Abstract
Epidemiological studies show that some nonsteroidal anti-inflammatory drugs, nonspecific inhibitors of the cyclooxygenase enzyme, reduce the incidence of Alzheimer’s disease (AD). We determined the impact of two nonsteroidal anti-inflammatory drugs on Aβ levels, deposition, and metabolism in a mouse model (the Tg2576) of AD-like amyloidosis. To this end, mice were treated with indomethacin and nimesulide continuously from 8 months of age until they were 15 months old. At the end of the study, indomethacin significantly reduced Aβ1-40 and Aβ1-42 levels in both cortex and hippocampus. This decrease was coincidental with a significant reduction of the nuclear factor (NF)-κB activity. By contrast, nimesulide had no effect on both Aβ peptides and NF-κB. Consistently, mice receiving indomethacin, but no nimesulide, showed a significant reduction in the amyloid burden compared with placebo. Neither drug had an effect on plasma levels of Aβ peptides or the Aβ precursor protein metabolism. In vitro studies confirmed that genetic absence of this factor reduces the anti-amyloidogenic effect of indomethacin. These findings indicate that chronic administration of indomethacin by blocking the activation of the NF-κB significantly reduces the amyloid pathology in Tg2576 mice, and provide insights into the mechanisms by which this drug could slow progression of AD.
Epidemiological studies have suggested that the use of nonsteroidal anti-inflammatory drugs (NSAIDs) may prevent or delay the clinical features of Alzheimer’s disease (AD).1 Traditional NSAIDs reversibly inhibit the cyclooxygenase (COX) activity of the enzyme prostaglandin H synthase, which converts arachidonic acid to prostaglandin H2, the immediate precursor of various prostaglandins and thromboxane.2 There are two known COX isoforms, which have highly structural identity, but are different in substrate and inhibitory selectivity, as well as intracellular localization. COX-1 is expressed constitutively in many tissues and its metabolic products are considered to be involved in cellular housekeeping functions.2 COX-2 has lower expression levels in tissues under normal circumstances and is increased during inflammatory responses.2 Because of this evidence, a lot of attention has recently been focused on the role that these two enzyme isoforms could play in AD pathogenesis. However, the reports regarding COX expression levels in AD have been conflicting.3–5 Nonetheless, it is still unclear how COX-1 and COX-2 expression levels and activities relate to amyloid β (Aβ) deposition, and in particular whether they are primary or secondary events to this accumulation or they are two independent processes.
Interestingly, in a small clinical study indomethacin, a nonselective COX-1 inhibitor, improved cognitive performance of mild to moderately impaired AD patients.6 Some studies indicated that nonselective COX, rather than selective COX-2-specific inhibitors, influence total brain amyloidosis in animal models of AD.7,8 However, these studies did not address the issue of a COX involvement in this inhibitory effect, or the question of a possible regional differential effect of these drugs on the soluble versus the insoluble fractions of the two Aβ isoforms (1-40 and 1-42). Further, in vitro studies performed in nonneuronal cells showed that very high concentrations of a subset of NSAIDs such as ibuprofen and indomethacin, all with prevalent inhibitory activity toward COX-1, but not COX-2 inhibitors, preferentially decreases Aβ1-42 but not Aβ1-40 peptide production by modulating the activity or specificity of γ-secretase.9,10 However, these results were not confirmed when the same drugs were tested in neuronal cell cultures.11
The aim of our study was to investigate whether chronic selective pharmacological inhibition of COX-1 activity by indomethacin,12 or COX-2 by nimesulide13 had an effect on amyloidosis in a mouse model of AD-like amyloidosis, the Tg2576.14 In particular, we sought to investigate if chronic administration of these drugs had a different effect on the soluble versus insoluble fractions of Aβ1-40 and Aβ1-42, on the Aβ precursor protein (APP) metabolism, the peripheral Aβ levels, and the amount of amyloid deposited.
Materials and Methods
Animals
The genotype and phenotypic features of the heterozygote Tg 2576 and wild-type (WT) littermates studied here have been already described elsewhere.14 Mice were weaned at 4 weeks of age, kept on a chow diet, and males were always separated from females for the entire study. In the first study, brain tissues were obtained from cohorts of mice at 4, 8, 12, and 15 months of age (three Tg and three WT for each time point). In a separate study, 8-month-old male and female Tg animals were divided in three groups (n = 10 each), and randomized to receive indomethacin (10 mg/L) or nimesulide (40 mg/L) in their drinking water, or placebo for 7 months before being sacrificed. Drinking water was always replaced every other day. Preliminary experiments demonstrated that the selected dose of indomethacin suppressed total COX-1 activity in vivo and ex vivo without concomitant significant reduction of COX-2.12,13 Similarly, the dose of nimesulide was chosen based on previous experiments that showed a significant reduction of total COX-2, but no effect on COX-1 activity.12,13 During the study, mice in all groups gained weight regularly, and no significant difference in weight was detected among the three groups. Macroscopic postmortem examination showed no sign of gastric lesions or effect on the overall health was observed in the animals receiving the active treatments. At the end of the study, urine samples were collected from both groups and analyzed for 2,3dinor-TxB2, an index of systemic COX-1 activity, and 2,3dinor-6-ketoPGF1α, an index of systemic COX-2 activity, by gas chromatography/mass spectrometry (GC/MS), as previously described.12,15
Tissue Preparation
Animals were anesthetized and euthanized following procedures recommended by the Panel on Euthanasia of the American Veterinary Medical Association (AVMA). They were always perfused intracardially for 30 minutes with ice-cold 0.9% phosphate-buffered saline (PBS), containing ethylenediaminetetraacetic acid (EDTA, 2 mmol/L) and butylated hydroxytoluene (2 mmol/L), pH 7.4. Brains were removed and one hemisphere was fixed by immersion in 4% paraformaldehyde in 0.1 mol/L PBS (pH 7.4) at 4°C overnight, blocked in the coronal plane, and embedded in paraffin as previously described for immunohistochemistry.16 The other hemisphere was gently rinsed in cold 0.9% PBS, then immediately dissected in three anatomical regions (cerebral cortex, hippocampus, and cerebellum) for biochemistry.
For PGE2, TxB2, and 8,12-iso-iPF2α-VI analyses, aliquots of the tissues were homogenized, lipids extracted, and their levels measured by a stable isotope dilution method using GC/MS, as previously described.12,15–17 Brain tissues were always analyzed in a coded manner.
Aβ1-40 and Aβ1-42 Levels
Sequential extraction of brain samples was performed with high-salt buffer and formic acid, respectively, to measure soluble and insoluble Aβ1-40 and Aβ1-42 levels, as previously described.16 Briefly, cerebral cortex, hippocampus, and cerebellum were serially extracted in high-salt Re-assembly buffer (0.1 mol/L Tris, 1 mmol/L EGTA, 0.5 nmol/L MgSO4, 0.75 mol/L NaCl, and 0.02 mol/L NaF, pH 7.0) containing protease inhibitor mixture (pepstatin A, leupeptin, N-tosyl-l-phenylalanine chloromethyl ketone, soybean trypsin inhibitor, each at 1 μg/ml in 5 mmol/L EDTA). Homogenates were centrifuged at 100,000 × g for 1 hour at 4°C. Supernatants were removed, pellets were resuspended in 70% formic acid and sonicated and centrifuged at 100,000 × g for 1 hour at 4°C. Supernatants were diluted 1:20 with 1 mol/L Tris base. Samples were mixed with buffer EC [0.02 mol/L sodium phosphate, 0.2 mmol/L EDTA, 0.4 mol/L NaCl, 0.2% bovine serum albumin, 0.05% CHAPS, 0.4% Block-ace (Dainippon, Suita, Osaka), 0.05% sodium azide, pH 7.0] and analyzed directly using the Ban50/BA27, for Aβ(1-40) or Ban50/BC-05 for Aβ (1-42/43) sandwich enzyme-linked immunosorbent assay systems as previously described.16,18 Results were expressed as pmol/g of tissue. The values were calculated by comparison with a standard curve of synthetic Aβ1-40 and Aβ1-42. Plasma Aβ levels were measured as previously described.19 Briefly, final blood was collected in 1% EDTA, centrifuged at 3000 rpm for 15 minutes and plasma collected. After the appropriate dilutions, samples were tested with the following systems: Ban50/BA27 for Aβ1-40, BNT-77/BC-05 for Aβ1-42. Analyses were always performed in duplicate and in a coded manner.
APP Biochemistry
Brain tissues were homogenized in RIPA buffer (0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate, 1% Nonidet P-40, 5 mmol/L EDTA in Tris-buffered saline, pH 8.0) in the presence of protease inhibitors (1 μg/ml of pepstatin A, leupeptin, l-1-tosylamido-2-phenylethyl chloromethyl ketone, 1-chloro-3-tosylamido-7-amino-2-heptanone, and soybean trypsin inhibitor, and 0.5 mmol/L phenylmethyl sulfonyl fluoride) and briefly sonicated as previously described.20 Samples were centrifuged at 100,000 × g for 30 minutes at 4°C, electrophoresed on 7.5% Tris-glycine polyacrylamide gels and transferred to nitrocellulose. Total APP, including full length APP and secreted APP isoforms, was detected using a goat polyclonal antibody, named Karen, raised against the N-terminal sAPP domain.20 sAPPβswe was probed with a rabbit polyclonal antibody, 54;21 β-tubulin was probed with a mouse monoclonal antibody, TUB2.1 (Sigma Chemical Co., St. Louis, MO). To detect only full-length APP, brain lysates were first immunoprecipitated with 5685, a rabbit polyclonal antibody raised against a synthetic peptide consisting of the last 40 amino acids of the C-terminus of APP.20 Immunoprecipitated material was electrophoresed as described above, and immunoblotted with Karen. Finally, C-terminal APP fragments were first immunoprecipitated with 5685, electrophoresed on 10/16.5% step gradient Tris-tricine polyacrylamide gels, and immunoblotted with 5685. Immunoblots were visualized by enhanced chemiluminescence (Perkin-Elmer Life Sciences, Boston, MA) after application of species-specific horseradish peroxidase-conjugated anti-IgG antibodies (Santa Cruz Biotechnology, Santa Cruz, CA) as previously described.20
Expression of COX-1 and COX-2 mRNA
Expression of COX-1 and COX-2 mRNA in brains was assessed by using a ribonuclease protection assay, as previously described.12 Briefly, a 300- to 400-bp fragment of interest cDNA was polymerase chain reaction amplified, a T7 promoter was added to the 3′ ending by polymerase chain reaction (Lig’n Scribe kit; Ambion, Austin, TX), and a radiolabeled RNA probe was synthesized by in vitro-transcription system (MAXIScript T7 kit, Ambion). The probe was hybridized to 10 to 20 μg of total RNA for ∼16 hours at 42°C (RPA III kit, Ambion), and the samples were digested with RNase A/T, precipitated, and loaded onto 7% denaturing polyacrylamide gel. The gel was dried and signals were detected by autoradiography and quantitated on a PhosphorImager (Molecular Dynamics). The following mouse-probe primers were used: for COX-1, forward primer 5′-GTTCCGAGCCCAGTTCCA-3′ and reverse primer 5′-CATCTCCTTCTCTCCTGTG-3′; and for COX-2, forward primer 5′-CCAGCACTTCACCCATCAG-3′ and reverse primer 5′-CTCATCACCCCACTCAGGA-3′. Both pairs of primers are hosted on C-terminal sequences, which diverge between the two isoforms.12
Nuclear Factor-κB
Nuclear extracts from brain tissues were dissociated with use of a mortar and pestle and extracted with NE-PER nuclear and cytoplasmic extraction reagents (Pierce Chemical Co., Rockford, IL). Protein concentrations were determined by the Bradford method (Bio-Rad, Richmond, CA). Double-stranded nuclear factor (NF)-κB consensus oligonucleotide (5′-AGTTGAGGGGGACTTTCCCAGGC-3′) (Promega Corp., Madison, WI) was used as probe after 5′-end labeling and purification. Binding reactions were performed as follows: nuclear extracts (10 μg protein) were incubated with radiolabeled DNA probes (70 fmol, 2.5 × 104 cpm) at room temperature for 30 minutes in 20 μl of binding buffer (4% glycerol, 1 mmol/L MgCl2, 0.5 mmol/L EDTA, 0.5 dithiothreitol, 0.5 mmol/L NaCl, 10 mmol/L Tris-HCl, pH 7.5, 0.05 mg/ml poly dI-dC). Competitor unlabeled oligonucleotide was added to the reaction at 50-fold molar excess. For supershift assay analyses, samples were incubated with anti-p50, anti-p65, anti-C-Rel, or anti-p52 rabbit polyclonal antibodies (Santa Cruz Biotechnology). The products of the binding reactions were electrophoresed through 5% nondenaturating polyacrylamide gels in Tris-buffered saline buffer. Gels were dried and analyzed by autoradiography. The assays were always performed in a blinded manner.
Immunoblot Analysis
Brain homogenates were electrophoresed on a 10% acrylamide gel under reducing conditions. Proteins were transferred to a polyvinylidene membrane before blocking in 10% nonfat dry milk for 2 hours. Blots were incubated with monoclonal antibody against glial fibrillary acidic protein (GFAP) (2.2B10) (1:1000), transforming growth factor (TGF)-β1 (sc-146, 1:200), or an anti-β actin (1:5000) antibody overnight at 4°C. After three rinses, blots were incubated with horseradish peroxidase-conjugated goat anti-mouse for 45 minutes before development with chemiluminescent detection system using enhanced chemiluminescence (Amersham, Piscataway, NJ). Bands were quantitated using densitometric software (Molecular Analyst, Sunnyvale, CA). The anti-GFAP is a monoclonal antibody, and its characterization has previously been published22 (Zymed, South San Francisco, CA). The anti-murine transforming growth factor-β1 and anti-β actin are from commercial sources (Santa Cruz Biotechnology and Novus Biological).
Amyloid Deposition
Serial 6-μm-thick paraffin sections were cut throughout each brain, and mounted on 3-aminopropyl triethoxy saline (APES)-coated slides. Sections were deparaffinized, hydrated, rinsed with PBS, and pretreated with formic acid (88%) for 10 minutes for antigen retrieval, and with 3% H2O2 in methanol for 30 minutes to eliminate endogenous peroxidase activity in the tissue and with the blocking solution (5% normal horse serum in Tris buffer, pH 7.6). Subsequently, sections were incubated with a biotinylated antibody against Aβ (4G8) (1:10,000 dilution), at 4°C overnight.16 Sections were then incubated with secondary antibody for 1 hour (dilution, 1:1000), then reacted with horseradish peroxidase-avidin-biotin complex (Vector Laboratories, Burlingame, CA), and immunocomplexes visualized by using 3,3′-diaminobenzidine as the chromogen. Finally, they were dehydrated with ethanol, cleared with xylene, and coverslipped with Cytoseal. As control, sections from the same group of animals were treated in the same manner, except for the primary antibody.16,18 Light microscopic images from the somatosensory cortex, perihippocampal cortex, and hippocampus were captured from eight series of sections using a Nikon Microphot-FXA microscope with a ×4 objective lens. The areas occupied by Aβ-immunoreactive products in the region of interest were measured, and the total area occupied by the outlined structures was measured to calculate: the total area with selected immunoreactive products and the percentage of the area occupied by immunoreactive products over the outlined anatomical area in the image, as previously described.16,18 Analyses were always performed in a coded manner.
Drug Levels
After addition of internal standards, samples were deproteinated and extracted. Extractions were then evaporated and reconstituted in 100 μl of mobile phase of which 10 μl were injected into the high performance liquid chromatograph fitted with a C-18 reverse phase column, and a 254-nm UV detector.23,24 Analyses were always performed in a coded manner.
In Vitro Studies
Control NIH3T3 fibroblasts and embryonic fibroblasts from p65/RelA knockout mice (a gift from Dr. C. Hunter, University of Pennsylvania, Philadelphia, PA) were maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum, penicillin (100 U/ml), streptomycin (100 μg/ml), and l-glutamine (2 mmol/L) in a 37°C, humidified incubator with 5% CO2. Cells were infected with Semliki Forest virus encoding Swedish mutant APP695 (multiplicity of infection = 2) for 1 hour, as previously described.25 Medium was changed and cells were treated with different concentrations of indomethacin (25 to 100 μmol/L) or vehicle for 24 hours. Conditioned medium was collected and assayed for levels of Aβ1-40 and Aβ1-42 as described above. Aβ1-40 and Aβ1-42 levels were normalized to total APP levels and expressed as a ratio of Aβ1-42/Aβ1-40. Cells were rinsed with PBS, harvested into RIPA buffer supplemented with protease inhibitors, and lysed using sonication. Lysates were centrifuged at 100,000 × g and supernatants collected for Western blot analysis followed by I125quantitation to assay total APP levels. Each experiment was repeated three times with similar results.
Statistic Analysis
Data are expressed as mean ± SE of mean (SEM). Unless otherwise specified, statistical significance was determined by a two-way analysis of variance , and subsequently by Student’s unpaired two-tailed t-test corrected for multiple comparisons. Significance was set at P < 0.05.
Results
COX-1 and COX-2 mRNA Expression Levels in Tg2576
Brain COX-1 and COX-2 mRNA expression levels were assayed by reverse transcriptase polymerase chain reaction analysis in total cerebral cortex and cerebellum homogenates from Tg2576 and WT mice at 4, 8, 12, and 15 months of age. We found that COX-1 mRNA was expressed at similar levels in both regions, and no difference was observed between Tg2576 and WT animals at any time point considered (Figure 1A). By contrast, while at 4, 8, and 12 months of age cerebral cortices from Tg2576 and WT mice showed no difference in COX-2 expression levels, a significant increase was found in 15-month-old Tg2576 but not in WT mice (Figure 1B). However, compared with WT mice COX-2 levels in cerebellum of Tg2576 did not change with aging (not shown). Further, we compared the relative expression of COX-1 to COX-2 mRNA in Tg2576 and found that while at 4, 8, and 12 months of age the ratio was 1, at 15 months the amount of COX-2 was higher than COX-1 (not shown).
Figure 1.
Late increase of COX-2 mRNA brain levels in cerebral cortex of Tg2576. COX-1 (A) and COX-2 (B) mRNA expression in total cerebral cortex from Tg2576 (filled bars) and WT littermates (open bars) at 4, 8, 12, and 15 months of age (*, P < 0.05; n = 3 per each group).
Effects on COX Activity and Expression Levels
The effect of indomethacin or nimesulide on brain COX activity was assayed by determining PGE2 and TxB2 levels in total cerebral cortex and hippocampus of these animals at the end of the study. At this time point, Tg2576 mice were 15 months old and had been treated with the drugs or placebo for 7 months. By the end of the study, body weight, total plasma cholesterol, triglycerides, and blood cell counts were not different among animals randomized to the three groups (not shown). Compared with mice on placebo, Tg2576 mice that received indomethacin had a significant reduction in PGE2 and a suppression of TxB2 levels (Table 1). Urinary levels of 2,3-dinorTxB2 and 2,3-dinor-6-ketoPGF1α, indices of systemic COX-1 and COX-2 activation, respectively, were also measured.12,15 Confirming previous results, we found that at the dosage used in our study, indomethacin suppressed total COX-1 by 95% and COX-2 activity by 30% (Table 1). Conversely, indomethacin had no effect on the expression levels of both COX-1 and COX-2 mRNA (not shown). Animals receiving nimesulide showed only a partial but not significant reduction in PGE2, and no effect on TxB2 levels (Table 1). Consistent with previous reports, they also had a significant reduction in 2,3-dinor-6-ketoPGF1α, but no effect on 2,3-dinor TxB2 levels (Table 1).12,15 Finally, plasma levels of indomethacin were 20 ± 5 μg/ml, whereas nimesulide were 12 ± 4 μg/ml, which are comparable to therapeutic plasma levels in humans.26
Table 1.
Effects of Indomethacin and Nimesulide on Total Brain Cortex Levels of PGE2, TxB2, and Urinary Levels of 2,3-dinor-TxB2 and 2,3-dinor-6-ketoPGF1α
| Placebo (n = 10) | Indomethacin (n = 10) | Nimesulide (n = 10) | |
|---|---|---|---|
| PGE2 (percentage) | 100 | 36 ± 3† | 85 ± 4 |
| TxB2 (percentage) | 100 | 13 ± 1† | 105 ± 5 |
| 2,3-Dinor-TxB2 (ng/mg creatinine) | 58 ± 10 | 4.5 ± 1.1† | 56 ± 9.1 |
| 2,3-Dinor-6ketoPGF1α (ng/mg creatinine) | 0.55 ± 0.08 | 0.38 ± 0.09* | 0.12 ± 0.05‡ |
Tg2576 mice received the drugs in the drinking water starting at 8 month of age until they were 15-month old.
Results are expressed as means ± SEM.
P = 0.03;
P < 0.01;
P < 0.001.
Effects on NF-κB
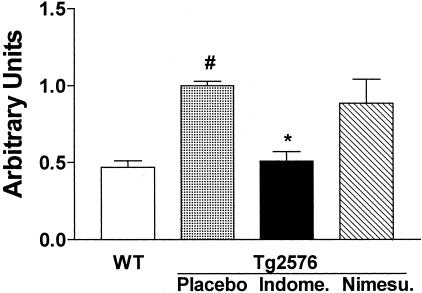
At the end of the study, nuclear extracts were isolated and assayed for NF-κB binding activity. Gel mobility shift assay results indicated the functional presence of NF-κB in total cerebral cortex and hippocampus nuclear extracts from all groups of mice. Compared with WT mice, Tg2576 on placebo showed a significant increase in NF-κB activity (Figure 2). This was significantly reduced in extract samples from hippocampi of indomethacin- but not nimesulide-treated Tg2576 mice (Figure 2). We also observed similar findings in the cortices of these mice (not shown). The identity of the NF-κB band was verified by competition studies in which a 50-fold excess unlabeled NF-κB probe completely blocked NF-κB binding activity (not shown). Supershift studies with antibodies against p65 and p50 and incubation with anti-p52 and anti-C-Rel further confirmed the specificity of NF-κB binding activity (not shown).
Figure 2.
Indomethacin reduces activation of brain NF-κB in Tg2576 mice. DNA binding activity of nuclear extracts from hippocampi of WT mice, Tg2576 on placebo, indomethacin, or nimesulide was tested by a labeled NF-κB consensus probe in an electrophoretic mobility shift assay. Results are expressed as arbitrary units. Statistical significance was determined by using a one-way analysis of variance (#, P < 0.01 versus WT; *, P = 0.03 versus placebo, n = 5 per group; age, 15 months old).
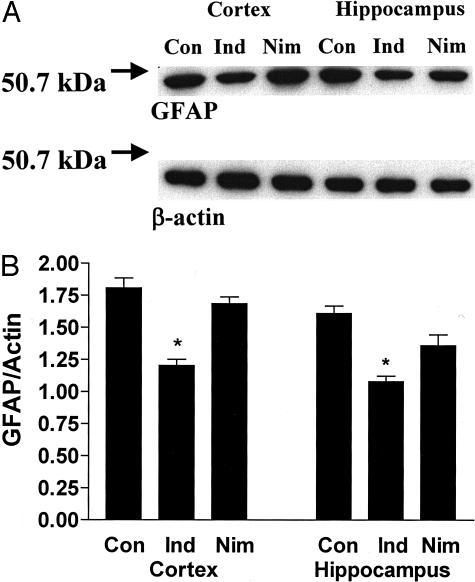
Effects on GFAP
Western blot analysis was used to determine the effect of the drug treatment on GFAP levels, a marker of astrocytosis that is elevated in Tg2576 mice.7 These levels were significantly lower in the indomethacin-treated than the placebo group, both in the total cerebral cortex as well as in the hippocampus (Figure 3). By contrast, in the nimesulide-treated group we observed a small reduction only in the hippocampus but not in the cortical regions (Figure 3). Both drugs did not have any significant effect on transforming growth factor-β1 levels (not shown).
Figure 3.
Effect of indomethacin and nimesulide on GFAP levels in Tg2576 brains. A: Representative immunoblots of GFAP and β-actin levels in total cerebral cortex and hippocampus homogenates of Tg2576 on placebo (Con), indomethacin (Ind), or nimesulide (Nim). B: Semiquantitative measurement of GFAP/β-actin ratio in the same experiments (*, P < 0.01, n = 5 per group; age, 15 months old).
Effects on Soluble and Insoluble Aβ Peptides and APP Metabolism
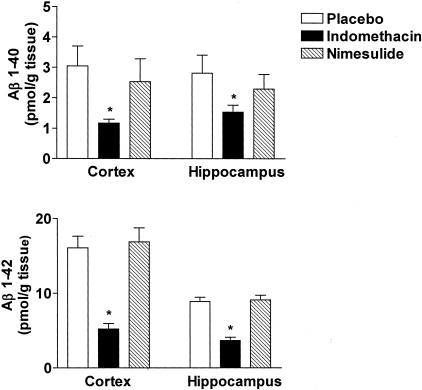
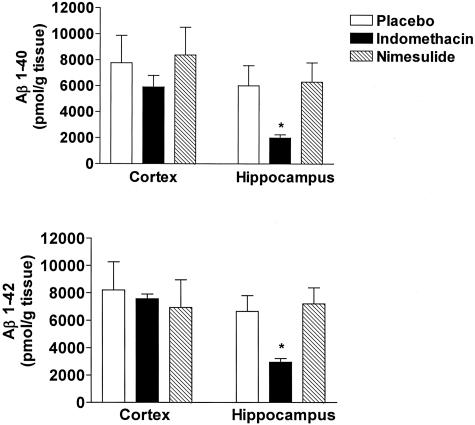
Next, we assessed the effect of indomethacin and nimesulide on brain levels of soluble and insoluble Aβ1-40 and Aβ1-42 by a sandwich enzyme-linked immunosorbent assay. As expected for their age, Tg2576 mice on placebo showed elevated levels of both soluble and insoluble Aβ1-40 and Aβ1-42 in their cerebral cortex as well as in hippocampus (Figures 4 and 5). By contrast, cerebellum had much lower levels of these peptides (not shown). Soluble Aβ1-40 and Aβ1-42 were significantly reduced in both cortex and hippocampus of Tg2576 treated with indomethacin (Figure 4). Despite the fact that an inhibitory trend was observed for insoluble Aβ1-40 in the cortex of Tg2576 treated with indomethacin, it did not reach statistical significance (Figure 5, top). However, a significant reduction in insoluble Aβ1-40 levels was observed in the hippocampus of these animals. Similar results were obtained for insoluble Aβ1-42 (Figure 5, bottom). By contrast, animals receiving nimesulide did not show any significant difference in both soluble and insoluble Aβ1-40 and Aβ1-42 compared with placebo (Figures 4 and 5). Finally, neither indomethacin nor nimesulide had an effect on both Aβ peptides in cerebellum of Tg2576 compared with control mice (not shown).
Figure 4.
Indomethacin and nimesulide effects on soluble Aβ. Levels of high salt-soluble Aβ1-40 and Aβ1-42 in total cerebral cortex and hippocampus homogenates from Tg2576 mice receiving placebo (open bars), indomethacin (filled bars), or nimesulide (hatched bars) for 7 months (*, P < 0.01; n = 10 each group; age, 15 months old).
Figure 5.
Indomethacin and nimesulide effects on insoluble Aβ. Levels of formic acid-soluble Aβ1-40 and Aβ1-42 in total cerebral cortex and hippocampus homogenates from mice receiving placebo (open bars), indomethacin (filled bars), or nimesulide (hatched bars) for 7 months (*, P < 0.01; n = 10 each group; age, 15 months old).
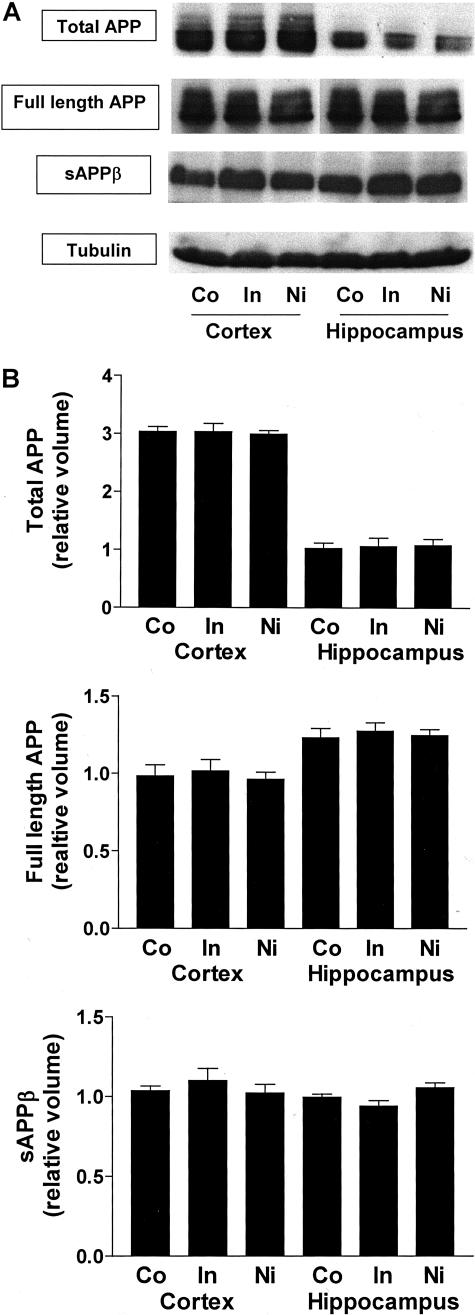
Next, we determined whether the drugs used in our studies could have altered APP processing in these mice. Western blot analyses did not show any change in the levels of total APP (including full-length APP and secreted APP isoforms), full length APP, sAPPβswe, or APP C-terminal fragments produced by α- or β-secretase (Figure 6).
Figure 6.
Lack of effect of indomethacin and nimesulide on APP metabolism. Representative gels (A) and semiquantitative analyses (B) of total APP levels; full length-APP levels; secreted APPβ levels in total cerebral cortex and hippocampus homogenates from Tg2576 receiving placebo (Co), indomethacin (In), or nimesulide (Nim). Results of B are mean ± SEM; n = 5 per group; age, 15 months old.
Effects on Aβ Plasma Levels
To better understand the effects of these drugs on the Aβ metabolism in vivo we assessed plasma concentration of Aβ1-40 and Aβ1-42 in these animals. Confirming a previous observation we found that at 15 months of age mice on placebo had high levels of both peptides (Figure 7). However, compared with this group no significant difference in the levels of both peptides was observed in either mice receiving the indomethacin or the nimesulide (Figure 7).
Figure 7.
Effects of indomethacin and nimesulide on Aβ plasma levels. Circulating levels of Aβ1-40 and Aβ1-42 in mice receiving placebo, indomethacin, or nimesulide. Results are mean ± SEM, n = 6 animals per group; age, 15 months old.
Effects of Amyloid Deposition
Amyloid deposits were widely present in the cerebral cortex and hippocampus of Tg2576 mice at 15 months of age, as reported previously.14,16,19 To determine the effects of these drugs on amyloid deposition, the areas occupied by 4G8-immunopositive reactions were analyzed in three brain regions: the somatosensory cortex, perihippocampal cortex, and hippocampus areas. Comparison of the burden of Aβ-positive deposits between placebo and indomethacin-treated groups revealed a reduction for the amyloid burden in the somatosensory cortex and hippocampus areas of treated Tg2576 mice (Figure 8). However, no significant change in amyloid burden between placebo and indomethacin groups was detected in perihippocampal cortex areas (Figure 8). By contrast, nimesulide-treated animals did not show any difference compared with placebo in the amount of amyloid deposited in all three regions considered (Figure 8).
Figure 8.
Indomethacin and nimesulide effect on amyloid deposition. Percentage area of the somatosensory cortex (SS), hippocampus (HIP), and perihippocampal cortex (PHC) occupied by Aβ immunoreactive deposits in Tg2576 receiving placebo (open bars), indomethacin (filled bars), or nimesulide (hatched bars) for 7 months (*, P < 0.01; n = 8 each group; age, 15 months old).
Effects on Oxidative Stress
Because oxidative stress is increased in the brains of Tg2576 mice16 and some NSAIDs have been reported to act as antioxidants,27 we investigated levels of 8,12-iso-iPF2α-VI, a specific marker of lipid peroxidation,17 in cerebral cortex and hippocampus of these mice. Although treatment with nimesulide had no effect in these brain regions (not shown), indomethacin only partially reduced total 8,12-iso-iPF2α-VI levels in the hippocampus (0.44 ± 0.06 ng/mg tissue versus 0.32 ± 0.07 ng/mg tissue, P = 0.02) but not in the cerebral cortex of Tg2576 mice (0.52 ± 0.06 ng/mg tissue versus 0.49 ± 0.07 ng/mg tissue, P > 0.05).
NF-κB-Dependent Anti-Amyloidogenic Effect of Indomethacin
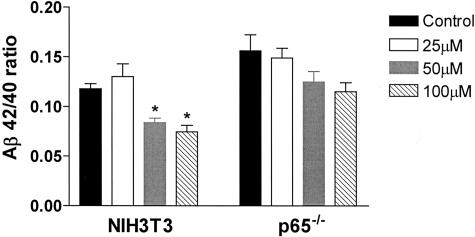
Because indomethacin, together with other NSAIDs, can inhibit the activation of NF-κB, we tested the hypothesis that its anti-amyloidogenic activity was linked to the presence of this transcription complex. Control NIH3T3 fibroblasts and p65/RelA knockout fibroblasts were infected with Swedish mutant APP695 Simliki Forest virus and then treated with different concentrations of indomethacin (25 to 100 μmol/L) for 24 hours. At this time point, we found that the drug already at 50 μmol/L significantly reduced the Aβ42/40 ratio in control cells (Figure 9). By contrast, indomethacin at the concentrations tested did not have any significant effect in embryonic fibroblasts generated from p65-knockout mice, suggesting that this factor is required to mediate the anti-amyloid effect of indomethacin (Figure 9).
Figure 9.
The amyloid-lowering effect of indomethacin requires p65/RelA. The ratio of Aβ42/40 in conditioned media of indomethacin-treated (25 to 100 μmol/L) control NHI3T3 fibroblasts, and fibroblasts genetically deficient for p65/RelA. The experiment was repeated three times, and a representative experiment is shown. Results are mean ± SEM, n = 3 per group; *, P < 0.05.
Discussion
In this study we demonstrated that chronic administration of indomethacin, but not nimesulide, reduces both Aβ1-40 and Aβ1-42 levels and Aβ amyloid plaque deposition in a mouse model of AD-like amyloidosis, the Tg2576.16 Indomethacin and nimesulide are members of the large family of NSAIDs, which have been associated with a reduced risk to develop AD.1 Previously, Lim and colleagues7 showed that ibuprofen, another NSAID, significantly reduced total Aβ levels and deposition in the same animal model. However, the authors did not investigate whether the drug had a differential effect on the two Aβ isoforms, or whether ibuprofen had an effect on brain prostanoid levels. Another group demonstrated that in cultured cells from peripheral or glial origin (ie, CHO, HEK293, H4 cells) a subset of NSAIDs, among which were ibuprofen and indomethacin, at concentrations much higher than in the present study, preferentially decreases the formation of Aβ1-42, but not Aβ1-40.9,10 The same authors showed that short-term high-dose ibuprofen treatment in the Tg2576 also reduces insoluble Aβ1-42 but not Aβ1-40.9 In a recent paper Yan and colleagues28 showed that long-term ibuprofen treatment results in a stronger reduction for sodium dodecyl sulfate-soluble Aβ1-42 than Aβ1-40. Based on these studies it has been proposed that the effects of Aβ1-42 lowering by NSAIDs are COX-independent and mediated by the modulation of a γ-secretase activity.
However, conflicting results have also been reported. Thus, some in vitro evidence would suggest that COX’s expression does influence Aβ generation;29 and a more recent study with neuronal cells failed to show any preferential effect of several NSAIDs on Aβ1-42 as well as on APP metabolism.11 Our different results with respect to a selective reduction of individual Αβ species could be justified by the fact that although our measurements were performed using distinct brain regions, in the other studies entire cerebral hemispheres were always used. However, the age of the mice, the length of the treatment, and the different NSAIDs used are also important factors to be considered. In the current investigation we found no sign of an increased clearance of peripheral pools of Αβ peptides in both groups. This finding would argue against any potential peripheral action of the drugs used. Moreover, the observed inhibitory effect by indomethacin was not mediated by an alteration of APP metabolism, because analyses of the full length APP, secretion of the APP ectodomain, and the APP C-terminal fragment levels were not modified.
Based on the reported beneficial effects that NSAIDs may have on the onset of AD, and that their main pharmacological property is the inhibition of COX, in recent years there has been an increasing interest in these enzymes and AD pathogenesis. However, conflicting reports regarding COX protein expression levels in AD have also been published.3–5 To the best of our knowledge only one study showed evidence for COX-2 immunoreactivity in a double Tg (APP/PS1) mouse model of AD, and no gross up-regulation of COX-2 was found in transgenic compared with WT littermates.30 Our study is the first to show that although COX-1 mRNA expression levels are not different between transgenic and WT mice and do not significantly change during the evolution of the disease, COX-2 manifests an increase in its mRNA levels only after amyloid is deposited. This finding suggests that COX-2 up-regulation in the Tg2576 mice is a secondary and late event in the course of the disease, and probably does not play an early pathogenic role in the formation of Aβ deposits in this animal model. This observation explains the failure of nimesulide in influencing the amyloidotic phenotype of these animals. Our conclusion is further supported by a report in which overexpression of COX-2 in double APP/PS Tg did not influence Aβ amyloidosis until the mice were 24 months old,31 and the negative results of two clinical trials in AD patients with selective COX-2 inhibitors.32,33
As expected, we found that indomethacin suppressed brain TxB2 levels, whereas nimesulide had no effect. Surprisingly, we observed that indomethacin also significantly reduced brain PGE2, whereas nimesulide had only a minor effect, suggesting that in this transgenic mouse model COX-2 is only partially responsible for PGE2 biosynthesis. We exclude that this observation is secondary to a low brain penetration of nimesulide, because the pKa of this drug is 6.5 and diffuses into the brain even more easily than indomethacin (pKa 4.5).34
Despite the fact that the main target of NSAIDs is the inhibition of COX activity, several alternative in vivo biological properties have been attributed to these drugs. Among them is the inhibition of the NF-κB transcription complex activity.35 Previous findings showed that this factor is increased in AD brains and regulates Aβ formation in neuronal cells.36,37 First, we found that activated NF-κB is increased in brains of Tg2576 compared with WT littermates. Second, we observed that indomethacin, but not nimesulide, significantly reduced the activity of this factor in the same regions where it induced a strong anti-amyloidogenic effect. Interestingly, this effect was also associated with a significant reduction of reactive astrocytosis, measured as GFAP levels. Finally, we observed that in vitro NF-κB activity is required to mediate the indomethacin-induced reduction of Aβ42/40 ratio. Transforming growth factor-β1 has been reported to potentiate Aβ generation,38 and another possible pathway of indomethacin effect on amyloid might have been through the inhibition of this factor. However, confirming a previous report we found that indomethacin did not influence this factor.39
Another reported biological activity of NSAIDs is an antioxidant capacity.27,40 Our data show that together with a reduction in brain PGE2 and TxB2 levels, indomethacin reduced 8,12-iso-iPF2α-VI levels but only in the hippocampus. These findings would not support a significant contribution of brain COX activation to lipid peroxidation, or a direct anti-oxidant activity of indomethacin. Another COX-independent mechanism of NSAIDs is the activation of the peroxisome proliferator-activated receptor γ (PPARγ). However, it seems unlikely that this mechanism is involved in the Aβ-lowering effect of NSAIDs, because a direct agonist for this receptor, pioglitazone, failed to influence amyloidosis in the same animal model.28 Finally, some NSAIDs, including indomethacin, at high concentrations modulate in vitro the multidrug resistance protein-4, and the Rho-Rock pathway, which are both known to regulate Aβ metabolism.41,42 Despite the fact that the drug levels in our study are too low to achieve these effects, we cannot completely exclude these mechanisms in our in vivo model, and further investigation is warranted to explore them.
An important caveat of our study is the limitation of the animal model used. Thus, the Tg2576 is a well-characterized model of AD-like amyloidosis, but it does not show neuronal loss or tau abnormalities.14 In conclusion, these data reveal a novel mechanism by which indomethacin, without directly interfering with the APP metabolism or Aβ clearance, influences the AD-like amyloidotic phenotype (Aβ formation and deposition) of the Tg2576 mice, and underline the critical role that the NF-κB pathway could hold in AD pathogenesis.
Acknowledgments
We thank Dr. Karen Hsiao for the generous gift of the Tg2576 mice and Dr. Chris Hunter for the p65/RelA knockout and WT cells.
Footnotes
Address reprint requests to Domenico Praticò, M.D., Center for Experimental Therapeutics, University of Pennsylvania, BRB 2/3, Room 812, 421 Cure Blvd., Philadelphia, PA 19104. E-mail: domenico@spirit.gcrc.upenn.edu.
Supported by the National Institutes of Health (grants AG-11542 and AG-22512), Alzheimer’s Association (grant IIRG-02-4010), and the Coins for Alzheimer Research Trust (CART) Fund.
References
- Stewart WF, Kawas C, Corrada M, Metter EJ. Risk of Alzheimer’s disease and duration of NSAID use. Neurology. 1997;48:626–632. doi: 10.1212/wnl.48.3.626. [DOI] [PubMed] [Google Scholar]
- Smith WL, DeWitt DL, Garavito RM. Cyclooxygenases: structural, cellular, and molecular biology. Annu Rev Biochem. 2000;69:145–182. doi: 10.1146/annurev.biochem.69.1.145. [DOI] [PubMed] [Google Scholar]
- Oka A, Takashima S. Induction of cyclo-oxygenase 2 in brains of patients with Down’s syndrome and dementia of Alzheimer type: specific localization in affected neurons and axons. Neuroreport. 1997;8:1161–1164. doi: 10.1097/00001756-199703240-00020. [DOI] [PubMed] [Google Scholar]
- Yermanova AV, Rollins J, Callahan LM, Rogers J, O’Banion MK. Cycloxygenase-1 in human Alzheimer and control brain: quantitative analysis of expression by microglia and CA3 hippocampal neurons. J Neuropathol Exp Neurol. 1999;58:1135–1146. doi: 10.1097/00005072-199911000-00003. [DOI] [PubMed] [Google Scholar]
- O’Banion MK, Chang JW, Coleman PD. Decreased expression of prostaglandin G/H synthase-2 (PGHS-2) in Alzheimer’s disease brain. Adv Exp Med Biol. 1997;407:171–174. doi: 10.1007/978-1-4899-1813-0_26. [DOI] [PubMed] [Google Scholar]
- Rogers J, Kirby L, Hempelman S, Berry D, McGeer P, Kaszniak A, Zalinski J, Cofield M, Mansukhani L, Willson P. Clinical trial of indomethacin in Alzheimer’s disease. Neurology. 1993;53:197–200. doi: 10.1212/wnl.43.8.1609. [DOI] [PubMed] [Google Scholar]
- Lim GP, Yang F, Chu T, Chen P, Beech W, Teter B, Tran T, Ubeda O, Hsiao Ashe K, Frautschy SA, Cole GM. Ibuprofen suppresses plaque pathology and inflammation in a mouse model of Alzheimer’s disease. J Neurosci. 2000;20:5709–5714. doi: 10.1523/JNEUROSCI.20-15-05709.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jantzen PT, Connor KE, Dicarlo G, Wenk GL, Wallace JL, Rojiani AM, Coppola D, Morgan D, Gordon MN. Microglial activation and β-amyloid deposit reduction caused by a nitric oxide-releasing nonsteroidal anti-inflammatory drug in amyloid precursor protein plus presenilin-1 transgenic mice. J Neurosci. 2002;22:2246–2254. doi: 10.1523/JNEUROSCI.22-06-02246.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weggen S, Eriksen JL, Das P, Sagi SA, Wang R, Pietrzik CU, Findlay KA, Smith TE, Murphy MP, Bulter T, Kang DE, Marquez-Sterling N, Golde TE, Koo EH. A subset of NSAIDs lowers amyloidogenic Aβ42 independently of cyclooxygenase activity. Nature. 2001;414:212–216. doi: 10.1038/35102591. [DOI] [PubMed] [Google Scholar]
- Weggen S, Eriksen JL, Sagi SA, Pietrzik CU, Ozols V, Fauq A, Golde TE, Koo EH. Evidence that nonsteroidal anti-inflammatory drugs decrease Aβ42 production by direct modulation of γ-secretase activity. J Biol Chem. 2003;278:31831–31837. doi: 10.1074/jbc.M303592200. [DOI] [PubMed] [Google Scholar]
- Gasparini L, Rusconi L, Xu H, del Soldato P, Ongini E. Modulation of β-amyloid metabolism by non-steroidal anti-inflammatory drugs in neuronal cell cultures. J Neurochem. 2004;88:337–348. doi: 10.1111/j.1471-4159.2004.02154.x. [DOI] [PubMed] [Google Scholar]
- Praticò D, Cyrus T, Zhang ZB, Li H, FitzGerald GA. Acceleration of atherogenesis by COX-1-dependent prostanoid formation in low density lipoprotein receptor knockout mice. Proc Natl Acad Sci USA. 2001;98:3358–3363. doi: 10.1073/pnas.061607398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah AA, Murray FE, Fitzgerald DJ. The in vivo assessment of nimesulide cycloxygenase-2 selectivity. Rheumatology. 1999;38:19–23. doi: 10.1093/rheumatology/38.suppl_1.19. [DOI] [PubMed] [Google Scholar]
- Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G. Correlative memory deficits, Abeta elevation, and amyloid plaque in transgenic mice. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- Praticò D, Cyrus T, Li H, FitzGerald GA. Endogenous biosynthesis of thromboxane and prostacyclin in 2 distinct murine models of atherosclerosis. Blood. 2000;96:3823–3826. [PubMed] [Google Scholar]
- Praticò D, Uryu K, Leight S, Trojanowski JQ, Lee VM-Y. Increased lipid peroxidation precedes amyloid plaque formation in an animal model of Alzheimer amyloidosis. J Neurosci. 2001;21:4183–4187. doi: 10.1523/JNEUROSCI.21-12-04183.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praticò D, Lawson JA, Rokach J, FitzGerald GA. The isoprostanes in biology and medicine. Tr Endocr Metabol. 2001;12:243–247. doi: 10.1016/s1043-2760(01)00411-8. [DOI] [PubMed] [Google Scholar]
- Uryu K, Laurer H, McIntosh T, Praticò D, Martinez D, Leight S, Lee VM-Y, Trojanowski JQ. Repetitive mild brain trauma accelerates Aβ deposition, lipid peroxidation, and cognitive impairment in a transgenic mouse model of Alzheimer amyloidosis. J Neurosci. 2002;22:446–454. doi: 10.1523/JNEUROSCI.22-02-00446.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawarabayashi T, Younkin LH, Saido TC, Shoji M, Hsiao Ashe K, Younkin SG. Age-dependent changes in brain CSF, and plasma amyloid β protein in the Tg2576 transgenic mouse model of Alzheimer’s disease. J Neurosci. 2001;21:372–381. doi: 10.1523/JNEUROSCI.21-02-00372.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EB, Skovronski DM, Abtahian F, Doms RW, Lee VM-Y. Secretion and intracellular generation of truncated Aβ in β-site amyloid-β precursor protein-cleaving enzyme expressing human neurons. J Biol Chem. 2003;278:4458–4466. doi: 10.1074/jbc.M210105200. [DOI] [PubMed] [Google Scholar]
- Siman R, Durkin JT, Husten EJ, Savage MJ, Murthy S, Mistertta S, Chatterjee S, Rotella D, Dembofsky B, Poorman R, Greenberg BD. 1995. Iqbal K, Mortimer JA, Winblad B, Wisnieski HM, editors. New York: John Wiley and Sons,; Research Advances in Alzheimer’s Disease and Related Disorders. 1995:pp 675–684. [Google Scholar]
- Kosik KS, Orechhio LD, Binder L, Trojanowski JQ, Lee VM, Lee G. Epitopes that span the tau molecule are shared with paired helical filaments. Neuron. 1988;1:817–825. doi: 10.1016/0896-6273(88)90129-8. [DOI] [PubMed] [Google Scholar]
- Liu S, Kamijo M, Takayasu T, Takayama S. Direct analysis of indomethacin in rat plasma using a column-switching high-performance liquid chromatography system. J Chromatogr. 2002;767:53–60. doi: 10.1016/s0378-4347(01)00532-1. [DOI] [PubMed] [Google Scholar]
- Gupta SK, Velpandian T, Mathur P, Sengupta S. Comparative analgesic activity of nimesulide and diclofenac by intramuscular route: correlation with pharmacokinetic profile of nimesulide. Pharmacology. 1998;56:137–143. doi: 10.1159/000028191. [DOI] [PubMed] [Google Scholar]
- Wilson CA, Doms RW, Zheng H, Lee VM-Y. Presenilins are not required for Aβ 42 production in the early secretory pathway. Nat Neurosci. 2002;5:849–855. doi: 10.1038/nn898. [DOI] [PubMed] [Google Scholar]
- Eriksen J, Sagi SA, Smith T, Weggen S, Das P, McLendon DC, Ozols VV, Jessing KW, Zavitz KH, Koo EH, Golde TE. NSAIDs and enantiomers of flurbiprofen target γ-secretase and lower Aβ42 in vivo. J Clin Invest. 2003;112:440–449. doi: 10.1172/JCI18162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilodeau JF, Wang M, Chung FL, Castonguay A. Effects of nonsteroidal anti-inflammatory drugs on oxidative pathways in A/J mice. Free Radic Biol Med. 1995;18:47–54. doi: 10.1016/0891-5849(94)00099-6. [DOI] [PubMed] [Google Scholar]
- Yan Q, Zhang J, Liu H, Babu-Khan S, Vassar R, Biere AL, Citron M, Landreth G. Anti-inflammatory drug therapy alters β-amyloid processing and deposition in an animal model of Alzheimer’s disease. J Neurosci. 2003;23:7504–7509. doi: 10.1523/JNEUROSCI.23-20-07504.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin W, Pompl PN, Peng Y, Zhao Z, Xiang Z, Robakis NK, Shioi J, Suh J, Pasinetti GM. Cyclooxygenase (COX)-2 and COX-1 potentiates β-amyloid peptide generation through mechanisms that involves γ-secretase activity. J Biol Chem. 2003;278:50970–50977. doi: 10.1074/jbc.M307699200. [DOI] [PubMed] [Google Scholar]
- Matsuoka Y, Picciano M, Malester B, LaFrancois J, Zehr C, Daeschner JM, Olschowka JA, Fonseca MI, O’Banion MK, Tenner AJ, Lemer CA, Duff K. Inflammatory responses to amyloidosis in a transgenic mouse model of Alzheimer’s disease. Am J Pathol. 2001;158:1345–1354. doi: 10.1016/S0002-9440(10)64085-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Z, Ho L, Yemul S, Zhao Z, Pompl P, Kelley K, Dang A, Qing W, Teplow D, Pasinetti MG. Cyclooxygenase-2 promotes amyloid plaque deposition in a mouse model of Alzheimer’s disease neuropathology. Gene Expr. 2002;10:271–278. doi: 10.3727/000000002783992352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aisen PS, Schafer KA, Grundman M, Pfeiffer E, Sano M, Davis KL, Farlow MR, Jin S, Thomas RG, Thal LJ. Effects of rofecoxib or naproxen vs placebo on Alzheimer disease progression: a randomized controlled trial. JAMA. 2003;289:2819–2826. doi: 10.1001/jama.289.21.2819. [DOI] [PubMed] [Google Scholar]
- Reines SA, Block GA, Morris JC, Liu G, Nessly ML, Lines CR, Norman BA, Baranak CC. Rofecoxib. No effect on Alzheimer’s disease in 1-year, randomized, blinded, controlled study. Neurology. 2004;62:66–71. doi: 10.1212/wnl.62.1.66. [DOI] [PubMed] [Google Scholar]
- Taniguchi Y, Yokohama K, Inui K, Deguchi Y, Furukawa K, Noda K. Inhibition of brain cyclooxygenase-2 activity and the antipyretic action of nimesulide. Eur J Pharmacol. 1997;330:221–229. doi: 10.1016/s0014-2999(97)00183-0. [DOI] [PubMed] [Google Scholar]
- Straus DS, Pascual G, Li M, Welch JS, Ricote M, Hsiang CH, Sengchathalangsy LL, Gosh G, Glass CK. 15-Deoxy-delta 12,14-prostaglandin J2 inhibits multiple steps in the NF-κB signaling pathway. Proc Natl Acad Sci USA. 2000;97:4844–4849. doi: 10.1073/pnas.97.9.4844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiyama Y, Arai K, Hattori T. Enhanced expression of I-κB with neurofibrillary pathology in Alzheimer’s disease. Neuroreport. 2001;12:2641–2645. doi: 10.1097/00001756-200108280-00011. [DOI] [PubMed] [Google Scholar]
- Tomita S, Fujita T, Kirino Y, Suzuki T. PDZ domain-dependent suppression of NF-κB/p65-induced Aβ42 production by a neuron-specific X11-like protein. J Biol Chem. 2000;275:13056–13060. doi: 10.1074/jbc.c000019200. [DOI] [PubMed] [Google Scholar]
- Lesne S, Docagne F, Gabriel L, Lahiri DK, Buee L, Plawisnki L, Delacourte A, MacKenzie ET, Buisson A, Vivien D. Transforming growth factor-β1 potentiates amyloid-β generation in astrocytes and in transgenic mice. J Biol Chem. 2003;278:18408–18418. doi: 10.1074/jbc.M300819200. [DOI] [PubMed] [Google Scholar]
- Chang JK, Chuang LY, Wang GJ. Effects of nonsteroidal anti-inflammatory drugs on transforming growth factor-β expression and bioactivity in rat osteoblast-enriched culture. Kaohsiung J Med Sci. 2003;19:278–288. doi: 10.1016/s1607-551x(09)70474-7. [DOI] [PubMed] [Google Scholar]
- Asanamura M, Nishibayashi-Asanamura S, Miyazaki I, Kohno M. Neuroprotective effects of non-steroidal anti-inflammatory drugs by direct scavenging of nitric oxide. J Neurochem. 2001;76:1895–1904. doi: 10.1046/j.1471-4159.2001.00205.x. [DOI] [PubMed] [Google Scholar]
- Reid G, Wielinga P, Zelcel N, van der Heijden I, Kuil A, de Haas M, Wijinholds J, Borst P. The human multidrug resistance protein MRP4 functions as a prostaglandin efflux transporter as is inhibited by nonsteroidal anti-inflammatory drugs. Proc Natl Acad Sci USA. 2003;100:9244–9249. doi: 10.1073/pnas.1033060100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Su Y, Baolin L, Liu F, Ryder JW, Wu X, Gonzalez-DeWhitt PA, Gelfanova V, Hale JE, May PC, Paul SM, Ni B. Nonsteroidal anti-inflammatory drugs can lower amyloidogenic Aβ42 by inhibiting rho. Science. 2003;302:1215–1217. doi: 10.1126/science.1090154. [DOI] [PubMed] [Google Scholar]