Abstract
The gyrA and gyrB genes of Chlamydia pneumoniae TW-183 were cloned, and their proteins were purified by use of a fusion system with a maltose-binding protein. The 50% inhibitory concentrations of garenoxacin, sparfloxacin, moxifloxacin, gatifloxacin, and levofloxacin were 10.1, 47.5, 39.6, 64.2, and 156.9 μg/ml, respectively, and the MICs against C. pneumoniae TW-183 were 0.008, 0.016, 0.063, 0.125, and 0.25 μg/ml, respectively.
Chlamydia pneumoniae is a frequent cause of community-acquired respiratory tract infections including pneumonia and bronchitis in adults and children (2, 11, 13). Quinolones have been recommended for the treatment of pneumonia caused by C. pneumoniae (1). The targets of quinolones are considered to be DNA gyrase and topoisomerase IV (7, 10), which are essential enzymes for controlling the topological state of DNA in DNA replication and transcription. DNA gyrase is composed of subunits A and B, encoded by the gyrA and gyrB genes, respectively. DNA gyrase catalyzes ATP-dependent negative supercoiling of DNA.
Quinolones developed recently, such as garenoxacin (T-3811, BMS-284756), moxifloxacin, and gatifloxacin, possess a wide antimicrobial spectrum (3, 8, 9, 18) and also have a potent activity against C. pneumoniae (5, 6, 14, 15, 16, 17). These are expected to be useful in the treatment of infectious diseases caused by C. pneumoniae. In this study, we cloned C. pneumoniae DNA gyrase genes, and examined the activities of quinolones against the recombinant proteins to investigate the relation between the 50% inhibitory concentrations (IC50) and the activities against C. pneumoniae.
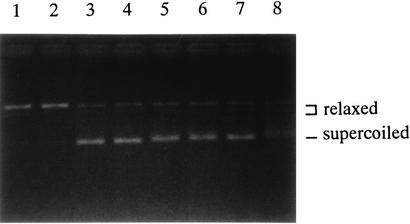
The gyrA and gyrB genes in C. pneumoniae CWL029 are denoted gyrA_1 and gyrA_2 and gyrB_1 and gyrB_2, respectively, in the GenBank (accession no.: AE001612 for gyrA_1 and gyrB_1 and AE001653 for gyrA_2 and gyrB_2). We first cloned the gyrA_1 and gyrB_1 genes from C. pneumoniae TW-183 and separately purified the encoded proteins (GyrA_1 and GyrB_1, respectively) by the use of a fusion system with a maltose-binding protein (MBP) and then examined enzymatic properties. For cloning gyrA_1 and gyrB_1 into the pT7Blue T vector, the C. pneumoniae TW-183 genome was isolated from chlamydial elementary bodies (104 inclusion-forming units). PCR amplification of gyrA_1 and gyrB_1 was performed with the CPGA-1-CPGA-2 and CPGB-1-CPGB-2 primer sets, respectively (Table 1). DNA was amplified for 35 cycles, in which the conditions were 1 min at 94°C, 1 min at 55°C, and 2 min at 72°C. Amplified PCR products for CPGA-1-CPGA-2 and CPGB-1-CPGB-2 primer pairs were about 2.5 and 2.4 kbp, respectively, and cloned genes were sequenced. Two sets of oligonucleotide primers (CPGA-3-CPGA-4 and CPGB-3-CPGB-4; Table 1) were designed for amplification of gyrA_1 and gyrB_1 genes and their subsequent insertion into BamHI and HindIII sites and BamHI and PstI sites, respectively, in the pMAL-c2 vector. The proteins obtained by fusion of MBP with GyrA_1 and GyrB_1 (GyrA_1-MBP and GyrB_1-MBP, respectively) were produced by the Escherichia coli DH5α overexpression system and were purified according to the method of Tanaka et al. (19). The molecular masses of GyrA_1-MBP and GyrB_1-MBP by sodium dodecyl sulfate-polyacrylamide gel electrophoresis were approximately 137 and 134 kDa, respectively. Reconstituted proteins showed ATP- and Mg2+-dependent supercoiling activity when GyrA_1-MBP and GyrB_1-MBP were used simultaneously (Fig. 1) but not decatenation (data not shown). Accordingly, it was suggested that gyrA_1 and gyrB_1 were the gyrA and gyrB genes, respectively.
TABLE 1.
Oligonucleotide primers used to amplify the DNA gyrase genes from C. pneumoniae TW-183 for protein purification
| Primer | Nucleotide sequencea (5′-3′) | Position | Length (kbp) |
|---|---|---|---|
| CPGA-1 | AGTCATGCTTTGTCCATTAGGATA | 34 bp upstream of start codon of gyrA | 2.5 |
| CPGA-2 | CCTTTGCTAAAGAACTTTTGCCAGACCCTTCGCC | 26 bp downstream of stop codon of gyrA | |
| CPGA-3 | GGGGATCCTTCAATAAAGATGAAATTATAGTCCC | bp 4-29 of gyrA | 2.5 |
| CPGA-4 | CCTTTGCTAAAGAAGCTTTGCCAGACCCTTCGCC | 26 bp downstream of stop codon of gyrA | |
| CPGB-1 | TCACGTGCAGATTAGGGAAATACAGT | 18 bp upstream of start codon of gyrB | 2.4 |
| CPGB-2 | TTTCATCTTTATTGAACATAGGGATTGTG | 6 bp downstream of stop codon of gyrB | |
| CPGB-3 | GGGGATCCGACCCAAAAGAAAAAAATTACGATGC | bp 4-29 of gyrB | 2.4 |
| CPGB-4 | AACTGCAGTTTCATCTTTATTGAACATAGGGATTGTG | 6 bp downstream of stop codon of gyrB |
The region engineered restriction enzyme (BamHI, HindIII, or PstI) sites are in boldface.
FIG. 1.
Enzymatic activities of purified recombinant DNA gyrase of C. pneumoniae TW-183. Lane 1, GyrA-MBP (4 U) and GyrB-MBP (4 U) without ATP; lane 2, GyrA-MBP (4 U) and GyrB-MBP (4 U) without Mg2+; lane 3, GyrA-MBP (8 U) and GyrB-MBP (8 U); lane 4, GyrA-MBP (4 U) and GyrB-MBP (4 U); lane 5, GyrA-MBP (8 U) and GyrB-MBP (3/2 U); lane 6, GyrA-MBP (3/2 U) and GyrB-MBP (8 U); lane 7, GyrA-MBP (2 U) and GyrB-MBP (2 U); lane 8, GyrA-MBP (1 U) and GyrB-MBP (1 U).
The following quinolones were employed in this study: garenoxacin, sparfloxacin, moxifloxacin, gatifloxacin, and levofloxacin. Garenoxacin, gatifloxacin, and moxifloxacin were synthesized in Research Laboratories, Toyama Chemical Co., Ltd., Toyama, Japan. Sparfloxacin and levofloxacin were purchased from their respective manufacturers. Susceptibility testing of C. pneumoniae TW-183 was performed by using a cell culture with HEp-2 cells grown in 24-well plates according to the method of the Japan Society of Chemotherapy (12). The IC50s of quinolones in a DNA-supercoiling assay were determined by using recombinant GyrA and GyrB of C. pneumoniae TW-183. The supercoiling activity of DNA gyrase was measured in a 20-μl solution containing 2 U of reconstituted GyrA-MBP and GyrB-MBP, drug solution, 50 mM Tris-HCl (pH 8.0), 5 mM MgCl2, 20 mM KCl, 5 mM dithiothreitol, 5 mM ATP, 350 mM potassium glutamate, 5 μg of bovine serum albumin, and 0.05 μg of relaxed pBR322 plasmid DNA. After incubation at 37°C for 1 h, reactions were terminated by the addition of a dye mixture, and then the products were analyzed by electrophoresis in 1% agarose. Gels were stained with ethidium bromide following electrophoresis. One unit of supercoiling activity was defined as the amount of GyrA-MBP and GyrB-MBP proteins that produces half-maximal supercoiling of 0.05 μg of relaxed pBR322 DNA in 1 h at 37°C, and the specific activities of GyrA-MBP and GyrB-MBP were determined as 1.7 × 105 and 6.9 × 103 U per mg, respectively. The DNA-supercoiling assay in the presence of an excess of one of the subunits is shown in Fig. 1. The IC50 for DNA gyrase supercoiling activity was defined as the concentration of drug that reduces the intensity of the most-supercoiled DNA band by one-half in the presence of enzyme proteins just sufficient for full supercoiling of the relaxed substrate DNA. The IC50s expressed are the averages of three trials.
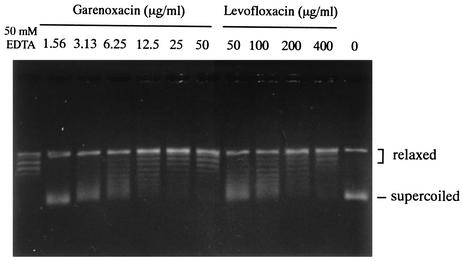
In the supercoiling inhibition assay, the effects of a range of garenoxacin and levofloxacin concentrations were determined (Fig. 2). The MICs for C. pneumoniae TW-183 and the IC50s for the DNA-supercoiling activity in three trials are shown in Table 2. The MICs of garenoxacin, sparfloxacin, moxifloxacin, gatifloxacin, and levofloxacin against C. pneumoniae TW-183 were 0.008, 0.016, 0.063, 0.125, and 0.25 μg/ml, respectively, and the mean IC50s were 10.1, 47.5, 39.6, 64.2, and 156.9 μg/ml, respectively. The MICs also roughly correlated with the IC50s (r2 = 0.91).
FIG. 2.
DNA-supercoiling activity of gyrase in the presence of garenoxacin and levofloxacin.
TABLE 2.
MIC and inhibitory activity against DNA gyrase
| Quinolone | MIC (μg/ml)a | IC50 (μg/ml)b |
|---|---|---|
| Garenoxacin | 0.008 | 10.1 ± 3.5 |
| Sparfloxacin | 0.016 | 47.5 ± 13.6 |
| Moxifloxacin | 0.063 | 39.6 ± 12.7 |
| Gatifloxacin | 0.125 | 64.2 ± 11.7 |
| Levofloxacin | 0.25 | 156.9 ± 35.6 |
MICs for C. pneumoniae TW-183 were determined according to the approved guidelines of the Japan Society of Chemotherapy.
IC50, concentration of drug that reduces the intensity of the most-supercoiled DNA band by one half in the presence of enzyme proteins just sufficient for full supercoiling of the relaxed pBR322 DNA. Values are means ± standard deviations.
The IC50 of sparfloxacin (47.5 μg/ml) was higher than that of moxifloxacin (39.6 μg/ml), although the MIC of sparfloxacin (0.016 μg/ml) was fourfold lower than that of moxifloxacin (0.063 μg/ml). These results might indicate that there are other factors that affect the antichlamydial activity of sparfloxacin, such as good permeability on the HEp-2 cell membrane and chlamydial outer membrane, less-efficient efflux in HEp-2 and chlamydia cells, and preferential action through topoisomerase IV rather than DNA gyrase. It has been reported that sparfloxacin preferentially inhibits DNA gyrase rather than topoisomerase IV in Chlamydia trachomatis (4). However, it has not been clarified whether the primary target of quinolones is DNA gyrase or topoisomerase IV in C. pneumoniae. More work will need to be done to resolve the enzymatic characterization of proteins encoded by the gyrA_2 and gyrB_2 genes (putative topoisomerase IV genes) of C. pneumoniae and to characterize the mutants selected with quinolones.
In summary, we obtained the recombinant DNA gyrase proteins of C. pneumoniae TW-183 and the IC50s against supercoiling activity of DNA gyrase roughly correlated with their MICs for the quinolones tested. Garenoxacin had the most-potent inhibitory activity against DNA gyrase.
Nucleotide sequence accession number.
The products of PCR amplification of gyrA_1 and gyrB_1 were entered into GenBank under accession no. AB103388.
Acknowledgments
Preliminary sequence data were obtained from the Chlamydia Genomes website (http://chlamydia-www.berkeley.edu:4231/). We thank to Brian Coffey for reviewing the manuscript.
REFERENCES
- 1.Barlett, J. G., R. F. Breiman, L. A. Mandell, and T. M. File, Jr. 1998. Community-acquired pneumonia in adults: guidelines for management. Clin. Infect. Dis. 26:811-838. [DOI] [PubMed] [Google Scholar]
- 2.Barlett, J. G., S. F. Dowell, L. A. Mandell, T. M. File, Jr., D. M. Musher, and M. J. Fine. 2000. Practice guidelines for the management of community-acquired pneumonia in adults. Clin. Infect. Dis. 31:347-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dalhoff, A., U. Petersen, and R. Endermann. 1996. In vitro activity of BAY 12-8039, a new 8-methoxyquinolone. Chemotherapy 42:410-425. [DOI] [PubMed] [Google Scholar]
- 4.Dessus-Babus, S., C. M. Bébéar, A. Charron, C. Bébéar, and B. de Barbeyrac. 1998. Sequencing of gyrase and topoisomerase IV quinolone-resistance-determining regions of Chlamydia trachomatis and characterization of quinolone-resistant mutants obtained in vitro. Antimicrob. Agents Chemother. 42:2474-2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donati, M., M. R. Fermepin, A. Olmo, L. D'Apote, and R. Cevenini. 1999. Comparative in-vitro activity of moxifloxacin, minocycline and azithromycin against Chlamydia spp. J. Antimicrob. Chemother. 43:825-827. [DOI] [PubMed] [Google Scholar]
- 6.Donati, M., G. M. Pollini, M. Sparacino, M. T. Fortugno, E. Laghi, and R. Cevenini. 2002. Comparative in vitro activity of garenoxacin against Chlamydia spp. J. Antimicrob. Chemother. 50:407-410. [DOI] [PubMed] [Google Scholar]
- 7.Ferrero, L., B. Cameron, B. Manse, D. Lagneaux, J. Crouzet, A. Famechon, and F. Blanche. 1994. Cloning and primary structure of Staphylococcus aureus DNA topoisomerase IV: a primary target of fluoroquinolones. Mol. Microbiol. 13:641-653. [DOI] [PubMed] [Google Scholar]
- 8.Fung-Tomc, J., B. Minassian, B. Kolek, T. Washo, E. Huczko, and D. Bonner. 2000. In vitro antibacterial spectrum of a new broad-spectrum 8-methoxy fluoroquinolone, gatifloxacin. J. Antimicrob. Chemother. 45:437-446. [DOI] [PubMed] [Google Scholar]
- 9.Fung-Tomc, J. C., B. Minassian, B. Kolek, E. Huczko, L. Aleksunes, T. Stickle, T. Washo, E. Gradelski, L. Valera, and D. P. Bonner. 2000. Antibacterial spectrum of a novel des-fluoro(6) quinolone, BMS-284756. Antimicrob. Agents Chemother. 44:3351-3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gellert, M. 1981. DNA topoisomerase. Annu. Rev. Biochem. 50:879-910. [DOI] [PubMed] [Google Scholar]
- 11.Grayston, J. T., L. A. Campbell, C.-C. Kuo, C. H. Mordhorst, P. Saikku, D. H. Thom, and S. P. Wang. 1990. A new respiratory tract pathogen: Chlamydia pneumoniae strain TWAR. J. Infect. Dis. 161:618-625. [DOI] [PubMed] [Google Scholar]
- 12.Japan Society of Chemotherapy. 1992. Method for in vitro determination of chlamydial susceptibility (minimum inhibitory concentration; MIC) to antimicrobial agents. Chemotherapy 40:308-314. [Google Scholar]
- 13.Kuo, C.-C., L. A. Jackson, L. A. Campbell, and J. T. Grayston. 1995. Chlamydia pneumoniae (TWAR). Clin. Microbiol. Rev. 8:451-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malay, S., P. M. Roblin, T. Reznik, A. Kutlin, and M. R. Hammerschlag. 2002. In vitro activities of BMS-284756 against Chlamydia trachomatis and recent clinical isolates of Chlamydia pneumoniae. Antimicrob. Agents Chemother. 46:517-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miyashita, N., Y. Niki, T. Kishimoto, M. Nakajima, and T. Matsushima. 1997. In vitro and in vivo activities of AM-1155, a new fluoroquinolone, against Chlamydia spp. Antimicrob. Agents Chemother. 41:1331-1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roblin, P. M., and M. R. Hammerschlag. 1998. In vitro activities of a new 8-methoxyquinolone, BAY12-8039, against Chlamydia pneumoniae. Antimicrob. Agents Chemother. 42:951-952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roblin, P. M., and M. R. Hammerschlag. 1999. In-vitro activity of gatifloxacin against Chlamydia trachomatis and Chlamydia pneumoniae. J. Antimicrob. Chemother. 44:549-551. [DOI] [PubMed] [Google Scholar]
- 18.Takahata, M., J. Mitsuyama, Y. Yamashiro, M. Yonezawa, H. Araki, Y. Todo, S. Minami, Y. Watanabe, and H. Narita. 1999. In vitro and in vivo antimicrobial activities of T-3811ME, a novel des-F(6)-quinolone. Antimicrob. Agents Chemother. 43:1077-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tanaka, M., Y. Onodera, Y. Uchida, K. Sato, and I. Hayakawa. 1997. Inhibitory activities of quinolones against DNA gyrase and topoisomerase IV purified from Staphylococcus aureus. Antimicrob. Agents Chemother. 41:2362-2366. [DOI] [PMC free article] [PubMed] [Google Scholar]