Abstract
Leukocyte recruitment into pancreatic islets is believed to play an important pathophysiological role in autoimmune diabetes. Previous reports have suggested that several different adhesion molecules may be involved in leukocyte recruitment during autoimmune diabetes, including members of the leukocyte β2 integrins. Here we report that a gene-targeted deficiency of the β2 integrin, CD18, protects against multiple low-dose streptozotocin-induced autoimmune diabetes. CD18 null mice displayed lower blood glucose values throughout the study, with only 10% of these mice eventually developing diabetes compared to 95% in the control group. Importantly, the development of insulitis was markedly absent in the CD18 null mice, suggesting that members of this integrin subfamily predominately regulate leukocyte infiltration into pancreatic islets. This study demonstrates that the β2 integrins play a key pathophysiological role in the development of multiple low-dose streptozotocin-induced autoimmune diabetes.
The exact pathogenic mechanisms of autoimmune diabetes are multifactoral and remain largely unknown. While causative factors are uncertain, it is thought that both genetic and environmental factors contribute to the etiology of disease. A strong linkage has been demonstrated between Type 1 diabetes and HLA class II immune recognition molecules.1,2 However, other immune modulating factors can also contribute to the development of autoimmune diabetes, such as leukocyte infiltration into pancreatic islets (ie, insulitis).
Recruitment of leukocytes is primarily dependent on cell adhesion molecules, and occurs through a concerted, multi-step process involving leukocyte rolling, adhesion, and migration across the vascular endothelium. As such, adhesion molecules have been suggested to be an attractive target for intervention in autoimmune diabetes.3,4 However, a limited number of studies have addressed the role of adhesion molecules during multiple low-dose streptozotocin (MLDSTZ) autoimmune diabetes. Studies have shown that combined inhibition of various adhesion molecules (ICAM-1, VLA-4, LFA-1, and Mac-1) with monoclonal antibodies may attenuate MLDSTZ diabetes.5–7 While these reports implicate a role for adhesion molecules, an understanding of the critical adhesion molecules involved in MLDSTZ diabetes has not been reported. Interestingly, a recent study by Martin and colleagues8 showed that a gene-targeted mutation of ICAM-1 did not confer protection against MLDSTZ diabetes. These findings suggest that a functional hierarchy may exist among adhesion molecules during autoimmune diabetes, requiring further investigation into the roles of these proteins.
Here we report that a gene-targeted CD18 null mutation significantly protects against MLDSTZ autoimmune diabetes. This protection was associated with an attenuation of leukocyte infiltration into the pancreatic islet. These data suggest that members of the β2 integrins are the predominant adhesion molecules involved in leukocyte recruitment during autoimmune diabetes. Targeted intervention against these molecules may be useful in therapeutic interventions aimed at the development of autoimmune diabetes.
Materials and Methods
Animals
Mice used for this study were bred and housed at the Association for Assessment and Accreditation of Laboratory Animal Care, International-accredited Louisiana State University Health Sciences Center-Shreveport animal resource facility and maintained according to the National Research Council’s Guide for Care and Use of Laboratory Animals. Twelve 12-week old male CD18 null−/− (Itgb2tm2Bay) C57BL/6 or wild-type C57BL/6 mice were used for this study. Breeder pairs were kindly provided by Daniel Bullard from the University of Alabama at Birmingham. The CD18 null−/− mice have been previously reported to completely lack CD18 expression and the 12th backcross generation was used for all experiments.9 All mice were housed under specific pathogen-free conditions in sterile micro-isolator cages with sterile bedding, chow, and acidified water.
Experimental Protocols
Sequential intraperitoneal injections of STZ (40 mg/kg) were performed over a 5-day period as originally reported.10 Blood glucose measurements were performed using the glucometer Elite system from Bayer (Elkhart, IN). Glucose readings of non-fasted mice were made before STZ injection and every 5 days thereafter for a total of 40 days. At the end of the study, mice were euthanized by cervical dislocation and pancreata immediately removed for histopathology.
CD18, CD4, and CD8 Immunohistochemistry
Pancreata from treated and untreated mice were frozen in OCT media and 5-μm sections fixed in 95% ethanol and 5% glacial acetic acid at −20°C for 30 minutes. Sections were then incubated in 0.3% H2O2 for 30 minutes followed by incubation in phosphate-buffered saline (PBS) with 4% rabbit serum plus 4 drops/ml avidin blocking solution (Vector Laboratories, Burlingame, CA) for 30 minutes. Sections were then incubated with rat anti-mouse primary antibody (eBiosciences, San Diego, CA), anti-CD18 (M18/2) at 1:200 dilution, anti-CD4 (L3T4) at 1:50 dilution, or anti-CD8a (Ly-2) at 1:50 dilution in PBS with 1% rabbit serum and 4 drops/ml biotin solution (Vector Laboratories) for 1 hour in a humidified chamber. This was followed by incubation with biotinylated rat IgG in PBS with 0.1% rabbit serum for 1 hour in a humidified chamber, and then incubation in VectaStain ABC (Vector Laboratories) for 1 hour. Sections were then stained with NovaRed (Vector Laboratories) for 30 minutes followed by hematoxylin for 3 minutes and mounted under glass coverslips.
Tissue Histopathology
Pancreata were harvested from all mice and fixed in 70% alcoholic formalin for 24 hours. Specimens were then transferred to 10% buffered formalin for 48 hours, embedded in paraffin, and sectioned. Sections were stained with hematoxylin and eosin and examined for leukocyte infiltration.
Results
Immunohistochemistry of CD18, CD4, and CD8 in MLDSTZ-Treated Mice
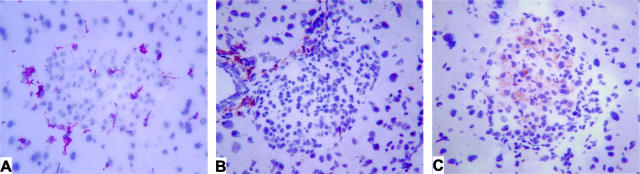
Immunostaining of MLDSTZ-treated wild-type pancreata with anti-CD18 antibody demonstrated positive-staining leukocytes infiltrating at the periphery of affected pancreatic islets with a limited number of positive cells within the center of the islet (Figure 1A). Staining with anti-CD4 revealed a peri-insulitis pattern with CD4+ lymphocytes infiltrating from local vasculature into the periphery of the islet (Figure 1B). There were numerous islets demonstrating this pattern of limited infiltrate of leukocytes staining positive for CD18 and CD4, but with minimal islet cell damage. Anti-CD8 immunostaining revealed CD8+ lymphocytes both at the periphery and distributed throughout the islet (Figure 1C). This is in contrast to the peri-islet staining observed in CD4-positive cells. Islets with heavy CD8-positive lymphocytic infiltrate also appeared to have more β cell destruction than those with lighter infiltrate.
Figure 1.
Immunohistochemistry for CD18, CD4, and CD8 in MLDSTZ-treated mice. A, B, and C, respectively, illustrate islet CD18, CD4, and CD8 staining in response to MLDSTZ treatment. Note the peri-insulitis appearance of CD18 and CD4 staining, while CD8 staining is observed throughout the islet.
Attenuation of MLDSTZ Hyperglycemia and Diabetes in CD18 Null Mice
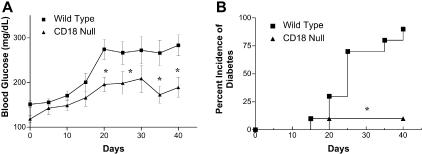
Both experimental and clinical reports have suggested a role for the leukocyte β2 integrins in autoimmune diabetes.4,11 However, no study has directly investigated MLDSTZ diabetes initiation and progression in mice that are genetically deficient for CD18. Figure 2A demonstrates that wild-type mice display increased blood glucose beginning at day 15 that peaks and remains elevated at day 20. MLDSTZ treatment of CD18 null mice does slightly increase blood glucose levels beginning at day 20, yet these levels are significantly reduced compared to wild-type mice and decrease toward the later period of the protocol.
Figure 2.
Hyperglycemia and diabetes incidence in CD18 null mice. A: Blood glucose levels throughout the MLDSTZ protocol for wild-type (▪) and CD18 null (▴) C57BL/6J mice. B: Percent incidence of diabetes between wild-type (▪) and CD18 null (▴) C57BL/6J mice. *, P < 0.05; n = 12.
The percent incidence of diabetes was determined after the completion of the 40-day experimental period. Mice were considered to be diabetic after two consecutive blood glucose measurements of ≥200 mg/dL. Figure 2B shows that 75% of wild-type mice are diabetic by day 25, whereas only 10% of CD18 null mice are diabetic. By the end of the 40-day period, 90% of wild-type mice and 10% of CD18 null mice are diabetic. These data clearly demonstrate that a genetic deficiency of CD18 significantly protects against MLDSTZ-induced diabetes.
Lack of Insulitis in MLDSTZ-Treated CD18 Null Mice
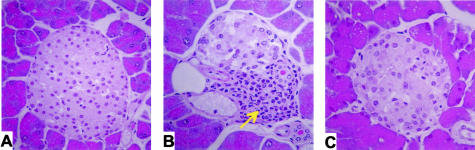
MLDSTZ has been reported to stimulate leukocyte recruitment into pancreatic islets resulting in insulitis.12 Pancreata were harvested at the end of the 40-day experimental period for histopathological analysis. Figure 3 illustrates islet histopathology and insulitis development between wild-type and CD18 null mice. Significant numbers of leukocyte infiltrates and insulitis can be observed in islets from wild-type mice on completion of the 40-day protocol (Figure 3B). Conversely, islet histopathology from CD18 null mice was devoid of infiltrating leukocytes and insulitis (Figure 3C). These data strongly suggest that the β2 integrins are primary mediators of leukocyte recruitment into pancreatic islets.
Figure 3.
MLDSTZ-mediated insulitis in CD18 null mice. A: Normal islet from a C57BL/6J mouse. B: Islet from a wild-type mouse treated with MLDSTZ. Arrow indicates prominent leukocyte infiltration. C: Demonstrates an islet from a CD18 null mouse treated with MLDSTZ. Note the lack of leukocyte infiltrates. Magnification, ×200.
Discussion
It is now well accepted that the initiation and development of IDDM (Type 1 diabetes) is largely due to an autoimmune response resulting in leukocyte-mediated destruction of insulin-producing β cells. A hallmark feature of autoimmune diabetes is the infiltration of T cells into the islet and the development of insulitis. T cell recruitment from the vasculature into tissue in response to an inflammatory stimulus is a regulated process involving rolling, adhesion, and migration across vascular endothelium that requires both adhesion proteins and chemoattractant/activating molecules. Several different adhesion molecules facilitate T cell-endothelial cell interactions during immune responses, including selectins, ICAM-1, VCAM-1, VLA-4, LFA-1, and others.13,14 Given this diversity, it has been reported that these molecules can display functional redundancy to maintain adequate immune responses.13,14
Several studies have examined adhesion molecule expression and function in patients with autoimmune diabetes. An early study by Hanninen and colleagues11 demonstrated pancreatic endothelial cell activation (up-regulation of ICAM-1 and VCAM-1 expression) in tissue specimens, and reported enhanced T cell adhesion to the activated endothelium. Another study by Lampeter et al15 examined levels of circulating adhesion molecules (an indication of inflammatory processes) in either patients recently diagnosed or at high risk for IDDM. Increased circulating ICAM-1 levels were found in both recent-onset IDDM patients and first-degree relatives at risk for IDDM (sharing the HLA-DR3 and/or DR4 haplotype with the diabetic relative). A later study from the same group showed that monoclonal antibodies against ICAM-1 suppressed autoreactive T cell proliferation from IDDM patients.16 Together, these reports implicate different adhesion molecules during the pathogenesis of autoimmune diabetes. However, the identification of key adhesion molecules in IDDM is lacking, and few reports have used gene-targeted knockout mice to precisely ascertain the specific adhesion molecule requirements in autoimmune diabetes models.
In this study, we determined the specific pathological importance of the β2 integrin CD18 in the MLDSTZ autoimmune diabetes model. The β2 integrin family, or CD18 integrins, consists of four different adhesion proteins LFA-1 (CD11a/CD18), Mac-1 (CD11b/CD18), p150/95 (CD11c/CD18), and CD11d/CD18.17 An important and dominant role for the β2 integrins has been illustrated by several studies using gene-targeted mutant mice of CD18 in various inflammatory disease models.13,14 Data from these and other studies indicates that the β2 integrins are important mediators of leukocyte adhesion and migration, co-stimulation and activation, and leukocyte-mediated tissue damage; yet, the importance of this molecule in autoimmune diabetes has not been investigated.9,18–23
Here we report that a deficiency of CD18 significantly protected against MLDSTZ-induced hypoglycemia and the development of frank diabetes. A small percentage (10%) of CD18 null mice did develop diabetes; however, this low incidence rate is similar to previous studies in T cell-deficient mice using the MLDSTZ protocol.24–26 It is well appreciated that even low doses of streptozotocin may be directly cytotoxic to β cells; therefore, some degree of diabetes development could be expected in CD18 null mice. Additionally, we observed a complete attenuation of insulitis in response to MLDSTZ. We regard these observations as quite striking since many studies have suggested an important role for other adhesion molecules (β1 integrins, eg, VLA-4) in mediating T cell adhesion to endothelium.11,27,28 Our data also demonstrate a hierarchical role of adhesion molecule function during MLDSTZ autoimmune diabetes, since CD18 deficiency was protective while loss of ICAM-1 (a CD18 counter ligand) was not.8 A likely explanation for this finding is that β2 integrins have other known ligands besides ICAM-1 (eg, ICAM-2 and fibrinogen) that could still facilitate T cell recruitment in ICAM-1-deficient mice.13 Together, these data strongly suggest that CD18 plays a dominant role in mediating MLDSTZ-dependent CD4+ T cell recruitment into pancreatic islets, which may in turn stimulate CD8+ T cell infiltration resulting in islet destruction.
In conclusion, we have shown that the β2 integrin, CD18, plays a key pathophysiological role during the development of autoimmune diabetes. While these data are insightful into the adhesion molecule requirements necessary for MLDSTZ diabetes, further experimentation is needed to precisely define the temporal manner in which CD18 mediates T cell recruitment and the role of individual β2 integrin family members during autoimmune diabetes.
Acknowledgments
We thank Mr. Lamar Jones for expert technical assistance.
Footnotes
Address reprint requests to Christopher G. Kevil, Ph.D., Assistant Professor, Department of Pathology, LSU Health Sciences Center, 1501 Kings Hwy, Shreveport, LA 71130. E-mail: ckevil@lsuhsc.edu.
Supported by the LSUHSC-S Center of Excellence for Arthritis and Rheumatology and National Institutes of Health grant DK43785.
References
- Notkins AL, Lernmark A. Autoimmune type 1 diabetes: resolved and unresolved issues. J Clin Invest. 2001;108:1247–1252. doi: 10.1172/JCI14257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenbarth GS. Type I diabetes mellitus: a chronic autoimmune disease. N Engl J Med. 1986;314:1360–1368. doi: 10.1056/NEJM198605223142106. [DOI] [PubMed] [Google Scholar]
- Yang XD, Michie SA, Mebius RE, Tisch R, Weissman I, McDevitt HO. The role of cell adhesion molecules in the development of IDDM: implications for pathogenesis and therapy. Diabetes. 1996;45:705–710. doi: 10.2337/diab.45.6.705. [DOI] [PubMed] [Google Scholar]
- Yang XD, Michie SA, Tisch R, Karin N, Steinman L, McDevitt HO. Cell adhesion molecules: a selective therapeutic target for alleviation of IDDM. J Autoimmun. 1994;7:859–864. doi: 10.1006/jaut.1994.1069. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Hashimoto S, Kameyama Y. Reduced streptozotocin-induced insulitis in CD-1 mice by treatment with anti-intercellular adhesion molecule-1 and anti-lymphocyte function associated antigen-1 monoclonal antibodies together with lactic dehydrogenase virus infection. Int J Exp Pathol. 1994;75:117–121. [PMC free article] [PubMed] [Google Scholar]
- Herold KC, Vezys V, Gage A, Montag AG. Prevention of autoimmune diabetes by treatment with anti-LFA-1 and anti-ICAM-1 monoclonal antibodies. Cell Immunol. 1994;157:489–500. doi: 10.1006/cimm.1994.1244. [DOI] [PubMed] [Google Scholar]
- Hutchings P, Rosen H, O’Reilly L, Simpson E, Gordon S, Cooke A. Transfer of diabetes in mice prevented by blockade of adhesion-promoting receptor on macrophages. Nature. 1990;348:639–642. doi: 10.1038/348639a0. [DOI] [PubMed] [Google Scholar]
- Martin S, Vinke A, Heidenthal E, Schulte B, van den Engel N. Development of low-dose streptozotocin-induced diabetes in ICAM-1-deficient mice. Horm Metab Res. 1999;31:636–640. doi: 10.1055/s-2007-978812. [DOI] [PubMed] [Google Scholar]
- Scharffetter-Kochanek K, Lu H, Norman K, van Nood N, Munoz F, Grabbe S, McArthur M, Lorenzo I, Kaplan S, Ley K, Smith CW, Montgomery CA, Rich S, Beaudet AL. Spontaneous skin ulceration and defective T cell function in CD18 null mice. J Exp Med. 1998;188:119–131. doi: 10.1084/jem.188.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Like AA, Rossini AA. Streptozotocin-induced pancreatic insulitis: new model of diabetes mellitus. Science. 1976;193:415–417. doi: 10.1126/science.180605. [DOI] [PubMed] [Google Scholar]
- Hanninen A, Salmi M, Simell O, Jalkanen S. Endothelial cell-binding properties of lymphocytes infiltrated into human diabetic pancreas: implications for pathogenesis of IDDM. Diabetes. 1993;42:1656–1662. doi: 10.2337/diab.42.11.1656. [DOI] [PubMed] [Google Scholar]
- Leiter EH. Multiple low-dose streptozotocin-induced hyperglycemia and insulitis in C57BL mice: influence of inbred background, sex, and thymus. Proc Natl Acad Sci USA. 1982;79:630–634. doi: 10.1073/pnas.79.2.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kevil C, Bullard D. Roles of leukocyte/endothelial cell adhesion molecules in the pathogenesis of vasculitis. Am J Med. 1999;106:677–687. doi: 10.1016/s0002-9343(99)00132-1. [DOI] [PubMed] [Google Scholar]
- Bullard D. Knockout mice in inflammation research. Ley K, editor. New York: Oxford; Physiology of Inflammation. 2000:pp 381–401. [Google Scholar]
- Lampeter ER, Kishimoto TK, Rothlein R, Mainolfi EA, Bertrams J, Kolb H, Martin S. Elevated levels of circulating adhesion molecules in IDDM patients and in subjects at risk for IDDM. Diabetes. 1992;41:1668–1671. doi: 10.2337/diab.41.12.1668. [DOI] [PubMed] [Google Scholar]
- Roep BO, Heidenthal E, de Vries RR, Kolb H, Martin S. Soluble forms of intercellular adhesion molecule-1 in insulin-dependent diabetes mellitus. Lancet. 1994;343:1590–1593. doi: 10.1016/s0140-6736(94)93055-4. [DOI] [PubMed] [Google Scholar]
- Harris ES, McIntyre TM, Prescott SM, Zimmerman GA. The leukocyte integrins. J Biol Chem. 2000;275:23409–23412. doi: 10.1074/jbc.R000004200. [DOI] [PubMed] [Google Scholar]
- Jung U, Norman KE, Scharffetter-Kochanek K, Beaudet AL, Ley K. Transit time of leukocytes rolling through venules controls cytokine-induced inflammatory cell recruitment in vivo. J Clin Invest. 1998;102:1526–1533. doi: 10.1172/JCI119893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walzog B, Scharffetter-Kochanek K, Gaehtgens P. Impairment of neutrophil emigration in CD18-null mice. Am J Physiol. 1999;276:G1125–G1130. doi: 10.1152/ajpgi.1999.276.5.G1125. [DOI] [PubMed] [Google Scholar]
- Walzog B, Weinmann P, Jeblonski F, Scharffetter-Kochanek K, Bommert K, Gaehtgens P. A role for beta(2) integrins (CD11/CD18) in the regulation of cytokine gene expression of polymorphonuclear neutrophils during the inflammatory response. FASEB J. 1999;13:1855–1865. doi: 10.1096/fasebj.13.13.1855. [DOI] [PubMed] [Google Scholar]
- Sixt M, Hallmann R, Wendler O, Scharffetter-Kochanek K, Sorokin LM. Cell adhesion and migration properties of beta 2-integrin negative polymorphonuclear granulocytes on defined extracellular matrix molecules: relevance for leukocyte extravasation. J Biol Chem. 2001;276:18878–18887. doi: 10.1074/jbc.M010898200. [DOI] [PubMed] [Google Scholar]
- Willeke T, Scharffetter-Kochanek K, Gaehtgens P, Walzog B. A role for beta2 integrin (CD11/CD18)-mediated tyrosine signaling in extravasation of human polymorphonuclear neutrophils. Biorheology. 2001;38:89–100. [PubMed] [Google Scholar]
- Grabbe S, Varga G, Beissert S, Steinert M, Pendl G, Seeliger S, Bloch W, Peters T, Schwarz T, Sunderkotter C, Scharffetter-Kochanek K. Beta2 integrins are required for skin homing of primed T cells but not for priming naive T cells. J Clin Invest. 2002;109:183–192. doi: 10.1172/JCI11703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerling IC, Friedman H, Greiner DL, Shultz LD, Leiter EH. Multiple low-dose streptozocin-induced diabetes in NOD-scid/scid mice in the absence of functional lymphocytes. Diabetes. 1994;43:433–440. doi: 10.2337/diab.43.3.433. [DOI] [PubMed] [Google Scholar]
- Reddy S, Wu D, Elliott RB. Low-dose streptozotocin causes diabetes in severe combined immunodeficient (SCID) mice without immune cell infiltration of the pancreatic islets. Autoimmunity. 1995;20:83–92. doi: 10.3109/08916939509001931. [DOI] [PubMed] [Google Scholar]
- Elliott JI, Dewchand H, Altmann DM. Streptozotocin-induced diabetes in mice lacking alphabeta T cells. Clin Exp Immunol. 1997;109:116–120. doi: 10.1046/j.1365-2249.1997.4241319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vennegoor CJ, van de Wiel-van Kemenade E, Huijbens RJ, Sanchez-Madrid F, Melief CJ, Figdor CG. Role of LFA-1 and VLA-4 in the adhesion of cloned normal and LFA-1 (CD11/CD18)-deficient T cells to cultured endothelial cells: indication for a new adhesion pathway. J Immunol. 1992;148:1093–1101. [PubMed] [Google Scholar]
- Yusuf-Makagiansar H, Anderson ME, Yakovleva TV, Murray JS, Siahaan TJ. Inhibition of LFA-1/ICAM-1 and VLA-4/VCAM-1 as a therapeutic approach to inflammation and autoimmune diseases. Med Res Rev. 2002;22:146–167. doi: 10.1002/med.10001. [DOI] [PubMed] [Google Scholar]