Abstract
Stromal cell-derived factor-1 is a chemokine that plays a major role during embryogenesis. Since stromal cell-derived factor-1 and its unique receptor CXCR4 are involved in the differentiation of progenitor cells, we studied the expression of this chemokine and of its receptor in hepatic regeneration from precursor oval cells. Hepatic regeneration was induced by treating rats with 2-acetylaminofluorene, and followed by partial hepatectomy. Oval cell accumulation, which predominated in periportal regions, reached a maximum at days 9 to 14 after hepatectomy and declined thereafter. Oval cells strongly expressed stromal cell-derived factor-1 protein and mRNA. CXCR4 mRNA hepatic level paralleled the number of oval cells and in situ hybridization showed CXCR4 mRNA expression by these cells. Treatment of rats with fucoidan, a sulfated polysaccharide which binds to stromal cell-derived factor-1 and blocks its biological effects, markedly decreased oval cell accumulation in five of the seven treated rats. In conclusion, our data demonstrate an expression of stromal cell-derived factor-1 and of its receptor CXCR4 in oval cells during hepatic regeneration and strongly suggest that stromal cell-derived factor-1 stimulates the proliferation of these precursor cells through an autocrine/paracrine pathway.
The liver is an organ with an almost unlimited capacity to regenerate from mature differentiated cells.1 However, in severe liver injury or after partial hepatectomy combined with inhibition of mature hepatocyte proliferation, regeneration is achieved through the expansion and differentiation of liver precursor cells, termed oval cells.2 These cells have been well characterized in rats in response to galactosamine D-induced massive necrosis,3 or after partial hepatectomy (PH) associated with treatment by 2-acetylaminofluorene (AAF).4 They have also been reported in humans in fulminant hepatitis and in chronic liver diseases.5,6 Oval cells are small, with a high nuclear-cytoplasmic ratio. They express hepatocellular and biliary markers, and can differentiate into hepatocytes3 or biliary cells.7 They initially expand in the periportal region where they form ductular structures, and radiate toward the center of the lobule. In the adult liver, they can be viewed as the counterpart of bipotent hepatoblasts which, in the fetus, differentiate into both types of epithelial cells. The origin of oval cells is not fully elucidated. It is generally accepted that oval cells are the progeny of quiescent facultative stem cells which persist in the portal region of adult liver in terminal hepatic ductules (Hering ducts).8 It has been suggested that oval cells also derive from bone marrow stem cells, most likely hematopoietic stem cells, which might migrate into the liver, and differentiate into oval cells to give rise to hepatocytes or biliary cells.9 However, this assumption has been challenged in a recent study showing that mouse oval cells do not originate in bone marrow.10 The mechanisms responsible for the activation, expansion, and differentiation of oval cells are still largely unknown, but soluble factors as well as direct contacts with their matricial and cellular microenvironment might dictate their fate.11–17
Stromal cell-derived factor-1 (SDF-1) is a member of the CXC chemokine family18 that binds to the seven-span transmembrane G-protein-coupled CXCR4 receptor, which has SDF-1 as its unique ligand.19 CXCR4 is expressed by most leukocyte populations, endothelial cells, as well as by epithelial and carcinomatous cells.18,19 Stromal cell-derived factor-1 is expressed in a broad range of tissues and is a potent chemo-attractant for a variety of cells including hematopoietic stem cells, lymphocytes, and monocytes.19 Targeted destruction of its gene as well as of its receptor has shown the role of the SDF-1/CXCR4 couple during embryogenesis and organogenesis.20 It might be involved in the differentiation of progenitor cells to a more mature form, which is illustrated by the supporting role of SDF-1 during B-lymphopoiesis20 and by the observation that, in epithelia renewing from precursor cells, CXCR4 is expressed by the less differentiated cells, eg, lung type II alveolar cells,19 basal crypt cells of the gut.21 Since hepatic precursor cells can give rise to mature epithelial cells in the adult liver, the aim of the present work was to study the expression and the role of the SDF-1/CXCR4 couple in a model of liver regeneration from oval cells.
Materials and Methods
Animals
Male Fischer F-344 rats (Charles River IFFA CREDO, L’arbresle, France) and male Wistar rats (Centre d’Elevage Roger Janvier, Le Genest Saint Isle, France) were purchased at the age of 7 weeks (170 g). Animal manipulations were performed according to the recommendations of the French ethical committee and under the supervision of authorized investigators.
Regeneration Protocols
Liver regeneration from oval cells was induced in Fischer F-344 rats by treating the animals with AAF followed by a PH (AAF/PH model), according to a protocol previously described.14 In brief, rats were fed a diet containing 0.02% AAF (Altromin, Lage, Germany) for 7 days followed by a two-third hepatectomy (day 0) under diethyl ether anesthesia. The administration of AAF was then continued for 5 days. Animals were sacrificed under sodium pentobarbital anesthesia by exsanguination before treatment, the day of surgery (day 0) and at days 1, 5, 9, 14, or 21 after PH. A second group of rats fed a standard pelleted chow underwent PH only (PH model). These animals were sacrificed at the days above indicated in the AAF/PH model. In both groups, the whole liver was divided in two parts. One piece was fixed in buffered formalin, then embedded in paraffin for histopathological, immunohistochemical, and in situ hybridization studies; the other part was quickly frozen in liquid nitrogen and stored at −70°C until use.
Fucoidan Treatment
Wistar rats fed an AAF-containing diet underwent a two-third hepatectomy after 7 days. Immediately after surgery, the animals received an i.p. injection of 25 mg/kg fucoidan (Sigma Chemical Company, St. Louis, MO) dissolved in sterile pyrogen-free phosphate-buffered saline. Injections were repeated at the same dosage 8 hours after surgery and twice daily thereafter. Control animals received only the solvent. The animals were maintained on the AAF diet and sacrificed 4 days after surgery. One piece of liver was fixed in buffered formalin, and then embedded in paraffin for histopathological and immunohistochemical evaluation.
Histopathological and Immunohistochemical Evaluation
Three μm-thick sections of paraffin-embedded liver tissue were stained with hematein-eosin and picrosirius red for collagen with hematein counterstain. Oval cells, when present, predominated in periportal areas. Their size was slightly larger than that of epithelial cells lining interlobular bile ducts and was smaller than that of hepatocytes (Figure 1b). They were isolated or formed ductular structures, tortuous or not, with either clearly visible or poorly defined lumens (Figure 1b). Oval cell accumulation was semi-quantitatively evaluated as minimal when either individual oval cells or ductular structures were strictly limited to periportal areas (ie, involvement of less than one-third of the lobules), marked when isolated oval cells or ductules extended into mediolobular and centrilobular areas (ie, involvement of at least one-half of the lobules), and moderate when the pattern was intermediate between minimal and marked.
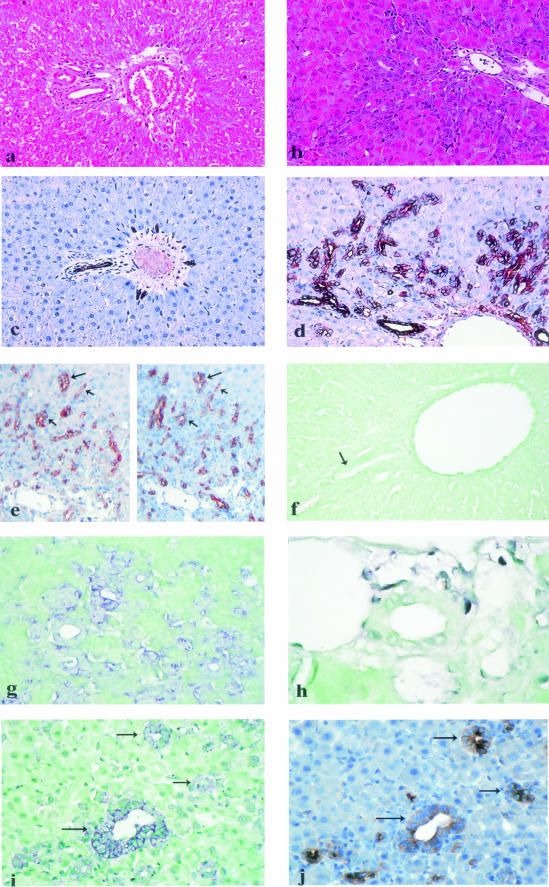
Figure 1.
Histopathological findings and stromal cell-derived factor-1 (SDF-1) protein and mRNA expression in normal liver and in hepatic regeneration from oval cells in rat [2-acetylaminofluorene (AAF)/partial hepatectomy (PH) model]. a: Normal liver (hematein-eosin stain, original magnification, ×200). b: Liver from AAF-treated rats (day 9 after PH); there is marked periportal oval cell accumulation (hematein-eosin stain, original magnification, ×400). c: Normal liver (same portal area as in Figure 1a); SDF-1 protein is immunohistochemically detected in interlobular bile duct epithelial cells and in rare periportal ductules (original magnification, ×200). d: Liver from AAF-treated rats (day 9 after PH); SDF-1 protein is strongly expressed by oval cells forming periportal ductular structures, with increased labeling at the periphery of the cell cytoplasm; SDF-1 protein is also expressed by interlobular bile duct epithelial cells (original magnification, ×400). e: Liver from AAF-treated rats (day 9 after PH); on serial sections, the same ductules (arrows), made of oval cells, express alphafetoprotein (left) as well as SDF-1 protein (right). (f) Portal area in a normal liver (an interlobular bile duct is indicated by an arrow); absence of SDF-1 mRNA detection with an antisense probe (original magnification, ×400). g: Liver from AAF-treated rats (day 9 after PH); SDF-1 mRNA is demonstrated by in situ hybridization with an antisense probe in periportal oval cells (original magnification, ×400). h: Liver from AAF-treated rats (day 9 after PH); absence of SDF-1 mRNA detection in an interlobular bile duct (original magnification, ×1000). i and j: Serial sections of a liver from AAF-treated rats (day 9 after PH); SDF-1 mRNA (i) as well as alphafetoprotein (j) are detected in the same ductules (arrows), made of oval cells.
For immunohistochemistry, antigen retrieval was performed in 0.01 mol/L citrate buffer at pH 6.0 in a 750 W microwave oven (3 × 5 minutes) after dewaxing and rehydration of 4 μm-thick sections. Endogenous peroxidase activity was blocked with 3% hydrogen peroxide. A three-step method using streptavidin-biotin-complexes (ABC) conjugated with peroxidase (Vectastain kit, Vector Laboratories, Burlingame, CA) and 3,3′-diaminobenzidine (DAB +, DAKO SA, Trappes, France) as a chromogen was performed according to the manufacturers’ instructions. Sections were incubated with an anti-SDF-1 mouse monoclonal antibody (K15C IgG 2a)22 (a gift from Dr. F. Arenzana-Seisdedos, Institut Pasteur, Paris, France) for 1 hour at room temperature at a final concentration of 20 μg/ml. This antibody, which recognizes an epitope encompassing the amino-terminal end of the chemokine, has been demonstrated to specifically react with SDF-1α and SDF-1β. Sections were counterstained with Mayer’s hematoxylin and mounted with an aqueous medium. Controls consisted in omission of the anti-SDF-1 antibody or its replacement by an isotype-matched control antibody (IgG 2a anti-human granzyme B, Novocastra Laboratories Ltd, Newcastle upon Tyne, UK). Rabbit polyclonal antibodies against alphafetoprotein (DAKO SA), an oval cell marker,23 and Ki67 (Novocastra Laboratories Ltd) were incubated for 1 hour at room temperature and revealed by the three-step method described above.
In Situ Hybridization
Complementary RNA probes were synthesized using a digoxigenin RNA labeling kit (Roche Diagnostics, Meylan, France) according to the manufacturer’s instructions. The complementary RNA probe specific for CXCR4 was obtained from the mouse I.M.A.G.E. cDNA clone 3385804 (UK HGMP Resource Centre, Cambridge, UK) digested with EcoRI (antisense probe) or NotI (sense probe) and transcribed from the T3 or T7 promoters, respectively. For SDF-1, probes were transcribed from an I.M.A.G.E cDNA clone 3483088 (UK HGMP Resource Centre) fragment obtained by PCR and subcloned into pCR4-TOPO plasmid. PCR was performed using forward 5′-CGCTGTGCTGGCCCTGGTGC-3′ and reverse 5′-CGCCCCCTGCAGGACAAGCC-3′, which map to positions 50–69 and 797–778 of the rat sequence (GI: 19923685). The amplified cDNA was ligated into pCR4-TOPO plasmid using a TOPO TA Cloning kit (Invitrogen, Cergy Pontoise, France) in the conditions recommended by the supplier. Double-strand sequencing of the insert was performed using the BigDyeTM Terminator Cycle Sequencing kit (Applied Biosystems, Foster City, CA) in an automated Applied Biosystems sequencer model 3100 Genetic Analyser (Applied Biosystems). The complementary RNA probes specific for SDF-1 were transcribed from T7 and T3 promoters of the plasmid digested with SpeI (antisense probe) or NotI (sense probe). The size of the RNA probes (750 bp for CXCR4 and 740 bp for SDF-1) was checked on 2% agarose gel. The digoxigenin-labeled RNA probes were applied to a nylon membrane positively charged to determine the labeling efficiency, using a DIG Nucleic Acid Detection kit (Roche) according to the supplier’s instructions.
Four μm-thick sections mounted on superfrost + slides (CML, Nemours, France) and stored at room temperature were treated as previously described.24 They were hybridized overnight at 52°C in a humid chamber with sense and antisense probes in parallel. Hybridization buffer contained 50% desionized formamide, 4X SSC, (0.6 mol/L NaCl, 60 mmol/L Na+ citrate) 1X Denhardt’s solution, 250 μg/ml yeast tRNA, and 5 ng/μl (CXCR4) or 8 ng/μl (SDF-1) digoxigenin-labeled oligonucleotide probes. After hybridization, sections were rinsed in 2X SSC and in 1X SSC for 30 minutes each at 52°C, then twice in 0.1X SSC for 30 minutes at 37°C. Detection of the probes was carried out using a DIG Nucleic Acid Detection kit (Roche). After a blocking step in bovine serum albumin and levamisole treatment, sections were incubated for 60 minutes with anti-digoxigenin Fab fragments conjugated with alkaline phosphatase. Enzymatic activity was revealed after 3 hours in nitroblue tetrazolium 5-bromo-4-chloro-3-indolylphosphate (NBT-BCIP). Sections were counterstained in light green and mounted with Eukitt.
Double-Staining of CXCR4 mRNA and Alphafetoprotein
Double-staining of CXCR4 mRNA and of alphafetoprotein was performed on the same section. After CXCR4 mRNA detection by in situ hybridization as above described, sections were incubated with the anti-alphafetoprotein rabbit polyclonal antibody. The immunohistochemical reaction was performed with the Envision Dual Link System (DAKO SA) conjugated with peroxidase for 30 minutes, 3,3′-diaminobenzidine being used as a chromogene during 10 minutes. CXCR4 mRNA signal was dark blue, whereas alphafetoprotein appeared in brown.
Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
Total RNA was isolated from rat liver.25 The amount and quality of RNA samples were determined by measuring optical density at 260 nm and analyzing 28S rRNA on an agarose gel, respectively.24 Total RNA (5 μg) was reverse-transcribed from an oligo(dT) primer using a first-strand synthesis kit (Stratagene Europe, Amsterdam, The Netherlands) in the conditions recommended by the supplier. Amplification of the first cDNA strand was carried out using a LightCycler Instrument and the LightCycler FastStart DNA Master SYBR Green I kit (Roche Diagnostics), as previously described.26 The following set of primers was used for CXCR4 cDNA amplification: forward 5′-CTGCATCATCATCTCCAAGC-3′ and reverse 5′-GGAAAGGATCTTGAGGCTGG-3′, which map to positions 648–667 and 981–962 of the rat sequence (GI: 1906612), two regions that are identical in rat and mouse. Negative controls without the RNA template or reverse transcriptase were included in each experiment. The melting curve, obtained at the end of each PCR, showed that the PCR products were free of primer dimers. In each PCR experiment, eight dilutions of mouse CXCR4 cDNA clone I.M.A.G.E. were used as external standards. The level of the CXCR4 sequence in cDNA samples was deduced from the standard curve. The amplified product was analyzed by hybridization to a 5′ end-labeled oligonucleotide (5′-CCCTCGCCTTCTTCCACTGTTGCCTGAACC-3′) complementary to CXCR4 mRNA. Hybridization was performed in Rapid-hyb buffer (Amersham Pharmacia Biotech Europe GmbH, Saclay, France). The blot was washed at 42°C in 0.1% sodium dodecyl sulfate, 0.1X SSC and visualized with a PhosphorFluorImager Storm 840 (Molecular Dynamics, Sunnyvale, CA).
Results
In Situ Expression of SDF-1 Protein and mRNA in Regenerating Liver
In the AAF/PH model, rare oval cells were observed in the periportal areas at day 1 after PH. Their number progressively increased with time. Oval cell accumulation was moderate at day 5 and marked at days 9 and 14 after PH, oval cells being individual or forming numerous periportal ductular structures extending into mediolobular areas (Figure 1b). Oval cell accumulation was milder at day 21 after PH. Biliary epithelial cells lining the interlobular bile ducts, individual oval cells as well as oval cells forming ductular structures strongly expressed SDF-1 protein, with an increased labeling at the periphery of the cell cytoplasm (Figure 1d). On serial sections, most SDF-1-labeled cells also expressed alphafetoprotein, confirming that they were indeed oval cells (Figure 1e). Stromal cell-derived factor-1 protein was also weakly detected in the cytoplasm of rare hepatocytes at days 9 and 14 after PH. No staining was detected in control sections incubated with a secondary antibody in the absence of anti-SDF-1 antibody or with an isotype-matched control antibody (data not shown). In situ hybridization with antisense SDF-1 riboprobe was performed in livers obtained at days 5, 9, and 14 after PH. There was marked cytoplasmic SDF-1 mRNA expression in individual intralobular oval cells as well as in oval cells forming periportal ductular structures (Figure 1, g and i). As shown above for the protein, SDF-1 mRNA-positive cells also expressed alphafetoprotein, as detected by in situ hybridization and immunohistochemistry performed on serial sections (Figure 1, i and j). By contrast, SDF-1 mRNA expression was not detected in biliary cells lining interlobular bile ducts (Figure 1h), and a low signal, barely detected in endothelial cells, could not be clearly distinguished from the background. In situ hybridization with sense SDF-1 riboprobe disclosed a very mild labeling of the structures labeled with antisense riboprobe.
In normal rat liver as well as in hepatectomized liver without AAF treatment (PH model) at days 1, 5, 9, 14, and 21 after surgery, SDF-1 was immunohistochemically detected on biliary cells lining interlobular bile ducts and in very rare periportal ductules (Figure 1c). Stromal cell-derived factor-1 was not expressed by sinusoidal cells, portal mesenchymal cells, or endothelial cells lining hepatic arterioles and portal or hepatic venules. Stromal cell-derived factor-1 was weakly detected in rare hepatocytes in livers obtained at days 9, 14, and 21 after PH. Scarce intrasinusoidal mononuclear cells were also positive. Controls after omission of anti-SDF-1 antibody were negative. In situ hybridization with antisense SDF-1 riboprobe did not disclose any labeling in normal rats as well as in the PH model at days 9, 14, and 21 after surgery (Figure 1f).
CXCR4 mRNA Expression in Regenerating Liver
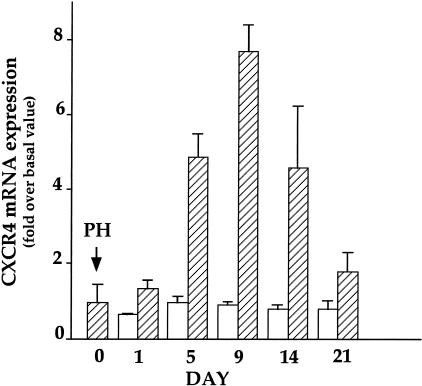
In the AAF/PH model, hepatic CXCR4 mRNA expression, assessed by quantitative RT-PCR, was low at day 0, increased to reach a maximal intensity at day 9 post-PH and decreased thereafter, thus paralleling the number of oval cells (Figure 2). In the PH model, CXCR4 mRNA expression remained low and constant throughout the study period.
Figure 2.
Hepatic CXCR4 mRNA expression in the partial hepatectomy (PH) (open bars) and 2-acetylaminofluorene/PH models (hatched bars). CXCR4 mRNA expression was measured by quantitative RT-PCR. Results are expressed as fold increase over basal value obtained in normal rat livers (57 ± 12 CXCR4 mRNA copies per ng total RNA). Note that liver at D0 in the 2-acetylaminofluorene/PH model is not considered as normal since the animals have been treated for 7 days with 2-acetylaminofluorene. D0 in both regeneration models refers to a specific group of animals that was sacrificed as described in Materials and Methods the day the other rats underwent the hepatectomy. Results are expressed as the mean ± 1 SE of seven (AAF/PH model) and three (PH model) experiments.
In situ hybridization with antisense CXCR4 riboprobes was performed in livers obtained in normal rats and at days 1, 9, and 14 after PH in the AAF/PH and PH models. In the AAF/PH model, no clear positive signal was observed at day 1. At days 9 and 14, there was marked cytoplasmic CXCR4 mRNA expression in individual intralobular oval cells as well as in oval cells forming ductular structures in periportal areas (Figure 3, a and b). Double-staining of CXCR4 mRNA and alphafetoprotein clearly showed that these cells corresponded to oval cells since CXCR4 mRNA and alphafetoprotein colocalized in occasional oval cells forming ductules (Figure 3f). This double-staining also demonstrated that many oval cells, predominantly located around portal areas and identified by their typical morphology and their organization in ductules, only expressed CXCR4 mRNA and not alphafetoprotein, whereas many oval cells forming ductular structures at distance from the portal areas only expressed alphafetoprotein and not CXCR4 mRNA (Figure 3e). CXCR4 mRNA expression could also be detected in the cytoplasm of some intrasinusoidal mononuclear cells. CXCR4 mRNA was not detected in biliary cells lining interlobular bile ducts (Figure 3a), and the low signal observed in endothelial cells was not clearly significant. In situ hybridization with sense CXCR4 riboprobe did not disclose any labeling (Figure 3c). In normal rats and in the PH model, no labeling could be detected in any cell type, either with the antisense or with the sense riboprobes (Figure 3d). However, these results do not exclude the existence in these livers of some CXCR4 mRNA-positive cells, for instance, inflammatory lymphoid cells not detected on the examined sections, which may account for the low but measurable level of CXCR4 mRNA detected by RT-PCR in the PH model (Figure 2).
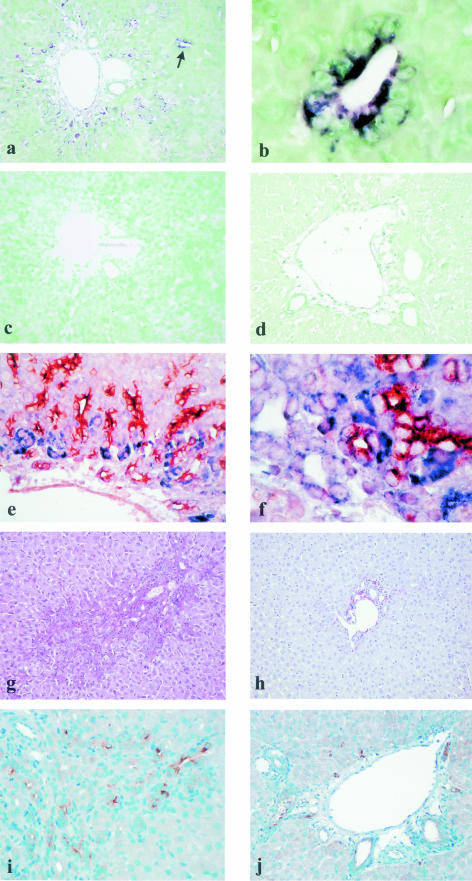
Figure 3.
CXCR4 mRNA detection by in situ hybridization in hepatic regeneration from oval cells in rat [2-acetylaminofluorene (AAF)/partial hepatectomy (PH) model], at day 9 after PH. a: CXCR4 mRNA can be detected with an antisense probe in periportal oval cells, which can form intralobular ductular structures (arrow); interlobular bile duct epithelial cells are not labeled (original magnification, ×200). b: CXCR4 mRNA is detected in intralobular oval cells forming a ductular structure indicated by the arrow in Figure 3a (original magnification, ×1000). c: There is no CXCR4 mRNA detection with a sense riboprobe in the same portal area as in Figure 3a (original magnification, ×200). d: Absence of CXCR4 mRNA expression with an antisense probe at day 9 after PH without AAF treatment (original magnification, ×200). e and f: Double-staining of CXCR4 mRNA and alphafetoprotein. e: CXCR4 mRNA expression (blue signal) without alphafetoprotein expression (brown signal) is mainly observed in periportal oval cells, whereas oval cells forming ductular structures at distance from the portal areas only express alphafetoprotein (original magnification, ×400). f: Colocalization of CXCR4 mRNA and alphafetoprotein in the same periportal oval cells forming ductular structures (original magnification, ×1000). g–j: Effect of fucoidan treatment on oval cell accumulation in hepatic regeneration from oval cells in rat [2-acetylaminofluorene (AAF)/partial hepatectomy (PH) model]. g: In untreated animals, periportal oval cell accumulation is obvious (picrosirius red and hematein, original magnification, ×200). h: After 4 days of fucoidan treatment, there is a low number of periportal oval cells (picrosirius red and hematein, original magnification, ×200). i: Immunodetection of alphafetoprotein in untreated animals (original magnification, ×200). j: In fucoidan-treated rats, the number of alphafetoprotein-positive oval cells is markedly reduced (original magnification, ×200).
Effect of Fucoidan on Oval Cell Proliferation
To determine whether the SDF-1/CXCR4 couple plays a role in oval cell accumulation, rats were injected for 4 days with fucoidan, a sulfated polysaccharide known to bind to SDF-1 and to inhibit its biological effects.27 These experiments were performed in Wistar rats because preliminary trials showed that this strain tolerated this compound better than Fischer rats. Livers were examined after 4 days of a treatment which was well tolerated, as judged by the absence of difference in the weight of treated and control animals at the end of the experiment. Seven rats fed an AAF-containing diet received fucoidan after PH. The presence and number of oval cells were compared to those observed in six AAF/PH rats which did not receive fucoidan. As estimated after picrosirius red and hematein-eosin stainings, a similar degree of oval cell accumulation was observed in all untreated animals (Figure 3g). In five of the seven rats treated with fucoidan, oval cell number decreased by about one-third in one rat, one-half in two rats, two-thirds in one rat, and could not be seen in one animal (Figure 3h). In the two remaining rats, oval cell number was the same as in untreated animals. Similar results were observed when oval cells were identified by immunodetection of alphafetoprotein (Figure 3, i and j). In both animal groups, only rare hepatocytes expressed Ki67, a cell proliferation marker, indicating that fucoidan-treated animals had indeed taken AAF.
Discussion
In this study, we examined the hepatic expression of SDF-1 and of its receptor CXCR4 during liver regeneration from precursor cells. This study was conducted in a well-characterized model consisting in an AAF treatment associated with PH. In this model and in agreement with previous reports,14 periportal oval cell accumulation was moderate at day 5 after PH, reached a peak at days 9 to 14, and then decreased. We demonstrate that oval cells synthesize SDF-1, as shown by in situ hybridization and by immunohistochemistry. Stromal cell-derived factor-1 immunolabeling has already been demonstrated in cholangiolar cells in a variety of human chronic liver diseases28 and in biliary ductal plate epithelial cells during development.22 However, in the latter study, it was not clear whether SDF-1 was synthesized by labeled cells or if it was of exogenous origin and bound to cell surface receptors or to plasma membrane-associated heparan sulfates.29 Our in situ hybridization findings in oval cells suggest that ductal plate cells might indeed synthesize SDF-1. Contrasting with our results, it was found in a regeneration model similar to the present one that SDF-1 protein was mainly expressed by hepatocytes located at proximity of oval cells.30 The reason for such a discrepancy is not clear, but our observation of a faint staining in rare hepatocytes suggests either that these cells could express the chemokine in certain conditions, or that they might be transitional hepatocytic cells recently derived from oval cells. The discrepancy might also be explained by the use of different antibodies. In the present study, SDF-1 protein but not its mRNA was detected in epithelial cells lining interlobular bile ducts. Although it cannot be excluded that SDF-1 mRNA might have a very short half life, this result strongly suggests that SDF-1 is not synthesized by these cells but only bound to their surface, possibly through plasma membrane-associated heparan sulfates.29
We also demonstrate that CXCR4 is expressed in the liver and that this expression is localized in oval cells. Indeed, in the AAF/PH model, CXCR4 mRNA hepatic expression varied in parallel with the number of oval cells, whereas it did not change in absence of oval cell accumulation (PH model). In situ hybridization showed that CXCR4 mRNA was expressed by cells which were formally identified as oval cells on the basis of the following arguments: their morphological characteristics, clearly distinct from those of mononuclear inflammatory cells, their organization in ductular structures, and the expression of an oval cell marker, ie, alphafetoprotein, in some of them.23 These results are in accordance with the findings of Hatch et al,30 who recently detected CXCR4 protein in oval cells. Thus, CXCR4, which is expressed by most leukocyte populations, can be added to various other hematopoietic cell markers already described in oval cells, such as c-kit, CD 34, and Thy-1.11,31,32 However, we noted that CXCR4 and alphafetoprotein were mutually exclusive in many cells; CXCR4-positive cells predominated around portal areas whereas cells expressing only alphafetoprotein were mainly found at distance from the portal areas, essentially in intralobular ductular structures, confirming the heterogeneity of the oval cell population.33 We did not detect CXCR4 mRNA in interlobular bile duct epithelial cells, suggesting that these cells do not synthesize this receptor. As SDF-1 is visualized in these structures by immunohistochemistry, this constitutes an additional argument favoring the binding of SDF-1 to plasma membrane-associated heparan sulfates29 rather than to its receptor. Altogether, our results demonstrate for the first time that oval cells express SDF-1 as well as its receptor CXCR4 during hepatic regeneration from precursor cells, and suggest that SDF-1 exerts its biological effect on oval cells through an autocrine/paracrine pathway.
Stromal cell-derived factor-1 is a chemokine that plays a critical role in lymphatic circulation and in bone marrow homing of hematopoietic stem cells.19 Besides these important functions, SDF-1 has also growth-promoting effects,34 participates to angiogenesis,35 and is involved in the differentiation of progenitor cells.19,36 Stromal cell-derived factor-1 binding to heparan sulfates at the outer surface of cell membranes increases its local concentration and facilitates receptor occupancy.37 To establish a role of SDF-1 in hepatic regeneration from oval cells, rats were injected with fucoidan. This sulfated polysaccharide binds to SDF-1, releases the chemokine from cell membrane-associated heparan sulfate proteoglycans,27 and could therefore inhibit its biological activity. Indeed, in our AAF/PH model, fucoidan markedly decreased oval cell accumulation in a majority of treated rats, suggesting that SDF-1 promotes the activation of Hering duct-located quiescent stem cells into oval cells and/or stimulates oval cell growth. However, SDF-1’s role in the differentiation of oval cells cannot be inferred from our results. The decreased number of oval cells seen in fucoidan-treated animals cannot be unambiguously attributed to SDF-1 inactivation since the polysaccharide is also known to bind growth factors such as fibroblast growth factor38 and transforming growth factor-β.39 An effect mediated by fibroblast growth factor is nonetheless unlikely since fucoidan has been shown to potentiate in vivo its proliferative effects.40 By contrast, we cannot exclude that fucoidan may have exerted its effects by increasing the antiproliferative activity of transforming growth factor-β.39 Use of specific pharmacological inhibitors of CXCR4 or of SDF-1, when available, will be mandatory to clarify the exact role played by the SDF-1/CXCR4 couple during liver regeneration from oval cells.
In conclusion, this study strongly suggests that the SDF-1/CXCR4 couple promotes the activation of quiescent hepatic stem cells into oval cells and/or stimulates oval cell proliferation through an autocrine/paracrine pathway.
Acknowledgments
We thank F. Arenzana-Seisdedos (Unité d’Immunologie Virale, Institut Pasteur, Paris) for supplying the anti-SDF-1 K15C monoclonal antibody. We also thank B. Coste (IFR 10, Hôpital Henri Mondor, Créteil, France) for performing SDF-1 sequencing.
Footnotes
Address reprint requests to Philippe Mavier, INSERM U581, Hôpital Henri Mondor, 94010 Créteil, France. E-mail: mavier@im3.inserm.fr.
References
- Fausto N. Hepatocytes break the rules of senescence in serial transplantation studies: is there a limit to their replicative capacity? Am J Pathol. 1997;151:1187–1189. [PMC free article] [PubMed] [Google Scholar]
- Sell S. Heterogeneity and plasticity of hepatocyte lineage cells. Hepatology. 2001;33:738–750. doi: 10.1053/jhep.2001.21900. [DOI] [PubMed] [Google Scholar]
- Dabeva MD, Shafritz DA. Activation, proliferation, and differentiation of progenitor cells into hepatocytes in the D-galactosamine model of liver regeneration. Am J Pathol. 1993;143:1606–1620. [PMC free article] [PubMed] [Google Scholar]
- Paku S, Schnur J, Nagy P, Thorgeirsson SS. Origin and structural evolution of the early proliferating oval cells in rat liver. Am J Pathol. 2001;158:1313–1323. doi: 10.1016/S0002-9440(10)64082-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowes KN, Brennan BA, Yeoh GC, Olynyk JK. Oval cell numbers in human chronic liver diseases are directly related to disease severity. Am J Pathol. 1999;154:537–541. doi: 10.1016/S0002-9440(10)65299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque S, Haruna Y, Saito K, Nalesnik MA, Atillasoy E, Thung SN, Gerber MA. Identification of bipotential progenitor cells in human liver regeneration. Lab Invest. 1996;75:699–705. [PubMed] [Google Scholar]
- Germain L, Noel M, Gourdeau H, Marceau N. Promotion of growth and differentiation of rat ductular oval cells in primary culture. Cancer Res. 1988;48:368–378. [PubMed] [Google Scholar]
- Theise ND, Saxena R, Portmann BC, Thung SN, Yee H, Chiriboga L, Kumar A, Crawford JM. The canals of Hering and hepatic stem cells in humans. Hepatology. 1999;30:1425–1433. doi: 10.1002/hep.510300614. [DOI] [PubMed] [Google Scholar]
- Petersen BE, Bowen WC, Patrene KD, Mars WM, Sullivan AK, Murase N, Boggs SS, Greenberger JS, Goff JP. Bone marrow as a potential source of hepatic oval cells. Science. 1999;284:1168–1170. doi: 10.1126/science.284.5417.1168. [DOI] [PubMed] [Google Scholar]
- Wang X, Foster M, Al-Dhalimy M, Lagasse E, Finegold M, Grompe M. The origin and liver repopulating capacity of murine oval cells. Proc Natl Acad Sci USA. 2003;100(Suppl 1):11881–11888. doi: 10.1073/pnas.1734199100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsusaka S, Tsujimura T, Toyosaka A, Nakasho K, Sugihara A, Okamoto E, Uematsu K, Terada N. Role of c-kit receptor tyrosine kinase in development of oval cells in the rat 2-acetylaminofluorene/partial hepatectomy model. Hepatology. 1999;29:670–676. doi: 10.1002/hep.510290304. [DOI] [PubMed] [Google Scholar]
- Park DY, Suh KS. Transforming growth factor-beta1 protein, proliferation and apoptosis of oval cells in acetylaminofluorene-induced rat liver regeneration. J Korean Med Sci. 1999;14:531–538. doi: 10.3346/jkms.1999.14.5.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy P, Bisgaard HC, Santoni-Rugiu E, Thorgeirsson SS. In vivo infusion of growth factors enhances the mitogenic response of rat hepatic ductal (oval) cells after administration of 2-acetylaminofluorene. Hepatology. 1996;23:71–79. doi: 10.1002/hep.510230111. [DOI] [PubMed] [Google Scholar]
- Hu Z, Evarts RP, Fujio K, Marsden ER, Thorgeirsson SS. Expression of hepatocyte growth factor and c-met genes during hepatic differentiation and liver development in the rat. Am J Pathol. 1993;142:1823–1830. [PMC free article] [PubMed] [Google Scholar]
- Fujio K, Evarts RP, Hu Z, Marsden ER, Thorgeirsson SS. Expression of stem cell factor and its receptor, c-kit, during liver regeneration from putative stem cells in adult rat. Lab Invest. 1994;70:511–516. [PubMed] [Google Scholar]
- Bustos M, Sangro B, Alzuguren P, Gil AG, Ruiz J, Beraza N, Qian C, Garcia-Pardo A, Prieto J. Liver damage using suicide genes: a model for oval cell activation. Am J Pathol. 2000;157:549–559. doi: 10.1016/S0002-9440(10)64565-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin L, Lynch D, Sell S. Participation of different cell types in the restitutive response of the rat liver to periportal injury induced by allyl alcohol. J Hepatol. 1999;31:497–507. doi: 10.1016/s0168-8278(99)80043-9. [DOI] [PubMed] [Google Scholar]
- Luster AD. Chemokines: chemotactic cytokines that mediate inflammation. N Engl J Med. 1998;338:436–445. doi: 10.1056/NEJM199802123380706. [DOI] [PubMed] [Google Scholar]
- Murdoch C. CXCR4: chemokine receptor extraordinaire. Immunol Rev. 2000;177:175–184. doi: 10.1034/j.1600-065x.2000.17715.x. [DOI] [PubMed] [Google Scholar]
- Ma Q, Jones D, Borghesani PR, Segal RA, Nagasawa T, Kishimoto T, Bronson RT, Springer TA. Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4- and SDF-1-deficient mice. Proc Natl Acad Sci USA. 1998;95:9448–9453. doi: 10.1073/pnas.95.16.9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan NJ, Kolios G, Abbot SE, Sinai MA, Thompson DA, Petraki K, Westwick J. Expression of functional CXCR4 chemokine receptors on human colonic epithelial cells. J Clin Invest. 1999;104:1061–1069. doi: 10.1172/JCI6685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulomb-L’Herminé A, Amara A, Schiff C, Durand-Gasselin I, Foussat A, Delaunay T, Chaouat G, Capron F, Ledee N, Galanaud P, Arenzana-Seisdedos F, Emilie D. Stromal cell-derived factor 1 (SDF-1) and antenatal human B cell lymphopoiesis: expression of SDF-1 by mesothelial cells and biliary ductal plate epithelial cells. Proc Natl Acad Sci USA. 1999;96:8585–8590. doi: 10.1073/pnas.96.15.8585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petropoulos CJ, Yaswen P, Panzica M, Fausto N. Cell lineages in liver carcinogenesis: possible clues from studies of the distribution of alpha-fetoprotein RNA sequences in cell populations isolated from normal, regenerating, and preneoplastic rat livers. Cancer Res. 1985;45:5762–5768. [PubMed] [Google Scholar]
- Holic N, Suzuki T, Corlu A, Couchie D, Chobert MN, Guguen-Guillouzo C, Laperche Y. Differential expression of the rat gamma-glutamyl transpeptidase gene promoters along with differentiation of hepatoblasts into biliary or hepatocytic lineage. Am J Pathol. 2000;157:537–548. doi: 10.1016/s0002-9440(10)64564-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Couchie D, Holic N, Chobert MN, Corlu A, Laperche Y. In vitro differentiation of WB-F344 rat liver epithelial cells into the biliary lineage. Differentiation. 2002;69:209–215. doi: 10.1046/j.1432-0436.2002.690414.x. [DOI] [PubMed] [Google Scholar]
- Sweeney EA, Lortat-Jacob H, Priestley GV, Nakamoto B, Papayannopoulou T. Sulfated polysaccharides increase plasma levels of SDF-1 in monkeys and mice: involvement in mobilization of stem/progenitor cells. Blood. 2002;99:44–51. doi: 10.1182/blood.v99.1.44. [DOI] [PubMed] [Google Scholar]
- Terada R, Yamamoto K, Hakoda T, Shimada N, Okano N, Baba N, Ninomiya Y, Gershwin ME, Shiratori Y. Stromal cell-derived factor-1 from biliary epithelial cells recruits CXCR4-positive cells: implications for inflammatory liver diseases. Lab Invest. 2003;83:665–672. doi: 10.1097/01.lab.0000067498.89585.06. [DOI] [PubMed] [Google Scholar]
- Amara A, Lorthioir O, Valenzuela A, Magerus A, Thelen M, Montes M, Virelizier JL, Delepierre M, Baleux F, Lortat-Jacob H, Arenzana-Seisdedos F. Stromal cell-derived factor-1alpha associates with heparan sulfates through the first beta-strand of the chemokine. J Biol Chem. 1999;274:23916–23925. doi: 10.1074/jbc.274.34.23916. [DOI] [PubMed] [Google Scholar]
- Hatch HM, Zheng D, Jorgensen ML, Petersen BE. SDF-1alpha/CXCR4: a mechanism for hepatic oval cell activation and bone marrow stem cell recruitment to the injured liver of rats. Cloning Stem Cells. 2002;4:339–351. doi: 10.1089/153623002321025014. [DOI] [PubMed] [Google Scholar]
- Omori N, Omori M, Evarts RP, Teramoto T, Miller MJ, Hoang TN, Thorgeirsson SS. Partial cloning of rat CD34 cDNA and expression during stem cell-dependent liver regeneration in the adult rat. Hepatology. 1997;26:720–727. doi: 10.1002/hep.510260325. [DOI] [PubMed] [Google Scholar]
- Petersen BE, Goff JP, Greenberger JS, Michalopoulos GK. Hepatic oval cells express the hematopoietic stem cell marker Thy-1 in the rat. Hepatology. 1998;27:433–445. doi: 10.1002/hep.510270218. [DOI] [PubMed] [Google Scholar]
- Yang L, Faris RA, Hixson DC. Phenotypic heterogeneity within clonogenic ductal cell populations isolated from normal adult rat liver. Proc Soc Exp Biol Med. 1993;204:280–288. doi: 10.3181/00379727-204-43664. [DOI] [PubMed] [Google Scholar]
- Lataillade JJ, Clay D, Dupuy C, Rigal S, Jasmin C, Bourin P, Le Bousse-Kerdiles MC. Chemokine SDF-1 enhances circulating CD34(+) cell proliferation in synergy with cytokines: possible role in progenitor survival. Blood. 2000;95:756–768. [PubMed] [Google Scholar]
- Salcedo R, Wasserman K, Young HA, Grimm MC, Howard OM, Anver MR, Kleinman HK, Murphy WJ, Oppenheim JJ. Vascular endothelial growth factor and basic fibroblast growth factor induce expression of CXCR4 on human endothelial cells: in vivo neovascularization induced by stromal-derived factor-1alpha. Am J Pathol. 1999;154:1125–1135. doi: 10.1016/s0002-9440(10)65365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayali AG, Van Gunst K, Campbell IL, Stotland A, Kritzik M, Liu G, Flodstrom-Tullberg M, Zhang YQ, Sarvetnick N. The stromal cell-derived factor-1alpha/CXCR4 ligand-receptor axis is critical for progenitor survival and migration in the pancreas. J Cell Biol. 2003;163:859–869. doi: 10.1083/jcb.200304153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela-Fernandez A, Palanche T, Amara A, Magerus A, Altmeyer R, Delaunay T, Virelizier JL, Baleux F, Galzi JL, Arenzana-Seisdedos F. Optimal inhibition of X4 HIV isolates by the CXC chemokine stromal cell-derived factor 1 alpha requires interaction with cell surface heparan sulfate proteoglycans. J Biol Chem. 2001;276:26550–26558. doi: 10.1074/jbc.M100411200. [DOI] [PubMed] [Google Scholar]
- Belford DA, Hendry IA, Parish CR. Investigation of the ability of several naturally occurring and synthetic polyanions to bind to and potentiate the biological activity of acidic fibroblast growth factor. J Cell Physiol. 1993;157:184–189. doi: 10.1002/jcp.1041570124. [DOI] [PubMed] [Google Scholar]
- McCaffrey TA, Falcone DJ, Borth W, Brayton CF, Weksler BB. Fucoidan is a non-anticoagulant inhibitor of intimal hyperplasia. Biochem Biophys Res Commun. 1992;184:773–781. doi: 10.1016/0006-291x(92)90657-7. [DOI] [PubMed] [Google Scholar]
- Luyt CE, Meddahi-Pelle A, Ho-Tin-Noe B, Colliec-Jouault S, Guezennec J, Louedec L, Prats H, Jacob MP, Osborne-Pellegrin M, Letourneur D, Michel JB. Low-molecular-weight fucoidan promotes therapeutic revascularization in a rat model of critical hindlimb ischemia. J Pharmacol Exp Ther. 2003;305:24–30. doi: 10.1124/jpet.102.046144. [DOI] [PubMed] [Google Scholar]