Abstract
Gender influences mediated by 17β-estradiol (E2) have been associated with susceptibility to and severity of autoimmune diseases such as diabetes, arthritis, and multiple sclerosis. In this regard, we have shown that estrogen receptor-α (Esr1) is crucial for the protective effect of 17β-estradiol (E2) in murine experimental autoimmune encephalitis (EAE), an animal model of multiple sclerosis. The expression of estrogen receptors among various immune cells (eg, T and B lymphocytes, antigen-presenting cells) suggests that the therapeutic effect of E2 is likely mediated directly through specific receptor binding. However, the target immune cell populations responsive to E2 treatment have not been identified. In the current study, we induced EAE in T-cell-deficient, severe combined immunodeficient mice or in immunocompetent mice with encephalitogenic T cells from wild-type Esr1+/+ or Esr1 knockout (Esr1−/−) donors and compared the protective E2 responses. The results showed that E2-responsive, Esr1+/+ disease-inducing encephalitogenic T cells were neither necessary nor sufficient for E2-mediated protection from EAE. Instead, the therapeutic response appeared to be mediated through direct effects on nonlymphocytic, E2-responsive cells and down-regulation of the inflammatory response in the central nervous system. These results provide the first demonstration that the protective effect of E2 on EAE is not mediated directly through E2-responsive T cells and raise the alternative possibility that nonlymphocytic cells such as macrophages, dendritic cells, or other nonlymphocytic cells are primarily responsive to E2 treatment in EAE.
A disproportionate number of women suffer from autoimmune diseases such as diabetes, arthritis, and multiple sclerosis.1–3 Gender bias in autoimmune disease and other evidence suggests that sex steroid hormones play a role in regulating immune responses.4,5 Immunomodulatory actions of estrogen appear to result from effects on immune cytokine production,6,7 leukocyte adherence to vascular endothelial cells,8–11 and impairment of macrophage function.12–14 Additionally, and of relevance to central nervous system (CNS) autoimmune disease, a direct neuroprotective effect of E2 has also been demonstrated.15–17 These effects of estrogen are mediated through specific receptors and depend on regulated expression and cellular distribution of these receptors.18 We have shown recently that estrogen receptor-α (Esr1) is crucial for the beneficial therapeutic effect of 17β-estradiol (E2) in murine experimental autoimmune encephalomyelitis (EAE), an animal model of multiple sclerosis.19 The expression of estrogen receptors among various immune cells (eg, T and B lymphocytes, antigen-presenting cells) suggests that the therapeutic effect of E2 is likely mediated directly through specific receptor binding. However, the target immune cell populations responsive to E2 treatment have not been identified. To understand the cellular basis of protective E2 responses, we induced EAE in lymphocyte-deficient severe combined immunodeficient (SCID) mice with encephalitogenic T cells from either wild-type (WT) Esr1+/+ or Esr1−/− (knockout) donors and compared the effects of E2 treatment. The results provide a demonstration of cellular requirements for Esr1 expression and suggest that immune modulation of autoreactive T cells by E2 is indirect and is mediated through direct or indirect effects of E2 on CNS cells expressing the phenotypic characteristics of antigen-presenting cells.
Materials and Methods
Mice
Female, 10- to 12-week-old, C57BL/6 (Esr1+/+, WT) and C57BL/6J-PrkdcSCID (Esr1+/+, SCID) mice were obtained from The Jackson Laboratory, Bar Harbor, ME. The mice were housed and cared for in the Veterinary Medical Unit of the Portland V.A. Medical Center according to institutional guidelines. B6.129 Esr1−/− (Esr1−/−, WT), estrogen receptor-α knockout mice were provided by Dr. Paul Cooke (University of Illinois, Urbana, IL).
Treatment of Mice with 17-β-Estradiol
For 17β-estradiol (E2) hormone therapy, a single 3-mm pellet containing 15 mg of E2 (Innovative Research of America, Sarasota, FL) was implanted (subcutaneously) on the back of each mouse 14 days before induction of EAE. The pellets provide continuous controlled release of a constant level of hormone throughout a period of 60 days. Control animals were left untreated. Preliminary experiments showed unequivocally that pellets containing saline did not affect the course of EAE (unpublished data). The expected serum concentration of 17-β-estradiol was verified in all mice from each group by radioimmunoassay.
Adoptive Transfer of EAE
To prepare T-cell lines specific for MOG-35-55 peptide, C57BL/6 mice were immunized subcutaneously at four sites above the flanks with a total of 0.2 ml of an emulsion containing 200 μg of MOG-35-55 peptide in complete Freund’s adjuvant containing 200 μg of heat-killed Mycobacterium tuberculosis H37RA (Difco Laboratories, Detroit, MI). Ten days after immunization, a single cell suspension of lymph node and spleen cells was cultured with MOG-35-55 peptide (20 μg/ml) at 8 × 106 cells/ml in stimulation medium (RPMI 1640 medium supplemented with nonessential amino acids, sodium pyruvate, 2-ME, and 10% fetal bovine serum) for 48 hours. Antigen (Ag) specificity of the T cells was evaluated in vitro by a thymidine incorporation proliferation assay after stimulation with MOG-35-55 peptide. For adoptive transfer, 20 × 106 cells per mouse were transferred by intraperitoneal injection. On the same day and 2 days after the cell transfer, mice were injected (intraperitoneally) with pertussis toxin (75 and 200 ng/mouse, respectively; List Biological Laboratories, Campbell, CA). Additionally, a portion of the T cells was expanded for 5 to 10 days after the first stimulation in medium containing interleukin (IL)-2 (100 U/ml). These cells were cultured with anti-mouse CD3 (1 μg/ml; clone 145-2C11; BD PharMingen, San Diego, CA) and anti-CD28 (1 μg/ml; clone 37.51; BD PharMingen) monoclonal antibodies immobilized on a tissue culture plate with IL-12 (20 ng/ml; R&D Systems, Minneapolis, MN) and IL-18 (25 ng/ml; MBL, Nagoya, Japan) added to the culture medium for 24 hours. After this time cells were washed three times with phosphate-buffered saline (PBS) and transferred intraperitoneally into naive B6 recipients (1 to 4 × 106 cells/mouse). Mice were injected with pertussigen on days 0 and 2 as above.
Evaluation of Clinical Severity
Mice were scored daily for development of EAE according to the following scale (0 to 6): 0, no signs; 1, limp tail; 1.5, moderate hind limb weakness with difficulty to right itself; 2, moderate hind limb weakness without ability to right itself; 2.5, moderate hind limb weakness without ability to right itself; 3, moderately severe hind limb weakness, walks upright only a few steps; 3.5, moderately severe hind limb weakness, paralysis of one limb; 4, severe hind limb weakness; 4.5, severe hind limb weakness with mild fore limb weakness; 5, paraplegia with no more than moderate fore limb weakness; 5.5, paraplegia with severe fore limb weakness (quadriplegia); 6, moribund condition.
Flow Cytometry
Three-color (fluorescein isothiocyanate, phycoerythrin, cychrome) fluorescence flow cytometry analyses were performed to determine the phenotypes of cells isolated from brain and spinal cord. Briefly, cells were washed with staining medium (PBS containing 0.1% NaN3 and 2% fetal calf serum) and preincubated with anti-mouse CD16/CD32 monoclonal antibody to block nonspecific binding to Fc receptors. All antibodies were purchased from PharMingen. Pooled cells were divided equally (1 to 5 × 105 cells per tube) and were stained with a combination of the following monoclonal antibodies: rat anti-mouse CD3, CD4, CD8, CD11b, CD11c, CD45, VLA-4, LFA-1 for 25 minutes on ice. After incubation cells were washed three times with staining medium and analyzed immediately with a FACScan using CellQuest (Becton-Dickinson, Mountain View, CA) software. Data represent 10,000 events.
Histological Analysis of Spinal Cords
Mice were randomly selected from each E2- and placebo-treated group. Spinal cords were isolated and fixed in 10% paraformaldehyde. Transverse paraffin sections of the spinal cords were stained with Luxol Fast Blue-periodic acid-Schiff reagent-hematoxylin (LFB-PAS-H). The slides were analyzed by light microscopy. Demyelination was detected in spinal cord sections as a decrease or loss of blue stain from the white matter. Inflammatory cells were detected as an accumulation of darkly stained (hematoxylin stained) nuclei.
Reverse Transcriptase-Polymerase Chain Reaction
For quantitative real-time polymerase chain reaction analysis, total RNA was extracted from spleen and spinal cord using Total Rneasy kit (Qiagen, Valencia, CA) according to manufacturer’s instructions, and cDNA was prepared with 2.5 μmol/L of random hexamer primers. The sequence-specific primers were designed using Primer Express software (Applied Biosystems, Inc., Foster City, CA).20 The levels of interferon (IFN)-γ, tumor necrosis factor (TNF)-α, IL-β, RANTES, MIP-2, IP-10, CCR1, CCR2, CCR6, CCR7, and CCR8 expression were quantified by real-time polymerase chain reaction using the ABI 7000 sequence detection system (Applied Biosystems, Inc.). Amplification was performed in a total volume of 25 μl for 40 cycles and products were detected using SYBR Green I dye (Molecular Probes, Eugene, OR). Samples were run in triplicate and their relative expression levels were determined by normalizing expression of each target to L32. Expression levels of normalized samples are displayed in relative expression units.
Results
Passive Transfer of EAE Using Either Esr1+/+ or Esr1−/− MOG-Specific T Cells Is Inhibited in SCID Mice Pretreated with 17β-Estradiol
A role for myelin antigen-specific T lymphocytes in the etiology of multiple sclerosis has been appreciated for many years21,22 and expression of Esr1 by these cells raises the question as to whether they are the primary E2-responsive cells in EAE. To evaluate this possibility, we induced EAE with E2-reposnsive Esr1+/+ encephalitogenic T cells in immunocompetent E2-responsive mice (Table 1, Esr1+/+ T cells → Esr1+/+ WT), in immunocompetent E2-nonresponsive mice (Table 1, Esr1+/+ T cells → Esr1−/− WT), and in E2-responsive T-cell-deficient SCID mice (Esr1+/+ T cells → Esr1+/+ SCID; Table 1). We also induced EAE with E2-nonresponsive Esr1−/− encephalitogenic T cells in E2-responsive T-cell-deficient SCID mice (Table 1, Esr1−/− T cells → Esr1+/+ SCID). E2 responses for each donor/recipient combination were detected by comparing E2-treated and untreated (placebo) animals (Table 1).
Table 1.
Susceptibility to Passively Transferred EAE Using Esr1+/+ and Esr1−/− Encephalitogenic T Cells among Untreated and E2-Treated Esr1+/+WT, Esr1−/−WT, and Esr1+/+ SCID Recipients
| Treatment | Incidence | Onset* | Peak* | CDI* | E2 (pg/ml) | Thymic Index (mg/g) | Uterine Index (mg/g) |
|---|---|---|---|---|---|---|---|
| Esr1+/+T cells → Esr1+/+WT | |||||||
| Placebo | 5/7 | 6.6 ± 1.2 | 4.7 ± 0.2 | 38.1 ± 11.7 | 36.8 ± 10.3 | 4.2 ± 1.1 | 5.6 ± 0.2 |
| E2 | 0/5† | –† | 0† | 0† | 4,171.5 ± 378.8† | 0.9 ± 0.3† | 12.0 ± 0.3† |
| Esr1+/+T cells → Esr1−/−WT | |||||||
| Placebo | 4/5 | 22.0 ± 2.31 | 4.0 ± 1.22 | 17.2 ± 13.7 | 25.0 ± 0.0 | 2.4 ± 0.5 | 6.8 ± 0.7 |
| E2 | 4/5 | 20.0 ± 0.0 | 3.7 ± 1.8 | 21.0 ± 11.5 | 4,384 ± 210.4‡ | 1.3 ± 0.2‡ | 4.9 ± 0.9 |
| Esr1+/+T cells → Esr1+/+SCID | |||||||
| Placebo | 10/11 | 15.3 ± 2.1 | 4.3 ± 0.7 | 27.0 ± 4.9 | 27.0 ± 3.4 | 2.8 ± 0.9 | 5.9 ± 0.6 |
| E2 | 1/7§ | 20§ | 1§ | 1§ | 4,900 ± 297§ | 2.0 ± 0.3 | 13.6 ± 6.6§ |
| Esr1−/−T cells → Esr1+/+SCID | |||||||
| Placebo | 9/12 | 0.5 ± 1.7 | 2.7 ± 0.9 | 21.0 ± 7.0 | 23.6 ± 1.7 | 2.1 ± 0.5 | 6.3 ± 0.9 |
| E2 | 0/13¶ | –¶ | 0¶ | 0¶ | 5,253 ± 93.5¶ | 1.7 ± 0.4 | 13.2 ± 4.2¶ |
Onset, peak, and CDI are presented as the mean trait value ± SEM. The CDI was determined by summing the daily clinical scores.
P value ≤ 0.05, as compared to Esr1+/+ T cells → Esr1+/+ WT without E2.
P value ≤ 0.05, as compared to Esr1+/+ T cells → Esr1−/− WT without E2.
P value ≤ 0.05, as compared to Esr1+/+ T cells → Esr1+/+ SCID without E2.
P value ≤ 0.05, as compared to Esr1−/− T cells → Esr1−/− SCID without E2.
As expected, Esr1+/+ animals with EAE induced by Esr1+/+ T cells (Table 1, Esr1+/+ T cells → Esr1+/+ WT) were highly susceptible to treatment with E2, because E2-treated animals had reduced incidence (none of five versus five of seven), reduced peak disease severity (0 versus 4.7 ± 0.2) and reduced CDI (0 versus 38.1 ± 11.7). Measures of serum E2, thymic index, and uterine index demonstrated that disease differences were associated with differences in systemic levels of E2. In contrast to the Esr1+/+ WT mice with EAE induced by Esr1+/+ T cells, Esr1−/− WT mice with EAE induced by Esr1+/+ T cells (Table 1, Esr1+/+ T cells → Esr −/− WT) developed severed EAE that did not respond to E2 treatment, indicating that Esr1 expression by transferred encephalitogenic T cells was not sufficient and indicating that Esr1 expression by recipient-derived cells was necessary for the E2 response in these immunocompetent mice.
To evaluate the role of E2-responsive recipient-derived cells, the E2 response was evaluated in placebo- and E2-treated immunodeficient Esr1+/+ SCID mice with EAE induced by Esr1+/+ T cells (Table 1, Esr1+/+ T cells→ Esr1+/+ SCID). Similar to immunocompetent Esr1+/+ WT mice, T-cell-deficient Esr1+/+-immunodeficient SCID mice were also susceptible to treatment with E2, displaying an absence of disease compared to placebo-treated mice. Esr1+/+ T-cell-deficient SCID mice with EAE induced by Esr1−/− T cells (Esr1−/− T cells → Esr1+/+ SCID; Table 1) also responded to E2 compared to placebo with complete absence of disease, demonstrating that Esr1 expression by pathogenic T cells was not necessary for the E2 protection even in the absence of all other T cells. As expected, all E2-treated Esr1+/+ mice had a significant, nearly threefold increase in uterine index weight [expressed as uterine weight (mg) divided by body weight (g)] compared to untreated mice. We observed the typical decrease in thymic index after E2 therapy in C57BL/6 (Esr1+/+ WT) mice and a more modest lowering in the thymic index in E2-treated SCID mice compared to placebo-treated mice, apparently because of an overall reduced thymic index in this immunodeficient strain. Radioimmunoassay measurement of E2 serum levels in mice implanted with 15-mg pellets showed the mean level ranged between 4000 to 5000 pg/ml, which was significantly higher than in placebo-treated mice (Table 1). Taken together, these results are consistent with previous findings19 regarding the requirement for E2 signaling through Esr1 and confirm that E2 protection in EAE does not require direct E2 signaling to pathogenic T cells expressing the estrogen receptor-α.
E2 Blocks Trafficking of MOG-Specific T Cells into CNS
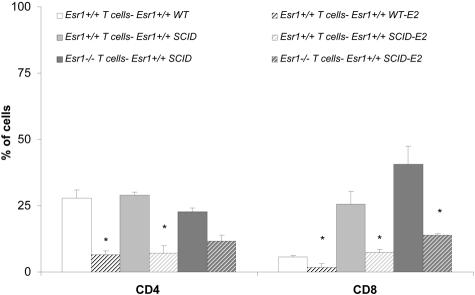
In addition to E2-mediated effects on clinical disease, we evaluated the effect of E2 on T-cell trafficking to the CNS. As shown in Figure 1, E2 treatment caused a reduction of inflammatory T cells in C57BL/6 and C57BL/6 SCID recipients of Esr1+/+ cells, with a marked reduction in CD4+ T cells in Esr1+/+ recipients as determined by fluorescence-activated cell sorting analysis. In C57BL/6 SCID recipients of Esr1−/− cells, we observed a modest decrease in CD4+ T cells and a substantial drop in CD8 cells in the CNS. The percentage of CD8+ T cells was substantially higher in CNS of untreated SCID recipients of Esr1+/+ or Esr1−/− cells when compared to untreated C57BL/6 recipients of Esr1+/+ T cells. This was most likely because of expansion of these cells within the SCID environment, as was reflected by the higher number of cells recovered per cord. However, the frequency of CD8+ cells in the CNS was significantly reduced after E2 therapy in each transfer group. Moreover, the number of CD3+ T cells co-expressing inflammatory adhesion markers VLA-4 and LFA-1 in the brain was reduced in E2-protected versus sick mice (Figure 2), indicating that E2 treatment blocks entry of T cells into the CNS indirectly and independent of E2 effects on adhesion molecule expression by the transferred encephalitogenic T cells. Although some of these changes did not achieve statistical significance, the lack of Esr1 on encephalitogenic T cells did not abrogate the protective effect of E2. Histological sections from brain of C57BL/6 (Figure 3, A and B) and C57BL/6 SCID (Figure 3, C and D) recipients of Esr1+/+ cells showed dramatic differences in pathological disease comparing E2-treated versus placebo-treated mice. C57BL/6 SCID recipients of Esr1−/− MOG-specific T cells also showed dramatically decreased severity of pathological disease with E2 treatment (Figure 3; E, F, K, L).
Figure 1.
E2 treatment decreased the frequency of encephalitogenic T cells in the CNS of Esr1+/+ WT and Esr1+/+ SCID recipients. MNCs were isolated from brain and spinal cord harvested 10 days after disease onset from five mice. Cells were stained with anti-mouse CD3 and anti-mouse CD4 and anti-mouse CD8. Data presented are mean from two independent experiments and indicate the percentage of total gated cells that were dual-positive for CD3 and CD4 or CD8. Significance between control and experimental groups were determined by Student’s t-test (*, P < 0.05).
Figure 2.
E2 treatment decreased the frequency of adhesion molecules on encephalitogenic T cells in the CNS of C57BL/6 and C57BL/6 SCID recipients. MNCs were isolated from brain and spinal cord harvested at 10 days after onset from five mice. Cells were stained with anti-mouse CD3 and anti-mouse VLA-4 and anti-mouse LFA-1 to identify expression of these adhesion molecules on T cells. Data presented are percentage of total gated cells that were dual-positive for CD3 and VLA-4 or LFA-1. Significance between control and experimental groups were determined by Student’s t-test (*, P < 0.05).
Figure 3.
Fixed paraffin-embedded spinal cord sections from control (A–F) or E2-treated (G–L) recipients. Spinal cord from control Esr1+/+ T cells → Esr1+/+ WT (A, B), Esr1+/+ T cells → Esr1+/+ SCID (C, D), and Esr1−/− T cells → Esr1+/+ SCID recipients showed dense mononuclear infiltration with apparent loss of myelin (blue stain, luxol fast blue) in the surrounding myelinated tissue. Spinal cord from E2-treated control Esr1+/+ T cells → Esr1+/+ WT (G, H), Esr1+/+ T cells → Esr1+/+ SCID (I, J) showed no visible signs of inflammation whereas in spinal cord of Esr1−/− T cells → Esr1+/+ SCID (K, L) single mononuclear cells were present without visible demyelination. Original magnification: ×50 (A, C, E, G, I, K); ×150 (B, D, F, H, J, L).
E2 Decreases CNS Macrophages and Dendritic Cells and Increases CNS Microglia
Resident microglia and/or infiltrating macrophages participate in disease pathogenesis through effects on blood brain barrier permeability, antigen presentation, immune regulation, and elaboration of cytotoxins (reactive oxygen and nitrogen, TNF-α).23–25 Because these cells express Esr1 in all of the E2-responsive transfer combinations used in this study, disease-modulating E2 effects on these cells were likely.26 To evaluate this possibility, cells isolated from the CNS were triple-stained with CD11b, CD11c, and CD45. The CNS of E2-treated mice contained fewer harvested mononuclear cells and fewer CD11b+ CD11c+ dendritic cells compared to untreated mice and this did not depend on T cell’s expression of Esr1 (Table 2). The CNS from untreated Esr1−/− T cells → Esr1+/+ SCID mice had the most total CNS mononuclear cells (40 × 104 per cord) and the highest proportion of CD11b+ CD11c+ dendritic cells (15 ± 3%), indicating that cellular infiltration into the CNS and CNS APC activity were vigorous during disease induced in SCID mice by Esr1−/− or Esr1+/+ T cells in the absence of E2 treatment. CD45+ CD11b− cells comprised primarily of T lymphocytes were similar among all untreated mice and were markedly decreased after E2 treatment. It is notable that the decrease in CD45+ CD11b− cells was not as great (74 ± 4, untreated; 44 ± 10, E2 treated) in mice with Esr1−/− T cells compared to either WT (C57BL/6) (76 ± 5, placebo; 22 ± 6, E2 treated) or SCID mice (72 ± 4, placebo; 18 ± 5, E2 treated) with Esr1+/+ T cells, suggesting a possible role for T cell Esr1 in the loss of T cells from the CNS of E2-treated mice.
Table 2.
The Frequency of DCs, Peripheral Macrophages, and Resident Microglia in CNS of Sick and Protected Mice
|
Esr1+/+ T cells → Esr1+/+ WT
|
Esr1+/+ T cells → Esr1+/+ SCID
|
B6 129 Esr1−/− T cells → Esr1+/+ SCID
|
||||
|---|---|---|---|---|---|---|
| No treatment (MFI)* | Treatment (MFI) | No treatment (MFI) | Treatment (MFI) | No treatment (MFI) | Treatment (MFI) | |
| Total cells harvested per mouse | 30 × 104 | 5 × 104 | 24 × 104 | 4 × 104 | 40 × 104 | 6 × 104 |
| Percentage of CD11b+CD11c+ (DC) among total | 6 ± 1 | 2 ± 1 | 9 ± 2 | 3 ± 2 | 15 ± 3 | 4 ± 2 |
| Percentage of CD45+CD11b− (T cells) among total | 76 ± 5 | 22 ± 6 | 72 ± 4 | 18 ± 5 | 74 ± 4 | 44 ± 10 |
| Percentage of CD45+CD11b+ (Mø) among total | 24 ± 4 | 78 ± 3 | 18 ± 2 | 72 ± 7 | 26 ± 3 | 56 ± 5 |
| Percentage of CD45+ among CD11b+ | 100 | 100 | 100 | 100 | 100 | 100 |
| Density of CD45 expression | ||||||
| CD45intr (microglia) | 30 ± 5 (671) | 69 ± 2 (560) | 23 ± 2 (790) | 84 ± 6 (351) | 35 ± 7 (897) | 72 ± 4 (585) |
| CD45high (Mø) | 70 ± 2 | 31 ± 1 | 77 ± 8 | 16 ± 8 | 65 ± 9 | 28 ± 2 |
MNCs were isolated from brain and spinal cord harvested at 10 days after disease onset from five mice. Cells were stained with anti-mouse CD11b, CD11c, and CD45. Data show the percentage of total gated cells that were positive for the indicated markers. The frequency of CD11b with different densities of CD45 were estimated among CD11b+ CD11c− (R2) as described in Material and Methods. The values presented are representative of one of two independent experiments.
Mean fluorescence intensity (MFI) for CD45 as measured among CD11b+ (R3).
In addition to the loss of dendritic cells and T cells with E2 treatment, analytical-negative gating of the CD11c+ cells (Table 2; Figure 4, R2 gate) permitted analysis of the CD11b+ CD11c− cells (microglia and macrophages) for expression of CD45, a marker for distinguishing CD11b+ resident microglia from infiltrating macrophages. In contrast to the decreases in total cells, dendritic cells, and T cells induced by E2, total CD45+ CD11b+ myelomonocytic cells increased with E2 treatment, reflecting a higher proportion of microglial cells because of decreased total inflammatory cells. This was also demonstrated by higher proportions of CD11b+ CD45intr cells (microglia) (Table 2; Figure 4, R3 gate) in E2 compared to untreated mice. As was found for dendritic cells, E2-treated mice had relatively fewer CD11b+ CD45high cells (macrophages) (Table 2; Figure 4, R4 gate) in the CNS compared to untreated mice and these differences were observed independent of Esr1 expression by the transferred T cells.
Figure 4.
CD45 expression of microglia/CNS-associated macrophages from control and E2-treated recipients. Microglia/CNS-associated macrophages were gated as CD11b+CD11c− cells (R2) to exclude the potential dendritic cells. All cells from R2 express CD45 and subdivide into two main populations: CD45intr (R3) and CD45high (R4).
E2 Therapy Decreased Expression of Cytokines, Chemokines, and Chemokine Receptors
To gain insights about the cellular and molecular mechanisms of E2 responses in the spinal cord, we evaluated relative expression levels of proinflammatory cytokines (IFN-γ, TNF-α, IL-1β) and various chemokines (RANTES, MIP-2, IP-10) and chemokine receptors (CCR1, CCR2, CCR6, CCR7, CCR8) known to be involved in inflammatory tissue reactions (Table 3). Comparisons between untreated and E2-treated spinal cords were performed (Table 3) and revealed striking differences in the effect of E2 that depended on the presence of recipient-derived nonspecific T cells (comparing WT versus SCID recipients of Esr1+/+ T cells) or that depended on T-cell expression of Esr1 (comparing E2 responses in Esr1+/+ SCID mice with disease induced by Esr1+/+ versus Esr1−/− T cells). E2-induced changes in gene expression in the spinal cord are expressed as the fold change of gene expression in E2-treated mice compared to untreated mice (Table 3). The comparison between Esr1+/+ T cells → Esr1+/+ WT recipients and Esr1+/+ T cells → Esr1+/+ SCID recipients revealed enhanced E2-induced down-regulation of IFN-γ expression in the absence of recipient T cells in Esr1+/+ SCID mice (Table 3, center column, −246) compared to Esr1+/+ WT mice (Table 3, left column, −42) suggesting that E2 down-regulates, either directly or indirectly IFN-γ expression by transferred encephalitogenic T cells. Enhanced down-regulation of TNF-α (−58 versus −12), MIP-2 (−166 versus −86) and CCR8 (−297 versus −134) in the presence of recipient-derived lymphocytes in Esr1+/+ recipients suggests that E2 down-regulates these mRNAs primarily on recipient-derived cells or cells recruited nonspecifically to the CNS. Other E2 effects on cytokines and receptor expression did not vary significantly depending on the presence or absence of recipient-derived lymphocytes when comparing Esr1+/+ WT versus Esr1+/+ SCID recipients.
Table 3.
Gene Expression Was Quantified by Real-Time PCR from Spinal Cord cDNA of E2-Treated Mice without Signs of Disease and Sick Mice with Moderate-Severe or Severe Clinical Signs of EAE
| GENE | Fold change
|
||
|---|---|---|---|
| Esr1+/+ T-cells → Esr1+/+ WT | Esr1+/+ T-cells → Esr1+/+ SCID | Esr1−/− T-cells → Esr1+/+ SCID | |
| IFN-γ | −42** | −246* | −90** |
| TNF-α | −58* | −12* | −267* |
| IL-1β | −13* | −17** | −11** |
| RANTES | −108* | −121** | −30* |
| MIP-2 | −166* | −86* | −302* |
| IP-10 | −13*** | −30* | −147** |
| CCR1 | −83 | −77 | −83* |
| CCR2 | −8* | −11* | −134* |
| CCR6 | −108* | −10* | −60* |
| CCR7 | −293* | −237* | −82* |
| CCR8 | −297* | −134* | −82* |
For all genes, relative expression level was determined by normalization to L32. Fold change (FC) in mRNA expression was calculated as x/y, where x = relative expression units detected in untreated recipient, y = relative expression units detected in E2-treated recipient. Decreased expression because of E2 treatment was assigned a negative value. Both x and y values were equal to the mean from two independent experiments. P values were determined using Student’s t-test and only P ≤ 0.05 was considered significant.
, P = 0.05–0.01,
, P = 0.0001,
, P ≤ 0.0001.
A comparison between Esr1+/+ T cells → Esr1+/+ SCID recipients and Esr1−/− T cells → Esr1+/+ SCID recipients revealed enhanced E2-induced down-regulation of TNF-α, MIP-2, IP-10, CCR2, and CCR6 in mice lacking Esr1 expression on T cells compared to mice with encephalitogenic Esr1+/+ T cells, and enhanced down-regulation of IFN-γ, RANTES, and CCR7 in mice with Esr1+/+ encephalitogenic T cells (Table 3). Therefore, the E2-induced gene expression changes in mice possessing Esr1+/+ T cells (Esr1+/+ T cells → Esr1+/+ WT and Esr1+/+ T cells → Esr1+/+ SCID) may include direct effects of E2 on T cell gene expression (eg, down-regulation of expressed proinflammatory genes in the CNS) and these direct effects on T-cell gene expression are not essential for E2-mediated protection. In contrast, E2-induced gene expression changes by nonlymphoid cells in SCID recipients of Esr1−/− T cells (eg, decreased TNF-α, MIP-2, IP-10, CCR2, and CCR6 mRNA expression) may result from direct effects of E2 on nonlymphoid cells whose E2 response is essential for protection.
Discussion
Previous reports showed effective treatment of clinical EAE with 17β-estradiol (E2).27 The protective effect of E2 was associated with diminished migration of encephalitogenic T cells into the CNS and effects on dendritic cells and macrophages.28,29 Recently, we also showed using knockout mice that Esr1 is required for the protective effect of E2 on EAE. The pathogenic role for myelin-specific T lymphocytes in EAE suggested that E2-mediated protection from EAE might be mediated through direct effects on Esr1-positive T lymphocytes. We examined this possibility by evaluating E2-protective effects in mice with EAE induced after adoptive transfer of Esr1+/+ versus Esr1−/− encephalitogenic T cells.
E2 treatment protected mice from EAE and did not depend on Esr1 expression by encephalitogenic myelin-specific T cells. This demonstrated that the disease-initiating lymphocytes are not the primary E2-responsive cells and implicates other cell types as the primary E2-responsive cells in EAE. The clinical response to E2 was associated with decreased mononuclear cells (total cells, CD4+ and CD8+ lymphocytes, and CD11b+ macrophages and dendritic cells) in the CNS. The numbers of T cells expressing inflammatory adhesion markers VLA-4 and LFA-1 in the brain was reduced in E2-protected versus sick mice (Figure 2), indicating that E2 treatment blocks entry of T cells into the CNS indirectly and independent of E2 effects on adhesion molecule expression by encephalitogenic T cells. An absence of inflammation and demyelination, pathological disease in all E2-protected mice regardless of Esr1 expression by encephalitogenic T cells further suggests that encephalitogenic T cells do not directly mediate the E2 treatment response and nonlymphoid cells are the primary E2-responsive cells.
The clinical response to E2 was also associated with a dramatic decrease in absolute numbers of CD11b+, myelomonocytic lineage-derived macrophages, and dendritic cells in the CNS. CD11c+ CD11b+ dendritic cells and CD45high CD11b+ macrophages decreased in absolute numbers and relative abundance in the CNS independent of Esr1 expression by T cells. CD45intr CD11b+ microglia remained relatively stable in absolute terms but increased in relative abundance because of the E2-induced drop in total cells within the CNS. These results suggest that nonlymphoid Esr1+/+ cells such as macrophages and dendritic cells, rather than T cells, are the primary E2-responsive cells.
Certain E2-induced gene expression changes detected by quantitative reverse transcriptase-polymerase chain reaction demonstrated that T cells expressing Esr1 appeared to respond directly to E2 in these experiments. Thus, E2 levels achieved with the duration, route, and dose used here were effective at inducing immunologically relevant responses by Esr1+/+ T cells. However, such direct E2 responses by T cells were not required to induce protection in response to E2 because Esr1−/− T cells also supported the E2 protective response in T-cell-deficient SCID mice. Other cell types, besides encephalitogenic T cells or other T cells are likely to be directly responsive to E2 and are therefore the primary cellular element for mediating protection. In contrast to T cells, nonlymphoid Esr1+/+ cells were required for down-regulated expression of TNF-α, MIP-2, IP-10, and CCR2 in response to E2 and this was independent of Esr1 expression by T cells. Thus, these E2-induced gene expression changes depended on nonlymphoid cells independent of Esr1 expression by T cells and are therefore implicated as crucial participants in the molecular mechanism of E2 protection.
Among the down-regulated expressed genes, down-regulation of TNF-α and MIP-2 appeared to be essential for E2-induced protection in EAE because substantial down-regulation of these gene products was uniformly observed in E2-protected mice. E2-induced down-regulation of TNF-α expression may prevent secondary induction of MIP-2 expression by TNF-α-responsive macrophages or astrocytes.30–32 Decreased MIP-2 expression would be expected to cause diminished recruitment of CCR2-expressing myelomonocytic lineage cells (blood monocytes and tissue macrophages) from the circulation.33–36 Therefore, our detection of reduced CCR2 mRNA expression in the spinal cord was likely because of the diminished recruitment of CCR2-expressing monocytes/macrophages because of decreased MIP-2. Although beyond the scope of the present study, future experiments evaluating the phenotype and expressed genes of blood cells before CNS infiltration should contribute additional useful information necessary for understanding the development of the inflammatory response during disease and E2 protection.
E2-induced down-regulation of certain gene products (IP-10, CCR2, and CCR6) appeared to occur to the greatest degree in SCID mice with EAE induced by Esr1−/− T cells and to a lesser degree in mice possessing T cells with Esr1 (eg, SCID mice with Esr1+/+ encephalitogenic T cells or immunocompetent WT mice). Because E2-induced down-regulation of these genes was mediated primarily through E2-responsive Esr1+/+ nonlymphoid cells, we expected to detect this primary E2 response even when E2-responsive T cells were present. Our inability to detect down-regulation of IP-10, CCR2, and CCR6 in mice with E2-responsive T cells to the same degree as in the absence of E2-responsive T cells may be related to differences between E2 responses expressed by lymphoid versus nonlymphoid cells. As an example of this, CCR8 was down-regulated to the greatest extent by E2 only in mice possessing Esr1+/+ T lymphocytes, even though all mice used in these studies possessed E2-responsive nonlymphoid cells. These observations suggest that changes in IP-10, CCR2, and CCR6 expression by nonlymphoid cells may be crucial for E2-induced protection despite an inability to detect such expression changes in the presence of E2-responsive lymphocytes to the same degree as in mice without E2-responsive lymphocytes.
In summary, 17β-estradiol protected mice from EAE induced by adoptively transferred myelin-specific T lymphocytes. Protection was observed as a virtually complete absence of clinical neurological deficits and an absence of inflammatory and other pathological changes in the CNS and did not depend on E2-reponsive lymphocytes. CD4+ and CD8+ lymphocytes, monocytes/macrophages, and CD11c+ dendritic cells were each present in greatly reduced numbers in the CNS of E2-protected mice compared to unprotected, untreated mice. Microglial cell numbers remained relatively stable with E2 treatment. E2-induced protection was mediated through nonlymphoid E2-responsive cells. E2-induced down-regulation of TNF-α and MIP-2 at the transcriptional level was consistently observed, suggesting a primary role for TNF-α-producing macrophages and dendritic cells in E2 protection.28 A sequential regulatory cascade in which TNF-α induces MIP-2 expression in the CNS may be primarily responsible for recruiting CCR2+ MIP-2-responsive monocyte/macrophage cells to the diseased CNS in the absence of E2. E2 induced a reduction in TNF-α, MIP-2, and CCR2 expression; prevented CNS inflammation; and protected mice from developing disease. Our results provide the first demonstration that the protective effect of E2 in EAE is mediated by nonlymphoid cells capable of modulating this T-cell-mediated autoimmune response. This raises the possibility that nonlymphoid cells such as bone marrow-derived dendritic cells and macrophages are the primary E2-responsive cells in EAE. Future experiments using Esr1+/+ and Esr1−/− bone marrow chimeric mice with EAE induced by Esr1−/− T cells will be conducted to identify the developmental origins of the primary E2-responsive cells.
Footnotes
Address reprint requests to Halina Offner, Dr. Med., Neuroimmunology Research R&D-31, Portland VA Medical Center, 3710 SW US Veterans Hospital Rd., Portland, OR 97239. E-mail: offnverva@ohsu.edu.
Supported by the National Institutes of Health (grants NS23444 and NS45445 to H.O., NS23221 to A.A.V., NS39122 to R.E.J., and ES11590 to P.C.), the National Multiple Sclerosis Society (to H.O., A.A.V.), The Nancy Davis MS Center Without Walls (to H.O., R.E.J.), and the Department of Veterans Affairs (to R.E.J., A.A.V.).
M.J.P and R.E.J. contributed equally to this work.
References
- Whitacre CC, Reingold SC, O’Looney PA. A gender gap in autoimmunity. Science. 1999;283:1277–1280. doi: 10.1126/science.283.5406.1277. [DOI] [PubMed] [Google Scholar]
- Jansson L, Holmdahl R. Estrogen-mediated immunosuppression in autoimmune diseases. Inflamm Res. 1998;47:290–301. doi: 10.1007/s000110050332. [DOI] [PubMed] [Google Scholar]
- Beeson PB. Age and sex associations of 40 autoimmune diseases. Am J Med. 1994;96:457–462. doi: 10.1016/0002-9343(94)90173-2. [DOI] [PubMed] [Google Scholar]
- Birk K, Ford C, Smeltzer S, Ryan D, Miller R, Rudick RA. The clinical course of multiple sclerosis during pregnancy and the puerperium. Arch Neurol. 1990;47:738–742. doi: 10.1001/archneur.1990.00530070026007. [DOI] [PubMed] [Google Scholar]
- Korn-Lubetzki I, Kahana E, Cooper G, Abramsky O. Activity of multiple sclerosis during pregnancy and puerperium. Ann Neurol. 1984;16:229–231. doi: 10.1002/ana.410160211. [DOI] [PubMed] [Google Scholar]
- Correale J, Arias M, Gilmore W. Steroid hormone regulation of cytokine secretion by proteolipid protein-specific CD4+ T cell clones isolated from multiple sclerosis patients and normal control subjects. J Immunol. 1998;161:3365–3374. [PubMed] [Google Scholar]
- Krishnan L, Guilbert LJ, Russell AS, Wegmann TG, Mosmann TR, Belosevic M. Pregnancy impairs resistance of C57BL/6 mice to Leishmania major infection and causes decreased antigen-specific IFN-gamma response and increased production of T helper 2 cytokines. J Immunol. 1996;156:644–652. [PubMed] [Google Scholar]
- Pare G, Krust A, Karas RH, Dupont S, Aronovitz M, Chambon P, Mendelsohn ME. Estrogen receptor-alpha mediates the protective effects of estrogen against vascular injury. Circ Res. 2002;90:1087–1092. doi: 10.1161/01.res.0000021114.92282.fa. [DOI] [PubMed] [Google Scholar]
- Caulin-Glaser T, Watson CA, Pardi R, Bender JR. Effects of 17beta-estradiol on cytokine-induced endothelial cell adhesion molecule expression. J Clin Invest. 1996;98:36–42. doi: 10.1172/JCI118774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhat MY, Lavigne MC, Ramwell PW. The vascular protective effects of estrogen. FASEB J. 1996;10:615–624. [PubMed] [Google Scholar]
- Mori M, Tsukahara F, Yoshioka T, Irie K, Ohta H. Suppression by 17beta-estradiol of monocyte adhesion to vascular endothelial cells is mediated by estrogen receptors. Life Sci. 2004;75:599–609. doi: 10.1016/j.lfs.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Mor G, Sapi E, Abrahams VM, Rutherford T, Song J, Hao XY, Muzaffar S, Kohen F. Interaction of the estrogen receptors with the Fas ligand promoter in human monocytes. J Immunol. 2003;170:114–12l. doi: 10.4049/jimmunol.170.1.114. [DOI] [PubMed] [Google Scholar]
- Wira CR, Rossoll RM. Antigen-presenting cells in the female reproductive tract: influence of sex hormones on antigen presentation in the vagina. Immunology. 1995;84:505–508. [PMC free article] [PubMed] [Google Scholar]
- Gulshan S, McCruden AB, Stimson WH. Oestrogen receptors in macrophages. Scand J Immunol. 1990;31:691–697. doi: 10.1111/j.1365-3083.1990.tb02820.x. [DOI] [PubMed] [Google Scholar]
- Bruce-Keller AJ, Keeling JL, Keller JN, Huang FF, Camondola S, Mattson MP. Antiinflammatory effects of estrogen on microglial activation. Endocrinology. 2000;141:3646–3656. doi: 10.1210/endo.141.10.7693. [DOI] [PubMed] [Google Scholar]
- Santizo RA, Anderson S, Ye S, Koenig HM, Pelligrino DA. Effects of estrogen on leukocyte adhesion after transient forebrain ischemia. Stroke. 2000;31:2231–2235. doi: 10.1161/01.str.31.9.2231. [DOI] [PubMed] [Google Scholar]
- Vegeto E, Belcredito S, Etteri S, Ghisletti S, Brusadelli A, Meda C, Krust A, Dupont S, Ciana P, Chambon P, Maggi A. Estrogen receptor-alpha mediates the brain antiinflammatory activity of estradiol. Proc Natl Acad Sci USA. 2003;100:9614–9619. doi: 10.1073/pnas.1531957100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrgregor JI, Jordan VC. Basic guide to the mechanism of antiestrogen rceptors. Steroids. 67:471–475. [Google Scholar]
- Polanczyk M, Zamora A, Subramanian S, Matejuk A, Hess DL, Blankenhorn EP, Teuscher C, Vandenbark AA, Offner H. The protective effect of 17beta-estradiol on experimental autoimmune encephalomyelitis is mediated through estrogen receptor-alpha. Am J Pathol. 2003;163:1599–1605. doi: 10.1016/s0002-9440(10)63516-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huan J, Subramanian S, Jones R, Rich C, Link J, Mooney J, Bourdette DN, Vandenbark AA, Burrows GG, Offner H. Monomeric recombinant TCR ligand reduces relapse rate and severity of experimental autoimmune encephalomyelitis in SJL/J mice through cytokine switch. J Immunol. 2004;172:4556–4566. doi: 10.4049/jimmunol.172.7.4556. [DOI] [PubMed] [Google Scholar]
- Zamvil SS, Steinman L. The T lymphocyte in experimental allergic encephalomyelitis. Annu Rev Immunol. 1990;8:579–621. doi: 10.1146/annurev.iy.08.040190.003051. [DOI] [PubMed] [Google Scholar]
- Paterson PY. Experimental allergic encephalomyelitis: role of fibrin deposition in immunopathogenesis of inflammation in rats. Fed Proc. 1976;35:2428–2434. [PubMed] [Google Scholar]
- Fischer HG, Reichmann G. Brain dendritic cells and macrophages/microglia in central nervous system inflammation. J Immunol. 2001;166:2717–2726. doi: 10.4049/jimmunol.166.4.2717. [DOI] [PubMed] [Google Scholar]
- Ford AL, Foulcher E, Lemckert FA, Sedgwick JD. Microglia induce CD4 T lymphocyte final effector function and death. J Exp Med. 1966;184:1737–1745. doi: 10.1084/jem.184.5.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold R, Schmied M, Tontsch U, Hartung HP, Wekerle H, Toyka KV, Lassmann H. Antigen presentation by astrocytes primes rat T lymphocytes for apoptotic cell death. A model for T-cell apoptosis in vivo. Brain. 1996;119:651–659. doi: 10.1093/brain/119.2.651. [DOI] [PubMed] [Google Scholar]
- Tran EH, Hoekstra K, van Rooijen N, Dijkstra CD, Owens T. Immune invasion of the central nervous system parenchyma and experimental allergic encephalomyelitis, but not leukocyte extravasation from blood, are prevented in macrophage-depleted mice. J Immunol. 1998;161:3767–3775. [PubMed] [Google Scholar]
- Bebo BF, Jr, Fyfe-Johnson A, Adlard K, Beam AG, Vandenbark AA, Offner H. Low-dose estrogen therapy ameliorates experimental autoimmune encephalomyelitis in two different inbred mouse strains. J Immunol. 2001;166:2080–2089. doi: 10.4049/jimmunol.166.3.2080. [DOI] [PubMed] [Google Scholar]
- Ito A, Buenafe AC, Matejuk A, Zamora A, Silverman M, Dwyer J, Vandenbark AA, Offner H. Estrogen inhibits systemic T cell expression of TNF-alpha and recruitment of TNF-alpha(+) T cells and macrophages into the CNS of mice developing experimental encephalomyelitis. Clin Immunol. 2002;102:275–282. doi: 10.1006/clim.2001.5175. [DOI] [PubMed] [Google Scholar]
- Liu HY, Buenafe AC, Matejuk A, Ito A, Zamora A, Dwyer J, Vandenbark AA, Offner H. Estrogen inhibition of EAE involves effects on dendritic cell function. J Neurosci Res. 2002;70:238–248. doi: 10.1002/jnr.10409. [DOI] [PubMed] [Google Scholar]
- Luo Y, Fischer FR, Hancock WW, Dorf ME. Macrophage inflammatory protein-2 and KC induce chemokine production by mouse astrocytes. J Immunol. 2000;165:4015–4023. doi: 10.4049/jimmunol.165.7.4015. [DOI] [PubMed] [Google Scholar]
- Karpus WJ, Ransohoff RM. Chemokine regulation of experimental autoimmune encephalomyelitis: temporal and spatial expression patterns govern disease pathogenesis. J Immunol. 1998;161:2667–2671. [PubMed] [Google Scholar]
- Babcock AA, Kuziel WA, Rivest S, Owens T. Chemokine expression by glial cells directs leukocytes to sites of axonal injury in the CNS. J Neurosci. 2003;23:7922–7930. doi: 10.1523/JNEUROSCI.23-21-07922.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaupp S, Pitt D, Kuziel WA, Cannella B, Raine CS. Experimental autoimmune encephalomyelitis (EAE) in CCR2(−/−) mice: susceptibility in multiple strains. Am J Pathol. 2003;162:139–150. doi: 10.1016/S0002-9440(10)63805-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izikson L, Klein RS, Charo IF, Weiner HL, Luster AD. Resistance to experimental autoimmune encephalomyelitis in mice lacking the CC chemokine receptor (CCR)2. J Exp Med. 2000;192:1075–1080. doi: 10.1084/jem.192.7.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuziel WA, Morgan SJ, Dawson TC, Griffin S, Smithies O, Ley K, Maeda N. Severe reduction in leukocyte adhesion and monocyte extravasation in mice deficient in CC chemokine receptor 2. Proc Natl Acad Sci USA. 1997;94:12053–12058. doi: 10.1073/pnas.94.22.12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma M, Wei T, Boring L, Charo IF, Ransohoff RM, Jakeman LB. Monocyte recruitment and myelin removal are delayed following spinal cord injury in mice with CCR2 chemokine receptor deletion. J Neurosci Res. 2002;68:691–702. doi: 10.1002/jnr.10269. [DOI] [PubMed] [Google Scholar]