Abstract
Vascular adhesion protein-1 (VAP-1) has been shown to mediate lymphocyte adhesion to endothelia at sites of inflammation, but its functional role in vivo has not been tested in any rodent model. Here we report the effects of VAP-1 blockade on rat liver allograft rejection. BN recipients of PVG liver allografts (known to develop acute rejection by day 7) were treated with 2 mg/kg anti-VAP-1 (a new anti-rat VAP-1 mAb 174–5) or isotype-matched irrelevant antibody (NS1) every other day (n = 6/group) and one group with anti-VAP-1 2 mg/kg daily (n = 7). On day 7, samples were collected for transplant aspiration cytology, histology, and immunohistochemistry. Lymphocyte infiltration to the graft was clearly affected by VAP-blockade. The total inflammation, mainly the number of active lymphoid cells, in transplant aspiration cytology was significantly decreased in animals treated with anti-VAP-1 (4.7 ± 1.0 and 2.4 ± 1.0 corrected increment units, respectively) compared to control (6.6 ± 1.0) (P < 0.05). In histology, the intensity of portal inflammation was significantly decreased (P < 0.05). The amount of T cells expressing activation markers diminished. This is the first demonstration in any prolonged in vivo model that VAP-1 plays an important role in lymphocyte infiltration to sites of inflammation, and, in particular, liver allograft rejection.
The hallmark of liver allograft rejection is the influx of inflammatory cells, mainly lymphocytes and monocytes/macrophages, into the graft. This process involves sequential adhesive interactions between the leukocyte and the endothelium. The complex process of adhesion and diapedesis of leukocytes into the tissue sites of inflammation is coordinated by several adhesion molecules.1 During the course of liver rejection, expression of adhesion molecules such as ICAM-1, VCAM-1, and E-selectin is induced on endothelial cells.2,3
Vascular adhesion protein-1 (VAP-1) is a dimeric endothelial transmembrane protein that has been demonstrated to mediate lymphocyte binding to peripheral lymph node high endothelial venules (HEV) and also to be induced at sites of inflammation.4–6 VAP-1 has been suggested to play a significant role in controlling entry of lymphocytes into sites of inflammation.5 However, due to lack of suitable reagents so far this has only been shown in vivo in a short-term (4-hour) treatment model of acute peritonitis in rabbits.7
We have previously shown that VAP-1 is up-regulated in acute liver allograft rejection in the rat.8 In man, VAP-1 expression is reported to be similar in both uninflamed livers and liver grafts with rejection, and in primary biliary cirrhosis.9 However VAP-1 was demonstrated by an in vitro adhesion assay to be important in mediating T-cell adhesion to endothelia in liver tissue9 and to cultured hepatic endothelial cells displaying charasteristics of sinusoidal endothelial cells.10 Serum levels of the soluble form of VAP-1 have been shown to be elevated in certain inflammatory liver diseases.11 Since sinusoids do not express selectins, VAP-1 could play a greater role in hepatic sinusoidal vascular bed than in other organs.12
The effect of prolonged VAP-1 blockade on the inflammatory response in the liver or, in fact in any in vivo model, has not previously been demonstrated. In this study we show that VAP-1 blockade significantly decreases the inflammatory response in rat liver allograft rejection.
Materials and Methods
Rats
A fully allogeneic donor-recipient combination of PVG (RT1c) into BN (RT1n) (both from Harlan, Horst, The Netherlands) was used. This strain combination is known to develop intense acute liver allograft rejection in approximately 1 week and has a mean survival of 36 days after liver transplantation.13,14 Development of tolerance has not been reported in this strain combination. The rats were fed with regular rat food and tap water ad libitum. The animals received humane care according to the criteria outlined in the “Guide for the Care and Use of Laboratory Animals” (National Institutes of Health). The study was approved by the committee for experimental research of the Helsinki University Hospital and the regional authorities.
Transplantation
Liver transplantation under ether anesthesia was performed using the technique introduced by Kamada.15 Briefly, cuff technique was used for the portal and infrahepatic vena cava anastomoses, and 7−0 continuous suture for the suprahepatic vena cava anastomosis. The anhepatic period lasted 20 minutes or less in all operations included in the study. The cold ischemia time did not exceed 1 hour.
Anti-VAP-1 Monoclonal Antibody
Mice were immunized subcutaneously with purified vessels from human peripheral lymph nodes to isolate new mAbs against endothelial antigens. The lymphocytes isolated from a draining lymph node were fused with SP2/0 myeloma cells using standard techniques. The resulting hybridomas were screened using immunohistochemical stainings of frozen sections as described. One of the new antibodies (174) appeared to stain VAP-1 in human tissues. This antibody was subcloned (174–5) and tested also with rat organs.
The specificity of the mAb against VAP-1 was ascertained using immunofluorescent staining of Ax cells stably transfected with VAP-1 cDNA or with the vector alone using published protocols.16 The function-blocking capacity of the mAb 174–5 was tested in a Stamper-Woodruff binding assay.17 In brief, frozen sections were overlaid with mAb 174–5 or control mAbs, and peripheral blood lymphocytes were then incubated on the sections under constant rotation (60 rpm) at 7°C for 30 minutes. The non-adherent cells were tilted off and the adherent cells fixed with glutaraldehyde. The number of lymphocytes binding to morphologically distinct endothelial cells of HEV was enumerated under dark-field microscopy.
174–5 (mouse IgG1) and an isotype-matched control mAb (NS-1) were grown in serum-free hybridoma medium. They were precipitated by ammonium sulfate treatment and dialyzed against phosphate-buffered saline (PBS). The protein concentration was determined spectrophotometrically and the purity of the mAbs in SDS-PAGE electrophoresis followed by Coomassie blue staining.
Anti-VAP-1 Antibody Administration
Based on previous observations in pigs and dogs,18 and our own preliminary experiments studying intraluminal VAP-1 expression (see “Immunohistochemistry” below), the antibody dose was first chosen to be 2 mg/kg every second day.
The first antibody dose of 2 mg/kg was given intravenously perioperatively and the following doses were administered by the intraperitoneal route. One group of animals (n = 6) received 2 mg/kg of anti-VAP-1 antibody every second day after the initial injection, and one group of animals (n = 7) received daily injections of the anti-VAP-1 antibody. A control group of six animals received irrelevant isotype-matched control antibody (NS1) 2 mg/kg every other day.
Fine-Needle Aspiration Biopsy
Fine-needle aspiration biopsy (FNAB) is an atraumatic method that has been used to diagnose acute rejection in clinical liver and kidney allografts.19,20,21 In this method a cellular aspirate is obtained from the graft. The intensity and type of the inflammatory response in the graft can be deduced from the amounts of different types of inflammatory cells present in the aspirate. The hallmarks of acute liver allograft rejection, demonstrated by aspiration cytology, are the appearance of lymphoid blasts and lymphocytosis in the graft.20 This is also the case in rat liver allograft rejection, and the method has been proven to be useful in the monitoring of intragraft inflammatory events of the experimental model of liver transplantation.14
The fine-needle aspirate was obtained from the graft using a small needle and placed into heparinized RPMI 1640 cell culture medium containing albumin. A blood sample was taken in parallel and processed similarly. The specimens were cytocentrifuged onto microscope slides and stained with May-Grünwald-Giemsa. The intensity of inflammation associated with rejection was quantified using the increment method as described previously.19,20,21 Briefly, in this method the amount of each inflammatory cell type in blood is first subtracted from the corresponding amount in the graft. Then the amount of each inflammatory cell type is multiplied by a correction factor, which reflects its diagnostic value in acute rejection. Lymphoid blasts, plasma cells, monoblasts, and macrophages have the highest correction factor, 1.0; activated lymphocytes are 0.5, large granular lymphocytes and monocytes 0.2, and lymphocytes and polymorphonuclear cells 0.1. The results are expressed in corrected increment units (CIU). Total inflammation associated with rejection is expressed as total corrected increment (TCI), which is a corrected sum of all counted inflammatory cells in the FNAB from which the blood background is subtracted.20
Graft Histology
The liver tissue specimens were fixed in 10% buffered formalin for 1 day and embedded in paraffin. Four-μm thick sections were cut and stained with hematoxylin and eosin.
The histopathological changes were blindly scored by one pathologist (L.K.). The changes looked for were those recommended by the International Working Party for Terminology of Hepatic Allograft Rejection.22 The scores were derived as follows: no change was designated 0, mild change 1, moderate 2, and severe changes 3. The following parameters were analyzed: (a) portal inflammation, which included both the area and the density of the portal mononuclear infiltrate. Significance was also attached to the number of large activated lymphoid cells, as compared to the number of small inactive lymphocytes. (b) Cholangitis, the degree of epithelial sloughing, and the presence of inflammatory cells beneath and between the epithelial cells. (c) Endothelitis, the presence of inflammatory cells beneath the endothelium and endothelial proliferation. (d) Parenchymal inflammation, the amount of inflammatory cells between the hepatocytes. (e) Parenchymal necrosis, the estimated percentage of the area of the section that was necrotic.
To further characterize the inflammatory infiltrate in the portal areas, 10 high-power fields (×1000) in each graft were analyzed. Whenever possible, the field was chosen in such a way that it was full of the leukocyte infiltrate. The numbers of lymphoid blasts, small lymphocytes, polymorphonuclear leukocytes, and macrophages were counted. The results were expressed as percentage of each inflammatory cell type of the total count.
Immunohistochemistry
A piece of fresh liver tissue was embedded in Tissue-Tek (Sakura Finetek Europe, The Netherlands), snap-frozen in liquid nitrogen and stored at −70°C. Five-μm thick sections were cut onto Superfrost slides using a cryostat, fixed in acetone, air-dried, and stored at −20°C until use.
To investigate the luminal expression of VAP-1, two normal rats and six allografted rats at day 7 after transplantation with no previous treatment, were given 2 mg/kg of anti-VAP-1 antibody intravenously. One normal and one allografted animal received the same dose of the irrelevant isotype control antibody. After 15 or 30 minutes animals were sacrificed and organs and tissues were harvested and processed as above. Sections were stained either by anti-VAP-1 antibody followed by secondary and tertiary peroxidase-conjugated antibodies, or left without the primary antibody step but processed similarly otherwise. Thus, any positive staining with the second-stage reagent alone indicates that the primary antibody has bound to the lumen of vessels during its circulation in vivo, ie, luminal expression of the given antigen in vivo. For Figure 2, a normal human liver from autopsy material was stained with anti-VAP-1 antibody TK10–79.23
Figure 2.
A: Expression of VAP-1 in normal human liver. Paraffin section, mAb TK10–79 as primary antibody. Original magnification, ×200. B: VAP-1 expression in normal rat liver. Frozen section, immunoperoxidase staining, mAb 174–5 as primary antibody. Original magnification, ×400. D: Rat liver allograft with acute rejection 7 days post-transplantation. Frozen section, immunoperoxidase staining, mAb 174–5 as primary antibody. Original magnification, ×400. C, D, and F: Intraluminal expression of VAP-1. Frozen sections, immunoperoxidase staining detecting the primary antibody given i.v. Original magnification, ×20. C: Normal liver, 30 minutes after i.v. injection of 2 mg/kg anti-VAP-1. E: Allografted liver, 7 days post-transplantation, 30 minutes after single i.v. injection of 2 mg/kg anti-VAP-1. Intraluminal antibody binding is inhibited. F: Allografted liver, 7 days post-transplantation, treated with daily injections of 2 mg/kg anti-VAP-1. There is clear intraluminal expression of VAP-1.
To characterize the effect of VAP-1 blockade on lymphocyte subgroups in the grafts, immunostainings of the frozen sections were performed. A three-layer indirect immunoperoxidase technique and monoclonal mouse antibodies against the following rat antigens was used: CD2 (Oxford Biomarketing, UK), CD3 (Research Diagnostics, Pleasant Hill, NJ), CD4 (MAS 1131, Sera-Lab, UK), CD8 (MAS 041, Sera-Lab), CD45RC (Pharmingen, San Diego, CA), IL-2-R (MAS 263, Sera-Lab), LFA-1 (BSA 3, R&D Systems Europe, Abingdon, UK), VLA-4 (Pharmingen), and anti-rat B cell (HM3004, HyCult Biotechnology, Uden, The Netherlands). The frozen liver tissue sections were first incubated with the monoclonal mouse antibody, then with peroxidase-conjugated rabbit anti-mouse antibody (Dako, Copenhagen, Denmark) and thereafter treated with a peroxidase-conjugated goat anti-rabbit antibody (Tago Inc, Burlingame, CA). The reaction was revealed using an AEC (3-amino-9-ethyl carbazole) solution containing hydrogen peroxide. Mayer’s hemalum was used as a counterstain.
The number of positive cells in the tissue sections were assessed by computerized image analysis. The whole tissue section was photographed, producing 4 to 16 images per slide depending on the size of the specimen. The relative amount of primary antibody-positive label per image was determined by computerized densitometry (Leica IM500 and Leica QWIN software; Leica Microsystems AG, Heerbrugg, Switzerland). The results were expressed as the percentage of the area staining positively. Due to the very small numbers of the B-cell marker-positive cells, these were counted manually from 10 portal fields/section and expressed as mean number of cells per field.
VAP-1 Enzyme Activity
The radiochemical assay to measure serum semicarbazide-sensitive amine oxidases (SSAO) was performed as described.24 Briefly, 25 μl of rat serum was incubated in the presence of 50 μmol/L cold benzylamine (SSAO substrate) and 35 000 dpm tracer 14C-benzylamine with or without 1 mmol/L semicarbazide (SSAO inhibitor), and the SSAO-specific formation of 14C-benzaldehyde (product) was quantified by scintillation counting. The SSAO activity is expressed as pmol of benzaldehyde formed by 100 μl of serum per hour.
In Vitro Binding Assay
Lymphocytes were isolated from rat peripheral lymph nodes and activated with PHA for 3 days to produce immunoblasts. The binding of these cells to vessels in liver sections was studied using a modification of Stamper-Woodruff assay.9 Briefly, frozen sections freshly cut from rejecting rat allografts (non-treated animals) were pre-treated in vitro with an anti-VAP-1 or control mAb and then 3 × 106 PHA-activated lymphocytes (per section) were overlaid onto them. After a 30-minute incubation at +7°C under rotatory conditions (60 rpm on an orbital shaker), the non-interacting cells were removed by tilting the slides and the adherent cells were fixed to tissues in a glutaraldehyde fixative. The number of cells binding to vessels in liver sections was counted under dark-field microscopy.
Statistics
In the box plots, median, the interquartile range and adjacent values are shown. For hypothesis testing, the non-parametric Kruskall-Wallis test was used, and Tukey-Kramer test for comparisons between the groups. P values <0.05 were considered significant.
Results
Characterization of a New mAb Against Rat VAP-1
One of the existing anti-human VAP-1 mAbs (TK8–110) cross-reacts faintly with rat VAP-1.8 To develop more useful reagents for experiments in rat, we tested new anti-human VAP-1 mAbs for their ability to react with rat VAP-1. One of the mAbs, 174–5, stained human tissues in an identical manner to the prototype anti-VAP-1 mAbs 2D10 and TK8–14. In the human PLN, for example, both 174–5 and 2D10 stain HEV-like venules (and dendritic cells in germinal centers, data not shown) while leaving other cell types negative (Figure 1A). mAb 174–5 also cross-reacted brightly with rat VAP-1. As shown in Figure 1B, it stains rat and human gut samples in an identical manner, and the same observation holds true in many other rat organs as well (data not shown).
Figure 1.
Characterization of an anti-rat VAP-1 mAb. A: Immunohistochemical staining of human peripheral lymph nodes with mAb 174–5 yields identical results with that of a prototype anti-VAP-1 mAb (both stain HEV). NS1 is a negative control mAb. 174–5 stains small vessels (white arrows) in the transverse sections of human and rat gut. L, lumen. Black arrows point to mast cells that are brown due to their endogenous peroxidase. B: FACS staining of Ax cells transfected with mock or VAP-1 plasmid with NS-1 negative control mAb and with 174–5. C: In HEV binding assay, mAb 174–5 inhibits significantly lymphocyte binding to HEV when compared to a negative control.
The specificity of 174–5 against VAP-1 was confirmed using immunofluorescence staining of stable transfectants. This mAb did not stain mock-transfected cells, but reacted positively with VAP-1 transfectants (Figure 1C). An isotype control NS1 did not stain either type of transfectant.
The function-blocking capacity of mAb 174–5 was tested in an in vitro frozen section binding assay. Pretreatment of the tissue section with mAb 174–5 inhibited lymphocyte binding to high endothelial venules by about 80%. This level of inhibition is higher than with many other blocking anti-VAP-1 mAbs. Thus, 174–5 is a novel, function-blocking mAb against human and rat VAP-1.
Luminal VAP-1 in Normal Rats and Rats with Liver Rejection
We first wanted to ascertain the luminal expression of VAP-1 in rats in vivo. To that end anti-VAP-1 was injected intravenously 15 to 30 minutes before harvesting the organs, and the tissue sections were stained with either anti-VAP-1 mAb or secondary antibody alone. In tissue sections from both normal rats and those with acute liver allograft rejection (7 days post-transplantation) primary VAP-1 antibody incubated with the tissue section in vitro stained blood vessels in the liver, spleen, kidney, heart, skin, skeletal muscle, gut, and brain. In the liver, strong vascular and faint sinusoidal staining was seen (Figure 2). Staining of nonvascular cells was absent or very rare. Control mAb revealed no specific staining.
When the intravenously administered mAbs were detected with the secondary antibody alone, the results were different between normal and transplanted rats. In normal rat vessels in liver, spleen and skin stained positive with the secondary antibody, indicating luminal VAP-1 expression (Figure 2). Vessels in skeletal muscle stained very faintly. Animals that had received the control mAb NS1 intravenously showed no specific staining.
Surprisingly, however, in the six rats with ongoing rejection, only one had positive vessel staining in the liver and the spleen when stained with the secondary antibody alone after single i.v. injection of anti-VAP-1 mAb. In the other five rats both the liver and the spleen were negative. Nevertheless, in the treatment groups (see below), with antibody injection given every other day or daily for a week the proportion of animals with positive vessel staining with a secondary antibody alone increased with the amount of antibody given. In the group with daily anti-VAP-1 injections every graft had blood vessels positive for luminal VAP-1 when grafts were harvested on day 7 (Figure 2). The animals that were treated with the control mAb showed no specific staining with the second stage reagent. We also tested the ability of sera from the treated rats to stain tissue sections previously known to be VAP-1 positive to demonstrate the level of free anti-VAP-1 mAb in the circulation. The staining intensity correlated positively with the dose of the antibody. These experiments indicated that at least the animals in the higher dose group and half of the rats in the lower dose group had levels of circulating antibody that could be detected with this relatively insensitive method.
Liver Allograft Rejection Increases Serum VAP-1 Enzyme (Semicarbazide-Sensitive Amine Oxidase) Activity
Due to the findings suggestive of increased soluble VAP-1 concentrations in rejection we performed assays of VAP-1 enzyme activity in sera of normal rats, and recipients of syngeneic and allogeneic liver transplants. In the animals without rejection (n = 3) the serum SSAO activity was 131 ± 58 pmol/h/100 μl serum, whereas in the animals with acute liver rejection (n = 16) the mean activity was 252 ± 68 pmol/h/100 μl serum (P = 0.01, t-test). Therefore, acute liver allograft rejection leads to significant increase in serum SSAO-activity, indicating higher concentration of soluble VAP-1.
Anti-VAP-1 Inhibits Lymphocyte Infiltration in Liver Allograft Rejection
Lymphocyte infiltration to the graft was clearly affected by VAP-blockade. (Figure 3). The total inflammation in transplant aspiration cytology was significantly decreased in animals treated with anti-VAP-1. In the control group the mean TCI value was 6.6 ± 1.0, consistent with clear acute rejection, while in the lower and higher dose anti-VAP1 groups it was 4.7 ± 1.0 and 2.4 ± 1.0, respectively. Most of the difference was explained by decreased numbers both of lymphoid blasts and activated lymphocytes. The number of lymphoid blasts in the graft was significantly decreased in both treatment groups when compared to control (Figure 3). Also, the number of activated lymphocytes and small lymphocytes was clearly, but not statistically significantly decreased by anti-VAP-1 treatment. VAP-1 blockade did not seem to influence the number of monocytes and macrophages in the rejecting graft.
Figure 3.
Effect of anti-VAP-1 treatment on the rejection response measured by transplant aspiration cytology on day 7 post-transplantation. The results are given in corrected increment units (CIU). In the box plots median, interquartile range (box) and adjacent values (whiskers) are shown. Outliers are shown as dots. Significant difference (P < 0.5) compared to control is indicated by asterisks.
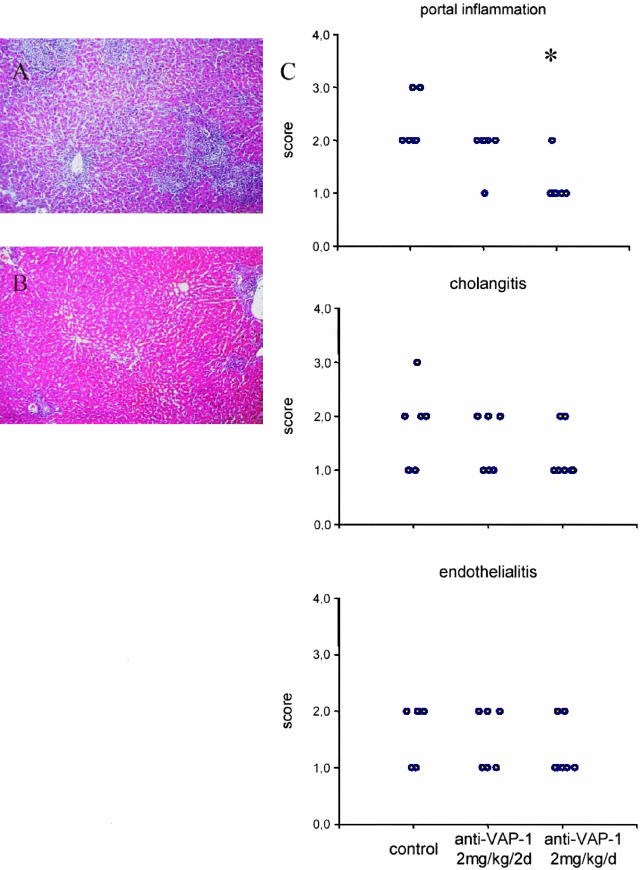
Treatment with Anti-VAP-1 Decreases Portal Inflammation in the Liver Allografts
In the histological analyses, the control group demonstrated signs of acute rejection: portal mononuclear inflammation, endothelitis, and cholangitis. In the group treated every other day with anti-VAP-1, the scores for portal inflammation and cholangitis were slightly lower than in control, but this was not significant.
In the group treated daily with anti-VAP-1 antibody the intensity of the portal inflammation was significantly decreased (Figure 4). In part of the grafts in this group the size of the portal inflammatory infiltrates was close to that in syngeneic grafts at the same time point (data not shown). The proportion of lymphoid blasts in the inflammatory infiltrate was clearly decreased by the higher dose of anti-VAP-1 (Table 1). Also the score for bile duct inflammation was lower in this treatment group but the difference was not significant. In other parameters (endothelitis, parenchymal inflammation, and necrosis) there was no significant difference.
Figure 4.
Histopathological findings 7 days after liver transplantation. Control antibody- (A) and anti-VAP-1- treated (2 mg/kg/d) (B) liver allografts. Original magnification, ×100. C. Dot plots show the individual scores of the grafts. For control antibody group n = 6, for anti-VAP-1 2 mg/kg/2 days group n = 6, and for anti-VAP-1 2 mg/kg/d group n = 7. Significant difference compared to control is indicated by asterisk.
Table 1.
Proportions of Leukocyte Subtypes in the Portal Inflammatory Infiltrates
| Lymphoid blasts | Small lymphocytes | Polymorphonuclear leukocytes | Macrophages | |
|---|---|---|---|---|
| Isotype control | 22.2 ± 10.9% | 61.4 ± 9.4% | 14.9 ± 8.8% | 0.3 ± 0.2% |
| Anti-VAP 2mg/kg/2 days | 22.5 ± 8.9% | 66.8 ± 8.1% | 10.6 ± 4.3% | 0.1 ± 0.1% |
| Anti-VAP | 12.5 ± 6.5% | 78.2 ± 8.1% | 9.1 ± 3.8% | 0.2 ± 0.3% |
| 2 mg/kg/day | (P = 0.0875) |
Ten high-power fields (×1000) maximally covered with infiltrate were counted in each liver.
Blockade of VAP-1 Significantly Decreases the Number of Activated T Cells in the Graft
Percentage of the area containing cells positive for lymphocyte lineage- and activation markers was measured in frozen tissue sections by image analyzer. The results are summarized in Figure 5.
Figure 5.
Expression of leukocyte surface markers CD3, CD4, CD8, IL-2 receptor, VLA-4, LFA-1, CD45RC, rat B-cell marker HY3004, and CD2 in the liver allografts. The results are expressed as percentage of positive label of the total surface area, except B cells as mean number of positive cells/field. In the box plots median, interquartile range (box) and adjacent values (whiskers) are shown. Outliers are shown as dots. Significant difference (P < 0.05) compared to control is indicated by asterisks.
The percentage of the area containing a positive signal for CD3 was significantly reduced by both doses of anti-VAP-1 antibody. The infiltration of CD2-positive lymphocytes was significantly decreased by the higher dose of anti-VAP-1, but not by the lower. The signals for both CD4- and CD8-positive lymphocytes were significantly decreased by both doses of anti-VAP-1 mAb, but the relative decrease of CD4-positive cells was more dramatic, up to 80% compared to control (Figure 5).
The infiltration of IL2-R-positive lymphocytes was significantly decreased by both doses of anti-VAP-1. Similarly, the number of VLA-4-positive cells in the graft was significantly decreased in both treatment groups compared to control. Signal for LFA-1 was significantly decreased only by the higher dose of anti-VAP-1. Anti-VAP-1 did not influence the amount of CD45RC signal in the graft. The amount of B cells in the infiltrates was low and did not change significantly by the treatment.
Immunoblast Adhesion to Vasculature of Rejecting Rat Liver Allografts Is Inhibited by Anti-VAP-1 in Vitro
To study the role of VAP-1 in the initial interactions (binding) between PHA-activated immunoblasts and vasculature in a rejecting liver, an in vitro frozen section assay was used. The binding was inhibited by 42 ± 2% (mean ± SEM, n = 3, each using a different liver, P < 0.01) in the presence of an anti-VAP-1 mAb when compared to a control mAb-treated sections. These data show that VAP-1 is important in binding of immunoblasts to liver vasculature. Together with the histological analyses, which take into account also the final transmigration step of the extravasation cascade (which also is VAP-1 dependent10), these results suggest that VAP-1 preferentially supports migration of immunoblasts to rejecting liver.
Discussion
We showed here that a 1-week treatment with an anti-VAP-1 mAb significantly reduces the infiltration of inflammatory cells into a liver undergoing acute rejection. This is the first description of the feasibility and efficacy of blocking VAP-1 function by repeated administration of anti-VAP-1 mAbs in any animal model in vivo.
We did not aim to assess the effect on postoperative survival in this study because of the previously known prolonged morbidity connected to untreated rejection in part of the animals in the control group.
In the transplant aspiration cytology, the significant decrease in the number of lymphoid blasts was the strongest contributing factor for the decrease in total inflammation score. Also in histology, the proportion of lymphoid blasts in the portal inflammatory infiltrate was decreased by the treatment. This might indicate that the receptor for VAP-1 on lymphoid cells (still uncharacterized) could be up-regulated by lymphocyte activation. The amount of IL2R-positive signal was also significantly decreased (by approximately 35%) in the anti-VAP-1 treated grafts. On the other hand, in immunohistological analysis, the total CD3-positive T-cell number was decreased by up to 70%, and, in the FNAB, the number of small lymphocytes was decreased. It is difficult to compare the absolute percentages of subpopulations of liver-infiltrating lymphoid cells to each other. This is mainly due to the intrinsically different avidity of different mAbs against their target antigen. Moreover, the lymphocytes may only start to express or up-regulate the expression of certain activation markers after they have extravasated into the graft and proliferate in the tissue. Based on our results, we conclude that anti-VAP-1 mAb inhibits the accumulation of activated T cells, perhaps more drastically those with a T-helper phenotype, during a liver graft rejection.
The difference between normal and rejecting rats in the staining for intraluminal VAP-1 led us to hypothesize that liver rejection could induce a soluble factor that blocks anti-VAP-1 binding to endothelia. The fact that increasing the cumulative dose of the anti-VAP-1 antibody led to reappearance of intraluminal VAP-1 positivity clearly supports this hypothesis. In the assay for the enzymatic activity of VAP-1 we saw a significant increase induced by liver rejection further supporting this. The soluble form of VAP-1 has been shown to be increased in a variety of liver diseases.11 This up-regulation seems to be quite specific for liver inflammation indicating liver as the major source of soluble VAP-1.11,24 Therefore, it would seem logical that the amount of soluble VAP-1 is also increased by liver allograft rejection. Soluble ICAM-1 and VCAM-1 are up-regulated by liver allograft rejection,25 and are generally thought to play an immunomodulatory role. Soluble VAP-1 was found to be active in adhesion assays, but instead of inhibiting lymphocyte adhesion it was found to enhance binding.11
Antibodies and antisense oligonucleotides targeting ICAM-1 have previously been shown to alleviate liver allograft rejection in the rat,26,27 and to decrease leukocyte recruitment to the graft.26 In our study the effect on histological changes of rejection was comparable to effects achieved by blocking ICAM-1. However, the expression of VAP-1 is much more restricted than that of ICAM-1, which is also expressed in leukocytes and epithelial cells, and therefore VAP-1 may be a more feasible target to prevent inapropriate lymphocyte infiltration into the liver. Moreover, VAP-1 belongs molecularly to semicarbazide-sensitive amine oxidases, and it also functionally possesses this enzymatic activity.23 It has been proposed that the SSAO activity is directly involved in the adhesive function of VAP-1, and that adhesion could be inhibited by SSAO inhibitors.28 In the future, a small molecular weight enzyme inhibitor would therefore be more practical as an anti-adhesive therapy when compared with monoclonal antibodies.
In conclusion, blockade of VAP-1 adhesion leads to significant decrease of T-lymphocyte infiltration and alleviation of histological changes of acute rejection in rat liver allografts. This indicates that VAP-1 indeed plays a significant role in the traffic of lymphocytes into the inflamed liver allograft. These data are also the first in vivo demonstration of the efficacy of a long-term treatment targeting VAP-1 in any animal model. Due to the restricted tissue expression of VAP-1, targeting it could offer possibilities for more liver-specific immunosuppression.
Acknowledgments
We thank Raisa Loginov, Mikael Heydari, and Saila Saarinen for excellent technical assistance and Kari Savelius for the animal care. We also thank Piet Finckenberg and Saara Merasto, Department of Pharmacology, University of Helsinki, for their advice on image analysis, and Heikki Irjala for the help with the fusion.
Footnotes
Address reprint requests to Timi Martelius M.D., Ph.D., Transplant Unit Research Laboratory, Helsinki University Hospital, P.O. Box 340, 00029 HUS, Helsinki, Finland. E-mail: timi.martelius@helsinki.fi.
Supported by grants from the Sigrid Juselius Foundation (to K.H. and I.L.) and Helsinki University Hospital research funds (EVO to I.L.). M.S. and S.J. have patent licensing arrangements with the BioTie Therapies Company, which is developing VAP-1-based therapeutics.
References
- Butcher EC, Williams M, Youngman K, Rott L, Briskin M. Lymphocyte trafficking and regional immunity. Adv Immunol. 1999;72:209–253. doi: 10.1016/s0065-2776(08)60022-x. [DOI] [PubMed] [Google Scholar]
- Steinhoff G, Behrend M, Schrader B, Pichlmayr R. Intercellular immune adhesion molecules in human liver transplants: overview on expression patterns of leukocyte receptor and ligand molecules. Hepatology. 1993;18:440–453. [PubMed] [Google Scholar]
- Lautenschlager I, Hockerstedt K, Taskinen E, von Willebrand E. Expression of adhesion molecules and their ligands in liver allografts during cytomegalovirus (CMV) infection and acute rejection. Transplant Int. 1996;9:S213–S215. doi: 10.1007/978-3-662-00818-8_54. [DOI] [PubMed] [Google Scholar]
- Salmi M, Jalkanen S. A 90-kilodalton endothelial cell molecule mediating lymphocyte binding in humans. Science. 1992;257:1407–1409. doi: 10.1126/science.1529341. [DOI] [PubMed] [Google Scholar]
- Salmi M, Kalimo K, Jalkanen S. Induction and function of vascular adhesion protein-1 at sites of inflammation. J Exp Med. 1993;178:2255–2260. doi: 10.1084/jem.178.6.2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvilommi AM, Salmi M, Jalkanen S. Organ-selective regulation of vascular adhesion protein-1 expression in man. Eur J Immunol. 1997;27:1794–1800. doi: 10.1002/eji.1830270730. [DOI] [PubMed] [Google Scholar]
- Tohka S, Laukkanen M, Jalkanen S, Salmi M. Vascular adhesion protein 1 (VAP-1) functions as a molecular brake during granulocyte rolling and mediates recruitment in vivo. FASEB J. 2001;15:373–382. doi: 10.1096/fj.00-0240com. [DOI] [PubMed] [Google Scholar]
- Martelius T, Salmi M, Wu H, Bruggeman C, Höckerstedt K, Jalkanen S, Lautenschlager I. Induction of vascular adhesion protein-1 (VAP-1) during liver allograft rejection and concomitant CMV infection in rats. Am J Pathol. 2000;157:1229–1237. doi: 10.1016/S0002-9440(10)64638-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNab G, Reeves JL, Salmi M, Hubscher S, Jalkanen S, Adams DH. Vascular adhesion protein 1 mediates binding of T-cells to human hepatic endothelium. Gastroenterology. 1996;110:522–528. doi: 10.1053/gast.1996.v110.pm8566600. [DOI] [PubMed] [Google Scholar]
- Lalor PF, Edwards S, McNab G, Salmi M, Jalkanen S, Adams DH. Vascular adhesion protein-1 mediates adhesion and transmigration of lymphocytes on human hepatic endothelial cells. J Immunol. 2002;169:983–992. doi: 10.4049/jimmunol.169.2.983. [DOI] [PubMed] [Google Scholar]
- Kurkijärvi R, Adams DH, Leino R, Möttönen T, Jalkanen S, Salmi M. Circulating form of human vascular adhesion protein-1 (VAP-1): increased serum levels in inflammatory liver diseases. J Immunol. 1998;161:1549–1557. [PubMed] [Google Scholar]
- Lalor PF, Shields P, Grant A, Adams DH. Recruitment of lymphocytes to the human liver. Immunol Cell Biol. 2002;80:52–64. doi: 10.1046/j.1440-1711.2002.01062.x. [DOI] [PubMed] [Google Scholar]
- Zimmermann FA, Davies HS, Knoll PP, Gokel JM, Schmidt T. Orthotopic liver allografts in the rat: influence of strain combination on the fate of the graft. Transplantation. 1984;37:406–410. doi: 10.1097/00007890-198404000-00019. [DOI] [PubMed] [Google Scholar]
- Martelius T, Mäkisalo H, Höckerstedt K, Taskinen E, Lautenschlager I. A rat model of monitoring liver allograft rejection. Transplant Int. 1997;10:103–108. doi: 10.1007/s001470050020. [DOI] [PubMed] [Google Scholar]
- Kamada N, Calne RY. Orthotopic liver transplantation in the rat: technique using cuff for portal vein anastomosis and biliary drainage. Transplantation. 1979;28:47–50. [PubMed] [Google Scholar]
- Smith DJ, Salmi M, Bono P, Hellman J, Leu T, Jalkanen S. Cloning of vascular adhesion protein 1 reveals a novel multifunctional adhesion molecule. J Exp Med. 1998;188:17–27. doi: 10.1084/jem.188.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamper HB, Jr, Woodruff JJ. Lymphocyte homing into lymph nodes: in vitro demonstration of the selective affinity of recirculating lymphocytes for high-endothelial venules. J Exp Med. 1976;144:828–833. doi: 10.1084/jem.144.3.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaakkola K, Nikula T, Holopainen R, Vahasilta T, Matikainen MT, Laukkanen ML, Huupponen R, Halkola L, Nieminen L, Hiltunen J, Parviainen S, Clark MR, Knuuti J, Savunen T, Kaapa P, Voipio-Pulkki LM, Jalkanen S. In vivo detection of vascular adhesion protein-1 in experimental inflammation. Am J Pathol. 2000;157:463–471. doi: 10.1016/S0002-9440(10)64558-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lautenschlager I, Hockerstedt K, Ahonen J, Eklund B, Isoniemi H, Korsbäck C, Pettersson E, Salmela K, Scheinin TM, von Willebrand E, Häyry B. Fine-needle aspiration biopsy in the monitoring of liver allografts: II. applications to human liver allografts. Transplantation. 1988;46:47–53. doi: 10.1097/00007890-198807000-00007. [DOI] [PubMed] [Google Scholar]
- von Willebrand E, Lautenschlager I. Organ transplantion. Gray W, McKee G, editors. London: Churchill-Livingstone,; Diagnostic Cytopathology. (ed 2) 2002:pp 551–562. [Google Scholar]
- Kwekkeboom J, Zondervan PE, Kuijpers MA, Tilanus HW, Metselaar HJ. Fine-needle aspiration cytology in the diagnosis of acute rejection after liver transplantation. Br J Surg. 2003;90:246–247. doi: 10.1002/bjs.4099. [DOI] [PubMed] [Google Scholar]
- Ludwig J. Terminology for hepatic allograft rejection: International Working Party. Hepatology. 1995;22:648–654. [PubMed] [Google Scholar]
- Bono P, Salmi M, Smith DJ, Jalkanen S. Cloning and characterization of mouse vascular adhesion protein-1 reveals a novel molecule with enzymatic activity. J Immunol. 1998;160:5563–5571. [PubMed] [Google Scholar]
- Kurkijärvi R, Yegutkin GG, Gunson BK, Jalkanen S, Salmi M, Adams DH. Circulating soluble vascular adhesion protein 1 accounts for the increased serum monoamine oxidase activity in chronic liver disease. Gastroenterology. 2000;119:1096–1103. doi: 10.1053/gast.2000.18163. [DOI] [PubMed] [Google Scholar]
- Fabrega E, Castro B, Crespo J, de la Pena J, Gomez-Fleitas M, Garcia-Unzueta MT, Amado JA, Pons-Romero F. Different time course of circulating adhesion molecules and hyaluran during hepatic allograft rejection. Transplantation. 2000;69:569–573. doi: 10.1097/00007890-200002270-00018. [DOI] [PubMed] [Google Scholar]
- Harihara Y, Sakamoto H, Sanjo K, Otsubo O, Watanabe G, Idezuki Y. Prolongation of hepatic allograft survival with antibodies to ICAM-1 and LFA-1. Transplant Proc. 1994;26:2258. [PubMed] [Google Scholar]
- Wong J, Kubes P, Zhang Y, Li Y, Urbanski SJ, Bennett CF, Lee SS. Role of ICAM-1 in chronic hepatic allograft rejection in the rat. Am J Physiol. 2002;283:G196–G203. doi: 10.1152/ajpgi.00222.2001. [DOI] [PubMed] [Google Scholar]
- Salmi M, Yegutkin GG, Lehvonen R, Koskinen K, Salminen T, Jalkanen S. A cell surface amine oxidase directly controls lymphocyte migration. Immunity. 2001;14:265–276. doi: 10.1016/s1074-7613(01)00108-x. [DOI] [PubMed] [Google Scholar]