Abstract
In pancreatic acini, the G-protein-activated phosphoinositide 3-kinase-γ (PI3Kγ) regulates several key pathological responses to cholecystokinin hyperstimulation in vitro. Thus, using mice lacking PI3Kγ, we studied the function of this enzyme in vivo in two different models of acute pancreatitis. The disease was induced by supramaximal concentrations of cerulein and by feeding mice a choline-deficient/ethionine-supplemented diet. Although the secretive function of isolated pancreatic acini was identical in mutant and control samples, in both models, genetic ablation of PI3Kγ significantly reduced the extent of acinar cell injury/necrosis. In agreement with a protective role of apoptosis in pancreatitis, PI3Kγ-deficient pancreata showed an increased number of apoptotic acinar cells, as determined by terminal dUTP nick-end labeling and caspase-3 activity. In addition, neutrophil infiltration within the pancreatic tissue was also reduced, suggesting a dual action of PI3Kγ, both in the triggering events within acinar cells and in the subsequent neutrophil recruitment and activation. Finally, the lethality of the choline-deficient/ethionine-supplemented diet-induced pancreatitis was significantly reduced in mice lacking PI3Kγ. Our results thus suggest that inhibition of PI3Kγ may be of therapeutic value in acute pancreatitis.
Clinical manifestations of acute pancreatitis vary in severity from mild to severe attacks, the latter being still associated with a mortality rate of 20 to 40%.1,2 The initial pathogenic event of pancreatitis is generally considered the intra-acinar cell conversion of inactive zymogens,3–6 followed by the activation of the proinflammatory transcription factor nuclear factor-κB.7,8 The generation and release of proinflammatory cytokines and chemokines, and the sequestration and activation of circulating inflammatory cells, especially neutrophils, have been shown to determine the severity of the injury to the gland, as well as to distant organs.2,9–12
An important signaling molecule, potentially implicated in the early phases of acute pancreatic damage as well as in neutrophil recruitment and activation, is class I phosphoinositide 3-kinase (PI3K).13 PI3K selectively phosphorylates the 3′-OH residue of phosphatidylinositol (4,5)-bisphosphate [PtdIns(4,5) P2], to form PtdIns(3,4,5) P3, which serves as a docking site for cytoplasmic proteins involved in multiple cellular processes, such as survival, proliferation, cytoskeletal remodeling, and membrane trafficking.14,15 Class I PI3Ks consist of dimers that are classified in two subfamilies depending on their composition and mechanism of activation.14,15 PI3Ks of the first subgroup (class IA) are formed by the catalytic subunit associated with a p85-like regulatory protein docking to phosphorylated tyrosines in YXXM motives. The other group is instead characterized by the interaction of the catalytic subunit with an adaptor called p101 and by the activation through βγ subunits of trimeric G proteins (class IB). The only class IB member, PI3Kγ, is highly expressed in white blood cells, but it can be detected at lower levels in other tissues, including exocrine pancreas.16–18 PI3Kγ may be activated by several growth factors and G-protein-coupled receptors (GPCRs),14,15 here potentially including also CCK and its analogue cerulein. We and others recently reported that PI3Kγ-deficient mice were viable and fertile, but displayed defective GPCR responses to chemoattractants in leukocytes,16,17,19 to ADP in platelets,20 and to adenosine in mast cells.21 Consequently, the severity of endotoxin-induced acute lung injury,22 platelet-dependent thromboembolic vascular occlusion,20 and passive anaphylaxis were markedly reduced in vivo in these animals.21 Recent studies have stressed the importance of PI3K involvement in the pathogenesis of acute pancreatitis, from the early phases of acinar cell injury to the development of multiple organ damage. Pharmacological PI3K inhibitors, wortmannin and LY294002, prevented indeed trypsinogen activation in vitro in CCK-stimulated acini and modulated the severity of acute pancreatitis in two different experimental models.23 Moreover, a very recent study by Gukovsky and colleagues24 showed that the isoform γ of PI3K regulates several key pathological responses to CCK hyperstimulation in vitro, namely, the sustained rise in free cytosolic Ca2+ concentrations, and the activation of trypsinogen and nuclear factor-κB. Using mice in which PI3Kγ expression was genetically ablated,16 we studied the role of PI3Kγ isoform in the development of acute pancreatitis in vivo in two different experimental models. We found that genetic ablation of PI3Kγ significantly reduced the severity of acute pancreatic damage induced by both supramaximally stimulating doses of cerulein and choline-deficient, ethionine-supplemented (CDE) diet. This protective effect was associated with preserved exocrine secretion from isolated pancreatic acini in vitro, increased apoptosis of acinar cells, and decreased neutrophil infiltration within the pancreatic tissue.
Materials and Methods
Animals
PI3Kγ-null mice were obtained as previously described.16 Both mutant and control animals were inbred 129 sv. Animals were bred and housed in standard cages in a climate-controlled room with an ambient temperature of 23 ± 2°C and a 12-hour light/dark cycle. They were fed standard laboratory chow, given water ad libitum, and randomly assigned to control or experimental groups. Animal experiments were performed in compliance with institutional guidelines as well as with Italian and Swiss government regulations.
Preparation of Acini
Dispersed acini were prepared by collagenase digestion of pancreas from PI3Kγ-deficient mice and wild-type animals, as described.25 The acini were suspended in Krebs-Ringer bicarbonate medium buffered to pH 7.4 with 12.5 mmol/L HEPES and containing 0.1% bovine serum albumin (control Krebs-Ringer bicarbonate medium).
In Vitro Cerulein-Induced Amylase Secretion
Acini from both PI3Kγ-null mice and wild-type animals were incubated at 37°C with varying concentrations of cerulein for 30 minutes. Amylase secretion into the medium and total amylase content of the samples were measured. Net stimulated amylase secretion during 30 minutes was calculated as the difference between the percentage of total amylase secreted in the presence and absence of cerulein.25
Induction of Pancreatitis
For in vivo experiments, PI3Kγ-deficient mice and wild-type mice (20 to 22 g) were administered 6 or 13 hourly intraperitoneal injections (0.2 ml) containing a supramaximally stimulating dose (50 μg/kg) of cerulein (Sigma Chemical Co., St Louis, MO) to elicit secretagogue-induced pancreatitis. Control mice (PI3Kγ-null mice and wild-type mice) were administered comparable injections of saline. One hour after the final cerulein or saline injection, animals were anesthetized with avertin and killed by cervical dislocation.
A well-described model of severe lethal, necrotizing pancreatitis was induced using a CDE diet as described previously.26 Briefly, young (6 weeks old) female mice were fasted for 24 hours and then fed either regular chow or CDE diet for 48 hours. To ensure equal exposure by all animals, the experimental diet was replaced with a clean, fresh diet every 12 hours. Randomly selected animals were then given regular chow to permit estimation of the 7-day mortality rate, whereas the other animals were fasted overnight, anesthetized with avertin, killed by cervical dislocation, and used for studies evaluating the severity of pancreatitis.
Quantitation of Cerulein- and CDE Diet-Induced Injuries
At the time the mice were killed, blood was withdrawn from the orbital plexus and centrifuged and the serum kept at −80°C until assayed. Serum amylase activity was measured as previously described using 4,6-ethylidene (G1)-p-nitrophenyl(G1)-d-malto-heptoside as the substrate.27 The pancreas was rapidly removed and divided in several parts. One part was used to quantitate the extent of pancreatic edema by measuring tissue water content. To this end, pancreatic tissue was weighed before and after desiccation at 95°C for 24 hours. The difference between the wet and dry tissue weights was calculated and expressed as a percentage of the tissue wet weight.27 The other parts were frozen in liquid nitrogen for later analysis. Inflammatory infiltration was evaluated by counting infiltrating neutrophils on tissue-sections stained with fluorescein isothiocyanate-labeled rabbit anti-rat polymorphonuclear leukocyte antibody (Accurate Chemical and Science Corp., Westbury, NY), as described.28 To evaluate injury/necrosis, the pancreas was fixed in 4% neutral buffered formalin, embedded in paraffin, and sectioned (5 μm). After staining with hematoxylin and eosin, sections were examined by a blinded experienced morphologist who was not aware of the sample identity. In the cerulein model, the extent of acinar cell injury/necrosis (defined by the presence of acinar cell ghosts, vacuolization and swelling of the acinar cells, and/or the destruction of the histoarchitecture of whole or parts of the acini) was quantitated by computer-assisted morphometry and expressed as a percentage of total acinar tissue, as previously described.29 On the contrary, in the CDE diet-model, which induces a severe necrotizing acute pancreatitis, the areas of pancreatic tissue occupied by coagulative necrosis were measured by computer-assisted morphometry and expressed as a percentage of total acinar tissue. Of note, in this model, because the definition of coagulative necrosis is comprehensive of inflammatory cell infiltration within the necrotic areas, only the neutrophils infiltrating the areas of acinar tissue with preserved morphology (nonnecrotic) were counted.
To evaluate the extent of lung injury, at the time of death, a polyvinyl catheter was secured in the trachea and used to instill 4% neutral buffered formalin into the lungs at a hydrostatic pressure of 30 cm H2O. Portions of the formalin-distended lungs were harvested, fixed, paraffin-embedded, sectioned (5 μm), stained, and examined by a morphologist who was not aware of the sample identity. The extent of alveolar-capillary membrane thickening and of neutrophil infiltration were estimated visually and scored from 0 to 4.
Quantitation of Apoptosis
The terminal dUTP nick-end labeling (TUNEL) method (Fluorescein-FragEL DNA Fragmentation detection kit; Oncogene Research Products, San Diego, CA) was used to histologically detect apoptosis in pancreatic acinar cells from mice treated with cerulein or saline. Briefly, sections from formol-fixed and paraffin-embedded pancreas were deparaffinized, rehydrated, and digested with 3 μg/ml of proteinase K for 15 minutes at room temperature. After washing with phosphate-buffered saline (PBS), sections were incubated in 25 mmol/L cobalt chloride, 0.01 mmol/L biotin-16-deoxyuridine triphosphate, and 25 U/ml terminal deoxynucleotidyltransferase. The positive nuclei were counted at high-power field (×400).
To discriminate apoptotic cells from necrotic cells, we used an additional method of detection by immunohistochemical labeling of active caspase-3. For these experiments, sections were incubated with anti-active caspase-3 antibodies (1:500; Cell Signaling Technology, Beverly, MA) overnight at 4°C. Slides were washed in PBS and incubated with anti-rabbit secondary antibodies (1:250) for 1 hour at room temperature, washed again, and incubated for another hour with streptavidin/horseradish peroxidase. The reaction was followed under the microscope and stopped 2 minutes after addition of 3-amino-g-ethylcarbazole (AEC) chromogen, rinsed, and mounted according to the manufacturer’s instructions (R&D Systems, Minneapolis, MN).
Protein Extraction and Western Blot Analysis
For Western blots, the pancreas was rapidly frozen and ground in liquid nitrogen, and proteins were extracted in ice-cold solubilization buffer containing 50 mmol/L Tris, pH 7.5, 150 mmol/L NaCl, and 0.5% Triton X-100. Protein extracts (60 μg) was resolved by 10% sodium dodecyl sulfate/polyacrylamide gel electrophoresis and electroblotted to nitrocellulose membranes using standard protocols. Filters were then incubated overnight at 4°C with anti-COX-2 rabbit polyclonal antibody (1 μg/ml; Upstate Ltd., Milton Keynes, UK) or anti-α-tubulin monoclonal antibody (1:1000; Sigma) in blocking solution, washed three times, and incubated with the appropriate peroxidase-labeled secondary antibody (1:5000 dilution for anti-rabbit IgG and 1:20,000 for anti-mouse IgG; Amersham, Arlington Heights, IL) for 1 hour at room temperature. The immune complexes were visualized using the enhanced chemiluminescent detection method (Amersham).
Analysis of Data
Data are expressed as the mean ± SEM. Comparisons used unpaired t-test for differences between wild-type and PI3Kγ-deficient mice, paired t-test, or one-way analysis of variance followed by Newman-Keul’s post hoc test, as appropriate. Mortality rates were analyzed using the method of Kaplan-Meier. A P value of 0.05 was considered significant.
Results
In Vitro Amylase Secretion from Acini Prepared from PI3Kγ-Null and Wild-Type Mice
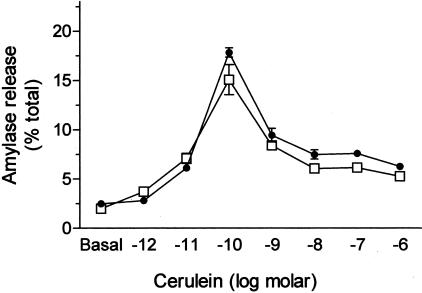
Cerulein induced a biphasic, dose-dependent, secretion response in isolated pancreatic acini prepared from wild-type animals. In PI3Kγ-null mice, the pattern of secretion was identical to the pattern observed in wild-type animals (Figure 1).
Figure 1.
Cerulein-stimulated in vitro amylase secretion from pancreatic acini. Acini were prepared from both wild-type (□) and PI3Kγ-deficient animals (•) and incubated with varying concentrations of cerulein for 30 minutes. Amylase release was quantitated as described in Materials and Methods. Results represent mean ± SEM for three animals in each group.
Effects of PI3Kγ Deficiency on the Severity of Cerulein-Induced Acute Pancreatitis
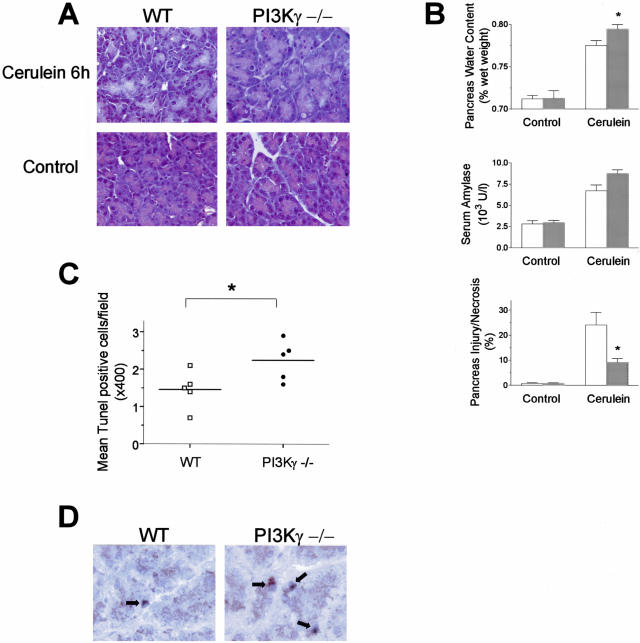
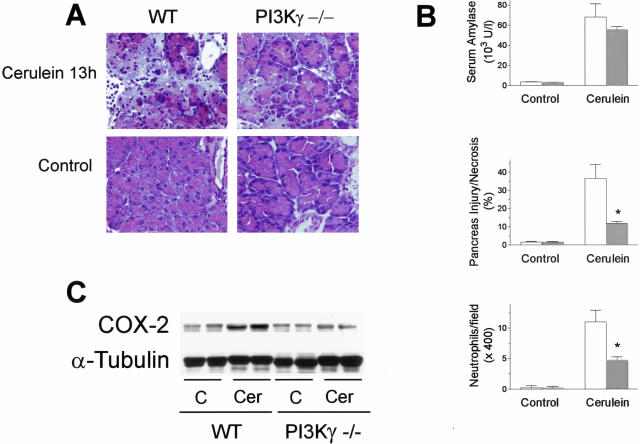
Genetic ablation of PI3Kγ did not result in alterations in serum amylase levels or in pancreas histology in basal conditions (not shown). To examine the contribution of PI3Kγ in the severity of acute pancreatitis, mice deficient for the PI3Kγ gene were treated with supramaximal doses of cerulein. Because PI3Kγ is known to affect neutrophil recruitment and activation,16,17,19 we studied the effects of PI3Kγ genetic ablation at two different time points, ie, at 6 hours, when, in preliminary experiments, only minimal neutrophil infiltration could be detected within the pancreatic tissue in our model, and at 13 hours, when an evident inflammatory infiltrate could be seen. The severity of cerulein-induced pancreatitis was evaluated by measuring the extent of acinar cell injury/necrosis by standard histological examination, serum amylase activity, pancreatic water content, and neutrophil infiltration. As shown in Figure 2, A and B, supramaximal stimulation with cerulein induced an increase in acinar cell injury/necrosis, serum amylase activity, and pancreatic water content after 6 hours from the start of cerulein administration. At this time-point PI3Kγ-deficient mice showed a partial but significant reduction in acinar cell necrosis compared to control animals (Figure 2, A and B). Serum amylase levels were markedly increased in both experimental groups, but no difference was seen between PI3Kγ-null and control mice (Figure 2B). Unexpectedly, pancreatic water content was higher in knockout mice than in controls (Figure 2B). Moreover, only minimal neutrophil infiltration was observed in our model at this time point, both in wild-type and PI3Kγ-deficient animals (Figure 2A). On the contrary, prolonged administration of cerulein for 13 hours further increased all of the parameters of acute pancreatic damage in wild-type mice (Figure 3, A and B). Furthermore, in this experimental set, evident sequestration of neutrophils within the pancreatic tissue and the presence of small foci of coagulative necrosis were observed (Figure 3A). At 13 hours, all of the parameters, except for amylase serum levels, of acute pancreatic damage was significantly reduced in PI3Kγ-deficient compared to wild-type mice (Figure 3B). Although secretagogue-induced pancreatitis has been previously associated with evidence of lung injury,2,12,22,23,27 neither thickening of the alveolar membrane nor neutrophil infiltration were seen in our model at both time points (not shown).
Figure 2.
Effects of PI3Kγ deletion on cerulein-induced acute pancreatitis. Mice were administered six intraperitoneal injections of cerulein and killed 1 hour after the last injection of cerulein. A: Representative micrographs of morphological alterations induced in exocrine pancreas by 6 hours of cerulein administration (cerulein 6 hours) compared with 6 hours of saline administration (control), in wild-type (WT) and PI3Kγ-deficient (PI3Kγ−/−) mice. B: Quantitation of cerulein-induced pancreatic injury. Pancreatic water content, serum amylase activity, and acinar cell injury/necrosis were measured as described in the text. Wild-type mice, open columns; PI3Kγ-deficient mice, shaded columns. Results are mean ± SEM values for five or more animals in each group. *, P < 0.05 versus control. C: Effects of PI3Kγ deletion on acinar cell apoptosis in cerulein-induced acute pancreatitis. Apoptotic acinar cells were detected by TUNEL as described in Materials and Methods. Wild-type mice (□) and PI3Kγ-deficient mice (•) were administered six intraperitoneal injections of cerulein and killed 1 hour after the last injection of cerulein. *, P < 0.05 PI3Kγ-deficient versus wild-type mice. D: Immunohistochemical analysis of caspase-3 activity in exocrine pancreas from wild-type (WT) and PI3Kγ-deficient (PI3Kγ−/−) mice after 6 hours of cerulein administration. Original magnifications: ×400 (A); ×1000 (D).
Figure 3.
Effects of PI3Kγ deletion on cerulein-induced acute pancreatitis. Mice were administered 13 intraperitoneal injections of cerulein and killed 1 hour after the last injection of cerulein. A: Representative micrographs of morphological alterations induced in exocrine pancreas by 13 hours of cerulein administration (cerulein 13h) compared with 13 hours of saline administration (Control), in wild-type (WT) and PI3Kγ-deficient (PI3Kγ−/−) mice. Thirteen hours of cerulein administration induced evident morphological alterations and massive neutrophil infiltration of the exocrine pancreas compared with controls. Foci of coagulative necrosis are also evident. Original magnifications, ×400. B: Quantitation of cerulein-induced pancreatic injury. Serum amylase activity, acinar cell injury/necrosis, and neutrophil infiltration were measured as described in the text. Wild-type mice, open columns; PI3Kγ-deficient mice, shaded columns. Results are mean ± SEM values for five or more animals in each group. *, P < 0.05 versus control. C: Effects of PI3Kγ deletion on pancreatic COX-2 expression during cerulein-induced acute pancreatitis. Pancreata from wild-type (WT) and PI3Kγ-deficient (PI3Kγ−/−) mice were harvested after 13 hours from the beginning of saline (C) or cerulein (Cer) administration, and analyzed by Western blot for COX-2 and α-tubulin expression, as described in Materials and Methods.
Determination of Apoptotic Acinar Cells in PI3Kγ-Null and Wild-Type Mice during Cerulein-Induced Acute Pancreatitis
In the cerulein model of pancreatitis, the number of apoptotic acinar cells increased throughout time. Apoptosis of acinar cells was then determined by TUNEL assay in wild-type and PI3Kγ-null mice injected with cerulein for 6 hours. As expected,30–32 the number of TUNEL-positive cells increased after administration of cerulein in exocrine pancreas of wild-type mice; however, the detection of apoptotic acinar cells was significantly increased in PI3Kγ-deficient animals compared to control littermates (Figure 2C). Quantitative evaluation of TUNEL-positive cells showed that the number of apoptotic acinar cells after treatment with cerulein was approximately twofold increased in pancreas from PI3Kγ-null mice compared with wild-type mice (Figure 2C).
To confirm the apparent increased sensitivity of PI3Kγ-deficient acinar cells to apoptosis, the activity of caspase 3 was detected by immunohistochemistry. Under control conditions, the number of caspase-3-positive acinar cells was not different between wild-type mice and their equivalent knockout animals (not shown). The number of caspase-3-positive acinar cells increased significantly after 6 hours of treatment with cerulein in wild-type animals, but it was much higher in PI3Kγ-null mice (Figure 2D).
Effects of PI3Kγ Deficiency on the Expression of COX-2 during Cerulein-Induced Acute Pancreatitis
Because COX-2 expression has been shown to increase after the induction of secretagogue-induced pancreatitis33 and to modulate the course of the disease,33–35 we studied COX-2 expression in pancreata harvested from PI3Kγ-deficient and wild-type animals subjected to repeated injections of saline (controls) or cerulein for 13 hours. Low levels of COX-2 expression were present in both PI3Kγ-deficient and wild-type animals after saline injection, whereas cerulein administration induced an increase in COX-2 expression that was more evident in wild-type than in PI3Kγ-null mice (Figure 3C). Thus, the protective effect of PI3Kγ ablation in acute pancreatitis was associated with reduced pancreatic COX-2 expression.
Effects of PI3Kγ Deficiency on the Severity of CDE Diet-Induced Acute Pancreatitis
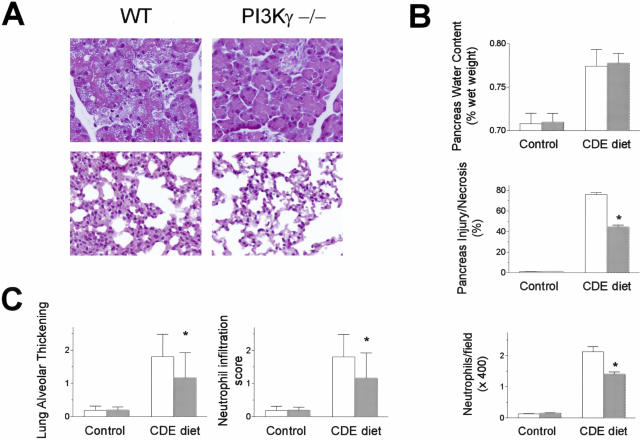
To assess whether the effects of PI3Kγ genetic ablation were specific to the secretagogue-induced model of acute pancreatitis or relevant to the pathogenesis of acute pancreatitis in general, we used a second, noninvasive model of experimental pancreatitis, based on the administration of a CDE diet to young female mice.26 CDE diet administration resulted in a severe, necrotizing acute pancreatitis characterized by pancreatic acinar cell vacuolization and necrosis, and neutrophil infiltration, in all mice (Figure 4, A and B). Also in this model, several parameters of pancreatic damage were attenuated in PI3Kγ-null compared to wild-type mice (Figure 4B). In particular, the extent of acinar cell injury/necrosis was 44.68 ± 4.53% in PI3Kγ-null mice compared to 76.01 ± 6.27% in wild-type mice (P < 0.0001). Also neutrophil infiltration, but not pancreatic edema, was significantly decreased (Figure 4B). Moreover, the extent of lung injury, evaluated in terms of thickening of the alveolar-capillary membrane and of neutrophil infiltration within lung tissue, was reduced in PI3Kγ-deficient compared to wild-type mice (Figure 4, A and C). Finally, the mortality rate was significantly lower in PI3Kγ-null mice (53%) than in control animals (83%, P < 0.03; Figure 5).
Figure 4.
Effects of PI3Kγ deletion on CDE diet-induced acute pancreatitis. Young female mice were fasted for 24 hours and then fed the CDE diet for 48 hours, as described in Materials and Methods. A: Representative micrographs of morphological alterations of exocrine pancreas (top) and lung (bottom) after CDE diet administration in wild-type (WT) and PI3Kγ-deficient (PI3Kγ−/−) mice. Original magnifications, ×400. B: Quantitation of CDE diet-induced pancreatic injury. Pancreatic water content, acinar cell injury/necrosis, and neutrophil infiltration were measured as described in the text. Wild-type mice, open columns; PI3Kγ-deficient mice, shaded columns. Results are mean ± SEM values for five or more animals in each group. *, P < 0.05 versus control. C: Quantitation of CDE diet-induced lung injury. The extent of alveolar-capillary membrane thickening and of neutrophil infiltration were measured as described in the text. Wild-type mice, open columns; PI3Kγ-deficient mice, shaded columns. Results are mean ± SEM values for five or more animals in each group. *, P < 0.05 versus control.
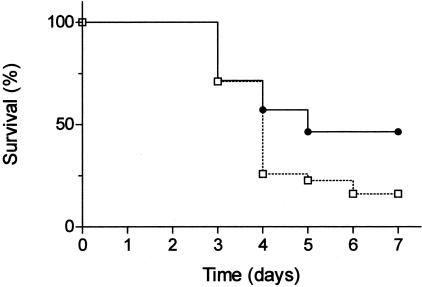
Figure 5.
Mortality curve for wild-type (□) and PI3Kγ-deficient animals (•) during CDE diet-induced acute pancreatitis. Young female mice were fed a CDE diet for 48 hours and then regular chow for the following days, and mortality was recorded, as described in Materials and Methods. P < 0.03 for PI3Kγ-deficient mice versus wild-type mice.
Discussion
The aim of this study was to investigate, using mice in which PI3Kγ expression was genetically ablated,16 the role of PI3Kγ in vitro on amylase secretion from isolated acini, and in vivo in two different experimental models of acute pancreatitis. Genetic ablation of PI3Kγ did not result in alterations in serum amylase levels or in pancreas histology in basal conditions. In addition, we observed an identical biphasic, dose-dependent, secretion response to cerulein in isolated pancreatic acini prepared from wild-type animals and PI3Kγ-null mice, indicating that the receptor remained functionally intact in these genetically modified animals.
At 6 hours from the beginning of cerulein administration, we observed a partial but significant reduction in the extent of acinar cell injury/necrosis. On the contrary, serum amylase levels were not decreased, and pancreatic water content was even increased in PI3Kγ-deficient compared to wild-type mice at 6 hours, suggesting that PI3Kγ does not mediate these events in the early phases of pancreatitis. A similar dissociation between hyperamylasemia and pancreatic edema and the other parameters of acute pancreatic damage has been previously described in other studies. For instance, blocking tumor necrosis factor-α with neutralizing antibody exerted little or no effect on pancreatic edema in the secretagogue-induced model of acute pancreatitis, but evident protective effect on inflammatory infiltration and necrosis.36,37 Similar effects were observed by either inhibiting platelet-activating factor (PAF), a mediator of inflammation involved in the pathogenesis of acute pancreatitis,9,10,38 with recombinant PAF acetylhydrolase,29 or COX-2.33 In our model, only minimal neutrophil infiltration was seen at time points as early as 6 hours. These data are partially in disagreement with others studies, which reported that neutrophil infiltration within the pancreatic tissue can be evident after 4 to 6 hours from the beginning of the experiment.12,27,29,32 Mouse strain differences might, however, account for this discrepancy.
Because no neutrophil infiltration was seen at 6 hours, the protective effect observed in PI3Kγ-deficient mice at this time point should be reasonably ascribed to the influence of the lack of PI3Kγ on the early intra-acinar cell events, as suggested by the data recently reported in vitro by Gukovsky and colleagues.24 Moreover, the number of apoptotic acinar cells, identified by TUNEL and caspase-3 activity, was markedly higher in PI3Kγ-null than in wild-type mice, suggesting that increased apoptosis may be an additional mechanism of protection during the course of acute pancreatitis in these animals. An inverse correlation between the extent of acinar cell apoptosis on the one hand and necrosis and the severity of acute pancreatitis on the other hand has been indeed reported.39,40 Our data suggest that PI3Kγ is involved in mediating the molecular and cellular processes leading to acinar cell apoptosis. Although several studies have shown that Akt/PKB is the major effector of PI3K survival signaling,41,42 conflicting results have been reported on the ability of CCK or cerulein to directly activate Akt/PKB in pancreatic cells.23,43–46 In the present study, we were not able to detect the activation of Akt/PKB ex vivo in pancreatic tissue (not shown). Since it was previously shown that supramaximal concentrations of CCK are able to directly induce apoptosis in pancreatic acini in vitro by several mechanisms, including caspase activation, cytochrome c release, and mitochondrial depolarization,47 we can hypothesize that PI3Kγ may interfere with some of these death signaling pathways.
Prolonged administration of cerulein for 13 hours further increased all of the parameters of acute pancreatic damage in wild-type mice. Moreover, in this experimental set, evident sequestration of neutrophils within the pancreatic tissue and the presence of small foci of coagulative necrosis were observed. A significant reduction of acinar cell injury/necrosis and neutrophil infiltration was observed in PI3Kγ-deficient compared to wild-type mice. It is widely accepted that neutrophils, activated and chemoattracted to the pancreas during the proinflammatory phase of the pathogenic process by a variety of soluble mediators produced by acinar cells themselves, resident macrophages, and endothelial cells, have an important role in regulating the severity of acute pancreatitis.2 Neutrophil depletion, indeed, reduced the severity of acute pancreatitis by several mechanisms, including decreasing trypsinogen activation, and shifting the balance between acinar cell necrosis and apoptosis.12,48,49 One of the mechanisms possibly implicated in the protective effect of PI3Kγ ablation in acute pancreatitis may therefore be related to the well-known ability of PI3Kγ to regulate neutrophil chemotaxis to the site of injury, as well as the respiratory burst after neutrophil activation.50,51 Moreover, an additional mechanism may be through enhancing neutrophil apoptosis, thereby increasing the removal of activated neutrophils from the pancreatic tissue.22 Finally, we showed here that the up-regulation of pancreatic COX-2 expression during acute pancreatitis is blunted in PI3Kγ-null animals. COX-2 expression has been indeed previously shown to increase after the induction of secretagogue-induced pancreatitis,33 and to modulate the course of the disease.33–35 In agreement with other studies that showed that PI3K signaling pathway is implicated in COX-2 up-regulation in endothelial cells52 and macrophages,53 our data suggest that PI3Kγ may have a role in mediating the increase in COX-2 expression during acute pancreatitis. Unexpectedly, and at variance from other studies,2,12,22,23,27 we did not observe thickening of the alveolar membrane or neutrophil infiltration in the cerulein model at both time points studied, may be because of variable response in different mouse strains.
In this study, we observed also a significant protective effect exerted by PI3Kγ genetic ablation on several parameters of pancreatic damage in a second, noninvasive, and lethal model of experimental pancreatitis, based on the administration of a CDE diet to young female mice.26 Therefore, these phenomena were relevant to the pathogenesis of acute pancreatitis in general, and not specific to the secretagogue-induced model. In addition, the mortality rate recorded in the CDE diet model was significantly lower in PI3Kγ-null mice than in control animals, suggesting that PI3Kγ ablation may affect also the development of injury to organs distant from the pancreas (primarily the lung). Thickening of the alveolar-capillary membrane and neutrophil infiltration within lung tissue were indeed reduced in PI3Kγ-null compared to wild-type mice. However, because the protective effect exerted by PI3Kγ genetic ablation was only partial, we must hypothesize that other PI3K isoforms and/or other signaling molecules, in addition to PI3Kγ, are involved in this process, as already suggested by other studies.23,24
The results of the present study partially differ from those previously reported using a pharmacological approach to inhibit PI3K that question the role of class I PI3Ks in pancreatitis.23 The unrelated PI3K inhibitors wortmannin and LY294002 were reported indeed to modulate the severity of the disease in two dissimilar experimental models, secretagogue- and duct injection-induced pancreatitis.23 However, in this study, concentrations of cerulein that induced ex vivo trypsinogen activation did not significantly increase PtdIns(3,4) P2 and PtdIns(3,4,5) P3 levels or induce phosphorylation of Akt/PKB, leading to the conclusion that class I PI3Ks were not involved.23 On the contrary, because wortmannin decreased the concentrations of phosphatidylinositol-3-phosphate, the authors rather suggested that class III PI3Ks, which are believed to be constitutive active, may be involved in this phenomenon, regulating intracellular vesicle trafficking and fusion of lysosomes and zymogen granules.23 The results just described differ from those reported by others groups, which showed instead that class I PI3Ks may be directly activated by cerulein treatment, and be involved in CCK-induced phosphorylation of p70S6 kinase and focal adhesion kinase, exocytosis, and trypsinogen activation.24,43–46 However, the PI3K isoforms involved in these phenomena have not been identified to date. Our results, in agreement with those recently reported by Gukovsky and colleagues,24 unequivocally showed that PI3Kγ has an important pathogenic role in vivo in acute pancreatitis. On the other hand, our data do not exclude the intervention of other PI3K isoform(s), in addition to PI3Kγ, in the in vivo pathogenic processes involved in acute pancreatitis. Indeed, PI3K inhibitors have been shown to further reduce Ca2+ signaling, and trypsinogen and nuclear factor-κB activation in acini isolated from PI3Kγ-deficient compared to wild-type mice.24 Additional studies will thus be required to assess the relative role of different PI3K isoforms in the course of acute pancreatitis.
In conclusion, our results show that genetic ablation of PI3Kγ significantly reduced the severity of acute pancreatic damage in two different experimental models, secretagogue- and CDE diet-induced acute pancreatitis. This protective effect was associated with preserved exocrine secretion from isolated pancreatic acini in vitro, increased apoptosis of acinar cells, and decreased neutrophil infiltration within the pancreatic tissue. The data in the present report indicate that PI3Kγ has a crucial role in the development of acute pancreatitis, possibly suggesting that modulation of PI3Kγ activity might provide new valuable therapeutic strategies to improve the clinical course of this disease.
Acknowledgments
We thank Reinhard Wetzker for kindly providing anti-PI3Kγ antibodies and Marc Chanson for helping us in setting-up the procedure of acini dispersion and for helpful discussion.
Footnotes
Address reprint requests to Enrico Lupia, M.D., Dipartimento di Fisiopatologia Clinica, Università di Torino, Via Genova 3, 10126 Torino, Italy. E-mail: elupia@molinette.piemonte.it.
Supported by the Human Frontier Science Project and the European Union Fifth Framework Programme (QLG1–2001-02171 to E.H. and M.P.W., Murst 60% to G.M. and G.E., Murst Cofin 2002 to E.H. and G.M., and FIRB 2002 to E.H. and G.M.).
E.H., G.M., and G.E. contributed equally.
References
- Steer ML. Etiology and pathophysiology of acute pancreatitis. Go VLV, Dimango EP, Gardner JD, Lebenthal E, Reber HA, Scheele GA, editors. New York: Raven; The PancreasBiology, Pathobiology, and Disease. 1993:pp 581–592. [Google Scholar]
- Steer M. Pancreatitis severity: who calls the shots? Gastroenterology. 2002;122:1168–1172. doi: 10.1053/gast.2002.32761. [DOI] [PubMed] [Google Scholar]
- Steer ML. Early events in acute pancreatitis. Bailliere’s Best Pract Res Clin Gastroenterol. 1999;13:213–225. doi: 10.1053/bega.1999.0020. [DOI] [PubMed] [Google Scholar]
- Hofbauer B, Saluja AK, Lerch MM, Bhagat L, Bhatia M, Lee HS, Frossard JL, Adler G, Steer ML. Intra-acinar cell activation of trypsinogen during caerulein-induced pancreatitis in rats. Am J Physiol. 1998;275:G352–G362. doi: 10.1152/ajpgi.1998.275.2.G352. [DOI] [PubMed] [Google Scholar]
- Raraty M, Ward J, Erdemli G, Vaillant C, Neoptolemos JP, Sutton R, Petersen OH. Calcium-dependent enzyme activation and vacuole formation in the apical granular region of pancreatic acinari cells. Proc Natl Acad Sci USA. 2000;97:13126–13131. doi: 10.1073/pnas.97.24.13126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger B, Albrecht E, Lerch MM. The role of intracellular calcium signaling in premature protease activation and the onset of pancreatitis. Am J Pathol. 2000;157:43–50. doi: 10.1016/S0002-9440(10)64515-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gukovsky I, Gukovskaya AS, Blinman TA, Zaninovic V, Pandol SJ. Early NF-κB activation is associated with hormone-induced pancreatitis. Am J Physiol. 1998;275:G1402–G1414. doi: 10.1152/ajpgi.1998.275.6.G1402. [DOI] [PubMed] [Google Scholar]
- Steinle AU, Weidenbach H, Wagner M, Adler G, Schmid RM. NF-κB/Rel activation in cerulein pancreatitis. Gastroenterology. 1999;116:420–430. doi: 10.1016/s0016-5085(99)70140-x. [DOI] [PubMed] [Google Scholar]
- Bhatia M, Brady M, Shokuhi S, Christmas S, Neoptolemos JP, Slavin J. Inflammatory mediators in acute pancreatitis. J Pathol. 2000;190:117–125. doi: 10.1002/(SICI)1096-9896(200002)190:2<117::AID-PATH494>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Norman J. New approaches to acute pancreatitis: role of inflammatory mediators. Digestion. 1999;60:57–60. doi: 10.1159/000051455. [DOI] [PubMed] [Google Scholar]
- Saluja AK, Steer M. Pathophysiology of pancreatitis. Role of cytokines and other mediators of inflammation. Digestion. 1999;60:27–33. doi: 10.1159/000051450. [DOI] [PubMed] [Google Scholar]
- Frossard JL, Saluja A, Bhagat L, Lee HS, Bhatia M, Hofbauer B, Steer ML. The role of intercellular adhesion molecule 1 and neutrophils in acute pancreatitis and pancreatitis-associated lung injury. Gastroenterology. 1999;116:694–701. doi: 10.1016/s0016-5085(99)70192-7. [DOI] [PubMed] [Google Scholar]
- Wymann MP, Pirola L. Structure and function of phosphoinositide 3-kinases. Biochim Biophys Acta. 1998;1436:127–150. doi: 10.1016/s0005-2760(98)00139-8. [DOI] [PubMed] [Google Scholar]
- Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- Vanhaesebroeck B, Leevers SJ, Ahmadi K, Timms J, Katso R, Driscoll PC, Woscholski R, Parker PJ, Waterfield MD. Synthesis and function of 3-phosphorylated inositol lipids. Annu Rev Biochem. 2001;70:535–602. doi: 10.1146/annurev.biochem.70.1.535. [DOI] [PubMed] [Google Scholar]
- Hirsch E, Katanaev VL, Garlanda C, Azzolino O, Pirola L, Silengo L, Sozzani S, Mantovani A, Altruda F, Wymann MP. Central role for G protein-coupled phosphoinositide 3-kinase gamma in inflammation. Science. 2000;287:1049–1053. doi: 10.1126/science.287.5455.1049. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Irie-Sasaki J, Jones RG, Oliveira-dos-Santos AJ, Stanford WL, Bolon B, Wakeham A, Itie A, Bouchard D, Kozieradzki I, Joza N, Mak TW, Ohashi PS, Suzuki A, Penninger JM. Function of PI3Kγ in thymocyte development, T cell activation, and neutrophil migration. Science. 2000;287:1040–1046. doi: 10.1126/science.287.5455.1040. [DOI] [PubMed] [Google Scholar]
- Bernstein HG, Keilhoff G, Reiser M, Freese S, Wetzker R. Tissue distribution and subcellular localization of a G-protein activated phosphoinositide 3-kinase. An immunohistochemical study. Cell Mol Biol. 1998;44:973–983. [PubMed] [Google Scholar]
- Li Z, Jiang H, Xie W, Zhang Z, Smrcka AV, Wu D. Roles of PLC-beta2 and -beta3 and PI3Kgamma in chemoattractant-mediated signal transduction. Science. 2000;287:1046–1049. doi: 10.1126/science.287.5455.1046. [DOI] [PubMed] [Google Scholar]
- Hirsch E, Bosco O, Tropel P, Laffargue M, Calvez R, Altruda F, Wymann M, Montrucchio G. Resistance to thromboembolism in PI3Kgamma-deficient mice. FASEB J. 2001;15:2019–2021. doi: 10.1096/fj.00-0810fje. [DOI] [PubMed] [Google Scholar]
- Laffargue M, Calvez R, Finan P, Trifilieff A, Barbier M, Altruda F, Hirsch E, Wymann MP. Phosphoinositide 3-kinase gamma is an essential amplifier of mast cell function. Immunity. 2002;16:441–451. doi: 10.1016/s1074-7613(02)00282-0. [DOI] [PubMed] [Google Scholar]
- Yum HK, Arcaroli J, Kupfner J, Shenkar R, Penninger JM, Sasaki T, Yang KY, Park JS, Abraham E. Involvement of phosphoinositide 3-kinases in neutrophil activation and the development of acute lung injury. J Immunol. 2001;167:6601–6608. doi: 10.4049/jimmunol.167.11.6601. [DOI] [PubMed] [Google Scholar]
- Singh VP, Saluja AK, Bhagat L, van Acker GJ, Song AM, Soltoff SP, Cantley LC, Steer ML. Phosphoinositide 3-kinase-dependent activation of trypsinogen modulates the severity of acute pancreatitis. J Clin Invest. 2001;108:1387–1395. doi: 10.1172/JCI12874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gukovsky I, Cheng JH, Nam KJ, Lee OT, Lugea A, Fischer L, Penninger JM, Pandol SJ, Gukovskaya AS. Phosphatidylinositide 3-kinase gamma regulates key pathologic responses to cholecystokinin in pancreatic acinar cells. Gastroenterology. 2004;126:554–566. doi: 10.1053/j.gastro.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Chanson M, Bruzzone R, Bosco D, Meda P. Effects of alcohols on junctional coupling and amylase secretion of pancreatic acinar cells. J Cell Physiol. 1989;139:147–156. doi: 10.1002/jcp.1041390121. [DOI] [PubMed] [Google Scholar]
- Lombardi B, Estes LW, Longnecker DS. Acute hemorrhagic pancreatitis (massive necrosis) with fat necrosis induced in mice by DL-ethionine fed with a choline-deficient diet. Am J Pathol. 1975;79:465–480. [PMC free article] [PubMed] [Google Scholar]
- Frossard JL, Kwak BR, Chanson M, Morel P, Hadengue A, Mach F. CD40L deficient mice are protected against cerulein-induced acute pancreatitis and pancreatitis-associated lung injury. Gastroenterology. 2001;121:184–194. doi: 10.1053/gast.2001.25483. [DOI] [PubMed] [Google Scholar]
- Gukovskaya AS, Gukovsky I, Zaninovic V, Song M, Sandoval D, Gukovsky S, Pandol SJ. Pancreatic acinar cells produce, release, and respond to tumor necrosis factor-alpha. Role in regulating cell death and pancreatitis. J Clin Invest. 1997;100:1853–1862. doi: 10.1172/JCI119714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofbauer B, Saluja AK, Bhatia M, Frossard JL, Lee HS, Bhagat L, Steer ML. Effect of recombinant platelet-activating factor acetylhydrolase on two models of experimental acute pancreatitis. Gastroenterology. 1998;115:1238–1247. doi: 10.1016/s0016-5085(98)70096-4. [DOI] [PubMed] [Google Scholar]
- Gukovskaya AS, Perkins P, Zaninovic V, Sandoval D, Rutherford R, Fitzsimmons T, Pandol SJ, Poucell-Hatton S. Mechanisms of cell death after pancreatic duct obstruction in the opossum and the rat. Gastroenterology. 1996;110:875–884. doi: 10.1053/gast.1996.v110.pm8608898. [DOI] [PubMed] [Google Scholar]
- Kaiser AM, Saluja AK, Sengupta A, Saluja M, Steer ML. Relationship between severity, necrosis, and apoptosis in five models of experimental acute pancreatitis. Am J Physiol. 1995;269:C1295–C1304. doi: 10.1152/ajpcell.1995.269.5.C1295. [DOI] [PubMed] [Google Scholar]
- Frossard JL, Rubbia-Brandt L, Wallig MA, Benathan M, Ott T, Morel P, Hadengue A, Suter S, Willecke K, Chanson M. Severe acute pancreatitis and reduced acinar cell apoptosis in the exocrine pancreas of mice deficient for the Cx32 gene. Gastroenterology. 2003;124:481–493. doi: 10.1053/gast.2003.50052. [DOI] [PubMed] [Google Scholar]
- Ethridge RT, Chung DH, Slogoff M, Ehlers RA, Hellmich MR, Rajaraman S, Saito H, Uchida T, Evers BM. Cyclooxygenase-2 gene disruption attenuates the severity of acute pancreatitis and pancreatitis-associated lung injury. Gastroenterology. 2002;123:1311–1322. doi: 10.1053/gast.2002.35951. [DOI] [PubMed] [Google Scholar]
- Song AM, Bhagat L, Singh VP, Van Acker GG, Steer ML, Saluja AK. Inhibition of cyclooxygenase-2 ameliorates the severity of pancreatitis and associated lung injury. Am J Physiol. 2002;283:G1166–G1174. doi: 10.1152/ajpgi.00370.2001. [DOI] [PubMed] [Google Scholar]
- Foitzik T, Hotz HG, Hotz B, Wittig F, Buhr HJ. Selective inhibition of cyclooxygenase-2 (COX-2) reduces prostaglandin E2 production and attenuates systemic disease sequelae in experimental pancreatitis. Hepatogastroenterology. 2003;50:1159–1162. [PubMed] [Google Scholar]
- Guice KS, Oldham KT, Remick DG, Kunkel SL, Ward PA. Anti-tumor necrosis factor antibody augments edema formation in caerulein-induced acute pancreatitis. J Surg Res. 1991;51:495–499. doi: 10.1016/0022-4804(91)90171-h. [DOI] [PubMed] [Google Scholar]
- Hughes CB, Gaber LW, Mohey el-Din AB, Grewal HP, Kotb M, Mann L, Gaber AO. Inhibition of TNF alpha improves survival in an experimental model of acute pancreatitis. Am Surg. 1996;62:8–13. [PubMed] [Google Scholar]
- Johnson CD. Platelet-activating factor and platelet-activating factor antagonists in acute pancreatitis. Dig Surg. 1999;16:93–101. doi: 10.1159/000018699. [DOI] [PubMed] [Google Scholar]
- Bhatia M, Wallig MA, Hofbauer B, Lee HS, Frossard JL, Steer ML, Saluja AK. Induction of apoptosis in pancreatic acinar cells reduces the severity of acute pancreatitis. Biochem Biophys Res Commun. 1998;246:476–483. doi: 10.1006/bbrc.1998.8519. [DOI] [PubMed] [Google Scholar]
- Bhatia M. Apoptosis versus necrosis in acute pancreatitis. Am J Physiol. 2004;286:G189–G196. doi: 10.1152/ajpgi.00304.2003. [DOI] [PubMed] [Google Scholar]
- Talapatra S, Thompson CB. Growth factor signaling in cell survival: implications for cancer treatment. J Pharmacol Exp Ther. 2001;298:873–878. [PubMed] [Google Scholar]
- Franke TF, Hornik CP, Segev L, Shostak GA, Sugimoto C. PI3K/Akt and apoptosis: size matters. Oncogene. 2003;22:8983–8998. doi: 10.1038/sj.onc.1207115. [DOI] [PubMed] [Google Scholar]
- Rivard N, Rydzewska G, Lods JS, Martinez J, Morisset J. Pancreas growth, tyrosine kinase, PtdIns 3-kinase, and PLD involve high-affinity CCK-receptor occupation. Am J Physiol. 1994;266:G62–G70. doi: 10.1152/ajpgi.1994.266.1.G62. [DOI] [PubMed] [Google Scholar]
- Bragado MJ, Groblewski GE, Williams JA. Regulation of protein synthesis by cholecystokinin in rat pancreatic acini involves PHAS-I and the p70 S6 kinase pathway. Gastroenterology. 1988;115:733–742. doi: 10.1016/s0016-5085(98)70153-2. [DOI] [PubMed] [Google Scholar]
- Nozu F, Owyang C, Tsunoda Y. Involvement of phosphoinositide 3-kinase and its association with pp60src in cholecystokinin-stimulated pancreatic acinar cells. Eur J Cell Biol. 2000;79:803–809. doi: 10.1078/0171-9335-00108. [DOI] [PubMed] [Google Scholar]
- Charland S, Boucher MJ, Houde M, Rivard N. Somatostatin inhibits Akt phosphorylation and cell cycle entry, but not p42/p44 mitogen-activated protein (MAP) kinase activation in normal and tumoral pancreatic acinar cells. Endocrinology. 2001;142:121–128. doi: 10.1210/endo.142.1.7908. [DOI] [PubMed] [Google Scholar]
- Gukovskaya AS, Gukovsky I, Jung Y, Mouria M, Pandol SJ. Cholecystokinin induces caspase activation and mitochondrial dysfunction in pancreatic acinar cells. Roles in cell injury processes of pancreatitis. J Biol Chem. 2002;277:22595–22604. doi: 10.1074/jbc.M202929200. [DOI] [PubMed] [Google Scholar]
- Sandoval D, Gukovsky AS, Reavey P, Gukovsky S, Sisk A, Braquet P, Pandol SJ. The role of neutrophils and platelet-activating factor in mediating experimental pancreatitis. Gastroenterology. 1996;111:1081–1091. doi: 10.1016/s0016-5085(96)70077-x. [DOI] [PubMed] [Google Scholar]
- Gukovskaya AS, Vaquero E, Zaninovic V, Gorelick FS, Lusis AJ, Brennan ML, Holland S, Pandol SJ. Neutrophils and NADPH oxidase mediate intrapancreatic trypsin activation in murine experimental acute pancreatitis. Gastroenterology. 2002;122:974–984. doi: 10.1053/gast.2002.32409. [DOI] [PubMed] [Google Scholar]
- Wymann MP, Bjorklof K, Calvez R, Finan P, Thomast M, Trifilieff A, Barbier M, Altruda F, Hirsch E, Laffargue M. Phosphoinositide 3-kinase gamma: a key modulator in inflammation and allergy. Biochem Soc Trans. 2003;31:275–280. doi: 10.1042/bst0310275. [DOI] [PubMed] [Google Scholar]
- Wymann MP, Sozzani S, Altruda F, Mantovani A, Hirsch E. Lipids on the move: phosphoinositide 3-kinases in leukocyte function. Immunol Today. 2000;21:260–264. doi: 10.1016/s0167-5699(00)01649-2. [DOI] [PubMed] [Google Scholar]
- Pedram A, Razandi M, Aitkenhead M, Hughes CC, Levin ER. Integration of the non-genomic and genomic actions of estrogen. Membrane-initiated signaling by steroid to transcription and cell biology. J Biol Chem. 2002;277:50768–50775. doi: 10.1074/jbc.M210106200. [DOI] [PubMed] [Google Scholar]
- Lee JY, Ye J, Gao Z, Youn HS, Lee WH, Zhao L, Sizemore N, Hwang DH. Reciprocal modulation of toll-like receptor-4 signaling pathway involving MyD88 and phosphatidylinositol 3-kinase/AKT by saturated and polyunsaturated fatty acids. J Biol Chem. 2003;278:37041–37051. doi: 10.1074/jbc.M305213200. [DOI] [PubMed] [Google Scholar]