Abstract
We used expression of the ganglioside A2B5 to isolate putative myelin progenitor cells from adult human central nervous system parenchyma and compared their phenotypic (expression of myelin lineage molecules) and functional (survival, proliferation) properties with mature oligodendrocytes (OLGs) derived from the same adult material and with A2B5+ cells isolated from human fetal brain. A2B5+ cells represented 3 to 5% of the total cell suspension derived from adult specimens. Results of protein (immunostaining) and RNA (polymerase chain reaction) analyses indicated that the adult A2B5+ cells were more committed to the OLG lineage than their fetal counterparts while continuing to retain properties of progenitor cells compared to the postmitotic mature OLGs. Although the adult A2B5+ cells retained the capacity to divide, albeit at a reduced rate compared to fetal A2B5+ cells, they showed reduced survival and process outgrowth compared not only to fetal cells but also to mature OLGs. Our results confirm the presence of progenitor cells committed to the OLG lineage in the adult human central nervous system but raise the issues regarding the intrinsic capacity of these cells to contribute to the process of remyelination that may be necessary during demyelinating diseases.
Recovery from relapses in multiple sclerosis (MS) is attributed at least in part to the extent of remyelination observed in early MS lesions.1–4 Many actively demyelinating MS lesions contain preserved numbers of mature, presumably previously myelinating oligodendrocytes (OLGs).2,5 Complete remyelination in experimental animal models of demyelination is ascribed to recruitment of progenitor cells that differentiate into myelinating OLGs.6–12 Cells bearing specific surface markers [O4, galactocerebroside (GalC), chondroitin sulfate proteoglycan (NG2), proteolipid protein (PLP), platelet-derived growth factor receptor-α (PDGF-Rα)] or morphological features characteristic of myelin progenitor cells, are described in MS lesions. Phenotypic profiles of such cells include: O4+ GalC−NG2−,13 PDGF-Rα+ GalC−,14,15 PDGF-Rα+, NG2+,16 and PLP+ pre-OLGs.17 These cells do not however appear to be overrepresented nor are they consistently proliferating in the lesions. In late chronic MS lesions, where remyelination is less widespread than in early lesions, the number of both progenitors and mature OLGs is decreased compared to early active lesions.
Roy and colleagues,18 reported that progenitor cells (ganglioside A2B5-positive) comprised 3 to 5% of the total cell population derived from human adult temporal lobe resections and could undergo a limited number of cell divisions in vitro. OLGs could be derived only from the A2B5+ population, although this population could also give rise to neurons and astrocytes.20–22 The purpose of our current study was to compare phenotypic (myelin lineage gene expression) and functional (in vitro survival, cell cycling, and process formation) properties of the adult A2B5+ cells with those of mature OLGs isolated from the same tissue samples. We further compared the properties of adult A2B5+ cells with their fetal counterparts providing insight for the observation that, when compared to fetal A2B5+ cells, transplanted adult A2B5+ cells produce fewer surviving cells but among those a higher proportion contribute to myelination of the dismyelinated mouse mutants, shiverer mice.19–21 Our results demonstrate that the adult A2B5+ cells retain properties of progenitor cells when compared to postmitotic mature human OLGs and are more committed to the OLG lineage than their fetal counterparts. Adult A2B5+ cells, however, have more limited cell survival even in a growth factor-supplemented environment, compared not only to fetal ones but also to the mature OLGs.
Materials and Methods
Adult Human Cell Preparations
Surgical resections were obtained from the treatment of nontumor-related intractable epilepsy, in accordance with the guidelines set by the Biomedical Ethics Unit of McGill University. White matter was obtained from regions distant from the main electrically active site. As previously described,22 tissue specimens were dissociated enzymatically with trypsin and DNase I, and mechanically. Cells were separated on a linear 30% Percoll density gradient (Pharmacia LKB, Baie D’Urfé, Canada). The derived cell population was put in minimal essential culture medium (MEM), containing 5% fetal calf serum, penicillin, streptomycin, glutamine, and glucose (complete MEM; all from Life Technologies, Burlington, Canada). The next day, the less adherent cells, containing the OLG/progenitor cell pool, were removed by pipetting, washed, and incubated on ice with anti-A2B5 IgM antibody (Ab) purified from the original hybridoma supernatant (gift from T. Kennedy, McGill University). Cells were then washed in magnetic cell sorting (MACS) buffer [phosphate-buffered saline (PBS), 2 mmol/L ethylenediaminetetraacetic acid, 5% fetal calf serum] and incubated with the microbead-conjugated rat anti-mouse IgM antibody (Miltenyi Biotech, Auburn, CA) following the manufacturer’s instructions. Cells were washed and separated using positive selection columns (Miltenyi Biotech). The A2B5+ cell fraction was then resuspended in Dulbecco’s modified Eagle’s medium (DMEM)-F12 N1 supplement (Sigma) media containing basic-fibroblast growth factor (bFGF) (20 ng/ml; Sigma, Oakville, Canada) and thyroid hormone, T3 (2 ng/ml, Sigma) and plated onto fibronectin/laminin-coated chamber slides (105 cells/well). Additional studies were done with cells grown in complete MEM medium or in DMEM/F12 added with PDGF (20 ng/ml, Sigma) and neurotrophic factor 3 (NT-3) (20 ng/ml, Sigma) and bFGF. The A2B5− fraction, obtained from adult human brain cells after sorting, was replated. The next day the floating cells, highly enriched for mature OLGs, were harvested, resuspended in complete MEM medium, and then plated on poly-l-lysine-coated chamber slides (105 cells/well).
Fetal Human Cell Preparation
Human fetal central nervous system (CNS) tissue obtained from 14- to 23-week-old embryos was provided either by Cindy Goodyer (Montreal Children’s Hospital) or by the Human Fetal Tissue Repository (Albert Einstein College of Medicine, Bronx, NY). The studies were approved by their and our institutional review boards. Brain tissue diced into ≤1-mm fragments was incubated with 0.25% trypsin and 25 μg/ml of DNase I and washed through a 132-μm nylon mesh and centrifuged. The cells were then washed and sorted using the MACS system as described for the adult cells. Both the positive and the negative fractions of the sorted cells were grown in the same media used for the adult cells for 1 to 2 weeks. Additional studies were done with cells grown in complete MEM medium and/or adding PDGF and NT-3 with bFGF as growth factors.
Immunostaining
Fetal and adult A2B5+ cells and mature OLGs were fixed at different time points with 4% paraformaldehyde and blocked in HHG (1 mmol/L HEPES buffer, 2% horse serum, and 10% goat serum in Hanks’ balanced salt solution). Cells were stained with mouse monoclonal antibodies (mAbs) specific for A2B5, CD68, NG2, O4, MAG (myelin-associated glycoprotein), MBP (myelin basic protein), GalC, βTub-III (β-tubulin-III), and the rabbit polyclonal Ab for GFAP (glial fibrillary acidic protein) (see Table 1 for details). Then cells were washed and incubated with the appropriate secondary Cy3 (Bio-Source International, Camarillo, CA) or fluorescein isothiocyanate-conjugated (Jackson Laboratories, Bar Harbor, ME) Abs. For cytoplasmic antigens, cells were permeabilized either with cold acetone (for GFAP and CD68), cold methanol (for MAG and MBP), or Triton X100 (for βTub-III). Corresponding isotypes were used as controls. For double staining Abs recognizing cytoplasmic antigens were applied first. Cultures were counterstained with Hoechst dye (33258) to label nuclei.
Table 1.
List of the Antibodies Used to Detect Mature and Immature Neural Cells
| Antigen/antibody | Cell type | Type | Species | Clonality | Source |
|---|---|---|---|---|---|
| A2B5 | Progenitor cells | IgM | Mouse | Mono | Hybridoma gift from T. Kennedy |
| GFAP | Astrocytes | IgG | Mouse | Poly | DAKO, Mississauga, Canada |
| MAG | Myelinating OLGs | IgG1 | Mouse | Mono | Chemicon Int., Temecula, CA |
| MBP | Myelinating OLGs | IgG1 | Mouse | Mono | Sternberger-Meyer, Jarrettsville, MD |
| GalC | Mature OLGs | IgG1 | Mouse | Mono | Supernatant gift from V.W. Yong, U. Calgary |
| O4 | OLGs progenitor | IgM | Mouse | Mono | Chemicon Int., Temecula, CA |
| β-Tub-III | Neurons | IgG2b | Mouse | Mono | Sigma, Oakville, Canada |
| MAP-2 | Mature neurons | IgG1 | Mouse | Mono | Sigma, Oakville, Canada |
| NG-2 | Progenitor cells | IgG2a | Mouse | Mono | Pharmingen, Mississauga, Canada |
| CD68 | Microglia | IgG1 | Mouse | Mono | DAKO, Mississauga, Canada |
Fetal A2B5+ cells were also analyzed by flow cytometry immediately after magnetic bead separation. For these studies both the A2B5+ and A2B5− fractions were stained with Abs specific for A2B5, NG2, O4, GalC, MAG, and CD68 (the latter two required permeabilization with saponin23), followed by incubation with secondary Abs conjugated to phycoerythrin or fluorescein isothiocyanate fluorochromes. Cells were acquired on a FACS Scan flow cytometer (Becton Dickinson, San Jose, CA) and analyzed with FlowJo (Treestar, Ashland, OR) software.
RNA-Based Characterization of A2B5+ Cells
Total RNA was isolated from adult and fetal unfractionated brain samples (ie, before cell dissociation), as well as from adult and fetal A2B5+ cell fractions immediately after magnetic bead separation, and from mature adult OLGs using Trizol reagent with an additional DNase step to eliminate genomic DNA. First-strand cDNA synthesis was performed using 3 μg of total RNA and mouse Maloney leukemia virus reverse transcriptase (Invitrogen, Burlington, Canada) according to the manufacturer’s instructions. One-tenth of the resulting cDNA was then used for each polymerase chain reaction (PCR) reaction. The primers (Table 2) were designed using OLIGO software Primer 3, Whitehead Ins., Cambridge according to sequences available on the GenBank (National Biosciences, Plymouth, MN) and synthesized by Invitrogen. PCRs were performed under the following conditions: primer (1 μmol/L of each), 2.5 U Taq polymerase (Invitrogen), 1.5 mmol/L MgCl2, and 0.2 mmol/L dNTP with the following reaction-cycling parameters: 94°C, 60 seconds; 60°C, 60 seconds; 72°C, 60 seconds for 25 cycles. Twenty-five cycles were used because it was in the linear range of PCR amplification and showed no saturation.
Table 2.
Primer Pairs Used to Amplify Myelin Lineage Gene Products
| Primer | Forward 5′ to 3′ | Reverse 5′ to 3′ | bp |
|---|---|---|---|
| PDGF-Rα | GAAGCTGTCAACCTGCATGA | CTTCCTTAGCACGGATCAGC | 187 |
| MOG | CTGGAGTGCTGGTTCTCCTC | TTGCCCTGCTAGTCTTCGAT | 257 |
| MBP | CTGGGCAGCTGTTAGAGTCC | CTGTGGTTTGGAAACGAGGT | 275 |
| PLP | GGCGACTACAAGACCACCAT | AGGTGGTCCAGGTGTTGAAG | 247* |
| NG2 | ACTGGCTAGGGGTGTCAATG | TCCTCAAGGTCCTGCTGAGT | 271 |
| Actin | GAGGGCATACCCCTCGTAGAT | CAGAAGGATTCCTATGTGGGC | 378 |
Predicted PCR product of 247 bp (PLP full length)-142 bp (DM-20).
Cell Cycling and Survival Studies
For the proliferation studies, right after isolation, cells were maintained for 48 hours in the culture media described above supplemented with 10 mmol/L BrdU (Sigma) and cultured for 5 to 6 additional days. The cells were immunostained for neural markers as described above and then permeabilized with ice-cold methanol:acetic alcohol (1:1), washed, incubated in HCl 2 N subsequently neutralized in 0.1 mol/L borate buffer (pH 8.5), and washed. Cells were blocked with HHG containing 0.3% Triton X-100 before being labeled with anti-BrdU antibody (Sigma). Cells were labeled with the appropriate secondary antibody and mounted with gelvatol.
Results
Comparison of Phenotypic Properties of Adult A2B5+ Cells with Primary Adult OLGs and Fetal A2B5+ Cells
For all our studies A2B5-expressing cells were derived using an immunomagnetic bead-based cell separation system. We refer to the cell fraction derived from the column as the “A2B5+ cell fraction” and the flow through cell fraction as the “A2B5− cell fraction.” The adult A2B5+ cell fraction comprised on average 2 to 3% (and never >5%) of the total adult CNS cell. The fetal A2B5+ cell fraction accounted for 30 to 60% of the total cell population, with the proportions being higher with younger specimens [60% for 14- to 16-week-old specimens; 30% for 22- to 24-week-old specimens (Table 3)]. Immunohistochemical studies were performed after 5 to 7 days in culture, allowing time for the A2B5 cells to firmly adhere to the culture dish.
Table 3.
Comparison of Immunophenotypic Properties of Adult and Fetal A2B5+ Cell Fractions
| Markers | Fetal A2B5+ (% of total cells) | Adult A2B5+ (% of total cells) | OLGs (% of total cells) |
|---|---|---|---|
| A2B5 in total CNS | 30–60%* | 3–5% | N/A |
| A2B5 ex vivo (Flow cytometry) | >90% | N/D | N/A |
| A2B5 in vitro§ | 80–85% | 80–85% | 10% |
| GalC+§ | None | 20% | >95% |
| MAG+§ | None | 20% | >95% |
| NG2+§ | >10%* | 5–10% | None |
| O4+§ | >10%* | Not detected | None |
| β-Tub III+§ | <5%* | None | None |
| GFAP+§ | 20–40%* | <5% | <5% |
| A2B5− CD68+§ | ∼10%* | ∼10% | <5% |
| A2B5+ CD68+§ | None | None | None |
Numbers vary with fetal age.
Measured by immunostaining.
N/D, Not done; N/A, not applicable.
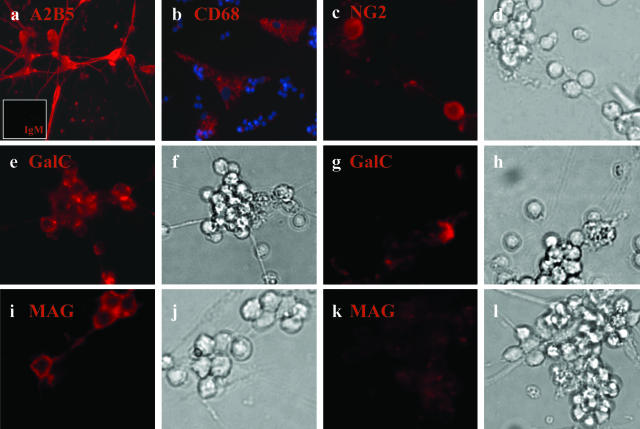
Over 80 to 85% of the A2B5 bead-selected adult CNS cells expressed detectable levels of A2B5 (Figure 1a and Table 3). The large majority of the non-A2B5 cells in this fraction were CD68+, a microglial cell marker (Figure 1b). Between 5 and 10% of the total A2B5+-selected population expressed NG2 (Figure 1, c and d), these were morphologically distinct from the CD68+ cells (Figure 1b). Astrocytes (GFAP+ cells) were rare (<2 to 3% of the total cell number). At this time in culture, we did not detect O4 or βTub-III+ cells. Among the A2B5+ cells 20 to 25% of them expressed GalC and MAG with some apparent concentration within aggregates (Figure 1; e to l).
Figure 1.
Immunocytochemical characterization of the A2B5+ cell fraction derived from the adult human CNS. After magnetic bead selection, A2B5+ cells derived from human adult CNS were grown in DMEM F12 supplemented with N1, bFGF, and T3, kept in culture for 7 days, and then immunostained for different neural markers; a: A2B5+ cells (red) with a small inset for the isotype control IgM (red); b: CD68+ cells (red) with nuclei double-stained with Hoechst (blue); c and d: NG2+ cells (red) and corresponding bright field; e–h: GalC+ cells (red) with corresponding bright fields; i–l: MAG+ cells (red) and bright fields. Both for GalC and MAG two different fields are shown to represent cell aggregates that are mostly positive (e and i) or mostly negative (g and k) for GalC and MAG. Original magnifications: ×40 (a, c–l); ×20 (b).
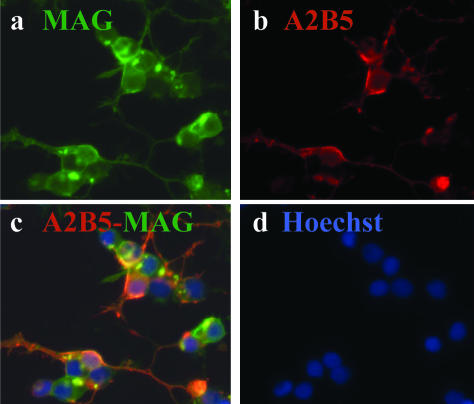
Primary adult OLGs were derived from the A2B5-negative fraction of the same adult brain. This fraction initially consisted of 35% microglia (CD68+), 60% mature OLGs (GalC/MAG+), and a small portion (∼5%) of astrocytes (GFAP+) and neurons (βTub-III+). In the nonadherent fraction of these cells, 95% expressed OLG markers (Figure 2; a to d) whereas OLGs were absent in the adherent fraction. In the OLG-enriched fraction up to 10% of the cells co-expressed A2B5 and MAG (Figure 2, b and c) suggesting either that during the maturation from progenitor to OLG, cells continue to bear A2B5 on their surface or that the cells are dedifferentiating.
Figure 2.
Immunocytochemical characterization of adult OLGs indicating that a fraction of these cells co-express A2B5. After 1 week in culture in MEM supplemented with 5% fetal calf serum, OLGs were co-stained for the myelin antigen MAG and for A2B5. Each staining is shown individually as well as the overlay: a: MAG+ cells (green); b: A2B5+ cells in red; c: overlay indicating co-staining; d: nuclear stain Hoechst for the same field. Original magnifications, ×40.
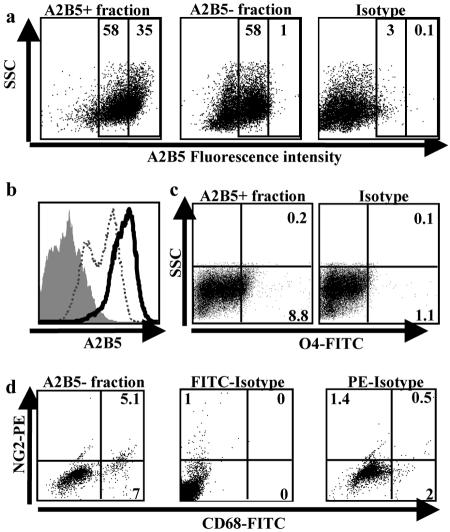
Fetal A2B5+ cells were analyzed by flow cytometry within 24 hours of cell isolation. Our results indicated that >90% of the positively selected cells expressed A2B5 above the isotype control (Figure 3, a and b; dot plot and histogram). A variable proportion of these cells expressed low levels of O4 (<10% in the sample illustrated in Figure 3c). Although the fetal A2B5− fraction still contained a significant proportion of cells with detectable levels of A2B5 (Figure 3, a and b; dot plot and histogram) above the isotype control, the fluorescence intensity was significantly lower than that found on A2B5+ cells in the positively selected fraction. None of these cells expressed O4 (data not shown), suggesting that cells of the OLG lineage are derived from A2B5+ high-expressing progenitors. The non-A2B5 cells consisted of microglia, astrocytes, and neurons; no mature OLGs could be detected (data not shown). Using double labeling for NG2 and CD68, we observed that NG2 was expressed on a fraction of the microglial cells present in the A2B5− fraction (Figure 3d).
Figure 3.
Flow cytometry analysis of fetal-derived A2B5+ and A2B5− cell fractions. Both positive and negative fractions were immunostained immediately after magnetic bead selection. a: Dot plot represents the same data shown in the histogram: A2B5 positively selected cell fraction, A2B5 negatively selected cell fraction, and isotype control. Numbers indicate percentage of cells with medium- or high-fluorescence intensity. b: Histogram of A2B5 expression. Histogram compares levels of A2B5 immunoreactivity (fluorescence intensity) of the positively selected cell fraction (black line) with the negatively selected cell fraction (dotted line) and the isotype control (solid gray). c: A2B5+ cell fraction immunostained for expression of O4. Numbers indicate percentage of positive cells. No O4 cells were found in the negative cell fraction (data not shown). d: A2B5− cell fraction double-stained for CD68 and NG2. Isotype controls (IgG1 for CD68 and IgG2a for NG2) are also shown.
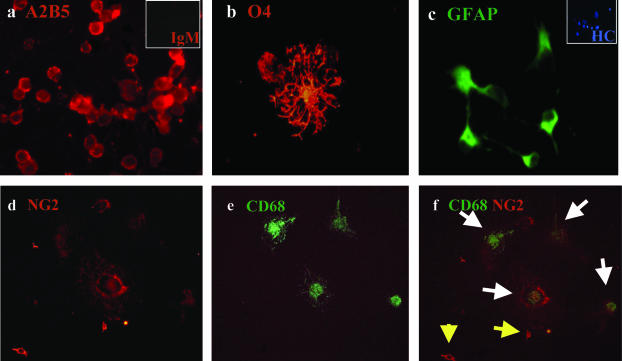
Immunocytochemistry analysis of the fetal A2B5+ fraction after 5 to 7 days in culture showed a high percentage of A2B5+ cells (>80%; Figure 4a and Table 3). O4+ cells could also be detected (Figure 4b), becoming more abundant in longer duration cultures. At least 20% (and up to 40%) of the A2B5+ cells expressed GFAP (Figure 4c). Among these A2B5+ cells we could detect NG2+/CD68− cells (Figure 4; d to f) displaying bipolar morphology typical of progenitor cells. No cells were found expressing mature OLG markers GalC or MAG.
Figure 4.
Immunocytochemical characterization of the A2B5+ cell fraction derived from the fetal CNS. After the magnetic bead selection, the A2B5+ cells derived from human fetal CNS were kept in culture in DMEM F12 supplemented with N1, bFGF, and T3 for 1 week and then immunostained for different neural markers: a: A2B5+ cells (red) with the small inset for the isotype control IgM (red); b: O4+ cell (red); c: GFAP+ cells (green) with small inset for Hoechst staining; d–f: double staining for NG2 (d, red) and CD68 (e, green). Overlay is shown in f. White arrows, cells co-expressing NG2 and CD68; yellow arrows, NG2 single positive cells. Original magnifications, ×40.
Comparison of Myelin Lineage-Related Gene Expression in Adult A2B5+ Cells with Primary Adult OLGs and Fetal A2B5+ Cells
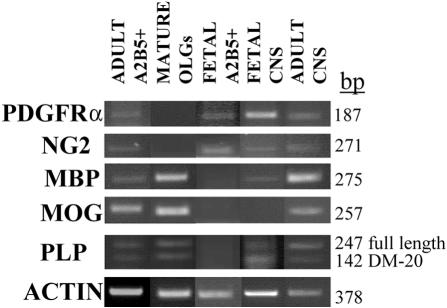
RNA extracted from both adult and fetal A2B5+ fractions and from adult cultured OLGs was analyzed by reverse transcriptase-PCR for multiple genes (Figure 5). RNA encoding for immature cell markers (PDGF-Rα and NG2) could be detected in adult and fetal A2B5+ cells but not in mature OLGs, whereas they were also detected in total tissue sample from both the adult and fetal CNS, confirming the presence of progenitor cells in CNS throughout life. MBP and MOG transcripts were expressed in the adult A2B5+ cells and mature OLGs but not in the fetal A2B5+ cells, suggesting that only adult A2B5+ cells had committed to the OLG lineage. The adult total CNS expressed more myelin mRNAs than the fetal counterpart. Both adult A2B5+ cells and mature OLGs (and adult CNS tissue), expressed the full length isoform of PLP more strongly than the DM20 isoform. Neither isoform was detected in the fetal A2B5+ cells. The DM20 isoform was the most apparent one in fetal CNS tissue.
Figure 5.
Gene expression of OLG lineage markers in adult and fetal A2B5+ cells, mature OLGs, and whole CNS tissues. Total RNA was extracted, transcribed into cDNA, and then amplified by PCR, as described in the Material and Methods section, using primers listed in Table 1. The genes tested were PDGF-Rα, NG2, MBP, MOG, PLP, and actin as control. RNA from adult A2B5+ cells, mature OLGs, fetal A2B5+ cells, fetal CNS, and adult CNS were analyzed. The size of PCR amplicons is indicated on the right.
Comparison of in Vitro Proliferative and Survival (Functional) Properties of Adult A2B5+ Cells with Primary Adult OLGs and Fetal A2B5+ Cells
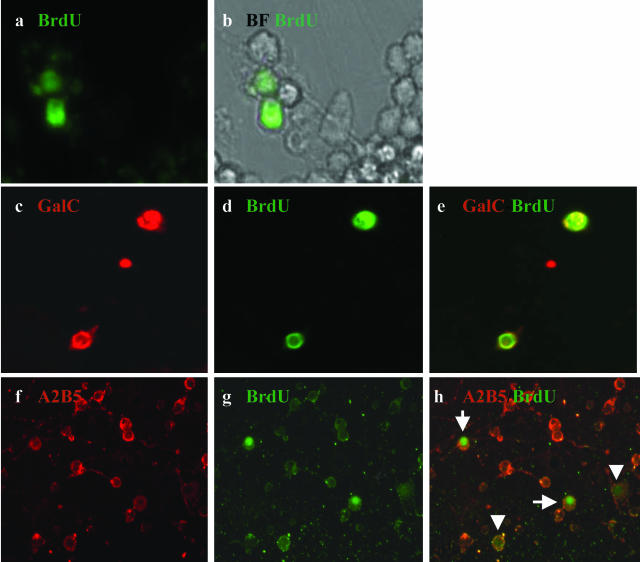
Among adult A2B5+ cells, a relatively small proportion (∼10 to 15%) was found to incorporate BrdU during the labeling period (Figure 6, a and b). A small proportion of the BrdU+ cells were also GalC+ (Figure 6; c to e) suggesting proliferation of progenitor cells committed to the OLG lineage. During the second week of culture, 20 to 30% of the adult A2B5+ fraction continued to express GalC and MAG whereas GFAP+ cells remained rare. The cells did not express extensive processes and did not survive for more than 2 weeks under our culture conditions or when supplemented with PDGF/NT3/bFGF or in the medium used for mature OLGs. No BrdU uptake was detected in pure cultures of mature OLGs. These cells continued to extend processes that were already apparent even after 1 week in culture in either complete MEM (Figure 2b) or bFGF/T3-supplemented medium (data not shown) and continued to survive for many weeks in culture. Approximately 30% of fetal A2B5+ cells incorporated BrdU (Figure 6; f to h). Some of the BrdU+ cells showed an intense staining whereas others showed a much weaker staining (greater dilution) suggesting differential rates of proliferation. The cultures could be maintained under these conditions for more than 5 weeks without significant cell death.
Figure 6.
Comparison of BrdU labeling of adult and fetal A2B5+ cell fractions. A2B5+ cells from adult and fetal material were incubated for 48 hours with BrdU and then cultured for 5 to 6 additional days before staining for BrdU and neural markers. a and b: Adult A2B5+-positive cell fraction stained for BrdU (a) with the respective bright field (b). c–e: Double staining of adult A2B5+ cells for BrdU and GalC. Single stains are shown (c, GalC; d, BrdU) as well as overlay of the two stains (e), indicating co-localization. f–h: Fetal-derived A2B5+ cells co-stained for A2B5 (f) and BrdU (g); overlay of stains is shown in h. Arrowheads show weakly positive cells; long arrows show cells that are strongly positive. Original magnifications: ×60 (a–e); ×40 (f–h).
Discussion
The current study was designed to delineate the properties of CNS parenchyma-derived progenitor cells implicated in the remyelination or lack thereof that occurs in MS. We used expression of the ganglioside A2B5 as a marker to identify and isolate such progenitor cells. Our study confirms the existence of such cells in the temporal lobe in numbers that closely coincide with those described by Roy and colleagues.18
Our phenotypic analysis of the adult A2B5+ cells indicate that such cells are further along the OLG lineage than their fetal counterparts but remain less differentiated in vitro than the mature OLGs isolated from the adult human CNS. This comparative analysis was performed after cells were maintained in culture for 5 to 7 days under relatively basal culture conditions supplemented with growth factors (bFGF and T3) that are considered to promote cell survival and proliferation rather than differentiation.24,25 A small proportion (5 to 10%) of the adult A2B5+ cell population morphologically distinct from the CD68 microglia expressed NG2+, which is found later in the OLG progenitor lineage than is A2B5, showing the persistence of the progenitor characteristic on these cells. This population was smaller than in fetal A2B5+ cells (10 to 20% of cells). Up to 20% of adult A2B5+ cells expressed OLG lineage markers (GalC, MAG) as determined by immunostaining; such expression was rarely seen in the fetal A2B5+ cells. Unlike the fetal A2B5+ population, the adult A2B5+ cells did not co-express, in our culture conditions, neuron (β-Tub-III) or astrocyte (GFAP) markers, again indicating that these A2B5+ cells were less pluripotential than their fetal counterparts. A small proportion of the mature OLGs expressed A2B5. Whether this represents persistence of A2B5 expression or dedifferentiation is not resolved; a similar cell phenotype has been described in the rodent system.26,27 The A2B5+ cells in the mature OLG fraction did extend processes similar to the non-A2B5-expressing cells.
The flow cytometric analysis conducted on the fetal A2B5+ cells indicated that the immunomagnetic bead procedure was selecting for cells with relatively high expression of A2B5 and that only this population contained cells co-expressing OLG lineage markers (O4). Our overall results derived from samples ranging from 14- to 23-week-old tissues, denote that the younger samples had fewer neurons and astrocytes, indicating the increased pluripotential capacity earlier in development. Our RNA-based analyses also support that the adult A2B5+ cells were further along in the myelin lineage than their fetal counterparts but yet retained progenitor properties compared to mature OLGs. We compared RNA collected immediately after magnetic bead isolation from adult and fetal materials with RNA derived from mature OLGs cultured for 5 to 7 days. Our results indicated that the adult and fetal A2B5+ cells, but not the mature OLGs, expressed the immature lineage genes NG2 and PDGF-Rα. Conversely, the adult A2B5+ cells expressed the mature myelin-associated genes MOG and MBP, that were also present in mature OLGs but not in the fetal A2B5+ cells. Moreover, the fetal A2B5+ cells did not express any isoform of PLP whereas the adult A2B5+ cells and mature OLGs expressed both the DM20 and the full length isoforms, with the later being dominant.
The functional properties (survival and differentiation) of the adult A2B5+ cells also indicated distinct properties that would reflect on their capacity to contribute to the process of remyelination. The adult A2B5+ cells retained the capacity to enter the cell cycle as assessed by BrdU uptake, but at a significantly lower level than the fetal A2B5+ cells. These observations are consistent with rodent studies showing that adult myelin progenitor cells have reduced proliferation and survival capacity than progenitors derived from the more immature brain28 and that with age the OLG precursors showed a declining ability to repopulate tissues.29 We did not observe any BrdU incorporation by the mature OLGs including the previously mentioned small proportion that expressed A2B5, consistent with the postmitotic characteristic of these cells. Unlike the adult A2B5 cells, these human mature OLGs survive indefinitely in culture. The long-term survival of the mature human OLGs in vitro would seem to contrast with experience using similar rodent-derived cells. Most available rodent data have been generated using OLGs that have been matured in vitro.28,30–32 Previously we33 reported, consistent with results of Armstrong and colleagues34 that immediately on isolation a proportion of cells within the mature OLG fraction express early myelin lineage markers (A007) and not mature markers (MBP). Armstrong and colleagues34 speculated this may represent dedifferentiation of the cells during the isolation procedure. The A2B5-expressing mature OLGs observed in this study showed sustained survival and process outgrowth.
Although adult A2B5+ cells continued to maintain their OLG lineage properties in vitro, they showed limited process outgrowth and survival under the relatively basal culture conditions used, compared both to the fetal cells and the mature OLGs. Our results indicated that fetal A2B5+ cells retained their relatively undifferentiated properties when maintained throughout several weeks under our culture conditions. In this regard, Dietrich and colleagues35 found that enhanced numbers of OLG lineage cells could be generated from fetal A2B5+ cells by adding bFGF followed by PDGF and T3. In addition, Wilson and colleagues36 using A2B5+ cells derived from fetal human spinal cord found that cells supplemented with PDGF, NT3, and GGF for 10 days expressed the highest proportion of myelin markers compared to an array of other culture conditions. Using the same supplements, we were unable to prolong the in vitro survival of adult A2B5+ cells significantly.
Our central findings, as summarized in Table 4, that the adult A2B5+ cells are committed to the OLG lineage but have limited survival and differentiation properties provide further insights into the remyelination process in adult humans. Our current results are consistent with those of Windrem and colleagues20 showing that although adult human A2B5+ cells transplanted into shiverer mice had reduced survival (as shown by significantly lower number of engrafted cells) compared to fetal A2B5+ cells, those that did survive could produce more myelin than their fetal counterparts. As mentioned, cells that are committed to the OLG lineage, including pre-OLGs, can be detected within MS lesions. The apparent failure of such cells to mediate successful remyelination could reflect a number of factors including receiving and responding to signals needed to engage the myelination program and/or integrity of axons. Our study further raises the issues of the intrinsic remyelination capacity of the putative myelin progenitor cells identified to date in the adult CNS parenchyma and how this capacity compares to previously myelinating cells that survive in the MS lesion. The challenge remains to identify the optimal cells and the environment they require to enhance the process of remyelination in MS.
Table 4.
Comparison of Phenotypic and Functional Properties of Adult A2B5+ Cells with Fetal A2B5+ Cells and with Mature Adult OLGs
| Fetal A2B5 | Adult A2B5 | Adult OLGs | |
|---|---|---|---|
| Commitment to OLG lineage | − | +++ | N/A* |
| Multipotency | +++ | − | N/A* |
| Proliferation rate | +++ | + | − |
| Survival in vitro | +++ | + | +++ |
| Process extension | ++ | + | +++ |
N/A, Not applicable.
Acknowledgments
We thank the institutional review board-approved Human Fetal Tissue Repository at Albert Einstein College of Medicine, Bronx, NY, for providing human fetal CNS tissue.
Footnotes
Address reprint requests to Jack P. Antel, Neuroimmunology Unit, Room 111, Montreal Neurological Institute, McGill University, 3801 University St., Montreal, Quebec H3A 2B4 Canada. E-mail: jack.antel@mcgill.ca.
Supported by the Foundation of the Multiple Sclerosis Society of Canada and the Canadian Institutes of Health Research (senior postdoctoral fellowship to N.A.).
References
- Prineas JW, Kwon EE, Goldenberg PZ, Ilyas AA, Quarles RH, Benjamins JA, Sprinkle TJ. Multiple sclerosis. Oligodendrocyte proliferation and differentiation in fresh lesions. Lab Invest. 1989;61:489–503. [PubMed] [Google Scholar]
- Raine CS, Wu E. Multiple sclerosis—remyelination in acute lesions. J Neuropathol Exp Neurol. 1993;52:199–204. [PubMed] [Google Scholar]
- Lucchinetti C, Bruck W, Parisi J, Scheithauer B, Rodriguez M, Lassmann H. Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann Neurol. 2000;47:707–717. doi: 10.1002/1531-8249(200006)47:6<707::aid-ana3>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Prineas JW, Barnard RO, Kwon EE, Sharer LR, Cho ES. Multiple sclerosis: remyelination of nascent lesions. Ann Neurol. 1993;33:137–151. doi: 10.1002/ana.410330203. [DOI] [PubMed] [Google Scholar]
- Lucchinetti C, Bruck W, Parisi J, Scheithauer B, Rodriguez M, Lassmann H. Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann Neurol. 2000;47:707–717. doi: 10.1002/1531-8249(200006)47:6<707::aid-ana3>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Rodriguez M, Pierce ML, Thiemann RL. Immunoglobulins stimulate central nervous system remyelination—electron-microscopic and morphometric analysis of proliferating cells. Lab Invest. 1991;64:358–370. [PubMed] [Google Scholar]
- Carroll WM, Jennings AR. Early recruitment of oligodendrocyte precursors in CNS demyelination. Brain. 1994;117:563–578. doi: 10.1093/brain/117.3.563. [DOI] [PubMed] [Google Scholar]
- Gensert JM, Goldman JE. Endogenous progenitors remyelinate demyelinated axons in the adult CNS. Neuron. 1997;19:197–203. doi: 10.1016/s0896-6273(00)80359-1. [DOI] [PubMed] [Google Scholar]
- Franklin RJM, Gilson JM, Blakemore WF. Local recruitment of remyelinating cells in the repair of demyelination in the central nervous system. J Neurosci Res. 1997;50:337–344. doi: 10.1002/(SICI)1097-4547(19971015)50:2<337::AID-JNR21>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Keirstead HS, Levine JM, Blakemore WF. Response of the oligodendrocyte progenitor cell population (defined by NG2 labelling) to demyelination of the adult spinal cord. Glia. 1998;22:161–170. [PubMed] [Google Scholar]
- Redwine JM, Armstrong RC. In vivo proliferation of oligodendrocyte progenitors expressing PDGF alpha R during early remyelination. J Neurobiol. 1998;37:413–428. doi: 10.1002/(sici)1097-4695(19981115)37:3<413::aid-neu7>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Di Bello IC, Dawson MR, Levine JM, Reynolds R. Generation of oligodendroglial progenitors in acute inflammatory demyelinating lesions of the rat brain stem is associated with demyelination rather than inflammation. J Neurocytol. 1999;28:365–381. doi: 10.1023/a:1007069815302. [DOI] [PubMed] [Google Scholar]
- Wolswijk G. Chronic stage multiple sclerosis lesions contain a relatively quiescent population of oligodendrocyte precursor cells. J Neurosci. 1998;18:601–609. doi: 10.1523/JNEUROSCI.18-02-00601.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scolding N, Franklin R, Stevens S, Heldin CH, Compston A, Newcombe J. Oligodendrocyte progenitors are present in the normal adult human CNS and in the lesions of multiple sclerosis. Brain. 1998;121:2221–2228. doi: 10.1093/brain/121.12.2221. [DOI] [PubMed] [Google Scholar]
- Maeda Y, Solanky M, Menonna J, Chapin J, Li WP, Dowling P. Platelet-derived growth factor-alpha receptor-positive oligodendroglia are frequent in multiple sclerosis lesions. Ann Neurol. 2001;49:776–785. doi: 10.1002/ana.1015. [DOI] [PubMed] [Google Scholar]
- Chang A, Nishiyama A, Peterson J, Prineas J, Trapp BD. NG2-positive oligodendrocyte progenitor cells in adult human brain and multiple sclerosis lesions. J Neurosci. 2000;20:6404–6412. doi: 10.1523/JNEUROSCI.20-17-06404.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A, Tourtellotte WW, Rudick R, Trapp BD. Premyelinating oligodendrocytes in chronic lesions of multiple sclerosis. N Engl J Med. 2002;346:165–173. doi: 10.1056/NEJMoa010994. [DOI] [PubMed] [Google Scholar]
- Roy NS, Wang S, Harrison-Restelli C, Benraiss A, Fraser RAR, Gravel M, Braun PE, Goldman SA. Identification, isolation, and promoter-defined separation of mitotic oligodendrocyte progenitor cells from the adult human subcortical white matter. J Neurosci. 1999;19:9986–9995. doi: 10.1523/JNEUROSCI.19-22-09986.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windrem MS, Roy NS, Wang J, Nunes M, Benraiss A, Goodman R, McKhann GM, Goldman SA. Progenitor cells derived from the adult human subcortical white matter disperse and differentiate as oligodendrocytes within demyelinated lesions of the rat brain. J Neurosci Res. 2002;69:966–975. doi: 10.1002/jnr.10397. [DOI] [PubMed] [Google Scholar]
- Windrem MS, Nunes MC, Rashbaum WK, Schwartz TH, Goodman RA, McKhann G, Roy NS, Goldman SA. Fetal and adult human oligodendrocyte progenitor cell isolates myelinate the congenitally dysmyelinated brain. Nat Med. 2004;10:93–97. doi: 10.1038/nm974. [DOI] [PubMed] [Google Scholar]
- Nunes MC, Roy NS, Keyoung HM, Goodman RR, McKhann G, Jiang L, Kang J, Nedergaard M, Goldman SA. Identification and isolation of multipotential neural progenitor cells from the subcortical white matter of the adult human brain. Nat Med. 2003;9:439–447. doi: 10.1038/nm837. [DOI] [PubMed] [Google Scholar]
- Yong VW, Antel JP. Culture of glial cells from human brain biopsies. Sedorf S, Richardson A, editors. New York: Plenum Publishing Corp.; Protocols for Neural Cell Culture. 1992:pp 81–96. [Google Scholar]
- Sergent-Tanguy S, Chagneau C, Neveu I, Naveilhan P. Fluorescent activated cell sorting (FACS): a rapid and reliable method to estimate the number of neurons in a mixed population. J Neurosci Methods. 2003;129:73–79. doi: 10.1016/s0165-0270(03)00210-3. [DOI] [PubMed] [Google Scholar]
- Jones SA, Jolson DM, Cuta KK, Mariash CN, Anderson GW. Triiodothyronine is a survival factor for developing oligodendrocytes. Mol Cell Endocrinol. 2003;199:49–60. doi: 10.1016/s0303-7207(02)00296-4. [DOI] [PubMed] [Google Scholar]
- Goddard DR, Berry M, Butt AM. In vivo actions of fibroblast growth factor-2 and insulin-like growth factor-I on oligodendrocyte development and myelination in the central nervous system. J Neurosci Res. 1999;57:74–85. doi: 10.1002/(SICI)1097-4547(19990701)57:1<74::AID-JNR8>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Li GL, Crang AJ, Rundle JL, Blakemore WF. Oligodendrocyte progenitor cells in the adult rat CNS express myelin oligodendrocyte glycoprotein (MOG). Brain Pathol. 2002;12:463–471. doi: 10.1111/j.1750-3639.2002.tb00463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood PM, Bunge RP. The origin of remyelinating cells in the adult central nervous system—the role of the mature oligodendrocyte. Glia. 1991;4:225–232. doi: 10.1002/glia.440040214. [DOI] [PubMed] [Google Scholar]
- Shi J, Marinovich A, Barres BA. Purification and characterization of adult oligodendrocyte precursor cells from the rat optic nerve. J Neurosci. 1998;18:4627–4636. doi: 10.1523/JNEUROSCI.18-12-04627.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chari DM, Crang AJ, Blakemore WF. Decline in rate of colonization of oligodendrocyte progenitor cell (OPC)-depleted tissue by adult OPCs with age. J Neuropathol Exp Neurol. 2003;62:908–916. doi: 10.1093/jnen/62.9.908. [DOI] [PubMed] [Google Scholar]
- Cammer W. Effects of TNFalpha on immature and mature oligodendrocytes and their progenitors in vitro. Brain Res. 2000;864:213–219. doi: 10.1016/s0006-8993(00)02178-8. [DOI] [PubMed] [Google Scholar]
- Baud O, Greene AE, Li J, Wang H, Volpe JJ, Rosenberg PA. Glutathione peroxidase-catalase cooperativity is required for resistance to hydrogen peroxide by mature rat oligodendrocytes. J Neurosci. 2004;24:1531–1540. doi: 10.1523/JNEUROSCI.3989-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholas RS, Stevens S, Wing MG, Compston DA. Microglia-derived IGF-2 prevents TNFalpha induced death of mature oligodendrocytes in vitro. J Neuroimmunol. 2002;124:36–44. doi: 10.1016/s0165-5728(02)00011-5. [DOI] [PubMed] [Google Scholar]
- Prabhakar S, D’Souza S, Antel JP, McLaurin J, Schipper HM, Wang E. Phenotypic and cell cycle properties of human oligodendrocytes in vitro. Brain Res. 1995;672:159–169. doi: 10.1016/0006-8993(94)01377-t. [DOI] [PubMed] [Google Scholar]
- Armstrong RC, Dorn HH, Kufta CV, Friedman E, Duboisdalcq ME. Preoligodendrocytes from adult human CNS. J Neurosci. 1992;12:1538–1547. doi: 10.1523/JNEUROSCI.12-04-01538.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich J, Noble M, Mayer-Proschel M. Characterization of A2B5+ glial precursor cells from cryopreserved human fetal brain progenitor cells. Glia. 2002;40:65–77. doi: 10.1002/glia.10116. [DOI] [PubMed] [Google Scholar]
- Wilson HC, Onischke C, Raine CS. Human oligodendrocyte precursor cells in vitro: phenotypic analysis and differential response to growth factors. Glia. 2003;44:153–165. doi: 10.1002/glia.10280. [DOI] [PubMed] [Google Scholar]