Abstract
The Meccano (or Lego) set approach to synthetic protein design envisages covalent assembly of prefabricated units of peptide secondary structure. Stereochemical control over peptide folding is achieved by incorporation of conformationally constrained residues like α-aminoisobutyric acid (Aib) or dPro that nucleate helical and β-hairpin structures, respectively. The generation of a synthetic sequence containing both a helix and a hairpin is achieved in the peptide BH17, Boc-Val-Ala-Leu-Aib-Val-Ala-Leu-Gly-Gly-Leu-Phe-Val-dPro-Gly-Leu-Phe-Val-OMe (where Boc is t-butoxycarbonyl), as demonstrated by a crystal structure determination. The achiral -Gly-Gly- linker permits helix termination as a Schellman motif and extension to the strand segment of the hairpin. Structure parameters for C89H143N17O20⋅2H2O are space group P21, a = 14.935(7) Å, b = 18.949(6) Å, c = 19.231(8) Å, β = 101.79(4)°, Z = 2, agreement factor R1 = 8.50% for 4,862 observed reflections > 4σ(F), and resolution of ≈0.98 Å.
Several approaches to de novo protein design envisioning successful construction of protein-like structural motifs have been investigated over the past 2 decades (1). The Meccano (or Lego) set approach to synthetic protein design is based on the covalent assembly of prefabricated modules of peptide secondary structures (2, 3). Stereochemical control over peptide folding may be achieved by incorporation of conformationally constrained residues, like α-aminoisobutyric acid (Aib) or dPro, that nucleate helical and β-hairpin structures, respectively (4). Nucleation of appropriate local conformations, together with the cooperative formation of several intramolecular hydrogen bonds in secondary structures, favors the population of stereochemically ordered segments in solvents that compete poorly for hydrogen bonding. The construction of helical segments of varying length has been achieved by using Aib residues for helix stabilization. Conformational characterization of peptide helices containing Aib has been documented extensively in a large number of single crystal x-ray diffraction studies (5–9). The use of dPro-Xxx segments to nucleate β-hairpin conformations is of more recent origin (10–13). β-Hairpins have been well characterized in solutions by using NMR spectroscopy, and the formation of three- and four-stranded β-sheets has been demonstrated in solution by incorporating multiple dPro-Xxx segments (14–18). Designed octapeptide β-hairpin modules Boc-L-V-V-dP-G-L-V-V-OMe (where Boc represents t-butoxycarbonyl and dP represents dPro; ref. 19) and Boc-L-V-V-dP-A-L-V-V-OMe (I.L.K., P.B., and S. K. Awasthi, unpublished work) have been characterized in the crystalline state. The availability of peptide building blocks that adopt helical and β-hairpin conformations has enabled the construction of a synthetic 17-residue sequence, Boc-V-A-L-U-V-A-L-G-G-L-F-V-dP-G-L-F-V-OMe (BH17, where U represents Aib), that contains both elements of secondary structures linked together by a Gly-Gly segment (residues 8 and 9). The positioning of the achiral Gly residue at position 8 facilitates termination of the potential helical segment (residues 1–7) by formation of a Schellman motif (20–23). Gly-9 is anticipated to be the sole conformationally flexible linking residue. We describe the structure of BH17 in crystals, establishing an approximately orthogonal arrangement of the two elements of secondary structures, as shown in Fig. 1 a and b.
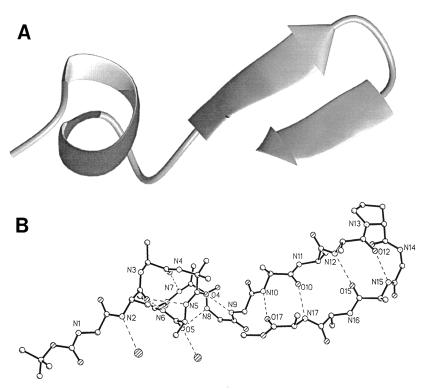
Figure 1.
(a) Ribbon drawing of BH17 showing the N-terminal extension, helix, linker, and β-hairpin (generated by the program molmol; ref. 31). (b) Backbone in BH17 drawn with x-ray structure coordinates. Dashed lines indicate the intramolecular hydrogen bonds.
Experimental Methods
The 17-residue peptide BH17 was synthesized by conventional solution phase procedures by using a fragment condensation strategy. Boc and methylester groups are used for N- and C-terminal protection. Peptide couplings were mediated by N,N′-dicyclohexylcarbodiimide and 1-hydroxybenzotriazole. The N-terminal fragment (residues 1–7) and the C-terminal fragment (residues 8–17) were synthesized independently, followed by final extension to the target sequence. The peptide was purified by medium pressure liquid chromatography on a reverse phase C18 (10- to 60-μm) column followed by HPLC on a C18 (5- to 10-μm) column with methanol–water gradients.
Crystals of BH17 were obtained by slow evaporation from methanol containing a small amount of water. The crystals were very thin and fragile. Initially, x-ray diffraction data were collected at −50°C from a crystal that was covered with microscope immersion oil immediately after removal from the mother liquor. The check reflections wandered in value during the data collection, and the number of data with observable intensity was somewhat limited. No structure solution was obtained. After 3 weeks of exposure to air, the same crystal yielded quite satisfactory data to a higher resolution. The x-ray data were measured at −60°C on a four-circle diffractometer (Broker P4, Madison, WI) with CuKα radiation (λ = 1.54178 Å). The θ/2θ scan mode was used with a 2.0° + 2θ(α1 − α2) scan width, 15°/min scan speed, and 2θmax = 105° (0.98-Å resolution). The crystal size was 0.07 × 0.80 × 0.90 mm. The crystal data are C89H143N17O20·2H2O, molecular weight = 1,771.2 + 36, space group = P21, a = 14.935(7) Å, b = 18.949(6) Å, c = 19.231(8) Å, β = 101.79(4)°, V = 5,327.6 Å3, Z = 2, and calculated d = 1.124 g/cm3. The structure was solved with the aid of a vector search and translation procedure contained in the patsee program (24). The search model consisted of 23 backbone and Cβ atoms that are contained in the middle of the α-helix in the acetyl carbonyl-V-A-L-α,α′-dipropylglycine-V-A-L-OMe molecule (25). The best rotation and second-best translation position yielded 84 atoms when subjected to the tangent formula expansion procedure (26). The remainder of the atoms were found in difference maps interspersed with least-squares refinements. The final least-squares refinement—performed on all 7,148 F2 values, with anisotropic thermal parameters, and 143 hydrogen atoms placed in idealized positions and riding with the C or N atoms to which they are bonded—resulted in R1 = 8.50% for 4,862 data with |Fo| > 4.0σ and 1,154 variables. (The least-squares program used was Siemens shelxtl, version 5.03, Iselin, NJ.)
Results and Discussion
Inspection of the backbone torsion angles in Table 1 indicates that residues 3–7 form about one and a half turns of a helix. As anticipated, Gly-8 adopts a left-handed helical (αL) conformation, resulting in the formation of a Schellman motif, which is characterized by a 6 → 1 hydrogen bond between Gly-9 NH and Aib-4 CO groups. Such a structural feature is a common mode of helix termination in proteins with Gly or Asn residues adopting the left-handed, helix-terminating conformation (20, 27, 28). Gly-9 is extended, allowing the polypeptide chain to progress comfortably into the N-terminal arm of the β-hairpin. Residues 10–17 form a classical β-hairpin nucleated by a type II′ β-turn at the dPro-13–Gly-14 segment (19). The β-hairpin segment has nearly ideal intrastrand hydrogen bonds as shown in Table 2.
Table 1.
Torsional angles in BH17
| Residue | Torsional angles, degrees
|
|||||||
|---|---|---|---|---|---|---|---|---|
| φ | ψ | ω | χ1 | χ2 | χ3 | χ4 | § | |
| Boc-0 | 178 | −177 | ||||||
| Val-1 | −129 | +119 | 178 | −58 | −178 | |||
| Ala-2 | −68 | +148 | −177 | |||||
| Leu-3 | −55 | −52 | −178 | 179 | −178, +60 | |||
| Aib-4 | −49 | −44 | −175 | |||||
| Val-5 | −71 | −39 | 178 | −63 | 175 | |||
| Ala-6 | −60 | −32 | −176 | |||||
| Leu-7 | −97 | +16 | 171 | −65 | 177, −57 | |||
| Gly-8 | +93 | +11 | −178 | |||||
| Gly-9 | +110 | +175 | −172 | |||||
| Leu-10 | −133 | +121 | 167 | −173 | 72, −166 | |||
| Phe-11 | −72 | +134 | 170 | −69 | −86 | 95 | ||
| Val-12 | −119 | +91 | −166 | 177 | −59 | |||
| dPro-13 | +60 | −131 | 178 | +8 | −28 | +38 | +16 | −33 |
| Gly-14 | −82 | −9 | −179 | |||||
| Leu-15 | −92 | 171 | −168 | −62 | 172, −58 | |||
| Phe-16 | −144 | +126 | −171 | −172 | −99, 83 | |||
| Val-17 | −136 | 174 | 174 | +73 | −51 | |||
Torsional angles φ, ψ, and ω for the backbone and χn for the side chains follow the convention presented in ref. 32. The estimated standard deviations are near 2.0°. The symbol § represents the torsion angle CδNCαCβ.
Table 2.
Hydrogen bonds
| Type | Donor | Acceptor** | Donor– acceptor, Å | Hydrogen*– acceptor, Å | CO⋅⋅⋅N angle, degrees |
|---|---|---|---|---|---|
| Intermol | N1 | O11† | 3.035 | 2.17 | 143 |
| Solvent | N2 | W1 | 2.887 | 2.00 | |
| Head-to-tail | N3 | O9† | 2.870 | 2.01 | 147 |
| Head-to-tail | N4 | O7† | 2.979 | 2.14 | 169 |
| Helix 4 → 1 | N5 | O2 | 2.975 | 2.41 | 123 |
| Transition | |||||
| Helix 5 → 1 | N6 | O2 | 2.956 | 2.10 | 166 |
| Helix 5 → 1 | N7 | O3 | 3.139 | 2.35 | 162 |
| Helix 4 → 1 | N8 | O5 | 3.046 | 2.18 | 116 |
| Helix reversal | N9 | O4 | 2.818 | 2.02 | 150 |
| β- | N10 | O17 | 3.001 | 2.16 | 158 |
| Intermol | N11 | O1‡ | 2.825 | 1.98 | 147 |
| β- | N12 | O15 | 2.914 | 2.05 | 128 |
| N13 (dPro) | |||||
| Intermol | N14 | O6§ | 2.889 | 2.002 | 158 |
| β- | N15 | O12 | 2.982 | 2.18 | 133 |
| N16 | No hydrogen bond | ||||
| β- | N17 | O10 | 2.858 | 1.99 | 154 |
| Solvent | W1 | W2¶ | 2.564 | ||
| Solvent | W2 | Oo∥ | 2.681 | ||
| Solvent | W2 | O5 | 2.773 |
*Hydrogen atoms were placed in idealized positions with N–H = 0.90 Å.
†At symmetry equivalent −x, ½ + y, −z.
‡At symmetry equivalent −x, −½ + y, −z.
§At symmetry equivalent −1 + x, y, −1 + z.
¶At symmetry equivalent 1 − x, ½ + y, −z.
∥At symmetry equivalent 1 − x, −½ + y, −z.
**O8, O13, O14, and O16 do not participate in hydrogen bonding.
A striking feature of the peptide in crystals is the partially unfolded N-terminal helix, with Val-1 and Ala-2 adopting extended (β) and semiextended conformations, respectively. Indeed, the heptapeptide module (residues 1–7) has been characterized as a helix in a large number of peptide crystal structures (29). However, in solution, this helix has been shown to be relatively fragile (30). Interestingly, in the present crystal structure, partial unfolding at the N terminus facilitates the formation of important intermolecular contacts.
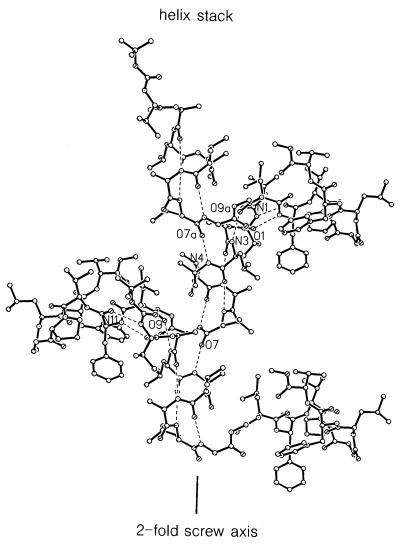
There are several distinct features that characterize the assembly of these molecules in the crystal. One characteristic feature is the head-to-tail hydrogen bonding between the helical segments of adjacent molecules (N4⋅⋅⋅O7), even although the helical segments are not free-standing but are in the interior of the sequence (Fig. 2). The stacking of the helical segments simulates an infinite helix with a sinusoidal helix axis.
Figure 2.
Three molecules of BH17 are shown winding around the b axis (vertical). The helical portions are stacked vertically over each other and linked by N4⋅⋅⋅O7a-type hydrogen bonds.
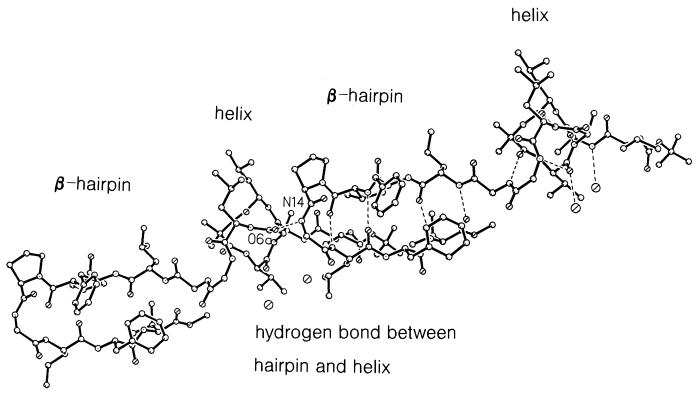
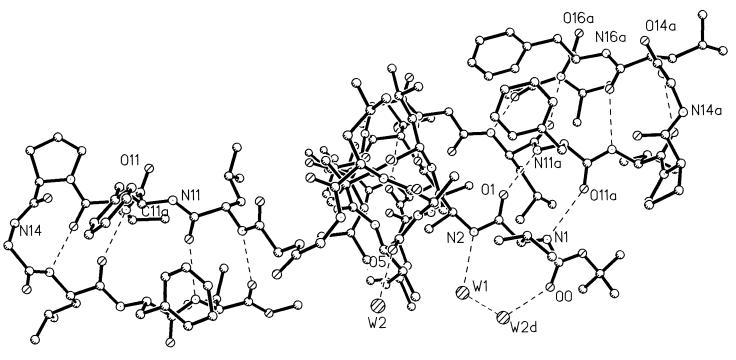
The two strands of the β-hairpin do not form an expected extended β-sheet by lateral intermolecular hydrogen bonding. N14 in the turn region of the β-hairpin forms a hydrogen bond with O6 in the helical portion of an adjacent molecule. The process is repeated by translation and forms an extended chain (Fig. 3). One arm of the β-hairpin is roughly antiparallel to the N-terminal extension of an adjacent molecule, as shown in Fig. 4, and a pair of hydrogen bonds are formed between the two structural elements (N1⋅⋅⋅O11a and N11a⋅⋅⋅O1). In this region, there is a limited extended β-sheet. Curiously, the other arm of the β-hairpin does not participate in any external hydrogen bonding. There is no acceptor for the N16H group. Atoms O14, O16, and N16H are directed into a hydrophobic pocket formed by methyl groups on the side chains of the helix and on the terminal methoxy group of several adjacent molecules. The closest approach is 3.35 Å between O16 and C3D2 of Leu-3.
Figure 3.
Two molecules of BH17 related by translation. A continuous chain is formed by hydrogen bonds between N14 (at the turn of the β-hairpin) and O6 in the helix of an adjacent molecule.
Figure 4.
Two molecules of BH17 with their helical portions stacked in a direction perpendicular to the page and oriented such that the N-terminal extension and its accompanying hydrogen bonds are visible. On the right side is illustrated a limited, extended β-sheet formed by the two strands of the β-hairpin in one molecule and the N-terminal extension (Boc–Val-1–Ala-2) of an adjacent molecule.
Two water molecules, W1 and W2, occupy a polar pocket along the extended N terminus on the other side from the β-hairpin (Fig. 4). W1 forms hydrogen bonds with N2 and W2, whereas W2 forms additional hydrogen bonds to O0 and O5 (in a symmetry-related molecule).
Conclusions
The crystal structure of the peptide BH17 validates the assumption that the structural domains within the molecule are indeed independent, suggesting that a modular assembly strategy may be viable for the construction of larger synthetic structures.
Acknowledgments
This research was partially supported in Bangalore by the program on Drug and Molecular Design of the Department of Biotechnology, Government of India, as well as by National Institutes of Health Grant GM30902 and by the Office of Naval Research.
Abbreviations
- Boc
t-butoxycarbonyl
- Aib
α-aminoisobutyric acid or α,α′-dimethylglycine
- V
lVal
- A
lAla
- G
Gly
- L
lLeu
- F
lPhe, U, Aib
- dP
dPro
Footnotes
Data deposition: The atomic coordinates have been deposited in the Cambridge Crystallographic Data Centre, www.ccdc.cam.ac.uk (ID code CCDC 139326).
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.070042697.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.070042697
References
- 1.DeGrado W F, Summa C M, Pavone V, Nastri F, Lombardi A. Annu Rev Biochem. 1999;68:779–819. doi: 10.1146/annurev.biochem.68.1.779. [DOI] [PubMed] [Google Scholar]
- 2.Balaram P. Pure Appl Chem. 1992;64:1061–1066. [Google Scholar]
- 3.Balaram P. J Peptide Res. 1999;54:195–199. doi: 10.1034/j.1399-3011.1999.00119.x. [DOI] [PubMed] [Google Scholar]
- 4.Kaul R K, Balaram P. Bioorg Med Chem. 1999;7:105–117. doi: 10.1016/s0968-0896(98)00221-1. [DOI] [PubMed] [Google Scholar]
- 5.Balaram P. Curr Opin Struct Biol. 1992;2:845–851. [Google Scholar]
- 6.Karle I L. Acc Chem Res. 1999;32:693–701. [Google Scholar]
- 7.Karle I L, Balaram P. Biochemistry. 1990;29:6747–6756. doi: 10.1021/bi00481a001. [DOI] [PubMed] [Google Scholar]
- 8.Toniolo C, Benedetti E. ISI Atlas Sci Biochem. 1988;1:225–230. [Google Scholar]
- 9.Toniolo C, Benedetti E. Trends Biochem Sci. 1991;16:350–353. doi: 10.1016/0968-0004(91)90142-i. [DOI] [PubMed] [Google Scholar]
- 10.Awasthi S K, Raghothama S, Balaram P. Biochem Biophys Res Commun. 1995;216:375–381. doi: 10.1006/bbrc.1995.2634. [DOI] [PubMed] [Google Scholar]
- 11.Haque T S, Little J C, Gellman S H. J Am Chem Soc. 1996;118:6975–6985. [Google Scholar]
- 12.Haque T S, Gellman S H. J Am Chem Soc. 1997;119:2303–2304. [Google Scholar]
- 13.Struthers M D, Cheng R P, Imperiali B. Science. 1996;271:342–345. doi: 10.1126/science.271.5247.342. [DOI] [PubMed] [Google Scholar]
- 14.Lacroix E, Kortemme T, de la Paz M L, Serrano L. Curr Opin Struct Biol. 1999;9:487–493. doi: 10.1016/s0959-440x(99)80069-4. [DOI] [PubMed] [Google Scholar]
- 15.Raghothama S R, Awasthi S K, Balaram P. J Chem Soc Perkin Trans. 1998;2:137–143. [Google Scholar]
- 16.Das C, Raghothama S, Balaram P. J Am Chem Soc. 1998;120:5812–5813. [Google Scholar]
- 17.Schenck H L, Gellman S H. J Am Chem Soc. 1998;120:4869–4870. [Google Scholar]
- 18.Das C, Raghothama S R, Balaram P. J. Chem. Soc. Chem. Commun. 1999. 967–968. [Google Scholar]
- 19.Karle I L, Awasthi S K, Balaram P. Proc Natl Acad Sci USA. 1996;93:8189–8193. doi: 10.1073/pnas.93.16.8189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gunasekaran K, Nagarajaram H A, Ramakrishnan C, Balaram P. J Mol Biol. 1998;215:917–932. doi: 10.1006/jmbi.1997.1505. [DOI] [PubMed] [Google Scholar]
- 21.Karle I L, Flippen-Anderson J L, Uma K, Balaram P. Int J Peptide Protein Res. 1993;42:401–410. [PubMed] [Google Scholar]
- 22.Datta S, Shamala N, Banerjee A, Pramanik A, Bhattacharjya S, Balaram P. J Am Chem Soc. 1997;119:9246–9251. [Google Scholar]
- 23.Banerjee A, Datta S, Pramanik A, Shamala N, Balaram P. J Am Chem Soc. 1996;118:9477–9483. [Google Scholar]
- 24.Egert E, Sheldrick G M. Acta Crystallogr A. 1985;41:262–268. [Google Scholar]
- 25.Vijayalakshmi S, Rao R B, Karle I L, Balaram P. Biopolymers. 2000;53:84–98. doi: 10.1002/(SICI)1097-0282(200001)53:1<84::AID-BIP8>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 26.Karle J. Acta Crystallogr B. 1968;24:182–186. [Google Scholar]
- 27.Schellman C. In: Protein Folding. Jaenicke R, editor. North–Holland, Amsterdam: Elsevier; 1980. pp. 53–61. [Google Scholar]
- 28.Richardson J S. Adv Protein Chem. 1981;34:164–339. doi: 10.1016/s0065-3233(08)60520-3. [DOI] [PubMed] [Google Scholar]
- 29.Karle I L, Gurunath R, Prasad S, Kaul R, Rao R B, Balaram P. J Am Chem Soc. 1995;117:9632–9637. [Google Scholar]
- 30.Karle I L, Flippen-Anderson J L, Uma K, Balaram P. Biopolymers. 1993;33:827–837. doi: 10.1002/bip.360330511. [DOI] [PubMed] [Google Scholar]
- 31.Koradi R, Billeter M, Wuthrich K. J Mol Graphics. 1996;14:51–55. doi: 10.1016/0263-7855(96)00009-4. [DOI] [PubMed] [Google Scholar]
- 32.IUPAC-IUB Commission on Biochemical Nomenclature. Biochemistry. 1970;9:3471–3479. doi: 10.1021/bi00820a001. [DOI] [PubMed] [Google Scholar]