Abstract
Injury of the tubular epithelium and TGF-β1-induced conversion of epithelial cells to α-smooth muscle actin (SMA)-expressing myofibroblasts are key features of kidney fibrosis. Since injury damages intercellular junctions and promotes fibrosis, we hypothesized that cell contacts are critical regulators of TGF-β1-triggered epithelial-to-mesenchymal transition (EMT). Here we show that TGF-β1 was unable to induce EMT in intact confluent monolayers, but three different models of injury-induced loss of epithelial integrity (subconfluence, wounding, and contact disassembly by Ca2+-removal) restored its EMT-inducing effect. This manifested in loss of E-cadherin, increased fibronectin production and SMA expression. TGF-β1 or contact disassembly alone only modestly stimulated the SMA promoter in confluent layers, but together exhibited strong synergy. Since β-catenin is a component of intact adherens junctions, but when liberated from destabilized contacts may act as a transcriptional co-activator, we investigated its role in TGF-β1-provoked EMT. Contact disassembly alone induced degradation of E-cadherin and β-catenin, but TGF-β1 selectively rescued β-catenin and stimulated the β-catenin-driven reporter TopFLASH. Moreover, chelation of free β-catenin with the N-cadherin cytoplasmic tail suppressed the TGF-β1 plus contact disassembly-induced SMA promoter activation and protein expression. These results suggest a β-catenin-dependent two-hit mechanism in which both an initial epithelial injury and TGF-β1 are required for EMT.
Epithelial-to-mesenchymal transition (EMT) plays a central role in development and carcinogenesis.1 Recently, the revolutionary concept has emerged that EMT is one of the key mechanisms underlying organ fibrosis including kidney.2–4 Indeed, tubulointerstitial fibrosis (TIF) is the common pathological pathway through which chronic kidney diseases progress to end-stage renal failure. TIF is characterized by the gradual loss of the tubular epithelium, concomitant with the progressive accumulation of fibroblasts and α-smooth muscle actin (SMA)-positive myofibroblasts, leading to an excessive production and deposition of extracellular matrix components.5 The key importance of myofibroblasts in TIF is further supported by the strong positive correlation between SMA expression and the loss of kidney functions.6
Both clinical studies and animal models of TIF indicate that fibroblasts and myofibroblasts can be derived from the tubular epithelium undergoing EMT7–9 in response to inflammatory cytokines, predominantly transforming growth factor-β1 (TGF-β1).10,11 The most compelling evidence for EMT in fibrosis has been provided by Iwano et al,12 who found that in mice with genetically tagged proximal tubular cells, 36% of renal fibroblasts originated from the epithelium in a TIF model.
To explore the underlying mechanisms, several groups including our own have developed cellular models of TGF-β1-induced epithelial-fibroblast/myofibroblast transition.13–15 These studies showed that the key features of EMT include the early loss of cell-cell contacts due to the down-regulation of ZO-1 and E-cadherin, reorganization of the cytoskeleton, acquisition of spindle-like morphology, and finally the expression of SMA, the hallmark of the myofibroblast phenotype. This process takes several days and requires the interplay of a multitude of TGF-β1-induced pathways, including SMAD proteins,16 integrin-linked kinase,17 and Rho-family GTPases.15,18
Extensive research in tumor and developmental biology has solidified the concept that intracellular contacts are not only passive targets, but also are active regulators of EMT. Accordingly, the loss of E-cadherin promotes EMT, while forced E-cadherin expression can restore the epithelial phenotype in transformed tumor cells.19–22 Furthermore, recent studies by Zeisberg and Kalluri23 provide strong support for the importance of the loss of E-cadherin in EMT in tubular cells. These authors showed that inhibition of the TGF-β1-induced down-regulation of E-cadherin reverses EMT. Accordingly, epithelial injury and subsequent repair, which involve damage or loss of intercellular contacts, are known to facilitate tissue fibrosis.24 Regarding the underlying mechanism, β-catenin, the intracellular binding partner of E-cadherin appears to be a good candidate to participate in contact-dependent regulation of EMT, because of its dual function. Namely, in cells with intact intercellular contacts, β-catenin is an integral component of the adherens junctions, however when freed from the contacts, it can act as a transcriptional co-activator by binding to members of the T cell factor/lymphoid enhancer factor (TCF/LEF) family of transcription factors.25 Indeed, β-catenin signaling has been implicated in EMT progression in tumor cells,26–28 although its role in promoting organ fibrosis remains to be defined. Consistent with its potential involvement, we and others have shown that TGF-β1 induces increased nuclear accumulation of β-catenin in tubular cells,15,29 and β-catenin has been reported to interact with SMAD proteins.30,31 Further, epithelial cells from patients with pulmonary fibrosis showed strong nuclear staining for β-catenin.32 Finally β-catenin targets certain genes, which have been implicated in EMT.33–35 Nonetheless, it remains controversial whether TGF-β1 induces β-catenin-dependent transcription in nonmalignant epithelial cells,29,30,36 and if so, whether such a process can impact on epithelial-myofibroblast transition. Accordingly, we hypothesized that the integrity of cell-cell contacts in general, and β-catenin signaling in particular might be important regulators of TGF-β1-induced myofibroblast generation.
Our present studies provide evidence that cell contacts are critical determinants of EMT susceptibility to TGF-β1. We show that a partial absence or disassembly of cell-cell junctions is a prerequisite for the TGF-β1-provoked EMT. Investigating the underlying mechanism, we found β-catenin plays an important role in the TGF-β1 and cell contact-dependent, synergistic regulation of the SMA promoter and protein expression. These findings suggest a two-hit model in which both an initial tissue injury and TGF-β1 are required for EMT.
Materials and Methods
Materials and Antibodies
The following primary antibodies were used: anti-β-catenin, anti-Rho (Santa Cruz Biotechnology, Santa Cruz, CA), anti-E-cadherin (Transduction Laboratories, Mississauga, ON), anti-SMA (1A4), anti-fibronectin, anti-β-actin, and anti-tubulin (Sigma, St. Louis, MO). Rhodamine-phalloidin was from Cytoskeleton (Denver, CO). Horseradish peroxidase-conjugated anti-mouse and anti-rabbit IgG antibodies were purchased from Amersham Biosciences (Baic d’Urfé, QC), and anti-goat antibody from Santa Cruz. Fluorescein isothiocyanate and Cy3-labeled anti-goat, anti-rabbit, and anti-mouse secondary antibodies were obtained from Jackson Immunoresearch Laboratories (West Grove, PA).
Cells
For most of our studies we used LLC-PK1 CL4 cells stably expressing the AT1 receptor, as we characterized EMT in detail in this proximal tubular cell line in our previous studies.15 However, the process of EMT was qualitatively and quantitatively similar in wild-type LLC-PK1 cells as well. Cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM), containing 10% fetal bovine serum (FBS), 1% penicillin/streptomycin (Gibco, Burlington, ON) at 37°C under 5% CO2 in a humidified incubator. Cells were grown to 30% or 100% confluence, and then treated with vehicle only (4 mmol/L HCl and 0.1% albumin) or 4 ng/ml human recombinant TGF-β1 (Sigma) for the indicated times. In certain experiments, the medium of confluent layers grown on plastic or permeable support (Corning, Acton, MA) was replaced with nominally Ca2+-free DMEM (Gibco) without FBS.
Western Blotting
After treatments, cells were scraped into Triton lysis buffer (30 mmol/L N-2-hydroxyethylpiperazine-N-2-ethanesulfonic acid (HEPES) (pH 7.4), 100 mmol/L NaCl, 1 mmol/L ethylene glycol-bis (2-aminoethylether)-N, N N′ N′-tetraacetic acid (EGTA), 20 mmol/L NaF, 1% Triton X-100, 1 mmol/L phenylmethylsulfonyl fluoride (PMSF), 20 μl/ml Protease Inhibitory Cocktail (Pharmingen BD Biosciences, San Diego, CA), and 1 mmol/L Na3VO4). A portion of the samples was saved as total lysates, then the cytosolic (Triton soluble) and cytoskeletal (Triton insoluble) fractions were separated by centrifugation. Samples were dissolved in Laemmli buffer and boiled for 5 minutes. Equal amount of protein were separated on SDS-polyacrylamide gels and transferred to nitrocellulose membranes (Bio-Rad, Hercules, CA). Immunoblotting was performed as described previously.15 Immunoreactive bands were visualized by enhanced chemiluminescence reaction kit (Amersham).
Preparation of GST-RBD Beads and Measurement of Rho Activity
Rho activity was determined as described previously15,37 using a pull-down affinity assay based on the ability of the Rho-binding domain (RBD) of Rhotekin to capture the GTP-loaded form of Rho. Recombinant glutathione-S-transferase (GST)-RBD fusion protein was prepared from E. coli, and adsorbed onto glutathione-covered beads. Confluent monolayers on 10-cm Petri dishes were serum-starved for 3 hours in normal or calcium-free DMEM, and then exposed to 10 ng/ml TGF-β1 for 10 minutes. Subsequently, cells were washed with ice-cold phosphate-buffered saline (PBS) and scraped into 800 μl lysis buffer supplemented with 0.1% SDS, 0.5% Na-Deoxycholate. The supernatants were incubated with GST-RBD beads for 45 minutes at 4°C. After washing, the beads were boiled in Laemmli buffer. Bead-associated Rho protein was detected by Western blotting.
Wound Assay
Confluent monolayers grown on 25-mm glass coverslips were wounded with a rubber policeman generating an ∼3-mm gap. After extensive washing, the cells were placed into DMEM, and treated with 4 ng/ml TGF-β1 or vehicle for 3 days.
Immunofluorescence Microscopy
Cells grown on coverslips were fixed in 4% paraformaldehyde, incubated with PBS containing 100 mmol/L glycine, and washed with PBS. Cells were permeabilized in PBS containing 0.1% Triton X-100, blocked with 5% albumin for 1 hour, and incubated with the primary antibodies for another hour. After extensive washing, fluorescently labeled secondary antibodies were added for 1 hour, the coverslips were washed again and mounted on slides using Fluorescence Mounting Medium (DAKO Diagnostics, Mississauga, ON). Samples were analyzed using a Nikon Eclipse TE200 microscope (×100 objective) (Nikon, Mississauga, ON) and a Hamamatsu cooled CCD camera (C4742–95) (Hamamatsu, Bridgewater, NJ) controlled by the Simple PCI software.
Plasmids
A 765-bp piece of the rat SMA promoter containing several cis-elements including the serum response element binding motifs (CArG A and CArG B boxes), a TGF-β1 control element (TCE), a TATA box, and two E-boxes was ligated into PA3-Luc luciferase vector (pSMA-Luc). The construct was a kind gift from Dr. R. A. Nemenoff (Department of Medicine, Renal Division, University of Colorado Health Sciences Center, Denver, CO).38 The LEF/TCF reporter plasmid, TopFLASH and its mutant control, FopFLASH were purchased from Upstate Biotechnology (Charlottesville, VA). The thymidine kinase-driven Renilla luciferase vector (pRL-TK, Promega, Madison, WI) was used as an internal control. The construct coding the cytoplasmic tail of N-cadherin ligated to GFP (N-cad-GFP) was a generous gift of Dr. B. Geiger (Department of Chemical Immunology, The Weizmann Institute of Science, Rehovot, Israel).39 Dominant-negative TCF4 construct (dN-TCF4) cloned into pcDNA3 vector was from Drs. O. Tetsu and F. McCormick (Cancer Reserch Institute, University of California, School of Medicine, San Francisco, CA).40 This construct encodes for a Myc-epitope-tagged truncation mutant of TCF-4, in which the region between amino acids 2–53 has been deleted. pEGFP vector was from Clontech Laboratories (Palo Alto, CA) and pcDNA3 was from Invitrogen (Burlington, ON).
Transient Transfection and Luciferase Assay
Cells plated onto 6-well plates were transfected at 100% or 30% of confluence using FuGENE6 (Roche Molecular Biochemicals, Indianapolis, IN; reagent:DNA ratio 2.5 μl/μg). For promoter activity measurements, cells were co-transfected with 0.5 μg promoter plasmid, 0.05 μg pRL-TK along with 2 μg of the specific inhibitory construct or pcDNA3 per well. After 16 hours, the cells were washed with Hank’s balanced salt solution (HBSS) and incubated in serum-free normal or low calcium DMEM for 3 hours, followed by treatment with vehicle or 10 ng/ml TGF-β1 for 24 hours. Subsequently, the cells were lysed in 250 μl Passive Lysate Buffer (Promega), exposed to freezing/thawing, and the samples were clarified by centrifugation. Firefly and Renilla luciferase activities were determined using the Dual-Luciferase Reporter Assay Kit (Promega) and a Berthold Lumat LB 9507 luminometer according to the manufacturers’ instructions. Results were normalized by dividing the Firefly luciferase activity with the Renilla luciferase activity of the same sample. Each transfection was done in duplicate, and determinations for each group were repeated at least three times.
Retroviral Infection
For generating the pFB-N-cad and pFB-GFP vectors, the coding region of N-cad-GFP and pEGFP plasmids were inserted into the pFB-Neo plasmid (Stratagene, La Jolla, CA) using EcoRI and Xho enzymes. To produce retroviruses, 293T packaging cells were transfected with pFB-N-cad, pFB-GFP, or pFB-Neo vectors, respectively, along with pVpack-GP and pVpack-VSVG. After 24 to 36 hours, supernatants were collected and filtered through a 0.45-μm filter. LLC-PK1 cells plated on 6-well plates were transduced with the virus at 20% confluence. After a 16-hour transduction, cells were further incubated in fresh medium for 36 hours and treated with vehicle or 4 ng/ml TGF-β1. Three days later, cell lysates were prepared and samples were analyzed by Western blot.
Statistical Analysis
Data are presented as representative blots from three similar experiments or as the means ± SEM for the number of experiments (n) indicated. Statistical significance was determined by Student’s t-test or analysis of variance (one-way analysis of variance, SPSS Inc., Chicago, IL), using Bonferroni and Tukey post-hoc tests. P < 0.05 value is accepted to be significant.
Results
Cell Confluence Is a Critical Determinant of TGF-β1-Induced EMT in LLC-PK1 Tubular Cells
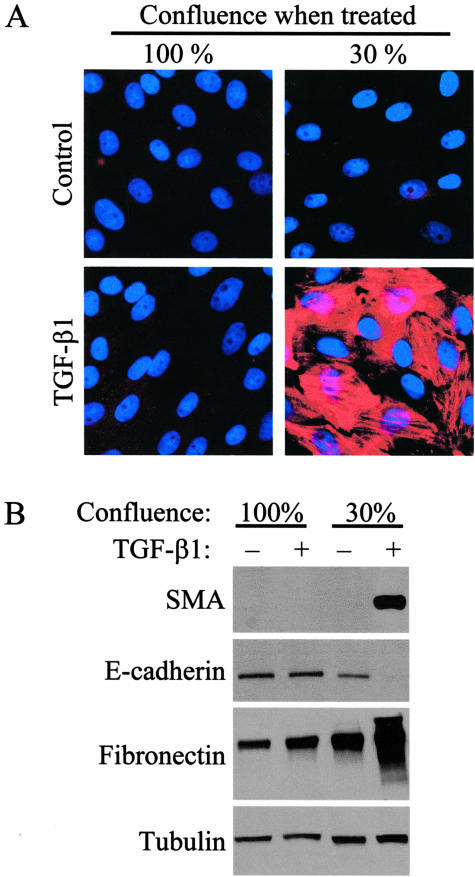
To assess the role of cell contacts in TGF-β1-induced EMT, we first compared the effect of the cytokine in confluent and sparse cultures, ie, under conditions where the cells have mature intercellular junctions or less well-developed contacts and free borders. In the absence of TGF-β1, LLC-PK1 cells possess an epithelial phenotype15 and did not express SMA, irrespective of the level of cell confluence (Figure 1A, top). However, cell confluence had a dramatic impact on the ability of TGF-β1 to induce EMT. Notably, when LLC-PK1 cells were seeded at 30% confluence and then treated with TGF-β1 for 3 days, ∼50% of the cells showed staining for SMA, the hallmark of myofibroblast phenotype. In contrast, no SMA-positive cells were observed when TGF-β1 was added to confluent layers for 3 days (Figure 1A, bottom). To substantiate this finding, we performed Western blot analysis to determine the expression of SMA and other proteins whose level shows characteristic changes during EMT. Remarkably, TGF-β1 treatment for as long as 5 days failed to induce any SMA expression in 100% confluent cultures, whereas it triggered robust SMA expression when the cells were exposed to the cytokine at 30% confluence (Figure 1B). Further, TGF-β1 induced only marginal increase in fibronectin in confluent cultures, while it strongly increased the abundance of this matrix protein when added at 30% confluence. Importantly, in confluent cultures TGF-β1 was unable to down-regulate the adherent junction protein E-cadherin, a classical epithelial cell marker. In contrast, no E-cadherin protein was detected in cells that had been treated with TGF-β1 before reaching confluence (Figure 1B). Together these results show that high cell density prevents or strongly suppresses a number of key features of TGF-β1-induced mesenchymal transition, including the loss of epithelial markers, increased extracellular matrix production, and expression of the myofibroblast marker, SMA.
Figure 1.
Cell density determines the transforming effect of TGF-β1 in LLC-PK1 cells. A: Cells were grown on coverslips to 100% or 30% confluence, and then treated with vehicle (Control) or 4 ng/ml TGF-β1 for 3 days. They were then stained with anti-SMA antibody (red) and the nuclear marker DAPI (to verify the presence of cells). B: Cells were grown on 6-well plates until 30% or 100% confluence and treated with vehicle (−) or TGF-β1 (+) for 5 days. Equal amounts of protein were subjected to SDS-PAGE, and analyzed for SMA, E-cadherin, and fibronectin by Western blotting. To test equal loading, the membrane was probed for tubulin.
Role of Cell-Cell Contacts in TGF-β1-Induced Epithelial-Myofibroblast Transition
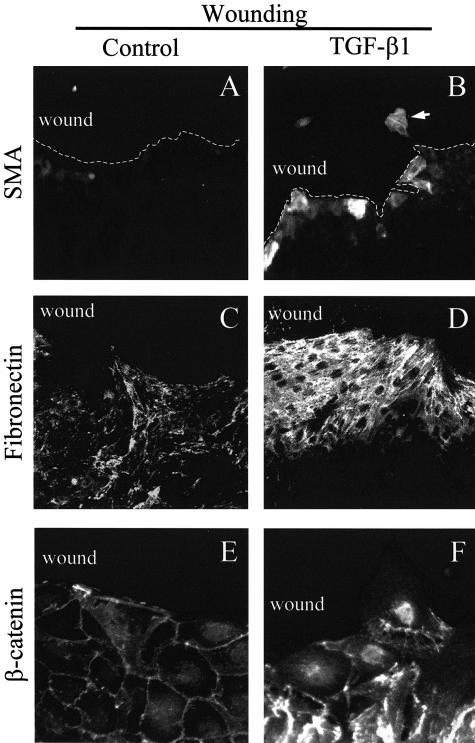
We sought to determine whether the ability of TGF-β1 to induce EMT was indeed related to the absence of intercellular contacts. To address this we generated a mechanical wound in otherwise confluent monolayers, and tested the effect of TGF-β1 on SMA expression. Wounding itself did not induce SMA production, while exposure to TGF-β1 resulted in SMA expression (Figure 2, A and B). Strikingly, SMA expression was restricted exclusively to cells located at the wound edge, ie, it occurred in cells, which partially lost their contacts with their neighbors. Similarly, enhanced fibronectin labeling exhibited a strong gradient starting a few cell rows behind the wound and sharply increasing toward the free edge (Figure 2, C and D). The disintegration of the cell-cell contacts in rows near the wound was clearly visualized by β-catenin staining. Cells lining the wound lost their β-catenin labeling at the free edge but kept most of the normal peripheral β-catenin distribution at cell-cell borders. When treated with TGF-β1, β-catenin became redistributed in cells near the wound. This manifested in the widening of the peripheral β-catenin staining, higher cytosolic labeling, and accumulation of β-catenin in the nuclei of cells located at the edge or migrating into the wound (Figure 2, E and F).
Figure 2.
Loss of cell-cell contacts by wounding restores the ability of TGF-β1 to induce EMT. Cells were allowed to grow until 100% confluence and then a wound was generated with a rubber policeman in the monolayer. Subsequently cells were treated with vehicle (Control; A, C, and E) or 4 ng/ml TGF-β1 (B, D, and F) for 3 days, then fixed, permeabilized, and stained for SMA (A and B), fibronectin (C and D), or β-catenin (E and F). The magnification was ×40 for A to D, and ×100 for E and F.
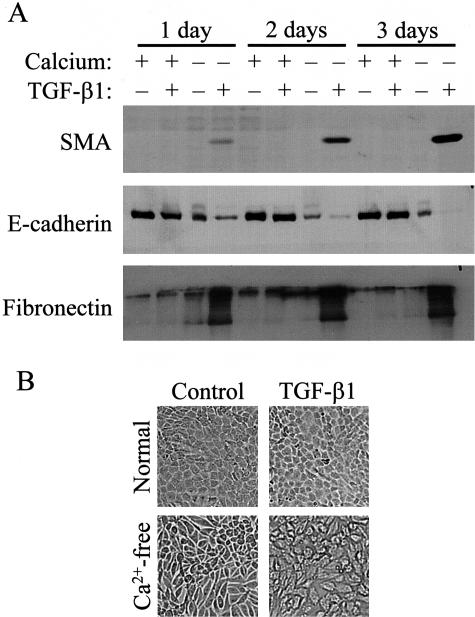
As a further method to test the role of cell-cell contacts in EMT, we used an approach that did not involve mechanical injury. We induced disassembly of mature cell-cell contacts in confluent cultures by Ca2+-removal, a maneuver known to disrupt the homotypic adhesion between cadherins.41 Remarkably, Ca2+-removal completely restored the ability of TGF-β1 to provoke EMT. During the course of a 3-day experiment in the presence of Ca2+, TGF-β1 neither induced SMA expression, nor caused a significant reduction in E-cadherin, as reported above (Figure 3A). Removal of Ca2+ without TGF-β1 addition did not cause SMA expression and only marginally affected fibronectin expression, but it induced a substantial reduction in E-cadherin. Importantly, TGF-β1 added in the absence of Ca2+ triggered a gradually increasing SMA expression, led to complete elimination of E-cadherin, and strongly stimulated fibronectin production (Figure 3A). In accordance with these findings, in a Ca2+-free medium epithelial cells still preserved their polygonal shape. However, exposure of these cells to TGF-β resulted in mesenchymal morphology (Figure 3B). Thus, the absence of stable intercellular junctions, irrespective of whether it is brought about by non-confluence or mechanical or chemical disruption of the cell contacts is a prerequisite for the EMT-inducing effect of TGF-β1.
Figure 3.
Disassembly of intercellular contacts by low calcium restores the transforming effect of TGF-β1 in confluent monolayers. A: Confluent cells were washed and incubated in serum-free normal or Ca2+-free DMEM, and 3 hours later treated with vehicle (Control) or 4 ng/ml TGF-β1 for the indicated times. Expression of SMA, E-cadherin, and fibronectin were determined by Western blotting. B: TGF-β1 treatment (24 hours) induced morphological changes in low calcium DMEM (phase-contrast microscopy, magnification, ×40).
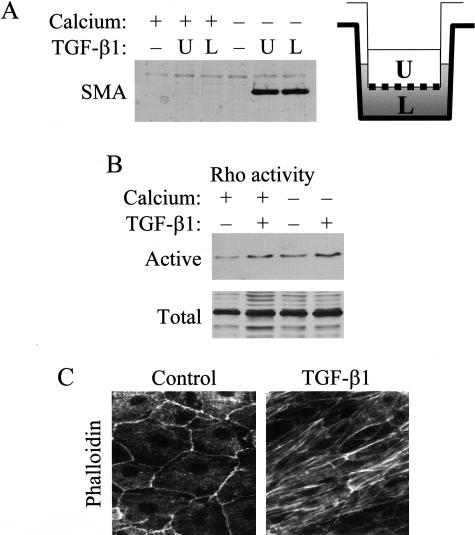
We considered the possibility that, in confluent layers, TGF-β1 cannot promote EMT when added from the apical side, because it cannot access the basolateral membrane, where TGF-β1 receptors might reside.29 To test this, we performed control experiments using LLC-PK1 cells grown on permeable support to provide free access to both membrane domains. As shown on Figure 4A, in confluent monolayers neither apically nor basolaterally added TGF-β1 induced SMA expression. However, when cell contacts were disassembled by Ca2+-removal, apical or basolateral administration of TGF-β1 provoked a robust and similar SMA response. These data precluded that resistance to TGF-β1 was due to differential accessibility. Next, we asked whether confluent cultures exhibited general unresponsiveness to TGF-β1, or only the transforming effect was suppressed. To address this, we determined if TGF-β1 remained able to stimulate Rho GTPase, an important signal for cytoskeletal remodeling. In agreement with our previous findings,15 TGF-β1 induced a sizable Rho activation in confluent cultures (Figure 4B), which was similar in the presence or absence of Ca2+. This suggests that contact disassembly was not required for this response. Consistent with these results, exposure of confluent layers to TGF-β1 stimulated stress fiber formation (Figure 4C). Together these data show that TGF-β1 signaling pathways are functional in confluent LLC-PK1 cells, suggesting that failed transformation is not due to general unresponsiveness, but rather due to the absence of cell contact-regulated permissive factor(s).
Figure 4.
Confluent cells remain responsive to TGF-β1. A: Cells were cultured until confluence on porous tissue culture inserts (pore size, 3 μm) then serum-deprived in normal (+) or low calcium medium (−). After 3 hours, cells were treated with vehicle (−) or 4 ng/ml TGF-β1 from the upper (U) or the lower side (L) for 3 days. Total cell lysates were prepared and SMA expression was examined by Western blots. B: To determine Rho activity were serum-starved for 3 hours in normal (+) or low Ca2+ (−) DMEM, and then treated with vehicle (−) or 10 ng/ml TGF-β1 (+) for 10 minutes. After lysis, active Rho was captured with GST-Rhotekin beads and detected by Western blotting. Aliquots of the same lysates were probed for total Rho. C: Confluent cultures were treated with vehicle (Control) or 4 ng/ml TGF-β1. F-actin was visualized by rhodamine phalloidin.
Cell-Cell Contacts Regulate the SMA Promoter
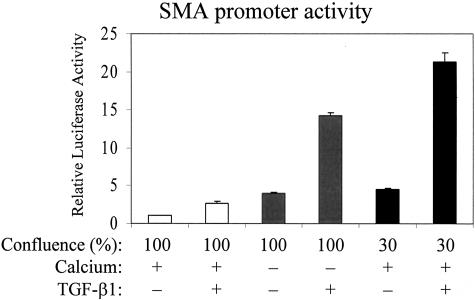
To assess whether cell contacts might impact the regulation of the SMA promoter, we investigated the effect of TGF-β1 on a SMA promoter luciferase construct under conditions where cell junctions were manipulated by the above-described means (Figure 5). In confluent cultures with stable cell contacts (100%, normal Ca2+ medium), TGF-β1 induced a modest ∼3-fold increase in the activity of the SMA promoter, confirming the basal responsiveness to TGF-β1 in confluent layers. Reduction of Ca2+ without TGF-β1 addition resulted in ∼4-fold rise in SMA promoter activity. Importantly, TGF-β1 added to confluent layers in which the cell junctions had been disassembled by Ca2+-removal elicited a 14-fold increase in SMA promoter activity. Thus, contact disassembly and TGF-β1 acted in a strongly synergistic manner as evidenced by the multiplicative effect. We next tested the impact of subconfluence in the presence of normal Ca2+. The basal activity of the SMA promoter was fivefold higher in 30% than in 100% confluent cultures. TGF-β1 addition to 30% confluent cells resulted in a 20-fold increase in SMA promoter activity, compared to confluent, unstimulated cells. These data show that the overall stimulation of the SMA promoter by TGF-β1 strongly depends on the state of cell-cell contacts in both models, irrespective of whether the contacts have been disassembled in already confluent layers or have not been fully formed yet in growing subconfluent cultures.
Figure 5.
Subconfluence or disassembly of cell-cell contacts potentiates the effect of TGF-β1 on SMA promoter activity. LLC-PK1 cells grown to 100% or 30% confluence were co-transfected with pSMA-Luc (0.5 μg/well), and the Renilla luciferase construct (pRL-TK, 0.05 μg/well). After 16 hours, cells were washed and incubated with serum-free normal (+) or low Ca2+ (−) DMEM for 4 hours, and then treated with vehicle (−) or 10 ng/ml TGF-β1 (+) for 24 hours. After lysis, Firefly and Renilla luciferase activities were determined by luminometry. Values were normalized to the basal activity measured in the 100% confluent vehicle-treated group in the presence of normal calcium (n = 6).
TGF-β1 Stimulates the Transcriptional Activity of β-Catenin in LLC-PK1 Tubular Cells
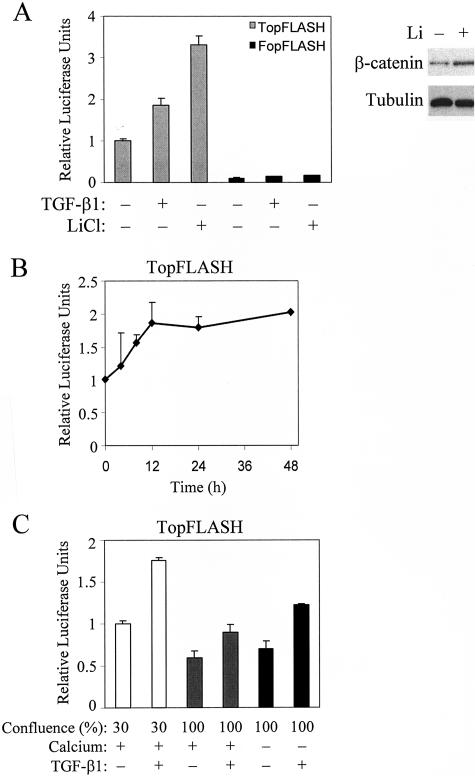
Since β-catenin has a dual function as an adherent junction component and a transcriptional co-activator, and it redistributes during EMT in LLC-PK1 cells (15 and Figure 2), it might act as a mediator of contact-dependent transcriptional responses. To address this possibility, we first determined whether TGF-β1 could stimulate β-catenin-dependent transcription. Cells at 30% confluence were transfected with the β-catenin-dependent reporter TopFLASH or its inactive mutant FopFLASH, and then exposed to vehicle or TGF-β1. The basal TopFLASH activity was 10-fold higher than FopFLASH, indicating that TopFLASH expression reflects β-catenin-specific transcription, and that there is readily detectable, β-catenin-dependent transcription even in non-stimulated, subconfluent cells (Figure 6A). Importantly, TGF-β1 induced a twofold increase in TopFLASH activity, while it had no significant effect on the marginal FopFLASH activity. Moreover, LiCl, an inhibitor of β-catenin degradation also stimulated TopFLASH activity. Western blot analysis verified that LiCl indeed increased the steady state level of β-catenin in LLC-PK1 cells (Figure 6A). Together these findings suggest that the continuous degradation of β-catenin limits β-catenin signaling in non-stimulated cells. We then examined the kinetics of the TGF-β1-induced TopFLASH response. Figure 6B shows that the TopFLASH activity gradually increased over the first 24 hours following TGF-β1 treatment, and then reached a plateau.
Figure 6.
TGF-β1 activates β-catenin-dependent TCF/LEF reporter construct TopFLASH. A: Cells grown to 30% confluence on 6-well plates were transfected either with TopFLASH (0.5 μg/well) or its inactive mutant, FopFLASH (0.5 μg/well), along with pRL-TK (0.05 μg/well). Sixteen hours after transfection, the medium was replaced with serum-free DMEM for 4 hours, followed by exposure to vehicle (Control), 10 ng/ml TGF-β1 or 30 mmol/L LiCl. Cells were lysed after 24 hours, and luciferase activities determined. Values were normalized to the relative luciferase activity measured in the TopFLASH-transfected control group, and expressed as means ± SEM (n = 8). The blot shows the effect of LiCl treatment on the steady state level of β-catenin. Equal loading was detected by probing the blot for tubulin. B: Thirty percent confluent cultures transfected with TopFLASH, as in A, were treated with vehicle or 10 ng/ml TGF-β1 and lysed at the indicated time points. C: The effect of subconfluence or the disassembly of cell-cell contacts on TopFLASH activity was examined by transfecting cells at the indicated confluence with DNA mixtures as above. After 16 hours, cells were washed and further incubated in serum-free normal (+) or low calcium medium (−) for 4 hours before treatment with vehicle or 10 ng/ml TGF-β1. Twenty-four hours later, cells were lysed, and luciferase activities were measured. Results are expressed as means ± SEM, normalized to the activity measured in the 30% confluent vehicle-treated group in normal calcium medium (n = 6).
In subsequent experiments, we tested whether the state of cell-cell contacts affected β-catenin-dependent transcription. After reaching confluence, the basal TopFLASH activity was ∼60% of the level observed in subconfluent cultures (Figure 6C). Addition of TGF-β1 to confluent layers induced only a slight (non-significant) increase in TopFLASH from this reduced baseline. Disassembly of junctions by removal of Ca2+ did not exert a significant effect itself, but it significantly potentiated the TGF-β1-induced increase in TopFLASH activity. Together these results show that TGF-β1 induced β-catenin-dependent transcription, and the amplitude of this effect was dependent on the state of intercellular contacts.
TGF-β1 Rescues β-Catenin But Not E-Cadherin after Destabilization of Cell Contacts
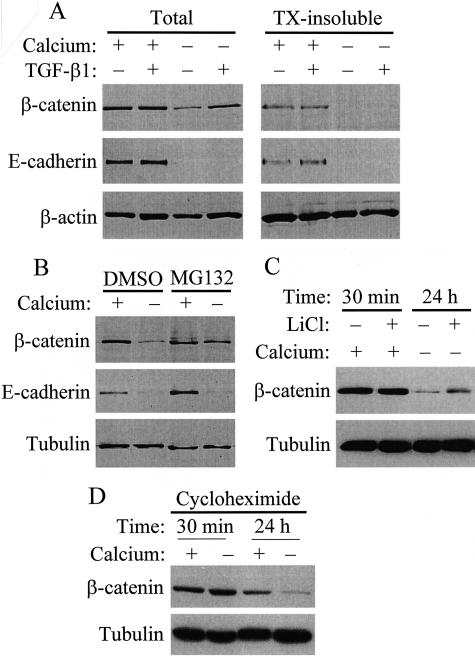
To explore the mechanism whereby TGF-β1 stimulates β-catenin-dependent transcription, we examined the fate of junctional proteins after disassembly of cell contacts. Ca2+-removal resulted in a dramatic reduction of E-cadherin and a substantial decrease in β-catenin protein, indicating that dissociation of the contacts promoted the degradation of their components (Figure 7A). In intact monolayers, TGF-β1 did not affect the level of these proteins. However, when TGF-β1 was added to monolayers kept in Ca2+-free medium, it exerted grossly different effects on the two junctional proteins: while it did not influence the degradation of E-cadherin, it largely prevented the loss of β-catenin. The massive down-regulation of E-cadherin, the transmembrane binding partner of β-catenin, together with the selective rescue of β-catenin should increase the level of free β-catenin. In agreement with this assumption, in intact cells β-catenin was present both in the cytosolic and the cytoskeletal (Triton-insoluble) fraction, while after low Ca2+ plus TGF-β1 treatment the rescued β-catenin was not bound to the cytoskeleton (Figure 7A, right).
Figure 7.
TGF-β1 rescues β-catenin but not E-cadherin from contact disassembly-induced degradation. A: Confluent LLC-PK1 cells were incubated in serum-free normal (+) or low calcium (−) DMEM for 16 hours, and then treated with vehicle (−) or 4 ng/ml TGF-β1 (+) for 24 hours. Subsequently cells were washed and lysed in Triton lysis buffer. Aliquots from total cell lysates and Triton-insoluble extracts were subjected to SDS-PAGE and the components of adherent junction were detected by Western blotting. Equal loading was verified by reprobing the membranes for β-actin. B: Confluent cells were treated with vehicle dimethylsulfoxide (DMSO) or proteasome inhibitor, MG132 (1 μmol/L) followed by incubation in normal (+) or low calcium (−) medium in the presence of DMSO or MG132 for 24 hours. β-catenin and E-cadherin were measured by Western blotting in total cell lysates. The membrane was reprobed for tubulin. C: Confluent cells were incubated in normal or low calcium (−) medium, in the presence or absence of 30 mmol/L LiCl for 30 minutes or 24 hours, as indicated. Subsequently the cells were lysed and β-catenin and tubulin (as loading control) were detected by Western blotting. D: To assess the role of contact disassembly in β-catenin degradation, protein synthesis was blocked in confluent monolayers by 100 μg/ml cycloheximide, and the cells were incubated for 30 minutes or 24 hours in the presence or absence of Ca2+. Note that Ca2+-removal strongly stimulated the loss of β-catenin also in the presence of cycloheximide, indicating that it accelerated the degradation of β-catenin.
To gain insight into the mechanism responsible for the decrease in β-catenin protein on contact disassembly, we tested if degradation by the proteasomal pathway contributes to this process (Figure 7B). MG132, a potent proteasome inhibitor strongly reduced the Ca2+-removal-induced degradation of β-catenin. Furthermore, LiCl, an inhibitor of glycogen synthase kinase-3β, the enzyme which phosphorylates and thereby facilitates proteasomal processing of β-catenin, also strongly reduced the Ca2+ removal-induced drop in β-catenin protein (Figure 7C). Together these findings suggest that proteasomal degradation is involved in β-catenin elimination, and TGF-β1 inhibits this process. Interestingly, MG132 did not inhibit the contact disassembly-induced loss of E-cadherin, suggesting that E-cadherin down-regulation may be due to alternative degradation pathways and/or the inhibition of its synthesis. Pharmacological inhibition of β-catenin degradation is expected to reduce the loss of β-catenin even if contact disassembly does not accelerate β-catenin degradation per se, but inhibits β-catenin synthesis. To address whether contact disassembly indeed impacts on β-catenin degradation, we inhibited protein synthesis by cycloheximide, and tested the effect of Ca2+ removal under these conditions, when any change should be due to a difference in degradation. Cycloheximide added alone modestly reduced the level of β-catenin after 24 hours. Importantly, Ca2+ removal strongly facilitated the loss of β-catenin even in the presence of cycloheximide, indicating that the disruption of the contacts indeed promotes degradation of β-catenin. These results do not exclude the possibility that altered synthesis also contributes to the reduction in β-catenin.
β-Catenin Is Involved in the TGF-β1-Induced SMA Promoter Activation and Protein Expression
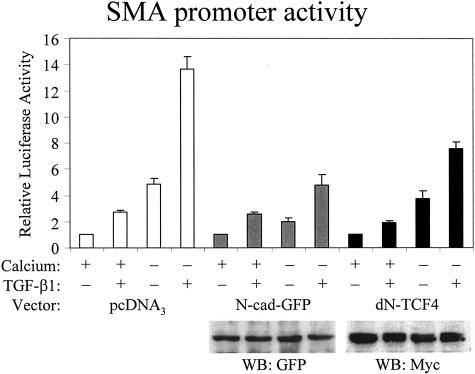
While these observations are consistent with a potential role for β-catenin in epithelial-myofibroblast transition, they do not provide evidence for the involvement of β-catenin in the process. Therefore, we intended to interfere with β-catenin, and test the effect of this manipulation on SMA promoter activity and protein expression. We used a construct that encodes a chimera of green fluorescence protein (GFP) and the cytosolic tail of N-cadherin. This construct offers a uniquely selective way to inhibit β-catenin-dependent signaling: it binds to free β-catenin but does not disturb the function of β-catenin in the cell junctions.39 Confluent cells were co-transfected with control or N-cad plasmid plus the SMA-luciferase reporter system, and the promoter activity was tested under conditions where contact integrity was manipulated with Ca2+ in the absence or presence of TGF-β1 (Figure 8). In the control group, the promoter exhibited the same highly synergistic behavior as shown in Figure 5. In N-cad-transfected cells, TGF-β1 alone caused weak activation similar as in empty plasmid-transfected controls, while the Ca2+-removal-induced activation was somewhat reduced. Importantly, N-cad suppressed the robust activation on Ca2+-removal plus TGF-β1 by 65%. To substantiate that interference with β-catenin signaling inhibits SMA promoter activation, we used another construct, dominant-negative dN-TCF4. This construct interferes with β-catenin-dependent transcription by competing with the endogenous TCF transcription factors.40 dN-TCF4 slightly reduced the SMA promoter activation induced by TGF-β1 or Ca2+-removal, and strongly (∼50%) suppressed promoter activation elicited by the combination of the two stimuli. Together these data suggest that β-catenin is involved in SMA promoter regulation during EMT, and show that inhibition of β-catenin signaling disrupts the synergism between the TGF-β1 and cell contact disassembly-induced SMA promoter activation.
Figure 8.
Interference with β-catenin signaling inhibits TGF-β1 plus low calcium-induced activation of the SMA promoter. Cells grown until confluence were transfected with pSMA-Luc (0.5 μg/well) along with pRL-TK (0.05 μg/well) and either empty vector (pcDNA3, 2 μg/well) or N-cad-GFP or dominant-negative TCF4 (dN-TCF4). A day after transfection, cells were washed and incubated in serum-free normal (+) or low calcium (−) DMEM for 4 hours and then exposed to vehicle (−) or 10 ng/ml TGF-β1 (+) for 24 hours. Subsequently, luciferase activities were determined and normalized to the activities measured in vehicle-treated cells in normal calcium medium in each group, transfected with either pcDNA3 or the different test plasmids (n = 6). Cell lysates were prepared from each transfected group and probed with the corresponding antibodies ie, anti-GFP for N-cad-GFP and anti-myc for the myc-tagged dN-TCF4. The treatments (TGF-β1, Ca2+-removal), which were initiated after 24 hours of transfection, did not affect the expression of the transfected constructs.
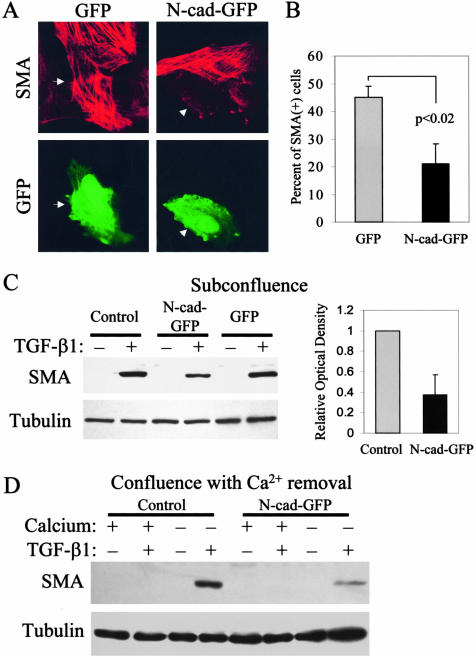
Next, we examined whether β-catenin signaling impacts on SMA protein as well. This is an important question, since robust (14- to 20-fold) but not modest (≤5-fold) activation of the promoter corresponded to SMA protein expression, suggesting the existence of a threshold. To follow SMA expression, 30% confluent cultures were transfected with GFP or N-cad-GFP, exposed to TGF-β1 and stained for SMA (Figure 9A). After a 3-day treatment with TGF-β1, ∼45% of the GFP-transfected cells were SMA-positive. In contrast, only 21% of N-cad-GFP-expressing cells stained for SMA, indicating that N-cad-GFP exerted a strong (> 50%) inhibition of SMA expression compared to GFP (Figure 9B).
Figure 9.
Chelation of β-catenin inhibits TGF-β1-induced SMA protein expression. A: Cells grown on coverslips were transfected with GFP alone or N-cad-GFP (2 μg/well) at 30% confluence, 16 hours before treatment with 4 ng/ml TGF-β1. After 3 days, cells were fixed, permeabilized, and stained for SMA. Successfully transfected cells were identified by GFP-positivity (GFP, bottom). Arrows indicate identical cells. No SMA expression was detected in cells transfected with either GFP or N-cad-GFP without the addition of TGF-β1 (not shown). B: For quantification, a hundred GFP-positive cells were counted in each of three independent experiments. Values are means ± SD, P < 0.02. C: N-cad-GFP was cloned into the pFB-neo retrovirus (pFB-N-cad-GFP), which provided at least 80% infection rate. Cells at 30% confluence were transduced with the pFB-Neo (Control) or pFB-GFP or pFB-N-cad-GFP. After 16 hours cells were treated with vehicle (−) or 4 ng/ml TGF-β1 (+) for 3 days, then lysed and equal amounts of total cell lysates were probed for SMA. The membrane was reprobed for tubulin to test equal loading. SMA protein was quantified by densitometry and normalized to the level of tubulin. D: Cells grown until 100% confluence were transduced with pFB-GFP or pFB-N-cad-GFP virus. After 16 hours cells were washed and incubated in normal (+) or low calcium (−) DMEM. After a 3-day treatment with vehicle (−) or TGF-β1 cells were lysed and SMA expression was measured.
We detected SMA expression biochemically as well. This approach is more accurate than counting transfected cells, because in the latter case the evaluation is based on an all-or-none response (SMA+ versus SMA−), and partially suppressed expression cannot be exactly assessed. To achieve this, we generated a retrovirus expressing N-cad-GFP. Viral transduction resulted in 80 to 90% efficiency of gene delivery as judged by GFP positivity (not shown). We used both model systems (subconfluence and Ca2+-removal) to test the effect of N-cad or GFP on SMA protein. Western blots shown in Figure 9, C and D demonstrate that infection with N-cad virus efficiently suppressed TGF-β1-induced SMA expression in both EMT models.
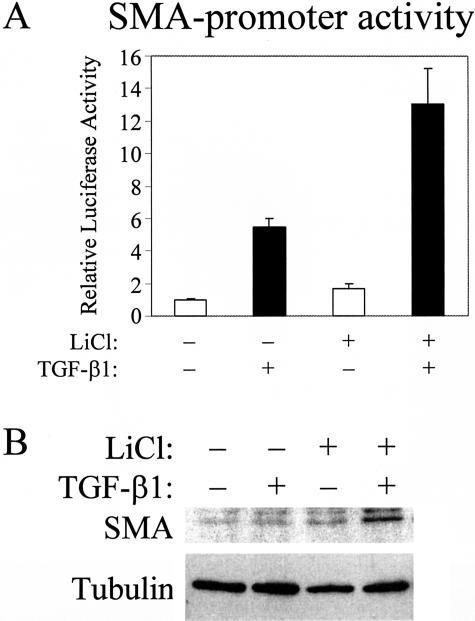
Finally, we considered whether insufficient β-catenin signaling in confluent cultures is an important factor for the reduced potency of TGF-β1 to stimulate the SMA promoter. If so, increasing free β-catenin by inhibition of its degradation should increase TGF-β1-induced SMA promoter activity. To test this idea, we used LiCl, as it stimulated TopFLASH activity even stronger than TGF-β1 (Figure 5). Confluent cells in normal Ca2+ medium were exposed to 30 mmol/L NaCl (as control) or LiCl in the absence or presence TGF-β1, and SMA promoter activity was determined (Figure 10A). While LiCl had only a modest effect in the absence of TGF-β1, it significantly potentiated the TGF-β1-induced activation of the SMA promoter. LiCl caused a 2- to 3-fold increase in the TGF-β1 effect, raising the overall activation of the promoter to 10- to 15-fold. Since this activation approached the level where SMA expression occurred after Ca2+-removal, we checked for SMA protein by Western blots. Remarkably, LiCl restored the ability of TGF-β1 to induce detectable SMA production in confluent layers, although the effect was substantially weaker than after complete disruption of the cell junctions (Figure 10B). Together these data show that specific inhibition of β-catenin function substantially reduces the TGF-β1-induced SMA promoter activity and protein expression, while enhancing β-catenin signaling restores promoter activity.
Figure 10.
The effect of LiCl on TGF-β1-induced SMA promoter activation and protein expression in confluent cultures. A: Confluent cells were transfected with pSMA-Luc and pRL-TK, and then exposed to 30 mmol/L NaCl (−, control) or LiCl (+) for 1 hour followed by a 24-hour treatment with vehicle or TGF-β1. SMA promoter activity was normalized to the NaCl and vehicle-treated group (n = 8). B: Confluent cells were treated as above except SMA protein was determined by Western blotting after a 3-day exposure to vehicle or TGF-β1.
Discussion
Our studies reveal novel insights into the pathophysiology of epithelial-myofibroblast transition, a process central to tubulointerstitial fibrosis. We provide evidence that an initial absence or loss of cell-cell adhesions is a prerequisite for TGF-β1 to induce complete myofibroblast transition, and that β-catenin is a key component of the contact-dependent regulation of EMT. Specifically, β-catenin signaling plays a strong potentiating and synergistic role in the TGF-β1-induced activation of the SMA promoter and protein expression. TGF-β1-has been shown to down-regulate key cell contact proteins13–15,42 and the TGF-β1-triggered loss of cell contacts has been identified as an early step in EMT.14 Moreover, our data suggest the existence of reciprocal regulatory relationship as well. Specifically, topical injury of cell junctions may be necessary for the initial transforming effect of TGF-β1. This notion is supported by our findings that while intact monolayers are resistant to full transformation by TGF-β1, three different methods that interfere with cell contacts (subconfluence, wounding, and Ca2+-removal) restore the ability of TGF-β1 to induce three major features of myofibroblast transition (E-cadherin down-regulation, enhanced fibronectin production, and SMA expression). Based on these observations, we propose a two-hit model for EMT. An initial loss of epithelial integrity occurs, which, in vivo, may be due to various insults known to trigger fibrosis, including immuncomplex deposition,8 hypoxic damage,43 ureteral obstruction or physical injury.44 When these injured sites are exposed to TGF-β1 (second hit), they serve as initial foci for EMT wherefrom it spreads in a self-augmenting manner. These local groups of cells undergo transition, leading to enhanced TGF-β1 production and extracellular matrix (ECM) deposition, which in turn disrupts neighboring areas. It is noteworthy that in other tubular cell systems TGF-β1 caused EMT in apparently confluent layers (eg, 10,13). However, in most of these studies TGF-β1 was added before the monolayer reached confluence, and it only became confluent by the end of the few-day exposure to TGF-β1. Interestingly Strutz et al45 remarked that the EMT in mouse proximal epithelial tubule (MCT) cells was more pronounced when TGF-β1 treatment started at lower density. Further, tissue fibroblasts have been shown to spontaneously differentiate into myofibroblasts with much higher frequency, when plated at low density.46 Our data together with these observations suggest that the contact-dependent sensitivity to TGF-β1 is a general phenomenon. However, it is conceivable that various tubular cells or segments are differentially sensitive to the inhibitory effect of contact integrity on the induction of EMT by TGF-β1 or other stimuli.47
We found that the inability of TGF-β1 to induce full transition in confluent cultures is not due to general unresponsiveness. These findings are in agreement with previous observations showing that certain features of EMT are present in apparently confluent cells.29 However, in our model, specific components required for the full effect are missing, and we have identified one of these as free β-catenin.
There is a substantial body of literature suggesting that β-catenin signaling plays important roles in EMT during tumorigenesis and metastasis (eg,48–50). Consistent with this notion, the loss of E-cadherin, which likely increases the level of free β-catenin, facilitates EMT, whereas the expression of E-cadherin can reverse the transformed phenotype.19–22 As a more direct indication for the role of β-catenin, Eger et al26 have shown that in mammary epithelial cells that express the Fos-estradiol receptor fusion protein, estradiol caused enhanced β-catenin-dependent transcription concomitant with EMT. A few days before the submission of the present study, the same group reported that β-catenin and TGF-β1 signaling cooperated to maintain the mesenchymal phenotype in the mammary tumor cells.28 Furthermore, overexpression of LEF-1 was found to provoke EMT in epithelial tumor cells.51 Recent studies have indicated that TGF-β1 inhibits E-cadherin expression at the transcriptional level also in kidney tubular cells.42 However, it remained questionable, and in fact controversial,29,30,36 whether in normal epithelial cells, without overexpression of signaling proteins, β-catenin contributes to TGF-β1-induced EMT, and especially to myofibroblast differentiation. Subconfluent cultures may contain less E-cadherin than mature fully confluent cultures, which itself may facilitate β-catenin-dependent signaling. However, to prove the involvement of β-catenin in TGF-β1-induced epithelial-myofibroblast transition one should demonstrate that the cytokine induces enhanced β-catenin signaling and that interference with β-catenin results in suppression of TGF-β1-induced transition. Regarding the first criterion, our previous15 and current results as well as Tian et al29 have shown that in normal kidney epithelial cells TGF-β1 induces β-catenin dissociation from contacts and translocation to the nucleus. Contact disassembly by Ca2+-removal also results in the release of β-catenin from the junctions, however this in itself does not lead to EMT. This fact is perfectly consistent with our finding that, in the absence of TGF-β1, both E-cadherin and β-catenin are rapidly degraded following contact disassembly. However, we have shown that TGF-β1 selectively rescues β-catenin, thereby allowing it to exert downstream effects. Recent studies offer plausible mechanisms whereby TGF-β1 may stabilize β-catenin; TGF-β1 has been shown to stimulate Akt kinase52 and integrin-linked kinase (ILK),17 both of which are involved in EMT. Recently TGF-β1-induced ILK activation has been found to be necessary and possibly sufficient for EMT in kidney tubular cells.17 Importantly, both kinases can activate β-catenin/LEF-dependent transcription, by inhibiting glycogen synthase kinase-3β, the enzyme that phosphorylates β-catenin, targeting it for degradation.53
We show that the TGF-β1-induced rise in free β-catenin is sufficient to exert TCF/LEF-dependent transcription (TopFLASH). This finding differs from those obtained in hepatoma30 and HK2 tubular cells,29 where TGF-β1 did not stimulate TopFLASH. There are, however, important differences between the experimental systems. Apart from the fact that hepatoma cells have abnormal β-catenin signaling, a notable point is that without transfection of LEF-1, TopFLASH activity was marginal in HK2 cells. Thus, in these cells, LEF-1 and not β-catenin is the limiting factor. This is obviously not the case in our model, where both TGF-β1 and LiCl activate TopFLASH, indicating that there is sufficient TCF/LEF present. In this respect, various tubular cells or tubule segments may show differences in their susceptibility to myofibroblast transition. A second important point is that the HK2 cells were confluent when challenged with TGF-β1, and, as we have shown, under these conditions the activation of β-catenin-dependent transcription is very weak. Accordingly, basal TopFLASH activity was inversely related to cell confluence, a finding consistent with recent studies showing that cell density regulates the cellular localization and transcriptional activity of β-catenin.54,55 Thus, the state of cell contacts determines the overall magnitude of TCF/LEF-dependent transcription induced by TGF-β1. To address whether β-catenin-dependent signaling impacts myofibroblast transition, we used two constructs, which interfere with β-catenin signaling: the N-cadherin cytosolic tail (N-cad) and dN-TCF4. Both of these strongly suppressed the synergistic effect exerted by cell contact disassembly plus TGF-β1 on the SMA promoter. These data, together with the finding that LiCl partially restores the effect of TGF-β1 in confluent cells provide evidence for the involvement of the β-catenin-TCF/LEF pathway in the regulation of the SMA promoter. However, it is worth noting that TGF-β1 may activate TCF/LEF signaling not only by enhancing the binding of β-catenin to TCF/LEF. In addition, TGF-β1 and β3 have been shown to stimulate the association of LEF-1 with SMAD proteins, and this complex may induce transcription through SMAD-binding elements.30,56 Moreover, TGF-β1 promotes the association of SMAD4 and 3 with β-catenin,31 which may also recruit TCF/LEF, initiating a cooperative regulation that involves both SMAD and TCF/LEF-dependent motifs. These interactions clearly point to a collaborative, synergistic relationship between the SMAD and β-catenin pathways. In accordance with this, while inhibition of β-catenin strongly reduced the effect of TGF-β1 on SMA promoter and protein expression, these actions were not complete. Interestingly, intact contacts had a stronger inhibitory effect than elimination of β-catenin, suggesting that other components of the cell junctions also participate in this regulation. In this regard, expression of truncation mutants of the tight junction protein ZO-1 has been reported to cause EMT48 and induce SMA expression.57 However, truncated ZO-1 may also act through β-catenin, as its transforming effect can be prevented by β-catenin down-regulation.48 Future studies should address which other junctional proteins contribute to EMT.
Finally, the central questions remains: how does β-catenin regulate SMA transcription? β-catenin may form a complex with SMAD proteins and act through SMAD-dependent motifs, and/or its effect is indirect. In agreement with the latter mechanism, the SMA promoter itself does not contain TCF/LEF binding motifs, and neither LiCl nor overexpression of β-catenin is sufficient to induce SMA expression in the absence of TGF-β1. However, there are a number of known β-catenin-dependent target genes whose products may be critically important for SMA expression. Of these, potential candidates are genes encoding fibronectin and metalloproteinases, which are regulated by β-catenin34,58 and have been suggested to play important roles in the regulation of SMA expression.59,60 Future studies are warranted to address these possibilities.
Once upregulated, β-catenin may modify the expression of a whole array of key proteins during EMT: for example, it can accelerate the down-regulation of E-cadherin,33 the up-regulation of vimentin35 and fibronectin.34 Indeed, Wnt proteins, the extracellular activators of the canonical β-catenin signaling have been implicated as mediators of renal fibrosis.44
In summary, we provide evidence that cell contact integrity and β-catenin signaling regulate SMA expression during TGF-β1-induced EMT. The β-catenin pathway may offer new therapeutic targets to lessen progressive organ fibrosis.
Acknowledgments
We thank Drs. K. Szaszi and B. Alman and Ms. C. Di Ciano-Oliveira for valuable discussions.
Footnotes
Address reprint requests to Dr. András Kapus, Toronto General Hospital, NUG-001, 200 Elizabeth Street, Toronto, Ontario, Canada, M5G 2C4. E-mail: akapus@uhnres.utoronto.ca.
Supported by grants from the Canadian Institutes of Health Research (CIHR) and Kidney Foundation of Canada (to A.K.), and the Hungarian Ministry of Education OTKA/T042651 (to I.M.) and OTKA/34409, ETT/564 (to R.L.). I.M. is a Békésy scholar and a recipient of NATO Scientific Fellowship 2006/NATO/02. A.K. is a CIHR scholar.
References
- Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- Strutz F, Okada H, Lo CW, Danoff T, Carone RL, Tomaszewski JE, Neilson EG. Identification and characterization of a fibroblast marker: FSP1. J Cell Biol. 1995;130:393–405. doi: 10.1083/jcb.130.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest. 2003;112:1776–1784. doi: 10.1172/JCI20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y. Epithelial to mesenchymal transition in renal fibrogenesis: pathologic significance, molecular mechanism, and therapeutic intervention. J Am Soc Nephrol. 2004;15:1–12. doi: 10.1097/01.asn.0000106015.29070.e7. [DOI] [PubMed] [Google Scholar]
- Eddy AA. Molecular basis of renal fibrosis. Pediatr Nephrol. 2000;15:290–301. doi: 10.1007/s004670000461. [DOI] [PubMed] [Google Scholar]
- Boukhalfa G, Desmouliere A, Rondeau E, Gabbiani G, Sraer JD. Relationship between alpha-smooth muscle actin expression and fibrotic changes in human kidney. Exp Nephrol. 1996;4:241–247. [PubMed] [Google Scholar]
- Ng YY, Huang TP, Yang WC, Chen ZP, Yang AH, Mu W, Nikolic-Paterson DJ, Atkins RC, Lan HY. Tubular epithelial-myofibroblast transdifferentiation in progressive tubulointerstitial fibrosis in 5/6 nephrectomized rats. Kidney Int. 1998;54:864–876. doi: 10.1046/j.1523-1755.1998.00076.x. [DOI] [PubMed] [Google Scholar]
- Jinde K, Nikolic-Paterson DJ, Huang XR, Sakai H, Kurokawa K, Atkins RC, Lan HY. Tubular phenotypic change in progressive tubulointerstitial fibrosis in human glomerulonephritis. Am J Kidney Dis. 2001;38:761–769. doi: 10.1053/ajkd.2001.27693. [DOI] [PubMed] [Google Scholar]
- Rastaldi MP, Ferrario F, Giardino L, Dell’Antonio G, Grillo C, Grillo P, Strutz F, Muller GA, Colasanti G, D’Amico G. Epithelial-mesenchymal transition of tubular epithelial cells in human renal biopsies. Kidney Int. 2002;62:137–146. doi: 10.1046/j.1523-1755.2002.00430.x. [DOI] [PubMed] [Google Scholar]
- Okada H, Danoff TM, Kalluri R, Neilson EG. Early role of Fsp1 in epithelial-mesenchymal transformation. Am J Physiol. 1997;273:F563–F574. doi: 10.1152/ajprenal.1997.273.4.F563. [DOI] [PubMed] [Google Scholar]
- Bottinger EP, Bitzer M. TGF-beta signaling in renal disease. J Am Soc Nephrol. 2002;13:2600–2610. doi: 10.1097/01.asn.0000033611.79556.ae. [DOI] [PubMed] [Google Scholar]
- Iwano M, Plieth D, Danoff TM, Xue C, Okada H, Neilson EG. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest. 2002;110:341–350. doi: 10.1172/JCI15518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan JM, Ng YY, Hill PA, Nikolic-Paterson DJ, Mu W, Atkins RC, Lan HY. Transforming growth factor-beta regulates tubular epithelial-myofibroblast transdifferentiation in vitro. Kidney Int. 1999;56:1455–1467. doi: 10.1046/j.1523-1755.1999.00656.x. [DOI] [PubMed] [Google Scholar]
- Yang J, Liu Y. Dissection of key events in tubular epithelial to myofibroblast transition and its implications in renal interstitial fibrosis. Am J Pathol. 2001;159:1465–1475. doi: 10.1016/S0002-9440(10)62533-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masszi A, Di Ciano C, Sirokmany G, Arthur WT, Rotstein OD, Wang J, McCulloch CA, Rosivall L, Mucsi I, Kapus A. Central role for Rho in TGF-beta1-induced alpha-smooth muscle actin expression during epithelial-mesenchymal transition. Am J Physiol. 2003;284:F911–F924. doi: 10.1152/ajprenal.00183.2002. [DOI] [PubMed] [Google Scholar]
- Li JH, Zhu HJ, Huang XR, Lai KN, Johnson RJ, Lan HY. Smad7 inhibits fibrotic effect of TGF-beta on renal tubular epithelial cells by blocking smad2 activation. J Am Soc Nephrol. 2002;13:1464–1472. doi: 10.1097/01.asn.0000014252.37680.e4. [DOI] [PubMed] [Google Scholar]
- Li Y, Yang J, Dai C, Wu C, Liu Y. Role for integrin-linked kinase in mediating tubular epithelial to mesenchymal transition and renal interstitial fibrogenesis. J Clin Invest. 2003;112:503–516. doi: 10.1172/JCI17913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhowmick NA, Ghiassi M, Bakin A, Aakre M, Lundquist CA, Engel ME, Arteaga CL, Moses HL. Transforming growth factor-beta1 mediates epithelial to mesenchymal transdifferentiation through a RhoA-dependent mechanism. Mol Biol Cell. 2001;12:27–36. doi: 10.1091/mbc.12.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vleminckx K, Vakaet L, Jr, Mareel M, Fiers W, van Roy F. Genetic manipulation of E-cadherin expression by epithelial tumor cells reveals an invasion suppressor role. Cell. 1991;66:107–119. doi: 10.1016/0092-8674(91)90143-m. [DOI] [PubMed] [Google Scholar]
- Hay ED, Zuk A. Transformations between epithelium and mesenchyme: normal, pathological, and experimentally induced. Am J Kidney Dis. 1995;26:678–690. doi: 10.1016/0272-6386(95)90610-x. [DOI] [PubMed] [Google Scholar]
- Cano A, Perez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, Portillo F, Nieto MA. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000;2:76–83. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- Auersperg N, Pan J, Grove BD, Peterson T, Fisher J, Maines-Bandiera S, Somasiri A, Roskelley CD. E-cadherin induces mesenchymal-to-epithelial transition in human ovarian surface epithelium. Proc Natl Acad Sci USA. 1999;96:6249–6254. doi: 10.1073/pnas.96.11.6249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisberg M, Kalluri R. The role of epithelial-to-mesenchymal transition in renal fibrosis. J Mol Med. 2004;82:175–181. doi: 10.1007/s00109-003-0517-9. [DOI] [PubMed] [Google Scholar]
- Saika S, Kono-Saika S, Ohnishi Y, Sato M, Muragaki Y, Ooshima A, Flanders KC, Yoo J, Anzano M, Liu CY, Kao WW, Roberts AB. Smad3 signaling is required for epithelial-mesenchymal transition of lens epithelium after injury. Am J Pathol. 2004;164:651–663. doi: 10.1016/S0002-9440(10)63153-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens J, von Kries JP, Kuhl M, Bruhn L, Wedlich D, Grosschedl R, Birchmeier W. Functional interaction of beta-catenin with the transcription factor LEF-1. Nature. 1996;382:638–642. doi: 10.1038/382638a0. [DOI] [PubMed] [Google Scholar]
- Eger A, Stockinger A, Schaffhauser B, Beug H, Foisner R. Epithelial mesenchymal transition by c-Fos estrogen receptor activation involves nuclear translocation of beta-catenin and upregulation of beta-catenin/lymphoid enhancer binding factor-1 transcriptional activity. J Cell Biol. 2000;148:173–188. doi: 10.1083/jcb.148.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockinger A, Eger A, Wolf J, Beug H, Foisner R. E-cadherin regulates cell growth by modulating proliferation-dependent beta-catenin transcriptional activity. J Cell Biol. 2001;154:1185–1196. doi: 10.1083/jcb.200104036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eger A, Stockinger A, Park J, Langkopf E, Mikula M, Gotzmann J, Mikulits W, Beug H, Foisner R. beta-Catenin and TGFbeta signalling cooperate to maintain a mesenchymal phenotype after FosER-induced epithelial to mesenchymal transition. Oncogene. 2004;23:2672–2680. doi: 10.1038/sj.onc.1207416. [DOI] [PubMed] [Google Scholar]
- Tian YC, Fraser D, Attisano L, Phillips AO. TGF-beta1-mediated alterations of renal proximal tubular epithelial cell phenotype. Am J Physiol. 2003;285:F130–F142. doi: 10.1152/ajprenal.00408.2002. [DOI] [PubMed] [Google Scholar]
- Labbe E, Letamendia A, Attisano L. Association of Smads with lymphoid enhancer binding factor 1/T cell-specific factor mediates cooperative signaling by the transforming growth factor-beta and wnt pathways. Proc Natl Acad Sci USA. 2000;97:8358–8363. doi: 10.1073/pnas.150152697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian YC, Phillips AO. Interaction between the transforming growth factor-beta type II receptor/Smad pathway and beta-catenin during transforming growth factor-beta1-mediated adherens junction disassembly. Am J Pathol. 2002;160:1619–1628. doi: 10.1016/s0002-9440(10)61109-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilosi M, Poletti V, Zamo A, Lestani M, Montagna L, Piccoli P, Pedron S, Bertaso M, Scarpa A, Murer B, Cancellieri A, Maestro R, Semenzato G, Doglioni C. Aberrant Wnt/beta-catenin pathway activation in idiopathic pulmonary fibrosis. Am J Pathol. 2003;162:1495–1502. doi: 10.1016/s0002-9440(10)64282-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann B, Gelos M, Siedow A, Hanski ML, Gratchev A, Ilyas M, Bodmer WF, Moyer MP, Riecken EO, Buhr HJ, Hanski C. Target genes of beta-catenin-T cell-factor/lymphoid-enhancer-factor signaling in human colorectal carcinomas. Proc Natl Acad Sci USA. 1999;96:1603–1608. doi: 10.1073/pnas.96.4.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradl D, Kuhl M, Wedlich D. The Wnt/Wg signal transducer beta-catenin controls fibronectin expression. Mol Cell Biol. 1999;19:5576–5587. doi: 10.1128/mcb.19.8.5576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilles C, Polette M, Mestdagt M, Nawrocki-Raby B, Ruggeri P, Birembaut P, Foidart JM. Transactivation of vimentin by beta-catenin in human breast cancer cells. Cancer Res. 2003;63:2658–2664. [PubMed] [Google Scholar]
- Letamendia A, Labbe E, Attisano L. Transcriptional regulation by Smads: crosstalk between the TGF-beta and Wnt pathways. J Bone Joint Surg Am. 2001;83:S31–S39. [PubMed] [Google Scholar]
- Di Ciano-Oliveira C, Sirokmany G, Szaszi K, Arthur WT, Masszi A, Peterson M, Rotstein OD, Kapus A. Hyperosmotic stress activates Rho: differential involvement in Rho kinase-dependent MLC phosphorylation and NKCC activation. Am J Physiol. 2003;285:C555–C566. doi: 10.1152/ajpcell.00086.2003. [DOI] [PubMed] [Google Scholar]
- Garat C, Van Putten V, Refaat ZA, Dessev C, Han SY, Nemenoff RA. Induction of smooth muscle alpha-actin in vascular smooth muscle cells by arginine vasopressin is mediated by c-Jun amino-terminal kinases and p38 mitogen-activated protein kinase. J Biol Chem. 2000;275:22537–22543. doi: 10.1074/jbc.M003000200. [DOI] [PubMed] [Google Scholar]
- Sadot E, Simcha I, Shtutman M, Ben-Ze’ev A, Geiger B. Inhibition of beta-catenin-mediated transactivation by cadherin derivatives. Proc Natl Acad Sci USA. 1998;95:15339–15344. doi: 10.1073/pnas.95.26.15339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetsu O, McCormick F. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398:422–426. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- Gumbiner BM. Regulation of cadherin adhesive activity. J Cell Biol. 2000;148:399–404. doi: 10.1083/jcb.148.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisberg M, Hanai J, Sugimoto H, Mammoto T, Charytan D, Strutz F, Kalluri R. BMP-7 counteracts TGF-beta1-induced epithelial-to-mesenchymal transition and reverses chronic renal injury. Nat Med. 2003;9:964–968. doi: 10.1038/nm888. [DOI] [PubMed] [Google Scholar]
- Manotham K, Tanaka T, Matsumoto M, Ohse T, Inagi R, Miyata T, Kurokawa K, Fujita T, Ingelfinger JR, Nangaku M. Transdifferentiation of cultured tubular cells induced by hypoxia. Kidney Int. 2004;65:871–880. doi: 10.1111/j.1523-1755.2004.00461.x. [DOI] [PubMed] [Google Scholar]
- Surendran K, McCaul SP, Simon TC. A role for Wnt-4 in renal fibrosis. Am J Physiol. 2002;282:F431–F441. doi: 10.1152/ajprenal.0009.2001. [DOI] [PubMed] [Google Scholar]
- Strutz F, Zeisberg M, Ziyadeh FN, Yang CQ, Kalluri R, Muller GA, Neilson EG. Role of basic fibroblast growth factor-2 in epithelial-mesenchymal transformation. Kidney Int. 2002;61:1714–1728. doi: 10.1046/j.1523-1755.2002.00333.x. [DOI] [PubMed] [Google Scholar]
- Masur SK, Dewal HS, Dinh TT, Erenburg I, Petridou S. Myofibroblasts differentiate from fibroblasts when plated at low density. Proc Natl Acad Sci USA. 1996;93:4219–4223. doi: 10.1073/pnas.93.9.4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuk A, Matlin KS, Hay ED. Type I collagen gel induces Madin-Darby canine kidney cells to become fusiform in shape and lose apical-basal polarity. J Cell Biol. 1989;108:903–919. doi: 10.1083/jcb.108.3.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichert M, Muller T, Hunziker W. The PDZ domains of zonula occludens-1 induce an epithelial to mesenchymal transition of Madin-Darby canine kidney I cells: evidence for a role of beta-catenin/Tcf/Lef signaling. J Biol Chem. 2000;275:9492–9500. doi: 10.1074/jbc.275.13.9492. [DOI] [PubMed] [Google Scholar]
- Lu Z, Ghosh S, Wang Z, Hunter T. Downregulation of caveolin-1 function by EGF leads to the loss of E-cadherin, increased transcriptional activity of beta-catenin, and enhanced tumor cell invasion. Cancer Cell. 2003;4:499–515. doi: 10.1016/s1535-6108(03)00304-0. [DOI] [PubMed] [Google Scholar]
- Oloumi A, McPhee T, Dedhar S. Regulation of E-cadherin expression and beta-catenin/Tcf transcriptional activity by the integrin-linked kinase. Biochim Biophys Acta. 2004;1691:1–15. doi: 10.1016/j.bbamcr.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Kim K, Lu Z, Hay ED. Direct evidence for a role of beta-catenin/LEF-1 signaling pathway in induction of EMT. Cell Biol Int. 2002;26:463–476. doi: 10.1006/cbir.2002.0901. [DOI] [PubMed] [Google Scholar]
- Bakin AV, Tomlinson AK, Bhowmick NA, Moses HL, Arteaga CL. Phosphatidylinositol 3-kinase function is required for transforming growth factor beta-mediated epithelial to mesenchymal transition and cell migration. J Biol Chem. 2000;275:36803–36810. doi: 10.1074/jbc.M005912200. [DOI] [PubMed] [Google Scholar]
- Delcommenne M, Tan C, Gray V, Rue L, Woodgett J, Dedhar S. Phosphoinositide-3-OH kinase-dependent regulation of glycogen synthase kinase 3 and protein kinase B/AKT by the integrin-linked kinase. Proc Natl Acad Sci USA. 1998;95:11211–11216. doi: 10.1073/pnas.95.19.11211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich C, Scherwat J, Faust D, Oesch F. Subcellular localization of beta-catenin is regulated by cell density. Biochem Biophys Res Commun. 2002;292:195–199. doi: 10.1006/bbrc.2002.6625. [DOI] [PubMed] [Google Scholar]
- Conacci-Sorrell M, Simcha I, Ben-Yedidia T, Blechman J, Savagner P, Ben-Ze’ev A. Autoregulation of E-cadherin expression by cadherin-cadherin interactions: the roles of beta-catenin signaling, Slug, and MAPK. J Cell Biol. 2003;163:847–857. doi: 10.1083/jcb.200308162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawshad A, Hay ED. TGFbeta3 signaling activates transcription of the LEF1 gene to induce epithelial mesenchymal transformation during mouse palate development. J Cell Biol. 2003;163:1291–1301. doi: 10.1083/jcb.200306024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryeom SW, Paul D, Goodenough DA. Truncation mutants of the tight junction protein ZO-1 disrupt corneal epithelial cell morphology. Mol Biol Cell. 2000;11:1687–1696. doi: 10.1091/mbc.11.5.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M, Tsunoda T, Seiki M, Nakamura Y, Furukawa Y. Identification of membrane-type matrix metalloproteinase-1 as a target of the beta-catenin/Tcf4 complex in human colorectal cancers. Oncogene. 2002;21:5861–5867. doi: 10.1038/sj.onc.1205755. [DOI] [PubMed] [Google Scholar]
- Serini G, Bochaton-Piallat ML, Ropraz P, Geinoz A, Borsi L, Zardi L, Gabbiani G. The fibronectin domain ED-A is crucial for myofibroblastic phenotype induction by transforming growth factor-beta1. J Cell Biol. 1998;142:873–881. doi: 10.1083/jcb.142.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S, Lovett DH. Gelatinase A (MMP-2) is necessary and sufficient for renal tubular cell epithelial-mesenchymal transformation. Am J Pathol. 2003;162:1937–1949. doi: 10.1016/S0002-9440(10)64327-1. [DOI] [PMC free article] [PubMed] [Google Scholar]