Abstract
To define the role of galectin-3 in breast cancer progression, we have used a novel three-dimensional co-culture system that recapitulates in vivo reciprocal functional breast epithelial-endothelial cell-cell and cell-matrix interactions, and examined the expression of galectin-3 mRNA and protein in human breast tumors and xenografts. Galectin-3 is required for the stabilization of epithelial-endothelial interaction networks because immunoneutralization with galectin-3 antibodies abolishes the interactions in a dose-dependent manner. Co-culture of epithelial cells with endothelial cells results in increase in levels of secreted galectin-3 and presence of proteolytically processed form of galectin-3 in the conditioned media. In contrast, intracellular galectin-3 predominantly exists in the intact form. This difference in sensitivity to proteolytic processing of secreted versus intracellular galectin-3 probably arises from differences in accessibility of protease-sensitive sites, levels, and/or type of activated protease(s), and may be indicative of different functional roles for intact and processed galectin-3. To determine whether the proteolytically cleaved galectin-3 retains its ability to bind to endothelial cells, binding assays were performed with the full-length and matrix metallopeoteinase-2-cleaved recombinant galectin-3. Although a dose-dependent increase in binding to human umbilical vein endothelial cells was observed with both full-length and cleaved galectin-3, proteolytically cleaved galectin-3 displayed ∼20-fold higher affinity for human umbilical vein endothelial cells as compared to the full-length protein. Examination of galectin-3 expression in breast tumors and xenografts revealed elevated levels of galectin-3 mRNA and protein in the luminal epithelial cells of normal and benign ducts, down-regulation in early grades of ductal carcinoma in situ (DCIS), and re-expression in peripheral tumor cells as DCIS lesions progressed to comedo-DCIS and invasive carcinomas. These data suggest that galectin-3 expression is associated with specific morphological precursor subtypes of breast cancer and undergoes a transitional shift in expression from luminal to peripheral cells as tumors progressed to comedo-DCIS or invasive carcinomas. Such a localized expression of galectin-3 in cancer cells proximal to the stroma could lead to increased invasive potential by inducing novel or better interactions with the stromal counterparts.
Galectins are a family of nonintegrin β-galactoside-binding lectins with related amino acid sequences.1 Galectin-3 formerly known as CBP35, Mac-2, and εBP, because of its affinity for IgE1 and HLB312 and because of its affinity for laminin is a 31 kd-galactoside-binding lectin and a member of the galectin family. Galectin-3 is widely expressed and secreted by myeloid and epithelioid cells3,4 and binds polylactosamine glycans.5 The lectin is associated with the plasma membrane and is secreted in the extracellular space6 where it presumably mediates cell-cell and cell-matrix interactions through its ability to bind to a variety of lactosamine-containing glycoconjugates.7,8 Galectin-3 is composed of an amino terminal half containing Gly-X-Tyr tandem repeats that are characteristic of collagens and a carboxyl terminal half containing the carbohydrate binding domain.1 The collagen-like N-terminal domain renders it susceptible to cleavage by matrix metalloproteinase (MMP)-2 or MMP-9 resulting in cleaved product size of ∼22 kd.9,10
Although the precise role of galectin-3 remains to be determined, several studies including ours have shown that expression of galectin-3 is positively correlated with the metastatic potential of several tumorigenic cell lines.11 However, the generality of these findings in relation to epithelial cell-derived human tumors is not clear as increases and decreases in galectin-3 have been reported during malignant progression of several cancers.11 In human colorectal carcinoma, galectin-3 has been reported to increase12 or decrease13,14 with progression to the metastatic state. Similarly, expression of galectin-3 is down-regulated in prostate,15 ovarian,16 and breast13,17 cancers.
We have previously reported that galectin-3 can induce endothelial cell morphogenesis in vitro and angiogenesis in vivo.18 Binding of galectin-3 to the endothelial cell surface is dependent on its carbohydrate domain as binding is specifically inhibited by the competitive disaccharide lactose or modified citrus pectin (MCP).18 Here, to further characterize the role of galectin-3 in endothelial morphogenesis and function, we have used a novel three-dimensional co-culture system of in vitro angiogenesis that permits reciprocal functional epithelial-endothelial cell-cell and cell-matrix interactions.19 Our results show that galectin-3 is important for stabilization of epithelial-endothelial interaction networks as immunoneutralization with galectin-3 antibodies specifically abolishes these interactions. Co-culture of epithelial cells with endothelial cells results in increase in levels of secreted galectin-3 and presence of proteolytically processed form of galectin-3 in the conditioned media. Binding assays performed with full-length and MMP-2-cleaved recombinant galectin-3 proteins showed that the proteolytically cleaved galectin-3 displays 20-fold greater affinity for human umbilical vein endothelial cells (HUVECs) as compared to the full-length protein. In situ hybridization and immunohistochemical analyses of galectin-3 mRNA and protein, respectively, showed that breast epithelium is the major source of galectin-3. Expression and distribution of galectin-3 mRNA and protein were also examined in cancerous breast tumors and in premalignant and comedo-ductal carcinoma in situ (DCIS) xenografts to further evaluate influence of galectin-3 on breast tumor growth and progression. Our findings show a switch in expression and distribution of galectin-3 from the luminal epithelium toward the periphery. These data suggest that such a transitional shift in galectin-3 expression, which is coincident with breast cancer progression, may facilitate novel galectin-3-mediated stromal-epithelial interactions that are probably required for invasion and metastatic progression.
Materials and Methods
Cell Lines and Xenografts
These studies used the following cell lines: MCF10AT1, MCF10AT1-EIII8, and MCF10DCIS.com cells. MCF10AT1 cells are T24 Ha-ras-transformed cells derived from normal-behaving MCF10A human breast epithelial cells and form persistent premalignant lesions in immune-deficient mice, whereas MCF10A cells do not grow in nude mice.20 Lesions formed by MCF10AT1 cells are composed of a heterogeneous spectrum of ductular tissues with a range of morphology that includes mild to moderate hyperplasia, atypical hyperplasia, DCIS, moderately differentiated carcinoma, undifferentiated carcinoma, as well as histologically normal ducts.21 MCF10AT1-EIII8 (referred to as EIII8) cells are premalignant epithelial cells that were derived from lesions of MCF10AT1 cells arising in 17-β-estradiol-supplemented animals and respond to estradiol with increased growth in vitro19 and in vivo.22,23 The MCF10DCIS.com cell line (referred as DCIS.com) was cloned from a cell culture derived from a MCF10AT xenograft after two successive trochar passages.24 Injection of DCIS.com cells into nude mice results in lesions that are predominantly of the comedo-DCIS subtype.24
MCF10AT1 and EIII8 cells were maintained in Dulbecco’s modified Eagle’s medium-F12 medium supplemented with 0.1 μg/ml cholera toxin, 0.02 μg/ml epidermal growth factor, 10 μg/ml insulin, 0.5 μg/ml hydrocortisone, 100 U/ml penicillin, 100 μg/ml streptomycin, and 5% horse serum.19 DCIS.com cells were maintained in Dulbecco’s modified Eagle’s medium-F12 medium supplemented with 5% horse serum.24 MCF10AT1 and DCIS.com xenografts were generated by injecting 1 × 107 MCF10AT122 or DCIS.com24 cells subcutaneously at orthotopic sites of female nude mice and lesions harvested at 100 (MCF10AT1), 22, 40, or 61 (DCIS.com) days. HUVECs (purchased from American Type Tissue Culture Collection, Rockville, MD) at passage 13 were maintained in endothelial serum-free basal growth medium (SFM; Life Technologies, Inc., Grand Island, NY) supplemented with 10 ng/ml of epidermal growth factor, 20 ng/ml of basic fibroblast growth factor, and 10 μg/ml of fibronectin.19 Cultures were maintained in a humidified atmosphere containing 5% CO2 in air.
Three-Dimensional Culture of EIII8-HUVECs (Heterotypic) on Reconstituted Basement Membrane
To understand the role of galectin-3 in mediation of functional heterotypic interactions between breast epithelial and endothelial cells, we have used a three-dimensional co-culture system that allows establishment of reciprocal and productive interactions between EIII8 and HUVECs.19 EIII8 cells (50 × 103) were mixed with an equal number of HUVECs and seeded onto chamber slides coated with reconstituted basement membrane (Matrigel) in SFM as described by Shekhar and colleagues.19 Twenty-four hours after seeding, 0.5 or 1 μg of rabbit polyclonal (HL-31) or rat monoclonal (TIB-166) anti-galectin-3 antibody was added. Control cultures were either untreated or treated with similar amounts of the corresponding normal rabbit or rat IgG. Effects of antibody or normal IgGs on EIII8-HUVEC interaction and growth were measured on day 5 by counting trypan blue-excluded cells from dispase-treated cultures.19
Preparation of Conditioned Media
EIII8 cells (50 × 103) were seeded alone or mixed with an equivalent number of HUVECs on Matrigel in SFM medium as described before. Cells were incubated for 6 hours to attach, and media were replaced with fresh SFM. Cultures were also treated with 0.01 or 0.1% of pH MCP or control citrus pectin (CP; Sigma Chemical Co., St. Louis, MO), prepared as described by Nangia-Makker and colleagues.25 Whereas CP is a noncompetitive polysaccharide and fails to influence galectin-3 interactions, MCP is a competitive polysaccharide and inhibits interactions between galectin-3 and its putative cell surface receptor.25 Culture media from homotypic and heterotypic cultures were removed on day 5, centrifuged to remove debris, and used for Western analysis. After removal of media, matrix containing the three-dimensional structures was either solubilized for sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blot analysis or fixed in buffered formalin for evaluation of galectin-3 mRNA and protein.
Western Blot Analysis
Steady-state levels of intact and cleaved galectin-3 proteins secreted into conditioned media and those present in EIII8-HUVEC cell lysates of untreated, MCP-treated, and CP-treated cultures at day 5 were determined by subjecting 20 or 50 μg of total protein, respectively, to sodium dodecyl sulfate-polyacrylamide gel electrophoresis on 7 to 15% gradient polyacrylamide gels and Western blot analysis. HL-31 polyclonal antibody recognizes both the intact 31-kd and cleaved galectin-3 proteins, whereas TIB-166 monoclonal antibody recognizes an epitope at the NH2-terminus and thus fails to recognize cleaved galectin-3. Blots containing cell lysates were stripped and reprobed with β-actin antibody for normalizing protein loading, whereas gels containing conditioned media samples were stained with Coomassie brilliant blue R250. Immunoreactive bands were visualized by chemiluminescence, and band intensities were quantitated with a model 300A densitometer (Molecular Dynamics, Sunnyvale, CA).
Proteolytic Cleavage of Galectin-3
Recombinant galectin-3 was prepared as described by Nangia-Makker and colleagues.18 Recombinant pro-MMP-2 (a gift from Dr. R. Fridman, Wayne State University, Detroit, MI), was activated as described earlier9 and used to cleave galectin-3 at enzyme:substrate ratio of 1:20 (weight:weight) for 30 minutes at 37°C.9 The reaction was stopped by the addition of 10 mmol/L ethylenediaminetetraacetic acid and the cleaved protein was purified by filtration through an Ultra Free-MC 10K NMWL filter unit (Millipore Corp., Bedford, MA).
Galectin-3 Binding Assay
To determine the binding affinities of full-length and cleaved galectin-3 to endothelial cells, full-length and cleaved recombinant galectin-3 proteins were iodinated in the presence of chloramine T, as described by Nangia-Makker and colleagues.18 Briefly, 1 μg of protein was incubated with 250 μCi of Na125I in the presence of 40 μg of chloramine T and 100 μl of H2O on ice for 1 minute. The reaction was stopped by the addition of 20 μl of 1 mol/L potassium iodide. To remove the unbound labeled iodine, the reaction mixture was spun through Ultra Free-CL centrifugal filters (Millipore) precoated with 0.1% bovine serum albumin in phosphate-buffered saline (PBS). Radiolabeled galectin-3 was resuspended in 50 μl of 0.1% bovine serum albumin in PBS. One day before the assay, HUVECs were plated at a density of 5 × 104 cells per well in 24-well plates. After washing, the cells were blocked with 0.1% bovine serum albumin in PBS for 30 minutes and incubated with 1 to 6 ng iodinated full-length or cleaved galectin-3 per well in the presence or absence of 50 mmol/L lactose, a specific disaccharide competitive inhibitor of galectin-3. After 2 hours of incubation at 4°C with constant shaking, the medium was removed, cells washed and lysed with 1 mol/L NaOH, and bound radioactivity was measured with a gamma counter.
Preparation of Digoxigenin-Labeled Galectin-3 Probes
A 353-bp fragment of human galectin-3 was amplified by polymerase chain reaction using primers specific for human galectin-3 corresponding to nucleotides 382 to 734 (GenBank Accession no. M57710) and subcloned into pCRII TA-cloning vector (Invitrogen, Carlsbad, CA). Plasmid DNAs were isolated from bacterial colonies and screened for orientation and sequence confirmation. Digoxigenin-labeled riboprobes specific for the sense or anti-sense strands of galectin-3 were synthesized from appropriately linearized plasmids using the DIG-RNA labeling kit (Roche, Indianapolis, IN) and the appropriate RNA polymerase (Promega Corp., Madison, WI) plus 10 U of recombinant RNase inhibitor. The specificity of probes was verified by hybridization to originating plasmids and negative controls.
In Situ Hybridization Analysis
Formalin-fixed paraffin-embedded sections prepared from EIII8-HUVEC three-dimensional co-cultures, MCF10AT1- and MCF10DCIS.com-derived xenografts, and human breast tumors were deparaffinized and used for detection of galectin-3 mRNA and galectin-3 protein by in situ hybridization and immunohistochemical analysis (see below), respectively. Two of the human breast tumors used for the study were ER+/PgR+/Her2-neu− and three were ER−/PgR−/Her2-neu+. In situ hybridization was performed as previously reported using digoxigenin-labeled cRNA probes.26,27 Tissue sections were processed consecutively, one slide being incubated with the sense probe and the other with the anti-sense probe. Four-μm-thick tissue sections were deparaffinized, rehydrated, and incubated in 0.2 N HCl for 20 minutes. Slides were rinsed with 2× standard saline citrate (SSC), and tissue sections were permeabilized with proteinase K at a concentration of 5 μg/ml for 15 minutes at 37°C. After fixation with 4% paraformaldehyde and washing in 2× SSC, the sections were prehybrizided for 2 hours at 42°C in buffer containing 5× SSC/50% formamide/5× Denhardt’s reagent and 250 μg/ml of yeast tRNA. Hybridization was performed overnight at the same temperature in 5× SSC/50% formamide/5× Denhardt’s reagent containing 500 μg/ml of yeast tRNA and 10% dextran sulfate. The final concentration of digoxigenin-labeled cRNA galectin-3 probes (sense and anti-sense) was ∼2 ng/μl. After hybridization, excess probe was removed by washing in 2× SSC and treatment with RNase (100 U/ml RNase T1 and 0.2 μg/ml DNase-free RNase A) at 37°C for 15 minutes. Slides were washed at 42°C in 2× SSC (15 minutes) and twice in 0.5× SSC (15 minutes each). The sections were incubated with an anti-digoxigenin antibody conjugated with horseradish peroxidase and color developed using the Vectastain ABC kit (Vector Laboratories, Burlingame, CA).
Immunohistochemistry
Galectin-3 protein was localized on corresponding deparaffinized sections of EIII8-HUVEC co-cultures, MCF10AT1- and MCF10DCIS.com-derived lesions, and human breast tumor tissues by immunohistochemistry using monoclonal TIB-166 galectin-3 antibody. The identity and functionality of endothelial and epithelial cells in the EIII8-HUVEC three-dimensional co-cultures were established by immunostaining the sections with cd31, factor VIII (endothelial-specific), or pan-cytokeratin (epithelial-specific) antibodies. Slides were overlaid with avidin-biotin-conjugated goat anti-mouse or anti-rat IgG, incubated in peroxidase substrate solution (3,3′-diaminobenzidine; VectaStain ABC kit), and counterstained with hematoxylin.
Statistical Analysis
Specific differences among treatments were examined using the Student’s t-test, and P ≤ 0.005 was accepted to be statistically significant.
Results
Galectin-3 Is Required for Maintenance of Stable Breast Epithelial-Endothelial Heterotypic Interactions
Using a novel three-dimensional co-culture system of premalignant EIII8 breast epithelial and HUVECs, we have previously reported the presence of an intimate interaction between epithelial and endothelial cell populations that is mutually beneficial as observed by the co-localization of branching ductal-alveolar outgrowths with endothelial cell-enriched regions.19 To evaluate the functional role of galectin-3 in mediation of stable heterotypic interactions between premalignant breast epithelial cells and endothelial cells, we tested the effects of galectin-3 antibodies on EIII8-HUVEC co-cultures. Results from Figure 1 show that whereas addition of the corresponding rat or rabbit normal IgGs failed to significantly influence growth and organization of EIII8-HUVEC co-cultures as compared to control untreated co-cultures, inclusion of equivalent amounts of polyclonal HL-31 (Figure 1a; A, B, and D) or monoclonal TIB-166 (Figure 1a, A and C) anti-galectin-3 antibodies produced a dose-dependent inhibition of growth and loss of organization in EIII8-HUVEC co-cultures. Addition of 0.5 or 1 μg of HL-31 anti-galectin-3 antibody caused ∼2- or 3.8-fold decrease in growth (P < 0.001), respectively, and complete loss of organization when compared to control untreated or normal IgG-treated cultures (Figure 1, a and b). Similar addition of 0.5 or 1 μg of the monoclonal TIB166 anti-galectin-3 antibody resulted in twofold to threefold decrease in growth (P < 0.005), respectively, and loss of organization as compared to control cultures (Figure 1, a and b). These data indicate that galectin-3 plays an important role in stabilization of heterotypic interactions between premalignant EIII8 and endothelial cells.
Figure 1.
Regulation of three-dimensional growth of EIII8-HUVEC co-cultures by galectin-3. Control cultures were untreated (control) or 24 hours after seeding treated with 0.5 or 1.0 μg of HL-31 or TIB166, or 1 μg of corresponding rat or rabbit nonimmune IgG. a: Phase-contrast morphology of heterotypic three-dimensional EIII8-HUVEC co-cultures. A and B: Co-cultures that were untreated or treated with 1.0 μg of normal rabbit IgG, respectively. C and D: Co-cultures that were treated with 1.0 μg of TIB166 or HL-31 galectin-3 antibody, respectively. Arrows indicate ductal alveolar outgrowths that co-localize with endothelial enriched regions. b: Cultures were treated with dispase to solubilize Matrigel- and trypan blue-excluded cells were counted by hemocytometer. Results obtained from three independent experiments performed in triplicate are expressed as mean ± SE. *, P < 0.001 and **, P < 0.005 are amounts of antibody that inhibited cell number significantly over corresponding control. Scale bar, 40 μm (a).
Co-Culture with Endothelial Cells Enhances Galectin-3 Secretion and Specific Proteolytic Cleavage of Secreted Galectin-3
Regulation by MCP and CP
Galectin-3 protein levels and its proteolytic processing in homotypic (EIII8) and heterotypic (EIII8-HUVEC) three-dimensional cultures treated with CP (a noncompetitive polysaccharide) or MCP (a competitive polysaccharide inhibitor) were determined by Western blot analysis of conditioned media and cell lysates. Immunoblot analysis of conditioned media with polyclonal HL-31 anti-galectin-3 antibody that is reactive to both intact and cleaved galectin-3 showed the presence of ∼31- and 27-kd bands, the latter indicative of proteolytic processing of galectin-3 (Figure 2A). That the 27-kd band represents proteolytic clipping of galectin-3 was confirmed by Western analysis with TIB-166 monoclonal antibody (reactive to only intact molecule), which revealed only the intact 31-kd galectin-3 protein (Figure 2B). Because MCP has been demonstrated to inhibit binding of galectin-3 to HUVECs,25 we evaluated the effects of CP and MCP on secretion and processing of galectin-3 in EIII8-HUVEC co-cultures. Consistent with previous data, results of Figure 2A show a 50 to 70% decrease in levels of total galectin-3 secreted in EIII8-HUVEC co-cultures treated with MCP as compared to those treated with CP or untreated cultures, respectively. The differences in levels of secreted galectin-3 observed between CP- and MCP-treated cultures are not because of loading differences because similar levels of Coomassie blue-stainable protein bands were present in all samples (Figure 2C). It is interesting to note that no differences in proteolytic processing of secreted galectin-3 were observed between control or treated EIII8-HUVECs, or homotypic EIII8 cultures. Analysis of corresponding lysates with HL-31 antibodies showed the presence of similar levels of intact 31-kd galectin-3 in CP- and MCP-treated cultures. Interestingly, the 31-kd protein was the major product in the cells and the smaller 27-kd product noted in the conditioned media was undetectable (Figure 2, A’ and A“). Longer exposure of the blots revealed the presence of a minor band at ∼22 kd in cultures treated with CP but were absent in MCP-treated cultures (Figure 2A’). Immunoblotting of cell lysates with TIB-166 antibody confirmed the presence of similar levels of the intact form of galectin-3 (Figure 2B’) relative to β-actin levels (Figure 2C’) in homotypic EIII8 and treated EIII8-HUVEC cultures. These results suggest that galectin-3 is primarily protected from proteolytic processing in the cells as opposed to the secreted protein that is highly sensitive to proteolytic cleavage at a site that is distinct from that of intracellular galectin-3.
Figure 2.
Three-dimensional EIII8-HUVEC co-cultures show higher steady-state levels of secreted galectin-3 and differences in protease sensitivity between secreted and intracellular galectin-3. Proteins, 20 or 50 μg, present in culture media and corresponding matrix fractions, respectively, at day 5 were analyzed by Western blotting. A and B: Galectin-3 levels in conditioned media of EIII8 or EIII8-HUVEC co-cultures that were untreated (control) or treated with 0.01 or 0.1% CP or MCP. A’ and B’: Galectin-3 detected in lysates of untreated, CP- or MCP-treated EIII8-HUVEC co-cultures, and EIII8 homotypic cultures. A and A’: Galectin-3 detected with polyclonal HL-31. B and B’: Detection with monoclonal TIB-166 galectin-3 antibody. A“: Short exposure of A’. C: Coomassie blue R250 staining of proteins in conditioned media; C’: Steady-state levels of β-actin in lysate samples. Arrows indicate positions of 62 (A)-, 27 (A)-, and 22 (A’)-kd bands.
Cleaved Galectin-3 Binds More Efficiently to Endothelial Cells than Full-Length Galectin-3
To determine whether the proteolytically cleaved galectin-3 retains its ability to bind to endothelial cells, binding assays were performed with various amounts of full-length and MMP-2-cleaved recombinant galectin-3 in the presence and absence of competitive disaccharide inhibitor lactose. A dose-dependent increase in binding to HUVECs was observed with both full-length and cleaved galectin-3 that reached saturation at 1.5 ng/5 × 104 cells (Figure 3). However, cleaved galectin-3 displayed ∼20 times greater affinity for HUVECs as compared to the full-length protein. Binding of both full-length and cleaved galectin-3 to endothelial cells involved carbohydrate domain as binding was competitively inhibited by lactose (Figure 3).
Figure 3.
MMP-2 cleaved galectin-3 displays greater affinity for endothelial cells. HUVECs were incubated with indicated amounts of radioiodinated full-length or MMP-2-cleaved recombinant galectin-3 protein in the absence or presence of 50 mmol/L lactose. A and B: Full-length galectin-3 in the absence (A) or presence (B) of lactose. C and D: Cleaved galectin-3 in the absence (C) or presence (D) of lactose.
Breast Epithelium Is the Major Source of Galectin-3
Immunocytochemical staining of formalin-fixed paraffin-embedded sections of EIII8-HUVEC co-cultures with cd31, Factor VIII, pan-cytokeratin, and proliferating cell nuclear antigen antibodies revealed the presence of functional endothelial cells juxtaposed to a proliferative epithelium (Figure 4). Immunostaining with TIB-166 galectin-3 antibody showed the presence of strong galectin-3-specific immunoreactivity exclusively in the epithelial compartment with discernibly higher reactivities in epithelial cells that are adjacent to endothelial cells (Figure 4, G and H).
Figure 4.
Formalin-fixed, paraffin-embedded sections of EIII8-HUVEC three-dimensional co-cultures were either stained with H&E (A and B) or with antibodies to cd31 (C), factor VIII (D), cytokeratins (E), proliferating cell nuclear antigen (F), or galectin-3 monoclonal TIB-166 antibody (G and H). Note the widespread immunoreactivity to cytokeratins in the branching end buds as opposed to the localized cd31 and factor VIII8-expressing endothelial cells. Also note the presence of numerous proliferating cells in branching end buds invading into the surrounding ECM. Note that galectin-3 staining is exclusively localized in epithelial buds with higher TIB-166 immunoreactivities in epithelial cells that are adjacent to endothelial cells (arrows, G and H). Original magnifications: ×25 (A–F); ×40 (H); ×10 (G).
In situ hybridization analysis of galectin-3 mRNA signals in serial sections of EIII8-HUVEC co-cultures showed the presence of galectin-3 mRNA signals at varying intensities throughout the proliferating epithelium but not in the endothelial compartment (Figure 5A). The in situ hybridization signals are specific to galectin-3 mRNA expression because it was detected only with the anti-sense probe. Hybridization with the sense probe failed to produce an in situ hybridization signal (Figure 5B) and pretreatment of slides with RNase abolished the hybridization signals. These data suggest that galectin-3 protein regulating heterotypic EIII8-HUVEC interactions, growth, and endothelial cell function is derived exclusively from galectin-3 messages transcribed in the epithelial compartment.
Figure 5.
Galectin-3 mRNA synthesis occurs only in the epithelial compartment of EIII8-HUVEC co-cultures. A and B: In situ hybridization analysis with anti-sense and sense DIG-labeled galectin-3 RNA probes, respectively. Note the presence of galectin-3 mRNA signals detected only with anti-sense galectin-3 probe and its exclusive presence in the epithelial compartment. Original magnifications, ×40.
Alterations in Galectin-3 Expression and Distribution in Premalignant Versus Comedo-DCIS Versus Invasive Breast Carcinomas Implicate Potential Differences in Galectin-3 Function during Progression
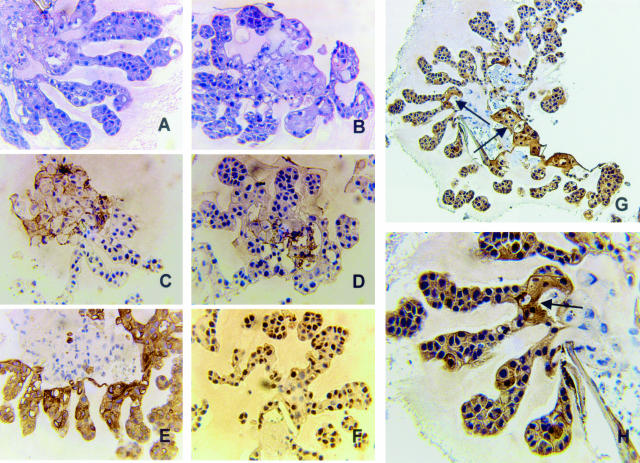
In situ hybridization analysis was performed to determine the site and levels of galectin-3 mRNA expression in premalignant (MCF10AT1 xenografts), comedo-DCIS (DCIS.com xenografts), and breast carcinomas. Intense signals for galectin-3 mRNAs were localized in luminal epithelial cells in normal and hyperplastic ducts of premalignant MCF10AT1 xenografts (Figure 6A) and in normal and hyperplastic areas of cancerous breast tissues (Figure 7, A and B). However, whereas only trace or negligible galectin-3 mRNA signals were detected in early comedo-DCIS, ie, before formation of central comedo-necrotic core (Figure 8A), advanced DCIS lesions with conspicuous central comedo necrosis exhibited moderate galectin-3 mRNA signals that were dispersed away from the comedo core, toward the periphery (Figure 8; B to F). Moderate to strong galectin-3 mRNA signals were also observed in fibroblasts of the extracellular matrix in and around the galactin-3-expressing infiltrating cancer cells in human breast tissues (Figure 7C).
Figure 6.
Galectin-3 mRNA and protein expression in premalignant lesions produced by MCF10AT1 xenografts. A and B: In situ hybridization with DIG-labeled anti-sense and sense galectin-3 RNA probes, respectively. C: Galectin-3 immunoreactivity to TIB-166 anti-galectin-3 antibody. Note the presence of strong galectin-3 protein expression in normal (thin arrow) and hyperplastic (block arrow) ducts. Also, note the presence of galectin-3 staining in the lumens of normal and hyperplastic ducts. Original magnifications, ×4.
Figure 7.
Galectin-3 mRNA and protein expression in human breast tumors. A–C and A’–C’: In situ hybridization with anti-sense and sense galectin-3 RNA probes, respectively. A”–C”: Galectin-3 protein staining with TIB-166 anti-galectin-3 antibody. A, A’, and A”: Normal areas of breast cancer tissue. B, B’, and B”: Papillary hyperplasias. C, C’, and C”: Infiltrating carcinoma cells. Note the mRNA signals detected only with anti-sense galectin-3 probe (A–C). Also note the presence of intense galectin-3 protein immunoreactivity in the lumens of normal and hyperplastic ducts and absence of staining in the adjacent stroma (A” and B”). In contrast, note the presence of very strong galectin-3 protein staining in invasive cancer cells and in fibroblasts and extracellular matrix surrounding them (C”). Original magnifications: ×25 (A, B, A’, B’, A”, B”); ×4 (C, C’, C”).
Figure 8.
Galectin-3 mRNA and protein expression exhibit alterations that are coincident with progression to comedo-DCIS of DCIS.com-derived xenografts. A and D: DCIS lesions harvested at 20 days. B, C, and F: Lesions harvested at 60 days. E: Lesions harvested at 40 days. A–C: In situ hybridization analysis with anti-sense galectin-3 RNA probe. D–F: Galectin-3 protein detected with TIB-166 galectin-3 antibody. Note the absence of detectable galectin-3 mRNA signals in early DCIS lesions, ie, before manifestation of comedo necrotic core (A). However, on progression to comedo-type, note the expression of galectin-3 mRNA in tumor cells away from the central necrotic core (B and C). Arrows in B and C indicate the comedo necrotic cores. Similarly, note that only very few tumor cells express galectin-3 protein in early DCIS lesions (D, arrow). By day 40, galectin-3 protein-staining peripheral cells are prevalent (E), and on manifestation of central comedo-necrosis, focally intense galectin-3-immunoreactive signals are observed in the tumor cells that are in close proximity to the stroma (F). Original magnifications: ×10 (A, C–E); ×4 (B, F).
The immunohistochemistry patterns of galectin-3 protein staining in normal, hyperplastic, comedo-DCIS, and invasive breast tumors were similar to the corresponding in situ hybridization patterns. Normal and hyperplastic ducts from human breast tissues and premalignant MCF10AT1 xenografts showed strong galectin-3 immunoreactivity in lumen and luminal epithelial cells (Figure 6C; Figure 7, “A and B”). Early grades of DCIS lesions (day 20), ie, before development of comedo necrosis, showed minimal and less prevalent galectin-3 immunoreactivity (Figure 8D). In contrast, DCIS lesions (mid-grade, harvested at 40 days) and advanced DCIS lesions (harvested at 60 days) with manifested central comedo necrosis displayed intense to focally intense galectin-3 immunoreactivity in cells situated at the periphery or proximal to the stromal microenvironment (Figure 8, E and F). Consistent with galectin-3 protein distribution in comedo-DCIS, a high-risk precursor subtype for progression, invasive breast carcinomas displayed strong galectin-3 immunoreactivity in the extracellular space around infiltrating tumor cells (Figure 7C“). The differential distribution patterns of galectin-3 observed in premalignant (six xenografts) versus DCIS-comedo (six xenografts) versus invasive breast carcinomas (five cases) were consistently seen in all of the samples examined. However, because the number of breast tumor specimens that were ER+/PgR+/Her2-neu− versus ER−/PgR−/Her2-neu+ was small, a statistical correlation between galectin-3 expression and receptor status could not be made. These data reveal a transitional shift in galectin-3 expression and distribution with breast cancer progression and suggest acquisition and/or assumption of novel galectin-3 mediated functional interactions with the stromal microenvironment that are probably necessary for progression.
Discussion
Galectin-3, a member of the β-galactoside-binding lectin family, has been implicated in several biological events and probably plays a role in tumor progression and metastasis by regulating homotypic and heterotypic cell-cell interactions and cell-matrix interactions. Galectin-3 has been positively and negatively correlated with tumor progression. For instance, expression of galectin-3 has been directly correlated with neoplastic potential and metastatic capacity in fibrosarcoma and melanoma cells,28 whereas galectin-3 levels were found to be down-regulated in advanced prostate15 and colon carcinomas14,29 when compared with their respective normal counterparts. Similarly, reduced expression of galectin-3 has been reported in advanced histological grades of breast cancer.13,17 These findings conflict with in vitro studies using MDA-MB-435 and BT-549 breast cancer cell lines in which a direct correlation between galectin-3 expression and metastatic and invasive potential was observed.30,31 Stable transfection of BT-549 (galectin-3 null) or MDA-MB-435 (galectin-3 expressing) cells with sense or anti-sense galectin-3, respectively, causes changes in tumorigenic phenotype that is commensurate with galectin-3 expression.30,31
Until now the majority of the studies have reported quantitative differences in galectin-3 protein expression between normal and cancerous tissues without ascribing alterations in expression to specific morphological subtypes that are precursors for breast cancer progression. To clarify the function of galectin-3 in breast cancer progression, we have characterized the role of galectin-3 in establishment and maintenance of stable functional heterotypic interactions between epithelial and endothelial cells using a three-dimensional co-culture system on reconstituted basement membrane,19 and performed detailed analysis of expression and distribution of galectin-3 mRNA and protein in human breast tissues, EIII8-HUVEC three-dimensional co-cultures, and xenografts that recapitulate morphological subtypes characteristic of human breast cancer evolution. Consistent with previous reports,13 our results show that normal and hyperplastic ducts express elevated levels of galectin-3 mRNA and protein in the luminal epithelial cells, and is down-regulated in early grades of DCIS. Interestingly, galectin-3 is re-expressed in the peripheral tumor cells as DCIS lesions evolve and/or progress to comedo-DCIS, the latter a precursor subtype conferring high risk for progression.32,33 This pattern of galectin-3 mRNA and protein expression in the peripheral tumor cells is maintained in invasive breast carcinomas. These data suggest that galectin-3 expression is not only associated with specific morphological precursor-subtypes of breast cancer but is also accompanied by a transitional shift in expression from luminal to peripheral epithelial cells that is coincident with acquisition of invasive potential. Such localized expression of galectin-3 in cancer cells proximal to the stroma34 could lead to an increase in invasive potential by inducing better interactions with the stromal counterparts. Our findings are in agreement with those of LeMarer and Hughes35 who have indicated that localized increase in threshold concentrations of galectin at the invasive sites is directly correlated with invasive capacity of a cell.
Up-regulation in galectin-3 could allow enhanced interactions with stromal cells and hence greater adhesion to target organ endothelial cells. To understand the role of galectin-3 in mediation of functional heterotypic interactions between breast epithelial and endothelial cells, we have used a three-dimensional co-culture system that allows establishment of reciprocal and productive interactions between EIII8 and HUVECs.19 Using this system, our data from galectin-3 immunoneutralization experiments have shown that galectin-3 is necessary for maintenance and stability of heterotypic interaction networks between EIII8 and HUVEC cells. Although galectin-3 is derived mainly from the epithelial compartment, it is interesting to note that co-culture of EIII8 cells with endothelial cells results in significant induction of secreted galectin-3 levels. Inclusion of MCP caused a significant decrease in the levels of secreted galectin-3. These results are in agreement with our previous studies that demonstrated the ability of MCP to effectively sequester galectin-3 and inhibit galectin-3-mediated functions such as homotypic tumor cell aggregation, galectin-3 binding by tumor endothelial cells, in vitro and in vivo tumor growth, and metastasis.25
Our findings also show that galectin-3 is protected from exogenous collagenases in the cells as majority of galectin-3 is found in its native 31-kd form in EIII8-HUVEC co-cultures. Galectin-3 secretion is probably slow in EIII8 cells and may account for the lower levels of secreted galectin-3 in EIII8 homotypic cultures. Interestingly, co-culture with endothelial cells results in a significant increase in levels of secreted 31-kd galectin-3, which is also reflected by the presence of detectable levels of a 62-kd galectin-3-immunoreactive band. Galectin-3 has been reported to exist as a monomeric protein at submicromolar concentrations and forms homodimers at high concentrations,36–39 thus suggesting that the 62-kd band may represent galectin-3 homodimers. After release, the secreted or soluble galectin-3 exists as 31- and 27-kd forms, the latter possibly a result of proteolytic processing at collagenase-sensitive cleavage site at amino acid residues Gly32-Ala33 (unpublished observation) with no immunoreactivity to TIB166 monoclonal galectin-3 antibody. Our previous studies have shown that a major breakdown product of galectin-3 cleavage in vitro by purified activated MMP-2 and MMP-9 is a 22-kd protein that ensues as a result of cleavage at Ala62-Tyr63.9 However, our present data indicate that secreted endogenous galectin-3 is insensitive to cleavage at Ala62-Tyr63, and that a site 5′ of this previously demonstrated MMP-2/MMP-9 sensitive site may be used. This difference in collagenase sensitivity observed in vitro with recombinant galectin-3 versus in vivo with endogenous galectin-3 probably arises from variations in accessibility of cleavage sites in vivo. Interestingly, cleavage at Ala62-Tyr63 occurs, albeit to a minor extent, inside the cells because only the 22-kd galectin-3 immunoreactive band is detected in the cell lysates. These data suggest that differences in proteolytic processing of secreted versus intracellular galectin-3 molecules probably arise from differences in accessibility of sensitive sites, levels, and/or type of activated protease(s). It is conceivable that the differentially processed galectin-3 molecules inside and outside the cells serve different functional roles because after initial cleavage the newly generated amino terminus is not further processed. It is intriguing to speculate that the 27-kd cleaved product is a result of proteolytic clipping by MMP-2 because we have previously demonstrated the presence of active MMP-2 in the conditioned media of EIII8-HUVEC co-cultures.19 Another interesting feature observed is that the ratios of secreted native 31-kd to cleaved 27-kd forms are maintained at constant levels, thus indicating that only a fixed proportion of secreted galectin-3 is accessible for proteolytic processing. The functional significance of cleaved galectin-3 has been recently proposed by John and colleagues.40 They suggested that after cleavage at the amino terminal end, galectin-3 loses its ability to dimerize but retains its ability to bind to glycoconjugates, thus competing with the intact galectin-3 for carbohydrate binding. Results from our binding assays using full-length and MMP-2-cleaved galectin-3 have demonstrated that cleaved galectin-3 binds more efficiently to HUVECs than intact galectin-3. These data are consistent with previous reports that cleavage of galectin-3 by metalloproteinases drastically improves its binding interactions with the β-galactoside containing laminin.41 Galectin-3 binding to HUVECs is competitively inhibited by disaccharide lactose; however, the inhibition was ∼50%. Although lactose has been demonstrated to specifically inhibit galectin-3 binding to its substrates, the extent of inhibition is variable. For example, lactose was demonstrated to completely inhibit galectin-3 binding to immobilized laminin42 or MCP,43 whereas in other studies lactose inhibited binding of galectin-3 to similar substrates by ∼50%.18,41 This variability in competition by lactose may be attributed to experimental conditions or to the possibility that galectin-3 may recognize other sugar residues that are not blocked by lactose. Regardless of variability of inhibition conferred by lactose on galectin-3/HUVEC-binding interactions, our data suggest that proteolytic removal of the amino terminal end that mediates galectin-3 homodimerization potentiates galectin-3 interactions with putative receptors on endothelial cells via carbohydrate recognition domains. Although we do not know the consequences of increased binding of cleaved galectin-3 to endothelial cells, based on our observations we speculate that it might play an important role in tumor invasion and angiogenesis.
In summary, our findings indicate that galectin-3 mRNA and protein expression is not only associated with specific morphological precursor-subtypes of breast cancer but also demonstrate alterations in expression from luminal to peripheral (proximal to stroma) epithelial cells that is coincident with acquisition of invasive potential. Our data from breast epithelial-endothelial co-culture experiments suggest that galectin-3 and its cleaved product play an important role in stabilization of epithelial-endothelial interactions. These data reinforce the importance of galectin-3 and proteolytically cleaved galectin-3 in stromal-epithelial interactions/angiogenesis/tumor invasion, and its utility as a marker for breast cancer progression and metastasis.
Footnotes
Address reprint requests to Dr. Malathy P.V. Shekhar, Breast Cancer Program, Room 2204, Prentis Building, Karmanos Cancer Institute, 110 East Warren Ave., Detroit, Michigan 48201. E-mail: shekharm@karmanos.org.
Supported by the United States Army Medical Research and Materiel Command (grants DAMD 17-02-I-0618 to M.P.V.S. and DAMD 17-00-I-0497 to A.R. and P.N.M.) and the National Institutes of Health (grant CA46120 to A.R.).
References
- Barondes SH, Cooper DN, Gitt MA, Leffler H. Galectins. Structure and function of a large family of animal lectins. J Biol Chem. 1994;269:20807–20810. [PubMed] [Google Scholar]
- Raz A, Carmi P, Raz T, Hogan V, Mohamed A, Wolman SR. Molecular cloning and chromosomal mapping of a human galactoside-binding protein. Cancer Res. 1991;51:2173–2178. [PubMed] [Google Scholar]
- Sato S, Burdett I, Hughes RC. Secretion of the baby hamster kidney 30-kDa galactose-binding lectin from polarized and nonpolarized cells: a pathway independent of the endoplasmic reticulum-Golgi complex. Exp Cell Res. 1993;207:8–18. doi: 10.1006/excr.1993.1157. [DOI] [PubMed] [Google Scholar]
- Hughes R. Mac-2: a versatile galactose-binding protein of mammalian tissues. Glycobiology. 1994;4:5–12. doi: 10.1093/glycob/4.1.5. [DOI] [PubMed] [Google Scholar]
- Sato S, Hughes RC. Binding specificity of a baby hamster kidney lectin for H type I and II chains, polylactosamine glycans, and appropriately glycosylated forms of laminin and fibronectin. J Biol Chem. 1992;267:6983–6990. [PubMed] [Google Scholar]
- Sato S, Hughes RC. Regulation of secretion and surface expression of Mac-2, a galactoside-binding protein of macrophages. J Biol Chem. 1994;269:4424–4430. [PubMed] [Google Scholar]
- Van den Brule FA, Castronovo V. Laminin binding lectins during cancer invasion and metastasis. Caron M, Steve AP, editors. Warsaw, Poland: Harwood Academic Publishers,; Lectins and Pathology. 2000:pp 79–121. [Google Scholar]
- Kasai K, Hirabayashi J. Galectins: a family of animal lectins that decipher glycocodes. J Biochem (Tokyo) 1996;119:1–8. doi: 10.1093/oxfordjournals.jbchem.a021192. [DOI] [PubMed] [Google Scholar]
- Ochieng J, Fridman R, Nangia-Makker P, Kleiner DE, Liotta LA, Stetler-Stevenson WG, Raz A. Galectin-3 is a novel substrate for human matrix metalloproteinases-2 and -9. Biochemistry. 1994;33:14109–14114. doi: 10.1021/bi00251a020. [DOI] [PubMed] [Google Scholar]
- Herrmann J, Turck CW, Atchison RE, Huflejt ME, Poulter L, Gitt MA, Burlingame AL, Barondes SH, Leffler H. Primary structure of the soluble lactose binding lectin L-29 from rat and dog and interaction of its non-collagenous proline-, glycine-, tyrosine-rich sequence with bacterial and tissue collagenase. J Biol Chem. 1993;268:26704–26711. [PubMed] [Google Scholar]
- Nangia-Makker P, Akahani S, Bresalier R, Raz A. The role of galectin-3 in tumor metastasis. Caron M, Steve AP, editors. Warsaw, Poland: Harwood Academic Publishers,; Lectins and Pathology. 2000:pp 67–77. [Google Scholar]
- Irimura T, Matsushita Y, Sutton RC, Carralero D, Ohannesian DW, Cleary KR, Ota DM, Nicolson GL, Lotan R. Increased content of an endogenous lactose-binding lectin in human colorectal carcinoma progressed to metastatic stages. Cancer Res. 1991;51:387–393. [PubMed] [Google Scholar]
- Castronovo V, Van Den Brule FA, Jackers P, Clausse N, Liu FT, Gillet C, Sobel ME. Decreased expression of galectin-3 is associated with progression of human breast cancer. J Pathol. 1996;179:43–48. doi: 10.1002/(SICI)1096-9896(199605)179:1<43::AID-PATH541>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Lotz MM, Andrews CW, Jr, Korzelius CA, Lee EC, Steele GD, Jr, Clarke A, Mercurio AM. Decreased expression of Mac-2 (carbohydrate binding protein 35) and loss of its nuclear localization are associated with the neoplastic progression of colon carcinoma. Proc Natl Acad Sci USA. 1993;90:3466–3470. doi: 10.1073/pnas.90.8.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacis RA, Pilat MJ, Pienta KJ, Wojno K, Raz A, Hogan V, Cooper CR. Decreased galectin-3 expression in prostate cancer. Prostate. 2000;44:118–123. doi: 10.1002/1097-0045(20000701)44:2<118::aid-pros4>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- van den Brule FA, Berchuck A, Bast RC, Liu FT, Gillet C, Sobel ME, Castronovo V. Differential expression of the 67-kD laminin receptor and 31-kD human laminin-binding protein in human ovarian carcinomas. Eur J Cancer. 1994;30A:1096–1099. doi: 10.1016/0959-8049(94)90464-2. [DOI] [PubMed] [Google Scholar]
- Idikio H. Galectin-3 expression in human breast carcinoma: correlation with cancer histologic grade. Int J Oncol. 1998;12:1287–1290. doi: 10.3892/ijo.12.6.1287. [DOI] [PubMed] [Google Scholar]
- Nangia-Makker P, Honjo Y, Sarvis R, Akahani S, Hogan V, Pienta KJ, Raz A. Galectin-3 induces endothelial cell morphogenesis and angiogenesis. Am J Pathol. 2000;156:899–909. doi: 10.1016/S0002-9440(10)64959-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shekhar MP, Werdell J, Tait L. Interaction with endothelial cells is a prerequisite for branching ductal-alveolar morphogenesis and hyperplasia of preneoplastic human breast epithelial cells: regulation by estrogen. Cancer Res. 2000;60:439–449. [PubMed] [Google Scholar]
- Basolo F, Elliott J, Tait L, Chen XQ, Maloney T, Russo IH, Pauley R, Momiki S, Caamano J, Klein-Szanto AJP. Transformation of human breast epithelial cells by c-Ha-ras oncogene. Mol Carcinog. 1991;4:25–35. doi: 10.1002/mc.2940040106. [DOI] [PubMed] [Google Scholar]
- Dawson PJ, Wolman SR, Tait L, Heppner GH, Miller FR. MCF10AT: a model for the evolution of cancer from proliferative breast disease. Am J Pathol. 1996;148:313–319. [PMC free article] [PubMed] [Google Scholar]
- Shekhar MP, Nangia-Makker P, Wolman SR, Tait L, Heppner GH, Visscher DW. Direct action of estrogen on sequence of progression of human preneoplastic breast disease. Am J Pathol. 1998;152:1129–1132. [PMC free article] [PubMed] [Google Scholar]
- Visscher DW, Nangia-Makker P, Heppner G, Shekhar PV. Tamoxifen suppresses histologic progression to atypia and DCIS in MCFIOAT xenografts, a model of early human breast cancer. Breast Cancer Res Treat. 2001;65:41–47. doi: 10.1023/a:1006490000659. [DOI] [PubMed] [Google Scholar]
- Miller FR, Santner SJ, Tait L, Dawson PJ. MCF10DCIS.com xenograft model of human comedo ductal carcinoma in situ. J Natl Cancer Inst. 2000;92:1185–1186. doi: 10.1093/jnci/92.14.1185a. [DOI] [PubMed] [Google Scholar]
- Nangia-Makker P, Hogan V, Honjo Y, Baccarini S, Tait L, Bresalier R, Raz A. Inhibition of human cancer cell growth and metastasis in nude mice by oral intake of modified citrus pectin. J Natl Cancer Inst. 2002;94:1854–1862. doi: 10.1093/jnci/94.24.1854. [DOI] [PubMed] [Google Scholar]
- Lu Z, Friess H, Graber HU, Guo X, Schilling M, Zimmermann A, Korc M, Buchler MW. Presence of two signaling TGF-beta receptors in human pancreatic cancer correlates with advanced tumor stage. Dig Dis Sci. 1997;42:2054–2063. doi: 10.1023/a:1018814416903. [DOI] [PubMed] [Google Scholar]
- Guo XZ, Friess H, Maurer C, Berberat P, Tang WH, Zimmermann A, Naef M, Graber HU, Korc M, Buchler MW. KAI1 is unchanged in metastatic and nonmetastatic esophageal and gastric cancers. Cancer Res. 1998;58:753–758. [PubMed] [Google Scholar]
- Meromsky L, Lotan R, Raz A. Implications of endogenous tumor cell surface lectins as mediators of cellular interactions and lung colonization. Cancer Res. 1986;46:5270–5275. [PubMed] [Google Scholar]
- Castronovo V, Campo E, van den Brule FA, Claysmith AP, Cioce V, Liu FT, Fernandez PL, Sobel ME. Inverse modulation of steady-state messenger RNA levels of two non-integrin laminin-binding proteins in human colon carcinoma. J Natl Cancer Inst. 1992;84:1161–1169. doi: 10.1093/jnci/84.15.1161. [DOI] [PubMed] [Google Scholar]
- Nangia-Makker P, Thompson E, Hogan C, Ochieng J, Raz A. Induction of tumorigenicity by galectin-3 in a non-tumorigenic human breast carcinoma cell line. Int J Oncol. 1995;7:1079–1087. doi: 10.3892/ijo.7.5.1079. [DOI] [PubMed] [Google Scholar]
- Honjo Y, Nangia-Makker P, Inohara H, Raz A. Down regulation of galectin-3 suppresses tumorigenicity of human breast carcinoma cells. Clin Cancer Res. 2001;7:661–668. [PubMed] [Google Scholar]
- Meyer JS. Cell kinetics of histologic variants of in situ breast carcinoma. Breast Cancer Res Treat. 1986;7:171–180. doi: 10.1007/BF01806247. [DOI] [PubMed] [Google Scholar]
- Lagios MD, Westdahl PR, Margolin FR, Rose MR. Duct carcinoma in situ. Relationship of extent of noninvasive disease to the frequency of occult invasion, multicentricity, lymph node metastases, and short-term treatment failures. Cancer. 1982;50:1309–1314. doi: 10.1002/1097-0142(19821001)50:7<1309::aid-cncr2820500716>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Nangia-Makker P, Sarvis R, Visscher DW, Bailey-Penrod J, Raz A, Sarkar FH. Galectin-3 and L1 retrotransposons in human breast carcinomas. Breast Cancer Res Treat. 1998;49:171–183. doi: 10.1023/a:1005913810250. [DOI] [PubMed] [Google Scholar]
- Le Marer N, Hughes RC. Effects of the carbohydrate-binding protein galectin-3 on the invasiveness of human breast carcinoma cells. J Cell Physiol. 1996;168:51–58. doi: 10.1002/(SICI)1097-4652(199607)168:1<51::AID-JCP7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Mehul B, Bawumia S, Hughes RC. Cross-linking of galectin 3, a galactose-binding protein of mammalian cells, by tissue-type transglutaminase. FEBS Lett. 1995;360:160–164. doi: 10.1016/0014-5793(95)00100-n. [DOI] [PubMed] [Google Scholar]
- Ochieng J, Platt D, Tait L, Hogan V, Raz T, Carmi P, Raz A. Structure-function relationship of a recombinant human galactoside-binding protein. Biochemistry. 1993;32:4455–4460. doi: 10.1021/bi00067a038. [DOI] [PubMed] [Google Scholar]
- Mehul B, Bawumia S, Martin SR, Hughes RC. Structure of baby hamster kidney carbohydrate-binding protein CBP30, an S-type animal lectin. J Biol Chem. 1994;269:18250–18258. [PubMed] [Google Scholar]
- Massa SM, Cooper DN, Leffler H, Barondes SH. L-29, an endogenous lectin, binds to glycoconjugate ligands with positive cooperativity. Biochemistry. 1993;32:260–267. doi: 10.1021/bi00052a033. [DOI] [PubMed] [Google Scholar]
- John CM, Leffler H, Kahl-Knutsson B, Svensson I, Jarvis GA. Truncated galectin-3 inhibits tumor growth and metastasis in orthotopic nude mouse model of human breast cancer. Clin Cancer Res. 2003;9:2374–2383. [PubMed] [Google Scholar]
- Ochieng J, Green B, Evans S, James O, Warfield P. Modulation of biological functions of galectin-3 by matrix metalloproteases. Biochim Biophys Acta. 1998;1379:97–106. doi: 10.1016/s0304-4165(97)00086-x. [DOI] [PubMed] [Google Scholar]
- Ochieng J, Gerold M, Raz A. Dichotomy in the laminin binding properties of soluble and membrane bound human galactoside binding protein. Biochem Biophys Res Commun. 1992;186:1674–1680. doi: 10.1016/s0006-291x(05)81601-8. [DOI] [PubMed] [Google Scholar]
- Inohara H, Raz A. Effects of natural complex carbohydrate (citrus pectin) on murine melanoma cell properties related to galectin-3 functions. Glycoconj J. 1994;11:527–532. doi: 10.1007/BF00731303. [DOI] [PubMed] [Google Scholar]