Abstract
Down-regulation of p27 is frequently observed in various cancers due to an enhancement of its degradation. Skp2 is required for the ubiquitination and consequent degradation of p27 protein. Another protein called Cks1 is also required for p27 ubiquitination in the SCFSkp2 ubiquitinating machinery. In the present study, we examined Cks1 expression and its correlation with p27 in oral squamous cell carcinoma (OSCC) derived from tongue and gingiva. By immunohistochemical analysis, high expression of Cks1 was present in 62% of OSCCs in comparison with 0% of normal mucosae. In addition, 65% of samples with low p27 expression displayed high Cks1 levels. Finally, Cks1 expression was well correlated with Skp2 expression and poor prognosis. To study the role of Cks1 overexpression in p27 down-regulation, we transfected Cks1 with or without Skp2 into OSCC cells. Cks1 transfection could not induce a p27 down-regulation by itself, but both Cks1 and Skp2 transfection strongly induced. Moreover, we inhibited Cks1 expression by small interference RNA (siRNA) in OSCC. Cks1 siRNA transfection induced p27 accumulation and inhibited the growth of OSCC cells. These findings suggest that Cks1 overexpression may play an important role for OSCC development through Skp2-mediated p27 degradation, and that Cks1 siRNA can be a novel modality of gene therapy.
p27, a cyclin-dependent kinase (Cdk) inhibitor, mediates G1 arrest induced by TGF-β, contact inhibition, or serum deprivation in epithelial cell lines.1,2 Although levels of p27 protein change during the cell cycle, with maximal levels occurring during G1 and quiescence (G0), p27 mRNA levels do not change during cell cycle progression. The increase in the cellular abundance of p27 on induction of cell quiescence is primarily due to a decrease in the rate of its degradation. p27 is polyubiquitinated both in vivo and in vitro, and a lower amount of p27 ubiquitinating activity is present in quiescent cells compared with proliferating cells.3 Furthermore, p27 ubiquitination requires its phosphorylation on threonine 187 (Thr187), a Cdk consensus site.4–7 It has been reported that reduced expression of p27 was frequently found in various cancers, and the lack of p27 is suggested to be due to an enhancement of its degradation.8,9 Aggressive human cancers express low levels of p27 because of its decreased stability.8 We also found that reduced expression of p27 was observed in 87% of oral squamous cell carcinoma (OSCC) cases and was well correlated with its malignancy.10 Importantly, reduced p27 levels represent a powerful prognostic marker for poor survival in cancer patients.
SCFSkp2 was identified as the E3 ubiquitin ligase that targets p27 for ubiquitination.11–13 SCF complexes represent an evolutionarily conserved class of E3 enzymes containing four subunits: Skp1, Cul1, one of many F-box proteins, and Roc1/Rbx1.9 Skp2, an F-box protein, is required for the ubiquitination and consequent degradation of p27 both in vivo and in vitro. Binding of Skp2 to p27 phosphorylated on Thr187 has been demonstrated both in vivo and in vitro. In addition, in vitro ubiquitination of recombinant p27 can be induced by the addition of purified Skp2 and cyclin E/cdk2 or cyclin A/cdk2 complexes to G1 cell extracts. Skp2 is frequently overexpressed in tumor cell lines, and forced expression of Skp2 in quiescent fibroblasts induces DNA synthesis.12,14 Skp2 expression was found to correlate inversely with p27 levels in epithelial dysplasias and OSCC.15,16 Furthermore, Skp2 expression increases significantly during malignant progression from epithelial dysplasia to invasive OSCC and is a good prognostic marker for OSCC.15,16 Skp2 overexpression is also found in other type of malignant tumors including lymphomas, breast, colorectal, lung, and gastric carcinomas.17–22 These findings indicate that Skp2 is an oncogene.9,23,24
It has been found that Cks1 acts as an accessory protein in the SCFSkp2 ubiquitinating machinery. The role of Cks1 in the ubiquitination and degradation of p27 was established by both biochemical reconstitution and gene knockout approach.25,26 Using a biochemical approach, fractions of HeLa extract were assayed for their ability to promote p27 ubiquitination in the presence of purified SCFSkp2, cyclin E/cdk2, Ubc3, and E1, and the factor responsible for this effect was purified and identified as Cks1.25 Accordingly, Cks1−/− cells contain elevated levels of p27 due to defective ubiquitination and degradation of this protein.26 Cks1 has three binding sites, for Cdk, anion, and Skp2, and all of the three binding sites are required for p27-ubiquitin ligation and for the association of Skp2 with Cdk-bound Thr187-phosphorylated p27.27 Cks1 may function as an adapter protein to bridge Skp2 with the phosphate group of Thr187-phosphorylated p27 or may alter the conformation of Skp2 to promote binding to phosphorylated p27. Initially, Cks1 was found as a binding protein of Cdc2, and Cks1 was found overexpressed in some cancer cells.28–30 Recently, it has been reported that Cks1 overexpression was found in gastric, lung, and colorectal carcinomas.31–33 Cks1 overexpression was well correlated with low expression of p27 and poor prognosis in gastric and colorectal cancer,31,33 while Cks1 overexpression had no such relationship with p27 in lung cancer although only 15 samples were analyzed in this study.32 The role of Cks1 overexpression in cancer is still unclear. Moreover, there is no report about the correlation between Cks1 and p27 expression in OSCC. In the present study, therefore, we examined the Cks1 expression and the role for p27 degradation in OSCC derived from tongue and gingiva.
Materials and Methods
Tissue Samples
Tissue samples of 10 normal oral mucosae and 63 OSCCs were retrieved from the Surgical Pathology Registry of Hiroshima University Hospital from 1976 to 2000 after the approval by the Ethical Committee of our institutions. At the time of diagnosis, age of the patients with OSCC ranged from 37 to 88 years (mean, 59.2). For the present analysis, only biopsied specimens from the tongue and gingiva, before radiochemotherapy, were selected to avoid possible influences of the treatment modalities on data. Tissues fixed in 10% buffered-formalin and embedded in paraffin were used for immunohistochemical examination. The histological grade and stage of tumor were classified according to the criteria of the Japan Society for Head and Neck Cancer.34
Immunohistochemistry
Immunohistochemical detection of Cks1, Skp2, or p27 was performed using a streptavidin-biotin peroxidase technique as described previously.10,16 The polyclonal antibody to human Cks1 (diluted 1:100) generated in collaboration with Zymed Inc. An anti-Skp2 monoclonal antibody (diluted 1:100, Zymed Inc., San Francisco, CA) and an anti-p27 monoclonal antibody (diluted 1:100, Transduction Laboratories, Lexington, KY) were used. Nuclear staining of Cks1, Skp2, and p27 was scored on a semi-quantitative scale (see below) by evaluating the percentage of stained nuclei within representative areas of each tumor. For superficial carcinomas, stained sections were observed throughout the lesion. For advanced large tumors, at least 10 fields including superficial, central, and deep invasive areas were observed and the number of stained cells and staining intensity were evaluated. In each field, we counted at least more than 300 cells using an eyepiece graticule to prevent recounting. Although qualitative differences in staining intensity were observed with considerable intratumoral heterogeneity, all positive cases showed obvious nuclear staining, at least focally. As several slides of OSCC cases include the normal oral mucosa, we used the staining of normal epithelial cells as a negative control of Cks1 and Skp2 or a positive control for p27 for assessment of intensity of staining. The expression of Cks1, Skp2 was graded as high (over 30% of tumor cells showed strong or diffuse immunopositivity) and low (less than 30% of tumor cells showed weak or focal immunopositivity or no staining). The expression of p27 was graded as high (over 30% of tumor cells showed strong or diffuse immunopositivity), moderate (5 to 30% of tumor cells showed moderate or patchy immunopositivity), and low (less than 5% of the tumor cells showed weak or focal immunopositivity or no staining).10 Three pathologists (Y.K., I.O., and T.T.) made all of the assessments.
Statistical Analysis
Possible correlation between variables of the analyzed tumor samples was tested for association by the Fisher’s exact test. Patient’s survival data were used to determine possible correlation between Cks1 and Skp2 expression levels and disease-free survival time. Curves for survival were drawn according to the Kaplan-Meier method, and differences between the survival rates of four groups with different Cks1 and Skp2 expression were examined. Statistical significance of these data was measured by the Cox-Mantel test. A P value <0.05 was required for significance.
Cell Culture
Six OSCC cell lines (HSC2, HSC3, HSC4, Ca9–22, Ho-1-U-1, and Ho-1-N-1) were examined. OSCC cell lines were provided by Japanese Cancer Research Resources Bank. They were routinely maintained in RPMI-1640 (Kyokuto Pharmaceutical Industrial Co., Tokyo, Japan) supplemented with 10% heat-inactivated fetal bovine serum (Boehringer Mannheim, Australia) and 100 U/ml penicillin-streptomycin (Gibco, Grand Island, NY) under conditions of 5% CO2 in air at 37°C. For experiments, they were grown to subconfluence in this medium. Cells were lysed as described below, and cell lysates were used for Western blot analysis.
Western Blot Analysis
To characterize Cks1 antibody, we examined the expression of Cks1 and Flag in Flag-Cks1 or Flag-Cks2 transfected HeLa cells by Western blot analysis. We also examined the expression of Cks1, Skp2, p27, and Cul1 protein in OSCC cell lines by Western blot analysis. Western blotting was carried out as we described previously.16 We used an anti-Cks1 polyclonal antibody (collaboration with Zymed), anti-Cks1/2 polyclonal antibody (FL-79, Santa Cruz, CA), anti-Skp2 monoclonal antibody (Zymed), anti-p27 monoclonal antibody (Transduction Laboratories), and anti-Flag monoclonal antibody (Sigma Chemical Co., St. Louis, MO). Thirty μg of protein was subjected to 12% polyacrylamide gel electrophoresis followed by electroblotting onto a nitrocellulose filter. For detection of the immunocomplex, the ECL Western blotting detection system (Amersham, Aylesbury, United Kingdom) was used.
Transfection
pcDNA-Flag-tagged Cks1 and pcDNA-Flag-tagged Cks2 were transfected into HeLa cells by using Lipofectin (Gibco). pcDNA3-Skp2 and pcDNA3-Cks1 were stably transfected into Ho-1-N-1 cells by using Fugene 6 (Roche Diagnostics Australia Pty. Ltd., Castle Hill, Australia). After 48 hours, cells were treated with G418 (Gibco) as a selective marker.
Plasmids and Synthetic siRNA
Vector pSUPPRESSOR generates biologically active siRNAs from the U6 promoter (Imagenex, San Diego, CA). A synthetic double-strand oligonucleotide (5′-GGGACATAGCCAAGCTGGTCgagtactgGACCAGCTTGGCTATGTCC-3′) was introduced into pSUPPRESSOR.Oligonucleotide sequences correspond to a 19-nt sequence from Cks1 (nucleotide 78–96), which are separated by a 9-nt linker (lower case letters) from the reverse complement of the same 19-nt sequence. We used a circular control plasmid, which contains a scrambled sequence that does not show significant homology to rat, mouse, or human gene sequences, as a control. Transfection was performed by using Fugene 6 (Roche). After 48 hours of transfection, cells were treated with G418 (Gibco) as a selective marker.
p27 Protein Stabilization and in Vitro p27 Protein Degradation Assay
For p27 protein stabilization, we measured its half-life by treatment with cycloheximide (CHX, Sigma) for 0, 30, and 60 minutes. After CHX treatment, cells were collected and we examined the expression of p27 protein by Western blot analysis as described above. Degradation assay of p27 was performed as described by Pagano et al.3 Cells were prepared by addition of 3 to 5 volumes of lysis buffer containing 20 mmol/L Tris-HCl (pH 8.5) and 1 mmol/L dithiothreitol (DTT) to a cell pellet. The following protease inhibitors were added: phenyl-methyl sulfonyl fluoride (PMSF), 0.1 mmol/L; leupeptin, 1 μg/ml; soybean trypsin inhibitor, 10 μg/ml; L-1 Chlor-3-(4-tosylamido)-4 Phenyl-2-butanon (TPCK), 10 μg/ml; L-1 Chlor-3-(4-tosylamido)-7-amino-2-heptanon-hydrochloride (TLCK), 10 μg/ml; and aprotinin, 1 μg/ml. The sample was frozen and thawed three times. The lysate was spun down at 13,000 rpm in an Eppendorf centrifuge for 15 minutes at 4°C. The supernatant was retrieved and stored at −80°C for the degradation assay. Purified histidine-tagged p27 was incubated at 37°C for different times in 30 μl of a degradation mix containing 100 μg of cell extract, 50 mmol/L Tris-HCl (pH 8.5), 5 mmol/L MgCl2, 1 mmol/L DTT, 2 mmol/L adenosine 5′-triphosphate (ATP), 10 mmol/L creatine phosphokinase (Sigma), 10 mmol/L creatine phosphate (Sigma), and 5 μmol/L ubiquitin (Sigma). Degradation of p27 was analyzed by immunoblotting with an anti-p27 antibody (Transduction Laboratories).
Cell Growth and BrdU Incorporation
Cells (5.0 × 103) were plated onto a 24-well multi-well plate (3047, Falcon, Franklin Lakes, NJ) and allowed to attach for 24 hours. The culture medium was then replaced with fresh medium, then the trypsinized cells were counted by Cell Counter (Coulter Z1) at days 0 and 6. For BrdU incorporation, cells growing on coverslips were incubated with 10 μmol/L bromodeoxyuridine (BrdU; Sigma) for 3 hours. The cells were fixed in cold methanol:acetone 1:1 for 10 minutes. In brief, the cells were sequentially incubated in 1.5 mol/L HCl for 10 minutes. Then cells were washed three times with PBS and incubated with the mouse anti-BrdU-fluorescein primary antibody (Roche) for 1 hour. The cells were washed four times with PBS. The nuclei were simultaneously stained with 10 μg/ml 4′ and 6-diamidino-2-phenylindole (DAPI). Cells with different BrdU-incorporation patterns were checked and counted with a conventional fluorescence microscope (Zeiss).
Tumorigenicity Assays
Briefly, cells (1.5 × 107) were injected s.c. into multiple sites in athymic (nude) mice. The protocol of the experiment was approved by Hiroshima University’s Committee of Research Facilities for Laboratory Animal Science. The animals were monitored for tumor formation every week and sacrificed 1 month later. Tumor length (L) and width (W) were measured at the end of the experiment, and tumor volume was calculated by the formula (L × W2)/2. For proliferation activity, the expression of proliferating cell nuclear antigen (PCNA) was examined by immunohistochemistry as described above. The anti-PCNA antibody (Novocastra Laboratories, Newcastle, UK) was used.
Terminal Deoxynucleotidyl Transferase-Mediated dUTP Nick End Labeling (TUNEL) Assay
Fragmented DNA was labeled with adigoxigenin-conjugated UTP by using terminal deoxytransferase. Positive nuclei were visualized by immunohistochemistry with an ApopTag labeling and detection kit (Intergen, Purchase, NY) according to manufacturer’s protocol, and nuclei were counterstained with hematoxylin. For quantification, 10 random fields per section were documented by photomicroscopy, and the percentage of TUNEL-positive epithelial cell nuclei relative to the total number of epithelial cell nuclei was calculated.
Results
Generation of a Cks1-Specific Antibody
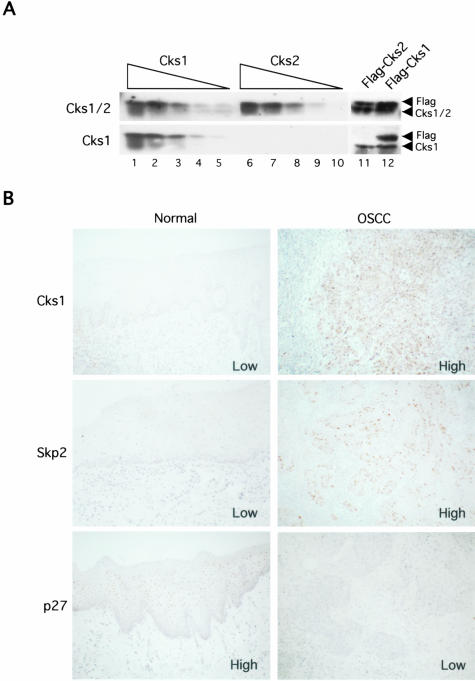
Cks1 has a homolog, Cks2, which is 81% identical and 90% similar to Cks1. Cks2 could not bind to Skp2 and substitute for Cks1 in SCFSkp2 reconstitution.26 In previous reports, Cks1 antibodies, which also react with Cks2, have been used. In the present study, therefore, we generated a specific antibody against human Cks1 using a peptide with high antigenic index, corresponding to the 10 amino acids at its extreme C-termini. Figure 1A shows that anti-Cks1 antibody only recognized recombinant purified Cks1. In contrast, the only commercially available antibody (Santa Cruz), which is also the only published antibody against mammalian Cks1, recognized both Cks1 and Cks2 proteins. Importantly, in immunoblots of whole cell lysates, Cks1 antibody recognized both endogenous and overexpressed Cks1. We used this antibody in the following study.
Figure 1.
Immunohistochemical expression of Cks1 and the correlation between Cks1 expression and survival rate in OSCCs. A: Characterization of anti-Cks1 antibody and recognition of human Cks1 in whole cell lysates. Lanes 1 to 5, decreasing amount of purified Cks1 (100–5 ng); Lanes 6 to 10, decreasing amount of purified Cks2 (100–5 ng); Lane 11, HeLa cells transfected with Flag-tagged Cks2; Lane 12, HeLa cells transfected with Flag-tagged Cks1. The top was immunoblotted with a commercially available antibody (Santa Cruz). The bottom was immunoblotted with our novel anti-Cks1 antibody. Overexpressed Cks proteins (lanes 11 and 12) run slightly slower than endogenous Cks proteins because of their Flag-tags. B: Immunohistochemical expression of Cks1, Skp2, and p27 in normal oral mucosae and OSCC.
High Expression of Cks1 in OSCC Cases
Ten normal oral mucosae and 63 OSCC cases were used for Cks1 expression by immunohistochemistry. The incidence of Cks1 expression in normal oral mucosae and OSCCs is summarized in Table 1. In normal oral mucosa, most of epithelial cells did not show Cks1 expression (Figure 1B and Table 1). In contrast to the normal oral mucosae, high expression of Cks1 was observed in 39 (62%) of 63 OSCC cases (Figure 1B and Table 1). We also compared the Cks1 expression with clinico-pathological findings (Table 1). High expression of Cks1 was significantly correlated with clinical stage (P = 0.0203), but not with histology (P = 0.0585) and metastasis (P = 0.1258). Though we also examined the influence of age, sex, or smoking habit of OSCC patients on the Cks1 expression, there was no correlation among them (data not shown).
Table 1.
Expression of Cks1 in OSCC and Its Correlation with Clinicopathologic Parameters
| No. of cases | Cks1 expression
|
P value | |||
|---|---|---|---|---|---|
| Low | High | ||||
| Normal oral mucosae | 10 | 10 (100%) | 0 (0%) | 0.0003 | |
| OSCC | 63 | 24 (38%) | 39 (62%) | ||
| Stage* | |||||
| I | 10 | 5 (50%) | 5 (50%) | 0.0203 | |
| II | 13 | 9 (69%) | 4 (31%) | ||
| III | 9 | 1 (11%) | 8 (89%) | ||
| IV | 31 | 9 (29%) | 22 (71%) | ||
| Histology* | |||||
| Well | 29 | 13 (45%) | 16 (55%) | 0.0585 | |
| Moderate | 26 | 11 (42%) | 15 (58%) | ||
| Poorly | 8 | 0 (0%) | 8 (100%) | ||
| Metastasis | |||||
| Negative | 37 | 17 (46%) | 20 (54%) | 0.1258 | |
| Positive | 26 | 7 (27%) | 19 (73%) | ||
| p27 expression | |||||
| High | 14 | 7 (50%) | 7 (50%) | 0.5757 | |
| Moderate | 18 | 6 (33%) | 12 (67%) | ||
| Low | 31 | 11 (35%) | 20 (65%) | ||
| Skp2 expression | |||||
| Low | 38 | 20 (53%) | 18 (47%) | 0.0034 | |
| High | 25 | 4 (16%) | 21 (84%) | ||
, Histological grade and stage of tumor were classified according to the criteria of the Japan Society for Head and Neck Cancer.
Next, we examined the correlation between Cks1 expression and p27 or Skp2 expression (Figure 1B and Table 1). In normal oral mucosae, all cases showed high expression of p27 and low expression of Cks1 and Skp2 (Figure 1B). In OSCCs, high expression of Cks1 was observed in 65% of the cases that showed low expression of p27 (Table 1). However, in 35% of cases both Cks1 and p27 levels were low, indicating that there are additional ways to down-regulate p27. Thus, there was no significant correlation between Cks1 and p27 expression (P = 0.5757). Interestingly, high expression of Cks1 was significantly correlated with high expression of Skp2 (P = 0.0034). High expression of Cks1 was observed in 84% of cases that showed high expression of Skp2.
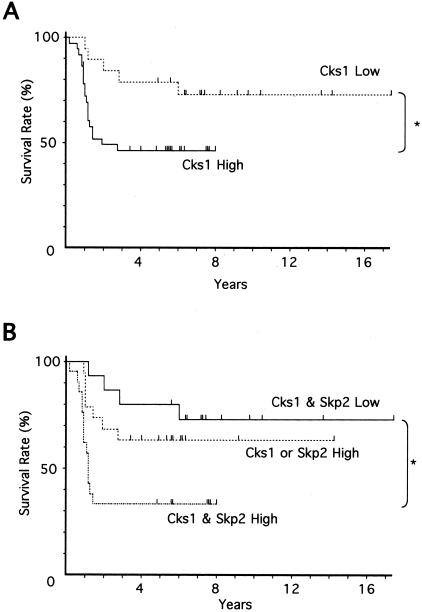
The relationship of Cks1 expression with survival rates of 55 OSCC patients with follow-up data were examined. Figure 2 shows the Kaplan-Meier survival curves of the patients grouped by the immunoreactivity of Cks1 in their tumors. The cumulative survival rate of the patients with high expression of Cks1 was lower than that with low expression of Cks1, and there was statistical significance between them (P = 0.0267) (Figure 2A). We also examined the relationship of Cks1 and Skp2 expression with survival rates. The patients with high expression of Cks1 and Skp2 showed a poor prognosis in comparison with the patients with high expression of Cks1 or Skp2 and the patients with low expression of Cks1 and Skp2. (Figure 2B). There was statistical significance between the patients with low expression of Cks1 and Skp2 and the patients with high expression of Cks1 and Skp2 (P = 0.0051). The type of treatment did not influence the correlation between Cks1 expression and survival (data not shown). However, since only univariate analysis was performed, the independent prognostic value of Cks1 expression was not assessed in this study.
Figure 2.
Relationship between high expression of Cks1 and poor survival. A: Relationship of Cks1 expression to survival. Prognosis of high expression of Cks1 (n = 36) and low expression of Cks1 (n = 19) were examined. Statistical significance of these data was measured by the Mantel-Cox test. B: Relationship of Cks1 and Skp2 expression to survival. Prognosis of Cks1 & Skp2 Low group (n = 15), Cks1 or Skp2 High group (n = 19) and Cks1 & Skp2 High group (n = 21) were examined. Statistical significance of these data were measured by the Mantel-Cox test.
Expression of Cks1 in OSCC Cell Lines
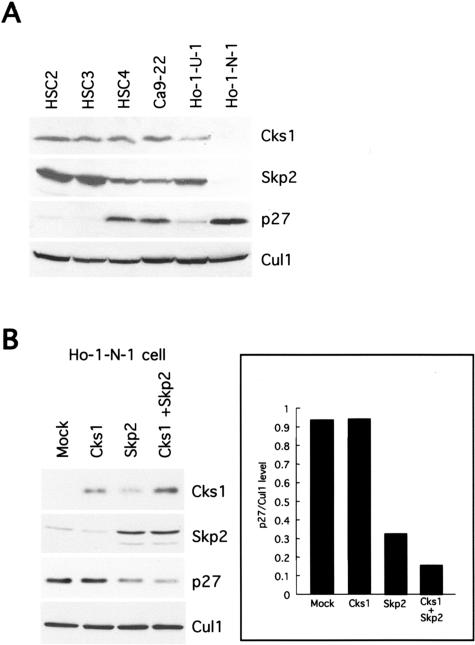
Expression of Cks1, p27, and Skp2 proteins in six OSCC cell lines was examined by Western blot analysis as shown in Figure 3A. Cks1 expression was observed in 5 of 6 OSCC cell lines, but there is no correlation between Cks1 and p27 expression. High expression of Skp2 was well correlated with low expression of p27 (Figure 3A). Interestingly, Ho-1-N-1 cell showed low expression of both Cks1 and Skp2 and high expression of p27. This finding is consistent with the immunohistochemical finding of OSCC cases.
Figure 3.
Expression of Cks1 protein and correlation with Skp2 or p27 expression in OSCC cell lines. A: Expression of Cks1, Skp2, and p27 protein in six OSCC cell lines (HSC2, HSC3, HSC4, Ca9–22, Ho-1-U-1, and Ho-1-N-1 cells). Thirty μg of protein was subjected to Western blot analysis as described in the Materials and Methods section. Cul1 expression was used as a loading control. B: Effect of Cks1, Skp2, and both Cks1 and Skp2 transfection on p27 protein levels in Ho-1-N-1 cells. Transfection was performed by using Fugene 6 (Roche). Thirty μg of protein was subjected to Western blot analysis as described in the Materials and Methods section. Cul1 expression was used as a loading control. p27/Cul1 level was measured by densitometry.
Cks1 Overexpression Enhanced Skp2-Mediated p27 Degradation
To understand the role of Cks1 overexpression for p27 down-regulation in OSCC, we transfected Cks1 gene with or without Skp2 gene and examined the expression of p27 protein (Figure 3B). Ho-1-N-1 cell, which showed low expression of Cks1 and Skp2 and high expression of p27 protein, was used in this experiment (Figure 3A). Although ectopic expression of Skp2 could induce down-regulation of p27 by itself, ectopic expression of Cks1 could not induce down-regulation of p27 by itself. Skp2 transfection induced up-regulation of Cks1. Interestingly, ectopic expression of both Cks1 and Skp2 induced down-regulation of p27 protein more than with Skp2 alone. The expression level of p27 protein in both Cks1 and Skp2 transfectant cells is lower than that in Skp2 transfectant cells. These results indicate that Cks1 enhances p27 down-regulation mediated by Skp2 overexpression.
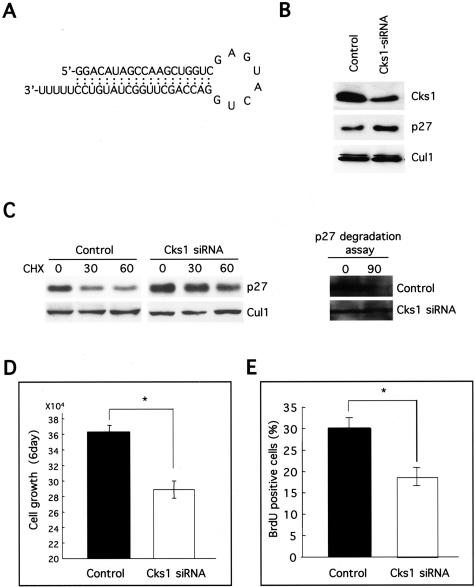
Cks1 siRNA Inhibited the Growth of OSCC Cells Both in Vitro and in Vivo
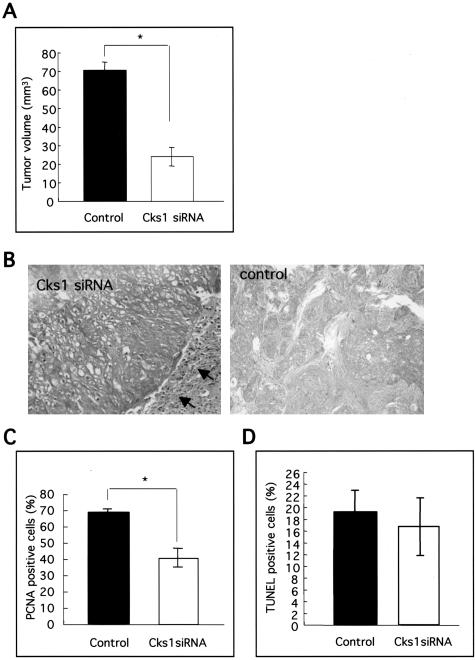
As shown above, Cks1 overexpression plays an important role for p27 down-regulation in OSCC. Moreover, Cks1 is specifically required for p27 ubiquitination.25,26 Therefore, to suppress the ubiquitin-mediated degradation of p27 frequently shown in OSCC, we inhibited Cks1 expression by siRNA in HSC4 cells. To block Cks1 expression selectively by siRNA, 19-nt target sequence, separated by a 9-nt spacer from its reverse complement sequence, was introduced into the siRNA generating pSUPPRESSOR vector system (Figure 4A). Cks1 siRNA transfection induced the down-regulation of Cks1 protein (Figure 4B). As we expected, accumulation of p27 protein was observed in Cks1 siRNA transfectant cells. Next, we examined the p27 protein stability and p27 degradation activity by measuring its half-life and in vitro p27 degradation assay in control siRNA and Cks1 siRNA transfectant cells. Interestingly, p27 protein rapidly degraded in control cells in comparison with Cks1 siRNA transfected cells after CHX treatment (Figure 4C). In addition, recombinant p27 protein degraded in control cells after a 90-minute incubation, but not in Cks1 siRNA transfected cells by in vitro p27 degradation assay (Figure 4C). Moreover, Cks1 siRNA transfectant cells grew slower in vitro and showed less BrdU-positive cells in comparison with control cells (Figure 4, D and E). Next, tumorigenicity of Cks1 siRNA transfectant cells was assayed in three nude mice by s.c. injection of 1.5 × 107 cells/injection site (Figure 5, A and B). The average of tumor size of Cks1 siRNA transfectant cells (24.0 ± 5.0 mm3) was significantly smaller, as compared to the control siRNA transfectant cells (70.3 ± 4.2 mm3) (Figure 5A). Microscopically, the tumors obtained by injection of Cks1 siRNA transfectant cells were generally well encapsulated by the fibrous connective tissue (Figure 5B). In contrast, the tumors obtained by injection of control, siRNA transfectant cells infiltrated the surrounded tissues without fibrous encapsulation (Figure 5B). In tumors obtained by injection of Cks1 siRNA transfectant cells, we confirmed less expression of Cks1 and more expression of p27 in comparison with tumors obtained by injection of control cells by immunohistochemistry (data not shown). Similar to the in vitro result, the number of PCNA-positive cells in tumors by injection of Cks1 siRNA transfected cells were significantly less than in those of control cells (Figure 5C). In addition, we examined the rate of apoptotic cells in tumors by TUNEL assay, but we could not find the difference between both types of tumors (Figure 5D).
Figure 4.
Cks1 siRNA transfection in OSCC. A: To suppress p27 degradation, we tried to inhibit the Cks1 expression by siRNA in HSC4 cells. A synthetic double-strand oligonucleotide 5′-GGGACATAGCCAAGCTGGTCgagtactg GACCAGCTTGGCTATGTCC-3′) was introduced into pSUPPRESSOR. Oligonucleotide sequences correspond to a 19-nt sequence from Cks1 (nucleotide 78–96), which are separated by a 9-nt linker (lower case letters) from the reverse complement of the same 19-nt sequence. Transfection was performed using Fugene 6. B: Cks1 siRNA transfection induced the p27 accumulation in HSC4 cells. In Cks1 siRNA transfectant cells, expression of Cks1 and p27 was examined by Western blot analysis. Thirty μg of protein was subjected to Western blot analysis as described in the Materials and Methods section. We used circular control plasmid, which contains a scrambled sequence that does not show significant homology to rat, mouse, or human gene sequences, as a control. Cul1 expression was used as a loading control. C: Half-life of p27 protein and p27 degradation activity in control and Cks1 siRNA transfected cells. To measure the half-life of p27 protein, we treated CHX (50 μg/ml) for 0, 30, and 60 minutes and then examined the expression of p27 by Western blot analysis. In vitro degradation assay of p27 was performed as described by Pagano et al3 One hundred μg of protein was subjected to Western blot analysis as described in the Materials and Methods section. D: The effect of Cks1 siRNA transfection on cell growth in vitro at day 6. Cells (5.0 × 103) were plated onto a 24-well multi-well plate and counted by Cell Counter at days 0 and 6. *, P < 0.05. Correlation was analyzed by Fisher’s exact test. E: BrdU incorporation in Cks1 siRNA transfected cells. For BrdU incorporation, cells growing on coverslips were incubated with 10 μmol/L BrdU for 3 hours. Incorporated BrdU was detected with antibodies as described in the Materials and Methods section. *, P < 0.05. Correlation was analyzed by Fisher’s exact test.
Figure 5.
Tumorigenicity of Cks1 siRNA transfected cells. A: Effect of Cks1 siRNA transfection on cell growth in vivo. Tumorigenicity of Cks1 siRNA transfected and control siRNA transfected cells were assayed in each three nude mice by s.c. injection of 1.5 × 107 cells/injection site. Tumor length (L) and width (W) were measured at the end of the experiment, and tumor volume was calculated by the formula (L × W2)/2. The average of tumor volume of Cks1 siRNA transfected cells was 24.0 ± 5.0 mm3 and that of control siRNA transfected cells was 70.3 ± 4.2 mm3. *, P < 0.05. Correlation was analyzed by Fisher’s exact test. B: Histology of tumors by injection of Cks1 siRNA and control siRNA transfected cells. Arrow shows encapsulation by the fibrous connective tissue. C: Proliferation activity in tumors by injection of Cks1 siRNA cells. For proliferation activity, the expression of PCNA was examined by immunohistochemistry in tumors by injection of Cks1 siRNA and control siRNA cells. Immunohistochemistry was performed as described in the Materials and Methods section. D: The rates of apoptosis in tumors by injection of Cks1 siRNA cells. The rates of apoptosis were examined by TUNEL assay. The percentage of and TUNEL-positive epithelial cell nuclei relative to the total number of epithelial cell nuclei was calculated in both tumors.
Discussion
Numerous studies have shown that loss or drastic reduction of p27 protein is frequently found in various aggressive cancers. In our previous study, reduced expression of p27 protein was frequently observed in OSCC cases and was well correlated with metastasis and poor prognosis.10 Moreover, we found that OSCC cell lines with low p27 protein levels displayed the high proteolytic activity toward recombinant p27 protein in vitro and accumulation of p27 protein after proteasome inhibitor treatment.10,35 Our previous findings are supported by several reports that aggressive human cancers such as colon cancers, lymphomas, and astrocytic brain tumors contain high ubiquitin-proteasome-mediated degradation activity specific for p27.36–39 SCFSkp2 was identified as the E3 ubiquitin ligase that targets p27 for ubiquitination.11–13 We and other groups found that Skp2 overexpression was frequently observed in epithelial dysplasia and OSCC, and that Skp2 expression correlates inversely with p27 levels.15,16 Cks1 was identified as an accessory protein in the SCFSkp2 ubiquitinating machinery.25,26 Three binding sites of Cks1 for Cdk, anion, and Skp2 are required for p27-ubiquitin ligation and for the association of Skp2 with Cdk-bound, Thr187-phosphorylated p27,27 suggesting that Cks1 may function as an adaptor protein to bridge Skp2 with the Thr187-phosphorylated p27 or may alter the conformation of Skp2 to promote binding to phosphorylated p27. In addition to the function of p27 ubiquitination, Cks1 is overexpressed in some cancer cells.30 Therefore, we hypothesize that Cks1 may act as an oncoprotein similarly to Skp2. In the present study, we found high expression of Cks1 in 62% of OSCC cases, but in 0% of normal oral mucosae. High expression of Cks1 was associated with low expression of p27, but we could not observe the statistical correlation between them (Table 1). Recently, three groups have reported that Cks1 overexpression was observed in gastric, non-small cell lung, and colon carcinoma.31–33 Cks1 overexpression was well correlated with reduced expression of p27 in gastric and colorectal carcinomas, but not in non-small cell lung carcinoma.31–33 Overall, Cks1 overexpression was frequently found in various cancers, but the role of Cks1 overexpression in cancer is still unclear. In the present study, we demonstrated that ectopic Cks1 expression did not induce down-regulation of p27 protein by itself, while ectopic Cks1 and Skp2 expression strongly induced down-regulation of p27 protein in comparison to ectopic Skp2 expression alone (Figure 3B). Surprisingly, Skp2 transfection induced up-regulation of Cks1 in Figure 3B. Although we do not know the reason of this phenomenon, it is possible that Skp2 transfection may increase the cell population in S-phase where Cks1 is more expressed. In S-phase, Cks1 protein is stabilized.40 These observations are consistent with the finding that high expression of Cks1 was well correlated with high expression of Skp2 by immunohistochemical analysis (Table 1). Interestingly, Cks1 overexpression was well correlated with poor prognosis, suggesting that Cks1 can be a good prognostic marker. We suggest that Cks1 overexpression may not induce p27 down-regulation by itself, and may be able to enhance p27 down-regulation caused by Skp2 overexpression in OSCC. Aberrant overexpression of Cks1 may play an important role for OSCC development and progression by enforced degradation of p27 through the Skp2 overexpression in OSCC.
Down-regulation of p27 is a common event with the high frequency in various malignant tumors including OSCC. As down-regulation of p27 is well known to correlate with malignant behavior in cancer, we believe that inhibition of p27 degradation can be a novel and powerful target of cancer therapy. It has been reported that Cks1−/− cells contain elevated levels of p27 due to defective ubiquitination and degradation of this protein, indicating that Cks1 is a specific factor for the ubiquitination and consequent degradation of p27.26 Therefore, we knocked-down Cks1 expression by siRNA to inhibit the p27 degradation (Figure 4). Cks1 siRNA transfection induced the down-regulation of Cks1 protein and the consequent accumulation of p27 (Figure 4B). Interestingly, Cks1 siRNA transfected cells showed lower activity of p27 degradation in comparison with control cells (Figure 4C). Moreover, knockdown of Cks1 expression actually inhibited the growth of OSCC cells both in cultured cells and in vivo (Figure 4D and 5A). These findings suggest that inhibition of Cks1 expression by using siRNA can be a novel modality of cancer gene therapy for suppression of p27 degradation.
It has recently been reported that degradation of Skp2 is mediated by ubiquitin ligase APC/CCdh1 (anaphase-promoting complex/cyclosome and its activator Cdh1).40,41 Cks1 degradation is also mediated by APC/CCdh1.40 As described above, overexpression of Skp2 and Cks1 is frequently observed and is associated with p27 down-regulation in OSCC. Although amplification of the Skp2 locus and overexpression Cks1 mRNA were found at least in small-cell lung tumors,21,32 the mechanism for the overexpression of Cks1 and Skp2 in cancer is not yet known. Therefore, there is a possibility that abnormalities in the degradation of Skp2 and/or Cks1 may be involved in high levels of these two proteins in human cancers. The clarification of this mechanism will be of interest.
Footnotes
Address reprint requests to Yasusei Kudo or Takashi Takata, Department of Oral Maxillofacial Pathobiology, Division of Frontier Medical Science, Graduate School of Biomedical Sciences, Hiroshima University, 1–2-3 Kasumi, Minami-ku, Hiroshima 734-8553, Japan. E-mail: ykudo@hiroshima-u.ac.jp or ttakata@hiroshima-u.ac.jp.
Supported in part by Grants-in-Aid from the Ministry of Education, Science and Culture of Japan (to Y.K. and T.T.), a fellowship from the New York State Breast Cancer Research and Education fund (to T.B.), and National Institutes of Health grants (to M.P.).
References
- Toyoshima H, Hunter T. p27, a novel inhibitor of G1 cyclin-Cdk protein kinase activity, is related to p21. Cell. 1994;78:67–74. doi: 10.1016/0092-8674(94)90573-8. [DOI] [PubMed] [Google Scholar]
- Polyak K, Lee MH, Erdjument-Bromage H, Koff A, Roberts JM, Tempst P, Massague J. Cloning of p27Kip1, a cyclin-dependent kinase inhibitor and a potential mediator of extracellular antimitogenic signals. Cell. 1994;78:59–66. doi: 10.1016/0092-8674(94)90572-x. [DOI] [PubMed] [Google Scholar]
- Pagano M, Tam SW, Theodoras AM, Beer-Romero P, Del Sal G, Chau V, Yew PR, Draetta GF, Rolfe M. Role of the ubiquitin proteasome pathway in regulating abundance of the cyclin-dependent kinase inhibitor p27. Science. 1995;269:682–685. doi: 10.1126/science.7624798. [DOI] [PubMed] [Google Scholar]
- Sheaff R, Groudine M, Gordon M, Roberts J, Clurman B. Cyclin E-Cdk2 is a regulator of p27Kip1. Genes Dev. 1997;11:1464–1478. doi: 10.1101/gad.11.11.1464. [DOI] [PubMed] [Google Scholar]
- Vlach J, Hennecke S, Amati B. Phosphorylation-dependent of the cyclin-dependent kinase inhibitor p27Kip1. EMBO J. 1997;16:5334–5344. doi: 10.1093/emboj/16.17.5334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagnoli A, Fiore F, Eytan E, Carrano AC, Draetta GF, Hershko A, Pagano M. Ubiquitination of p27 is regulated by Cdk-dependent phosphorylation and trimeric complex formation. Genes Dev. 1999;13:1181–1189. doi: 10.1101/gad.13.9.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen H, Gitig DM, Koff A. Cell-free degradation of p27Kip1, a G1 cyclin-dependent kinase inhibitor, is dependent on CDK2 activity and the proteasome. Mol Cell Biol. 1999;19:1190–1201. doi: 10.1128/mcb.19.2.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slingerland JM, Pagano M. Regulation of the cdk inhibitor p27 and its deregulation in cancer. J Cell Physiol. 2000;183:10–17. doi: 10.1002/(SICI)1097-4652(200004)183:1<10::AID-JCP2>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Bloom J, Pagano M. Deregulated degradation of the cdk inhibitor p27 and malignant transformation. Semin Cancer Biol. 2003;13:41–47. doi: 10.1016/s1044-579x(02)00098-6. [DOI] [PubMed] [Google Scholar]
- Kudo Y, Takata T, Yasui W, Ogawa I, Miyauchi M, Takekoshi T, Tahara E, Nikai H. Reduced expression of cyclin-dependent kinase inhibitor p27Kip1 is an indicator of malignant behavior of oral squamous cell carcinomas. Cancer. 1998;83:2447–2455. doi: 10.1002/(sici)1097-0142(19981215)83:12<2447::aid-cncr7>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Carrano AC, Eytan E, Hershko A, Pagano M. SKP2 is required for ubiquitin-mediated degradation of the CDK inhibitor p27. Nat Cell Biol. 1999;1:193–199. doi: 10.1038/12013. [DOI] [PubMed] [Google Scholar]
- Sutterluty H, Chatelain E, Marti A, Wirbelauer C, Senften M, Muller U, Krek W. p45SKP2 promotes p27Kip1 degradation and induces S phase in quiescent cells. Nat Cell Biol. 1999;1:207–214. doi: 10.1038/12027. [DOI] [PubMed] [Google Scholar]
- Tsvetkov LM, Yeh KH, Lee S, Sun H, Zhang H. p27Kip1 ubiquitination and degradation is regulated by the SCFSkp2 complex through phosphorylated Thr187 in p27. Curr Biol. 1999;9:661–664. doi: 10.1016/s0960-9822(99)80290-5. [DOI] [PubMed] [Google Scholar]
- Zhang H, Kobayashi R, Galaktionov K, Beach D. p19Skp-1 and p45Skp-2 are essential elements of the cyclin A-Cdk2 S phase kinase. Cell. 1995;82:915–925. doi: 10.1016/0092-8674(95)90271-6. [DOI] [PubMed] [Google Scholar]
- Gstaiger M, Jordan R, Lim M, Catzavelos C, Mestan J, Slingerland J, Krek W. Skp2 is oncogenic and overexpressed in human cancers. Proc Natl Acad Sci USA. 2001;98:5043–5048. doi: 10.1073/pnas.081474898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo Y, Kitajima S, Sato S, Miyauchi M, Ogawa I, Takata T. High expression of S-phase kinase-interacting protein 2, human F-box protein, correlates with poor prognosis in oral squamous cell carcinomas. Cancer Res. 2001;61:7044–7047. [PubMed] [Google Scholar]
- Latres E, Chiarle R, Schulman B, Pavletich N, Pellicer A, Inghirami G, Pagano M. Role of the F-box protein Skp2 in lymphomagenesis. Proc Natl Acad Sci USA. 2001;98:2515–2520. doi: 10.1073/pnas.041475098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiarle R, Fan Y, Piva R, Boggino H, Skolnik J, Novero D, Palestro G, De Wolf-Peeters C, Chilosi M, Pagano M, Inghirami G. S-phase kinase-associated protein 2 expression in non-Hodgkin’s lymphoma inversely correlates with p27 expression and defines cells in S phase. Am J Pathol. 2002;160:1457–1466. doi: 10.1016/S0002-9440(10)62571-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signoretti S, Dimarcotullio L, Richardson A, Ramaswamy S, Isaac B, Rue M, Monti F, Loda M, Pagano M. Oncogenic role of the ubiquitin ligase subunit Skp2 in human breast cancer. J Clin Invest. 2002;110:633–641. doi: 10.1172/JCI15795. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Hershko D, Bornstein G, Ben-Izhak O, Carrano A, Pagano M, Krausz MM, Hershko A. Inverse relation between levels of p27Kip1 and of its ubiquitin ligase subunit Skp2 in colorectal carcinomas. Cancer. 2001;91:1745–1751. doi: 10.1002/1097-0142(20010501)91:9<1745::aid-cncr1193>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Yokoi S, Yasui K, Saito-Ohara F, Koshikawa K, Iizasa T, Fujisawa T, Terasaki T, Horii A, Takahashi T, Hirohashi S, Inazawa J. A novel target gene, SKP2, within the 5p13 amplicon that is frequently detected in small cell lung cancers. Am J Pathol. 2002;161:207–216. doi: 10.1016/S0002-9440(10)64172-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda TA, Inoue H, Sonoda H, Mine S, Yoshikawa Y, Nakayama K, Mori M. Clinical and biological significance of S-phase kinase-associated protein 2 (Skp2) gene expression in gastric carcinoma: modulation of malignant phenotype by Skp2 overexpression, possibly via p27 proteolysis. Cancer Res. 2002;62:3819–3825. [PubMed] [Google Scholar]
- Philipp-Staheli J, Payne SR, Kemp CJ. p27Kip1: regulation and function of a haploinsufficient tumor suppressor and its misregulation in cancer. Exp Cell Res. 2001;264:148–168. doi: 10.1006/excr.2000.5143. [DOI] [PubMed] [Google Scholar]
- Pagano M, Benmaamar R. When protein destruction runs amok, malignancy is on the loose. Cancer Cell. 2003;4:251–256. doi: 10.1016/s1535-6108(03)00243-5. [DOI] [PubMed] [Google Scholar]
- Ganoth D, Bornstein G, Ko TK, Larsen B, Tyers M, Pagano M, Hershko A. The cell-cycle regulatory protein Cks1 is required for SCFSkp2-mediated ubiquitinylation of p27. Nat Cell Biol. 2001;3:321–324. doi: 10.1038/35060126. [DOI] [PubMed] [Google Scholar]
- Spruck C, Strohmaier H, Watson M, Smith A, Ryan A, Krek W, Reed S. A CDK-independent function of mammalian Cks1: targeting of SCFSkp2 to the CDK inhibitor p27. Mol Cell. 2001;7:639–650. doi: 10.1016/s1097-2765(01)00210-6. [DOI] [PubMed] [Google Scholar]
- Sitry D, Seeliger MA, Ko TK, Ganoth D, Breward SE, Itzhaki LS, Pagano M, Hershko A. Three different binding sites of Cks1 are required for p27-ubiquitin ligation. J Biol Chem. 2002;277:42233–42240. doi: 10.1074/jbc.M205254200. [DOI] [PubMed] [Google Scholar]
- Hayles J, Beach D, Durkacz B, Nurse P. The fission yeast cell cycle control gene cdc2: isolation of a sequence suc1 that suppresses cdc2 mutant function. Mol Gen Genet. 1986;202:291–293. doi: 10.1007/BF00331653. [DOI] [PubMed] [Google Scholar]
- Hadwiger JA, Wittenberg C, Mendenhall MD, Reed SI. The Saccharomyces cerevisiae CKS1 gene, a homolog of the Schizosaccharomyces pombe suc1+ gene, encodes a subunit of the Cdc28 protein kinase complex. Mol Cell Biol. 1989;9:2034–2041. doi: 10.1128/mcb.9.5.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne Y, Watson MH, Hickey MJ, Holmes W, Rocque W, Reed SI, Tainer JA. Crystal structure and mutational analysis of the human CDK2 kinase complex with cell cycle-regulatory protein CksHs1. Cell. 1996;84:863–874. doi: 10.1016/s0092-8674(00)81065-x. [DOI] [PubMed] [Google Scholar]
- Masuda T, Inoue H, Nishida K, Sonoda H, Yoshikawa Y, Kakeji Y, Utsunomiya T, Mori M. Cyclin-dependent kinase 1 gene expression is associated with poor prognosis in gastric carcinoma. Clin Cancer Res. 2003;9:5693–5698. [PubMed] [Google Scholar]
- Inui N, Kitagawa K, Miwa S, Hattori T, Chida K, Nakamura H, Kitagawa M. High expression of Cks1 in human non-small cell lung carcinomas. Biochem Biophys Res Commun. 2003;303:978–984. doi: 10.1016/s0006-291x(03)00469-8. [DOI] [PubMed] [Google Scholar]
- Shapira M, Ben-Izhak O, Bishara B, Futerman B, Minkov I, Krausz M, Pagano M, Hershko D. Alterations in the expression of the cell cycle regulatory protein Cks1 in colorectal carcinoma. Cancer. 2004;100:1615–1621. doi: 10.1002/cncr.20172. [DOI] [PubMed] [Google Scholar]
- Japan Society for Head and Neck Cancer Tokyo: Kanehara; General Rules for Clinical and Pathological Studies on Head and Neck Cancer. (ed 2) 1991 [Google Scholar]
- Kudo Y, Kitajima S, Sato S, Ogawa I, Miyauchi M, Takata T. Transfection of p27Kip1 threonine residue 187 mutant type gene, which is not influenced by ubiquitin-mediated degradation, induces cell cycle arrest in oral squamous cell carcinoma cells. Oncology. 2002;63:398–404. doi: 10.1159/000066222. [DOI] [PubMed] [Google Scholar]
- Loda M, Cukor B, Tam S, Lavin P, Fiorentino M, Draetta G, Jessup J, Pagano M. Increased proteasome-dependent degradation of the cyclin-dependent kinase inhibitor p27 in aggressive colorectal carcinomas. Nat Med. 1997;3:231–234. doi: 10.1038/nm0297-231. [DOI] [PubMed] [Google Scholar]
- Tan P, Cady B, Wanner M, Worland P, Cukor B, Magi-Galluzzi C, Lavine P, Draetta G, Pagano M, Loda M. The cell cycle inhibitor p27 is an independent prognostic marker in small (T1a, b) invasive breast carcinomas. Cancer Res. 1997;57:1259–1263. [PubMed] [Google Scholar]
- Piva R, Cancelli I, Cavalla P, Bortolotto S, Dominguez J, Draetta GF, Schiffer D. Proteasome-dependent degradation of p27/kip1 in gliomas. J Neuropathol Exp Neurol. 1999;58:691–696. doi: 10.1097/00005072-199907000-00002. [DOI] [PubMed] [Google Scholar]
- Chiarle R, Budel LM, Skolnik J, Frizzera G, Chilosi M, Corato A, Pizzolo G, Magidson J, Montagnoli A, Pagano M, Maes B, De Wolf-Peeters C, Inghirami G. Increased proteasome degradation of cyclin-dependent kinase inhibitor p27 is associated with a decreased overall survival in mantle cell lymphoma. Blood. 2000;95:619–626. [PubMed] [Google Scholar]
- Bashir T, Dorrello NV, Amador V, Guardavaccaro D, Pagano M. Control of the SCFSkp2-Cks1 ubiquitin ligase by the APC/CCdh1 ubiquitin ligase. Nature. 2004;428:190–193. doi: 10.1038/nature02330. [DOI] [PubMed] [Google Scholar]
- Wei W, Ayad NG, Wan Y, Zhang GJ, Kirshner MW, Kaelin WG., Jr Degradation of the SCF component Skp2 in cell-cycle phase G1 by the anaphase-promoting complex. Nature. 2004;428:194–198. doi: 10.1038/nature02381. [DOI] [PubMed] [Google Scholar]