Scholar]
- Tai JT, Brooks EE, Liang S, Somogyi R, Rosete JD, Lawn RM, Shiffman D. Determination of temporal expression patterns for multiple genes in the rat carotid artery injury model. Arterioscler Thromb Vasc Biol. 2000;20:2184–2191. doi: 10.1161/01.atv.20.10.2184. [DOI] [PubMed] [Google Scholar]
- Jain M, He Q, Lee WS, Kashiki S, Foster LC, Tsai JC, Lee ME, Haber E. Role of CD44 in the reaction of vascular smooth muscle cells to arterial wall injury. J Clin Invest. 1996;97:596–603. doi: 10.1172/JCI118455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster LC, Arkonac BM, Sibinga NE, Shi C, Perrella MA, Haber E. Regulation of CD44 gene expression by the proinflammatory cytokine interleukin-1beta in vascular smooth muscle cells. J Biol Chem. 1998;273:20341–20346. doi: 10.1074/jbc.273.32.20341. [DOI] [PubMed] [Google Scholar]
- Kinscherf R, Wagner M, Kamencic H, Bonaterra GA, Hou D, Schiele RA, Deigner HP, Metz J. Characterization of apoptotic macrophages in atheromatous tissue of humans and heritable hyperlipidemic rabbits. Atherosclerosis. 1999;144:33–39. doi: 10.1016/s0021-9150(99)00037-4. [DOI] [PubMed] [Google Scholar]
- Wuttge DM, Eriksson P, Sirsjo A, Hansson GK, Stemme S. Expression of interleukin-15 in mouse and human atherosclerotic lesions. Am J Pathol. 2001;159:417–423. doi: 10.1016/S0002-9440(10)61712-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuff CA, Kothapalli D, Azonobi I, Chun S, Zhang Y, Belkin R, Yeh C, Secreto A, Assoian RK, Rader DJ, Pure E. The adhesion receptor CD44 promotes atherosclerosis by mediating inflammatory cell recruitment and vascular cell activation. J Clin Invest. 2001;108:1031–1040. doi: 10.1172/JCI12455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evanko SP, Angello JC, Wight TN. Formation of hyaluronan- and versican-rich pericellular matrix is required for proliferation and migration of vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 1999;19:1004–1013. doi: 10.1161/01.atv.19.4.1004. [DOI] [PubMed] [Google Scholar]
- Cichy J, Bals R, Potempa J, Mani A, Pure E. Proteinase-mediated release of epithelial cell-associated CD44. Extracellular CD44 complexes with components of cellular matrices. J Biol Chem. 2002;277:44440–44447. doi: 10.1074/jbc.M207437200. [DOI] [PubMed] [Google Scholar]
- Sukhova GK, Williams JK, Libby P. Statins reduce inflammation in atheroma of nonhuman primates independent of effects on serum cholesterol. Arterioscler Thromb Vasc Biol. 2002;22:1452–1458. doi: 10.1161/01.atv.0000030360.72503.56. [DOI] [PubMed] [Google Scholar]
- Brown KL, Maiti A, Johnson P. Role of sulfation in CD44-mediated hyaluronan binding induced by inflammatory mediators in human CD14(+) peripheral blood monocytes. J Immunol. 2001;167:5367–5374. doi: 10.4049/jimmunol.167.9.5367. [DOI] [PubMed] [Google Scholar]
- Trochon V, Mabilat C, Bertrand P, Legrand Y, Smadja-Joffe F, Soria C, Delpech B, Lu H. Evidence of involvement of CD44 in endothelial cell proliferation, migration and angiogenesis in vitro. Int J Cancer. 1996;66:664–668. doi: 10.1002/(SICI)1097-0215(19960529)66:5<664::AID-IJC14>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Bennett KL, Jackson DG, Simon JC, Tanczos E, Peach R, Modrell B, Stamenkovic I, Plowman G, Aruffo A. CD44 isoforms containing exon V3 are responsible for the presentation of heparin-binding growth factor. J Cell Biol. 1995;128:687–698. doi: 10.1083/jcb.128.4.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopman G, Taher TE, Mazzucchelli I, Keehnen RM, van der Voort R, Manten-Horst E, Ricevuti G, Pals ST, Das PK. CD44 isoforms, including the CD44 V3 variant, are expressed on endothelium, suggesting a role for CD44 in the immobilization of growth factors and the regulation of the local immune response. Biochem Biophys Res Commun. 1998;245:172–176. doi: 10.1006/bbrc.1998.8295. [DOI] [PubMed] [Google Scholar]
- Jones M, Tussey L, Athanasou N, Jackson DG. Heparan sulfate proteoglycan isoforms of the CD44 hyaluronan receptor induced in human inflammatory macrophages can function as paracrine regulators of fibroblast growth factor action. J Biol Chem. 2000;275:7964–7974. doi: 10.1074/jbc.275.11.7964. [DOI] [PubMed] [Google Scholar]
- Seiter S, Engel P, Fohr N, Zoller M. Mitigation of delayed-type hypersensitivity reactions by a CD44 variant isoform v3-specific antibody: blockade of leukocyte egress. J Invest Dermatol. 1999;113:11–21. doi: 10.1046/j.1523-1747.1999.00635.x. [DOI] [PubMed] [Google Scholar]
- Goshen R, Ariel I, Shuster S, Hochberg A, Vlodavsky I, de Groot N, Ben-Rafael Z, Stern R. Hyaluronan, CD44 and its variant exons in human trophoblast invasion and placental angiogenesis. Mol Hum Reprod. 1996;2:685–691. doi: 10.1093/molehr/2.9.685. [DOI] [PubMed] [Google Scholar]
- Li L, Yoon SO, Fu DD, Zhang X, Choi YS. A novel follicular dendritic cell molecule, 8D6 collaborates with CD44 in supporting lymphomagenesis by a Burkitt lymphoma cell line L3055. Blood. 2004;104:815–821. doi: 10.1182/blood-2004-01-0292. [DOI] [PubMed] [Google Scholar]
- Rouschop KM, Sewnath ME, Claessen N, Roelofs JJ, Hoedemaeker I, van der Neut R, Aten J, Pals ST, Weening JJ, Florquin S. CD44 deficiency increases tubular damage but reduces renal fibrosis in obstructive nephropathy. J Am Soc Nephrol. 2004;15:674–686. doi: 10.1097/01.asn.0000115703.30835.96. [DOI] [PubMed] [Google Scholar]
- Yasuda M, Tanaka Y, Fujii K, Yasumoto K. CD44 stimulation down-regulates Fas expression and Fas-mediated apoptosis of lung cancer cells. Int Immunol. 2001;13:1309–1319. doi: 10.1093/intimm/13.10.1309. [DOI] [PubMed] [Google Scholar]
- Fujita Y, Kitagawa M, Nakamura S, Azuma K, Ishii G, Higashi M, Kishi H, Hiwasa T, Koda K, Nakajima N, Harigaya K. CD44 signaling through focal adhesion kinase and its anti-apoptotic effect. FEBS Lett. 2002;528:101–108. doi: 10.1016/s0014-5793(02)03262-3. [DOI] [PubMed] [Google Scholar]
- Termeer C, Averbeck M, Hara H, Eibel H, Herrlich P, Sleeman J, Simon JC. Targeting dendritic cells with CD44 monoclonal antibodies selectively inhibits the proliferation of naive CD4+ T-helper cells by induction of FAS-independent T-cell apoptosis. Immunology. 2003;109:32–40. doi: 10.1046/j.1365-2567.2003.01617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKallip RJ, Do Y, Fisher MT, Robertson JL, Nagarkatti PS, Nagarkatti M. Role of CD44 in activation-induced cell death: CD44-deficient mice exhibit enhanced T cell response to conventional and superantigens. Int Immunol. 2002;14:1015–1026. doi: 10.1093/intimm/dxf068. [DOI] [PubMed] [Google Scholar]
- Xu H, Manivannan A, Liversidge J, Sharp PF, Forrester JV, Crane IJ. Involvement of CD44 in leukocyte trafficking at the blood-retinal barrier. J Leukoc Biol. 2002;72:1133–1141. [PubMed] [Google Scholar]
- Guy R, Yefenof E, Naor D, Dorogin A, Zilberman Y. CD44 co-stimulates apoptosis in thymic lymphomas and T cell hybridomas. Cell Immunol. 2002;216:82–92. doi: 10.1016/s0008-8749(02)00505-1. [DOI] [PubMed] [Google Scholar]
- Marhaba R, Bourouba M, Zoller M. CD44v7 interferes with activation-induced cell death by up-regulation of anti-apoptotic gene expression. J Leukoc Biol. 2003;74:135–148. doi: 10.1189/jlb.1202615. [DOI] [PubMed] [Google Scholar]
- Kulseth MA, Kolset SO, Ranheim T. Stimulation of serglycin and CD44 mRNA expression in endothelial cells exposed to TNF-alpha and IL-1alpha. Biochim Biophys Acta. 1999;1428:225–232. doi: 10.1016/s0304-4165(99)00096-3. [DOI] [PubMed] [Google Scholar]
- Levesque MC, Haynes BF. Cytokine induction of the ability of human monocyte CD44 to bind hyaluronan is mediated primarily by TNF-alpha and is inhibited by IL-4 and IL-13. J Immunol. 1997;159:6184–6194. [PubMed] [Google Scholar]
- Mohamadzadeh M, DeGrendele H, Arizpe H, Estess P, Siegelman M. Proinflammatory stimuli regulate endothelial hyaluronan expression and CD44/HA-dependent primary adhesion. J Clin Invest. 1998;101:97–108. doi: 10.1172/JCI1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafi-Janajreh AQ, Chen D, Schmits R, Mak TW, Grayson RL, Sponenberg DP, Nagarkatti M, Nagarkatti PS. Evidence for the involvement of CD44 in endothelial cell injury and induction of vascular leak syndrome by IL-2. J Immunol. 1999;163:1619–1627. [PubMed] [Google Scholar]