Abstract
The presence of gastrin and cholecystokinin-2 (CCK2) receptors in human preneoplastic and neoplastic gastrointestinal lesions suggests a role in cancer development. In addition to the growth-promoting action of gastrin, recently a role of the cholecystokinin-2/gastrin receptor (CCK2-R) modulating cellular morphology in cultured epithelial cells has been shown. Here, we have investigated in transgenic (ElasCCK2) mice whether ectopic expression of human CCK2-R in the exocrine pancreas affected epithelial differentiation. Cellular localization of cell adhesion molecules, differentiation markers, and transcription factors was determined using immunofluorescence techniques. Before tumor formation, expression and subcellular localization of proteins of the adherens junction complex, differentiation markers, and transcription factors were altered in ElasCCK2 exocrine pancreas, indicating an evolution from an acinar to a ductal phenotype. Loss of cell polarity, defective secretion, and loss of intercellular adhesion in acini of ElasCCK2 mice was confirmed by ultrastructural analysis. Finally, expression of the transgene in mice treated with the carcinogen azaserine resulted in enhanced size of preneoplastic lesions as well as an increased degree of acinar-ductal transdifferentiation. Thus, these data represent the first evidence for the CCK2-R modulating intercellular adhesion and cell fate in vivo and show that these alterations may contribute to enhanced sensitivity of ElasCCK2 pancreas to chemical carcinogens.
The gastrointestinal peptide hormone gastrin is a potent stimulant of gastric acid secretion from the parietal cells of the stomach and an important growth/differentiation factor for the gastric mucosa. The role of gastrin as a key regulator in the gastric mucosa has been confirmed in mice knockouts for its gene1–3 or for the gene of its receptor, the gastrin/cholecystokinin-2 receptor (CCK2-R).4–6 Indeed, mice knockouts for the CCK2-R display an important decrease in gastric acid secretion, atrophy of the oxyntic mucosa, and an altered gastric differentiation. In correlation with these data, gastrin-deficient mice present a reduced proliferation of parietal and enterochromaffin-like (ECL) cells reversed with the perfusion of amidated gastrin. Besides gastric secretion and proliferation, gastrin has been implicated in a number of effects in several cellular systems, such as stress fiber assembly, anti-apoptosis, branching morphogenesis, and migration.7–14 The CCK2-R mediating the effects of amidated gastrin is a G protein-coupled receptor that is coupled to a variety of transduction pathways including phospholipase C, c-src-like tyrosine kinases, p125FAK, phosphatidylinositol 3-kinase (PI 3-kinase), and the MAPKs, as well as epidermal growth factor (EGF) receptor transactivation.9,15–18
Several lines of evidence support the role of both CCK2-R and amidated gastrin in pancreatic cancer formation: 1) up-regulation of gastrin and its receptor in human pancreatic adenocarcinoma suggests autocrine or paracrine stimulation.19–21 2) A novel splice variant of the CCK2-R has recently been identified, which has constitutive activity and is exclusively expressed in certain human colon and pancreatic cancers, although controversy exists about its role in cancer formation.22–24 3) Importantly, long-term studies with ElasCCK2 transgenic mice expressing functional human CCK2-R under the control of the elastase promoter in pancreatic exocrine cells have described the appearance of hyperplasia and tumors in the exocrine pancreas of transgenic mice.25
So far, the action of gastrin/CCK2-R has been essentially evaluated with respect to proliferation, but direct effects on cell differentiation and cell adhesion in the living organism (in vivo) have been elusive. However, coinciding with cell growth deregulation, malignant transformation results in the loss of epithelial differentiation, a crucial event in the development of cancer and a putative target of cancer therapy.26 Importantly, dedifferentiation and invasiveness appear after loss of intercellular adhesion, as observed after loss or modulation of adhesion molecules.27,28
In the exocrine pancreas, the functional units, acini, are composed of highly differentiated, polarized acinar cells, tightly bound together by characteristic adhesive cell junctions at many points of cell-cell and cell-matrix contact. Besides their role in tissue architecture and stability, adhesive cell junctions are further important for other cellular functions including epithelial differentiation, cell polarity, control of cell motility, apoptosis, and anchorage dependence of cell proliferation.29 Because intercellular adhesion can be regulated by extrinsic signals arising from the classical growth factor receptors, it is believed, that during malignant development, activation of signaling pathways, possibly those activating proliferation, might result in the modifications of cell-cell and cell-substrate adhesion.30
We and others have recently demonstrated, that prolonged activation of the CCK2-R by amidated gastrin results in modifications of epithelial differentiation including altered morphology, loss of cell-cell adhesion, as well as enhanced motility in nontransformed epithelial cells.12–14
We therefore, hypothesized that overexpression of CCK2-R may lead to modification of cellular differentiation and result in morphological alterations and development of preneoplastic lesions. Thereby, the CCK2-R may play a role in initiating events leading to cancer. To further investigate whether this contributed to sensitize the pancreas to a carcinogen, we have examined the effects of CCK2-R overexpression on cellular morphology, adhesion, and differentiation in the exocrine pancreas of transgenic ElasCCK2 mice, alone and in conjunction with the chemical carcinogen, azaserine.
Materials and Methods
Animals
ElasCCK2 transgenic mice have previously been described.31 Control mice were littermates of the same genetic background as that of ElasCCK2 mice. Homozygous ElasCCK2 and control mice of 6 months age were used for the chemical carcinogenesis protocol. The animal care committee of the IFR31 approved all procedures.
Chemical Carcinogenesis
Twenty-five ElasCCK2 mice and 25 control mice received intraperitoneally 30 mg of azaserine (Sigma)/kg body weight, once a week during 5 consecutive weeks, after preliminary verification that this dose did not suppress growth of the animals or induce lethality during the injection period.32 Untreated and treated mice were similarly housed, their body weight and health examined weekly, and after 2 months were killed.
Genotypic and Histological Analysis of Transgenic Pancreas
Mice were genotyped by polymerase chain reaction analysis as previously described.25 For histology, homozygous ElasCCK2 and control mice of 4 months of age were killed, the pancreas isolated and fixed in Bouin’s solution or in 4% paraformaldehyde (Sigma) in phosphate-buffered saline (PBS) at 4°C overnight, washed 1 hour in PBS at room temperature, dehydrated in graded alcohols, embedded in paraffin (Histowachs, Reichert-Jung), sectioned at 7 μm, and stained with hematoxylin and eosin (H&E) or toluidine blue and analyzed on a light microscope (Nikon E400, Melville, NY).25 Three pancreas per genotype and experiment were evaluated. Acinar lesions were quantified by morphometric analysis of H&E-stained sections. Sections were selected at various levels of the paraffin block to be representative of the entire embedded pancreas. Measurements of the areas of pancreatic sections and lesions (pseudoductular complexes and adenomas) were performed on a minimum of 10 sections using Biocom VisioLab 2000 system. Statistical analysis was performed using Student’s t-test or Mann-Whitney test.
Immunohistochemistry
For cryosectioning, organs were washed twice in PBS, embedded in Tissue-Tek OCT (Histolab, Göteborg, Sweden), frozen on dry ice, and transferred in liquid nitrogen. Cryostat sections of 15-μm thickness were prepared as previously described and heated in a microwave oven (only for N-cadherin antibody).33 After blocking in Tris-buffered saline-Ca2+ (10 mmol/L Tris, pH 7.6, 150 mmol/L NaCl, and 1 mmol/L CaCl2) supplemented with 5% skim milk for 30 minutes at room temperature, incubation with first antibody in Tris-buffered saline-Ca2+ supplemented with 5% skim milk was performed overnight at 4°C. Secondary antibodies, fluorescein isothiocyanate- or Cy3-coupled, were added for 60 minutes each. After washing twice, nuclei were stained for 5 minutes with 4′,6-diamino-2-phenylindole (Sigma) (1:2000) in PBS. Slides were mounted in PBS/glycerol or Mowiol (Calbiochem) and analyzed on a Nikon E400 fluorescence microscope with a Sony DXC 950 camera and Visiolab 2000 software. Images were further assembled using Adobe Photoshop software. For semiquantitative comparisons, identical volumes of antibody mix were used for all samples and identical exposure times taken. Three pancreata per genotype and experiment were evaluated.
Immunoreagents
The following primary antibodies were used: anti-amylase (1:5000, Calbiochem), anti-glucagon (1:500, DAKO), anti-keratin 8 (TROMA-1), anti-K18 (TROMA-2), anti-K19 (TROMA-3) (1:20; gift from Dr. R. Kemler, Department of Molecular Embryology, Max-Planck Institute of Immunobiology, Freiburg, Germany), anti-E-cadherin, DECMA-1 (1:1600, Sigma), anti-β-catenin (1:125, C19220; Signal Transduction Laboratories), anti-α-catenin (1:1000, C2081; Sigma) or (1:125, Signal Transduction Laboratories), anti-N-cadherin (1:100, Santa Cruz, Santa Cruz, CA) or (1:125, Signal Transduction Laboratories, anti ZO-1 (1:20, Ozyme/Chemicon), anti-PTF1-p48 (1:30, gift from Dr. F.X. Real, Unitat de Biologia Cellular i Molecular, Institute Municipal d’Investigacio Medica, Barcelona, Spain), and anti-Mist1 (1:250, gift from Dr. S.F. Konieczny, Department of Biological Sciences, Purdue University, West Lafayette, IN). Alexa Fluor 488 phalloidin (A-12379, Molecular Probes, Eugene, OR) was used to stain F-actin according to the manufacturer’s instructions. Secondary antibodies coupled to CY-3, fluorescein isothiocyanate, or Alexa Fluor 488 were purchased from Sigma, Jackson Immunoresearch Laboratories, and Molecular Probes and used at 1 μg/ml.
Transmission Electron Microscopic Analysis
Specimens were fixed overnight in 2.5% glutaraldehyde and 2% paraformaldehyde in 0.5 mol/L sodium cacodylate buffer, pH 7.2, at 4°C, rinsed in sodium cacodylate buffer, and postfixed in 0.5% OsO4 and 1% potassium ferrocyanide in 0.5 mol/L sodium cacodylate buffer at 4°C for 1 hour. After rinsing in water, specimens were treated en bloc with 0.5% uranyl acetate. Specimens were further dehydrated in graded alcohol/propylenoxid solutions, followed by propylenoxid/epoxy resin infiltration and embedded in epoxy resin. Ultrathin sections were cut on a LKB ultratome. The sections were placed on gold grids, stained with uranyl acetate and lead citrate, and examined in a Jeol 100 CX electron microscope. Three pancreata per genotype and experiment were evaluated.
Western Blot Analysis
For study of β-catenin expression, acini were prepared using collagenase as previously described.31 Cytoplasmic proteins were prepared after Nonidet P-40 lysis and total proteins after lysis in Laemmli buffer of acini. Sixty μg of soluble cytoplasmic proteins or 50 μg of total protein extracts were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and blotted on polyvinylidene difluoride membrane (Perkin-Elmer). Western blot analysis was performed using a mouse anti-β-catenin antibody (1:500, Transduction Laboratories). Proteins were visualized using 125I-protein A as previously described.14 To demonstrate equivalent protein loading, membranes were reprobed with a mouse anti-GAPDH antibody (1:500, Chemicon International Inc.).
Results
Disrupted Acinar Cell Compartment in the Pancreas of ElasCCK2 Mice
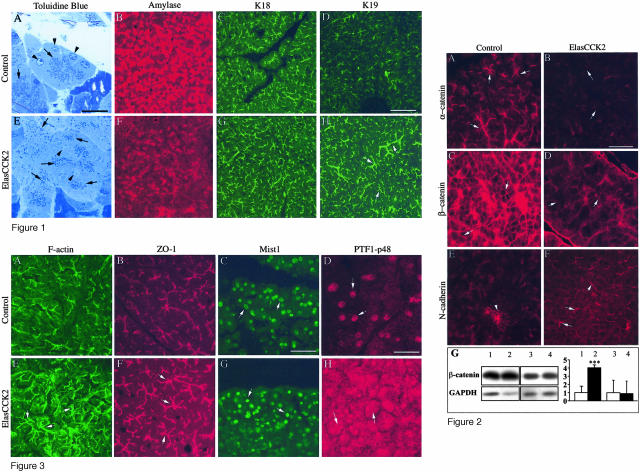
In the ElasCCK2 mouse model, cell-type-specific expression of the human CCK2-R in acinar cells is achieved through the regulatory region of the elastase1 promoter, and has been shown to induce pancreatic hyperplasia, pancreatic lesions, and cancer after 18 months age (15%).25,31 On the other hand, we have recently demonstrated in vitro that prolonged activation of the CCK2-R results in the modification of crucial processes of epithelial differentiation such as cellular morphology, adhesion, and motility of epithelial cells.14 To investigate the type of cellular processes affected by CCK2-R expression in vivo that might be part of a multistage process of carcinogenesis, pancreata of transgenic mice and control mice of 4 months age were examined microscopically. At this stage neither control nor ElasCCK2 pancreata contained tumors. H&E- and toluidine blue-stained sections through exocrine pancreata of control mice revealed the typical high inter- and intracellular organization of polarized acinar cells into acini (Figure 1A). However, pancreatic sections of ElasCCK2 mice showed that exocrine compartments displayed significant structural defects. In ElasCCK2 mice, whole acini of ElasCCK2 pancreata were significantly bigger in size, and consisted of disorganized, enlarged, sometimes binuclear cells (Figure 1E). The nucleus was no longer restricted to the basal position when compared to that of controls (Figure 1A), but found throughout the cell bodies (Figure 1E) and the secretory granules (zymogen granules) no longer restricted to apical cell compartment as in the controls (Figure 1A) but found throughout the cells (Figure 1E), suggesting, that these cells had lost cell polarity. Thus, acinar cell disorganization in ElasCCK2 mice was accompanied by cellular and nuclear dysplasia. To examine whether acinar final differentiation was altered in ElasCCK2 mice, the pancreas was processed for cryosections and immunofluorescence tests with antibodies against the acinar digestive enzyme, amylase, and pancreatic cytokeratins K8, K18, and K19. The exocrine pancreas of ElasCCK2 mice displayed greatly reduced expression of amylase (Figure 1F) as compared to the strong expression in the exocrine pancreas of control mice (Figure 1B). Decrease of amylase expression in transgenic exocrine pancreas was accompanied by enhanced expression of cytokeratin K19, specific for duct and centroacinar cells. Notably, staining of the latter revealed enlarged structures, namely distended acinis, lumens, and ducts in the ElasCCK2 pancreas (Figure 1, H and D). No differences between control and ElasCCK2 pancreases were observed in the staining patterns of acinar-specific cytokeratins K8 and K18 (Figure 1, C and G; and data not shown). Taken together, this set of data presents evidence for morphological and structural alterations of the acinar phenotype in the exocrine pancreas resulting from expression of the CCK2-R.
Figure 1.
Histological analysis of exocrine pancreas from adult wild-type and ElasCCK2 mice. A and E: Toluidine blue stainings of semithin epoxy resin sections of control (A) and ElasCCK2 (E) mice showing individual exocrine acini. Acinar cells in ElasCCK2 mice are disorganized, enlarged, often contain more than one nucleus, which, in addition, is not restricted basally (arrowheads) and zymogen granules are not localized apically but distributed throughout the cell (arrows). B–H: Cryostat sections from pancreas of wild-type (B, C, D) and ElasCCK2 mice (F, G, H) are stained with antibodies against acinar differentiation markers as indicated. Amylase and K18, keratin 18, for acinar cells, K19, keratin 19, for ductal cells. Strongly reduced expression of amylase as well as enhanced expression of keratin 19 is detected in ElasCCK2 exocrine pancreas (compare F to B and H to D). Note enlarged K19-positive duct-like structures (arrows) in ElasCCK2 exocrine pancreas (H). Scale bars: 20 μm (A, E); 120 μm (B–D, F–H).
Alterations in Cellular Adhesion and Cellular Organization in ElasCCK2 Mice
The adherens junction complex has been implicated as an important mediator of cellular polarity through maintaining cell-cell interactions and stabilizing the cytoskeleton.34 Recently, molecules involved in cell adhesion and junction formation have been identified as putative targets of the CCK2-R signal transduction pathway in cultured epithelial cells.14 Thus, to examine the underlying cause of acinar cell disorganization and cellular hypertrophy in ElasCCK2 mice, proteins involved in adherens and tight junction formation were analyzed on pancreas cryosections using antibodies against adherens junction proteins α-catenin, β-catenin, E-cadherin, and N-cadherin, the tight junction protein, ZO-1, and the actin cytoskeleton.
α-Catenin and β-catenin were no longer localized to the apical cell border of acini (Figure 2, A and C), and their staining was greatly diminished or diffusely localized around the basal membrane domain (Figure 2, B and D). Moreover, although expression of β-catenin was not modified in the acini of ElasCCK2 mice compared to controls, it was detected at significantly higher levels (approximately fourfold increase) in the soluble cytoplasmic proteins as compared with acini of control mice (Figure 2G). This shows that the subcellular localization of adherens junction proteins was clearly affected in the exocrine pancreas of ElasCCK2 mice. Notably, although E-cadherin staining was only slightly reduced in acini of transgenics (data not shown), staining of its homolog, N-cadherin, which is typically expressed during pancreatic embryogenesis and at cell contacts of Langerhans islets cells (Figure 2E), was augmented at cell-cell borders in the acinar compartment of ElasCCK2 mice (Figure 2F). Additionally, enhanced phalloidin staining in the ElasCCK2 exocrine pancreas cytoskeleton revealed an increased filamental (F-) actin network (Figure 3, A and E). Because F-actin normally localizes to the apico-lateral membrane of acinar, but especially of centroacinar and duct cells, delineating the cortical side of the lumens in the center of acinar cell clusters in the controls, this staining pattern is indicative of actin cytoskeleton reorganization and an enlargement of F-actin-positive structures, such as distended acini, lumens, and ducts. Analysis of the tight junction protein ZO-1 showed that its localization to presumptive apical borders of acinar cells is maintained in ElasCCK2 pancreas. Similarly to F-actin staining, ZO-1 staining was also enhanced in ElasCCK2 pancreas and revealed expanded structures (ducts), decorating distended lumens in the center of acinar cell clusters (Figure 3, B and D). In summary, these data show modifications in the localization of adherens junction and cytoskeleton proteins in cells expressing the CCK2 receptor. Thus, although tight junctions continue to form, the altered catenin expression pattern may account for the eventual loss of the acinar cell phenotype in ElasCCK2 mice.
Figure 2.
Immunohistochemistry for adhesion proteins on cryostat sections of wild-type and ElasCCK2 pancreas using antibodies against adherens junction proteins. A and B: α-Catenin, C and D: β-catenin, and E and F: N-cadherin. In ElasCCK2 exocrine pancreas expression of α-catenin and β-catenin at cell-cell contacts is strongly reduced (arrows in B and D) and some residual β-catenin diffusively localized (arrows in D), whereas N-cadherin staining at acinar cell-cell contacts is increased in ElasCCK2 exocrine pancreas (arrows in F). The arrowhead in E points to cell borders between pancreatic islet cells, which normally are strongly positive for N-cadherin in wild-type as well as in ElasCCK2 mice. G: Western blot analysis from soluble (lanes 1 and 2) and total (lanes 3 and 4) proteins of control (lanes 1 and 3) and of ElasCCK2 acini (lanes 2 and 4) and corresponding densitometric analysis of data. Western blot studies were performed using an anti-β-catenin antibody and, to normalize to equivalent protein amount, the blot was reprobed with an anti-GAPDH antibody. One Western blot experiment representative of four is shown. Results of quantification represent means ± SEM of four independent experiments and are expressed as fold control with control expression set to one. White bars, control; black bars, ElasCCK2. ***, Significance at P < 0.001 determined using the Student’s t-test. Scale bar, 60 μm.
Figure 3.
Immunohistochemistry of cell junctional components and acinar transcription factors on cryostat sections of wild-type and ElasCCK2 pancreas. A–H: Sections are stained with Alexa 488-phalloidin to detect F-actin (A, E), and with antibodies against ZO-1 (B, F), Mist1 (C, G), and PTF1-p48 (D, H). In ElasCCK2 exocrine pancreas, enhanced F-actin staining (compare E with A) as well as enlarged F-actin- and ZO-1-positive duct-like structures (arrows in E and F) are observed. Although Mist1 expression and localization is unaffected in ElasCCK2 mice (arrows in G), a striking amount of PTF1-p48 is seen in the cytoplasm, besides its normal nuclear localization, in ElasCCK2 exocrine pancreas (arrows in H). Note nuclear dysplasia in ElasCCK2 pancreas (G). Scale bars: 60 μm (A–C, E–G); 30 μm (D, H).
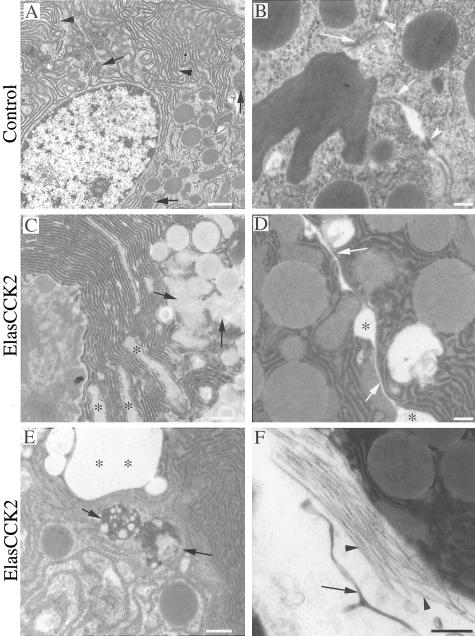
Acinar Cells in ElasCCK2 Mice Display Ultrastructural Defects Reflecting Abnormal Intracellular Organization
The disorganization of the exocrine pancreas in ElasCCK2 mice as well as modulation of cytoskeleton and adherens junction proteins suggested that intracellular organization and physiological functions of acinar cells might be altered. To investigate this possibility we analyzed acini of control and ElasCCK2 mice by transmission electron microscopy. Electron micrographs confirmed alterations of the acinar phenotype of ElasCCK2 mice revealing numerous ultrastructural defects in the intracellular organization. 1) Adherens junctions, easily identified as characteristic electron-dense plaques along the apical cell-cell border in controls (Figure 4B), were absent at acinar cell contacts of transgenics (Figure 4D) and lumens and intercellular spaces were dramatically enlarged (Figure 4D). 2) Additionally, the basal lamina was detached from acini (Figure 4F) in ElasCCK2 exocrine pancreas, altogether indicating that cell-cell adhesion between acinar cells and cell-substrate adhesion were greatly reduced. 3) A further important feature of acinar cells concerned the secretion phenotype. In ElasCCK2 exocrine pancreas zymogen granules appeared heterogeneous and were not properly targeted to the apical cell membrane, instead found throughout the cell (Figure 4, C and D) suggesting defects in secretion. 4) Notably, in ElasCCK2 acinar cells, organelles were often fusing to one another, mitochondria degraded (Figure 4D) and cells contained large autophagocytic vesicles (Figure 4E), suggesting that cells were undergoing intracellular degradation. 5) Finally, acinar cells of ElasCCK2 mice displayed nuclei polymorphism (anisokaryosis), with nuclei not being located basally (nuclear dysplasia), dilated endoplasmic reticulum (Figure 4C, asterisks), as well as fibrosis and matrix accumulation (Figure 4F), altogether indicating that both, acinar cell phenotype as well as acinar cell function (activity and turnover of organelles) were severely impaired in acini of ElasCCK2 mice.
Figure 4.
Ultrastructure of wild-type and ElasCCK2 exocrine pancreas. A–F: Electron photomicrographs of ultrathin epoxy resin sections through exocrine pancreas of wild-type (A, B) and ElasCCK2 mice (C–F). A: A typical acinar cell with basally located nucleus, endoplasmic reticulum (arrowheads), mitochondria (arrows), and zymogen granules is shown. B: Numerous adherens junctions and desmosomes (arrows and arrowheads) are found between neighbor acinar cells in the wild-type animals. Both types of junctions are missing at the cell-cell contacts between neighbor acinar cells in ElasCCK2 exocrine pancreas (arrows in D) and increased gaps between the cells observed (asterisks). C: Note dilatations of the endoplasmic reticulum (asterisks) and organelles fusing with mitochondria (arrows) in ElasCCK2 exocrine pancreas. E: In acinar cells of ElasCCK2 pancreas commonly autophagocytic bodies (arrows) and big gap (asterisks) are observed. F: Note enhanced fibrosis (arrowheads) and detachment of basal membrane (arrow) from acini in ElasCCK2 mice. Scale bars: 1.2 μm (A, C); 0.6 μm (B, E); 0.3 μm (D, F).
Defects in the Acinar Differentiation Pathway Are Associated with the ElasCCK2 Phenotype
To investigate whether expression of the CCK2-R in acinar cells affected the acinar differentiation program, exocrine pancreas cryosections were examined for the expression and localization of the pancreatic transcription factors Mist1 and PTF1-p48. PTF1-p48 is the earliest crucial transcription factor known to be required for exocrine pancreas development and function, Mist1, on the other hand, appears later during pancreas development and in other tissues displaying exocytosis and secretion. It is required for acinar function, stability, and identity because Mist1 knockout mice exhibit extensive disorganization of exocrine tissue and intracellular enzyme activation.35 Immunostaining for Mist1 in transgenic and control exocrine pancreas revealed, that for this protein, expression and localization were not affected by the expression of CCK2-R because expression was confined to the nuclei of all acinar cells of control (Figure 3C) and ElasCCK2 exocrine pancreases (Figure 3G). Note that nuclei in ElasCCK2 acinar cells, were no longer restricted to the basal cell body and therefore did not all appear in plane, confirming alterations in cell polarity previously described (Figure 3G, Figure 1E). Very much in contrast, immunostaining for PTF1-p48 showed that expression of CCK2-R clearly affects expression and localization of this transcription factor. Indeed, PTF1-p48 in ElasCCK2 pancreas was no longer exclusively localized to the nuclei of acinar cells as in the controls (Figure 3D), but detected to a high extent in the cytoplasm of acinar cells of ElasCCK2 mice (Figure 3H). Ductal cells, as well as Langerhans islet cells, were negative for Mist1 and for PTF1-p48 expression in control and ElasCCK2 pancreases (data not shown).
ElasCCK2 Mice Are More Susceptible to Develop Preneoplastic Lesions in the Exocrine Pancreas on Azaserine Treatment
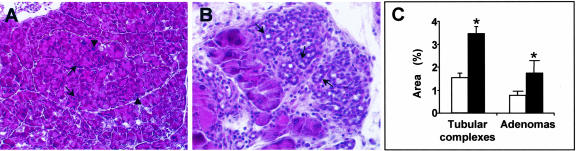
Our results, so far, demonstrate that expression of the CCK2-R induces alterations in the molecular acinar differentiation pathway as well as morphological and structural modifications in the acinar cell phenotype. Because ElasCCK2 mice, further on, generate preneoplastic lesions and tumors, these data support the idea that CCK2-R expression, by inducing alterations in acinar differentiation pathway, lead to morphologically and structurally modified acinar cells, which will give rise to preneoplastic lesions and tumors. On the other hand, azaserine is an established procarcinogenic cellular stress, which specifically targets the acinar compartment inducing hyperplasia and acinar tumors.36–38 Thus, we further asked, whether the conjunction of both stresses, CCK2-R expression and azaserine treatment, may result in additive effects, such as increased frequency of (pre-) neoplastic lesions and/or cell dedifferentiation and finally, whether this may result from the activation of separate or overlapping pathways. Control and ElasCCK2 mice were both treated with azaserine and surveys serially performed after the treatment. A detailed histological description of pathologies appearing at consecutive time points (kinetics) after azaserine treatment will be published elsewhere (Mathieu A, Clerc P, Portolan G, Bierkamp C, Lulka H, Pradayrol L, Seva C, Fourmy D, Dufresne M, manuscript in preparation). Pancreas was subjected to microscopical histological analysis and cryosections immunostained with antibodies recognizing the acinar-specific markers amylase, cytokeratins, adherens junction proteins, cytoskeleton proteins, and transcription factors 2 months after the end of treatment. No tumors were detected in any of the pancreata of the azaserine-treated ElasCCK2 or control animals and endocrine compartments were unaffected in azaserine-treated transgenics and in controls at this stage. However, H&E staining of histological sections through pancreas revealed important acinar lesions in the pancreas of both azaserine-treated nontransgenic as well as ElasCCK2 mice (Figure 5). One type of lesion, adenoma, consists of enlarged acinis composed of enlarged, swollen often plurinuclear cells (acinar hyperplasia), that exhibit cellular dysplasia and nuclear polymorphism (Figure 5A). The other type of lesion, metaplasia, consists of cells forming duct-like structures, with an enlarged centro-acinar lumen and is described as tubular or pseudoductular complexes (Figure 5B). Areas of both types of lesions were quantified and found significantly larger in pancreas of ElasCCK2 mice than in pancreas of controls (Figure 5C).
Figure 5.
Acinar lesions in the pancreas of azaserine-treated mice. A: Adenoma (delineated by a dashed line) in ElasCCK2 pancreas 2 months after the end of azaserine treatment exhibits disorganized acinar structure with larger (arrowheads) and more numerous (arrows) nuclei compared to adjacent acinar tissue (H&E). B: Ductal structures also called tubular or pseudoductular complexes (arrows) develop 2 months after the end of carcinogen treatment (H&E). C: Quantification of areas of tubular complexes and adenoma formation using morphometric analysis of azaserine-treated ElasCCK2 (black bars) and control (white bars) pancreas at 2 months after the end of carcinogen treatment. Results represent means ± SD and are expressed as percentage of the pancreatic section areas. *, Significance at P from 0.01 to 0.05, comparing ElasCCK2 values versus control values. Original magnifications: ×20 (A); ×40 (B).
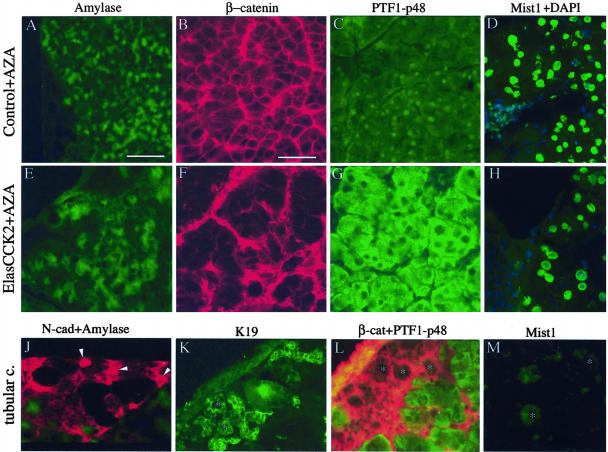
Examination and comparison of the distribution of the acinar digestive enzyme, amylase, in pancreas of azaserine-treated control (Figure 6A) and ElasCCK2 mice (Figure 6E) showed that in the latter, acini displayed less regular and occasional loss of staining of this protein. Notably, striking differences in accumulation and localization of β-catenin, and PTF1-p48 were observed in acini dependent on whether the treated mice were transgenic or control mice. In azaserine-treated controls, a large amount of PTF1-p48 was found in the cytoplasm, beside its nuclear localization, in most acinar cells (Figure 6C), whereas no modification was observed in the expression and localization of both, β-catenin to acinar cell-cell borders and Mist1 in most acinar nuclei (Figure 6, B and D). In contrast, in the pancreas of azaserine-treated ElasCCK2 mice, β-catenin staining along the acinar cell-cell borders had totally disappeared (Figure 6F), and PTF1-p48 was exclusively localized in the cytoplasm, acinar nuclei being devoid of PTF1-p48 (Figure 6G). No major change was observed in the expression of Mist1 in acinar nuclei (Figure 6H). So, taken together, these data present evidence that expression of CCK2-R together with a carcinogen treatment results in stronger morphological changes accompanied by an enhancement of molecular changes in the differentiation program. Cells in the tubular complexes, independent of CCK2-R expression, did not express amylase, PTF1-p48, or Mist1 (Figure 6; J, L, and M), but extensively expressed N-cadherin, K19, and β-catenin (Figure 6; J, K, and L). This is indicating that these cells expressed an altered cell fate in agreement with their ductal phenotype.
Figure 6.
Immunohistochemistry for acinar differentiation markers of wild-type and ElasCCK2 exocrine pancreas after azaserine treatment. A–H: Cryostat sections through exocrine pancreas from control (A–D) and ElasCCK2 (E–H) animals using antibodies recognizing the amylase (A, E), adherens junction protein β-catenin (B, F), and the acinar transcription factors PTF1-p48 (C, G) and Mist1 (D, H). In azaserine-treated exocrine pancreas amylase staining is weak and irregular (E), β-catenin staining is absent from the cell-cell contacts between acinar cells but localized basally (F), and PTF1-p48 localization exclusively cytoplasmic, nuclei being devoid of PTF1-p48 (G). H: Mist1 expression (green) appears normal in acinar nuclei in ElasCCK2 acini as visualized by double staining with 4′,6-diamino-2-phenylindole (blue). Note enlarged acinar cells (cellular dysplasia) and swollen nuclei (anisokaryosis) in ElasCCK2 pancreas (F, G, H). J–M: Cryostat sections through pancreatic periphery, where tubular complex formation (metaplasia) is found, stained with antibodies against N-cadherin (red), amylase (green) (J), K19 (K), β-catenin (red), PTF1-p48 (green) (L), and Mist1 (M). Cells in and around tubular complexes express N-cadherin, ductal K19 as well as β-catenin, but are devoid of amylase, PTF1-p48 and Mist1. Arrowheads in J point to cells expressing N-cadherin, asterisks in (K, L, M) denote tubular complexes. Note the small size of cells from tubular complexes in comparison to acinar cells (K, L). Scale bars: 120 μm (A, E, J); 60 mm (B–D, F–H, K–M).
Discussion
Transgenic ElasCCK2 mice expressing CCK2-R in acini display enhanced pancreatic growth and appearance of tumors.25 On the other hand, prolonged CCK2-R activation in stably transfected Madin-Darby canine kidney cells induces changes typical of an epithelial-mesenchymal transition, reflecting a state of dedifferentiation and malignancy induced in epithelial cells.14 Based on these previous results, we hypothesized that expression of the CCK2-R in vivo may, besides stimulating proliferation, lead to modifications of differentiation, alterations in morphology, and may further sensitize the organism to chemical carcinogenesis. In the present work we have investigated the effects of CCK2-R expression alone and in conjunction with a chemical carcinogen on cellular morphology, adhesion, and differentiation in the animal model ElasCCK2.
First, our results show that expression of CCK2-R leads to alterations in acinar cell morphology, cell adhesion, and cell differentiation affecting the entire exocrine pancreas of all animals analyzed. Importantly, these alterations are found before the development of metaplasia and preneoplastic lesions in ElasCCK2 mice. It is noteworthy to mention, that loss of acinar cell phenotype, indicative of acinar cell dedifferentiation, occurs in several different pancreatic diseases.39 Moreover, mice overexpressing transforming growth factor-α and mice deficient for Mist1 progressively develop lesions in the exocrine pancreas that also lead to a reversion from acinar to a ductal cell phenotype.35,40 Because in ElasCCK2 pancreas Mist1 expression in acinar nuclei was not altered, it rather seems, that CCK2-R expression and Mist1 expression are not directly linked. There are also striking similarities in the global cellular response observed in the ElasCCK2 model that had previously been described for the Madin-Darby canine kidney cellular model in culture stably expressing the CCK2-R and stimulated with gastrin:14 1) altered cell morphology that also includes in ElasCCK2 mice nuclear dysplasia and loss of cell polarity; 2) reorganization of the cytoskeleton that leads to enlargement of centro-acinar lumens in ElasCCK2 pancreas, as well as altered cell substrate adhesion; 3) modulation of adherens junction molecules β-catenin and α-catenin together with an important reduction of adherens junctions between acinar cells. Because functional cell-cell adhesion requires the correct assembly of the entire adherens junction complexes (cadherin-catenin) with F-actin, such modulation is indicative of impaired cell adhesion. This has been confirmed at the ultrastructural level by the reduction of adherens junctions found between acinar cells. In ElasCCK2 mice these modifications may result in disruption of cellular architecture accounting for the loss of acinar cell phenotype and defective secretion. However, future studies will be required to determine the factors initiating and promoting the alterations of the acinar cell phenotype and defective secretion. Because adherens junction molecules play important roles in epithelial differentiation and as tumor suppressor proteins, modulation of these proteins, as seen here in ElasCCK2 mice, may be suggestive of epithelial dedifferentiation or development of malignancy.28
Several different possibilities might explain the modulation of cell adhesion through ectopic CCK2-receptor expression: 1) signal transduction pathway activation resulting in kinase activation that induces posttranslational modifications of the adherens junction complex;14,18,41,42 2) signal transduction pathway activation resulting in metalloproteinase-mediated EGF receptor transactivation through cleavage of HB-EGF or production of multiple paracrine mediators including EGF and fibroblast growth factor;9,13,43,44 3) alteration of signal transduction pathway resulting from expression of the CCK2-receptor on cells that normally express CCK1-receptor.31 Indeed, heterodimerization of the two CCK receptors subtypes when co-expressed in the same cell has been reported with consequences on calcium responses, receptor trafficking, and cellular growth. Although they were not tested one can speculate that heterodimerization modulates other signaling pathways.45 Additionally, we recently reported overexpression and activation of the PLCγ1 in response to gastrin in ElasCCK2 acini, giving further support to a modification of signal transduction in these cells.46 Interestingly, besides its known role as an early effector in signal transduction phospholipase Cγ1 signaling is also postulated to be linked to cytoskeletal alterations, cellular morphological changes, and migration and therefore may contribute to the observed changes in cellular adhesion and differentiation;47–49 4) finally, our recent demonstration of an association of phospholipase Cγ1 with the C-terminal sequence of the CCK2-receptor raises the possibility of proteins complexes interacting with the CCK2 receptor, including scaffolding molecules and proteins of the cytoskeleton, for modification of cell adhesion.46,50
Second, in the exocrine pancreas of ElasCCK2 mice we find cellular and nuclear dysplasia accompanied by reduced expression of acinar-specific markers (amylase), ultrastructural defects in the secretion pathway, and the up-regulation of ductal markers (K19). These results show that, although still expressing some components of acinar cell fate, yet at reduced levels, these cells, concomitantly co-express components of ductal cells. Because during pancreatic ontogeny cells originating from ductal precursor cells develop cell polarity and further differentiate into acinar cells, loss of cell polarity and an evolution from acinar to ductal phenotype are signs of dedifferentiation.51 Thus our data suggest that on CCK2-R expression acinar cells may be reverting to a duct cell phenotype, and this process could participate in the development of premalignant lesions such as metaplasia. In good correlation with our data is the fact that, re-expression of the ductal cytokeratin K19 in acini has been observed in human cancers and cancer cell lines.52,53 In addition, delocalization of PTF1-p48 from the acinar nucleus to the cytoplasm in ElasCCK2 mice suggests that PTF1-p48, which is absolutely required for the maintenance of acinar cell phenotype, is no longer functional as in wild-type acinar cells in agreement with an altered cell fate.54 Interestingly, cytoplasmic distribution of PTF1-p48 has also been reported for a human acinar tumor as well as for several cancer cell lines, again providing support for the idea that expression of CCK2-R could be participating to the development of premalignant lesions.55 Future studies will be required to unravel the mechanisms on CCK2-R expression resulting in cytoplasmic retention of PTF1-p48 and the consequences for the acinar cell fate. One further interesting aspect concerns the enhanced accumulation of N-cadherin in exocrine pancreas of ElasCCK2 mice. Normally, N-cadherin is especially expressed during embryonic development of the pancreas, where it is required as a survival and differentiation factor, as well as on islet cells.56 Moreover, enhanced N-cadherin expression has been observed in breast cancer cells in correlation with acquirement of malignancy and been related to changes of cell adhesion and invasiveness, and has also been found here in tubular complexes of azaserine-treated mice.57 Therefore, enhanced N-cadherin expression in ElasCCK2 exocrine pancreas may also be related to an alteration of acinar cell differentiation.
Third, our results on acinar morphology, adhesion molecules, and differentiation markers highlight a striking synergistic action of CCK2-R expression and azaserine, which results in increased frequency, increased size of preneoplastic lesions, as well as in higher degree of cellular dedifferentiation. Notably, treatment of control mice with azaserine clearly does not alter β-catenin expression or localization showing that this carcinogen affects processes other than β-catenin regulation, such as PTF1-p48 localization. Because the latter is also affected by CCK2-R expression, this may explain the resulting direct additive effect between CCK2-R expression and carcinogen treatment in enhanced dedifferentiation. Moreover, because adherens junction molecules have established tumor suppressor roles, their absence promotes malignant development. In good correlation it has been found that loss of E-cadherin together with adenomatous polyposis coli mutations, results in synergistic enhanced intestinal tumor initiation.58 Therefore, it is tempting to speculate that in ElasCCK2 mice, modulation of adherens junction molecules induced by CCK2-R expression, may act in concert with alterations in the differentiation program induced by chemical carcinogens to also promote synergy.
Even though acinar ductal transition is not complete in the untreated ElasCCK2 model, tubular complexes are readily found after azaserine treatment. It is well established that the latter constitute transdifferentiation foci of acinar cells. Because these structures contain the precursor cells, which after transformation can generate ductal adenocarcinoma, tubular complexes are considered precursor lesions of ductal adenocarcinoma.40,59 Because the frequency and surface of these structures is also increased in azaserine-treated ElasCCK2 mice, this shows that ElasCCK2 mice are more susceptible to carcinogenic influences, possibly resulting in an increased rate of pancreatic cancer later on. Since here, we have focused on very early, initial cellular events, additional studies, focusing on long-term effects, will be required to see whether in ElasCCK2 mice overexpression of CCK2-R in conjunction with chemical carcinogenesis will definitely result in increased rate of pancreatic cancer.
Our results, indicate that expression of CCK2-R in acini is of pathophysiological importance with regard to differentiation of pancreatic acinar cells. They give strong support to the hypothesis that CCK2-R expression is incompatible with the maintenance of a fully differentiated acinar phenotype and shifts the phenotype to ductal differentiation. This goes in line with a recent report showing CCK2-R up-regulation in human chronic pancreatitis and the correlation of chronic pancreatitis with increased risk to develop pancreatic cancer.60–62 Indeed, the fact that acinar-ductal transition, or transdifferentiation, is a typical lesion of chronic pancreatitis, together with experimental evidence for the presence of the CCK2 receptor and gastrin in transdifferentiated acinar cells leads to take a role for this receptor in initiation steps of carcinogenesis into account (Mathieu et al, in preparation).63
In conclusion, we demonstrate here a novel biological role for the CCK2-R in vivo in modulation of cellular processes such as morphology, adhesion, and differentiation and propose an implication in initial steps of cancer formation in conjunction with chemical carcinogens.
Acknowledgments
We thank Drs. R. Kemler (Freiburg, Germany), F.X. Real (Barcelona, Spain), and S.F. Konieczny (West Lafayette, IN) for antibodies; Prof. N. Gas and Dr. N. Benmeradi for access to the technical plateau of electron microscopy of the IEFG (Institut d’Exploration Fonctionnelle des Genomes); H. Lulka for excellent technical assistance in the animal facility; G. Portolan in the histotechnology facility; and all the members of INSERM U531 for their interest and stimulating discussions.
Footnotes
Address reprint requests to Dr. Marlène Dufresne, INSERM U531, IFR31, Hospital Rangueil, TSA 50032, 31059 Toulouse Cedex 9, France. E-mail: dufresne@toulouse.inserm.fr.
Supported by the Association pour la Recherche contre le Cancer (grants 4430 and 4514 to C.B.), La Ligue Contre le Cancer, and the Ministry of Research (Action Concertée Incitative Biologie du Développement grant FNS 2001 1A067G).
References
- Koh TJ, Goldenring JR, Ito S, Mashimo H, Kopin AS, Varro A, Dockray GJ, Wang TC. Gastrin deficiency results in altered gastric differentiation and decreased colonic proliferation in mice. Gastroenterology. 1997;113:1015–1025. doi: 10.1016/s0016-5085(97)70199-9. [DOI] [PubMed] [Google Scholar]
- Friis-Hansen L, Sundler F, Li Y, Gillespie PJ, Saunders TL, Greenson JK, Owyang C, Rehfeld JF, Samuelson LC. Impaired gastric acid secretion in gastrin-deficient mice. Am J Physiol. 1998;274:G561–G568. doi: 10.1152/ajpgi.1998.274.3.G561. [DOI] [PubMed] [Google Scholar]
- Koh TJ, Dockray GJ, Varro A, Cahill RJ, Dangler CA, Fox JG, Wang TC. Overexpression of glycine-extended gastrin in transgenic mice results in increased colonic proliferation. J Clin Invest. 1999;103:1119–1126. doi: 10.1172/JCI4910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata A, Ito M, Iwata N, Kuno J, Takano H, Minowa O, Chihara K, Matsui T, Noda T. G protein-coupled cholecystokinin-B/gastrin receptors are responsible for physiological cell growth of the stomach mucosa in vivo. Proc Natl Acad Sci USA. 1996;93:11825–11830. doi: 10.1073/pnas.93.21.11825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langhans N, Rindi G, Chiu M, Rehfeld JF, Ardman B, Beinborn M, Kopin AS. Abnormal gastric histology and decreased acid production in cholecystokinin-B/gastrin receptor-deficient mice. Gastroenterology. 1997;112:280–286. doi: 10.1016/s0016-5085(97)90000-7. [DOI] [PubMed] [Google Scholar]
- Chen D, Zhao CM, Al-Haider W, Hakanson R, Rehfeld JF, Kopin AS. Differentiation of gastric ECL cells is altered in CCK(2) receptor-deficient mice. Gastroenterology. 2002;123:577–585. doi: 10.1053/gast.2002.34746. [DOI] [PubMed] [Google Scholar]
- Wang TC, Dockray GJ. Lessons from genetically engineered animal models. I. Physiological studies with gastrin in transgenic mice. Am J Physiol. 1999;277:G6–G11. doi: 10.1152/ajpgi.1999.277.1.G6. [DOI] [PubMed] [Google Scholar]
- Koh TJ, Chen D. Gastrin as a growth factor in the gastrointestinal tract. Regul Pept. 2000;93:37–44. doi: 10.1016/s0167-0115(00)00176-2. [DOI] [PubMed] [Google Scholar]
- Varro A, Noble PJ, Wroblewski LE, Bishop L, Dockray GJ. Gastrin-cholecystokinin(B) receptor expression in AGS cells is associated with direct inhibition and indirect stimulation of cell proliferation via paracrine activation of the epidermal growth factor receptor. Gut. 2002;50:827–833. doi: 10.1136/gut.50.6.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi T, Takaishi K, Murayama T, Ito M, Iwata N, Chihara K, Sasaki T, Takai Y, Matsui T. Cholecystokinin-B/gastrin receptors mediate rapid formation of actin stress fibers. Oncogene. 1996;12:1357–1360. [PubMed] [Google Scholar]
- Todisco A, Ramamoorthy S, Witham T, Pausawasdi N, Srinivasan S, Dickinson CJ, Askari FK, Krametter D. Molecular mechanisms for the antiapoptotic action of gastrin. Am J Physiol. 2001;280:G298–G307. doi: 10.1152/ajpgi.2001.280.2.G298. [DOI] [PubMed] [Google Scholar]
- Pagliocca A, Wroblewski LE, Ashcroft FJ, Noble PJ, Dockray GJ, Varro A. Stimulation of the gastrin-cholecystokinin(B) receptor promotes branching morphogenesis in gastric AGS cells. Am J Physiol. 2002;283:G292–G299. doi: 10.1152/ajpgi.00056.2002. [DOI] [PubMed] [Google Scholar]
- Noble PJ, Wilde G, White MR, Pennington SR, Dockray GJ, Varro A. Stimulation of gastrin-CCKB receptor promotes migration of gastric AGS cells via multiple paracrine pathways. Am J Physiol. 2003;284:G75–G84. doi: 10.1152/ajpgi.00300.2002. [DOI] [PubMed] [Google Scholar]
- Bierkamp C, Kowalski-Chauvel A, Dehez S, Fourmy D, Pradayrol L, Seva C. Gastrin mediated cholecystokinin-2 receptor activation induces loss of cell adhesion and scattering in epithelial MDCK cells. Oncogene. 2002;21:7656–7670. doi: 10.1038/sj.onc.1205999. [DOI] [PubMed] [Google Scholar]
- Rozengurt E, Walsh JH. Gastrin CCK, signaling, and cancer. Annu Rev Physiol. 2001;63:49–76. doi: 10.1146/annurev.physiol.63.1.49. [DOI] [PubMed] [Google Scholar]
- Dehez S, Bierkamp C, Kowalski-Chauvel A, Daulhac L, Escrieut C, Susini C, Pradayrol L, Fourmy D, Seva C. c-Jun NH(2)-terminal kinase pathway in growth-promoting effect of the G protein-coupled receptor cholecystokinin B receptor: a protein kinase C/Src-dependent-mechanism. Cell Growth Differ. 2002;13:375–385. [PubMed] [Google Scholar]
- Daulhac L, Kowalski-Chauvel A, Pradayrol L, Vaysse N, Seva C. Gastrin stimulates the formation of a p60Src/p125FAK complex upstream of the phosphatidylinositol 3-kinase signaling pathway. FEBS Lett. 1999;445:251–255. doi: 10.1016/s0014-5793(99)00129-5. [DOI] [PubMed] [Google Scholar]
- Daulhac L, Kowalski-Chauvel A, Pradayrol L, Vaysse N, Seva C. Src-family tyrosine kinases in activation of ERK-1 and p85/p110-phosphatidylinositol 3-kinase by G/CCKB receptors. J Biol Chem. 1999;274:20657–20663. doi: 10.1074/jbc.274.29.20657. [DOI] [PubMed] [Google Scholar]
- Tamiolakis D, Venizelos I, Simopoulos C, Kotini A, Jivannakis T, Papadopoulos N. Does neoplastic gastrin expression remodel the embryonal pattern of the protein? A study in human pancreas. Hepatogastroenterology. 2004;51:249–252. [PubMed] [Google Scholar]
- Caplin M, Savage K, Khan K, Brett B, Rode J, Varro A, Dhillon A. Expression and processing of gastrin in pancreatic adenocarcinoma. Br J Surg. 2000;87:1035–1040. doi: 10.1046/j.1365-2168.2000.01488.x. [DOI] [PubMed] [Google Scholar]
- Goetze JP, Nielsen FC, Burcharth F, Rehfeld JF. Closing the gastrin loop in pancreatic carcinoma: coexpression of gastrin and its receptor in solid human pancreatic adenocarcinoma. Cancer. 2000;88:2487–2494. doi: 10.1002/1097-0142(20000601)88:11<2487::aid-cncr9>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Hellmich MR, Rui XL, Hellmich HL, Fleming RY, Evers BM, Townsend CM., Jr Human colorectal cancers express a constitutively active cholecystokinin-B/gastrin receptor that stimulates cell growth. J Biol Chem. 2000;275:32122–32128. doi: 10.1074/jbc.M005754200. [DOI] [PubMed] [Google Scholar]
- Ding WQ, Kuntz SM, Miller LJ. A misspliced form of the cholecystokinin-B/gastrin receptor in pancreatic carcinoma: role of reduced cellular U2AF35 and a suboptimal 3′-splicing site leading to retention of the fourth intron. Cancer Res. 2002;62:947–952. [PubMed] [Google Scholar]
- Schmitz F, Otte JM, Stechele HU, Reimann B, Banasiewicz T, Folsch UR, Schmidt WE, Herzig KH. CCK-B/gastrin receptors in human colorectal cancer. Eur J Clin Invest. 2001;31:812–820. doi: 10.1046/j.1365-2362.2001.00870.x. [DOI] [PubMed] [Google Scholar]
- Clerc P, Leung-Theung-Long S, Wang TC, Dockray GJ, Bouisson M, Delisle MB, Vaysse N, Pradayrol L, Fourmy D, Dufresne M. Expression of CCK2 receptors in the murine pancreas: proliferation, transdifferentiation of acinar cells, and neoplasia. Gastroenterology. 2002;122:428–437. doi: 10.1053/gast.2002.30984. [DOI] [PubMed] [Google Scholar]
- Birchmeier W, Behrens J. Cadherin expression in carcinomas: role in the formation of cell junctions and the prevention of invasiveness. Biochim Biophys Acta. 1994;1198:11–26. doi: 10.1016/0304-419x(94)90003-5. [DOI] [PubMed] [Google Scholar]
- Behrens J, Mareel MM, Van Roy FM, Birchmeier W. Dissecting tumor cell invasion: epithelial cells acquire invasive properties after the loss of uvomorulin-mediated cell-cell adhesion. J Cell Biol. 1989;108:2435–2447. doi: 10.1083/jcb.108.6.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nollet F, Berx G, van Roy F. The role of the E-cadherin/catenin adhesion complex in the development and progression of cancer. Mol Cell Biol Res Commun. 1999;2:77–85. doi: 10.1006/mcbr.1999.0155. [DOI] [PubMed] [Google Scholar]
- Varner JA, Cheresh DA. Integrins and cancer. Curr Opin Cell Biol. 1996;8:724–730. doi: 10.1016/s0955-0674(96)80115-3. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Reynolds AB. Tyrosine phosphorylation and cadherin/catenin function. Bioessays. 1997;19:883–891. doi: 10.1002/bies.950191008. [DOI] [PubMed] [Google Scholar]
- Saillan-Barreau C, Clerc P, Adato M, Escrieut C, Vaysse N, Fourmy D, Dufresne M. Transgenic CCK-B/gastrin receptor mediates murine exocrine pancreatic secretion. Gastroenterology. 1998;115:988–996. doi: 10.1016/s0016-5085(98)70271-9. [DOI] [PubMed] [Google Scholar]
- Roebuck BD, Longnecker DS. Response of two rodents, Mastomys natalensis and Mystromys albicaudatus, to the pancreatic carcinogen azaserine. J Natl Cancer Inst. 1979;62:1269–1271. [PubMed] [Google Scholar]
- Butz S, Larue L. Expression of catenins during mouse embryonic development and in adult tissues. Cell Adhes Commun. 1995;3:337–352. doi: 10.3109/15419069509081018. [DOI] [PubMed] [Google Scholar]
- Conacci-Sorrell M, Zhurinsky J, Ben-Ze’ev A. The cadherin-catenin adhesion system in signaling and cancer. J Clin Invest. 2002;109:987–991. doi: 10.1172/JCI15429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pin CL, Rukstalis JM, Johnson C, Konieczny SF. The bHLH transcription factor Mist1 is required to maintain exocrine pancreas cell organization and acinar cell identity. J Cell Biol. 2001;155:519–530. doi: 10.1083/jcb.200105060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurlo J, Longnecker DS, Cooney DA, Kuhlmann ET, Curphey TJ. Studies of pancreatic nodule induction and DNA damage by D-azaserine. Cancer Lett. 1981;12:75–80. doi: 10.1016/0304-3835(81)90040-9. [DOI] [PubMed] [Google Scholar]
- Kawabata TT, Wick DG, Guengerich FP, Baron J. Immunohistochemical localization of carcinogen-metabolizing enzymes within the rat and hamster exocrine pancreas. Cancer Res. 1984;44:215–223. [PubMed] [Google Scholar]
- Roebuck BD, Baumgartner KJ, Longnecker DS. Growth of pancreatic foci and development of pancreatic cancer with a single dose of azaserine in the rat. Carcinogenesis. 1987;8:1831–1835. doi: 10.1093/carcin/8.12.1831. [DOI] [PubMed] [Google Scholar]
- Steer ML. Pathogenesis of acute pancreatitis. Digestion. 1997;58(Suppl 1):46–49. doi: 10.1159/000201525. [DOI] [PubMed] [Google Scholar]
- Schmid RM, Kloppel G, Adler G, Wagner M. Acinar-ductal-carcinoma sequence in transforming growth factor-alpha transgenic mice. Ann NY Acad Sci. 1999;880:219–230. doi: 10.1111/j.1749-6632.1999.tb09526.x. [DOI] [PubMed] [Google Scholar]
- Piedra J, Miravet S, Castano J, Palmer HG, Heisterkamp N, Garcia de Herreros A, Dunach M. p120 catenin-associated Fer and Fyn tyrosine kinases regulate beta-catenin Tyr-142 phosphorylation and beta-catenin-alpha-catenin interaction. Mol Cell Biol. 2003;23:2287–2297. doi: 10.1128/MCB.23.7.2287-2297.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollande F, Lee DJ, Choquet A, Roche S, Baldwin GS. Adherens junctions and tight junctions are regulated via different pathways by progastrin in epithelial cells. J Cell Sci. 2003;116:1187–1197. doi: 10.1242/jcs.00321. [DOI] [PubMed] [Google Scholar]
- Yan Y, Shirakabe K, Werb Z. The metalloprotease Kuzbanian (ADAM10) mediates the transactivation of EGF receptor by G protein-coupled receptors. J Cell Biol. 2002;158:221–226. doi: 10.1083/jcb.200112026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer B, Gschwind A, Ullrich A. Multiple G-protein-coupled receptor signals converge on the epidermal growth factor receptor to promote migration and invasion. Oncogene. 2004;23:991–999. doi: 10.1038/sj.onc.1207278. [DOI] [PubMed] [Google Scholar]
- Cheng ZJ, Harikumar KG, Holicky EL, Miller LJ. Heterodimerization of type A and B cholecystokinin receptors enhance signaling and promote cell growth. J Biol Chem. 2003;278:52972–52979. doi: 10.1074/jbc.M310090200. [DOI] [PubMed] [Google Scholar]
- Arnould M, Tassa A, Ferrand A, Archer E, Esteve JP, Penalba V, Portolan G, Escherich A, Moroder L, Fourmy D, Seva C, Dufresne M. The G-protein-coupled CCK2 receptor associates with phospholipase Cgamma1. FEBS Lett. 2004;568:89–93. doi: 10.1016/j.febslet.2004.05.012. [DOI] [PubMed] [Google Scholar]
- Piccolo E, Innominato PF, Mariggio MA, Maffucci T, Iacobelli S, Falasca M. The mechanism involved in the regulation of phospholipase Cgamma1 activity in cell migration. Oncogene. 2002;21:6520–6529. doi: 10.1038/sj.onc.1205821. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Kim E, Ryu SH, Suh PG. The mechanism of phospholipase C-gamma1 regulation. Exp Mol Med. 2000;32:101–109. doi: 10.1038/emm.2000.18. [DOI] [PubMed] [Google Scholar]
- Wells A, Ware MF, Allen FD, Lauffenburger DA. Shaping up for shipping out: pLCgamma signaling of morphology changes in EGF-stimulated fibroblast migration. Cell Motil Cytoskeleton. 1999;44:227–233. doi: 10.1002/(SICI)1097-0169(199912)44:4<227::AID-CM1>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Bockaert J, Marin P, Dumuis A, Fagni L. The ‘magic tail’ of G protein-coupled receptors: an anchorage for functional protein networks. FEBS Lett. 2003;546:65–72. doi: 10.1016/s0014-5793(03)00453-8. [DOI] [PubMed] [Google Scholar]
- Kim SK, Hebrok M. Intercellular signals regulating pancreas development and function. Genes Dev. 2001;15:111–127. doi: 10.1101/gad.859401. [DOI] [PubMed] [Google Scholar]
- Hall PA, Lemoine NR. Rapid acinar to ductal transdifferentiation in cultured human exocrine pancreas. J Pathol. 1992;166:97–103. doi: 10.1002/path.1711660203. [DOI] [PubMed] [Google Scholar]
- Schussler MH, Skoudy A, Ramaekers F, Real FX. Intermediate filaments as differentiation markers of normal pancreas and pancreas cancer. Am J Pathol. 1992;140:559–568. [PMC free article] [PubMed] [Google Scholar]
- Krapp A, Knofler M, Ledermann B, Burki K, Berney C, Zoerkler N, Hagenbuchle O, Wellauer PK. The bHLH protein PTF1–p48 is essential for the formation of the exocrine and the correct spatial organization of the endocrine pancreas. Genes Dev. 1998;12:3752–3763. doi: 10.1101/gad.12.23.3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adell T, Gomez-Cuadrado A, Skoudy A, Pettengill OS, Longnecker DS, Real FX. Role of the basic helix-loop-helix transcription factor p48 in the differentiation phenotype of exocrine pancreas cancer cells. Cell Growth Differ. 2000;11:137–147. [PubMed] [Google Scholar]
- Esni F, Johansson BR, Radice GL, Semb H. Dorsal pancreas agenesis in N-cadherin-deficient mice. Dev Biol. 2001;238:202–212. doi: 10.1006/dbio.2001.0405. [DOI] [PubMed] [Google Scholar]
- Bhowmick NA, Ghiassi M, Bakin A, Aakre M, Lundquist CA, Engel ME, Arteaga CL, Moses HL. Transforming growth factor-beta1 mediates epithelial to mesenchymal transdifferentiation through a RhoA-dependent mechanism. Mol Biol Cell. 2001;12:27–36. doi: 10.1091/mbc.12.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits R, Ruiz P, Diaz-Cano S, Luz A, Jagmohan-Changur S, Breukel C, Birchmeier C, Birchmeier W, Fodde R. E-cadherin and adenomatous polyposis coli mutations are synergistic in intestinal tumor initiation in mice. Gastroenterology. 2000;119:1045–1053. doi: 10.1053/gast.2000.18162. [DOI] [PubMed] [Google Scholar]
- Bockman DE, Guo J, Buchler P, Muller MW, Bergmann F, Friess H. Origin and development of the precursor lesions in experimental pancreatic cancer in rats. Lab Invest. 2003;83:853–859. doi: 10.1097/01.lab.0000074918.31303.5a. [DOI] [PubMed] [Google Scholar]
- Reubi JC, Waser B, Gugger M, Friess H, Kleeff J, Kayed H, Buchler MW, Laissue JA. Distribution of CCK1 and CCK2 receptors in normal and diseased human pancreatic tissue. Gastroenterology. 2003;125:98–106. doi: 10.1016/s0016-5085(03)00697-8. [DOI] [PubMed] [Google Scholar]
- Maisonneuve P, Lowenfels AB. Chronic pancreatitis and pancreatic cancer. Dig Dis. 2002;20:32–37. doi: 10.1159/000063165. [DOI] [PubMed] [Google Scholar]
- Cavestro GM, Comparato G, Nouvenne A, Sianesi M, Di Mario F. The race from chronic pancreatitis to pancreatic cancer. Journal of the Pancreas. 2003;4:165–168. [PubMed] [Google Scholar]
- Rooman I, Lardon J, Flamez D, Schuit F, Bouwens L. Mitogenic effect of gastrin and expression of gastrin receptors in duct-like cells of rat pancreas. Gastroenterology. 2001;121:940–949. doi: 10.1053/gast.2001.27998. [DOI] [PubMed] [Google Scholar]