Abstract
Advanced glycation end-products (AGEs) play a critical role in diabetic nephropathy by stimulating extracellular matrix (ECM) synthesis. Connective tissue growth factor (CTGF) is a potent inducer of ECM synthesis and increases in the diabetic kidneys. To determine the critical role of CTGF in AGE-induced ECM accumulation leading to diabetic nephropathy, rats were given AGEs by intravenous injection for 6 weeks. AGE treatment induced a significant renal ECM accumulation, as shown by increases in periodic acid-Schiff-positive materials, fibronectin, and type IV collagen (Col IV) accumulation in glomeruli, and a mild renal dysfunction, as shown by increases in urinary volume and protein content. AGE treatment also caused significant increases in renal CTGF and transforming growth factor (TGF)-β1 mRNA and protein expression. Direct exposure of rat mesangial cells to AGEs in vitro significantly induced increases in fibronectin and Col IV production, which could be completely prevented by pretreatment with anti-CTGF antibody. AGE treatment also significantly increased both TGF-β1 and CTGF mRNA expression; however, inhibition of TGF-β1 mRNA expression by shRNA or neutralization of TGF-β1 protein by anti-TGF-β1 antibody did not significantly prevent AGE-increased expression of CTGF mRNA and protein. These results suggest that AGE-induced CTGF expression, predominantly through a TGF-β1-independent pathway, plays a critical role in renal ECM accumulation leading to diabetic nephropathy.
Diabetes is the leading cause of end-stage renal disease and 10 to 21% of all people with diabetes have nephropathy, a frequent complication of both type 1 and type 2 diabetes.1 However, mechanisms underlying the pathogenesis of diabetic nephropathy are not completely understood.1 Advanced glycation end-products (AGEs), which are biochemical end-products of nonenzymatic glycation and are formed irreversibly in serum and tissues of diabetes,2–4 were found to play a critical role in the development of diabetic nephropathy. Drugs that either inhibit AGE formation or break the AGE crosslink showed a protective effect on experimental diabetic nephropathy.2,5–10
In response to AGEs or high levels of glucose, a potent profibrotic growth factor transforming growth factor-β1 (TGF-β1) significantly increases and leads to fibrotic consequence.11,12 To prevent a diabetes-caused fibrotic effect, a series of new approaches toward interference with TGF-β1 expression has been explored in the past decade.11–13 However, TGF-β1 also possesses other important functions such as anti-inflammation and anti-proliferation; therefore, a more specific anti-fibrotic target in its downstream has been sought.13
Connective tissue growth factor (CTGF) is a recently identified peptide and acts as a downstream mediator of TGF-β1-induced fibrosis.14–16 The critical role of CTGF in diabetes-induced renal extracellular matrix (ECM) accumulation and fibrosis has been implicated.10,14,15,17 For instance, a significant increase in renal CTGF mRNA expression along with significant glomerulosclerosis in the db/db diabetic mice or streptozotocin (STZ)-induced diabetic rats was observed.10,14,15,18 By immunohistochemical method or autoradiography, increased renal CTGF protein expression was also evident in the renal cortex of NOD diabetic mice and STZ-induced diabetic rats.10,18,19 Exposure of cultured human or murine mesangial cells (MCs) to high levels of glucose caused a significant induction of CTGF mRNA and protein expression with fibrotic effect, ie, fibronectin (FN) production.14,19 More importantly, the production of FN in human MCs caused by exposure to high levels of glucose can be prevented by CTGF anti-sense,19 suggesting the important role of CTGF in the diabetes-induced fibrotic effect.
Although AGEs were known to play a critical role in initiating the development of diabetic nephropathy, whether up-regulated CTGF initiates renal fibrosis through AGE formation in the diabetes remains unclear. In the present study, therefore, we aimed to determine whether AGEs directly cause renal CTGF up-regulation along with renal fibrotic effect, for which both in vivo experiments using AGE-treated rat model and in vitro experiments using AGE-exposed primary cultures of rat MCs were used; and whether AGE-induced CTGF up-regulation is mediated by TGF-β1.
Materials and Methods
AGE Synthesis
AGE-bovine serum albumin (AGE-BSA) and AGE-rat serum albumin (AGE-RSA) were prepared by incubating BSA and RSA (fraction V, low-endotoxin; Sigma, St. Louis, MO) with 500 mmol/L of d-glucose under aerobic conditions for 10 weeks at 37°C in the presence of protease inhibitors and antibiotics based on published methods.2,5 Unmodified BSA and RSA for control were prepared under the same conditions without the addition of sugar. All preparations were extensively dialyzed in phosphate-buffered saline (PBS), and then condensed in polyethylene glycol (molecular weight, 20,000). AGE content in the preparations was assessed by means of fluorescence photometer (at excitation wavelength of 370 nm and emission of 440 nm with slit width of 10 nm in arbitrary fluorescence units per mg protein) as 19.8 ± 1.3 for control BSA, 46.8 ± 5.6 for AGE-BSA, 18.6 ± 2.1 for control RSA, and 101.5 ± 12.1 for AGE-RSA. All reagents were prepared under endotoxin-free conditions. Each preparation was tested by Limulus amoebocyte lysate assay (Zhanjiang A&C Biological Ltd., Zhanjiang, Guangdong, Peoples’ Republic of China) for endotoxin content (<0.8 EU endotoxin per ml).
Animal Treatments
Twenty-four male Wister rats (6 to 7 weeks old) were purchased from the Jilin University Experimental Animal Center, and randomly divided into four groups: the control group, given tail vein injection with sterile PBS; the RSA group, given tail vein injections with RSA (100 mg/kg daily) for 6 weeks; the AGE group, given tail injections with AGE-RSA (100 mg/kg daily) for 6 weeks; and the AGE-AG group, given with AGE-RSA (100 mg/kg daily) followed by oral administration of AGE-crosslink inhibitor aminoguanidine (AG, 40 mg/kg daily) for 6 weeks. The dose of AGE-RSA was based on previous studies. To develop a typical diabetic nephropathy, oral supplementation with AGE-RSA at 25 mg/kg daily for 5 months has been used previously;5 however, to investigate molecule or gene regulation in response to AGE, subacute supplementation with AGE-RSA at 100 mg/kg daily for rats2 or 240 mg/kg daily for mice6 for 4 to 8 weeks also has been used. AG was used because it has been extensively indicated to react with Amdaori-derived products to form stable compounds to avoid the formation of reactive AGEs, consequently preventing AGE-protein cross-linking.2,5,6,10,20 All these procedures have been approved by the University Animal Care and Use Committee.
Primary Cultures of Rat MCs and Treatments
Rat MCs were isolated from rat glomeruli. In brief, rats were anesthetized with ether. The kidneys were collected under sterile conditions for obtaining renal cortices and minced to a fine paste with a razor blade and then pressed through serial stainless steel sieves (nos. 75, 150, and 200). Glomeruli were collected from the top of the 75-μm sieve. By this process, > 98% pure glomeruli were obtained. The glomeruli then were pelleted and resuspended in Dulbecco’s modified Eagle’s medium supplemented with 20% fetal bovine serum and 100 UI/ml penicillin and 100 μg/ml streptomycin. The glomerular suspensions were plated onto tissue flasks and incubated at 37°C in 5% CO2. Primary cultures were allowed to grow for 3 to 4 weeks, at which time the MCs were confluent. MCs between passages 5 and 10 were used for experiments.
After trypsinization, cells were grown in six-well plates for 5 days in their respective media with 10% fetal bovine serum until confluent. Cells were then incubated in Dulbecco’s modified Eagle’s medium with 0.5% fetal bovine serum for 24 hours followed by a change of same fresh medium and treatment with AGE-BSA as day 0. Cells and the media of these cells were harvested at different times after treatments. For experiments involving use of neutralizing antibodies, cells were preincubated with antibodies for 2 hours under 0.5% fetal bovine serum medium before adding AGE-BSA or control BSA directly to the medium. The concentration used for neutralization was 10 μg/ml for both anti-CTGF and anti-TGF-β according to published studies.8,16
Assessments of Renal Function
The animals were placed in individual metabolic cages for 24 hours to collect urine samples after receiving only access to tap water on the day before the experimental rats were killed. No difference for total water intake among groups was observed. Total urinary volume and urinary protein (grams per L) were measured and the urinary protein excretion was calculated as before.21
Measurement of Serum and Tissue AGE by Enzyme-Linked Immunosorbent Assay
When animals were sacrificed, blood samples were obtained to prepare serum for AGE measurement. Kidneys were removed and prepared as described by published methods.22 Briefly, kidney tissue was rinsed with PBS and finely minced with scissors. Lipids were extracted with acetone/chloroform (1:l), dried by vacuum centrifugation, and resuspended in 0.2 mol/L NaPO4 buffer (pH 7.4). Samples were digested with collagenase (type VII) and centrifuged at 15,000 × g, and the clear supernatants were collected for AGE measurements. The procedure to measure AGEs in serum and tissue suspension was exactly followed the published enzyme-linked immunosorbent assay method,22 by which the major moiety should be carboxymethy-lysine.
Light and Electron Microscope Examination
One-fourth of the kidneys were immersion-fixed in 10% buffered formalin and embedded in paraffin for a light microscopic study. Two sections of 4 μm thickness (an interval of 100 μm) per animal were stained with periodic acid-Schiff (PAS) reagent. For electron microscope examination, the renal cortex was cut into small pieces and prefixed in 2.5% glutaraldehyde (0.2mol/L cacodylate buffer, pH 7.4) for 4 hours, postfixed in 1% buffered sodium tetroxide for 1 hour and embedded in Epon 812. Ultra-thin sections were examined using a JEM-1200 EX electron microscope.
Mesangial matrix expansion was determined as PAS-positive materials presented in the mesangial region excluding cellular elements. Percentage of PAS-positive area in each glomerulus was analyzed using Leica Q500MC image analysis software. Ten glomeruli, randomly selected in the two slides from each rat (total six rats in each group), were evaluated by two investigators without knowledge of the origin of the slides.
Immunohistochemical Study
Renal tissue sections at 4 μm were used to perform immunohistochemical staining for FN, Col IV, TGF-β1, and CTGF based on our published method21 with the following specific antibodies: monoclonal mouse anti-rat Col IV antibody, polyclonal rabbit anti-rat FN antibody (Boster Biological Technology Co., Ltd., Wuhan, Peoples Republic of China), and polyclonal rabbit anti-rat TGF-β1 and goat anti-rat CTGF antibodies (Santa Cruz Biotechnology, Santa Cruz, CA). Color was developed by incubating with diaminobenzidine and counterstaining with hematoxylin. Controls were obtained by replacing the primary antibody with PBS. Semiquantitative analysis of the percentage of positive staining area in the glomerulus was evaluated by computer imaging analysis system, as described above.
Western Blot Assay
Protein samples (40 μg protein/lane) were electrophoresed through a 7.5% polyacrylamide gel and then transferred to nitrocellulose membranes (Amersham SA, Orsay, France). The membranes were blocked in 5% skim milk powder in PBS before overnight incubation in a 1:200 dilution of a polyclonal rabbit anti-rat TGF-β1 or goat anti-rat CTGF antibody (Santa Cruz Biotechnology). Proteins were visualized using a horseradish peroxidase-conjugated IgG (Amersham SA) and an enhanced chemiluminescence kit (Amersham SA).
Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
The total RNA was extracted from cultured MCs and the renal cortical tissues using TRIzol reagent (Gibco Corp., Beijing, Peoples Republic of China). Primers for TGF-β1, CTGF, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were designed and synthesized based on published sequence of these genes.23,24 The upstream and downstream of these primers are: 1) TGF-β1, 5′ GCTAATGGTGGACCGCAACAACG, 3′ CTTGCTGTACTGTGTGTCCAGGC, by which 682 bp of TGF-β1 cDNA would be synthesized;23 2) CTGF, 5′-GCTAAGA CCTGTGGAATGGGC-3′ and 5′-CTCAAAGATGTCATTGCCCCC-3′, by which 383 bp of CTGF cDNA would be synthesized;23 and 3) GAPDH, 5′-ACCACAGTCCATGCCATCAC-3′ and 5′-TCCACCA CCCTGTGCT GTA-3′, by which 452 bp of GAPDH cDNA would be synthesized. Total RNA (0.5 μg) was amplified using Titan One Tube RT-PCR kit (Boehringer-Mannheim, Shanghai, Peoples Republic of China). Twenty-five cycles of replication and five PCR tubes for each sample were used. The products were separated by agarose gel electrophoresis and visualized by ethidium bromide staining. Bands were digitized by a Tanon-1000 Gel Image System (Shanghai, Peoples Republic of China). Means of the ratio of TGF-β1 or CTGF band density to GAPDH band density in various groups were presented. GAPDH was used as control gene based on previous studies.8,16,18,19
Preparation and Transfection of Short Hairpin RNA (shRNA)
TGF-β-specific and scramble shRNAs were produced and purified by MessageMuter shRNAi production kit (Epicentre).25 The shRNA-specific sequences used for targeting TGF-β1 were as following: sense, 5′CAAUUCCUGGCGUUACCUU 3′; anti-sense, 5′AAGGUAACGCCAGGAAUUGGC 3′ (GenBank X5248, nucleotides 940 to 958). The sequence shRNA duplexes were synthesized by Dharmacon (Lafayette, IN), and searched with the National Center for Biotechnology Information database (http://www.ncbi.nlm.nih.gov/BLAST/) to ensure that only the TGF-β1 gene was targeted. The shRNA targeting luciferase (shRNA-luc) was used as negative control.25 MCs at density of 3 × 105 cells/well were plated for at least 24 hours before transfection, and transfected with 5 μg of shRNA using LipofectAMINE 2000 (Qiagen) according to the manufacturer’s protocol. Twenty-four hours after transfection, these MCs were exposed to 100 μg/ml of AGEs for another 24 hours, and then RNA was extracted from these cells for analysis of TGF-β1, CTGF, and GAPDH mRNA by the RT-PCR method.
Measurements of FN and Col IV by Enzyme-Linked Immunosorbent Assays
Mouse anti-rat monoclonal FN or Col IV antibody (5 μg/ml; Boster Biological Technology Co., Ltd.) in coating buffer were absorbed to 96-well microplates (Gibco BRL) by a 20-hour incubation at 4°C. The unbounded antibody was removed and the wells were blocked by incubation with 150 μl of PBS/Tween 20 containing 0.5% (w/v) BSA for 2 hours at 37°C. After three further washes with PBS/Tween 20, collected media (100 μl/ml) from treated MC cultures were incubated at 37°C for 2 hours. Each sample was repeated five times. The plates were washed, and then incubated with rabbit anti-rat polyclonal FN or Col IV antibody (1:1000 dilution, 100 μl) for 2 hours at 37°C. After washing, the plate was incubated with 100 μl of goat anti-rabbit IgG conjugated to horseradish peroxidase (1:1000 dilution; Boster Biological Technology Co., Ltd.) for 45 minutes at 37°C. A final wash was followed by color development using the colorimetric reagent [0.6 mg/ml diaminobenzidine in 0.01 mol/L Tris-Cl (pH7.6) containing 0.3% H2O2]. The reaction was stopped by the addition of 2 mol/L H2SO4, and the absorbance was measured at 490 nm using a microplate reader. The content of FN or Col IV was represented in OD units rather than concentrations.
Statistical Analysis
Data were presented as mean ± SD from at least six samples (rats) for in vivo study and five samples for in vitro experiments. One-way analysis of variance and Student’s t-test were used for statistical analysis. Differences were considered to be significant at P < 0.05.
Results
AGE-Induced Renal Dysfunction and Morphological Changes
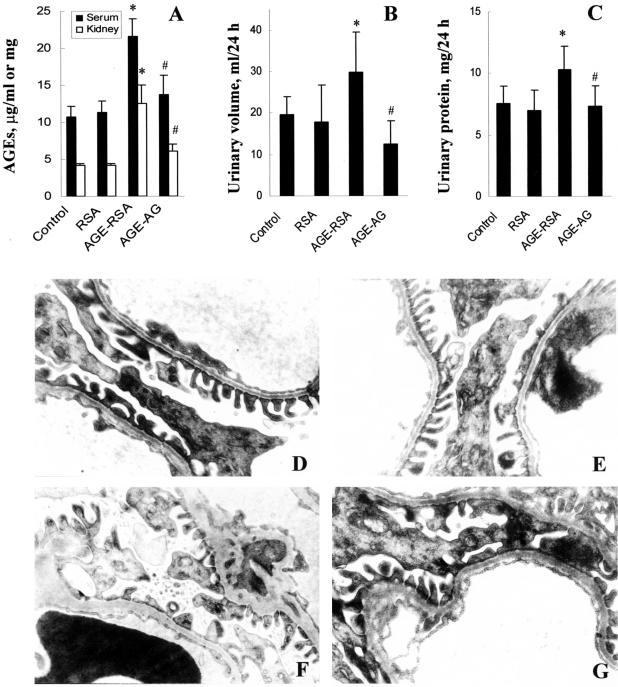
All animals treated with AGE-RSA showed a high level of AGEs in serum and kidney tissue (Figure 1A). A mild renal dysfunction was observed, shown by increases in urinary volume and protein excretion (Figure 1, B and C). Co-administration of AGEs with AG partially (Figure 1A) or significantly (Figure 1, B and C) prevented AGE renal deposition and AGE-induced renal dysfunction.
Figure 1.
AGE-caused renal dysfunction and histological changes. Rats were treated with AGE-RSA for 6 weeks and then sacrificed for evaluation of AGE decomposition in serum and renal tissue (A), total urinary volume (B), and urinary protein (C). AGE-induced ultrastructural changes in the kidney of control (D), RSA-treated (E), AGE-RSA-treated (F), and AGE-AG-treated (G) rats were examined by electron microscope. RSA, rat serum albumin; AGE-RSA, glycated RSA; AGE-AG, co-administration of AG with AGE-RSA. *, P < 0.05 versus control; #, P < 0.05 versus AGE-RSA.
Glomeruli morphological changes were assessed by electron microscopy (Figure 1; D to G). Segmental thickness of glomerular basement membranes, widely fused podocytes, and excessively deposited mesangial matrix were observed in the rats treated with AGE-RSA (Figure 1F). No significant abnormalities were observed in the RSA group and AG treatment partially prevented AGE-induced ultrastructural abnormalities (Figure 1, E and G).
AGE-Induced Renal ECM Accumulation and Expression of TGF-β1 and CTGF mRNA and Protein
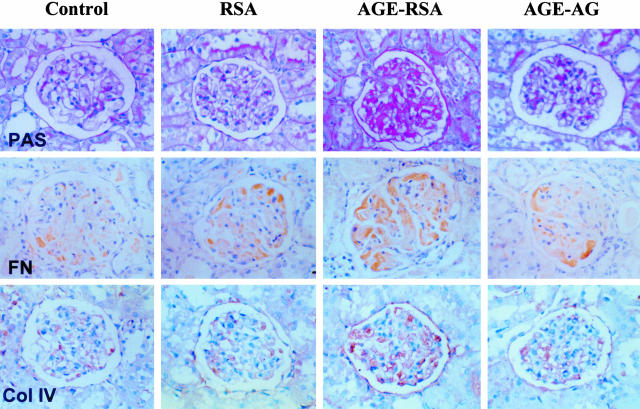
For ECM accumulation, PAS-positive materials were found to be increased in the kidneys of rats treated with AGE-RSA (Figure 2). Quantitative analysis for the percentage of PAS in the glomerulus using computer image analysis system is summarized in Table 1. Examination of FN and Col IV by immunohistochemical staining indicated that staining of both could be lightly observed in the normal glomeruli: FN was observed mainly in the intraglomerular mesangium and Col IV staining was located mainly in the glomerular basement membrane (Figure 2). The intensity and area of FN and Col IV staining, however, significantly increased in the glomeruli of AGE-treated rats as compared to control and RSA groups, and AG treatment partially prevented these effects (Table 1).
Figure 2.
AGE-induced renal ECM accumulation. Rats were treated with AGE-RSA for 6 weeks and then sacrificed for evaluation of renal ECM accumulation by examination of staining for PAS-positive material staining (first row) and immunohistochemical staining for FN (second row) and Col IV (third row).
Table 1.
Morphometric Analysis of PAS Staining and Immunohistochemical Staining for TGF-β1, CTGF, FN, and Col IV (%)
| PAS | FN | Col IV | TGF-β1 | CTGF | |
|---|---|---|---|---|---|
| Control | 11.4 ± 2.1 | 5.7 ± 1.7 | 1.0 ± 0.4 | 1.3 ± 0.5 | 0.7 ± 0.2 |
| RSA | 13.2 ± 1.4 | 5.7 ± 2.4 | 1.0 ± 0.6 | 1.2 ± 0.5 | 0.5 ± 0.1 |
| AGE-RSA | 19.5 ± 3.8* | 19.4 ± 5.7* | 4.3 ± 1.2* | 9.5 ± 1.1* | 1.9 ± 0.6* |
| AGE-AG | 9.3 ± 1.2† | 7.4 ± 1.8† | 2.8 ± 0.8 | 2.0 ± 0.6† | 1.0 ± 0.4 |
P < 0.05 versus control or RSA.
P < 0.05 versus AGEs.
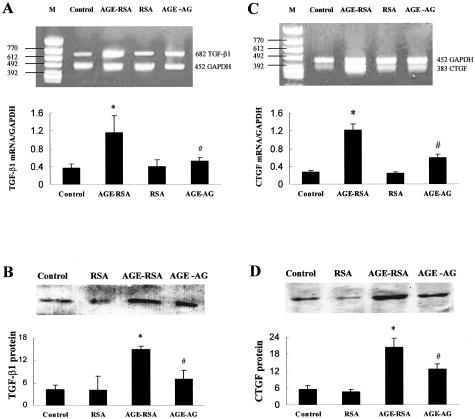
In the kidney cortex of AGE-RSA rats, expression of both TGF-β1 and CTGF mRNA, measured by the RT-PCR method, were significantly increased relative to control (Figure 3, A and C). Correspondingly, TGF-β1 and CTGF protein contents, measured by Western blotting, were also significantly increased (Figure 3, B and D). Immunohistochemical staining for both TGF-β1 and CTGF showed significantly increased expression and was mainly located in the glomeruli (Figure 4, Table 1), suggesting that TGF-β1 and CTGF are correlated to ECM accumulation in glomeruli. AG treatment partially prevented the AGE-enhanced expression of TGF-β1 (almost completely) and CTGF (partially) (Figures 3 and 4).
Figure 3.
AGE-induced renal TGF-β1 and CTGF mRNA and protein expression. Rats were treated with AGE-RSA for 6 weeks and then renal cortices of different groups were collected to analyze TGF-β1 and CTGF mRNA expression by RT-PCR method, as described in Materials and Methods. A and C: Representative gel profiles of TGF-β1 and CTGF mRNA by RT-PCR method and the quantitatively results of these band densities. B and D: Representative gel profiles of TGF-β1 and CTGF protein expression by Western blot method and the quantitative results of these band densities. *, P < 0.05 versus control; #, P < 0.05 versus AGE-RSA.
Figure 4.
Localization of AGE-induced renal TGF-β1 and CTGF expression. Rats were treated with AGE-RSA for 6 weeks and then sacrificed for evaluation the localization of TGF-β1 and CTGF protein expression by immunohistochemical staining, as labeled in the figure.
CTGF Plays a Pivotal Role in AGE-Induced Fibrotic Effects in MCs
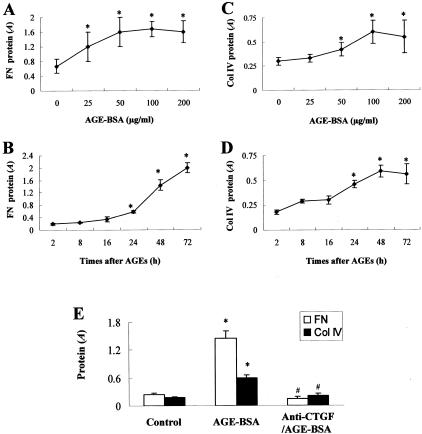
To determine the direct link of AGE in the kidney cortex to fibrotic effects, primary cultures of rat MCs were treated with soluble AGE-BSA. Because the concentration of serum AGEs in the rats treated with AGE-RSA at 100 mg/kg daily for 6 weeks was ∼25 μg/ml (Figure 1A), we investigated the effect of AGEs on FN and Col IV production in cultured MCs at 25 μg/ml and above (Figure 5, A and C). Consistent with the results obtained from the in vivo study, AGEs significantly induced MC production of FN and Col IV in a dose-dependent manner until 100 μg/ml (Figure 5, A and C). In previous in vitro studies,8,10,15,26 AGE-BSA was used mostly at 50 to 200 μg/ml. Therefore, the concentration of AGE-BSA at 100 μg/ml was used in our time-course studies, which showed significant time-dependent increases in AGE-induced FN and Col IV production in MCs (Figure 5, B and D). To define whether AGE-induced effects are directly related to CTGF expression, MCs were preincubated with anti-CTGF antibody (10 μg/ml) for 2 hours and then exposed to AGE-BSA (100 μg/ml) for 48 hours. Preincubation with anti-CTGF antibody completely prevented AGE-BSA-induced FN and Col IV production (Figure 5E).
Figure 5.
AGE-induced fibrotic effect in the primary cultures of rat MCs. Fibrotic effects and FN and Col IV overproduction were measured in the MCs in vitro directly exposed to AGEs, as described in Material and Methods. A and C: The dose-response of AGEs (48 hours) on FN and Col IV overproduction. B and D: The time-response of AGEs (100 μg/ml) on FN and Col IV overproduction. E: The effect of CTGF neutralization on AGE-induced overproduction of FN and Col IV. In the AGE-BSA group, MCs were exposed to 100 μg/ml of AGE-BSA for 48 hours. In the anti-CTGF group, MCs were pretreated with goat anti-CTGF (10 μg/ml) for 2 hours and then co-incubated with 100 μg/ml of AGE-BSA for 48 hours. *, P < 0.05 versus control; #, P < 0.05 versus AGE-BSA.
AGE-Induced CTGF Expression Predominantly through TGF-β1-Independent Pathways
Because in the kidney of AGE-treated rats, both TGF-β1 and CTGF mRNA and protein were up-regulated, the next experiment was to investigate the characteristics of both CTGF and TGF-β1 mRNA expression of the MCs in response to AGEs. After primary cultures of rat MCs were exposed to AGEs, both TGF-β1 and CTGF mRNA expressions were significantly increased (Figure 6; A to F). However, TGF-β1 mRNA expression was increased at a dose-dependent manner only within a narrow dose range (50 to 100 μg/ml, Figure 6B), whereas CTGF mRNA expression was increased at a dose-dependent manner within a wide dose range (50 to 200 μg/ml, Figure 6E). Time-course studies showed that the enhanced expression of TGF-β1 mRNA (Figure 6C) occurred early compared to CTGF mRNA expression (Figure 6F).
Figure 6.
AGE-induced expression of TGF-β1 and CTGF mRNA in the primary cultures of rat MCs. TGF-β1 and CTGF mRNA expressions were measured by RT-PCR, as described in Material and Methods, and representative gel profiles are given in A and D. Summarized results for the time-course and dose-effect of AGE-induced TGF-β1 mRNA expression (B, C) and CTGF mRNA expression (E, F) are presented. For the time-course study, MCs were treated with 100 μg/ml of AGE-BSA for the indicated times and for the dose-response study, MCs were treated with AGE-BSA at the indicated doses for 48 hours. *, P < 0.05 versus control.
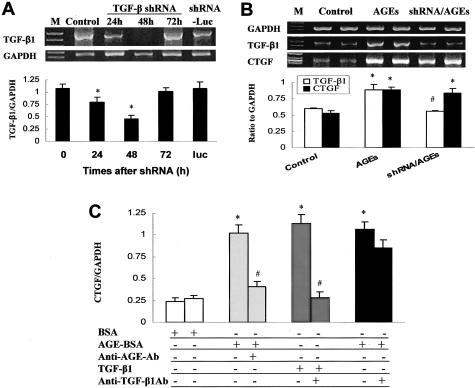
To determine whether AGE-increased CTGF mRNA expression is mediated by TGF-β1, TGF-β1 mRNA expression was silenced by incubation with TGF-β1 shRNA (Figure 7A). After MCs were incubated with TGF-β1 shRNA, TGF-β1 mRNA expression in these cells was significantly inhibited starting at 24 hours, reached the maximal inhibition at 48 hours, and then was restored to control level at 72 hours (Figure 7A). However, incubation of the MCs with the negative control shRNA (shRNA-luc) for 48 hours did not change TGF-β1 mRNA expression (Figure 7A). Therefore, MC cultures were preincubated with TGF-β1 shRNA for 24 hours and then co-incubation with AGEs for 24 hours (Figure 7B). Results showed that 24-hour treatment with AGEs for 24 hours significantly increased both TGF-β1 and CTGF mRNA expression. More importantly, the same treatment with AGEs in shRNA-treated MCs did not increase TGF-β1 mRNA expression but still significantly increased CTGF mRNA expression. Furthermore, the fact that inhibition of TGF-β1 did not affect AGE-induced CTGF expression was further confirmed by neutralization of TGF-β1 protein. MC cultures were preincubated with anti-AGE antibody (100 μg/ml) or anti-TGF-β1 antibody (10 μg/ml) for 2 hours and then were incubated with AGEs or TGF-β1 for 48 hours. AGEs and TGF-β1 both induced a significant increase in CTGF mRNA expression in the cells without preincubation of anti-AGE or anti-TGF-β1 antibody, whereas preincubation of MCs with anti-AGE antibody almost completely prevented AGE-induced CTGF mRNA expression. However, preincubation of MCs with anti-TGF-β1 antibody completely blocked only TGF-β1-induced CTGF mRNA expression but had almost no affect on AGE-induced CTGF mRNA expression (Figure 7C).
Figure 7.
Effect of silencing TGF-β1 mRNA expression or neutralization of TGF-β1 protein on AGE-induced CTGF expression. MCs were treated with shRNA for the indicated times and TGF-β1 mRNA expression was analyzed by RT-PCR, as described in Material and Methods. TGF-β1 mRNA expression in MCs was significantly inhibited starting at 24 hours and reaching maximal levels at 48 hours after incubation with TGF-β1 shRNA, but not changed until 48 hours after incubation with luciferase shRNA (shRNA-luc) (A); therefore, MCs were incubated with TGF-β1 shRNA for 24 hours and then added with 100 μg/ml of AGEs for another 24 hours (total 48 hours for TGF-β1 shRNA incubation). Expression of TGF-β1, CTGF, and GAPDH mRNA was analyzed by RT-PCR (B). C: The results of AGE- or TGF-β1-induced CTGF mRNA expression with and without neutralizing AGEs or TGF-β1 protein by anti-AGE or anti-TGF-β1 neutralizing antibody. In this experiment, MCs were treated with anti-AGE antibody (100 μg/ml) and anti-TGF-β1 antibody (10 μg/ml) for 2 hours before exposure to AGEs (100 μg/ml) or TGF-β1 (10 ng/ml) for 48 hours. *, P < 0.05 versus control; #, P < 0.05 versus AGE-BSA.
Discussion
Diabetic nephropathy is characterized by ECM accumulation in the glomerular mesangium and tubulointerstitium.1,8,14,18 AGEs have been documented to play an important role in the pathogenesis of diabetic nephropathy by stimulating cytokine and growth factor synthesis leading to ECM accumulation.2–6,10,20 In the present study, renal ECM accumulation along with an increased expression of CTGF and TGF-β1 mRNA and protein was significantly observed in AGE-treated rats. These pathological changes led a mild renal dysfunction. The only mild renal dysfunction noted in the present study may be because of the relative short term after AGE treatment (only 6 weeks). Consistent with previous studies,2,5–7,10,20 however, these fibrotic effects can be prevented by simultaneous supplementation with the AGE formation inhibitor AG, suggesting the importance of AGEs in the pathogenesis of diabetic nephropathy.
The importance of TGF-β in the pathogenesis of diabetic nephropathy has been extensively addressed because it promoted renal cell hypertrophy and stimulates ECM.8,11–13,26 In both tissue culture and animal studies, cellular matrix production were stimulated by high levels of glucose, along with an increase in TGF-β expression, and the matrix stimulatory effects of high glucose were prevented by anti-TGF-β therapy.8,11–13,26 However, there also was documentation indicating that TGF-β1 neutralization could not totally block FN and Col IV overproduction in MCs in response to high glucose.27,28 Recent studies29–31 further confirmed that renal fibrosis often occurred via TGF-β-independent pathways. They demonstrated that exposure of human renal fibroblasts to high glucose significantly increased FN secretion, but the kinetics of high glucose-increased secretion was significantly delayed compared to that of TGF-β1-induced FN. Type III collagen was up-regulated by high glucose, but not by TGF-β1. Furthermore, treatment with neutralizing anti-TGF-β antibody could not attenuate the effects of glucose.
The importance of CTGF as one of the downstream factors mediating fibrotic activity of TGF-β1 has been implicated.14–19 For instance, directly transferring the CTGF gene to human MCs produced a significant amount of FN.19 In contrast, the production of FN or collagens in response to high levels of glucose or TGF-β1 can be prevented by suppression of CTGF mRNA expression with CTGF anti-sense19,32 or CTGF-specific small interference RNA (siRNA).33 These studies suggest that CTGF plays a pivotal role in the fibrotic effects.7,13–19,32 The findings that direct exposure of human dermal fibroblasts to AGE in vitro caused a CTGF-mediated overproduction of FN15 and that AGE breaker can reduce renal CTGF expression in diabetic animals10 suggest that the AGE-caused fibrotic effect in diabetic kidney may also be mediated by CTGF. That AGE-induced fibrotic effect can be prevented by CTGF neutralization (Figure 5E) provides the direct evidence that CTGF plays a critical, pivotal role in AGE-induced ECM accumulation in MCs.
Another important and novel finding of this study is that AGEs induced CTGF up-regulation in rat MCs predominantly through the TGF-β1-independent pathway. Although many studies have shown the importance of CTGF as a downstream mediator of TGF-β1 in mediating the profibrotic effects,13–19 how TGF-β1 affects CTGF mRNA and protein expression in the diabetic condition remains unclear. Murphy and colleagues23 reported that CTGF expression induced by high glucose in human MCs was partially TGF-β1-dependent, whereas Riser and colleagues14 demonstrated that anti-TGF-β1 antibody completely prevented CTGF expression induced by high glucose in rat MCs. No information is available for the effect of TGF-β1 on CTGF expression in kidneys or in MCs in response to AGEs. We demonstrated in the present study that TGF-β1 and CTGF mRNA expression in the kidneys of AGE-treated rats and in AGE-treated MCs were both significantly up-regulated. However, silencing of TGF-β1 mRNA expression by TGF-β1-specific shRNA or blockage of TGF-β1 protein by a neutralizing antibody in cultured MCs did not significantly affect AGE-induced CTGF mRNA expression (Figure 7), suggesting that although AGE induces both TGF-β1 and CTGF expression, AGE-induced CTGF expression is predominantly TGF-β1-independent.
The CTGF gene contains several regulatory elements such as activator protein (AP-1), plasminogen activator inhibitor-1 (PAI-1), tumor necrosis factor-α, endothelin-1 (ET-1), and TGF-β1-response element; therefore, in addition to TGF-β1, CTGF gene expression is also regulated by tumor necrosis factor-α, vascular endothelial growth factor, cAMP, thrombin, prostaglandin E2, drugs such as iloprost and statins, as well as with cytomegalovirus infection.34–36 In the present study, we provide the first evidence that AGE-induced CTGF in MCs is predominantly TGF-β1-independent. To date, several other previous studies have also documented that CTGF expression could be up-regulated through TGF-β-independent pathways under other conditions.16,36–39 For instance, Way and colleagues37 reported that in transgenic mice overexpressing PKC-β2, CTGF mediated cardiac fibrosis and dysfunction independently from TGF-β activation. Candido and colleagues38 also found the overexpression of CTGF in STZ-induced diabetic hearts without TGF-β expression. In the study on skin-wound healing, accumulated evidence indicated that in the normal skin-wound healing response, the up-regulation of CTGF is dependent on the TGF-β response element of the CTGF promoter, whereas in pathological fibrosis, the expression of CTGF in the lesion is independent of the TGF-β response element of the CTGF promoter.39 Because ET-1 is up-regulated during pulmonary fibrosis, Xu and colleagues36 recently directly exposed normal lung fibroblasts to ET-1, resulting in a significant increase in CTGF gene expression without TGF-β expression. All these studies further support the early observation by Murphy and colleagues22 that CTGF up-regulation in human MCs in response to high glucose was partially inhibited by neutralization of endogenous TGF-β.
In summary, the present study presents the evidence that CTGF plays a pivotal role in mediating the AGE-induced fibrotic effect, predominantly through a TGF-β1-independent pathway, and targeting CTGF expression may provide a more specifically anti-fibrotic pathogenesis in the diabetic kidney to reserve the desirable actions of TGF-β1. Although we still do not know how AGEs induce CTGF expression (if not through TGF-β1-dependent pathway), this will be pursued in the future studies.
Acknowledgments
We thank X.Y. Yu from the Institute of Frontier Medical Science, Jilin University, for technical assistance; and Dr. Ping Wang and Jianxun Wang for their insightful discussion.
Footnotes
Address reprint requests to Dr. Lu Cai, Department of Medicine, the University of Louisville, 511 South Floyd St., MDR 533, Louisville, KY 40202. E-mail: L0cai001@gwise.louisville.edu or Dr. Cai Li, E-mail: licaijia@public.cc.jl.cn.
Supported in part by the National Natural Science Foundation of China (grant N39870312 to C.L.), the American Diabetes Association, and Philip Morris USA, Inc. (grants ADA020667 and PM020187 to L.C.).
References
- Caramori ML, Mauer M. Diabetes and nephropathy. Curr Opin Nephrol Hypertens. 2003;12:273–282. doi: 10.1097/00041552-200305000-00008. [DOI] [PubMed] [Google Scholar]
- Vlassara H, Fuh H, Makita Z, Krungkrai S, Cerami A, Bucala R. Exogenous advanced glycosylation end products induce complex vascular dysfunction in normal animals: a model for diabetic and aging complications. Proc Natl Acad Sci USA. 1992;89:12043–12047. doi: 10.1073/pnas.89.24.12043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beisswenger PJ, Makita Z, Curphey TJ, Moore LL, Jean S, Brinck-Johnsen T, Bucala R, Vlassara H. Formation of immunochemical advanced glycosylation end products precedes and correlates with early manifestation of renal and retinal disease in diabetes. Diabetes. 1995;44:824–829. doi: 10.2337/diab.44.7.824. [DOI] [PubMed] [Google Scholar]
- Jerums G, Panagiotopoulos S, Forbes J, Osicka T, Cooper M. Evolving concepts in advanced glycation, diabetic nephropathy, and diabetic vascular disease. Arch Biochem Biophys. 2003;419:55–62. doi: 10.1016/j.abb.2003.08.017. [DOI] [PubMed] [Google Scholar]
- Vlassara H, Striker LJ, Teichberg S, Fuh H, Li YM, Steffes M. Advanced glycation end products induce glomerular sclerosis and albuminuria in normal rats. Proc Natl Acad Sci USA. 1994;91:11704–11708. doi: 10.1073/pnas.91.24.11704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CW, Vlassara H, Peten EP, He CJ, Striker GE, Striker LJ. Advanced glycation end products up-regulated gene expression found in diabetic glomerular disease. Proc Natl Acad Sci USA. 1994;91:9436–9440. doi: 10.1073/pnas.91.20.9436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes JM, Thallas V, Thomas MC, Founds HW, Burns WC, Jerums G, Cooper ME. The breakdown of preexisting advanced glycation end products is associated with reduced renal fibrosis in experimental diabetes. FASEB J. 2003;17:1762–1764. doi: 10.1096/fj.02-1102fje. [DOI] [PubMed] [Google Scholar]
- Ohashi S, Abe H, Takahashi T, Yamamoto Y, Takeuchi M, Arai H, Nagata K, Kita T, Okamoto H, Yamamoto H, Doi T. Advanced glycation end products increase collagen-specific chaperone protein in mouse diabetic nephropathy. J Biol Chem. 2004;279:19816–19823. doi: 10.1074/jbc.M310428200. [DOI] [PubMed] [Google Scholar]
- Wilkinson-Berka JL, Kelly DJ, Koerner SM, Jaworski K, Davis B, Thallas V, Cooper ME. ALT-946 and aminoguanidine, inhibitors of advanced glycation, improve severe nephropathy in the diabetic transgenic (mREN-2) 27 rat. Diabetes. 2002;51:3283–3289. doi: 10.2337/diabetes.51.11.3283. [DOI] [PubMed] [Google Scholar]
- Twigg SM, Cao Z, McLennan SV, Burns WC, Brammar G, Forbes JM, Cooper ME. Renal connective tissue growth factor induction in experimental diabetes is prevented by aminoguanidine. Endocrinology. 2002;143:4907–4915. doi: 10.1210/en.2002-220619. [DOI] [PubMed] [Google Scholar]
- Park I, Kiyomoto H, Abboud SL, Abboud HE. Expression of transforming growth factor-beta and type IV collagen in early streptozotocin-induced diabetes. Diabetes. 1997;46:473–480. doi: 10.2337/diab.46.3.473. [DOI] [PubMed] [Google Scholar]
- Sharma K, Ziyadeh FN, Alzahabi B, McGowan TA, Kapoor S, Kurnik BR, Kurnik PB, Weisberg LS. Increased renal production of transforming growth factor-beta1 in patients with type II diabetes. Diabetes. 1997;46:854–859. doi: 10.2337/diab.46.5.854. [DOI] [PubMed] [Google Scholar]
- Gore-Hyer E, Shegogue D, Markiewicz M, Lo S, Hazen-Martin D, Greene EL, Grotendorst G, Trojanowska M. TGF-beta and CTGF have overlapping and distinct fibrogenic effects on human renal cells. Am J Physiol. 2002;283:F707–F716. doi: 10.1152/ajprenal.00007.2002. [DOI] [PubMed] [Google Scholar]
- Riser BL, Denichilo M, Cortes P, Baker C, Grondin JM, Yee J, Narins RGP. Regulation of connective tissue growth factor activity in cultured rat mesangial cells and its expression in experimental diabetic glomerulosclerosis. J Am Soc Nephrol. 2000;11:25–38. doi: 10.1681/ASN.V11125. [DOI] [PubMed] [Google Scholar]
- Twigg SM, Joly AH, Chen MM, Tsubaki J, Kim HS, Hwa V, Oh Y, Rosenfeld RG. Connective tissue growth factor/IGF-binding protein-related protein-2 is a mediator in the induction of fibronectin by advanced glycosylation end-products in human dermal fibroblasts. Endocrinology. 2002;143:1260–1269. doi: 10.1210/endo.143.4.8741. [DOI] [PubMed] [Google Scholar]
- Lam S, van der Geest RN, Verhagen NA, van Nieuwenhoven FA, Blom IE, Aten J, Goldschmeding R, Daha MR, van Kooten C. Connective tissue growth factor and IGF-I are produced by human renal fibroblasts and cooperate in the induction of collagen production by high glucose. Diabetes. 2003;52:2975–2983. doi: 10.2337/diabetes.52.12.2975. [DOI] [PubMed] [Google Scholar]
- Liu BC, Chen Q, Luo DD, Sun J, Phillips AO, Ruan XZ, Liu NF. Mechanisms of irbesartan in prevention of renal lesion in streptozotocin-induced diabetic rats. Acta Pharmacol Sin. 2003;24:67–73. [PubMed] [Google Scholar]
- Makino H, Mukoyama M, Sugawara A, Mori K, Suganami T, Yahata K, Fujinaga Y, Yokoi H, Tanaka I, Nakao K. Roles of connective tissue growth factor and prostanoids in early streptozotocin-induced diabetic rat kidney: the effect of aspirin treatment. Clin Exp Nephrol. 2003;7:33–40. doi: 10.1007/s101570300004. [DOI] [PubMed] [Google Scholar]
- Wahab NA, Yevdokimova N, Weston BS, Roberts T, Li XJ, Brinkman H, Mason RM. Role of connective tissue growth factor in the pathogenesis of diabetic nephropathy. Biochem J. 2001;359:77–87. doi: 10.1042/0264-6021:3590077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soulis-Liparota T, Cooper M, Papazoglou D, Clarke B, Jerums G. Retardation by aminoguanidine of development of albuminuria, mesangial expansion, and tissue fluorescence in streptozocin-induced diabetic rat. Diabetes. 1991;40:1328–1334. doi: 10.2337/diab.40.10.1328. [DOI] [PubMed] [Google Scholar]
- Song Y, Li C, Cai L. Fluvastatin prevents nephropathy likely through suppression of connective tissue growth factor-mediated extracellular matrix accumulation. Exp Mol Pathol. 2004;76:66–75. doi: 10.1016/j.yexmp.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Makita Z, Vlassara H, Cerami A, Bucala R. Immunochemical detection of advanced glycosylation end products in vivo. J Biol Chem. 1992;267:5133–5138. [PubMed] [Google Scholar]
- Murphy M, Godson C, Cannon S, Kato S, Mackenzie HS, Martin F, Brady HR. Suppression subtractive hybridization identifies high glucose levels as a stimulus for expression of connective tissue growth factor and other genes in human mesangial cells. J Biol Chem. 1999;274:5830–5834. doi: 10.1074/jbc.274.9.5830. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Takagawa S, Katayama I, Yamazaki K, Hamazaki Y, Shinkai H, Nishioka K. Animal model of sclerotic skin. I: Local injections of bleomycin induce sclerotic skin mimicking scleroderma. J Invest Dermatol. 1999;112:456–462. doi: 10.1046/j.1523-1747.1999.00528.x. [DOI] [PubMed] [Google Scholar]
- Paddison PJ, Caudy AA, Sachidanandam R, Hannon GJ. Short hairpin activated gene silencing in mammalian cells. Methods Mol Biol. 2004;265:85–100. doi: 10.1385/1-59259-775-0:085. [DOI] [PubMed] [Google Scholar]
- Kim YS, Kim BC, Song CY, Hong HK, Moon KC, Lee HS. Advanced glycosylation end products stimulate collagen mRNA synthesis in mesangial cells mediated by protein kinase C and transforming growth factor-beta. J Lab Clin Med. 2001;138:59–68. doi: 10.1067/mlc.2001.115494. [DOI] [PubMed] [Google Scholar]
- Riser BL, Cortes P. Connective tissue growth factor and its regulation: a new element in diabetic glomerulosclerosis. Ren Fail. 2001;23:459–470. doi: 10.1081/jdi-100104729. [DOI] [PubMed] [Google Scholar]
- Sharma K, Jin Y, Guo J, Ziyadeh FN. Neutralitration of TGF-beta by anti-TGF-beta antibody attenuates kidney hypertrophy and the enhanced extracellular matrix gene expression in STZ-induced diabetic mice. Diabetes. 1996;45:522–530. doi: 10.2337/diab.45.4.522. [DOI] [PubMed] [Google Scholar]
- Lam S, Verhagen NA, Strutz F, van der Pijl JW, Daha MR, van Kooten C. Glucose-induced fibronectin and collagen type III expression in renal fibroblasts can occur independent of TGF-beta1. Kidney Int. 2003;63:878–888. doi: 10.1046/j.1523-1755.2003.00824.x. [DOI] [PubMed] [Google Scholar]
- Weigert C, Brodbeck K, Brosius FC, III, Huber M, Lehmann R, Friess U, Facchin S, Aulwurm S, Haring HU, Schleicher ED, Heilig CW. Evidence for a novel TGF-beta1-independent mechanism of fibronectin production in mesangial cells overexpressing glucose transporters. Diabetes. 2003;52:527–535. doi: 10.2337/diabetes.52.2.527. [DOI] [PubMed] [Google Scholar]
- Lam S, Van Der Geest RN, Verhagen NA, Daha MR, Van Kooten C. Secretion of collagen type IV by human renal fibroblasts is increased by high glucose via a TGF-{beta}-independent pathway. Nephrol Dial Transplant. 2004;19:1694–1701. doi: 10.1093/ndt/gfh235. [DOI] [PubMed] [Google Scholar]
- Yokoi H, Mukoyama M, Nagae T, Mori K, Suganami T, Sawai K, Yoshioka T, Koshikawa M, Nishida T, Takigawa M, Sugawara A, Nakao K. Reduction in connective tissue growth factor by antisense treatment ameliorates renal tubulointerstitial fibrosis. J Am Soc Nephrol. 2004;15:1430–1440. doi: 10.1097/01.asn.0000130565.69170.85. [DOI] [PubMed] [Google Scholar]
- Wang JF, Olson ME, Ma L, Brigstock DR, Hart DA. Connective tissue growth factor siRNA modulates mRNA levels for a subset of molecules in normal and TGF-beta 1-stimulated porcine skin fibroblasts. Wound Repair Regen. 2004;12:205–216. doi: 10.1111/j.1067-1927.2004.012113.x. [DOI] [PubMed] [Google Scholar]
- Blom IE, Goldschmeding R, Leask A. Gene regulation of connective tissue growth factor: new targets for antifibrotic therapy? Matrix Biol. 2002;21:473–482. doi: 10.1016/s0945-053x(02)00055-0. [DOI] [PubMed] [Google Scholar]
- Ruperez M, Ruiz-Ortega M, Esteban V, Lorenzo O, Mezzano S, Plaza JJ, Egido J. Angiotensin II increases connective tissue growth factor in the kidney. Am J Pathol. 2003;163:1937–1947. doi: 10.1016/S0002-9440(10)63552-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu SW, Howat SL, Renzoni EA, Holmes A, Pearson JD, Dashwood MR, Bou-Gharios G, Denton CP, du Bois RM, Black CM, Leask A, Abraham DJ. Endothelin-1 induces expression of matrix-associated genes in lung fibroblasts through MEK/ERK. J Biol Chem. 2004;279:23098–23103. doi: 10.1074/jbc.M311430200. [DOI] [PubMed] [Google Scholar]
- Way KJ, Isshiki K, Suzuma K, Yokota T, Zvagelsky D, Schoen FJ, Sandusky GE, Pechous PA, Vlahos CJ, Wakasaki H, King GL. Expression of connective tissue growth factor is increased in injured myocardium associated with protein kinase C beta2 activation and diabetes. Diabetes. 2002;51:2709–2718. doi: 10.2337/diabetes.51.9.2709. [DOI] [PubMed] [Google Scholar]
- Candido R, Forbes JM, Thomas MC, Thallas V, Dean RG, Burns WC, Tikellis C, Ritchie RH, Twigg SM, Cooper ME, Burrell LM. A breaker of advanced glycation end products attenuates diabetes-induced myocardial structural changes. Circ Res. 2003;92:785–792. doi: 10.1161/01.RES.0000065620.39919.20. [DOI] [PubMed] [Google Scholar]
- Leask A, Denton CP, Abraham DJ. Insights into the molecular mechanism of chronic fibrosis: the role of connective tissue growth factor in scleroderma. J Invest Dermatol. 2004;122:1–6. doi: 10.1046/j.0022-202X.2003.22133.x. [DOI] [PubMed] [Google Scholar]