Abstract
Our laboratory and others have shown an important role of metalloelastase (MMP-12) in the pathogenesis of acute and chronic lung injury. Because chronic asthma is characterized by airway inflammation and alterations in the airway extracellular matrix, we explored the role of metalloelastase in a model of allergic airway inflammation induced by cockroach antigen (CRA). Using MMP-12-deficient mice we found a significant reduction in CRA-induced inflammatory injury, as evidenced by fewer peribronchial leukocytes, significantly less protein in the bronchoalveolar lavage (BAL) fluid, and a significant reduction in the number of infiltrating neutrophils, eosinophils, and macrophages, relative to wild-type mice. Although we did not find a significant reduction in the number of T cells in the injured MMP-12-deficient animals as compared to controls, levels of the chemotactic factors interleukin-5, macrophage inflammatory protein-1α, monocyte chemoattractant protein-1, thymus activation regulated chemokine, and the proinflammatory cytokine tumor necrosis factor-α were significantly reduced in the bronchoalveolar lavage fluid of CRA-challenged MMP-12-deficient mice, relative to CRA-challenged control animals. These studies indicate that MMP-12 plays an important proinflammatory role in the development of allergic inflammation in the CRA model. Alterations in the levels of chemotactic factors and other proinflammatory cytokines in the MMP-12-deficient mice may underlie the decrease in leukocyte recruitment into inflamed lungs.
Most studies of the pathogenesis of asthma have historically concentrated on the reversible airway obstruction, which is a hallmark of this disease.1 However, recent studies have also emphasized that chronic asthma is an inflammatory process characterized by neutrophils, eosinophils, macrophages, and lymphocytes accumulating around the airways.2–4 These inflammatory infiltrates are associated with airway structural changes including remodeling of the extracellular matrix with subepithelial collagen deposition. This remodeling is thought to be responsible for the residual airway obstruction observed in many patients.5,6
Matrix metalloproteinases (MMPs), as a group of zinc-containing neutral proteinases, are known to have the capacity to degrade every component of the extracellular matrix.7,8 As normal turnover and remodeling of the extracellular matrix are known to be on of the roles of MMPs, it is possible that extensive remodeling of the airway extracellular matrix by infiltrating leukocytes may in part be caused by increased levels of matrix metalloproteinases. Indeed, increased levels of certain MMPs (such as gelatinases, collagenases, and stromelysin) are present in the diseased tissues of arthritis, bullous skin diseases, and periodontitis,9–12 and it is felt that the infiltrating leukocytes are the primary source of the MMPs.
There is also evidence that MMPs are involved in pulmonary inflammation. In humans with adult respiratory distress syndrome or with chronic lung injury, increased levels of gelatinase B (MMP-9) and collagenase (MMP-1) have been described.13–18 Experimental models of acute and chronic lung injury have shown an important role for MMPs in the development of the lung injury.13–18
Increased levels of MMP-9 have been found in the bronchoalveolar lavage (BAL) of patients with asthma, as well as an upregulation of gelatinase and stromelysin-1 (MMP-3) in the bronchial mucosal tissue, which correlates with the degree of remodeling of the airway extracellular matrix.5,19,20 Experimental models of allergic inflammation have demonstrated an increase in the levels of gelatinase B and other MMPs.15,16 Additionally, using tissue inhibitors of metalloproteinases (TIMP),21 MMP-induced injury can be inhibited. Of interest, eosinophils, which are a hallmark of allergic inflammation, produce high levels of MMPs.22
Metalloelastase is the main elastolytic activity in macrophages including alveolar macrophages, which are known to play an important role in acute and chronic lung inflammation.18,23,24 Using mice deficient in metalloelastase (MMP-12−/−) we have found that these animals develop less severe acute lung injury, relative to their wild-type counterparts, expressing normal levels of metalloelastase (MMP-12+/+).16 In terms of chronic lung injury, Hautamaki and colleagues18 using MMP-12−/− mice have found that metalloelastase is involved in the pathogenesis of experimental smoking-induced emphysema. Because asthma represents a type of chronic inflammation with macrophage infiltration and airway remodeling, we wanted to assess the role of this macrophage-derived MMP in the pathogenesis of allergic airway injury. We present evidence that MMP-12 is critically involved in the pathogenesis of inflammation associated with a model of allergic airway inflammation.
Materials and Methods
Metalloelastase-Deficient Mice
Female and male 129SvEv mice possessing a homozygous deletion of exons 1 and 2 of MMP-12−/− were developed in one of our laboratories (Dr. Steven Shapiro) as described in previous publications.16,18 The wild-type 129 SvEv mice (MMP-12+/+) were purchased from Taconic (Germantown, NY). The mice used in these studies were 6 to 8 weeks of age and were housed in pathogen-free conditions.
Experimental Model of Allergen-Induced Airway Inflammation
As described in recent publications this model of allergic lung injury is induced in mice sensitized and challenged with cockroach antigen (CRA) to induce a Th2-type response.25,26 Mice were initially immunized (subcutaneously and intraperitoneally) with 10 μg of CRA (Bayer, Elkhart, IN) in incomplete Freund’s adjuvant (Sigma Chemical Co, St. Louis, MO) on day 0. To localize the response to the lung, mice were subsequently challenged with an intratracheal dose of 10 μg of CRA suspended in 50 μl of phosphate-buffered saline (PBS) on day 14. Mice were again challenged intratracheally on days 20 and 22 with the same concentration of allergen. Measurements of airway hyperreactivity (AHR) were taken in some of the animals 24 hours after the final challenge and then the animals were humanely sacrificed using a lethal injection of Ketamine (Fort Dodge Labs, Fort Dodge, IA) for histological examination and BAL studies. The University Committee on the Use and Care of Animals approved these studies.
Histology
At the time of sacrifice, lungs, heart, and associated vasculature were removed en bloc. The lungs were inflated with 4% paraformaldehyde, embedded in paraffin, and 5-μm sections were routinely processed and stained with hematoxylin and eosin (H&E) for light microscopic analysis.
Analysis of Leukocyte Accumulation in the Airways and Lung
To assess airway accumulation of leukocytes, mice were first sacrificed, the trachea exposed in situ and a total of 2 ml (in 0.5-ml aliquots) of PBS was used to wash and recover cells from the alveolar spaces. Cells were washed, pelleted onto glass slides using a cytospin (Shandon Scientific, Runcorn, UK), and differentially stained with Wright-Giemsa stain and counted on a light microscope. For T cells and macrophages, lungs were first inflated with OCT and then snap-frozen in liquid nitrogen. Six-μm-thick sections were cut on a cryostat (Leica Microsystems, Bannockburn, IL) and direct immunofluorescence analysis of the leukocytes was performed using a fluorescein-labeled anti-CD3 antibody for T cells and a rhodamine-labeled BS-1 lectin for the macrophages (Sigma Chemical Co.). These cells were quantified at ×40 using a Zeiss fluorescence microscope (Carl Zeiss Inc., Thornwood, NY). In situ morphometric quantitation of leukocyte (neutrophils, eosinophils, macrophages, and lymphocytes) accumulation in the lung parenchyma was performed using 5-μm sections. Neutrophil, macrophage, and lymphocyte determination was performed using H&E- and Trichrome-stained sections at ×40, whereas eosinophil quantification was performed on Congo Red-stained sections at ×100. For these studies at least 100 fields per animal were examined. Finally, eosinophil peroxidase (EPO) levels were assessed in the BAL, as described by Campbell and colleagues,25 50 μl of BAL fluid was mixed with o-phenylenediamine substrate (OPD) in a 96-well microtiter plate. The plates were incubated at 37°C for 30 minutes, the reaction was stopped with sulfuric acid and the optical density read (490 nm) using a Bio Kinetic ELISA plate reader (Bio-Tek Instruments, Winooski, VT).
Assessment of MMP Activity
BAL fluid was assessed for MMP activity, specifically gelatinase A and gelatinase B, by using substrate-embedded enzymography (sodium dodecyl sulfate-polyacrylamide gel electrophoresis). Briefly, as previously described,15,16 sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels were prepared for mini gels from 30:1 acrylamide/bis with the final concentrations of the other components of the gels being Tris-HCl at pH 8.8 (325 mmol/L), sodium dodecyl sulfate (0.1%), ammonium persulfate (0.05%), and TEMED (0.05%). Gelatin (1 mg/ml) was incorporated into the gel at the time of casting. The gels were routinely 7.5% acrylamide. Various denatured but nonreduced samples and standards were then electrophoresed into the gels at constant voltage (150 V) in an ice bath under nonreducing conditions. When the dye front reached a point ∼0.5 cm from the bottom, the gels were removed and subjected to the following washing protocol: 2×, 15 minutes in 50 mmol/L Tris buffer (containing 1 mmol/L Ca2+ and 0.5 mmol/L Zn2+) with 2.5% Triton X-100; 1×, 5 minutes in Tris buffer alone; and finally overnight in Tris buffer with 1% Triton X-100. The gels were stained the following morning with Coomassie Brilliant Blue 250-R. After destaining, zones of enzyme activity were detected as regions of negative staining. The zymograms were converted to negative images and digitized. Quantification was accomplished by determining the number of pixels in the negative images.
Permeability Measurement
Total protein content in the BAL fluid was used as a marker of lung permeability resulting from lung injury. Total protein content was assessed using the BCA protein assay.15,16 Briefly, protein levels of 100 μl of diluted BAL fluid were determined for injured and control animals using the BCA protein assay kit (Pierce, Rockford, IL) and quantified using a bovine serum albumin standard curve.
Cytokine and Chemokine Determination
The levels of the murine cytokines and chemokines, macrophage inflammatory protein (MIP)-1α, monocyte chemoattractant protein (MCP)-1, tumor necrosis factor (TNF)-α, and interleukin (IL)-5 were assessed in the BAL fluid and thymus activation-regulated chemokine (TARC) quantified in lung homogenates of challenged and control mice by enzyme-linked immunosorbent assay (ELISA). Using commercially available reagents from R&D Systems (Minneapolis, MN), ELISA plates (Immulon 4; Dynex Technologies, Inc., Chantilly, VA) were coated with 10 μg/ml of capture antibody in carbonate buffer (pH 9.6) and allowed to bind overnight at 4°C. The plates were then washed with PBS containing 0.02% Tween 20 (Bio-Rad, Hercules, CA) and then 50 μl of either sample or standard was added to appropriate wells. The plates were then incubated for 2 hours at room temperature, washed twice, and 50 μl of biotinylated secondary antibody was added. The plates were then incubated for 1 hour at room temperature, washed twice, and 50 μl of streptavidin-conjugated horseradish peroxidase (Zymed, San Francisco, CA) diluted 1:3000 added to each well. The plates were then incubated at room temperature for 30 minutes, washed twice, and 200 μl of OPD substrate (Sigma Chemical Co.) was added and color allowed to develop. The reaction was stropped with 50 μl of 3 mol/L sulfuric acid and the optical density (490 nm) determined using an ELISA plate reader (Bio-Tek Instruments).
Macrophage Isolation and Culture
Elicited macrophages were harvested from the peritoneum of mice (MMP-12+/+, MMP-12−/−) 96 hours after an intraperitoneal injection of thioglycollate solution (Life Technologies, Inc., Grand Island, NY). Cells were washed, resuspended in Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum (Life Technologies, Inc.), and 1 × 105 cells added to each well of a 12-well tissue culture plate. One hour later, nonadherent cells were removed, fresh media added to each well, and cells were allowed to go quiescent overnight. Wells were used as either unstimulated controls or stimulated with lipopolysaccharide (25 μg/ml) and allowed to incubate for 48 hours before the supernatant was harvested, frozen at −80°C, and analyzed for cytokines and chemokines by ELISA.
Measurement of Airway Hyperreactivity
Airway hyperreactivity was measured using a Buxco mouse plethysmograph, which is specifically designed for the low tidal volumes (Buxco, Troy, NY). As described in previous publications25,26 mice were anesthetized with sodium pentobarbital and intubated via cannulation of the trachea with an 18-gauge metal tube, subsequently ventilated with a Harvard pump ventilator (Harvard Apparatus, Inc., Holliston, MA) and a 27-gauge needle inserted into the tail vein for injection of methylcholine challenge. Animal were placed in the plethysmograph and readings monitored by computer. A second transducer was used to measure the pressure swings at the opening of the trachea tube (Paw), referenced to the body box (ie, pleural pressure), and to provide a measure of Tran pulmonary pressure (Ptp = Paw − Pbox). The trachea transducer was calibrated at a constant pressure of 20 cm H2O. Resistance is calibrated by the Buxco software by dividing the change in pressure (Ptp) by the change in flow (F) (δPtp/δF; units = cm H2O/ml/second) at two time points from the volume curve, based on a percentage of the inspiratory volume. Baseline levels for each mouse were determined (5-minute readings) and then a methylcholine challenge was given via tail vein and the peak airway resistance was recorded as a measure of airway hyperreactivity.
Statistical Analysis
Data sets were analyzed using two-way analysis of variance (Graph Pad Prism 3.0; Graph Pad, San Diego, CA) performing an all-way comparison with statistical significance set at P < 0.05 to determine interactions. Individual group comparisons were performed using the Bonferroni test with statistical significance set at P < 0.025 (P < 0.05/n = 2). The sample size of each parameter tested was a minimum of five animals.
Results
Metalloelastase Is Not Present in the Metalloelastase Gene-Deleted Mice
To confirm the absence of metalloelastase in the MMP-12−/− mice, supernatants from lipopolysaccharide-stimulated peritoneal macrophages from MMP-12−/− and MMP-12+/+ animals were analyzed for metalloelastase using Western blotting. Using an antibody to murine MMP-12, a 22-kd band corresponding to the molecular weight of MMP-12 was present in the supernatants of macrophages isolated from MMP-12+/+, whereas no such band was present in the macrophage supernatants from MMP-12−/− animals (data not shown). Thus as expected, there was no evidence of MMP-12 in the knockout animals.
CRA-Induced Lung Alterations in Normal and Metalloelastase-Deficient Mice
Allergic lung airway inflammation was induced in MMP-12+/+ and MMP-12−/− mice by sensitization and subsequent rechallenge with CRA. The intensity of the lung inflammatory response in these two groups was assessed by lung permeability changes, BAL leukocyte counts, and histology. Vascular permeability is a consistent marker of injury in several models of lung injury when used in conjunction with other measurements of lung injury, such as BAL fluid analysis and histology.15,16 After rechallenge with CRA on day 22, animals were sacrificed 24 hours later and the degree of lung permeability evaluated by assessing total protein content in BAL fluid. As shown in Figure 1 the BAL protein levels from uninjured MMP-12+/+ and MMP-12−/− mice were similar with a low background level of protein found in the BAL. When MMP-12+/+ mice were repeatedly challenged with CRA allergen there was a marked increase in protein content as compared to the uninjured animals (167.5 + 46 μg/ml versus 37.15 + 11.79 μg/ml, respectively). BAL fluid from MMP-12−/− mice challenged with CRA also showed an increase in the amount of protein as compared to the saline controls (61.55 + 11.79 μg/ml versus 37.15 + 13.47 μg/ml, respectively). However, the amount of protein leakage into the airway was reduced by 63%, relative to that present in the BAL of CRA-challenged MMP-12+/+ animals (P < 0.018). Thus, the MMP-12−/− mice exhibit lower vascular permeability, consistent with less lung inflammation than the MMP-12+/+ animals with normal levels of this MMP.
Figure 1.
Protein levels in BAL of MMP-12+/+ versus MMP-12−/− mice after CRA-induced inflammation (n = 15). Mean +/− SEM.
Histological Examination of CRA-Challenged Lungs
Histological findings from the CRA-challenged lungs of MMP-12+/+ and MMP-12−/− animals were compared to assess the degree of injury and the intensity of the inflammatory response in the lung. As shown in Figure 2A the lungs from the CRA-challenged MMP-12+/+ mice show evidence of significant inflammation in the lung that is primarily peribronchial and consists of mononuclear inflammatory cells (lymphocytes, macrophages) as well as significant numbers of neutrophils and eosinophils (inset). Some airway and alveolar inflammation were also present. When the intensity of the airway inflammation was assessed in the MMP-12−/− mice there were obvious differences as compared to the MMP-12+/+ animals with a marked reduction in the intensity of the inflammatory response in the MMP-12−/− mice as compared to the MMP-12+/+ controls (Figure 2B). Thus, the degree of lung inflammation in the MMP-12−/− animals is reduced and is associated with a marked reduction in the amount of the peribronchial inflammatory infiltrate. These studies support the BAL permeability studies, which revealed less protein leakage in the MMP-12−/− mice, consistent with less inflammation and injury.
Figure 2.
Lung sections (H&E) of MMP-12+/+ CRA (A) and MMP-12−/− (B) mice after CRA-induced inflammation (n = 5). Eosinophils indicated by arrows. Original magnifications: ×40 (A, B); ×100 (insets).
Quantification of Leukocyte Infiltration into CRA-Challenged Lungs
To further assess the reduction in inflammation seen in the MMP-12−/− mice quantitation of leukocytes was performed in the BAL and lungs of the MMP-12+/+ and MMP-12−/− mice. In the first series of studies we compared the numbers of BAL leukocytes (neutrophils, macrophages, and lymphocytes) between the two groups. As would be expected, neutrophil influx (Figure 3A) in both saline-treated groups (MMP-12+/+ and MMP-12−/−) was approximately the same minimal number of cells (3.0 × 104 + 0.4 cells/ml). There were some macrophages present in the BAL of the controls (1.1 × 104 + 0.3 cells/ml) but this represents the normal alveolar macrophage population in the lung (Figure 3B), as was also the case with minimal numbers of lymphocytes in the control lungs (Figure 3C) (0.14 × 104 + 0.06 cells/ml). In the CRA-challenged mice, there was a marked increase in the number of neutrophils present in the BAL of the MMP-12+/+ mice (4.89 × 105 + 4.6 cells/ml) as well as an increase in the numbers of macrophages (1.97 × 104 + 1.19 cells/ml) and lymphocytes (1.51 × 104 + 0.51 cells/ml). In CRA-challenged MMP-12−/− mice there was a 64% reduction in the number of neutrophils present in the BAL as compared to the MMP-12+/+ animals (1.77 × 105 + 0.75 versus 4.89 × 105 + 4.6 cells/ml, respectively) (P < 0.0197). As shown in Figure 3B there was also a smaller (nonsignificant) drop of 37% in the numbers of macrophages in the BAL of the CRA-challenged MMP-12−/− mice as compared to the MMP-12+/+ controls (1.24 × 104 + 0.77 versus 1.97 × 104 + 1.19 cells/ml, respectively). In addition, there was a significant (P < 0.0001) reduction (72%) in the number of infiltrating lymphocytes in the CRA-challenged MMP-12−/− mice (0.43 × 104 + 0.25 cells/ml) as compared to the MMP-12+/+ controls (1.51 × 104 + 0.51 cells/ml). These differences cannot be attributed to the deficiency in MMP-12 affecting circulating leukocytes because there was no significant difference in the numbers of peripheral leukocytes between wild-type and MMP-12−/− mice under control conditions or after CRA induction of airway inflammation (data not shown). Thus, the BAL leukocyte studies support the BAL permeability and histological observations that the intensity of the inflammatory response is diminished in the MMP-12−/− mice.
Figure 3.
Recovery of neutrophils (A), macrophages (B), and lymphocytes (C) from the lungs of CRA-challenged MMP-12+/+ versus MMP-12−/− mice (n = 12). Mean +/− SEM.
Because many of the peribronchial inflammatory cells are not in the airways or alveoli, studies were performed to quantify the leukocyte influx into the lung parenchyma. As would be expected, neutrophils in both MMP-12+/+ and MMP-12−/− mice, within the parenchyma and on the immediate alveolar wall were significantly increased after CRA challenge (Figure 4A) (12 + 2 versus 98 + 4 and 11.2 + 2 versus 36 + 5 neutrophils/field, respectively), relative to saline-treated controls. However, in the MMP-12−/− mice there was a 63% reduction in the neutrophil influx (P < 0.0001), relative to MMP-12+/+ mice. In terms of macrophages in the lung parenchyma (Figure 4B) morphometric quantitation of BS-1-labeled cells revealed that the lungs of the CRA-challenged MMP-12+/+ mice had an increase in the number of tissue macrophages as compared to the saline-treated control animals (11.02 + 2.05 macrophages/field versus 5.35 + 1.73 macrophages/field, respectively). In the MMP-12−/− mice there was also an increase in the number of tissue macrophages in the CRA-challenged animals as compared to the saline control animals (6.79 + 1.89 macrophages/field versus 4.69 + 2.13 macrophages/field, respectively) but the degree of macrophage influx in these animals was 38% less, as compared to the CRA-challenged MMP-12+/+ control animals (P < 0.021).
Figure 4.
Number of neutrophils (n = 10) periairway and surrounding alveoli (A), macrophages (B), and T cells (C) found periairway in the lungs of MMP-12+/+ control versus MMP-12−/− mice after CRA-induced inflammation (n = 18). Neutrophils were determined by H&E-stained sections. Macrophages and T cells were determined by immunofluorescence microscopy. Mean +/− SEM. Original magnifications, ×40.
Similar studies were performed to quantify the numbers of T lymphocytes in the lung using the CD-3 marker (Figure 4C). As expected, the numbers of lymphocytes increased in the lungs of the CRA-challenged MMP-12+/+ and MMP-12−/− mice as compared to the saline controls. There was a 20% reduction in the numbers of T lymphocytes present in the lungs of the CRA-challenged MMP-12−/− animals as compared to the injured MMP-12+/+ controls (15.36 + 6.14 lymphocytes/field versus 18.73 + 6.23 lymphocytes/field, respectively). However, there was no statistical difference in the numbers of lymphocytes between these two groups. Thus, quantification of tissue mononuclear cells in the lungs of the challenged mice revealed that the MMP-12−/− mice had less macrophages than the MMP-12+/+ animals but there were no statistical differences in the numbers of T lymphocytes.
Studies were also done to quantify the numbers of eosinophils present in the lungs of the CRA-challenged mice because this is a key inflammatory cell in allergic inflammation. The numbers of eosinophils in the lungs of these animals were quantified in two ways as shown in Figure 5. First, the numbers of eosinophils were counted in the lungs using oil immersion ×100 magnification of Congo Red-stained lung sections of CRA-challenged MMP-12+/+ and MMP-12−/− mice. As shown in Figure 5A there is a marked increase in the numbers of eosinophils present in the lungs of the MMP-12+/+ mice after CRA challenge as compared to the saline control animals (4.34 + 0.87 eosinophils/high-power field versus 0.22 + 0.05 eosinophils/high-power field, respectively). In the MMP-12−/− mice challenged with CRA there was an 80% reduction in the number of eosinophils present in the lungs as compared to the MMP-12+/+ CRA-challenged animals (0.85 + 0.32 eosinophils/high-power field versus 4.34 + 0.87 eosinophils/high-power field, respectively) (P < 0.0024). This finding was verified by assessing total lung EPO activity, which is a quantifiable way of assessing eosinophil numbers in tissues. As shown in Figure 5B there is a marked increase in EPO activity in the lungs of the MMP-12+/+ CRA-challenged animals as compared to the saline control values that were used as the baseline for 100% activity (1.0 relative activity). In the CRA-challenged MMP-12−/− animals there was a 60% reduction in the amount of EPO activity in the lung as compared to the CRA-challenged MMP-12+/+ animals (97 + 11% versus 245 + 36%, respectively) (P < 0.0033). In fact, the levels of EPO in the CRA-challenged MMP-12−/− animals were actually lower than in the saline control MMP-12−/− animals. Thus, by morphometric quantification and by EPO analysis the numbers of eosinophils present in the lungs of the MMP-12−/− mice challenged with CRA are markedly reduced as compared to the MMP-12+/+ animals.
Figure 5.
A: Number of eosinophils found periairway in the BAL fluid of MMP-12+/+ control versus MMP-12−/− mice after CRA-induced inflammation (n = 8). Determined by H&E microscopically. B: EPO values in the BAL fluid of MMP-12+/+ and MMP-12−/− mice (n = 8). Mean +/− SEM. Original magnifications, ×100.
Gelatinolytic Activities in the CRA-Challenged Lungs
Because previous studies from our laboratory and others have shown an increase in gelatinase A (MMP-2) and MMP-9 in models of lung injury (including allergic inflammation),15,16 we wanted to determine the levels of these MMPs in this chronic model of allergic airway inflammation. BAL fluid from normal and CRA-challenged animals was analyzed for MMP gelatinolytic activity by zymography. As shown in Figure 6, both MMP-2 and MMP-9 levels were elevated in the BAL fluid of CRA-challenged MMP-12+/+ mice, whereas there was nominal activity in unchallenged MMP-12+/+ and MMP-12−/− mice. MMP-12−/− mice challenged with CRA demonstrated only a nominal increase in MMP-2 and MMP-9 relative to that of MMP-12+/+ mice. Thus, gelatinolytic activities were increased in this model of CRA-induced allergic airway inflammation whereas MMP-12−/− mice showed little upregulation of gelatinolytic activity.
Figure 6.
Zymography of BAL fluid from injured and uninjured mouse lungs for gelatinolytic activity. MMP-2 (A) and MMP-9 (B) activity from pooled BAL of five mice of MMP-12+/+ control and MMP-12−/− mice. Quantitation of the bands was performed by densitometry.
Alterations in Levels of Inflammatory Mediators in the Metalloelastase-Deficient Mice
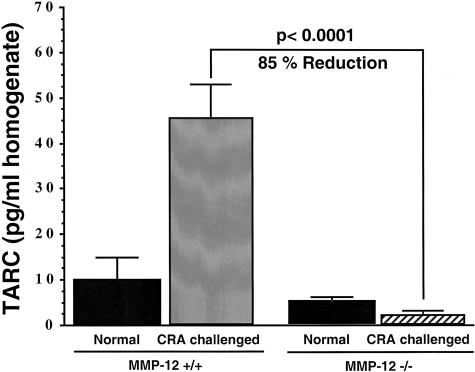
Previous studies have demonstrated a number of cytokines and chemokines, which participate in allergic airway responses.25–29 Levels of MIP-1α and MCP-1 in the BAL fluid of CRA-challenged MMP-12−/− mice (Figure 7A) were significantly decreased (P < 0.0065 and P < 0.001, respectively) relative to the increase seen in MMP-12+/+ CRA-challenged mice. The data in Figure 7B illustrates that in CRA-challenged MMP-12−/− mice there was a significant decrease in pulmonary TNF-α and IL-5 (P < 0.001 and P < 0.0059, respectively) levels in BAL as compared to CRA-challenged MMP-12+/+ control mice. Reduction of these two cytokines was nearly abrogated to background levels in the MMP-12−/− animals when compared to the large increase seen in the MMP-12+/+ animals after CRA challenge. There was no significant change in the amounts of interferon-γ or MIP-2 in the MMP-12−/− relative to the MMP-12+/+ animals (data not shown). Previous studies have demonstrated that both TNF-α and IL-5 are key cytokines responsible for activation and elicitation of the eosinophilic airway responses.27,28 In addition, there was a significant decrease of TARC (P < 0.0001) in the lung homogenate of MMP-12−/− mice relative to MMP-12+/+ CRA-challenged mice (Figure 8). MCP-1 and MIP-1α have been previously shown to play a role in airway hyperactivity and eosinophil attraction, respectively, in this model.25,26 TARC has also been postulated to play a role in eosinophilic recruitment in allergic inflammation.30 Taken together, these studies clearly reveal a reduction in the levels of proinflammatory cytokines and chemokines that are known to be involved in the pathogenesis of allergic inflammation. The reduction in the levels of these inflammatory mediators provides a potential mechanism for the reduced inflammatory response seen in the MMP-12−/− animals.
Figure 7.
Levels of MIP-1α and MCP-1 (A) and TNF-α and IL-5 (B) in the BAL fluid of MMP-12+/+ control versus MMP-12−/− mice after CRA-induced inflammation (n = 5). Determined by ELISA. Mean +/− SEM.
Figure 8.
Level of TARC in the BAL of MMP-12+/+ control versus MMP-12−/− mice after CRA-induced inflammation (n = 10). Determined by ELISA. Mean +/− SEM.
Macrophage Cytokine Generation in Normal and Metalloelastase-Deficient Mice
Because macrophages are known to produce many of the cytokines and chemokines that are upregulated in allergic inflammation, studies were done to determine whether isolated macrophages from the MMP-12−/− produced similar levels of cytokines in vitro as compared to those from MMP-12+/+ animals. Owing to the minimal number of macrophages recovered from alveolar isolation and the large number of cells required for multiple replicates, we have chosen to use peritoneal macrophage in these studies. Previous work in our laboratory has demonstrated their ability to produce very similar cytokine and chemokine expression patterns with similar agonists (data not shown). Supernatants from lipopolysaccharide-stimulated and unstimulated macrophages (MMP-12+/+ and MMP-12−/−) were assayed by ELISA and demonstrated that for both MCP-1 (11.9 + 4.25 ng/ml versus 10.84 + 1.36 ng/ml, respectively) and MIP-1α (8.11 + 1.25 ng/ml versus 7.36 + 0.94 ng/ml, respectively) there was no significant differences. Levels of TNF-α and IL-5 were both below the level of detection for the assays. These studies demonstrate that macrophages from MMP-12−/− animals appear to maintain their normal functional ability to produce inflammatory mediators. Thus, a defect in macrophages does not appear to be the cause of the reduced cytokines observed in vivo with the MMP-12−/− animals.
Measurement of Airway Hyperreactivity
The assessment of airway hyperreactivity allows the measurement of a physiologically relevant parameter that may be representative of the clinical manifestations of allergic inflammation similar to those found in asthma. Although there was a 31% reduction in airway resistance between MMP-12+/+ and MMP-12−/− mice after repeated CRA challenges, we found no statistical differences between these groups, even after running a total of 40 animals (Figure 9). The lack of statistical difference between unchallenged MMP-12+/+ and MMP-12−/− mice and the increase in airway resistance in MMP-12+/+ animals after CRA challenges would indicate that both the model and the phenotypes had no influence on the outcomes. Therefore, in these studies there was no correlation between the alteration in inflammatory infiltrate and the elicitation of methacholine-induced airway hyperreactivity.
Figure 9.
Methacholine-induced airway hyperactivity (cm H2O/ml/second) of MMP-12+/+ control versus MMP-12−/− mice after CRA-induced inflammation (n = 10). Mean +/− SEM.
Discussion
Our findings indicate for the first time that macrophage MMP-12 plays an important role in the development of the inflammatory response in a model of CRA-induced allergic lung injury. Mice deficient in MMP-12, have a marked reduction in the intensity of the airway inflammation in association with a reduction in proinflammatory cytokines and chemokines. In this model, the responses were found to resemble those seen in human asthma with airway eosinophilia and airway hyperreactivity. As a result of rechallenge with the CRA antigen there was an increase in peribronchial leukocyte infiltration, including macrophages, lymphocytes, neutrophils, as well as eosinophils. Curiously, although there was a substantial decrease in overall inflammation in the metalloelastase-deficient mice, there was no significant alteration in the AHR response. This latter issue may relate directly to the fact that although the total number of lymphocytes are reduced and the total numbers of T lymphocytes are not statistically reduced, T cells have been shown to play an important role in the AHR response. In addition, it has been our experience that this strain of mice has less of an AHR response that the B6 or Balb C and therefore the lack of a significant difference may be, in part, because of this. However, no data to date has demonstrated that an alteration in levels of MMP-12 has such an effect.
The finding of a role for MMP-12 in the development of this type of lung inflammation is not unexpected, given the evidence from our previous studies and others showing a role for MMPs generally and MMP-12 specifically in other types of lung injury.15–18 Our previous studies have shown important roles for these proteases in the development of acute lung injury.15,16 Specifically, we have found evidence that MMP-3, MMP-9, and MMP-12 are involved in the pathogenesis in a model of immune complex-mediated acute lung injury in rodents.15,16 In terms of chronic lung injury, studies from our laboratories have also found a critical role for metalloelastase in the development of a murine model of emphysema.18
Another metalloproteinase, MMP-9, has also been described as being upregulated in humans with asthma as well as in experimental models of allergic inflammation. It is postulated to be critical in the evolution of the airway remodeling observed in chronic asthma with degradation or reorganization of collagen and elastin resulting in decreased airway wall stiffness and resulting airway narrowing and collapse.21 In patients with uncontrolled chronic asthma there is increased MMP-9 production as compared to the TIMP-1 levels, with free gelatinase activity in the BAL associated with alterations in the periairway extracellular matrix.19 This study confirms that gelatinases are upregulated in experimental allergic inflammation that is correlated with the intensity of the allergic inflammation. This again is not surprising, given that MMP-9 is upregulated in many types of inflammatory lung injury and does appear to play a role in the damage to the lung extracellular matrix in models of acute lung injury.15 Because the leukocytes are a source of the enzyme31 it would be logical that the gelatinase levels would be markedly diminished in the MMP-12−/− mice with their marked reduction in leukocyte influx. In our previous studies with acute inflammation, gelatinases, unlike MMP-12, do not appear to be involved in the recruitment of leukocytes.15
Studies examining alterations in the extracellular matrix surrounding the airway in allergic inflammation have demonstrated a reduction in the amount of elastin fibers resulting in the diminished elastic recoil of the lung.5,32 Thus, macrophage-derived MMP-12, by its selective degradation of elastin, may be critical in the airway remodeling and subsequent thickening that occurs in chronic allergic inflammation. Although we have only seen a modest reduction in airway hyperactivity in this 22-day model of CRA-induced chronic allergy in the MMP-12−/− mice, it is possible that additional time would account for significant injury and remodeling and thus subsequent loss of pulmonary function. Indeed, we found a twofold increase in fibronectin and laminin fragments in the BAL fluid of MMP-12+/+ mice after CRA exposure and a decrease in these fragments found in MMP-12−/− mice after CRA exposure (data not shown).
There is also evidence from this study and from the other models of lung injury that MMP-12 appears to play a critical role in leukocyte recruitment into the lung.16 This was seen in models of acute lung injury as well in a model of emphysema in which reductions in the numbers of leukocytes were observed in the lungs of the MMP-12−/− animals.16–18 There are several possible mechanisms for this reduction in leukocyte accumulation in the lung that includes alterations in the levels of chemokines as well as other proinflammatory cytokines. In addition, there may also be a requirement for MMP-12 in leukocyte diapodesis.33 The studies to date suggest that alterations in the levels of chemotactic factors may be critical. In the model of emphysema there were reductions in the intensity of the macrophage response in the lung associated with a reduction in the levels of MCP-1.18 Alterations in the levels of fragments of elastin, which are chemotactic for macrophages, were also described. The MMP-12−/− mice had significant reductions in the levels of MIP-1α, MCP-1, TARC, and IL-5, which have all been shown to be important in the recruitment of inflammatory cells into the lung. MIP-1α and IL-5 are thought to play pivotal roles in the recruitment of neutrophils and eosinophils, and MCP-1 in the recruitment of macrophage.25–29 In addition, TARC has been postulated to be involved in eosinophil and lymphocyte recruitment in allergic inflammation but was found not to be involved in a Th-2-dependent model of allergic inflammation similar to this model.34 Taken together, the reductions observed in the amounts of these chemokines in the lungs of the CRA-challenged MMP-12−/− animals could explain the reduction in leukocyte infiltration.
There is also evidence in this study of the down-regulation of other proinflammatory mediators. TNF-α is an early response, proinflammatory cytokine and important in the development of acute lung injury by inducing the activation of adhesive interactions between endothelial cells and leukocytes.35 Previous studies have recognized the role of TNF-α in asthmatic responses clinically as well as in animal models of disease.35,36 Thus the marked reduction of TNF-α levels in the MMP-12−/− mice would also be expected to reduce the level of the inflammatory response.
The exact mechanism(s) leading to a reduction in inflammatory mediators in MMP-12−/− animals is unclear. Our in vitro studies using macrophages from the MMP-12−/− animals demonstrated no difference in the ability of these cells to produce a number of inflammatory mediators, relative to cells from control animals. It is therefore likely that another mechanism, rather than the inability of macrophages to produce chemoattractant factors would appear to cause the alterations in inflammatory cell recruitment. It may well be the difference in the activation state of the macrophages themselves that are responsible for the differences observed. Future studies are needed to explore whether this, or other mechanisms are responsible for the observed reduction of inflammation in the absence of MMP-12.
These studies demonstrate for the first time a role for macrophage MMP-12 in the pathogenesis of allergen-induced lung airway inflammation. Evidence suggests that MMP-12 is capable of modulating the intensity of the lung inflammatory response by alternating levels of chemotactic factors as well as proinflammatory cytokines. This results in altered lymphocyte recruitment into the alveolar spaces through the parenchymal layer and subsequent damage caused by the release of matrix-degrading proteases by the recruited lymphocytes. Such protease release can result in extensive remodeling of the peribronchial lung matrix and may lead to a significant loss of airway function. These studies suggest that selective inhibition of MMPs generally and specifically MMP-12 may be beneficial in the treatment of chronic asthma.
Footnotes
Address reprint requests to Kent J. Johnson, M.D., Department of Pathology, The University of Michigan, 1301 Catherine Rd., Box 0602, Ann Arbor, MI 48109. E-mail: kjjkjj@umich.edu.
Suported by the National Institutes of Health (grants RO1-HL-48889 and PO1-HL-31963).
References
- Arm JP, Lee TH. The pathobiology of bronchial asthma. Adv Immunol. 1992;51:323–382. doi: 10.1016/s0065-2776(08)60491-5. [DOI] [PubMed] [Google Scholar]
- Ulrik CS, Backer V, Dirksen A. A 10 year follow up of 180 adults with bronchial asthma: factors important to the decline in lung function. Thorax. 1992;47:8–14. doi: 10.1136/thx.47.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay AB. Asthma and inflammation. J Allergy Clin Immunol. 1991;87:893–910. doi: 10.1016/0091-6749(91)90408-g. [DOI] [PubMed] [Google Scholar]
- Corrigan CJ, Kay AB. T cells and eosinophils in the pathogenesis of asthma. Immunol Today. 1992;13:501–507. doi: 10.1016/0167-5699(92)90026-4. [DOI] [PubMed] [Google Scholar]
- Bousquet J, Lacoste J, Chanez P, Vic P, Godard PP, Michel F. Bronchial elastic fibers in normal subjects and asthmatic patients. Am J Respir Crit Care Med. 1996;153:1648–1654. doi: 10.1164/ajrccm.153.5.8630616. [DOI] [PubMed] [Google Scholar]
- Mautino G, Capony F, Bousquet J, Vignola AM. Balance in asthma between matrix metalloproteinases and their inhibitors. J Allergy Clin Immunol. 1999;104:530–533. doi: 10.1016/s0091-6749(99)70319-2. [DOI] [PubMed] [Google Scholar]
- Woessner JF., Jr The family of matrix metalloproteinases. Ann NY Acad Sci. 1994;732:11–21. doi: 10.1111/j.1749-6632.1994.tb24720.x. [DOI] [PubMed] [Google Scholar]
- Cawston TE. Metalloproteinase inhibitors and the prevention of connective tissue breakdown. Pharmacol Ther. 1996;3:163–182. doi: 10.1016/0163-7258(96)00015-0. [DOI] [PubMed] [Google Scholar]
- Liu Z, Shipely JM, Vu TH, Zhou X, Diaz LA, Werb Z, Senior RM. Gelatinase B-deficient mice are resistant to experimental bulbous pemphigoid. J Exp Med. 1998;188:475–482. doi: 10.1084/jem.188.3.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang MX, Qin X, Mudgett JS, Ferguson TA, Senior RM, Welgus HG. Matrix metalloproteinases deficiencies affect contact hypersensitivity: stromelysin-1 deficiencies prevents the response and gelatinase B deficiency prolongs the response. Proc Natl Acad Sci USA. 1999;96:6885–6889. doi: 10.1073/pnas.96.12.6885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawston TE. Proteinases and connective tissue breakdown. Henderson B, Edwards JCW, Pettiper ER, editors. London: Academic Press,; Mechanisms and Models in Rheumatoid Arthritis. 1995:pp 333–376. [Google Scholar]
- Reynolds JJ, Hembry RM, Meikle MC. Connective tissue degradation in health and periodontal tissue and the roles of matrix metalloproteinases and their natural inhibitors. Adv Dental Res. 1994;8:312–319. doi: 10.1177/08959374940080022701. [DOI] [PubMed] [Google Scholar]
- Ricov B, Nicod L, Lacraz S, Welgus HG, Suter PM, Dayer J. Matrix metalloproteinases and TIMP in acute respiratory distress syndrome. Am J Respir Crit Care Med. 1996;154:346–352. doi: 10.1164/ajrccm.154.2.8756805. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Stetler-Stevenson WG, Fleming M. Immunohistochemical study of metalloproteinases and their tissue inhibitors in the lungs of patients with diffuse alveolar damage and idiopathic pulmonary fibrosis. Am J Pathol. 1996;149:1241–1256. [PMC free article] [PubMed] [Google Scholar]
- Warner RL, Beltran L, Younkin EM, Lewis CS, Weiss SJ, Varani J, Johnson KJ. Role of stromelysin-1 and gelatinase B in experimental acute lung injury. Am J Cell Mol Biol. 2001;24:537–544. doi: 10.1165/ajrcmb.24.5.4160. [DOI] [PubMed] [Google Scholar]
- Warner RL, Lewis CS, Beltran L, Younkin EM, Varani J, Johnson KJ. The role of metalloelastase in immune complex-induced acute lung injury. J Pathol. 2001;158:2139–2144. doi: 10.1016/S0002-9440(10)64685-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson JJ, Senior RM. Matrix metalloproteinase 9 in lung remodeling. Am J Respir Cell Mol Biol. 2003;28:12–24. doi: 10.1165/rcmb.2002-0166TR. [DOI] [PubMed] [Google Scholar]
- Hautamaki RD, Kobayashi DK, Senior RM, Shapiro SD. Requirement for macrophage elastase from cigarette smoke-induced emphysema in mice. Science. 1997;227:2002–2004. doi: 10.1126/science.277.5334.2002. [DOI] [PubMed] [Google Scholar]
- Mautino G, Oliver N, Chanez P, Bousquet J, Capony F. Increased release of matrix metalloproteinases-9 in bronchoalveolar lavage fluid and by alveolar macrophages of asthmatics. Am J Respir Cell Mol Biol. 1997;17:583–591. doi: 10.1165/ajrcmb.17.5.2562. [DOI] [PubMed] [Google Scholar]
- Dahlen B, Shute J, Howarth P. Immunohistochemical localization of the matrix metalloproteinases MMP-3 and MMP-9 within the airway in asthma. Thorax. 1999;54:590–596. doi: 10.1136/thx.54.7.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai K, Ohno I, Okada S, Ohkawara G, Suzuki K, Shinya T, Nagase K, Iwata K, Shirato K. Inhibition of matrix metalloproteinases prevents allergen-induced airway inflammation in a murine model of asthma. J Immunol. 1999;162:4212–4219. [PubMed] [Google Scholar]
- Ohno I, Ohtani H, Nitta Y, Suzuki J, Hoshi H, Honma M, Isoyama S, Tanno J, Tamura G, Yamauchi K, Nagura H, Shirato K. Eosinophils as a source of metalloproteinase 9 in asthmatic airway inflammation. Am J Respir Cell Mol Biol. 1997;16:212–219. doi: 10.1165/ajrcmb.16.3.9070604. [DOI] [PubMed] [Google Scholar]
- Banda MJ, Werb Z. Mouse macrophage elastase purification and characterization as a metalloproteinases. Biochem J. 1981;193:589–605. doi: 10.1042/bj1930589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freestone T, Turner RJ, Coady A, Higman DJ, Greenhalgh R, Mand J, Powell T. Inflammation and matrix metalloproteinases in the enlarging abdominal aortic aneurysm. Arterioscler Thromb Vasc Biol. 1995;15:1145–1151. doi: 10.1161/01.atv.15.8.1145. [DOI] [PubMed] [Google Scholar]
- Campbell EM, Kunkel SL, Strieter RM, Lukacs NW. Temporal role of cytokines in a murine model of cockroach-antigen induced airway hyperreactivity and eosinophilia. J Immunol. 1998;161:7047–7053. [PubMed] [Google Scholar]
- Campbell EM, Charo IF, Kunkel SL, Strieter RM, Boring L, Gosling J, Lukacs N. Monocyte chemoattractant protein-1 mediates cockroach allergen-induced bronchial hyperreactivity in normal but not CCR2−/− mice: the role of mast cells. J Immunol. 1999;163:2160–2167. [PubMed] [Google Scholar]
- Shah A, Church MK, Holgate ST. Tumor necrosis factor α: a potential mediator of asthma. Clin Exp Allergy. 1995;25:1038–1044. doi: 10.1111/j.1365-2222.1995.tb03249.x. [DOI] [PubMed] [Google Scholar]
- Lee J, McGarry M, Farmer S, Denzler K, Larson K, Carrigan P, Brenneise I, Horton M, Haczku M, Gelfand E, Leikauf G, Lee N. Interleukin-5 expression in the lung epithelium of transgenic mice leads to pulmonary changes pathognomonic of asthma. J Exp Med. 1997;185:2143–2156. doi: 10.1084/jem.185.12.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster PS, Hogan SP, Ramsey AJ, Matthaei KI, Young IG. Interleukin 5 deficiency abolishes eosinophilia, airway reactivity and lung damage in a mouse asthma model. J Exp Med. 1996;183:195–201. doi: 10.1084/jem.183.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoval-Lopez G, Teran LM. TARC: novel mediator of allergic inflammation. Clin Exp Allergy. 2001;31:1809–1812. doi: 10.1046/j.1365-2222.2001.01268.x. [DOI] [PubMed] [Google Scholar]
- Gibbs DF, Warner RL, Weiss SJ, Johnson KJ, Varani J. Characterization of matrix metalloproteinases produced by rat alveolar macrophages. Am J Respir Cell Mol Biol. 1999;20:1136–1144. doi: 10.1165/ajrcmb.20.6.3483. [DOI] [PubMed] [Google Scholar]
- Roche WR, Beasley R, Williams JH, Holgate ST. Subepithelial fibrosis in the bronchi of asthmatics. Lancet. 1989;1:520–524. doi: 10.1016/s0140-6736(89)90067-6. [DOI] [PubMed] [Google Scholar]
- Shipely MJ, Wesselschmidt RL, Kobayashi DK, Ley TJ, Shapiro SD. Metalloelastase is required for macrophage-mediated proteolysis and matrix invasion in mice. Proc Natl Acad Sci USA. 1996;93:3942–3946. doi: 10.1073/pnas.93.9.3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chvatchko Y, Hoogewerf AJ, Meyer A. A key role for CC chemokine receptor 4 in lipopolysaccharide-induced endotoxic shock. J Exp Med. 2000;191:1755–1764. doi: 10.1084/jem.191.10.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan MM, Vaporciyan AA, Miyasaka M, Tamatani T, Ward PA. Tumor necrosis factor alpha regulates in vivo intrapulmonary expression of ICAM-1. Am J Pathol. 1993;142:1739–1749. [PMC free article] [PubMed] [Google Scholar]
- Shah A, Church MK, Holgate ST. Tumor necrosis factor alpha: a potential mediator of asthma. Clin Exp Allergy. 1995;25:1038–1044. doi: 10.1111/j.1365-2222.1995.tb03249.x. [DOI] [PubMed] [Google Scholar]