Abstract
Tumor necrosis factor (TNF) is critical for the control of visceral leishmaniasis caused by Leishmania donovani. However, the role of the related cytokine lymphotoxin (LT) α in this infection is unknown. Here we report that C57BL/6 mice deficient in TNF (B6.TNF−/−) or LTα (B6.LTα−/−) have increased susceptibility to hepatic L. donovani infection. Furthermore, the outcome of infection in bone marrow chimeric mice is dependent on donor hematopoietic cells, indicating that developmental defects in lymphoid organs were not responsible for increased susceptibility to L. donovani. Although both LTα and TNF regulated the migration of leukocytes into the sinusoidal area of the infected liver, their roles were distinct. LTα was essential for migration of leukocytes from periportal areas, an event consistent with LTα-dependent up-regulation of VCAM-1 on liver sinusoid lining cells, whereas TNF was essential for leukocyte recruitment to the liver. During visceral leishmaniasis, both cytokines were produced by radio-resistant cells and by CD4+ T cells. LTα and TNF production by the former was required for granuloma assembly, while production of these cytokines by CD4+ T cells was necessary to control parasite growth. The production of inducible nitric oxide synthase was also found to be deficient in TNF- and LTα-deficient infected mice. These results demonstrate that both LTα and TNF are required for control of L. donovani infection in noncompensatory ways.
Tumor necrosis factor (TNF) and lymphotoxin α (LTα) are structurally related members of the TNF superfamily.1 Along with LTβ, these cytokines are encoded by closely linked genes within the major histocompatibility complex gene region.2–5 TNF and LTα form soluble homotrimers that bind both TNFRI (p55) and TNFRII (p75).1 TNF has also been observed as a membrane-bound complex that is also able to bind both TNF receptors,6 while LTα can also exist as a membrane-bound heterotrimer with LTβ (LTα1β2) that is recognized by LTβR, but not TNFRI or TNFRII.7–10 Recently, soluble LTα was also found to bind the herpesvirus entry mediator receptor.11 Studies with mice deficient in TNF,12–14 LTα,15,16 LTβ,8,16,17 TNFRI,18,19 TNFRII,20 and LTβR9,10 have identified distinct functions for TNF and LTα in vivo. TNF plays an important role in host defense against intracellular pathogens13,21,22 and the regulation of inflammatory pathways.1,23 In addition, membrane-bound TNF supports the development of lymphoid structures, but plays a limited role in inflammation.6 The LTα1β2 complex is critical for the development of lymph nodes and Peyer’s patches and for the organization of the white pulp region of the spleen.8,9,24 Soluble LTα has been shown to participate in inflammatory responses, including the activation of macrophages25 and endothelial cells.26,27 Also, transgenic expression of the LTα gene under the control of the rat insulin promoter in mice was shown to cause chronic inflammation at sites of transgene expression.27 Recently, the role of LTα in infectious diseases has started to be defined. LTα was shown to play an important role in resistance to murine Mycobacterium tuberculosis28 and Toxoplasma gondii29 infections, and was found to be critical in the development of murine cerebral malaria.30 However, in murine Trypanosoma brucei infection, control of parasite growth was improved in the absence of LTα.31
Visceral leishmaniasis (VL) caused by Leishmania donovani is an important human disease.32 Parasites are found in macrophages in the spleen, liver, and bone marrow (BM).33,34 Despite the spleen and BM becoming sites of persistent infection, the liver is a site for an acute, but resolving infection.35,36 Mice infected with L. donovani that received a neutralizing anti-TNF antibody were unable to resolve infection in the liver.37 Similarly, mice deficient in TNF were unable to control parasite growth, had impaired hepatic granuloma formation, and ultimately died after 6 weeks of infection.21 However, we have recently observed that TNF also contributes to the pathology associated with murine VL.38,39 In particular, TNF is involved in the loss of marginal zone macrophages38 and stromal cells from the periarteriolar lymphoid sheath39 in the spleen after L. donovani infection.
To define the role of LTα in murine VL, and distinguish this role from that of TNF, we have analyzed the response to L. donovani infection in LTα- and TNF-deficient mice, as well as in chimeras generated using LTα−/− and TNF−/− BM cells. Data indicate that TNF and LTα are both important for host resistance to L. donovani in the liver in the first 14 days of infection, whereas later in infection, TNF but not LTα is critical for host survival. LTα and TNF production by radio-resistant cells was found to be required for efficient granuloma formation, although each cytokine appeared to be necessary for different stages in the recruitment of leukocytes. Furthermore, TNF and LTα produced by CD4+ T cells had nonredundant roles in host resistance and ultimately host survival.
Materials and Methods
Mice and Parasites
C57BL/6 mice were purchased from Tuck and Co. (Essex, UK) and were housed under conventional conditions. Mice deficient in TNF (B6.TNF−/−)12 and LTα−/− (LTα−/−)16,40 were obtained from Bantin & Kingman (Hull, UK). B6.CD45.1 mice were obtained from Charles River (IFFA Credo, Saint Germain Sur L’Arbresle, France). B6.RAG-1−/− mice were obtained from The Jackson Laboratory (Bar Harbor, ME). All mouse strains were bred at the London School of Hygiene and Tropical Medicine under barrier conditions. Mice used in all experiments were sex-matched and used at 6 weeks of age. Chimeric mice were prepared by irradiating animals twice (48 hours apart) with 5.5 Gy and then engrafting with 107 fresh BM cells intravenously via the lateral tail vein within 2 hours of the second radiation exposure. Mice were maintained on antibiotics for 4 weeks after engraftment and infected with parasites 8 weeks after receiving BM. L. donovani (LV9) was maintained by passage in Syrian hamsters and amastigotes were isolated from infected spleens, as previously described.34 Mice were infected at 6 weeks of age by injecting 2 × 107 amastigotes intravenously via the lateral tail vein. Mice were killed at times indicated in the text by cervical dislocation and bled by severing the aorta. Livers and spleens were removed and parasite burden was determined from Giemsa-stained impression smears.41 Parasite burden was expressed in Leishman-Donovan units, in which Leishman-Donovan unit is the number of amastigotes per 1000 host nuclei, multiplied by the organ weight.34
Histological Response to Hepatic Infection
Liver sections from infected mice were stained with hematoxylin and eosin and the granulomatous response was assessed in two ways. First, granuloma density was determined from 25 fields of view per mouse liver (×40 magnification; n = 3 mice/group) and second, the maturation of granulomas was scored around infected Kupffer cells, as described elsewhere.42
Fluorescence-Activated Cell Sorting (FACS) Analysis
Hepatic mononuclear cells were isolated by passing livers through a 200-μm sieve and washing twice with phosphate-buffered saline supplemented with 2% (v/v) fetal calf serum (wash buffer). The cell pellet was resuspended in 33% (v/v) Percoll and centrifuged at 693 × g for 12 minutes at room temperature. Supernatant containing hepatocytes was removed and the leukocyte pellet was washed once in wash buffer, depleted of red blood cells using Red Cell Lysis Buffer (Sigma, Poole, UK), according to the manufacturer’s instructions, underlayed with fetal calf serum, and centrifuged at 443 × g for 5 minutes. Cell pellets were washed once more with wash buffer and cells counted. Cells were labeled with antibody and analyzed by FACS as previously described.43 Antibodies used for FACS included rat anti-mouse CR3 (5C6), rat anti-mouse GR-1 (RB6 8C5), and PECy5-conjugated anti-mouse CD4 and CD8 (BioLegend, CA). Nonconjugated rat antibodies were detected with Alexa 488-conjugated goat anti-rat IgG (Molecular Probes, Eugene, OR).
Immunohistochemistry
Antibodies used for histology included rat anti-mouse ICAM-1 (KAT-1), rat anti-mouse VCAM-1 (M/K-2) (both from Serotec, Kidlington, UK), and rabbit anti-mouse inducible nitric oxide synthase (NOS-2) (Calbiochem, La Jolla, CA). ICAM-1 and VCAM-1 staining was conducted on 6-μm acetone-fixed liver sections, and primary antibodies were detected with appropriate secondary detection reagents according to the manufacturer’s instructions (Vector Laboratories, Peterborough, UK), and as previously described.34 NOS-2 staining was performed on 6-μm paraformaldehyde-fixed liver sections, and the primary antibody detected with an alkaline phosphatase-conjugated goat anti-rabbit antibody (Jackson Laboratories, West Grove, PA). Alkaline phosphatase was visualized using appropriate detection reagents according to the manufacturer’s instructions (Vector Laboratories). Sections were dehydrated and mounted before microscopic examination. In some cases, sections were counterstained with hematoxylin (Sigma) before dehydration and mounting.
Reconstitution of B6.RAG-1−/− Mice with CD4+ T Cells
Spleens were passed through a 20-μm sieve and red blood cells were lysed in Gey’s solution (130 mmol/L NH4Cl, 5 mmol/L KCl, 8.4 mmol/L Na2HPO4, 180 μmol/L KH2PO4, 5.6 mmol/L d-glucose, 0.001% (w/v) phenol red, 1 mmol/L MgCl2·6H2O, 280 μmol/L MgSO4.7H2O, 1.5 mmol/L CaCl2, 13 mmol/L NaHCO3) for 8 minutes at room temperature and washed twice in RPMI 1640. Splenic mononuclear cells were then enriched over Histopaque 1083 (1700 rpm, 15 minutes, room temperature; Sigma), washed, and resuspended in complete culture media. CD4+ T cells were positively selected from naïve splenocyte preparations using magnetic-activated cell sorting according to protocols recommended by the manufacturers of the metallo-conjugated anti-CD4 antibodies, and positive selection columns (Miltenyi Biotec, Bergisch Gladbach, Germany). Cells isolated by this procedure were greater than 98% pure, as assessed by FACS. B6.RAG-1−/− mice were reconstituted with 1 × 106 CD4+ T cells in 200 μl of RPMI 1640 via the lateral tail vein 24 hours before infection with 2 × 107 L. donovani amastigotes. The efficiency of reconstitution was assessed by FACS at the termination of experiments (day 14 after infection) with quantum red-conjugated anti-CD4 (H129.19) monoclonal antibody (Sigma).
Real-Time Reverse Transcriptase-Polymerase Chain Reaction
RNA was isolated from liver tissue using Tri Reagent (Sigma), and an RNAeasy mini kit with on-column DNase digestion (Qiagen, Valencia, CA), according to the manufacturers’ instructions. RNA was reverse-transcribed into cDNA as described previously.30 The number of NOS-2, LTα, TNF, and HPRT (housekeeping gene) cDNA molecules in each sample was calculated by real-time reverse transcriptase-polymerase chain reaction using TaqMan probes in an Assay-on-Demand Gene Expression kit (Applied Biosystems, Foster City, CA) and a Corbett Research RG-3000 Rotor Gene, according to the manufacturers instructions. Standard curves were constructed with known amounts of NOS-2, LTα, TNF, and HPRT cDNA, and the number of cytokine molecules per 1000 HPRT molecules in each sample was calculated.
Statistics
The statistical significance of differences between different groups was tested using the unpaired Student’s t-test with SigmaPlot software (SPSS Inc., Richmond, CA), except when the distribution of hepatic histological responses were compared using χ2 analysis with Microsoft Excel software (Microsoft Corp., Seattle, WA). All data are presented as the mean values ± standard errors unless otherwise stated.
Results
Different Roles for LTα and TNF during Hepatic L. donovani Infection
To distinguish the roles of LTα and TNF in the liver during VL, we infected mice deficient in these cytokines (B6.LTα−/− and B6.TNF−/−, respectively) with L. donovani and followed the course of infection throughout 56 days. In C57BL/6 mice, hepatic parasite burden peaked between days 14 to 28 after infection before resolving (Figure 1A). The liver parasite burdens in both LTα- and TNF-deficient mice were significantly increased early in infection (day 14 after infection), suggesting a critical role for both these cytokines in early control of L. donovani growth in the liver. However, after day 14 of infection, distinct patterns of parasite growth emerged in LTα- and TNF-deficient mice. B6.LTα−/− mice were able to control infection, whereas parasite growth continued in the livers of B6.TNF−/− mice, and these animals died between days 42 to 56 after infection, as previously reported.21 Whether the TNF-deficient mice died because of progressive infection because of the absence of anti-leishmanial mechanisms or an uncontrolled hyperinflammatory reaction, as suggested by others,21 is unknown.
Figure 1.
TNF and LTα are required for early control of L. donovani growth in the liver. A: The course of L. donovani infection in the liver of C57BL/6 (filled circle), B6.TNF−/− (open circle), and B6.LTα−/− (inverted filled triangle) mice. B6.TNF−/− mice died between days 42 to 56 after infection. Data represent the mean ± SE of Leishman-Donovan unit values in the liver from four mice per group at each time point. Parasite burdens in B6.TNF−/− and B6.LTα−/− mice were significantly greater than in C57BL/6 mice at day 14 after infection (*, P < 0.001). At day 28 after infection, B6.TNF−/− mice had statistically greater parasite loads than C57BL/6 mice (n = 4 mice per group; #, P < 0.001). B: B6.CD45.1 mice engrafted with BM from C57BL/6 (filled circle), B6.TNF−/− (open circle), and B6.LTα−/− (inverted filled triangle) mice. Mice engrafted with BM from B6.TNF−/− mice died between days 42 to 56 after infection. Parasite burdens in B6.TNF−/−→B6.CD45.1 and B6.LTα−/−→B6.CD45.1 mice were significantly greater than in B6→B6.CD45.1 mice at days 14 and 28 after infection (n = 3 mice per group at each time point; *, P < 0.001). These data are from one of four (A) and two (B) experiments conducted in the same way with similar results.
Tissue Structure Defects Associated with TNF- and LTα-Deficient Mice Do Not Account for Their Different Responses to L. donovani Infection
One possible explanation for the different responses of TNF- and LTα-deficient mice to L. donovani infection is that the aberrant lymphoid organogenesis associated with deficiency of these cytokines might impede the generation of effective immunity.6,8,9 To address this, we generated BM chimeric mice by irradiating congenic B6.CD45.1 mice and engrafting them with BM from C57BL/6 mice or mice lacking TNF or LTα (all express the CD45.2 allele). After engraftment, chimeric mice contained fewer than 2% CD45.1-positive leukocytes in the spleen and peripheral blood (data not shown). In addition, marginal zone macrophages and distinct B- and T-cell regions were present in the spleen of all chimeric animals (data not shown). The chimeric mice responded to hepatic L. donovani infection in accordance with their source of BM (Figure 1B). Both B6.TNF−/−→B6.CD45.1 and B6.LTα−/−→B6.CD45.1 mice had higher initial hepatic parasite burdens compared to B6→B6.CD45.1 chimeras, and whereas B6.LTα−/−→B6.CD45.1 mice eventually controlled infection, all B6.TNF−/−→B6.CD45.1 mice died between days 42 to 56 after infection. These results indicate that tissue structure defects and lymphoid tissue deficiencies associated with TNF- and LTα-deficient mice do not explain the different responses of these animals to L. donovani infection. Furthermore, these data indicate that TNF and LTα production by hematopoietic cells is a critical determinant of disease outcome. We next studied TNF- and LTα-dependent events in the liver in the first 14 days of L. donovani infection.
Liver Granuloma Formation Is Delayed in TNF- and LTα-Deficient Mice during L. donovani Infection
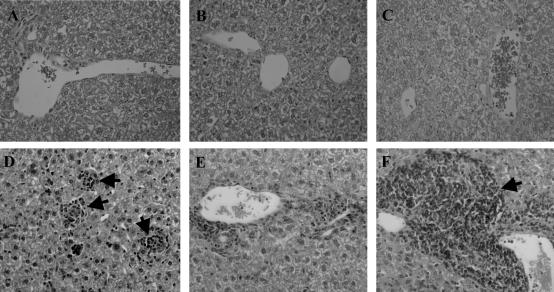
The formation of granulomas around infected Kupffer cells in the liver is a key event in the control of hepatic L. donovani,35 and TNF was previously shown to play a role in the efficient development of granulomas during VL.21 To investigate whether LTα is also involved in granuloma formation, we next evaluated granuloma formation in L. donovani-infected C57BL/6, B6.TNF−/−, and B6.LTα−/− mice. As previously reported,21 the development of hepatic granulomas was delayed in TNF-deficient mice with a lower frequency of mature granulomas observed at day 14 after infection (Table 1). Similarly, the development of hepatic granulomas was also delayed in LTα-deficient mice at day 14 after infection (Table 1). A striking feature of the livers of the L. donovani-infected B6.LTα−/− mice was the periportal accumulation of leukocytes, suggesting the recruitment of cells to the liver, but a failure to migrate deeper into the sinusoidal area (Figure 2). In B6.TNF−/− mice, there was little periportal accumulation of leukocytes and given the impaired granuloma development in these mice (Table 1), this suggests that leukocytes fail to be recruited into the periportal regions of the liver in the absence of TNF. FACS analysis of hepatic leukocytes at day 14 after infection showed that the total number of cells in the liver did not differ significantly between infected C57BL/6 and B6.LTα−/− mice (4.6 × 107± 1.1 × 107 versus 6.4 × 107± 2.0 × 107, respectively), but was lower in TNF-deficient mice at the same time (3.2 × 107± 0.8 × 107). Furthermore, the percentage of complement receptor 3 (CR3)-positive cells (recruited monocytes, macrophages, and neutrophils), GR-1-positive cells (recruited neutrophils), CD4+ and CD8+ T cells did not change between the different mouse strains studied (20.3 ± 2.9, 25.0 ± 0.7, 34.3 ± 1.5 and 16.0 ± 1.4, respectively). In addition, tissue staining revealed that all of the above cell populations accumulated in the periportal regions of LTα-deficient mice, indicating no selective accumulation of specific cell types in these mice after L. donovani infection (data not shown). A previous study on L. donovani infection in TNF-deficient mice reported a complete absence of hepatic inflammatory responses at day 14 after infection,21 whereas our studies indicate some cellular recruitment may occur at this time (Table 1). The different results may reflect the mixed C57BL/6 × 129/Sv background used in previous studies21 and the C57BL/6 background of mice used in this study. In contrast to some reports,10,15,44 periportal infiltration was not observed in age-matched naïve B6.LTα−/− mice in this study (Figure 2). The reason for this difference may result from the use of significantly older mice in the above studies (older than 3 months), whereas naïve mice used in the present studies were 6 to 8 weeks of age. These data indicate that both TNF and LTα are required for efficient granuloma formation in the liver during L. donovani infection, but that each cytokine is involved in different stages of cellular activation and recruitment.
Table 1.
Liver Granuloma Formation in L. donovani-Infected Mice at Day 14 after Infection*
| Tissue response | Percent infected foci showing the indicated cellular response†
|
|||||
|---|---|---|---|---|---|---|
| C57BL/6 | B6.TNFα−/− | B6.LTα−/− | B6 →B6.CD45.1 | B6.TNFα−/− →B6.CD45.1 | B6.LTα−/− →B6.CD45.1 | |
| KC | 19.7 ± 2.0 | 41 ± 2.0‡ | 22.2 ± 0.4‡ | 24.7 ± 2.1 | 25.9 ± 3.5 | 19.9 ± 1.7§ |
| IG | 57 ± 1.5 | 59 ± 1.8 | 74.4 ± 0.9 | 37.5 ± 5.9 | 47.4 ± 1.8 | 61.8 ± 1.4 |
| MG | 22 ± 3.0 | 0.2 ± 0.2 | 3.4 ± 0.4 | 35.6 ± 3.1 | 26.7 ± 1.8 | 18.3 ± 3.0 |
| SG | 1.1 ± 0.4 | 0 | 0 | 2.2 ± 1.0 | 0 | 0 |
| Total no. infected foci per 25 fields | 233 ± 5.3 | 360 ± 6.4¶ | 345 ± 11.6¶ | 182 ± 3.2 | 240 ± 18.5∥ | 232 ± 12.4∥ |
Granuloma counts performed on liver cryosections stained with hamster anti-L. donovani (LV9) Ab, then counterstained with hemotoxylin.
KC, infected KC with no surrounding leukocytes; IG, infected KC surrounded by some leukocytes, but not completely; MG, infected KC completely surrounded by leukocyte mantle; SG, sterile (empty) granuloma.
The distribution of tissue responses is significantly different (P < 0.001) than that observed in L. donovani-infected C57BL/6‡ and B6 → B6.CD45.1§ mice.
The number of infected foci is significantly different (P < 0.001¶ and P < 0.05∥) than that observed in L. donovani-infected C57BL/6¶ and B6 → B6.CD45.1∥ mice.
Figure 2.
Hepatic granuloma assembly is inhibited in L. donovani-infected mice deficient in TNF and LTα. A–C: H&E-stained tissue sections from naïve C57BL/6 (A), B6.TNF−/− (B), and B6.LTα−/− (C) mice, and corresponding sections taken at day 14 after infection (D–F). Mature and immature granulomas can be seen in infected C57BL/6 (D) mice (arrows), whereas minimal granuloma development can be seen in B6.TNF (E) mice and massive leukocyte accumulation can be observed in the perivascular regions in B6.LTα−/− (F) mice (arrow). Original magnifications, ×400.
Granuloma formation was also examined in BM chimeric mice at day 14 after infection. Compared to the deficiency of granuloma maturation observed in B6.TNF−/− and B6.LTα−/− mice, granuloma maturation was restored in B6.TNF−/−→B6.CD45.1 and B6.LTα−/−→B6.CD45.1 mice, although not to the full extent of that seen in B6→B6.CD45.1 mice (Table 1). These data indicate that radio-resistant cells are an important source of both TNF and LTα in the liver during L. donovani infection, but that TNF and LTα derived from BM-derived hematopoietic cells are required for optimal granuloma formation.
VCAM-1 Expression in the Liver During L. donovani Infection Requires LTα
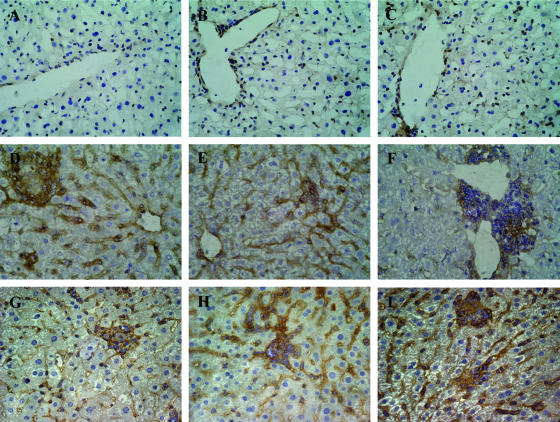
Both ICAM-1 and VCAM-1 play roles in the migration of leukocytes.45,46 Therefore, we next analyzed the expression of these adhesion molecules in liver sections taken from L. donovani-infected C57BL/6, B6.TNF−/−, and B6.LTα−/− mice at day 14 after infection. No difference in ICAM-1 expression was found in the different mouse strains studied (data not shown). In contrast, VCAM-1 expression was found to differ in C57BL/6, B6.TNF−/−, and B6.LTα−/− mice. Minimal VCAM-1 expression was observed in naïve mice, regardless of strain (Figure 3; A to C). However, at day 14 after infection, VCAM-1 expression was up-regulated on the sinusoids of C57BL/6 and B6.TNF−/− mice (Figure 3, D and E). In contrast, VCAM-1 expression in the livers of L. donovani-infected B6.LTα−/− mice was only observed in areas of periportal leukocyte accumulation (Figure 3F). Expression of VCAM-1 was also studied in chimeric mice. Strikingly, B6.LTα−/−→B6.CD45.1 mice up-regulated VCAM-1 in liver sinusoids to the same extent as B6→B6.CD45.1 and B6.TNF−/−→B6.CD45.1 mice (Figure 3; G to I). Together, these data indicate that LTα, but not TNF, is required for optimal up-regulation of VCAM-1 during L. donovani infection and that LTα from radio-resistant cells regulates this process.
Figure 3.
VCAM-1 expression on hepatic sinusoids is regulated by LTα during L. donovani infection. The expression of VCAM-1 (brown) was analyzed in naïve C57BL/6 (A), B6.TNF−/− (B), and B6.LTα−/− (C) mice, and corresponding sections taken at day 14 after infection (D–F), as well as B6.CD45.1 mice engrafted with BM from C57BL/6 (G), B6.TNF−/− (H), and B6.LTα−/− (I) mice at the same time point. Note the absence of VCAM-1 expression in all naïve tissue samples (A–C) and L. donovani-infected B6.LTα−/− (F) mice. Original magnifications, ×400 (hematoxylin counterstained).
LTα Produced by CD4+ T Cells Is Critical for Early Hepatic Control of L. donovani Infection
The increased parasite burden in B6.TNF−/−→B6.CD45.1 and B6.LTα−/−→B6.CD45.1 chimeric mice (Figure 1B) indicated that hematopoietic cells were a critical source of TNF and LTα for control of hepatic L. donovani infection. CD4+ T cells are significant sources of TNF and LTα,47 and are critical for early control of L. donovani infection in the liver.35,36 Therefore, we next tested if CD4+ T cells were an important source of TNF and LTα during the first 14 days of VL by adoptive transfer into B6.RAG-1−/− mice.43 As previously reported,43 parasite growth in the liver of B6.RAG-1−/− mice is rapid and significantly greater than in L. donovani-infected C57BL/6 mice (Figure 4). The transfer of CD4+ T cells from C57BL/6 mice into B6.RAG-1−/− mice before L. donovani infection significantly lowered parasite burden (Figure 4). However, B6.RAG-1−/− mice that received CD4+ T cells from B6.LTα−/− or B6.TNF−/− mice showed no control of parasite growth (Figure 4). Thus, TNF and LTα produced by CD4+ T cells are both critical for early control of hepatic L. donovani infection, and neither cytokine appears able to compensate for the absence of the other.
Figure 4.
CD4+ T cells require TNF and LTα expression to control L. donovani growth in the liver. B6.RAG-1−/− mice were reconstituted with CD4+ T cells (hatched bars) from C57BL/6, B6.TNF−/− and B6.LTα−/− mice, as indicated. Data represent the mean ± SE of Leishman-Donovan unit values in the liver from three mice per group at day 14 after infection. Parasite burdens in donor mice and nonreconstituted B6.RAG-1−/− mice (open bars) in the same experiment are also shown. Statistically differences of P < 0.05 (asterisk), relative to parasite burdens in C57BL/6 mice are indicated. These data are from one of two experiments conducted in the same way with similar results.
LTα Produced by CD4+ T Cells Is Not Required for Hepatic Granuloma Formation during L. donovani Infection
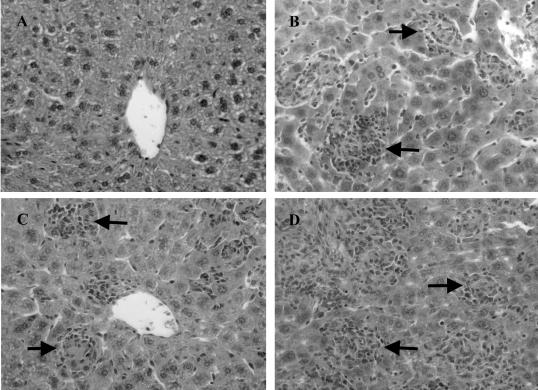
Given the requirement of LTα for efficient granuloma formation during L. donovani infection (Figure 2, Table 1), and the key role of CD4+ T cell-derived LTα for early control of hepatic parasite growth (Figure 4), we next investigated the role of LTα produced by CD4+ T cells in granuloma formation during the first 14 days of L. donovani infection using the adoptive transfer model described above. As previously reported,43 the transfer of CD4+ T cells from C57BL/6 mice into B6.RAG-1−/− mice before L. donovani infection significantly increased the rate of granuloma formation (Figure 5, Table 2). A similar increase in granuloma formation was also observed in B6.RAG-1−/− mice that received CD4+ T cells from B6.TNF−/− or B6.LTα−/− mice, despite relatively high parasite burdens (Figure 5, Table 2). These data indicate that granuloma formation can occur without control of parasite growth in the liver during VL. In addition, these results show that TNF and LTα produced by CD4+ T cells either have minimal roles in hepatic granuloma formation in mice infected with L. donovani or that both cytokines produced by these cells have redundant roles in this process. In contrast, both TNF and LTα produced by CD4+ T cells are critical for the control of parasite growth in the liver.
Figure 5.
Production of TNF and LTα by CD4+ T cells is not required for granuloma development after L. donovani infection. H&E-stained liver sections from B6.RAG-1−/− mice (A), and B6.RAG-1−/− mice reconstituted with CD4+ T cells from C57BL/6 (B), B6.TNF−/− (C), and B6.LTα−/− (D) mice taken at day 14 after infection. Note the advanced granuloma development (both numbers and state of maturation) in reconstituted B6.RAG-1−/− mice, regardless of the source of CD4+ T cells (arrows), and the total absence of granulomas in nonreconstituted B6.RAG-1−/− mice. Original magnifications, ×400.
Table 2.
Liver Granuloma Formation in L. donovani-Infected Mice at Day 14 after Infection*
| Tissue response | Percent infected foci showing the indicated cellular response†
|
|||
|---|---|---|---|---|
| B6.RAG-1−/− | B6.RAG-1−/− reconstituted with CD4+ T cells from
|
|||
| C57BL/6 | B6.TNFα−/− | B6.LTα−/−‡ | ||
| KC | 100 ± 0.0‡ | 4.2 ± 1.6 | 3.1 ± 1.0 | 2.0 ± 0.8‡ |
| IG | 0 | 15.8 ± 1.0 | 18.8 ± 3.0 | 8.9 ± 1.4 |
| MG | 0 | 59.3 ± 4.0 | 58.4 ± 3.4 | 63.4 ± 1.6 |
| SG | 0 | 20.6 ± 4.4 | 19.7 ± 1.0 | 25.8 ± 1.1 |
| Total no. infected foci per 25 fields | 72 ± 12§ | 141 ± 12.1 | 129 ± 20.1 | 105 ± 4.3¶ |
Granuloma counts performed as described in Table 1.
Granuloma scores as described in Table 1.
The distribution of tissue responses is significantly different (P < 0.05) than that observed in L. donovani-infected B6.RAG-1−/− reconstituted with CD4+ T cells from C57BL/6 mice.
The number of infected foci is significantly different (P < 0.001§¶ and P < 0.05¶) than that observed in L. donovani-infected B6.RAG-1−/− reconstituted with CD4+ T cells from C57BL/6 mice.
Inducible Nitric Oxide Synthase Production Is Reduced in TNF- and LTα-Deficient Mice after L. donovani Infection
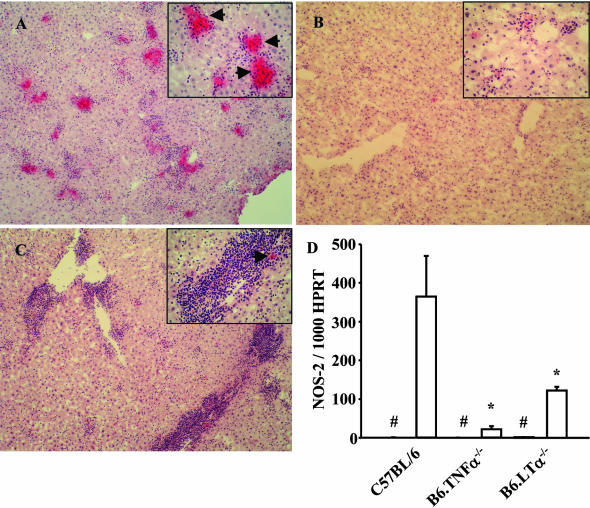
NOS-2 generates reactive nitrogen intermediates that are leishmanicidal.48 To determine whether reactive nitrogen intermediate generation was altered in the liver of TNF- and LTα-deficient mice after L. donovani infection, hepatic NOS-2 protein expression was analyzed by immunohistochemistry and NOS-2 mRNA levels were measured by real-time reverse transcription polymerase chain reaction (Figure 6). NOS-2 was detected in the granulomas of C57BL/6 mice at day 14 after infection (Figure 6A), but rarely observed in B6.TNF−/− mice at the same time point (Figure 6B), and only occasionally seen in the periportal regions of B6.LTα−/− mice (Figure 6C). NOS-2 mRNA levels correlated with protein expression patterns with highest levels detected in C57BL/6 mice and lowest levels in TNF-deficient mice (Figure 6D). We also observed a similar pattern of TNF mRNA expression with 242 ± 77 and 78 ± 21 TNF mRNA molecules per 1000 HPRT mRNA molecules detected in the livers of L. donovani-infected C57BL/6 and B6.LTα−/− mice at day 14 after infection, respectively. No TNF mRNA was detected in B6.TNF−/− mice, as expected. We were only unable to detect LTα mRNA in the liver by real-time reverse transcriptase-polymerase chain reaction in infected C57BL/6 mice, although we have previously shown that TNF-deficient mice can make this cytokine in the brain during murine cerebral malaria.30 These data show that the major leishmanicidal mechanism of reactive nitrogen intermediate production is deficient in mice lacking TNF or LTα early in infection. However, whether this deficiency arises from the inefficient recruitment of leukocytes into the liver, the need for TNF and LTα for the production of NOS-2, or possibly both, is unknown.
Figure 6.
Inducible nitric oxide synthase (NOS-2) generation is inhibited in L. donovani-infected mice deficient in TNF and LTα. A–C: NOS-2-stained liver sections from C57BL/6 (A), B6.TNF−/− (B), and B6.LTα−/− (C) mice taken at day 14 after infection. Arrows in insets indicate NOS-2-positive staining. Note the strong staining in granulomas found in C57BL/6 mice. D: The number of NOS-2 mRNA molecules per 1000 HPRT molecules found in the livers of C57BL/6, B6.TNF−/−, and B6.LTα−/− mice, as indicated. #, Indicates where the data for naïve mice is shown (all levels were below 2 NOS-2 mRNA per 1000 HPRT mRNA molecules). Statistically differences of P < 0.05 (asterisk), relative to NOS-2 mRNA levels in C57BL/6 mice are indicated. Original magnifications: ×100; ×400 insets (A–C).
Discussion
TNF and LTα are potent cytokines with multiple functions.1 Only recently have efforts been made to distinguish between the roles of these molecules during infectious diseases. Here we show that TNF and LTα are both required for control of hepatic L. donovani infection in the first 14 days of infection, whereas only TNF is critical for the ultimate survival of infected mice. Studies were focused on defining responses in the early stage (first 14 days) of L. donovani infection in the liver. At least two important sources of TNF and LTα early in L. donovani infection were identified. Radio-resistant cells play a key role in granuloma assembly, as revealed by studies in chimeric mice. In contrast, adoptive transfer experiments indicated that CD4+ T cells expressing both TNF and LTα are needed for efficient killing of parasites within assembled granulomas.
The roles of TNF and LTα early in L. donovani infection can be distinguished in at least two ways. First, LTα is critical for efficient migration of leukocytes from perivascular areas of the liver to infected Kupffer cells. In TNF-deficient mice, periportal accumulation of leukocytes was minimal, associated with reduced granuloma formation. This indicated that in the absence of TNF, leukocytes do not enter the periportal areas as they migrate through the liver, unlike leukocytes in the absence of LTα, suggesting that TNF-dependent mechanisms of leukocyte recruitment in the liver during VL are different from those involving LTα. Whether these TNF-dependent events occur during T cell activation or regulate the expression of hepatic homing receptors remains to be determined. As reported previously for TNF-deficient mice,21 granuloma assembly did improve in both B6.TNF−/− and B6.LTα−/− mice after day 14 after infection, although never to the same extent as in C57BL/6 mice (data not shown). A second distinction between the roles of TNF and LTα early in L. donovani infection was that LTα was required for expression of VCAM-1 on sinusoidal endothelium, whereas TNF was not. A central role for both TNF and LTα in up-regulating expression of VCAM-1 on various cell populations is well documented.26,49,50 However, our results show that during L. donovani infection, VCAM-1 up-regulation on hepatic sinusoids can occur in the absence of TNF, but not LTα. Furthermore, the LTα required for up-regulation of VCAM-1 expression derived from a radio-resistant cells, as indicated by an intact response in L. donovani-infected B6.LTα−/−→B6.CD45.1 chimeric mice. Whether the failure of leukocytes to migrate from the periportal area was caused by lack of VCAM-1 expression in B6.LTα−/− mice infected with L. donovani is unknown at present. However, previous studies have shown that IL-12-induced recruitment of hepatic NK and T cells was blocked by treatment with an anti-VCAM-1 antibody,46 suggesting that at least these two cell populations require VCAM-1 expression on hepatic endothelium for recruitment. Interestingly, in M. tuberculosis infection of LTα-deficient mice, leukocytes were recruited to the lungs, but also failed to migrate from perivascular and peribronchial regions to form pulmonary granulomas,28 suggesting similar LTα-dependent cellular recruitment pathways in pulmonary M. tuberculosis and hepatic L. donovani infection. Notably, however, in B6.LTα−/−→B6.RAG-1−/− chimeric mice infected with M. tuberculosis, the cellular response was similar to that seen in B6.LTα−/− mice,28 indicating that depending on the infection studied, radio-resistant cells may (L. donovani) or may not (M. tuberculosis) play a significant role in aiding leukocyte recruitment. However, one difference in the above studies was the use of B6.CD45.1 mice as recipients of BM grafts in our studies and the use of B6.RAG-1−/− mice as recipients in studies on M. tuberculosis. Whether this accounts for the different results is unknown.
In addition to radio-resistant cells, CD4+ T cells are a critical source of LTα during L. donovani infection. CD4+ T cells produce significant amounts of LTα.47 Interestingly, the LTα produced by CD4+ T cells was not required for granuloma assembly during L. donovani infection, but instead, played a key role in the generation of leishmanicidal mechanisms in and around infected foci. Although these data point to the cellular source of LTα as being of importance, it is difficult to formally rule out that different thresholds of LTα signaling govern up-regulation of VCAM-1 and the induction of leishmanicidal activity, with the low levels of LTα produced by endothelial cells possibly being sufficient for the former response but not the latter.
Importantly, CD4+ T cells producing both TNF and LTα are critical for killing parasites, with these cytokines acting in a noncompensatory manner in our adoptive transfer model. The nonredundant roles of TNF and LTα during murine L. donovani infection suggest that these molecules may function through different receptors after infection. Although both cytokines can signal via TNFRI and TNFRII,1,51 LTα is also able to form a membrane-bound LTα1β2 heterotrimer that binds LTβR7–10 and can also bind the herpesvirus entry mediator receptor.11 The roles of LTα1β2, LTβR, and herpesvirus entry mediator receptor in VL are currently unknown. An alternate possibility is that TNF and LTα may signal via the same receptors but the timing and anatomical location of signaling events may differ, resulting in different biological outcomes.
The importance of LTα in the control of many infectious diseases is becoming clear. However, the role that LTα plays in the immune response appears to depend on the pathogen studied. As discussed above, leukocyte recruitment to the lungs during M. tuberculosis infection is affected in a similar way to cellular recruitment in the liver during L. donovani infection. However, in the latter model, a radio-resistant cell is the critical source of LTα, whereas in the M. tuberculosis model hematopoietic cells are the important source of this cytokine.28 In other models, such as murine T. gondii infections, LTα appears to play a limited role in cellular recruitment to the brain, but is critical for control of infection in this tissue and host survival.29 However, in murine cerebral malaria caused by Plasmodium berghei ANKA, LTα is a key mediator of pathology leading to the death of infected mice.30 In this infection, recruitment of leukocytes to the brain is limited in LTα-deficient mice and cerebral malaria is prevented. Interestingly, a radio-resistant cell population in the brain is a critical source of LTα for induction of pathology.30 Therefore, the tissue microenvironment where LTα is produced appears to be critical for many of the outcomes of infectious diseases.
The data described in this study add to the growing body of work showing the importance of LTα in infectious diseases, and may enable strategies to be devised to either stimulate the production of this cytokine in appropriate tissue microenvironments to control infections or prevent its production in circumstances in which it mediates pathology. Significantly, recent genetic studies suggest that single nucleotide polymorphisms found in the human LTα gene can have profound effects on the transcription of this gene,52,53 and the function of transcribed product.52 In addition, susceptibility to diseases as diverse as myocardial infarction,52 cerebral infarction,54 asthma,55 type I diabetes mellitus,56 multiple sclerosis,57 rheumatoid arthritis,58 celiac disease,59 schizophrenia,60 and hepatitis C61 can all be related to polymorphisms associated with the LTα gene in humans. The influence of LTα single nucleotide polymorphisms on infectious diseases such as VL, tuberculosis, toxoplasmosis, and malaria remains unknown. However, knowledge gained from infectious disease models such as murine VL will enable rational predictions to be made about the functional consequences of such polymorphisms, and to provide a basis to devise strategies to counter any adverse effects associated with them.
Acknowledgments
We thank the staff of the Biological Services Facility for assistance in the breeding and maintenance of mouse colonies.
Footnotes
Address reprint requests to Dr. Christian Engwerda, Immunology and Infection Laboratory, Queensland Institute of Medical Research, 300 Herston Rd., Herston, Queensland, Australia 4029. E-mail: chrise@qimr.edu.au.
Supported by the Wellcome Trust, the British Medical Research Council, the United Nations Development Programme World Bank/WHO Special Programme for Research and Training in Tropical Diseases, and the Australian National Health and Medical Research Council.
C.E. is a National Health and Medical Research Council Career Development Fellow and M.A. is a recipient of a Wellcome Trust International Fellowship.
References
- Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2001;104:487–501. doi: 10.1016/s0092-8674(01)00237-9. [DOI] [PubMed] [Google Scholar]
- Muller U, Jongeneel CV, Nedospasov SA, Lindahl KF, Steinmetz M. Tumour necrosis factor and lymphotoxin genes map close to H-2D in the mouse major histocompatibility complex. Nature. 1987;325:265–267. doi: 10.1038/325265a0. [DOI] [PubMed] [Google Scholar]
- Browning JL, Ngam-ek A, Lawton P, DeMarinis J, Tizard R, Chow EP, Hession C, O’Brine-Greco B, Foley SF, Ware CF. Lymphotoxin beta, a novel member of the TNF family that forms a heteromeric complex with lymphotoxin on the cell surface. Cell. 1993;72:847–856. doi: 10.1016/0092-8674(93)90574-a. [DOI] [PubMed] [Google Scholar]
- Lawton P, Nelson J, Tizard R, Browning JL. Characterization of the mouse lymphotoxin-beta gene. J Immunol. 1995;154:239–246. [PubMed] [Google Scholar]
- Pokholok DK, Maroulakou IG, Kuprash DV, Alimzhanov MB, Kozlov SV, Novobrantseva TI, Turetskaya RL, Green JE, Nedospasov SA. Cloning and expression analysis of the murine lymphotoxin beta gene. Proc Natl Acad Sci USA. 1995;92:674–678. doi: 10.1073/pnas.92.3.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruuls SR, Hoek RM, Ngo VN, McNeil T, Lucian LA, Janatpour MJ, Korner H, Scheerens H, Hessel EM, Cyster JG, McEvoy LM, Sedgwick JD. Membrane-bound TNF supports secondary lymphoid organ structure but is subservient to secreted TNF in driving autoimmune inflammation. Immunity. 2001;15:533–543. doi: 10.1016/s1074-7613(01)00215-1. [DOI] [PubMed] [Google Scholar]
- Crowe PD, VanArsdale TL, Walter BN, Ware CF, Hession C, Ehrenfels B, Browning JL, Din WS, Goodwin RG, Smith CA. A lymphotoxin-beta-specific receptor. Science. 1994;264:707–710. doi: 10.1126/science.8171323. [DOI] [PubMed] [Google Scholar]
- Alimzhanov MB, Kuprash DV, Kosco-Vilbois MH, Luz A, Turetskaya RL, Tarakhovsky A, Rajewsky K, Nedospasov SA, Pfeffer K. Abnormal development of secondary lymphoid tissues in lymphotoxin beta-deficient mice. Proc Natl Acad Sci USA. 1997;94:9302–9307. doi: 10.1073/pnas.94.17.9302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koni PA, Sacca R, Lawton P, Browning JL, Ruddle NH, Flavell RA. Distinct roles in lymphoid organogenesis for lymphotoxins alpha and beta revealed in lymphotoxin beta-deficient mice. Immunity. 1997;6:491–500. doi: 10.1016/s1074-7613(00)80292-7. [DOI] [PubMed] [Google Scholar]
- Futterer A, Mink K, Luz A, Kosco-Vilbois MH, Pfeffer K. The lymphotoxin beta receptor controls organogenesis and affinity maturation in peripheral lymphoid tissues. Immunity. 1998;9:59–70. doi: 10.1016/s1074-7613(00)80588-9. [DOI] [PubMed] [Google Scholar]
- Mauri DN, Ebner R, Montgomery RI, Kochel KD, Cheung TC, Yu GL, Ruben S, Murphy M, Eisenberg RJ, Cohen GH, Spear PG, Ware CF. LIGHT, a new member of the TNF superfamily, and lymphotoxin alpha are ligands for herpesvirus entry mediator. Immunity. 1998;8:21–30. doi: 10.1016/s1074-7613(00)80455-0. [DOI] [PubMed] [Google Scholar]
- Korner H, Cook M, Riminton DS, Lemckert FA, Hoek RM, Ledermann B, Kontgen F, Fazekas de St Groth B, Sedgwick JD. Distinct roles for lymphotoxin-alpha and tumor necrosis factor in organogenesis and spatial organization of lymphoid tissue. Eur J Immunol. 1997;27:2600–2609. doi: 10.1002/eji.1830271020. [DOI] [PubMed] [Google Scholar]
- Marino MW, Dunn A, Grail D, Inglese M, Noguchi Y, Richards E, Jungbluth A, Wada H, Moore M, Williamson B, Basu S, Old LJ. Characterization of tumor necrosis factor-deficient mice. Proc Natl Acad Sci USA. 1997;94:8093–8098. doi: 10.1073/pnas.94.15.8093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasparakis M, Alexopoulou L, Episkopou V, Kollias G. Immune and inflammatory responses in TNF alpha-deficient mice: a critical requirement for TNF alpha in the formation of primary B cell follicles, follicular dendritic cell networks and germinal centers, and in the maturation of the humoral immune response. J Exp Med. 1996;184:1397–1411. doi: 10.1084/jem.184.4.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks TA, Rouse BT, Kerley MK, Blair PJ, Godfrey VL, Kuklin NA, Bouley DM, Thomas J, Kanangat S, Mucenski ML. Lymphotoxin-alpha-deficient mice. Effects on secondary lymphoid organ development and humoral immune responsiveness. J Immunol. 1995;155:1685–1693. [Google Scholar]
- De Togni P, Goellner J, Ruddle NH, Streeter PR, Fick A, Mariathasan S, Smith SC, Carlson R, Shornick LP, Strauss-Schoenberger J, Russell JH, Karr R, Chaplin DD. Abnormal development of peripheral lymphoid organs in mice deficient in lymphotoxin. Science. 264:703–707. doi: 10.1126/science.8171322. [DOI] [PubMed] [Google Scholar]
- Kuprash DV, Alimzhanov MB, Tumanov AV, Grivennikov SI, Shakhov AN, Drutskaya LN, Marino MW, Turetskaya RL, Anderson AO, Rajewsky K, Pfeffer K, Nedospasov SA. Redundancy in tumor necrosis factor (TNF) and lymphotoxin (LT) signaling in vivo: mice with inactivation of the entire TNF/LT locus versus single-knockout mice. Mol Cell Biol. 2002;22:8626–8634. doi: 10.1128/MCB.22.24.8626-8634.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer K, Matsuyama T, Kundig TM, Wakeham A, Kishihara K, Shahinian A, Wiegmann K, Ohashi PS, Kronke M, Mak TW. Mice deficient for the 55 kd tumor necrosis factor receptor are resistant to endotoxic shock, yet succumb to L. monocytogenes infection. Cell. 1993;73:457–467. doi: 10.1016/0092-8674(93)90134-c. [DOI] [PubMed] [Google Scholar]
- Rothe J, Bluethmann H, Gentz R, Lesslauer W, Steinmetz M. Genomic organization and promoter function of the murine tumor necrosis factor receptor beta gene. Mol Immunol. 1993;30:165–175. doi: 10.1016/0161-5890(93)90088-s. [DOI] [PubMed] [Google Scholar]
- Erickson SL, de Sauvage FJ, Kikly K, Carver-Moore K, Pitts-Meek S, Gillett N, Sheehan KC, Schreiber RD, Goeddel DV, Moore MW. Decreased sensitivity to tumour-necrosis factor but normal T-cell development in TNF receptor-2-deficient mice. Nature. 1994;372:560–563. doi: 10.1038/372560a0. [DOI] [PubMed] [Google Scholar]
- Murray HW, Jungbluth A, Ritter E, Montelibano C, Marino MW. Visceral leishmaniasis in mice devoid of tumor necrosis factor and response to treatment. Infect Immun. 2000;68:6289–6293. doi: 10.1128/iai.68.11.6289-6293.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm P, Ritter U, Labbow S, Donhauser N, Rollinghoff M, Bogdan C, Korner H. Rapidly fatal leishmaniasis in resistant C57BL/6 mice lacking TNF. J Immunol. 2001;166:4012–4019. doi: 10.4049/jimmunol.166.6.4012. [DOI] [PubMed] [Google Scholar]
- Sedgwick JD, Riminton DS, Cyster JG, Korner H. Tumor necrosis factor: a master-regulator of leukocyte movement. Immunol Today. 2000;21:110–113. doi: 10.1016/s0167-5699(99)01573-x. [DOI] [PubMed] [Google Scholar]
- Beutler B, van Huffel C. Unraveling function in the TNF ligand and receptor families. Science. 1994;264:667–668. doi: 10.1126/science.8171316. [DOI] [PubMed] [Google Scholar]
- Ding AH, Nathan CF, Stuehr DJ. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J Immunol. 1988;141:2407–2412. [PubMed] [Google Scholar]
- Cuff CA, Sacca R, Ruddle NH. Differential induction of adhesion molecule and chemokine expression by LTalpha3 and LTalphabeta in inflammation elucidates potential mechanisms of mesenteric and peripheral lymph node development. J Immunol. 1999;162:5965–5972. [PubMed] [Google Scholar]
- Sacca R, Cuff CA, Lesslauer W, Ruddle NH. Differential activities of secreted lymphotoxin-alpha3 and membrane lymphotoxin-alpha1beta2 in lymphotoxin-induced inflammation: critical role of TNF receptor 1 signaling. J Immunol. 1998;160:485–491. [PubMed] [Google Scholar]
- Roach DR, Briscoe H, Saunders B, France MP, Riminton S, Britton WJ. Secreted lymphotoxin-alpha is essential for the control of an intracellular bacterial infection. J Exp Med. 2001;193:239–246. doi: 10.1084/jem.193.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluter D, Kwok LY, Lutjen S, Soltek S, Hoffmann S, Korner H, Deckert M. Both lymphotoxin-alpha and TNF are crucial for control of Toxoplasma gondii in the central nervous system. J Immunol. 2003;170:6172–6182. doi: 10.4049/jimmunol.170.12.6172. [DOI] [PubMed] [Google Scholar]
- Engwerda CR, Mynott TL, Sawhney S, De Souza JB, Bickle QD, Kaye PM. Locally up-regulated lymphotoxin alpha, not systemic tumor necrosis factor alpha, is the principle mediator of murine cerebral malaria. J Exp Med. 2002;195:1371–1377. doi: 10.1084/jem.20020128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magez S, Stijlemans B, Caljon G, Eugster HP, De Baetselier P. Control of experimental Trypanosoma brucei infections occurs independently of lymphotoxin-alpha induction. Infect Immun. 2002;70:1342–1351. doi: 10.1128/IAI.70.3.1342-1351.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryceson ADM. Leishmaniasis. Cook GC, editor. London: W. B. Saunders,; Manson’s Tropical Diseases. 1996:pp 1213–1245. [Google Scholar]
- Wilson ME, Sandor M, Blum AM, Young BM, Metwali A, Elliott D, Lynch RG, Weinstock JV. Local suppression of IFN-gamma in hepatic granulomas correlates with tissue-specific replication of Leishmania chagasi. J Immunol. 1996;156:2231–2239. [PubMed] [Google Scholar]
- Smelt SC, Engwerda CR, McCrossen M, Kaye PM. Destruction of follicular dendritic cells during chronic visceral leishmaniasis. J Immunol. 1997;158:3813–3821. [PubMed] [Google Scholar]
- McElrath MJ, Murray HW, Cohn ZA. The dynamics of granuloma formation in experimental visceral leishmaniasis. J Exp Med. 1988;167:1927–1937. doi: 10.1084/jem.167.6.1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray HW, Squires KE, Miralles CD, Stoeckle MY, Granger AM, Granelli-Piperno A, Bogdan C. Acquired resistance and granuloma formation in experimental visceral leishmaniasis. Differential T cell and lymphokine roles in initial versus established immunity. J Immunol. 1992;148:1858–1863. [PubMed] [Google Scholar]
- Tumang MC, Keogh C, Moldawer LL, Helfgott DC, Teitelbaum R, Hariprashad J, Murray HW. Role and effect of TNF-alpha in experimental visceral leishmaniasis. J Immunol. 1994;153:768–775. [PubMed] [Google Scholar]
- Engwerda CR, Ato M, Cotterell SE, Mynott TL, Tschannerl A, Gorak-Stolinska PM, Kaye PM. A role for tumor necrosis factor-alpha in remodeling the splenic marginal zone during Leishmania donovani infection. Am J Pathol. 2002;161:429–437. doi: 10.1016/s0002-9440(10)64199-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ato M, Stager S, Engwerda CR, Kaye PM. Defective CCR7 expression on dendritic cells contributes to the development of visceral leishmaniasis. Nat Immunol. 2002;3:1185–1191. doi: 10.1038/ni861. [DOI] [PubMed] [Google Scholar]
- Riminton SD, Korner H, Strickland DH, Lemckert FA, Pollard JD, Sedgwick JD. Challenging cytokine redundancy: inflammatory cell movement and clinical course of experimental autoimmune encephalomyelitis are normal in lymphotoxin-deficient, but not tumor necrosis factor-deficient, mice. J Exp Med. 1998;187:1517–1528. doi: 10.1084/jem.187.9.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engwerda CR, Murphy ML, Cotterell SE, Smelt SC, Kaye PM. Neutralization of IL-12 demonstrates the existence of discrete organ-specific phases in the control of Leishmania donovani. Eur J Immunol. 1998;28:669–680. doi: 10.1002/(SICI)1521-4141(199802)28:02<669::AID-IMMU669>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Murphy ML, Cotterell SE, Gorak PM, Engwerda CR, Kaye PM. Blockade of CTLA-4 enhances host resistance to the intracellular pathogen, Leishmania donovani. J Immunol. 1998;161:4153–4160. [PubMed] [Google Scholar]
- Alexander CE, Kaye PM, Engwerda CR. CD95 is required for the early control of parasite burden in the liver of Leishmania donovani-infected mice. Eur J Immunol. 2001;31:1199–1210. doi: 10.1002/1521-4141(200104)31:4<1199::aid-immu1199>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Chin RK, Lo JC, Kim O, Blink SE, Christiansen PA, Peterson P, Wang Y, Ware C, Fu YX. Lymphotoxin pathway directs thymic Aire expression. Nat Immunol. 2003;4:1121–1127. doi: 10.1038/ni982. [DOI] [PubMed] [Google Scholar]
- Fogler WE, Volker K, McCormick KL, Watanabe M, Ortaldo JR, Wiltrout RH. NK cell infiltration into lung, liver, and subcutaneous B16 melanoma is mediated by VCAM-1/VLA-4 interaction. J Immunol. 1996;156:4707–4714. [PubMed] [Google Scholar]
- Fogler WE, Volker K, Watanabe M, Wigginton JM, Roessler P, Brunda MJ, Ortaldo JR, Wiltrout RH. Recruitment of hepatic NK cells by IL-12 is dependent on IFN-gamma and VCAM-1 and is rapidly down-regulated by a mechanism involving T cells and expression of Fas. J Immunol. 1998;161:6014–6021. [PubMed] [Google Scholar]
- Ware CF, Crowe PD, Grayson MH, Androlewicz MJ, Browning JL. Expression of surface lymphotoxin and tumor necrosis factor on activated T, B, and natural killer cells. J Immunol. 1992;149:3881–3888. [PubMed] [Google Scholar]
- Murray HW, Nathan CF. Macrophage microbicidal mechanisms in vivo: reactive nitrogen versus oxygen intermediates in the killing of intracellular visceral Leishmania donovani. J Exp Med. 1999;189:741–746. doi: 10.1084/jem.189.4.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassalli P. The pathophysiology of tumor necrosis factors. Annu Rev Immunol. 1992;10:411–452. doi: 10.1146/annurev.iy.10.040192.002211. [DOI] [PubMed] [Google Scholar]
- Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- Hochman PS, Majeau GR, Mackay F, Browning JL. Proinflammatory responses are efficiently induced by homotrimeric but not heterotrimeric lymphotoxin ligands. J Inflamm. 1995;46:220–234. [PubMed] [Google Scholar]
- Ozaki K, Ohnishi Y, Iida A, Sekine A, Yamada R, Tsunoda T, Sato H, Hori M, Nakamura Y, Tanaka T. Functional SNPs in the lymphotoxin-alpha gene that are associated with susceptibility to myocardial infarction. Nat Genet. 2002;32:650–654. doi: 10.1038/ng1047. [DOI] [PubMed] [Google Scholar]
- Knight JC, Keating BJ, Rockett KA, Kwiatkowski DP. In vivo characterization of regulatory polymorphisms by allele-specific quantification of RNA polymerase loading. Nat Genet. 2003;33:469–475. doi: 10.1038/ng1124. [DOI] [PubMed] [Google Scholar]
- Um JY, An NH, Kim HM. TNF-alpha and TNF-beta gene polymorphisms in cerebral infarction. J Mol Neurosci. 2003;21:167–171. doi: 10.1385/JMN:21:2:167. [DOI] [PubMed] [Google Scholar]
- Wang T, Chen W, Chen C, Huang L, Ko Y. Gene-gene synergistic effect on atopic asthma: tumour necrosis factor-alpha-308 and lymphotoxin-alpha-NcoI in Taiwan’s children. Clin Exp Allergy. 2004;34:184–188. doi: 10.1111/j.1365-2222.2004.01867.x. [DOI] [PubMed] [Google Scholar]
- Bouqbis L, Akhayat O, Garchon HJ, Calafell F, Izaabel H. TNFA-TNFB haplotypes modify susceptibility to type I diabetes mellitus independently of HLA class II in a Moroccan population. Tissue Antigens. 2003;61:72–79. doi: 10.1034/j.1399-0039.2003.610106.x. [DOI] [PubMed] [Google Scholar]
- Fernandes Filho JA, Vedeler CA, Myhr KM, Nyland H, Pandey JP. TNF-alpha and -beta gene polymorphisms in multiple sclerosis: a highly significant role for determinants in the first intron of the TNF-beta gene. Autoimmunity. 2002;35:377–380. doi: 10.1080/0891693021000021549. [DOI] [PubMed] [Google Scholar]
- Newton J, Brown MA, Milicic A, Ackerman H, Darke C, Wilson JN, Wordsworth BP, Kwiatkowski D. The effect of HLA-DR on susceptibility to rheumatoid arthritis is influenced by the associated lymphotoxin alpha-tumor necrosis factor haplotype. Arthritis Rheum. 2003;48:90–96. doi: 10.1002/art.10719. [DOI] [PubMed] [Google Scholar]
- Garrote JA, Arranz E, Telleria JJ, Castro J, Calvo C, Blanco-Quiros A. TNF alpha and LT alpha gene polymorphisms as additional markers of celiac disease susceptibility in a DQ2-positive population. Immunogenetics. 2002;54:551–555. doi: 10.1007/s00251-002-0498-9. [DOI] [PubMed] [Google Scholar]
- Jun TY, Pae CU, Chae JH, Bahk WM, Kim KS, Han H, Serretti A. TNFB polymorphism may be associated with schizophrenia in the Korean population. Schizophr Res. 2003;61:39–45. doi: 10.1016/s0920-9964(02)00303-1. [DOI] [PubMed] [Google Scholar]
- Goyal A, Kazim SN, Sakhuja P, Malhotra V, Arora N, Sarin SK. Association of TNF-beta polymorphism with disease severity among patients infected with hepatitis C virus. J Med Virol. 2004;72:60–65. doi: 10.1002/jmv.10533. [DOI] [PubMed] [Google Scholar]