Abstract
Blood neutrophils (PMN) are usually unresponsive to CC chemokines such as monacyte chemotactic protein-1 and macrophage inflammatory protein-1α. In rodents, the lung buildup of PMN as determined by myeloperoxidase (MPO) activity after airway instillation of bacterial lipopolysaccharide (LPS) was independent of MCP-1 and MIP-1α. In striking contrast, during sepsis following cecal ligation and puncture (CLP), blood PMN demonstrated mRNA for CC chemokine receptors. Furthermore, PMN from CLP, but not from sham rodents, bound MCP-1 and MIP-1α and responded chemotactically in vitro to both MCP-1 and MIP-1α. In CCR2−/− mice or WT mice treated in vivo with antibodies to either MCP-1 or MIP-1α, MPO activity was greatly attenuated in CLP animals. In CLP mice, increased serum IL-6 levels were found to be dependent on CCR2, MCP-1, and MIP-1α. When PMN from CLP rodents were incubated in vitro with either MCP-1 or MIP-1α, release of IL-6 was also shown. These findings suggest that sepsis fundamentally alters the trafficking of PMN into the lung in a manner that now engages functional responses to CC chemokines.
Blood neutrophils (PMN) trafficking during inflammation is a complex process which involves endothelial and PMN adhesion molecules1 and involvement of several types of chemotactic factors which may include lipids,2 complement activation products,3,4 and especially CXC chemokines.5,6 Initially, PMNs interact with endothelial selectins (E, P), resulting in PMN rolling along the endothelial surface. This rolling process appears to allow PMN to become activated (“primed”) by chemokines and other mediators secreted by the endothelium, resulting in their firm adhesion to endothelial adhesion molecule (ICAM-1) via the β2-integrins7 and possibly α48–11 and β1-integrins in conditions of sepsis.8 In general, CXC chemokines, particularly macrophage inflammatory protein (MIP)-2 and KC, appear to be involved in mediating PMN influx into tissues, while CC chemokines interact predominately with macrophages and monocytes.12 Recent findings suggest that under certain inflammatory conditions or in response to specific inflammatory stimuli, PMN may also directly interact with CC chemokines.13–17
To date, 28 CC chemokines have been identified,18 the cellular responses to them being mediated through binding to cognate receptors. Ten different CC family chemokine receptors (CCRs) have been identified.19 Promiscuity is known to exist among CC chemokines, involving the binding of a specific chemokine to more than one receptor. For instance, MIP-1α is known to bind both CC chemokine receptors 1 (CCR1) and 5 (CCR5). However, monocyte chemoattractant protein (MCP)-1 has been shown to bind solely to the CC chemokine receptor 2 (CCR2). In addition to binding MCP-1, CCR2 also serves as a receptor for four other MCP’s (MCP-1, MCP-3, -4, and -5) and is known to be expressed on monocytes and activated T cells. CCR1 and CCR5 are known to express on human peripheral blood lymphocytes as well as monocytes.
MIP-1α has been shown to regulate lung PMN migration after systemic exposure to lipopolysaccharide (LPS), MIP-1α being thought to mediate its effect indirectly by modulating the activity of macrophages or endothelial cells such as their release of TNFα or expression of ICAM-1, respectively.20 Recent in vitro studies show CCR1 can be induced on blood PMN after stimulation with specific cytokines,15,16 suggesting the ability of PMN to respond directly to MIP-1α, which is a major ligand for CCR1. In a recent novel study, blood PMN were shown to respond to exogenous MCP-1 in a mouse model of chronic adjuvant-induced arthritis and to bind antibody to CCR2 suggesting the presence of CCR2 receptors on these PMN.13 MCP-1 is known to be present in the lungs of patients during several lung inflammatory disorders, including sepsis and acute respiratory distress syndrome (ARDS),21,22 its presence correlating significantly with lung injury and mortality.21
Based on the above findings, we evaluated the ability of MCP-1 and MIP-1α to participate in PMN accumulation in lung after cecal ligation and puncture (CLP) or intratracheal administration of LPS. The results of the current study show that MCP-1 and MIP-1α mediate PMN accumulation in lungs of CLP mice, but not after airway instillation of LPS. In CLP mice, blood PMN were found to express mRNA for several CC chemokine receptors (CCR1, CCR2, and CCR5), to bind MCP-1 and MIP-1α, and to respond chemotactically in vitro to these chemokine receptors. In addition, serum IL-6 levels in CLP mice were found to be dependent on both MCP-1 and MIP-1α. This study suggests that neutrophil trafficking during sepsis is aberrant and that MCP-1 and MIP-1α play an important role in lung accumulation of PMN during sepsis.
Materials and Methods
Reagents
Myeloperoxidase (MPO) assay reagents were purchased from Sigma Aldrich (St. Louis, MO). A monoclonal antibody to mouse PMN specific antigen 7/4 was obtained from Caltag Laboratories (Burlingame, CA). Mouse JE (MCP-1) and MIP-1α biotin conjugates, recombinant mouse MIP-1α, antibodies to mouse MIP-1α and IL-6 together with flow cytometry reagents were purchased from R&D Systems, Inc. (Minneapolis, MN). Rat recombinant MCP-1 and MIP-1α, mouse recombinant MCP-1, as well as antibodies to mouse MCP-1 were purchased from BD Biosciences (San Diego, CA).
CLP-Induced Sepsis Model
All mice were on a C57BL/6 background. CCR2−/− male mice were generated as previously described and back-crossed >10 generations.23 Age-matched wild-type (WT) male pathogen-free mice (20 to 25 g) were purchased from Jackson Laboratories (Bar Harbor, ME) and male Long Evans pathogen-free rats (250 to 300 g) were purchased from Charles River (Wilmington, MA). All animal experiments were performed in compliance with the relevant laws and guidelines as set forth by the University of Michigan’s Committee on Use and Care of Animals. CLP was performed as previously described for rats24 and modified for mice. Anesthesia was induced in rats by intraperitoneal administration of 100 mg/kg ketamine HCl and 43 μg/kg xylazine HCl (150 mg/kg and 65 μg/kg, respectively, for mice). Using sterile conditions, the rat cecum was exposed through a 3 cm (1.5 cm for mice) incision and the cecum ligated below the ileocecal valve with a 4−0 silk suture without causing bowel obstruction and then punctured through and through with a 21-gauge needle. The cecum was repositioned and the abdominal incision was closed in layers with 4−0 surgical sutures (Ethicon Inc., Somerville, NJ) and metallic clips. In some experiments, MCP-1 or MIP-1α was blocked by intravenous administration of antibodies to either MCP-1 or MIP-1α (20 μg in 200 μl), together or alone, at the time of CLP. Control mice received 20 μg isotype-matched IgG.
LPS-Induced Lung Injury Model
Lung injury was induced by intratracheal administration of LPS as previously described.17 C57BL/6 male mice (20 to 25 g) from Jackson Laboratories were anesthetized by intraperitoneal injection of 150 mg/kg ketamine HCl and 65 μg/kg xylazine hydrochloride. LPS from E. coli (serotype 0111.B4; Sigma Aldrich) was instilled intratracheally (25 μg in 50 μl sterile saline) during inspiration. Before LPS treatment, mice were treated intravenously (via the penile vein) with antibodies to either MCP-1 or MIP-1α (20 μg in 200 μl phosphate-buffered saline (PBS)). Control mice received 20 μg isotype-matched IgG. Six hours after LPS instillation, mice were euthanized.
Determination of Lung MPO Activity
At the time points indicated, mouse lungs were perfused through the right ventricle with 3 ml of sterile PBS, snap-frozen in liquid nitrogen and stored at −70°C. To measure MPO activity, whole lungs were homogenized and sonicated in 50 mmol/L potassium phosphate buffer containing 0.5% hexadecyltrimethylammonium bromide (HTAB) and 5 mmol/L ethylene diaminetetraacetic acid (EDTA). After centrifugation at 12,000 × g for 10 minutes at 4°C, the supernatant fluids containing MPO were incubated in a 50 mmol/L potassium phosphate buffer containing the substrate, H2O2 (1.5 mol/L) and o-dianisidine dihydrochloride (167 μg/ml; Sigma Aldrich). The enzymatic activity was determined spectrophotometrically by measuring the change in absorbance at 460 nm over 3 minutes using a 96-well plate reader (Molecular Devices, Sunnyvale, CA). Values represent the change in OD/min per gram tissue weight.
Isolation of Peritoneal and Whole Blood PMN
Mouse peritoneal PMNs were isolated 6 hours after CLP or after peritoneal injection with 0.5 ml of 1.5% thioglycollate medium by lavaging the peritoneum three times with 4 ml of PBS. The cells were collected by centrifugation at 300 × g for 8 minutes at room temperature and the red blood cells eliminated by hypotonic lysis in water. The remaining PMNs were washed twice with PBS and resuspended in either PBS/0.1% bovine serum albumin (BSA) for chemotaxis and in vitro stimulation assays or Trizol for mRNA analysis. Peritoneal cell populations were found, in all experiments, to contain at least 95% PMNs as demonstrated by cytospin and differential stain analysis.
PMNs were isolated from rat blood as previously described.25 Briefly, blood was drawn from the inferior vena cava into syringes containing the anticoagulant ACD (Baxter Health Care, Deerfield, IL), mixed 1:1 with PBS and layered over Ficoll-Paque (Amersham Biosciences, Piscataway, NJ) in 50-ml centrifuge tubes. The tubes were centrifuged at 400 × g for 30 minutes at room temperature and the bottom pellet containing the PMNs and red blood cells was resuspended in 1% dextran (Amersham Biosciences) for 45 minutes to allow sedimentation of the PMN. Contaminating red blood cells were eliminated by hypotonic lysis in water and the remaining PMNs were either resuspended in PBS/0.1% BSA for chemotaxis and in vitro stimulation assays or in Trizol for mRNA analysis.
Assessment of Chemokine Binding to PMN by Flow Cytometry
Single channel flow cytometry was used to analyze binding of MCP-1 or MIP-1α to mouse peritoneal PMNs. Briefly, isolated PMNs were incubated for 60 minutes on ice with biotin-labeled mrMCP-1 or mrMIP-1α followed by 30 minutes incubation with fluorescein-conjugated avidin. Negative controls were incubated with a biotin-labeled soybean trypsin inhibitor. Cells were washed, fixed in 2% formaldehyde and analyzed by flow cytometry. To test for specificity of chemokine binding to cells, in some experiments, cells were pretreated with an anti-mrMCP-1 or anti-mrMIP-1α blocking antibody (R&D Systems, Inc.) for 15 minutes before addition of the biotin-labeled mrMCP-1.
Dual channel flow cytometry analysis was used to simultaneously analyze binding of MCP-1 or MIP-1α to blood PMN expressing the PMN specific antigen 7/4 (Caltag Laboratories). Whole blood was isolated from the inferior vena cava of mice into syringes containing the anticoagulant ACD (Baxter Health Care). Aliquots (100 μl) of whole blood were incubated with either biotin-labeled mrMCP-1, mrMIP-1α, or a soybean trypsin inhibitor (negative control) for 60 minutes followed by a 45-minute incubation with either phycoerythrin (PE)-conjugated anti-7/4 mAb or isotype-matched control IgG and fluorescein-conjugated avidin. All steps were performed on ice. Cells were washed, depleted of red blood cells by hypotonic lysis using FACS lysing solution (BD BioSciences), fixed in 2% formaldehyde and analyzed by flow cytometry.
All samples were analyzed on an EpicsXL flow cytometer (Coulter Corp., Miami, FL). MCP-1 and MIP-1α binding to peritoneal PMNs was detected by single channel flow cytometry. Cells were gated on forward and side scatter characteristics followed by analysis of MCP-1 in the fluorescein isothiocyanate (FITC) channel. For dual channel flow cytometry of MCP-1 or MIP-1α binding to 7/4+ blood PMNs, cells were gated on forward and side scatter characteristics followed by analysis in both the FITC (MCP-1 or MIP-1α) and PE (antigen 7/4) channels.
Chemotaxis of PMN to MCP-1 and MIP-1α
Chemotactic activity of isolated rat PMNs to MCP-1 or MIP-1α was monitored using a 96-well AB96 chemotaxis chamber according to the manufacturer’s instructions (Neuro Probe, Inc., Gaithersburg, MD). Briefly, PMNs were fluorescein-labeled with 2′,7′-bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein, acetoxymethyl ester (Molecular Probes, Eugene, OR) for 30 minutes at 37°C and loaded into the top of the polycarbonate filter (3 μm) at a concentration of 3.75 × 105 cells/well in Hanks’ balanced salt solution (HBSS) containing 0.1% BSA. The bottom wells of the chamber were loaded with 30 μl of either HBSS containing 0.1% BSA (negative control), formyl-methionyl-leucyl-phenylalanine (FMLP) (10−6 M; positive control) or various concentrations of rat recombinant MCP-1 or MIP-1α diluted in HBSS/BSA. The chamber was incubated for 30 minutes at 37°C and 5% CO2 (humidified), the filter was removed, and the non-migrated cells washed off by gentle scraping with a small squeegee followed by brief rinsing with PBS. PMN migration through the filter was detected by measuring the fluorescence of the filter using a SpectraMax fluorescent plate reader (Molecular Devices).
RT-PCR Analysis of CC Chemokine Receptor Expression
Total RNA was extracted from isolated PMNs using Trizol reagent (Life Technologies, Grand Island, NY) according to the manufacturer’s instructions and any contaminating DNA was degraded by a 30-minute incubation with RQ1 RNase-free DNase (Promega, Madison, WI). Reverse transcription was performed with 1 μg RNA using SuperScript II Rnase H-Reverse Transcriptase (Invitrogen Life Technologies, Carlsbad, CA) according to the manufacturer’s instructions. PCR was performed using the following sense and anti-sense oligonucleotide primers for CCR1, CCR2, CCR5, and the housekeeping gene GAPDH (control; rat and mouse):
Thermal cycling was performed under the following conditions: denaturation for 5 minutes at 94°C, followed by 40 cycles at 94°C for 1 minute, 60°C for 1 minute, and 72°C for 1 minute, with a final extension period at 72°C for 8 minutes. Product sizes were 425, 477, and 483 bp for mouse CCR1, CCR2, and CCR5, respectively. For rat, product sizes were 554, 597, and 465 bp for CCR1, CCR2, and CCR5, respectively. GAPDH product size was 400 bp. The products were analyzed by agarose gel (1.2%) electrophoresis in the presence of ethidium bromide and photographed digitally using the BioRad imaging system (Hercules, CA). Control RT-PCR experiments were also performed with the samples in the absence of reverse transcriptase to confirm the lack of contaminating genomic DNA. PCR was also performed using different cycle numbers to confirm DNA detection within the linear part of the amplification curve for all sets of primers.
In Vitro Stimulation of Peritoneal PMN
Mouse peritoneal PMN from 6-hour CLP animals were seeded at a density of 4 × 106 cells/ml and plated into 12-well plates at 0.5 ml/well in RPMI medium (Gibco-BRL) containing 10% fetal bovine serum (FBS). The cells were stimulated for 4 hours with MCP-1 (5 ng/ml), MIP-1α (5 ng/ml), and LPS (20 ng/ml), in various combinations or alone. After a 4-hour stimulation, the supernatants were removed, centrifuged at 300 × g for 10 minutes to remove remaining cells, and stored at −80°C for cytokine analysis.
Quantification of Chemokine and Cytokine Production by ELISA
Cytokine levels in serum or cell supernatants and MCP-1 or MIP-1α levels in whole lung homogenates were measured by ELISA. To measure chemokine levels in lung, whole lungs were homogenized in RIPA buffer containing 1X Complete EDTA-free protease inhibitor cocktail (Roche Diagnostics, Mannheim, Germany). Samples were extracted on ice for 30 minutes and centrifuged at 10,000 × g for 10 minutes at 4°C to clarify lysate. Immulon ELISA plates were coated overnight with capture antibodies to MCP-1, MIP-1α, or IL-6 at concentrations recommended by the manufacturers (see Reagents section). The plates were washed and blocked for at least 1 hour with PBS containing 3% bovine serum albumin. Various dilutions of samples with appropriate standards were added to the wells and incubated for 2 hours, followed by washing and incubation in appropriate biotinylated secondary antibody for 1 hour. Wells were washed and streptavidin-peroxidase was added for 30 minutes followed by washing and incubation in OPD substrate (Sigma Aldrich) for 10 minutes. The reaction was stopped by addition of 0.5 mol/L sulfuric acid. Absorbance was measured at 490 nm using a Molecular Devices plate reader. The detection limit for all chemokines ranged between 15 and 30 pg/ml.
Statistical Analysis
All numerical results are expressed as mean ± SEM. For these assays, statistical analysis was performed by one-way repeated-measures analysis of variance followed by a multiple comparison procedure with the Student-Newman Keuls method. A value of ≤0.05 was considered significant.
Results
MPO and Chemokine Content in Lungs of CLP Mice
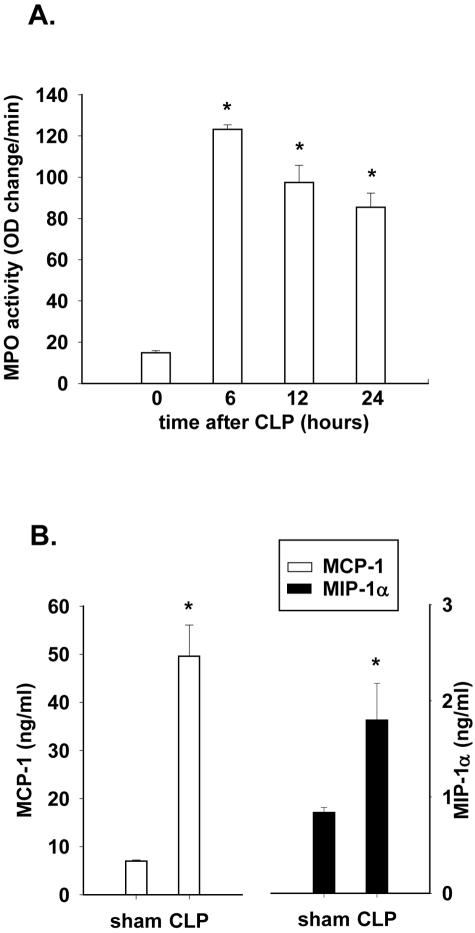
The time course for MPO buildup in lungs after CLP was determined in mice. Six hours after CLP, MPO content in lungs was increased nearly sixfold when compared to values in sham-operated mice, with slowly decreasing levels at later time points (Figure 1A). MPO activity was increased 4- to 5-fold at 12 and 24 hours when compared to MPO in lungs of sham-operated control mice (time 0).
Figure 1.
MPO activity and chemokine content in lungs of CLP mice. A: Time course of lung PMN accumulation (MPO content) after CLP. B: Measurements of MCP-1 and MIP-1α in whole lung extracts 6 hours after CLP. Results are mean ± SEM of two experiments with at least five mice per group, where * is P < 0.05 compared to sham-operated (time 0) control mice.
Increases in PMN accumulation at the 6-hour time point corresponded with an increase in lung MCP-1 and MIP-1α content as determined by ELISA (Figure 1B). Low levels of MCP-1 and MIP-1α were detectable in lung homogenates from control mice. However, 6 hours after CLP, both chemokines were significantly increased in content.
Role of MCP-1 and MIP-1α in MPO Buildup in Lungs of CLP Lungs
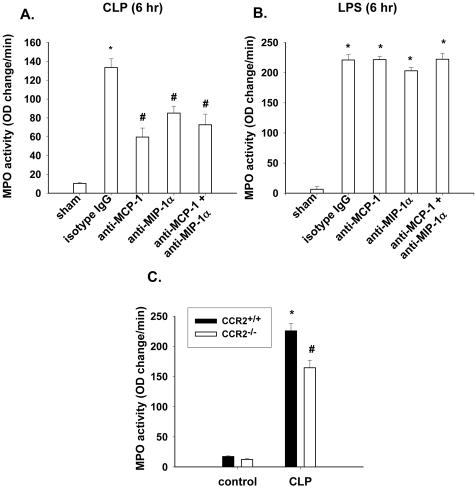
To assess roles for MCP-1 and MIP-1α in mediating MPO activity in CLP mice, we targeted blockade of either MCP-1 or MIP-1α, or both, using blocking antibodies to MCP-1 and MIP-1α (20 μg each), alone and in combination, or with an isotype-matched IgG at the onset of CLP. Blocking antibodies were infused intravenously and effects on lung MPO content were determined. As expected, 6 hours after CLP, MPO levels were significantly (P < 0.05) increased compared to lungs from sham-operated mice (Figure 2A). Treatment of CLP mice with anti-MCP-1 or anti-MIP-1α significantly reduced MPO activity by 55 and 36%, respectively, compared to IgG-treated CLP mice treated with isotype-matched IgG. The combination of both antibodies did not significantly reduce MPO content when compared to the use of either antibody alone.
Figure 2.
A: Antibody to MCP-1 and MIP-1α, alone or in combination, reduces lung buildup of MPO after CLP, but not after direct LPS-induced lung injury as determined by MPO activity in whole lung (B). C: MPO activity is also reduced in septic mouse lungs from CCR2−/− mice compared to CCR2+/+ mice. Results are mean ± SEM of two experiments with at least four mice per group, where * is P < 0.05 compared to uninjured and # is P < 0.05 compared to CLP + isotype group or CCR2+/+ CLP group.
To assess if decreased buildup of lung MPO in CLP mice was due to a change in blood PMN content in mice treated with antibody to MCP-1, blood PMN (assessed by flow cytometric analysis of 7/4+ cells) were evaluated in CLP mice given IgG or anti-MCP-1. Total neutrophil counts were found to be similar in the two groups (1476 ± 47 cells/μl for CLP mice treated with isotype-matched IgG and 2005 ± 222 cells/μl for anti-MCP-1-treated CLP mice), indicating that the antibodies did not induce neutropenia.
To determine whether MPO buildup in lungs of non-CLP mice is CC chemokine-dependent, airway instillation of LPS was done in the absence or presence of antibodies to either MCP-1 or MIP-1α, or both, and lung MPO activity measured. Six hours after airway instillation of LPS, MPO activity in mice given isotype IgG intravenously was significantly increased compared to sham-treated mice (Figure 2B). In the presence of antibodies to either MCP-1 or MIP-1α, or both, there was no significant reduction in MPO activity when compared to mice treated with isotype-matched IgG. Thus, after CLP but not after LPS-induced lung injury, lung buildup of MPO is dependent on MCP-1 and MIP-1α.
The ability of anti-MCP-1 to reduce MPO activity after CLP suggests a role for CCR2 in the buildup of MPO in lung. To assess this possibility, CCR2−/− mice underwent CLP and lung MPO activity was measured and compared to that in CCR2+/+ mouse lungs. In sham-operated mice, MPO levels were low and not significantly different in CCR2−/− and CCR2+/+ mice (Figure 2C). Six hours after CLP, there was a significant decrease (27%) in MPO activity in CCR2−/− mice when compared to values in CCR2+/+ mice. Thus, it appears after CLP that CCR2 is involved in the accumulation of MPO in lung.
Expression of CC Chemokine Receptor mRNA after CLP and Responsiveness of PMN to MCP-1 and MIP-1α
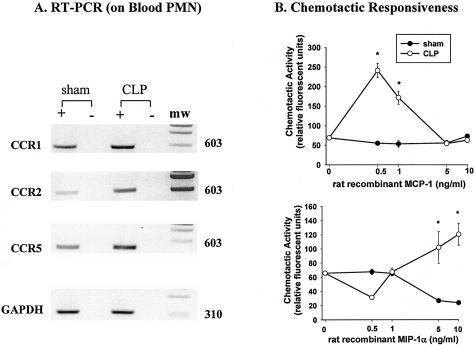
The results described above indicate that, after CLP, MPO buildup in lung is both MCP-1- and MIP-1α-dependent, suggesting that PMN acquire CCRs, which would allow them to respond to MCP-1 and MIP-1α. To assess this possibility, we analyzed mRNA in rat blood PMN from sham-operated or CLP rats, since adequate numbers of mouse blood PMN could not be obtained for such studies. Using RT-PCR analysis, blood PMN from sham rats demonstrated bands for CCR1, 2, and 5 (Figure 3A). Six hours after CLP, more intense bands for each of the three CCRs were found, especially for CCR2. In the presence of increased CCR expression in CLP rats, blood PMN responded chemotactically in vitro to both MIP-1α (a common ligand for both CCR1 and CCR5) and the ligand of CCR2, MCP-1 (Figure 3B). At a concentration of 0.5 ng/ml, MCP-1 induced peak migration of PMN isolated from rat blood 6 hours after CLP-induced sepsis, while PMN from sham-operated rats failed to respond chemotactically. For MIP-1α, a concentration of 5 ng/ml induced migration of septic rat blood PMN while no responses were found in blood PMN from sham-treated rats. The lack of reliable antibodies to these CCRs precluded direct protein measurements on PMN.
Figure 3.
Expression of CC chemokine receptor mRNA and in vitro chemotactic responsiveness to CC chemokines. A: RT-PCR analysis of CCR1, CCR2, and CCR5 expression in PMN 6 hours after CLP as compared to PMN from sham animals. Samples were run in the absence (−) and presence (+) of reverse transcriptase to confirm lack of DNA contamination. Blots are representative of two separate experiments where n = 4 rats per group. B: Chemotaxis of blood PMN to MCP-1 and MIP-1α obtained from rats 6 hours after CLP or from sham animals. Results are the mean ± SEM of two experiments performed in triplicate with n = 4 rats per group.
Binding of MCP-1 and MIP-1α to Blood PMN from CLP Mice
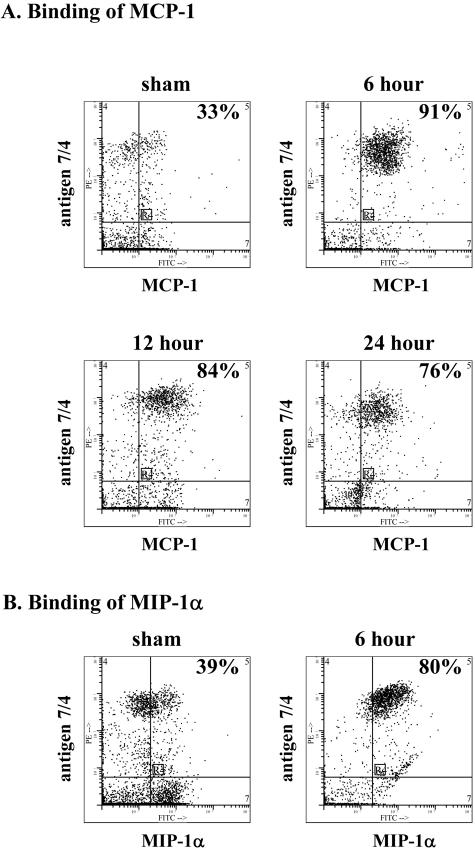
To assess binding of MCP-1 and MIP-1α to blood PMN from CLP mice, we analyzed peripheral blood PMN from CLP (at 6, 12, and 24 hours) and compared the results using sham-operated (at time 0) mice for the ability of PMN to bind MCP-1. Using a dual-labeling procedure to identify PMN, a limited percentage (33%) of blood PMN from sham-operated mice (control) showed binding of MCP-1 (Figure 4A), which is consistent with the PCR data in Figure 3. However, after CLP, there was a dramatic increase in the number of PMN binding MCP-1. The percentage of PMN binding MCP-1 rose at 6, 12, and 24 hours after CLP to values of 91%, 84%, and 76%, respectively (Figure 4A). Similarly, blood PMN were evaluated for binding of MIP-1α. Blood PMN from sham mice bound MIP-1α (39%), whereas this value rose to 80% in blood PMN obtained 6 hours after CLP (Figure 4B). Interestingly, in a separate experiment, there was no increase in binding of MCP-1 or MIP-1α to blood PMN obtained from sham mice (13 + 4 and 14 + 5%, respectively) when compared to blood PMN obtained from mice after intraperitoneal injection of 1.5% thioglycollate (15 + 4 and 17 + 2%, respectively), indicating that a localized peritonitis does not induce systemic changes in PMN CCRs.
Figure 4.
Binding of MCP-1 and MIP-1α to peripheral blood PMN of mice. A: In sham-operated mice, 33% of PMN (7/4+) showed evidence of MCP-1 binding whereas 6 to 24 hours after CLP there were dramatic increases in the percentage of PMN binding MCP-1. B: Increased binding of MIP-1α to blood PMN was also found 6 hours after CLP. Profiles are representative of two experiments with n = 5 mice per group.
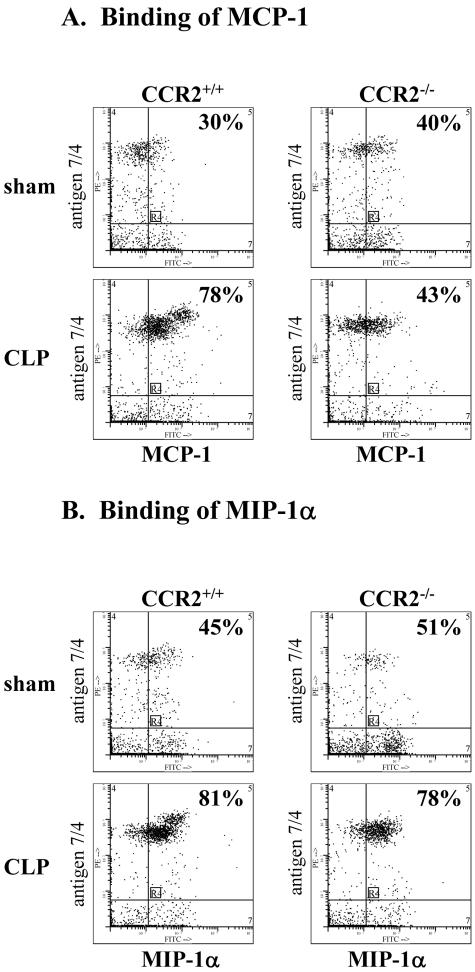
The lack of increased binding of MCP-1 to CCR2−/− blood PMN from CLP mice was also demonstrated (Figure 5A). Blood PMN from sham-operated CCR2+/+ and CCR2−/− mice showed limited binding of MCP-1 (30% and 40%, respectively). Six hours after CLP, this value rose to 78% in CCR2+/+ blood PMN, but no significant increase in MCP-1 binding was observed for CCR2−/− blood PMN after CLP (when compared to sham PMN (43% and 40%, respectively), confirming specificity of MCP-1 for CCR2 on these PMN. Binding of MIP-1α to these PMN was also evaluated (Figure 5B). Blood PMN from sham CCR2+/+ and CCR2−/− mice bound MIP-1α (45 and 51%, respectively), whereas these values rose dramatically in both CCR2+/+ and CCR2−/− blood PMN obtained 6 hours after CLP (81 and 78%, respectively). These data are consistent with the fact that MIP-1α binds to receptors different from CCR2.
Figure 5.
A and B: Binding of labeled MCP-1 and MIP-1α to blood PMN from CCR2+/+ and CCR2−/− mice 6 hours after CLP. MIP-1α binding to PMN from CCR2+/+ and CCR2−/− mice was similar for sham-operated mice with increases 6 hours after CLP. Profiles are representative of three experiments with n = 4 mice per group.
Expression of CC Chemokine Receptor mRNAs and Binding of MCP-1 and MIP-1α to Peritoneal Exudate PMN
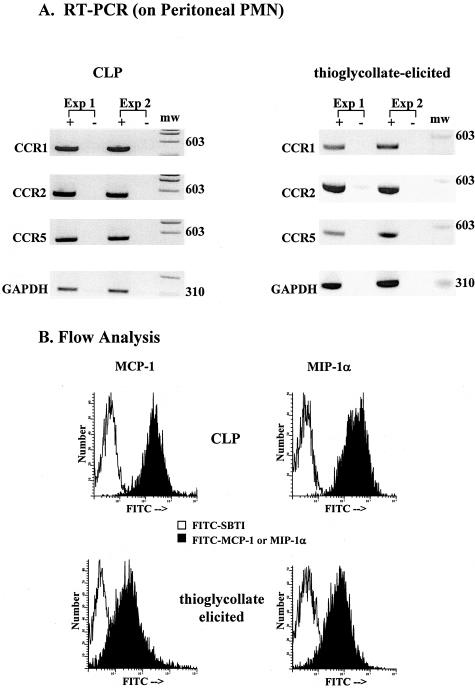
To determine whether exudate PMN express CC chemokine receptors and bind CC chemokines, we isolated mouse PMN from the peritoneum 6 hours after CLP or thioglycollate injection and analyzed cell content of CCR1, CCR2, and CCR5 expression by RT-PCR. As shown in Figure 6, mouse peritoneal PMN from both CLP- and thioglycollate-injected mice demonstrated strong mRNA band patterns for CCR1, 2, and 5 (Figure 6A). Furthermore, the binding of MCP-1 and MIP-1α to these PMN was very high (mean fluorescent units: 316.8 and 397.8, respectively, for CLP PMN, and 103.0 and 108.2, respectively, for thioglycollate-elicited PMN) (Figure 6B). Little or no binding for irrelevant biotinylated peptide (SBTI) of similar molecular weight was demonstrated (mean fluorescent units: 13.9 and 22.3 for CLP PMN, and 4.4 and 5.7 for thioglycollate-elicited PMN). These data suggest that peritoneal exudate PMN acquire mRNA for CCR1, 2, and 5 and demonstrate binding of MCP-1 and MIP-1α.
Figure 6.
Expression of CCR 1, 2, 5 and binding of CC chemokines to peritoneal PMN from CLP-operated or thioglycollate-treated mice. A: RT-PCR analysis of CCR1, CCR2, and CCR5 expression in PMN after 6 hours CLP or thioglycollate treatment. Samples were run in the absence (−) and presence (+) of reverse transcriptase to confirm lack of DNA contamination. Blots are from two separate experiments each where n = 4 mice per experiment. B: Binding of PMN to labeled MCP-1 and MIP-1α after 6 hours CLP or thioglycollate treatment. FITC-SBTI was used as a control peptide for binding studies. Histograms are representative of two experiments performed in duplicate with n = 4 mice per experiment.
Regulation of IL-6 by MCP-1 and MIP-1α after CLP
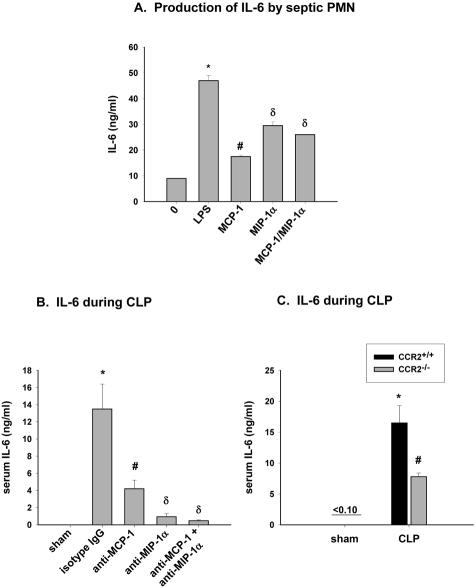
IL-6 is known to play a role in the response to sepsis. Recent studies indicate IL-6 production by blood PMN after in vitro exposure to C5a and LPS.26 The role of MCP-1 and MIP-1α in regulating IL-6 production by mouse PMN during sepsis was evaluated. After 6 hours CLP, peritoneal PMN were isolated and stimulated with 5 ng/ml MCP-1 or MIP-1α, or the combination. IL-6 production was measured by ELISA with LPS stimulated PMN representing a positive control. IL-6 production was low in non-stimulated PMN (Figure 7A). However, after incubation with MCP-1 or MIP-1α, the release of IL-6 was increased. Exposure of PMN to the combination of MCP-1 and MIP-1α did not induce further increases in levels of released IL-6.
Figure 7.
Role of MCP-1 and MIP-1α on serum IL-6 levels during CLP. A: Peritoneal PMN were isolated from CLP mice 6 hours after CLP and stimulated for 4 hours with either 20 ng/ml LPS (positive control) or 5 ng/ml MCP-1 and MIP-1α, each alone or together, and IL-6 release into supernatant was measured. Values represent mean ± SEM of two experiments performed in triplicate, with n = 4 mice per experiment, where * and # is P < 0.05 compared to no treatment, and δ is P < 0.05 compared to MCP-1-treated. B: Serum IL-6 levels in CLP mice treated with antibodies to MCP-1 and MIP-1α, each alone or together. Six hours after CLP, serum was collected, and IL-6 measured by ELISA. Values represent mean ± SEM of two experiments with n = 4 mice per treatment group, where * and # is P < 0.05 compared to sham-operated and δ is P < 0.05 compared to anti-MCP-1-treated. C: IL-6 was measured in serum from CCR2+/+ and CCR2−/− mice 6 hours after CLP. Values represent the mean ± SEM of two experiments with n = 4 mice per group where * is P < 0.05 compared to sham-operated and # is P < 0.05 compared to CCR2+/+ CLP group.
The ability of MCP-1 and MIP-1α to affect blood PMN IL-6 levels after CLP was examined in CLP mice that had been treated with antibodies to either MCP-1 or MIP-1α, or the combination. After 6 hours CLP, IL-6 serum levels in CLP mice with isotype matched IgG were significantly elevated when compared to levels in sham-operated mice (Figure 7B). Treatment with anti-MCP-1 or anti-MIP-1α significantly reduced IL-6 levels in the CLP mice by 70 and 93%, respectively, when compared to IgG-treated CLP mice. No further reduction in IL-6 levels after CLP was observed in the presence of both antibodies when compared to anti-MCP-1 or anti-MIP-1α treatment alone. Serum levels of IL-6 were also measured in CCR2−/− mice 6 hours after CLP and compared to that of CCR2+/+ mice (Figure 7C). In sham-operated mice, IL-6 levels were undetectable in both CCR2+/+ and CCR2−/− mice. However, 6 hours after CLP, IL-6 levels were significantly decreased in CCR2−/− (54%) when compared to CCR2+/+ CLP levels. Thus, it appears that, under conditions of sepsis, MCP-1 and MIP-1α enhance serum levels of IL-6 in CLP mice. Furthermore, in the absence of CCR2, the serum levels of IL-6 are diminished.
Discussion
In this study, we show expression of the CC chemokine receptors (CCR1, CCR2, and CCR5) on PMN from septic mice. To date, only macrophages, T-cells, lymphocytes, and mast cells have been shown to naturally possess these receptors in vivo. PMN are not thought to express these receptors. However, previous studies have shown that GM-CSF or IFNγ can induce expression of CCR1 or CCR3 in PMN.15,16 To date, no single agonist has been shown to induce CCR2 expression on PMN. We have been unable to induce CCR2 expression on human PMN stimulated with LPS, TNFα, or INFγ (data not shown). Binding of MCP-1 or MIP-1α to peripheral blood PMN from mice after intraperitoneal injection of thioglycollate failed to occur, although peritoneal PMN did express increased binding of these CC chemokines. Thus, it is possible that CC chemokine receptor (CCR1, CCR2, and CCR5) expression on peripheral blood PMN may be limited to the CLP condition.
While CC chemokine effects are currently thought to occur through their ability to facilitate monocyte influx and to activate macrophages to release inflammatory mediators, induction of CC chemokine receptors on PMN suggest another pathway by which the CC chemokines are able to enhance the PMN inflammatory response in sepsis. Expression of CC chemokine receptors on blood PMN from septic mice occurred along with increased lung CC chemokine levels and elevated MPO content. Furthermore, septic CCR2−/− mice or septic mice treated with antibodies to either MCP-1 or MIP-1α displayed reduced lung buildup of MPO compared to sham-treated mice, suggesting that they may play a role in mediating PMN recruitment. These results are in agreement with previous studies involving septic models where blockade of MIP-1α or MCP-1 was shown to reduce neutrophil recruitment. Anti-MIP-1α reduced lung PMN build-up after systemic exposure to LPS.20 More recently, blockade of MCP-1 was shown to reduce peritoneal PMN recruitment after acute septic peritonitis.27 In these studies, MCP-1 and MIP-1α have been postulated to mediate their effects on PMN accumulation indirectly by modulating the activity of macrophages or endothelial cells, via release of early inflammatory mediators (leukotriene B4, TNFα) or expression of ICAM-1, respectively, which would favor PMN recruitment. In the present study, treatment of septic mice with antibodies to MCP-1 or MIP-1α also reduced serum IL-6 levels. IL-6 is an early inflammatory mediator known to induce ICAM-1 expression on endothelial cells.28 Our recent studies have indicated that in the setting of sepsis, IL-6 induces up-regulation of C5aR in various organs in vivo and corresponds with decreased survival.29 Also, in vitro exposure of endothelial cells to IL-6 causes increases in mRNA for C5aR as well as C5aR protein.30 These data suggest that, in sepsis, MCP-1 and MIP-1α may have adverse effects by inducing up-regulation of IL-6.
Recent studies with neutrophil-depleted mice have suggested that PMN may be major contributors to serum IL-6 levels during sepsis.26 Treatment of septic peritoneal PMN in the present study with MCP-1 or MIP-1α induced IL-6 production. Therefore, in addition to its chemotactic role in mediating PMN migration, MCP-1 and MIP-1α may also regulate PMN migration indirectly by stimulating their release of IL-6. Interestingly, in the previous study,26 treatment of septic mice with antibodies to C5a also reduced serum IL-6 levels which leads one to speculate a possible connection between C5a/C5aR activation/internalization and induction of CC chemokine receptor expression on septic PMN. In fact, C5a by itself was unable to induce IL-6 production from isolated rat blood neutrophils, suggesting a possible dependence of C5a on MCP-1 or MIP-1α signaling through their respective receptors to induce IL-6 production. Studies addressing this possible scenario are currently ongoing in our lab.
Consistent with the expression of CC chemokine receptors on PMN, the ability of blood PMN to respond to CC chemokines may be unique to the septic condition. In LPS-induced acute lung injury, blockade of MIP-1α or MCP-1 does not affect the buildup of MPO in lung (Figure 2). This is consistent with previous studies in which MCP-1 or CCR2 could not be shown to influence PMN buildup in lung after lung deposition of LPS or IgG immune complex injury.14,17 However, a contrasting report has suggested that MIP-1α is capable of mediating PMN migration in a rat model of acute lung LPS injury which may indicate a species-specific difference in PMN responsiveness to CC chemokines.31
The data in the current report suggest that CLP-induced sepsis orchestrates a series of changes on blood PMN, resulting in their ability to bind MCP-1 and MIP-1α, implying induction of CCR1, CCR2, and CCR5. Such changes have also been found on peritoneal PMN induced by thioglycollate but not on blood PMN from the same animals. These results are consistent with previous studies showing the ability of thioglycollate-elicited PMN to respond in a chemotactic manner to MIP-1α.32 The presence of these CC chemokine receptors on peritoneal PMN, but not on naïve blood PMN, implies that CC chemokine receptor expression on peritoneal PMN can occur and may amplify innate immune responses. CCR2, but not CCR5, functions as a pro-inflammatory mediator responsible for promoting fibrosis in a bleomycin-induced pulmonary fibrosis model.33 MCP-1/CCR2 has also been shown to play a role in mediating tumor angiogenesis both in vitro and in vivo independent of monocyte recruitment.34,35 Recent progress in the area of apoptosis also suggests that CCR2/MCP-1 interaction delays neuronal and astrocytic apoptosis.36,37 Delayed apoptosis of PMN has been described in patients with severe sepsis.38 Evidence from animal studies supports this finding.39 However, the pathophysiological mechanisms responsible for extending the life span of the neutrophil during sepsis is poorly described. GM-CSF, which is present in high levels in serum from septic patients, has been shown to delay in vitro apoptosis of PMN.40 Previous studies have also demonstrated CCR1 up-regulation on PMN in response to GM-CSF.16 Therefore, MIP-1α binding and subsequent activation of these CC chemokine receptors on PMN in response to GM-CSF may be a mechanism by which GM-CSF inhibits apoptosis during sepsis.
In summary, the acquisition of MCP-1 and MIP-1α binding to blood PMN during sepsis leads to engagement of a CC pathway for PMN recruitment. This pathway, which does not normally appear to be operative, allows increased PMN (defined by MPO content) accumulation in lung. In addition, peritoneal PMN have been shown to express these receptors, implying that CC chemokine receptor activation on PMN may amplify PMN responses in tissues. Further studies aimed at defining the individual roles of these PMN-specific CC chemokine receptors in mediating lung accumulation and activation of PMN may provide novel insight into the mechanism(s) involved in the pathogenesis of sepsis.
Acknowledgments
We thank Ronald Craig for his assistance with the flow cytometric analyses and Beverly Schumann for her clerical assistance in the preparation of this manuscript.
Footnotes
Address reprint requests to Peter A. Ward, M.D., Department of Pathology, University of Michigan Medical School, 1301 Catherine Road, Ann Arbor, MI 48109-0602. E-mail: pward@umich.edu.
Supported by National Institutes of Health Grant GM-029507.
References
- Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- Hakansson L, Venge P. Inhibition of neutrophil and eosinophil chemotactic responses to PAF by the PAF-antagonists WEB-2086, L-652,731, and SRI-63441. J Leukoc Biol. 1990;47:449–456. doi: 10.1002/jlb.47.5.449. [DOI] [PubMed] [Google Scholar]
- Mulligan MS, Schmid E, Beck-Schimmer B, Till GO, Friedl HP, Brauer RB, Hugli TE, Miyasaka M, Warner RL, Johnson KJ, Ward PA. Requirement and role of C5a in acute lung inflammatory injury in rats. J Clin Invest. 1996;98:503–512. doi: 10.1172/JCI118818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid E, Warner RL, Crouch LD, Friedl HP, Till GO, Hugli TE, Ward PA. Neutrophil chemotactic activity and C5a following systemic activation of complement in rats. Inflammation. 1997;21:325–333. doi: 10.1023/a:1027302017117. [DOI] [PubMed] [Google Scholar]
- Lukacs NW, Hogaboam C, Campbell E, Kunkel SL. Chemokines: function, regulation, and alteration of inflammatory responses. Chem Immunol. 1999;72:102–120. doi: 10.1159/000058729. [DOI] [PubMed] [Google Scholar]
- Rollins BJ. Chemokines. Blood. 1997;90:909–928. [PubMed] [Google Scholar]
- von Andrian UH, Chambers JD, McEvoy LM, Bargatze RF, Arfors KE, Butcher EC. Two-step model of leukocyte-endothelial cell interaction in inflammation: distinct roles for LECAM-1 and the leukocyte beta 2 integrins in vivo. Proc Natl Acad Sci USA. 1991;88:7538–7542. doi: 10.1073/pnas.88.17.7538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo RF, Riedemann NC, Laudes IJ, Sarma VJ, Kunkel RG, Dilley KA, Paulauskis JD, Ward PA. Altered neutrophil trafficking during sepsis. J Immunol. 2002;169:307–314. doi: 10.4049/jimmunol.169.1.307. [DOI] [PubMed] [Google Scholar]
- Ibbotson GC, Doig C, Kaur J, Gill V, Ostrovsky L, Fairhead T, Kubes P. Functional alpha4-integrin: a newly identified pathway of neutrophil recruitment in critically ill septic patients. Nat Med. 2001;7:465–470. doi: 10.1038/86539. [DOI] [PubMed] [Google Scholar]
- Kubes P, Niu XF, Smith CW, Kehrli ME, Jr, Reinhardt PH, Woodman RC. A novel beta 1-dependent adhesion pathway on neutrophils: a mechanism invoked by dihydrocytochalasin B or endothelial transmigration. FASEB J. 1995;9:1103–1111. [PubMed] [Google Scholar]
- Reinhardt PH, Elliott JF, Kubes P. Neutrophils can adhere via alpha4beta1-integrin under flow conditions. Blood. 1997;89:3837–3846. [PubMed] [Google Scholar]
- Olson T, Ley K. Chemokines and chemokine receptors in leukocyte trafficking. Am J Physiol. 2002;283:R7–R28. doi: 10.1152/ajpregu.00738.2001. [DOI] [PubMed] [Google Scholar]
- Johnston B, Burns AR, Suematsu M, Issekutz TB, Woodman RC, Kubes P. Chronic inflammation upregulates chemokine receptors and induces neutrophil migration to monocyte chemoattractant protein-1. J Clin Invest. 1999;103:1269–1276. doi: 10.1172/JCI5208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bless NM, Huber-Lang M, Guo RF, Warner RL, Schmal H, Czermak BJ, Shanley TP, Crouch LD, Lentsch AB, Sarma V, Mulligan MS, Friedl HP, Ward PA. Role of CC chemokines (macrophage inflammatory protein-1 beta, monocyte chemoattractant protein-1, RANTES) in acute lung injury in rats. J Immunol. 2000;164:2650–2659. doi: 10.4049/jimmunol.164.5.2650. [DOI] [PubMed] [Google Scholar]
- Bonecchi R, Polentarutti N, Luini W, Borsatti A, Bernasconi S, Locati M, Power C, Proudfoot A, Wells TN, Mackay C, Mantovani A, Sozzani S. Up-regulation of CCR1 and CCR3 and induction of chemotaxis to CC chemokines by IFN-gamma in human neutrophils. J Immunol. 1999;162:474–479. [PubMed] [Google Scholar]
- Cheng SS, Lai JJ, Lukacs NW, Kunkel SL. Granulocyte-macrophage colony stimulating factor up-regulates CCR1 in human neutrophils. J Immunol. 2001;166:1178–1184. doi: 10.4049/jimmunol.166.2.1178. [DOI] [PubMed] [Google Scholar]
- Speyer CL, Neff TA, Warner RL, Guo RF, Sarma JV, Riedemann NC, Murphy ME, Murphy HS, Ward PA. Regulatory effects of iNOS on acute lung inflammatory responses in mice. Am J Pathol. 2003;163:2319–2328. doi: 10.1016/S0002-9440(10)63588-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy PM, Baggiolini M, Charo IF, Hebert CA, Horuk R, Matsushima K, Miller LH, Oppenheim JJ, Power CA. International union of pharmacology: XXII. Nomenclature for chemokine receptors. Pharmacol Rev. 2000;52:145–176. [PubMed] [Google Scholar]
- Murphy PM. International Union of Pharmacology: XXX. Update on chemokine receptor nomenclature. Pharmacol Rev. 2002;54:227–229. doi: 10.1124/pr.54.2.227. [DOI] [PubMed] [Google Scholar]
- Standiford TJ, Kunkel SL, Lukacs NW, Greenberger MJ, Danforth JM, Kunkel RG, Strieter RM. Macrophage inflammatory protein-1 alpha mediates lung leukocyte recruitment, lung capillary leak, and early mortality in murine endotoxemia. J Immunol. 1995;155:1515–1524. [PubMed] [Google Scholar]
- Goodman RB, Strieter RM, Martin DP, Steinberg KP, Milberg JA, Maunder RJ, Kunkel SL, Walz A, Hudson LD, Martin TR. Inflammatory cytokines in patients with persistence of the acute respiratory distress syndrome. Am J Respir Crit Care Med. 1996;154:602–611. doi: 10.1164/ajrccm.154.3.8810593. [DOI] [PubMed] [Google Scholar]
- Bossink AW, Paemen L, Jansen PM, Hack CE, Thijs LG, Van Damme J. Plasma levels of the chemokines monocyte chemotactic proteins-1 and -2 are elevated in human sepsis. Blood. 1995;86:3841–3847. [PubMed] [Google Scholar]
- Kuziel WA, Morgan SJ, Dawson TC, Griffin S, Smithies O, Ley K, Maeda N. Severe reduction in leukocyte adhesion and monocyte extravasation in mice deficient in CC chemokine receptor 2. Proc Natl Acad Sci USA. 1997;94:12053–12058. doi: 10.1073/pnas.94.22.12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czermak BJ, Sarma V, Pierson CL, Warner RL, Huber-Lang M, Bless NM, Schmal H, Friedl HP, Ward PA. Protective effects of C5a blockade in sepsis. Nat Med. 1999;5:788–792. doi: 10.1038/10512. [DOI] [PubMed] [Google Scholar]
- Riedemann NC, Guo RF, Bernacki KD, Reuben JS, Laudes IJ, Neff TA, Gao H, Speyer C, Sarma VJ, Zetoune FS, Ward PA. Regulation by C5a of neutrophil activation during sepsis. Immunity. 2003;19:193–202. doi: 10.1016/s1074-7613(03)00206-1. [DOI] [PubMed] [Google Scholar]
- Riedemann NC, Guo RF, Hollmann TJ, Gao H, Neff TA, Reuben JS, Speyer CL, Sarma JV, Wetsel RA, Zetoune FS, Ward PA. Regulatory role of C5a in LPS-induced IL-6 production by neutrophils during sepsis. FASEB J. 2004;18:370–372. doi: 10.1096/fj.03-0708fje. [DOI] [PubMed] [Google Scholar]
- Matsukawa A, Hogaboam CM, Lukacs NW, Lincoln PM, Strieter RM, Kunkel SL. Endogenous monocyte chemoattractant protein-1 (MCP-1) protects mice in a model of acute septic peritonitis: cross-talk between MCP-1 and leukotriene B4. J Immunol. 1999;163:6148–6154. [PubMed] [Google Scholar]
- Romano M, Sironi M, Toniatti C, Polentarutti N, Fruscella P, Ghezzi P, Faggioni R, Luini W, van Hinsbergh V, Sozzani S, Bussolino F, Poli V, Ciliberto G, Mantovani A. Role of IL-6 and its soluble receptor in induction of chemokines and leukocyte recruitment. Immunity. 1997;6:315–325. doi: 10.1016/s1074-7613(00)80334-9. [DOI] [PubMed] [Google Scholar]
- Riedemann NC, Neff TA, Guo RF, Bernacki KD, Laudes IJ, Sarma JV, Lambris JD, Ward PA. Protective effects of IL-6 blockade in sepsis are linked to reduced C5a receptor expression. J Immunol. 2003;170:503–507. doi: 10.4049/jimmunol.170.1.503. [DOI] [PubMed] [Google Scholar]
- Laudes IJ, Chu JC, Huber-Lang M, Guo RF, Riedemann NC, Sarma JV, Mahdi F, Murphy HS, Speyer C, Lu KT, Lambris JD, Zetoune FS, Ward PA. Expression and function of C5a receptor in mouse microvascular endothelial cells. J Immunol. 2002;169:5962–5970. doi: 10.4049/jimmunol.169.10.5962. [DOI] [PubMed] [Google Scholar]
- Shanley TP, Schmal H, Friedl HP, Jones ML, Ward PA. Role of macrophage inflammatory protein-1 alpha (MIP-1 alpha) in acute lung injury in rats. J Immunol. 1995;154:4793–4802. [PubMed] [Google Scholar]
- Gao JL, Wynn TA, Chang Y, Lee EJ, Broxmeyer HE, Cooper S, Tiffany HL, Westphal H, Kwon-Chung J, Murphy PM. Impaired host defense, hematopoiesis, granulomatous inflammation and type 1-type 2 cytokine balance in mice lacking CC chemokine receptor 1. J Exp Med. 1997;185:1959–1968. doi: 10.1084/jem.185.11.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore BB, Paine R, 3rd, Christensen PJ, Moore TA, Sitterding S, Ngan R, Wilke CA, Kuziel WA, Toews GB. Protection from pulmonary fibrosis in the absence of CCR2 signaling. J Immunol. 2001;167:4368–4377. doi: 10.4049/jimmunol.167.8.4368. [DOI] [PubMed] [Google Scholar]
- Ito WD, Arras M, Winkler B, Scholz D, Schaper J, Schaper W. Monocyte chemotactic protein-1 increases collateral and peripheral conductance after femoral artery occlusion. Circ Res. 1997;80:829–837. doi: 10.1161/01.res.80.6.829. [DOI] [PubMed] [Google Scholar]
- Salcedo R, Ponce ML, Young HA, Wasserman K, Ward JM, Kleinman HK, Oppenheim JJ, Murphy WJ. Human endothelial cells express CCR2 and respond to MCP-1: direct role of MCP-1 in angiogenesis and tumor progression. Blood. 2000;96:34–40. [PubMed] [Google Scholar]
- Muessel MJ, Klein RM, Wilson AM, Berman NE. Ablation of the chemokine monocyte chemoattractant protein-1 delays retrograde neuronal degeneration, attenuates microglial activation, and alters expression of cell death molecules. Brain Res Mol Brain Res. 2002;103:12–27. doi: 10.1016/s0169-328x(02)00158-4. [DOI] [PubMed] [Google Scholar]
- Eugenin EA, D’Aversa TG, Lopez L, Calderon TM, Berman JW. MCP-1 (CCL2) protects human neurons and astrocytes from NMDA or HIV-tat-induced apoptosis. J Neurochem. 2003;85:1299–1311. doi: 10.1046/j.1471-4159.2003.01775.x. [DOI] [PubMed] [Google Scholar]
- Weiss M, Elsharkawi M, Welt K, Schneider EM. Transient leukocytosis, granulocyte colony-stimulating factor plasma concentrations, and apoptosis determined by binding of annexin V by peripheral leukocytes in patients with severe sepsis. Ann NY Acad Sci. 2003;1010:742–747. doi: 10.1196/annals.1299.134. [DOI] [PubMed] [Google Scholar]
- Keel M, Ungethum U, Steckholzer U, Niederer E, Hartung T, Trentz O, Ertel W. Interleukin-10 counter-regulates proinflammatory cytokine-induced inhibition of neutrophil apoptosis during severe sepsis. Blood. 1997;90:3356–3363. [PubMed] [Google Scholar]
- Colotta F, Re F, Polentarutti N, Sozzani S, Mantovani A. Modulation of granulocyte survival and programmed cell death by cytokines and bacterial products. Blood. 1992;80:2012–2020. [PubMed] [Google Scholar]