Abstract
We tried to identify a novel marker characteristic for rat hepatocellular preneoplastic and neoplastic lesions, undetectable by well established cytochemical markers. Glutathione S-transferase placental (GST-P)-negative hepatocellular altered foci (HAF), hepatocellular adenoma (HCA), and hepatocellular carcinoma (HCC) were generated by two initiation-promotion models with N-nitrosodiethylamine (NDEN) and peroxisome proliferators, Wy-14,643 and clofibrate. Total RNAs isolated from laser-microdissected GST-P-negative HAF (amphophilic cell foci) and adjacent normal tissues were applied to microarray analysis. As a result, five up-regulated genes were identified, and further detailed examinations of the gene demonstrating most fluctuation, ie, that for α2-macroglobulin (α2M) were performed. In reverse transcriptase-polymerase chain reaction, α2M mRNA was overexpressed not only in amphophilic GST-P-negative HAF but also in amphophilic GST-P-negative HCA and HCC. In situ hybridization showed accumulation of α2M mRNA to be evenly distributed within GST-P-negative HAF (predominantly amphophilic cell foci). Distinctive immunohistochemical staining for α2M could be consistently demonstrated in GST-P-negative HAF, HCA, and HCC induced not only by peroxisome proliferators but also N-nitrosodiethylamine alone. Thus our findings suggest that α2M is an important novel cytochemical marker to identify hepatocellular preneoplastic and neoplastic lesions, particularly amphophilic cell foci, undetectable by established cytochemical markers and is tightly linked to rat hepatocarcinogenesis.
During the process of hepatocarcinogenesis, some small proportion of hepatocellular altered foci (HAF) develop into hepatocellular adenomas (HCAs) and hepatocellular carcinomas (HCCs). Many preneoplastic lesions regress after withdrawal of a proliferative stimulus,1–4 whereas others persist and demonstrate stable growth.5,6 The molecular mechanisms underlying these phenomena are unclear. The phenotypic diversity demonstrated by foci7 means that markers detectable for all foci have advantages for elucidating the process of hepatocarcinogenesis. Although γ-GT3,4,6–11 and particularly glutathione S-transferase placental (GST-P)12,13 have become established as marker enzymes for hepatocarcinogenesis in rats, they have shortcomings regarding detection of some types of HAF, predominantly amphophilic cell foci induced by peroxisome proliferators.14–16 Amphophilic cell foci, characterized by increased granular acidophilia and randomly scattered cytoplasmic basophilia, demonstrate alterations in mitochondrial enzymes.16,17 However, none of the cytochemical markers that are widely used such as γ-GT or GST-P are positive particularly in the small foci not readily evident in routine hematoxylin and eosin (H&E) staining.
Recent technical development and advances in tools for molecular biology such as cDNA microarrays and the availability of laser microdissection now allow us to monitor gene expression comprehensively in pure cell populations such as those in histopathological lesions in tissue sections. In addition, application of RNA linear amplification techniques for very limited quantities of RNA isolated from microdissected cells greatly facilitates examination of transcripts specific for microlesions and nonhomogeneous tissues. In the present study, we made use of these techniques to identify a novel marker specific for hepatocellular preneoplastic or neoplastic lesions undetectable with the hitherto available cytochemical markers for rat hepatocarcinogenesis.
Rat α2-macroglobulin (α2M), a homotetrameric major acute-phase glycoprotein18,19 is a typical member of the pan-proteinase inhibitors of the α2M family, capable of inhibiting a wide spectrum of proteinases from all classes by means of steric entrapment and covalent binding.20 It also plays a role as a carrier protein and regulator for various growth factors, polypeptide hormones, and cytokines.21–26
A number of authors have reported up-regulation of serum α2M in association with HCC in humans, being significantly raised as compared to liver cirrhosis and amoebic liver abscess.27 Poon and colleagues28 included α2M as a candidate serological marker for the diagnosis of HCC and a recent investigation revealed α2M to be overexpressed in HCCs with a background of hepatitis C virus as compared to nontumorous liver tissues.29 On the other hand, although elevated concentrations of α2M have been also found in the sera of rats bearing HCC30 or exposed to hepatocarcinogens,31 Hudig and colleagues32 concluded that this was not correlated with tumor development. Rather, they suggested that the previously observed increases in serum α2M concentrations during hepatocarcinogenesis and in animals bearing hepatic tumors were because of secretion by the host liver of α2M as an acute-phase reactant in response to inflammatory injury. Therefore, as contrast to the case in humans, so far it remains equivocal whether up-regulation of serum α2M is linked to hepatocarcinogenesis in rats.
We hereby demonstrate that α2M is a novel candidate cytochemical marker for identification of hepatocellular preneoplastic and neoplastic lesions, undetectable by hitherto established cytochemical markers, and may be tightly linked to rat liver lesion development from the initial stage through to tumor progression.
Materials and Methods
Animals and Chemicals
N-nitrosodiethylamine (NDEN) and clofibrate (>98%) were purchased from Tokyo Kasei Kogyo Co., Ltd. (Tokyo, Japan), and Wy-14,643 (>98%) was obtained from ChemSyn Laboratories (Lenexa, KS). A total of 54 male 5-week-old F344 rats were purchased from Charles River Japan, Inc. (Atsugi, Japan) and housed in suspended aluminum cages (three rats in a cage) in a room kept at 24 ± 2°C temperature and 40 to 70% humidity with a 12-hour light/dark cycle. They received CRF-1 laboratory chow (Charles River Japan, Inc.) as basal diet in experiments 1 and 2 ad libitum. The animals were observed daily and were used after a 1-week acclimation period for the experiments. Body weights were measured every week.
Experimental Protocol
All experiments were performed in accordance with the Guide for Animal Care and Use of Sumitomo Chemical Co. Ltd. In experiment 1, an in vivo medium-term bioassay13 with a minor modification as to the treatment period for the peroxisome proliferators was used. Briefly, at the age of 6 weeks, 24 male F344 rats were divided into four groups (six animals per group). Animals were given a single intraperitoneal injection of NDEN (200 mg/kg body weight) dissolved in saline to initiate hepatocarcinogenesis and after a 2-week recovery period, received clofibrate (3000 ppm, group 1) or Wy-14,643 (1000 ppm, group 2) in the basal diet. The rats were subjected to two-thirds partial hepatectomy at week 3. Animals in group 3 were given NDEN and partial hepatectomy in the same manner as for groups 1 and 2 without administration of any other chemicals, and animals in group 4 were treated in the same manner as for group 3 except injection of saline instead of NDEN. All animals were sacrificed at week 12.
In experiment 2, an initiation-promotion model in which multiple administrations of NDEN were given in place of partial hepatectomy in experiment 1 was used. In light of our experience, however, the dose of NDEN was set at 100 mg/kg not to impose too heavy a burden on the rats. Briefly, at the age of 6 weeks, 30 male F344 rats were divided into three groups (10 animals per group). Animals in groups 1 and 2 were injected with NDEN (100 mg/kg body weight) intraperitoneally once a week for 2 weeks, and after a 1-week recovery period, received clofibrate (3000 ppm, group 1) or the basal diet (group 2). Animals in group 3 were injected with saline instead of NDEN solution without subsequent administration of any chemicals. Sacrifice was at weeks 26 and 36. In both experiments, all animals in the each group were exsanguinated and sacrificed under ether anesthesia, and the liver tissues were obtained and treated with some appropriate procedures for the following examinations.
Laser Microdissection and Total RNA Isolation
Frozen liver tissues embedded in OCT compound (Sakura Finetech, Tokyo, Japan) were sectioned at ∼8 μm to get several sets of seven serial sections, and the first and last sections in each suite were applied to routine H&E staining and immunohistochemical staining for rat GST-P to identify lesions histopathologically for microdissection. The remaining sections were applied to casual H&E staining to block RNA from degradation, ie, the sections were 70% ethanol-fixed for 1 minute, immersed in RNase-free hematoxylin for 5 minutes, rinsed by RNase-free water several times, immersed in RNase-free phosphate-buffered saline for 5 minutes, and immersed in RNase-free eosin for 1 minute. In experiment 1, GST-P-positive and -negative HAF, and corresponding adjacent normal tissues were microdissected with the use of a laser microdissection system (Leica Microsystems Japan, Tokyo, Japan) from the following groups: GST-P-positive HAF (clear cell foci) in groups 1 and 3, and GST-P-negative HAF (amphophilic cell foci) in group 2. In experiment 2, GST-P-negative lesions [HAF, HCA, and HCC (amphophilic phenotype)], GST-P-positive lesions [HCA and HCC (clear and acidophilic phenotype)], and corresponding adjacent normal tissues were microdissected in the same manner from group 1. Subsequently, total RNAs were isolated from the microdissected tissues in accordance with the protocol for RNeasy Protect mini kit (Qiagen, Tokyo, Japan) with a minor modification using poly(C) (Amersham Bioscience, Buckinghamshire, UK) as a carrier. The total RNA pool was used for the following microarray analysis and reverse transcriptase-polymerase chain reaction (RT-PCR) assays.
High-Density Oligonucleotide Microarray Analysis
Rat genome U34A arrays, which contained 9000 probes for known rat genes or expressed sequence tags, were purchased from Affymetrix (Santa Clara, CA). For microarray probing, reverse transcription, second-strand synthesis, and probe generation were all accomplished following the technical notes of the Small Sample Labeling Protocol version 2 (Affymetrix). Briefly, from 100 ng of total RNA, first-strand cDNA was synthesized with SuperScript II reverse transcriptase (Invitrogen, Groningen, The Netherlands) and a T7-(dT)24 primer (Amersham Bioscience) and then double-strand cDNA was synthesized with Escherichia coli RNase H, E. coli DNA polymerase I, and E. coli DNA ligase (Invitrogen). From the double-strand cDNA, cRNA was prepared using aMEGAscript T7 kit (Ambion, Austin, TX). After a second cycle of amplification and biotin labeling with a BioArray high-yield RNA transcript labeling kit (Enzo Diagnostics, Farmingdale, NY), 20 μg of labeled cRNA was fragmented. The RGU34A arrays were hybridized as described in the Gene Chip Expression Analysis Technical Manual (Affymetrix) and stained for use with a GeneArray scanner (Agilent Technologies, Palo Alto, CA). The derived signal value was globally normalized and targeted to all probe sets equal to 100 before comparative analysis.
Microarray Data Analysis
To examine gene expression differences between GST-P-negative or -positive HAF, and the corresponding adjacent normal tissue, we performed comparison analysis using the Affymetrix data suite system, MAS 5.0. The genes (probe sets) showing greater than twofold alteration in value with a change of I or D were chosen as changed genes.
Semiquantitative RT-PCR Assay
Adequate amounts of total RNA that had not been amplified were reverse-transcribed in a 20-μl reaction mixture using the ThermoScript RT-PCR system (Invitrogen). Semiquantitative PCR conditions were optimized to obtain reproducible and reliable amplification within the logarithmic phase of the reaction. The cycling amplifications were conducted with 10-μl of reaction mixture using a Program Temp control system PC-800 (Astec, Fukuoka, Japan) programmed for 95°C for 5 minutes and then 23 cycles of denaturation at 95°C for 30 seconds, annealing at 60°C for 45 seconds, and extension at 72°C for 30 seconds (for 5 minutes in a final cycle). Alignments of primers were as follows: α2M forward, 5′-GGCCATTGCCTATCTCAATACG-3′, reverse, 5′-TTATCCCCAAAGGCGCTGTA-3′; β-actin forward, 5′-GACAGGATGCAGAAGGAGATTACTG-3′, reverse, 5′-AGAGCCACCAATCCACACAGA-3′. Signals stained by GelStar (FMC, Rockland, ME) were detected using a luminescent image analyzer (LAS-1000 plus; Fuji Photo Film, Tokyo, Japan).
In Situ Hybridization for α2M mRNA
In situ hybridization of α2M mRNA was performed in 4% paraformaldehyde-fixed liver sections consecutive to those applied to H&E staining and immunohistochemical staining for α2M and GST-P. Briefly, at first, cRNA probe for α2M mRNA was prepared by in vitro transcription of the cDNA fragment (∼1000 bp; 3315 to 4349) generated from the same cDNA pool as for the above-described semiquantitative RT-PCR assay, and labeled using a fluorescein RNA labeling mix (Roche Molecular Biochemicals, Mannheim, Germany). After several pretreatments including target retrieval using Target Retrieval Solution (DAKO Co., Carpinteria, CA), 4-μm sections were hybridized with cRNA probe overnight at 60°C, and signals were detected using the In Situ Hybridization Detection system (DAKO Co.). The numbers of foci positive for α2M mRNA signals (>0.1 mm in diameter) were counted under a light microscope in the groups of animals sacrificed at week 26 in experiment 2 (all of the sections counted were applied to in situ hybridization for α2M mRNA simultaneously). The total area of the liver sections was measured using an IPAP image analyzer (Sumika Technoservice, Osaka, Japan). Counts of the numbers of the positive foci for mRNA signals were repeated at least three times for confirmation in a blinded manner and an average value of the three counts was used as the final data.
Immunohistochemistry for α2M and GST-P
In the present study, immunohistochemical staining for α2M was conducted only for a qualitative analysis, because there was concern that this serum protein might be released from the lesions because of an inadequate fixation as mentioned in the Discussion. Immunohistochemical examination of localization of α2M and GST-P proteins was performed respectively in 4% paraformaldehyde-fixed liver sections consecutive to those applied for in situ hybridization, using the avidin-biotin complex method. Briefly, after deparaffinization (and target retrieval using Target Retrieval Solution in the case of α2M), the sections were treated sequentially with 3% H2O2, normal goat serum, primary antibody, ie, goat anti-rat GST-P (1:2000, room temperature, 1 hour; MBL Co., Ltd., Nagoya, Japan) or goat anti-rat α2M (1:2000, room temperature, 2 hours; Santa Cruz Biotechnology, Inc., Santa Cruz, CA), biotin-labeled goat anti-rabbit IgG and avidin-biotin-peroxidase complex (ABC kit; Funakoshi Co., Ltd., Tokyo, Japan). The sites of peroxidase binding were demonstrated by the diaminobenzidine method and light counterstaining with hematoxylin was performed to facilitate orientation. The numbers of GST-P-positive foci (>0.1 mm in diameter) were counted under a light microscope in experiments 1 and 2. Additionally, the numbers of GST-P-negative foci (>0.1 mm in diameter), which were detected by routine H&E staining and subsequently checked stainability against antibody to GST-P by immunohistochemical staining of the sections consecutive to those stained with H&E, were also counted by the same manner. The total area of the liver sections was measured using an IPAP image analyzer (Sumika Technoservice). Counts of the numbers of GST-P-positive or -negative foci were repeated at least three times for confirmation in a blinded manner and an average value of the three counts was used as the final data.
Results
Histopathology and Laser Microdissection
Histopathological examination and quantitative analysis of GST-P-positive and -negative foci were conducted with reference to earlier articles16,33 and a diagnostic guide34 and the results are shown in Tables 1 and 2. Several frozen cell populations were harvested specifically from GST-P-positive and/or-negative HAF, HCAs, and HCCs using laser microdissection, and total RNA was isolated from each lesion in experiments 1 and 2. GST-P-negative HAF, ie, amphophilic cell foci, were not clearly detectable in the frozen sections stained by H&E in group 1 of experiment 1, so that amphophilic GST-P-negative HAFs were microdissected from group 2 in experiment 1 and group 1 in experiment 2 (Table 3).
Table 1.
Histopathological Findings in Liver
| Histopathological findings* | Breeding term Group† | Experiment 1
|
Experiment 2
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 12W
|
26W
|
36W
|
|||||||||
| 1 | 2 | 3 | 4 | 1 | 2 | 3 | 1 | 2 | 3 | ||
| No. of animals examined | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | |
| Centrilobular hepatocellular hypertrophy | + | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2+ | 0 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Hepatocellular altered foci | 5‡ | 5§ | 5¶ | 0 | 5∥ | 5** | 0 | 5∥ | 5** | 1†† | |
| Hepatocellular adenoma | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 2 | 2 | 0 | |
| Hepatocellular carcinoma | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 4 | 1 | 0 | |
Grading: +−, slight; +, mild; 2+, moderate; 3+, severe.
Experiment 1: 1, NDEN + clofibrate; 2, NDEN + Wy-14,643; 3, NDEN alone; 4, no treatment. Experiment 2: 1, NDEN + clofibrate; 2, NDEN alone; 3, no treatment.
Predominantly clear cell foci, and small number of amphophilic cell foci.
Amphophilic cell foci and clear cell foci.
Predominantly clear cell foci and small number of amphophilic cell foci (basophilic).
Clear and acidophilic cell foci and amphophilic cell foci.
Predominantly clear and acidophilic cell foci, and small number of amphophilic cell foci (basophilic).
Clear cell foci.
Table 2.
Quantitative Analysis of GST-P-Positive and -Negative Foci Larger than 0.1 mm in Diameter
| No. of group | Treatment | No. of foci (No./cm2)*
|
||
|---|---|---|---|---|
| GST-P-Positive | GST-P-Negative† | |||
| Experiment 1 | ||||
| 1 | NDEN+ clofibrate | 9.61 ± 2.82 | 1.21 ± 0.85 | |
| 2 | NDEN+ Wy-14,643 | 8.81 ± 2.65 | 11.74 ± 2.65 | |
| 3 | NDEN alone | 16.15 ± 7.46 | 1.74 ± 1.11 | |
| 4 | No treatment | 0.09 ± 0.21 | 0.00 ± 0.00 | |
| Experiment 2 (26W) | ||||
| 1 | NDEN+ clofibrate | 33.17 ± 5.31 | 10.30 ± 2.10 | |
| 2 | NDEN alone | 41.09 ± 9.84 | 5.69 ± 2.40 | |
| 3 | No treatment | 0.24 ± 0.22 | 0.00 ± 0.00 | |
| Experiment 2 (36W) | ||||
| 1 | NDEN+ clofibrate | 52.02 ± 11.33 | 14.07 ± 2.84 | |
| 2 | NDEN alone | 75.66 ± 10.19 | 9.34 ± 2.72 | |
| 3 | No treatment | 0.25 ± 0.37 | 0.00 ± 0.00 | |
Except hepatocellular adenoma and carcinoma.
The foci, predominantly amphophilic cell foci, were detected by staining with H&E, and subsequently checked stainability against antibody to GST-P by using the serial sections.
Table 3.
Area of the Lesion Microdissected and Quantity of the Isolated Total RNA
| No. of group | Treatment | Microdissected lesion* | GST-P | Area of lesion microdissected† (mm2) | Total area microdissected (mm2) | Quantity of RNA (ng)‡ |
|---|---|---|---|---|---|---|
| Experiment 1 | ||||||
| 1 | NDEN+ clofibrate | HAF | Positive | 0.32∥ | 20.44 | 588 |
| 2 | NDEN+ Wy-14,643 | HAF | Negative¶ | 0.08∥ | 18.44 | 492 |
| 3 | NDEN alone | HAF | Positive | 0.23∥ | 20.00 | 644 |
| Experiment 2 | ||||||
| 1 | NDEN+ clofibrate | HAF | Negative¶ | 0.62∥ | 18.72 | 881 |
| 1 | NDEN+ clofibrate | HCA§ | Positive | 1.59 | 23.11 | 591 |
| 1 | NDEN+ clofibrate | HCA§ | Negative¶ | 2.51 | 21.87 | 606 |
| 1 | NDEN+ clofibrate | HCC§ | Positive | 3.84 | 23.06 | 759 |
| 1 | NDEN+ clofibrate | HCC§ | Negative¶ | 3.95 | 21.34 | 482 |
HAF, hepatocellular altered foci; HCA, hepatocellular adenoma; HCC, hepatocellular carcinoma.
In HCA and HCC, the tissue was microdissected from the same one lesion. In HAF, however, several lesions were pooled, since the size of each lesion was too small to get sufficient quantity of RNA from one lesion. Namely, 4 foci from group 1, 23 foci from group 2, and 6 foci from group 3 in experiment 1, and 3 foci from group 1 in experiment 2 were pooled, respectively.
One hundred ng of total RNA was used for linear amplification.
Total RNA isolated from the lesions were used only in RT-PCR assay.
Amphophilic phenotype.
The values show average area of the lesions microdissected.
Microarray Analysis
GST-P-negative HAF (amphophilic cell foci) induced by NDEN + Wy-14,643 or NDEN + clofibrate and GST-P-positive HAF (clear cell foci) induced by NDEN alone or NDEN + clofibrate were compared with the corresponding adjacent normal tissues. The numbers of up- and down-regulated genes obtained from those comparisons are listed in Table 4. In the up-regulated genes extracted from amphophilic GST-P-negative HAF, five genes were commonly overexpressed in the lesions induced by two different peroxisome proliferators (Table 5). That demonstrating greatest fluctuation, the α2M gene, showed overexpression specific for amphophilic GST-P-negative HAF (not up-regulated in GST-P-positive HAF). On the other hand, of the down-regulated genes extracted from amphophilic GST-P-negative HAF, two demonstrated a decrease in common in HAF induced by two different peroxisome proliferators (Table 5), but none were specific only for amphophilic GST-P-negative HAF. In the present study, microarray analysis revealed increased γ-GT mRNA in GST-P-positive HAF but not amphophilic GST-P-negative HAF.
Table 4.
The Number of Dysregulated Genes
| No. of group | Treatment | Microdissected lesions | GST-P | No. of dysregulated genes*
|
|
|---|---|---|---|---|---|
| Up | Down | ||||
| Experiment 1 | |||||
| 1 | NDEN+ clofibrate | Hepatocellular altered foci† | Positive | 279 | 67 |
| 2 | NDEN+ Wy-14,643 | Hepatocellular altered foci‡ | Negative | 67 | 7 |
| 3 | NDEN alone | Hepatocellular altered foci† | Positive | 137 | 71 |
| Experiment 2 | |||||
| 1 | NDEN+ clofibrate | Hepatocellular altered foci‡ | Negative | 110 | 51 |
Number of dysregulated genes in the lesion as compared to adjacent normal tissue.
Clear cell foci.
Amphophilic cell foci.
Table 5.
Common Dysregulated Genes in Amphophilic GST-P-Negative Foci Induced by Two Peroxisome Proliferators
| Access no. | Gene name | GST-P-negative foci
|
GST-P-positive foci
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| NDEN + WY*
|
NDEN + CF†
|
NDEN alone
|
NDEN + CF
|
||||||
| Signal‡ | Ratio§ | Signal | Ratio | Signal | Ratio | Signal | Ratio | ||
| Up-regulated genes | |||||||||
| M23566 | α2-macroglobulin | 385.7 | 1.4 | 3507.5 | 5.3 | ND¶ | – | ND | – |
| X61654 | ad1-antigen | 568.0 | 1.3 | 1019.5 | 1.8 | ND¶ | – | ND | – |
| X60767 | cdc2 promoter | 30.1 | 1.3 | 19.4 | 1.2 | 18.7 | 1.0 | 18.3 | 1.1 |
| AA860039 | EST | 22.6 | 1.1 | 7.8 | 2.9 | 28.1 | 2.1 | 16.7 | 1.8 |
| AI070295 | EST | 88.2 | 3.1 | 58.8 | 1.2 | ND | – | 58.0 | 0.8 |
| Down-regulated genes | |||||||||
| J00738 | α-2u globulin | 3.1 | −1.4 | 175.0 | −1.9 | 1110.8 | −0.6 | 54.9 | −1.8 |
| M18363 | P-450(M-1) | 3.9 | −2.7 | 512.0 | −1.4 | 403.8 | −1.2 | 233.7 | −1.6 |
WY; Wy-14,643.
CF; clofibrate.
Signal represents intensity of gene expression.
Ratio represents the log2 of the ratio between the experimental (GST-P-negative or -positive foci) and the control (adjacent normal liver tissue) samples.
ND represents “not detectable.”
Semiquantitative Analysis of α2M mRNA
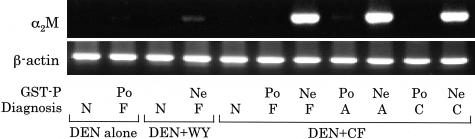
To validate expression of α2M mRNA observed in microarray analysis, semiquantitative RT-PCR was conducted. As shown in Figure 1, clear increased α2M mRNA expression was detected in amphophilic GST-P-negative HAF induced by NDEN + Wy-14,643 and NDEN + clofibrate as compared to the respective adjacent normal tissues, and also pronounced in amphophilic GST-P-negative HCA and HCC induced by NDEN + clofibrate. In the present study, however, elevation of α2M mRNA was less pronounced in amphophilic GST-P-negative HAF induced by NDEN + Wy-14,643 as compared to the lesions after exposure to NDEN + clofibrate. We consider that this variability is because of differences in progression of the lesions, because amphophilic GST-P-negative HAF microdissected in the NDEN + Wy-14,643 group were much smaller than the lesions microdissected in the group receiving NDEN + clofibrate (ie, 0.08 mm2 in the former and 0.62 mm2 in the latter as shown in Table 3).
Figure 1.
Semiquantitative RT-PCR for α2M mRNA in HAF, HCA, HCC, and adjacent normal liver tissues. Po, GST-P-positive; Ne, GST-P-negative (amphophilic phenotype); N, normal tissue; F, hepatocellular altered foci; A, hepatocellular adenoma; C, hepatocellular carcinoma; WY, Wy-14,643; CF, clofibrate.
Localization of α2M mRNA
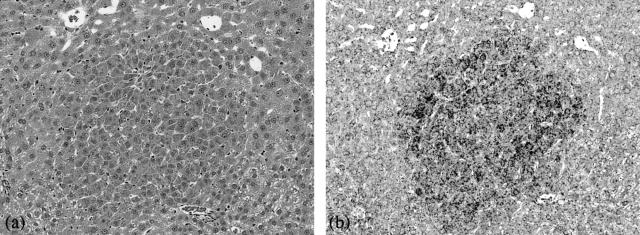
To examine the localization of α2M mRNA in the GST-P-negative HAF, in situ hybridization was performed (Figure 2). Specific increased accumulation of α2M mRNA was consistently and evenly distributed within amphophilic GST-P-negative HAF, including small number of foci composed of homogeneous cells whose cytoplasms were poor in glycogen and diffusely and relatively intense basophilic, induced by NDEN alone [described as amphophilic cell foci (basophilic) in Table 1]. Furthermore, the distinctive accumulation was observed not only in large but also very small foci, for which diagnosis only by H&E staining was difficult. Subsequently, the total number of foci positive for α2M mRNA signals was counted to investigate sensitivity as a marker of GST-P-negative foci. In situ hybridization was conducted for the animals sacrificed at week 26 in experiment 2, in which relatively large and many GST-P-negative foci were induced. As shown in Table 6, the total number of foci positive for α2M mRNA signals was higher than the total number of GST-P-negative foci (predominantly amphophilic cell foci) detected only by H&E staining shown in Table 2 (α2M mRNA-positive foci [19.06 ± 3.63 and 10.80 ± 2.26] versus GST-P-negative foci detected by H&E staining [10.30 ± 2.10 and 5.69 ± 2.40] in the groups of NDEN + clofibrate and NDEN alone, respectively).
Figure 2.
In situ hybridization for α2M mRNA in GST-P-negative HAF (amphophilic cell foci) induced by NDEN + clofibrate. a: H&E staining. b: Accumulation of α2-M mRNA. Original magnifications, ×125.
Table 6.
Quantitative Analysis of the Foci Positive for α2M mRNA Signals Larger than 0.1 mm in Diameter (Animals Sacrificed at Week 26 in Experiment 2)
| No. of group | Treatment | Total number of foci positive for α2M mRNA (No./cm2) |
|---|---|---|
| 1 | NDEN+ clofibrate | 19.06 ± 3.63 |
| 2 | NDEN alone | 10.80 ± 2.26 |
| 3 | No treatment | 0.00 ± 0.00 |
Localization of α2M Protein
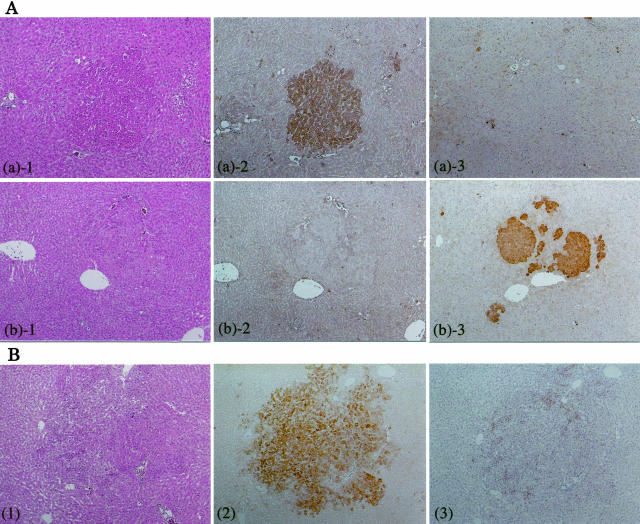
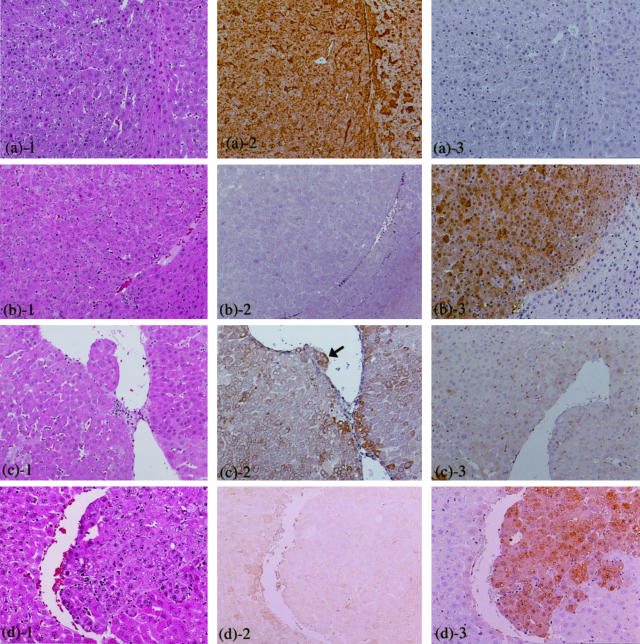
Strong immunohistochemical staining of α2M protein was observed characteristically in amphophilic GST-P-negative HAF, HCAs, and HCCs induced by NDEN + PPs (Figures 3 and 4). Interestingly, GST-P-negative HAF induced by NDEN alone also showed distinctive reactivity to anti-α2M antibody (Figure 3B). Furthermore, in contrast to amphophilic GST-P-negative HAF and HCAs, heterogeneous staining was shown within amphophilic GST-P-negative HCC, ie, the invasive tumor cells were more distinctly stained especially as compared to the other tumor cells (Figure 4c, arrow). Detailed observation, however, revealed that nearly same sized GST-P-negative HAF did not necessarily take on equivalent staining properties, and some foci with α2M mRNA signals showed no clearly positive staining.
Figure 3.
Immunohistochemistry for α2M and GST-P. A: HAF induced by NDEN + clofibrate. a: Amphophilic GST-P-negative HAF: 1, H&E staining; 2, α2-M; 3, GST-P. b: GST-P-positive HAF: 1, H&E staining; 2, α2-M; 3, GST-P. B: GST-P-negative HAF induced by NDEN alone. 1, H&E staining; 2, α2-M; 3, GST-P. Original magnifications: ×40 (A); ×30 (B).
Figure 4.
Immunohistochemistry for α2M and GST-P in liver tumors induced by NDEN + clofibrate. a and c: GST-P-negative HCA and HCC (amphophilic phenotype), respectively. b and d: GST-P-positive HCA and HCC, respectively. 1, H&E staining; 2, α2-M; 3, GST-P. Arrow illustrates invasive tumor cells into blood vessel in c2. Original magnifications, ×85.
Discussion
In the present study, microarray analysis revealed α2M to be characteristically overexpressed in amphophilic GST-P-negative HAF, in which mRNA overexpression for γ-GT was also undetectable as compared to adjacent normal tissues. Further detailed examinations demonstrated the same characteristics observed not only in amphophilic GST-P-negative HAF induced by peroxisome proliferators, but also amphophilic GST-P-negative HCAs and HCCs induced by peroxisome proliferators, and small number of foci composed of homogeneous cells whose cytoplasms were poor in glycogen and diffusely and relatively intense basophilic, induced by NDEN alone. Additionally, no obviously different immunohistochemical properties for α2M were observed among morphologically different types of GST-P-negative HAF, such as amphophilic cell foci (weakly basophilic or basophilic). Members in the group of Bannasch16,17 earlier reported in detail that enzyme histochemical examination revealed alterations of some mitochondrial enzymes in amphophilic cell foci. In the present study, however, characteristic accumulation of α2M mRNA was observed even in HAF not evident on routine H&E staining because of the small size or equivocal morphological character. Quantitative analysis of the total number of foci positive for α2M mRNA signals showed quantitative assay using α2M as a marker is possibly sensitive as compared to one only by routine H&E staining in evaluation of GST-P-negative foci. At present, the quantitative assay using GST-P as the indicator for carcinogenicity, eg, in vivo medium-term bioassay for carcinogens,13 is one of the most sensitive assays and makes it possible to evaluate earlier as compared to assessment only by routine H&E staining. Thus, in addition to hitherto markers such as GST-P, application of α2M to the above assay possibly accomplishes more accurate safety assessment. On the contrary of characteristic accumulation of α2M mRNA, however, immunohistochemical staining of α2M protein was not necessarily observed at all of the corresponding lesions. Smorenburg and colleagues35 reported only weak immunostaining of α2M protein present in cancer cells in the metastases of colon cancer, although high levels of the mRNA were found in cancer cells of all metastases, suggesting immediate release of α2M after being produced. Therefore, discrepancy between accumulation of mRNA and immunohistochemical staining of α2M in the HAF might be because of the same reason, so that if some appropriate procedures, eg, liver perfusion with an appropriate fixative, are conducted to prevent α2M being released, the inconvenience observed in the immunohistochemistry might be improved.
Furthermore, α2M increased with progression from HAF to HCA. As noted above, α2M functions as a carrier protein and regulator for various growth factors and cytokines such as transforming growth factor-β, interleukin-1, and tumor necrosis factor-α.21 transforming growth factor-β1 is known to be involved in the onset of hepatocyte apoptosis and that peroxisome proliferators can impinge on this cell death pathway.36 Furthermore, α2M partially counteracts the inhibitory effects of transforming growth factor-β1 or -β2 on proliferation of neoplastic hepatocytes,37 suggesting that under some conditions, α2M can promote hepatocarcinogenesis by perturbing transforming growth factor-β-induced apoptosis. Additionally, interleukin-1 and tumor necrosis factor-α mediate hepatocyte DNA synthesis and suppression of apoptosis induced by peroxisome proliferators,38 suggesting that α2M can also exert an influence by up-regulating some growth factors and cytokines such as interleukin-1 and tumor necrosis factor-α. Several investigators32,39 reported no elevations of serum α2M level during rat hepatocarcinogenesis induced by NDEN, acetylaminofluorene, and 3′-methyl-4-dimethyl aminoazobenzene except at the late stage. We consider that this is probably because most of all lesions induced by these agents were GST-P-positive lesions.13
The preferential localization of α2M protein was observed in the invasive and/or perivascular tumor cells, as opposed to homogeneous accumulation of α2M mRNA observed in our preliminary experiment (data not shown). Several experiments have been undertaken to clarify what role α2M might play in invasion or metastasis of tumor cells and it has been found to inhibit invasive growth because of its function as pan-protease inhibitor.40,41 However, Asplin and colleagues42 recently suggested that receptor-recognized forms of α2M behave like a growth factor in highly metastatic human prostate carcinoma cell line, 1-LN, and the 1-LN metastatic phenotype may result, in part, from aberrant expression of α2M signaling receptor, indicating the possible involvement of receptor-recognized forms of α2M in tumor progression. In addition, Smorenburg and colleagues35 indicated that α2M might be related to tumorigenicity, ie, they speculate α2M produced by metastatic colon cancer cells captures inflammatory cytokines, thereby inhibiting the defensive reaction against cancer cell spread, because colon cancer cells in metastases of all different stages were shown to produce α2M mRNA and seem to release the protein effectively. They further observed that although stromal cells surrounding metastases were low in α2M mRNA expression, they were positively stained for the protein. They speculated the stromal cells seem to be involved in endocytosis of α2M. In the present study, it seems likely that α2M produced by the tumor cells is trapped by the invasive tumor cells and tumor cells surrounding the blood vessels via the α2M receptor or low-density lipoprotein receptor-related protein.43 Taking all of the findings together, however, the significance of specific overexpression of α2M protein in tumor cells at the invasive sites and surrounding blood vessels remains unclear.
In conclusion, the present study demonstrated that α2M is a novel and an important candidate cytochemical marker for identification of HAF, in particular amphophilic cell foci, which are undetectable by established cytochemical markers for rat hepatocarcinogenesis, and could be applied to in vivo medium term bioassay for carcinogens13 in addition to GST-P. In addition, α2M may be tightly linked to the hepatocarcinogenesis from the initial stage through to tumor progression. However, it remains to be clarified why up-regulation of α2M is characteristic for GST-P-negative lesions and how α2M makes a contribution to rat hepatocarcinogenesis, invasion, or metastasis. Elevated α2M is also observed in humans with some type of HCC, so that it must give beneficial information for clinical diagnosis, prevention of liver tumor, and risk assessment for chemical carcinogens to elucidate what role α2M plays in rat hepatocarcinogenesis.
Acknowledgments
We thank Ms. M. Yamaguchi-Katsura and Ms. K. Tanaka-Iwai for expert assistance with histotechnology and genetic analysis, respectively.
Footnotes
Address reprint requests to Tokuo Sukata, Environmental Health Science Laboratory, Sumitomo Chemical Co., Ltd., 1-98, 3-chome, Kasugade-Naka, Konohana-ku, Osaka 554-8558, Japan. E-mail: sukata@sc.sumitomo-chem.co.jp).
References
- Ito N, Hananouchi M, Sugihara S, Shirai T, Tsuda H, Fukushima S, Nagasaki H. Reversibility and irreversibility of liver tumors in mice induced by the α isomer of 1,2,3,4,5,6-hexachlorocyclohexane. Cancer Res. 1976;36:2227–2234. [PubMed] [Google Scholar]
- Moore MA, Hacker H-J, Bannasch P. Phenotypic instability in focal and nodular lesions induced in a short-term system in the rat liver. Carcinogenesis. 1983;4:595–603. doi: 10.1093/carcin/4.5.595. [DOI] [PubMed] [Google Scholar]
- Tatematsu M, Nagamine Y, Farber E. Redifferentiation as a basis for remodeling of carcinogen-induced hepatocyte nodules to normal appearing liver. Cancer Res. 1983;43:5049–5058. [PubMed] [Google Scholar]
- Takahashi S, Lombardi B, Shinozuka H. Progression of carcinogen-induced foci of gamma-glutamyltranspeptidase-positive hepatocytes to hepatomas in rats fed a choline-deficient diet. Int J Cancer. 1982;29:445–450. doi: 10.1002/ijc.2910290414. [DOI] [PubMed] [Google Scholar]
- Scherer E, Emmelot P. Kinetics of induction and growth of precancerous liver-cell foci, and liver tumour formation by diethylnitrosamine in the rat. Eur J Cancer. 1975;11:689–696. doi: 10.1016/0014-2964(75)90042-0. [DOI] [PubMed] [Google Scholar]
- Moore MA, Mayer D, Bannasch P. The dose dependence and sequential appearance of putative preneoplastic populations induced in the rat liver by stop experiments with N-nitrosomorpholine. Carcinogenesis. 1982;3:1429–1436. doi: 10.1093/carcin/3.12.1429. [DOI] [PubMed] [Google Scholar]
- Ogawa K, Solt DB, Farber E. Phenotypic diversity as an early property of putative preneoplastic hepatocyte populations in liver carcinogenesis. Cancer Res. 1980;40:725–733. [PubMed] [Google Scholar]
- Enomoto K, Farber E. Kinetics of phenotypic maturation of remodelling of hyperplastic nodules during liver carcinogenesis. Cancer Res. 1982;42:2330–2335. [PubMed] [Google Scholar]
- Solt DB, Medline A, Farber E. Rapid emergence of carcinogen-induced hyperplastic lesions in a new model for the sequential analysis of liver carcinogenesis. Am J Pathol. 1977;88:595–618. [PMC free article] [PubMed] [Google Scholar]
- Ito N, Tsuda H, Hasegawa R, Imaida K. Sequential observation of pathomorphologic alterations in preneoplastic lesions during the promotion stage of hepatocarcinogenesis and the development of a short-term test system for hepatopromoters and hepatocarcinogens. Toxicol Pathol. 1982;10:37–49. doi: 10.1177/019262338201000207. [DOI] [PubMed] [Google Scholar]
- Tatematsu M, Kaku T, Medline A, Frikson L, Roomi W, Sharma RN, Murray RK, Farber E. Markers of liver neoplasm—real or fictional? Milman H, Sell S, editors. New York: Plenum,; 1983:pp 25–42. [Google Scholar]
- Sato K, Kitahara A, Satoh K, Ichikawa T, Tatematsu M, Ito N. The placental form of glutathione S-transferase as a new marker protein for preneoplasia in rat chemical carcinogenesis. Gann. 1984;75:199–202. [PubMed] [Google Scholar]
- Ito N, Tsuda H, Tatematsu M, Inoue T, Tagawa Y, Aoki T, Uwagawa S, Kagawa M, Ogiso T, Masui T, Imaida K, Fukushima S, Asamoto M. Enhancing effect of various hepatocarcinogens on induction of preneoplastic glutathione S-transferase placental form positive foci in rats—an approach for a new medium-term bioassay system. Carcinogenesis. 1988;9:387–394. doi: 10.1093/carcin/9.3.387. [DOI] [PubMed] [Google Scholar]
- Rao MS, Tatematsu M, Subbarao V, Ito N, Reddy JK. Analysis of peroxisome proliferator-induced preneoplastic and neoplastic lesions of rat liver for placental form of glutathione S-transferase and gamma-glutamyltranspeptidase. Cancer Res. 1986;46:5287–5290. [PubMed] [Google Scholar]
- Rao MS, Nemali MR, Usuda N, Scarpelli DG, Makino T, Pitot HC, Reddy JK. Lack of expression of glutathione-S-transferase P, gamma-glutamyl transpeptidase, and alpha-fetoprotein messenger RNAs in liver tumors induced by peroxisome proliferators. Cancer Res. 1988;48:4919–4925. [PubMed] [Google Scholar]
- Weber E, Moore MA, Bannasch P. Enzyme histochemical and morphological phenotype of amphophilic foci and amphophilic/tigroid cell adenomas in rat liver after combined treatment with dehydroepiandrosterone and N-nitrosomorpholine. Carcinogenesis. 1988;9:1049–1054. doi: 10.1093/carcin/9.6.1049. [DOI] [PubMed] [Google Scholar]
- Mayer D, Metzger C, Loenetti P, Beier K, Benner A, Bannasch P. Differential expression of key enzymes of energy metabolism in preneoplastic and neoplastic rat liver lesions induced by N-nitrosomorpholine and dehydroepiandrosterone. Int J Cancer. 1998;79:232–240. doi: 10.1002/(sici)1097-0215(19980619)79:3<232::aid-ijc4>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Schreiber G, Howlett G, Nagashima M, Millership A, Martin H, Urban J, Kotler L. The acute phase response of plasma protein synthesis during experimental inflammation. J Biol Chem. 1982;257:10271–10277. [PubMed] [Google Scholar]
- Northemann W, Andus T, Gross V, Nagashima M, Schreiber G, Heinrich PC. Messenger RNA activities of four acute phase proteins during inflammation. FEBS Lett. 1983;161:319–322. doi: 10.1016/0014-5793(83)81033-3. [DOI] [PubMed] [Google Scholar]
- Barret AJ, Starkey PM. The interaction of α2-macroglobulin with proteinase. Biochem J. 1973;133:709–724. doi: 10.1042/bj1330709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James K. Interactions between cytokines and α2-macroglobulin. Immunol Today. 1990;11:163–166. doi: 10.1016/0167-5699(90)90067-j. [DOI] [PubMed] [Google Scholar]
- Chu CT, Rubenstein DS, Enghild JJ, Pizzo SV. Mechanism of insulin incorporation into α2-macroglobulin: implication for the study of peptide and growth factor binding. Biochemistry. 1991;30:1551–1560. doi: 10.1021/bi00220a016. [DOI] [PubMed] [Google Scholar]
- Dennis PA, Saksela O, Harpel P, Rifkin DB. α2-Macroglobulin is a binding protein for basic fibroblast growth factor. J Biol Chem. 1989;264:7210–7216. [PubMed] [Google Scholar]
- O’Conner-McCourt MD, Wakefield LM. Latent transforming growth factor-β in serum. J Biol Chem. 1987;262:14090–14099. [PubMed] [Google Scholar]
- Huang JS, Huang SS, Deuel TF. Specific covalent binding of platelet-derived growth factor to human plasma α2-macroglobulin. Proc Natl Acad Sci USA. 1984;81:342–346. doi: 10.1073/pnas.81.2.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang SS, O’Grady P, Huang JS. Human transforming growth factor-β-α2-macroglobulin complex is a latent form of transforming growth factor β. J Biol Chem. 1988;263:1535–1541. [PubMed] [Google Scholar]
- Kar P, Gandhi BM, Irshad M, Gupta H, Tandon BN. Alpha-2 macroglobulin: an additional marker for diagnosis of hepatocellular carcinoma. J Assoc Physicians India. 1987;35:288–289. [PubMed] [Google Scholar]
- Poon TC, Chan AT, Zee B, Ho SK, Mok TS, Leung TW, Johnson PJ. Application of classification tree and neural network algorithms to the identification of serological liver marker profiles for the diagnosis of hepatocellular carcinoma. Oncology. 2001;61:275–283. doi: 10.1159/000055334. [DOI] [PubMed] [Google Scholar]
- Kotaka M, Chen GG, Lai PB, Lau WY, Chan PK, Leung TW, Li AK. Analysis of differentially expressed genes in hepatocellular carcinoma with hepatitis C virus by suppression subtractive hybridization. Oncol Res. 2002;13:161–167. [PubMed] [Google Scholar]
- Beaton GH, Selby AE, Veen MJ, Wright AM. Starch gel electrophoresis of rat serum proteins. II. Slow alpha 2-globulin and other serum proteins in pregnant, tumor bearing, and young rats. J Biol Chem. 1961;236:2005–2008. [PubMed] [Google Scholar]
- Stanislawski-Birencwajg M, Uriel J, Grabar P. Association of embryonic antigens with experimentally induced hepatic lesions in the rat. Cancer Res. 1967;27:1990–1997. [PubMed] [Google Scholar]
- Hudig D, Sell S, Newell L, Becker FF. Rat alpha-macrofetoprotein (acute-phase alpha 2-macroglobulin) during hepatocarcinogenesis. Cancer Res. 1979;39:3715–3719. [PubMed] [Google Scholar]
- Hasegawa R, Yoshida T, Mizoguchi Y, Futakuchi M, Kim DJ, Cui L, Ito N. Phenotypic alteration of hepatocellular foci in rats treated with clofibrate and phenobarbital. Cancer Lett. 1994;83:89–95. doi: 10.1016/0304-3835(94)90303-4. [DOI] [PubMed] [Google Scholar]
- Jones TC, Mohr U, Hunt RD, editors. Berlin: Springer,; Monographs on Pathology of Laboratory Animals Sponsored by the International Life Sciences Institute, Digestive System. 1997:pp 3–37. [Google Scholar]
- Smorenburg SM, Griffini P, Tiggelman AB, Moorman AF, Boers W, Van Noorden JF. Alpha2-macroglobulin is mainly produced by cancer cells and not by hepatocytes in rats with colon carcinoma metastases in liver. Hepatology. 1996;23:560–570. doi: 10.1002/hep.510230323. [DOI] [PubMed] [Google Scholar]
- Strange J, Roberts RA. Reduced expression of mature TGF beta 1 correlates with the suppression of rat hepatocyte apoptosis by the peroxisome proliferator, nafenopin. Mutat Res. 1996;372:107–113. doi: 10.1016/S0027-5107(96)00173-X. [DOI] [PubMed] [Google Scholar]
- Wollenberg GK, LaMarre J, Semple E, Farber E, Gauldie J, Hayes MA. Counteracting effects of dexamethasone and alpha 2-macroglobulin on inhibition of proliferation of normal and neoplastic rat hepatocytes by transforming growth factors-beta type 1 and type 2. Int J Cancer. 1991;47:311–316. doi: 10.1002/ijc.2910470223. [DOI] [PubMed] [Google Scholar]
- West DA, James NH, Cosulich SC, Holden PR, Brindle R, Rolfe M, Roberts RA. Role for tumor necrosis factor alpha receptor 1 and interleukin-1 receptor in the suppression of mouse hepatocyte apoptosis by the peroxisome proliferator nafenopin. Hepatology. 1999;30:1417–1424. doi: 10.1002/hep.510300612. [DOI] [PubMed] [Google Scholar]
- Dolezalova V, Stratil P, Simickova M, Kocent A, Nemecek R. Alpha-fetoprotein (AFP) and alpha 2-macroglobulin (alpha 2-M) as the markers of distinct responses of hepatocytes to carcinogens in the rat: carcinogenesis. Ann NY Acad Sci. 1983;417:294–307. doi: 10.1111/j.1749-6632.1983.tb32873.x. [DOI] [PubMed] [Google Scholar]
- Katerinaki E, Evans GS, Lorigan PC, MacNeil S. TNF-alpha increases human melanoma cell invasion and migration in vitro: the role of proteolytic enzymes. Br J Cancer. 2003;89:1123–1129. doi: 10.1038/sj.bjc.6601257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H, Miyazaki M, Nishimura F, Nakajima N. Secretion of extracellular matrix (fibronectin), growth factor (transforming growth factor beta) and protease (cathepsin D) by hepatoma cells. Oncology. 2000;58:261–270. doi: 10.1159/000012110. [DOI] [PubMed] [Google Scholar]
- Asplin IR, Misra UK, Gawdi G, Gonzalez-Gronow M, Pizzo SV. Selective upregulated expression of the alpha2-macroglobulin signaling receptor in highly metastatic 1-LN prostate carcinoma cells. Arch Biochem Biophys. 2000;383:135–141. doi: 10.1006/abbi.2000.2052. [DOI] [PubMed] [Google Scholar]
- Strickland DK, Kounnas MZ, Argraves WS. LDL-receptor-related protein: a multiligand receptor for lipoprotein and proteinase catabolism. FASEB J. 1995;9:890–898. doi: 10.1096/fasebj.9.10.7615159. [DOI] [PubMed] [Google Scholar]