Abstract
Stem cell factor (SCF) and endothelin-1 (ET-1) have been reported to be up-regulated at the protein and gene levels in human epidermis after ultraviolet B (UVB) irradiation and to play central roles in UVB-induced pigmentation. However, little is known about the time sequence of SCF and ET-1 expression in UVB-exposed human epidermis and the coordination of their roles during epidermal pigmentation. To clarify such parameters in UVB-exposed human skin, we measured the expression patterns of SCF and ET-1 (as well as of their corresponding receptors) at the gene level at various times during UVB-induced human pigmentation. When human forearm skin was exposed to UVB radiation at two minimal erythemal doses, the expression of SCF mRNA transcripts was significantly enhanced at 3 days after irradiation with an early decrease and subsequently constant expression of SCF receptor (c-KIT) mRNA transcripts. In contrast, up-regulation of ET-1 and endothelin B receptor (ETBR) mRNA expression was synchronized at 5 to 10 days after irradiation in concert with an increased expression of tyrosinase mRNA transcripts and the increase in pigmentation. In parallel the expression of tyrosinase and ETBR proteins as well as ET-1 was up-regulated at 7 to 10 days after irradiation, whereas KIT protein decreased at 3 days after irradiation and returned to the nonirradiated control level at 5 days after irradiation. When cultured human melanocytes were treated with human recombinant SCF, ETBR protein expression and the binding of 125I-labeled ET-1 to the ETBR were significantly increased, further suggesting the preferential and coordinated role of early expression of SCF in UVB-induced melanogenesis. These findings suggest that SCF/KIT signaling is predominantly involved in the early phase of UVB-induced human pigmentation during which it stimulates the ET-1/ETBR linkage that is associated with the later phase of UVB-induced melanogenesis.
On exposure of human skin to UVB radiation, pigmentation is elicited with a lag time of a few days after inflammation. In the pigmented human epidermis that results, there are increased numbers of pigment cells (melanocytes), increased amounts of melanin within them, as well as increased numbers of melanin granules within keratinocytes. Thus, the process of UV-induced hyperpigmentation in human epidermis is composed of three major steps; the first step is the proliferation of melanocytes,1 followed by the synthesis and activation of tyrosinase needed to increase melanogenesis,2,3 and finally the transfer of melanosomes to keratinocytes.4 During the first two steps, a complex network exists in the epidermis for secreting and responding to autocrine and paracrine cytokines by keratinocytes and melanocytes, respectively.5–22 Corresponding receptors, which are also regulated in their expression by various cytokines, participate in the complex network in which there is cross-talk in signaling between cytokines to support the enhanced proliferation of melanocytes and the activation of melanogenesis within the cells. These paracrine cytokines include basic fibroblast growth factor,5 endothelin-1 (ET-1),7–13 α-melanocyte-stimulating hormone,6,14–17,20 stem cell factor (SCF),7,18 and nitric oxide.19 The secretion of ET-1 and α-melanocyte-stimulating hormone or nitric oxide-associated signal transduction have been reported to be triggered by primary inflammatory cytokines such as interleukin (IL)-1α and tumor necrosis factor (TNF)-α, which are released by UVB-exposed keratinocytes.11,20,21
The pigmentary system in mouse and in rat skin is completely different from human skin because of the absence of functional melanocytes in the epidermis. Focusing on human skin in vivo, we found that the expression of ET-1 and SCF is remarkably enhanced at the gene and protein levels in UVB-exposed human epidermis.12,18 Based on evidence that ET-1 and SCF are potent mitogens and melanogens for cultured human melanocytes,7,22 our findings suggest that ET-1 and SCF are intrinsically involved in regulating the proliferation and melanogenesis of human melanocytes in vivo during UVB-induced hyperpigmentation of human skin. The specific relevance of both signaling cascades, ie, the ET-1/endothelin B receptor (ETBR) and the SCF/KIT, to UVB-induced pigmentation is corroborated by the fact that blocking either ligand/receptor interaction (by an ET receptor antagonist or by a monoclonal antibody to KIT) results in a significant reduction or a complete loss of pigmentation in vivo.8,18 However, little is known about the coordinated roles and the time sequence of expression of ligands and receptors in both cascades after UV irradiation.
ET-1 was first identified in the culture media of porcine endothelial cells and is a 21-residue peptide with a potent vasoconstrictive activity.23 To date, three distinct genes encoding three closely related peptides, ET-1, ET-2, and ET-3, have been identified.24 ETs have been reported to bind to two types of receptors, endothelin A receptor and ETBR, which bind all three ETs with similar affinities.25–27 It has been reported that ETs have hormonal regulatory activities in various types of cells, including melanocytes, and in target organs via a receptor-mediated biochemical mechanism.28–31
SCF is also known as steel factor, kit ligand, or mast cell growth factor and is encoded by the steel (Sl) locus; its receptor c-kit is encoded by the dominant white spotting (W) locus. Mutations in either of those loci elicit very similar phenotypes that are characterized by the loss of neural crest-derived pigment cells, hematopoietic stem cells, and primordial germ cells.32–38 The involvement of SCF/c-kit signaling in melanocyte development at embryonal stages has been delineated by phenotype analysis of Sl and W mice. Experiments using a monoclonal c-kit antibody (ACK2), an antagonistic blocker of c-kit function, also demonstrated the importance of SCF/c-kit signaling in the development of murine melanocytes.39–41
Piebaldism, a disorder presenting at birth that is characterized by amelanotic patches on acral and/or ventral skin surfaces, but apparently lacking detectable defects in germ cells or in the hematological system, are caused by mutations in the genes encoding c-kit42–44 or ETBR.45,46 In type II Waardenburg syndrome, an autosomal dominant disorder characterized by pigmentary abnormalities and sensorineural deafness, ∼15% of patients are heterozygous for mutations in the gene encoding microphthalmia-associated transcription factor.47,48 Microphthalmia-associated transcription factor expression is modulated by SCF through the mitogen-activated protein kinase pathway and stimulates transcription of the gene encoding tyrosinase, the rate-limiting enzyme in melanin synthesis.49,50 Furthermore, type IV Waardenburg syndrome (Shah-Waardenburg syndrome with Hirschsprung disease) is caused by mutations in the genes encoding ET-3 or ETBR.48
In addition to their significance during the development of melanocytes, ET-1 and/or SCF are involved in melanocyte activation in several human pigmentary disorders such as lentigo senilis, dermatofibroma, café-au-lait macules, and seborrehoic keratosis.51–55 Especially in lentigo senilis, which is similar in histological aspects to UVB-melanosis, the enhanced expression of SCF and ET-1 occurs concomitantly with an increased expression of ETBR in the lesional pigmented epidermis.
Such evidence indicates that SCF/c-kit and ET-1/ETBR signaling use some common pathways and may play coordinated roles in regulating human epidermal melanogenesis. In this respect, SCF and ET-1 synergistically increase the proliferation of human melanocytes7 and this synergistic cross-talk between SCF and ET-1 signaling is initiated by phosphorylation of c-kit in the signaling cascades. This results in activation of the Shc-Grb2 complex, which is in turn followed by the synergistic stimulation of mitogen-activated protein kinase signaling in human melanocytes.22 However, this might not reflect the mechanism(s) underlying the activation of melanocytes in vivo, because those results were derived from experiments in which SCF and ET-1 were added to human melanocytes at the same time. Therefore, it is of particular interest to characterize the dynamics of SCF/c-kit signaling and of ET-1/ETBR signaling during melanocyte activation after UVB-irradiation. If one pathway is stimulated at an early phase of UVB-induced pigmentation while the other occurs later, it would then be important to determine whether the earlier signaling pathway affects the later one. Here we report that SCF/c-kit signaling between keratinocytes and melanocytes is predominantly involved in the early phase of UVB-induced human pigmentation, whereas ET-1/ETBR signaling is associated with the later phase.
Materials and Methods
Materials
Normal human melanocytes and keratinocytes and serum-free melanocyte medium were purchased from Sankou Pure Chemicals (Tokyo, Japan). TRIzol, Moloney murine leukemia virus reverse transcriptase, serum-free keratinocyte medium, and RPMI 1640 medium were purchased from Life Technologies, Inc. (Grand Island, NY). Polyclonal rabbit anti-sera against human ET-1, SCF, and c-kit were obtained from Immuno-Biological Laboratories (Gunma, Japan). Other chemicals were of reagent grade.
Cell Cultures
Human primary cultured melanocytes were maintained in melanocyte growth medium supplemented with 1 ng/ml recombinant basic fibroblast growth factor, 5 μg/ml insulin, 0.5 μg/ml hydrocortisone, 10 ng/ml phorbol 12-myristate 13-acetate, 50 μg/ml streptomycin, and 0.2% (v/v) bovine pituitary extract at 37°C with 5% CO2, as previously described.10 Human primary cultured keratinocytes were maintained in serum-free keratinocyte medium supplemented with 5 ng/ml epidermal growth factor and 50 μg/ml bovine pituitary extract at 37°C with 5% CO2, as previously described.10
UVB Irradiation
Irradiations were performed using UVB lamps (Toshiba SE lamps for UVB with a peak of emission near 312 nm) on the volar forearms of normal human volunteers after previous determination of their minimal erythemal dose. One area (∼1.0 cm2) on the forearm was then irradiated with two minimal erythemal doses of UVB.
Suction Blister Formation
Human epidermal sheets (blister roofs) were obtained from healthy volunteers using the suction blister technique, as described elsewhere.56 Briefly, suction blisters were produced on volar forearm skin 3 to 25 days after exposure to UVB using a 1-ml or a 2.5-ml plastic syringe with negative pressure. Within 1 hour, blisters 10 mm in diameter were raised, and the epidermal sheet (∼0.8 cm2) was removed with a surgical blade. Blisters were also obtained from the nonirradiated (control) sites.
Real-Time Quantitative Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
After UVB irradiation, the expression of SCF, c-kit, ET-1 (as preproendothelin-1), ETBR, and tyrosinase in human epidermis was examined using real-time quantitative RT-PCR normalized against glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Total RNA from each epidermal sheet was isolated using a single-step guanidine thiocyanate-phenol-chloroform method with TRIzol (Life Technologies, Inc.). cDNA was then synthesized by reverse transcription of 1 μg of total RNA, using oligo dT and Moloney murine leukemia virus reverse transcriptase.
The following sets of oligonucleotide primers were used in this study: SCF (Stratagene, La Jolla, CA): 5′-GAT-GTT-TTG-CCA-AGT-CAT-TGT-TGG-3′, which corresponds to nucleotides 367 to 390 of SCF cDNA,57 and 5′-ACT-GAC-TCT-GGA-ATC-TTT-CTC-AGG-3′, which is complementary to nucleotides 694 to 717 of SCF cDNA; c-kit: 5′-GCT-GAG-CTT-TTC-TTA-CCA-GGT-GG-3′, which corresponds to nucleotides 2328 to 2350 of c-kit cDNA,58 and 5′-TAT-GTC-ATA-CAT-TTC-AGC-AGG-TGC-3′, which is complementary to nucleotides 2704 to 2727 of c-kit cDNA; prepro-ET-1 (preproendothelin-1): 5′-TTC-CCA-CAA-AGG-CAA-CAG-ACC-G-3′, which corresponds to nucleotides 545 to 566 of prepro-ET-1 cDNA,59 and 5′-GAC-AGG-CCC-CGA-AGT-CTG-TCA-3′, which is complementary to nucleotides 889 to 909 of prepro-ET-1 cDNA; ETBR: 5′-CGA-GCT-GTT-GCT-TCT-TGG-AGT-AG-3′, which corresponds to nucleotides 838 to 860 of ETBR cDNA,60 and 5′-AAC-GGA-AGT-TGT-CAT-ATC-CGT-GAT-3′, which is complementary to nucleotides 1517 to 1541 of ETBR cDNA; tyrosinase: 5′-TAC-TGC-CTG-CTG-TGG-AGT-TT-3′, which corresponds to nucleotides 521 to 540 of tyrosinase cDNA,61 and 5′-TTC-ATT-TGG-CCA-TAG-GTC-CC-3′, which is complementary to nucleotides 962 to 981 of tyrosinase cDNA; GAPDH (BD Biosciences, Palo Alto, CA): 5′-ACC-ACA-GTC-CAT-GCC-ATC-AC-3′, which corresponds to nucleotides 586 to 605 of GAPDH cDNA,62 and 5′-TCC-ACC-ACC-CTG-TTG-CTG-TA-3′, which is complementary to nucleotides 1018 to 1037 of GAPDH cDNA.
Real-time quantitative RT-PCR with SYBR Green was performed using SYBR Green PCR Core Reagents (PE Biosystems, Warrington, UK) in an ABI Prism 7700 sequence detection system (Perkin-Elmer, Foster City, CA) as described elsewhere.63 Briefly, the 20-μl reaction volume contained 1× SYBR Green PCR buffer; 0.2 U of uracil-N-glycosylase (AmpErase UNG); 0.5 U of AmpliTaq Gold DNA polymerase; 200 nmol/L of each primer; 200 μmol/L (each) dATP, dCTP, and dGTP; 500 μmol/L dUTP; 3 mmol/L MgCl2; and 2 μl of the template. Amplification in the ABI Prism 7700 sequence detection system after initial denaturation at 94°C for 2 minutes was performed for 50 cycles at 94°C for 30 seconds, 60 or 62°C for 1 minute, and 72°C for 45 seconds.
Immunohistology
Epidermis from UVB-irradiated and from nonirradiated sites on human volar forearms was fixed in formalin and then embedded in paraffin. ET-1 immunoreactivity was assessed using polyclonal rabbit anti-sera against human ET-1 (Immuno-Biological Laboratories) and the OmniTags Plus universal streptavidin/biotin immunoperoxidase detection system (Thermo Shandon, Pittsburgh, PA). Negative controls were performed using normal rabbit IgG (Sigma, St. Louis, MO) in place of the primary antibody. As an additional negative control for ET-1 expression, the primary antibody was preincubated with soluble ET-1 peptides (Sigma).
Western Blotting
The protein expression of c-kit, ETBR, and tyrosinase in human epidermis after UVB exposure was examined using Western blotting. In each experiment, except for the analysis of ETBR, the amount of protein loaded was normalized against a control protein, β-actin, with a monoclonal antibody specific for β-actin (Sigma).
To clarify the protein expression of c-kit and tyrosinase in human epidermis after UVB exposure, 15 μg of protein solubilized in RIPA buffer [consisting of 0.1 mol/L Tris-HCl, pH 7.2, 0.1% sodium dodecyl sulfate (SDS), 1% Nonidet P-40, and a protease inhibitor cocktail (Boehringer-Mannheim, Mannheim, Germany)] were separated on 7.5% SDS gels. They were then transferred to polyvinylidene difluoride membranes (Immobilon-P; Millipore Corp., Bedford, MA) and incubated with a purified polyclonal rabbit antibody specific for c-kit (Immuno-Biological Laboratories) or with a purified polyclonal rabbit antibody specific for tyrosinase (Wako Pure Chemical Industries, Ltd., Osaka, Japan) diluted to 10 μg/ml. Subsequent visualization of antibody binding was performed using Enhanced ChemiLuminescence (Amersham Corp., Arlington Heights, IL) according to the manufacturer’s instructions.
To determine ETBR expression in human epidermis after UVB irradiation, epidermal sheets were homogenized in liquid nitrogen, sonicated for 1 minute with a sonicator (model Branson Sonifier 150; Central Scientific Commerce, Inc., Tokyo, Japan) in 0.1 mol/L sodium phosphate buffer (pH 6.8) and centrifuged at 10,000 × g for 20 minutes at 4°C. The supernatants were then further centrifuged at 100,000 × g for 60 minutes at 4°C. The resulting precipitates were dissolved in RIPA buffer. Five μg of protein solubilized in RIPA buffer were separated, transferred to membranes, and visualized as described above except for using a purified polyclonal sheep antibody specific for ETBR (Research Diagnostics, Inc., Flanders, NJ) diluted to 10 μg/ml. To measure ETBR expression in human cultured melanocytes after incubation with human recombinant SCF (Sigma), 10 μg of protein solubilized in RIPA buffer were separated on 7.5% SDS gels. They were then transferred to membranes and visualized as described above. Because loading control using antibodies to β-actin in Western blotting for ETBR using membrane fraction cannot be performed, protein staining was concomitantly performed with Coomassie brilliant blue according to standard procedures to confirm tissue or cell extract quality, protein concentration, and transfer efficiency.
To assess SCF expression after incubation of human keratinocytes with IL-1α or TNF-α (both from Pepro Tech, Inc., Rocky Hill, NJ) at concentrations ranging from 0.05 to 0.5 nmol/L, 10 μg of protein solubilized in Nonidet P-40/SDS buffer were separated on 10% SDS gels as described above. They were then transferred to polyvinylidene difluoride membranes and incubated with a purified polyclonal rabbit antibody specific for SCF (Immuno-Biological Laboratories) diluted at 5 μg/ml. Subsequent visualization of antibody binding was performed as described above.
Radiolabeling and Ligand-Binding Assay
The ET-1 binding assay was performed as described elsewhere.64 Briefly, human recombinant ET-1 (Sigma) was labeled with 125I (Amersham Corp) using IODO-GEN iodination reagent (Pierce, Rockford, IL). For the binding assay, subconfluent melanocytes in 24-well plates were washed with PBS and were then incubated for 90 minutes at room temperature with 10 nmol/L of 125I-ET-1 in 300 μl of binding buffer (RPMI 1640 medium containing 25 mmol/L Hepes, pH 7.5, and 0.05% bovine serum albumin). The dishes were rinsed four times with ice-cold PBS, the cells were solubilized in 500 μl of 2 mol/L NaOH and radioactivity was counted in a Υ-counter (Canberra Packard GmbH, Frankfurt, Germany). Nonspecific binding was determined by parallel binding experiments in the presence of a 100-fold excess of unlabeled ET-1.
Measurement of Skin Color
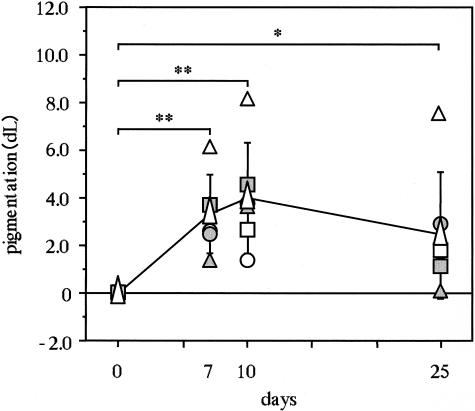
The intensity of UVB-induced pigmentation was measured using a color difference meter (Murakami Color Research Laboratory, Tokyo, Japan) and is expressed as the delta L value 7, 10, and 25 days after exposure to UVB. Pigmentation was not measured at day 3 because the degree of erythema remaining at that time prevented an accurate determination. Therefore, instead of day 3, it was measured at day 7 when the erythema had returned to baseline level.
Statistics
A nonparametric one-way analysis of variance (Kruskal-Wallis test) was used to evaluate differences between groups. Where appropriate, a nonparametric post hoc multiple comparison test (Steel-Dwass test) was performed to evaluate differences between the groups. A P value <0.05 is considered statistically significant.
Results
UVB Irradiation Significantly Increases Expression of SCF and Tyrosinase in Human Epidermis
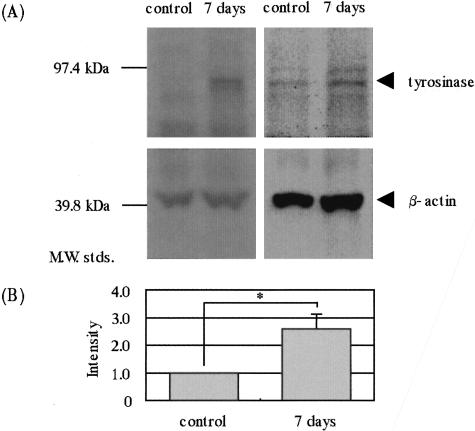
Real-time quantitative RT-PCR analysis of UVB-exposed human epidermis demonstrated that the expression of SCF mRNA transcripts had significantly increased 3 days after the irradiation with a peak on day 5 compared with the nonexposed epidermis, after which it returned to the nonirradiated control level by day 25 (Figure 1). In a parallel study, pigmentation levels increased significantly by day 7 and reached a plateau at day 10 (Figure 2). Real-time quantitative RT-PCR analysis of UVB-exposed human epidermis demonstrated that expression of tyrosinase mRNA transcripts was not changed by day 3, but had increased at day 5 with a plateau at day 10 (Figure 3). This time course of expression of tyrosinase paralleled the pigmentation levels. Western blotting analysis revealed that tyrosinase protein expression was significantly augmented 7 days after the UV exposure (Figure 4).
Figure 1.
Time course of the expression of SCF transcripts in human epidermis after UVB irradiation. Volar forearms of healthy human volunteers were exposed to two minimal erythemal doses of UVB, followed by suction blister biopsies to allow real-time quantitative RT-PCR analysis of SCF mRNA transcript levels. Real-time quantitative RT-PCR was performed using an ABI Prism 7700 sequence detection system as detailed in Materials and Methods. The expression of SCF transcripts in the UVB-exposed epidermis was normalized against the expression of GAPDH. The values reported represent means ± SD from two to eight volunteers. Symbols for circles, triangles, and squares correspond to each volunteer. White isosceles triangles represent the average of volunteers. *, P < 0.05; **, P < 0.01.
Figure 2.
Time course of visible pigmentation in human forearm skin after UVB irradiation. The intensity of UVB-induced pigmentation was measured by a color difference meter (Murakami Color Research Laboratory) and is expressed as the delta L value. Symbols for circles, triangles, and squares correspond to each volunteer. White isosceles triangles represent the average of volunteers. *, P < 0.05; **, P < 0.01.
Figure 3.
Time course of the expression of tyrosinase mRNA transcripts in human epidermis after UVB irradiation. Epidermal sheets after UVB exposure were obtained by suction blister biopsy, as detailed in Materials and Methods, followed by real-time quantitative RT-PCR analysis of tyrosinase mRNA transcripts. The expression of tyrosinase transcripts in UVB-exposed epidermis was normalized against the expression of GAPDH. The values reported represent means ± SD from two to eight volunteers. Symbols for circles, triangles, and squares correspond to each volunteer. White isosceles triangles represent the average of volunteers. *, P < 0.05.
Figure 4.
UVB irradiation stimulates the expression of tyrosinase protein in human epidermis. A: Western blotting. B: Densitometric analysis. Epidermal sheets after UVB exposure were obtained by suction blister biopsy, followed by Western blotting analysis of tyrosinase protein as detailed in Materials and Methods. The bands show two representatives of Western blotting analyses that were repeated three times with similar results. *, P < 0.05. The values reported represent means ± SD from three experiments.
The Expression of ET-1 Is Remarkably Increased in Human Epidermis 5 to 10 Days after UVB Irradiation
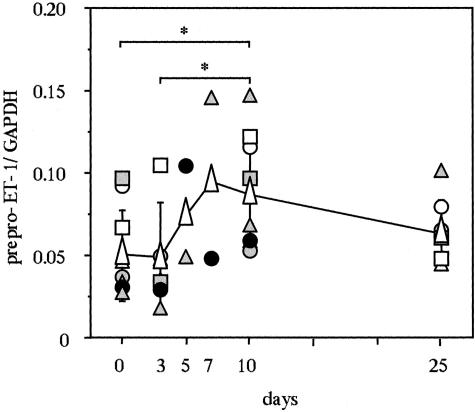
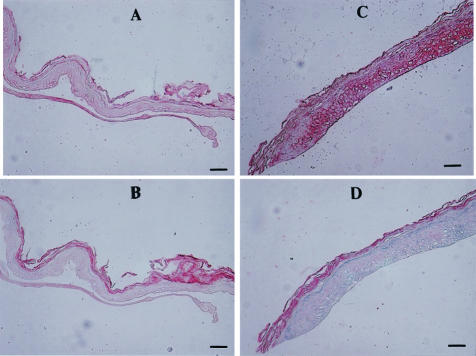
Real-time quantitative RT-PCR analysis of UVB-exposed human epidermis demonstrated that the expression of prepro-ET-1 mRNA transcripts was not changed by day 3, but had significantly increased 5 to 10 days after the UV irradiation compared with the nonexposed epidermis (Figure 5), the dynamics of which are very close to the increase in pigmentation (Figure 2). Immunohistochemistry of the UVB-exposed human skin using ET-1 antibodies revealed a strong immunostaining of ET-1 that was located in the basal layer and in the stratum spinosum layer, which was accompanied by epidermal hyperplasia at day 7 compared with the nonexposed epidermis (Figure 6). As a control, there was no immunostaining with the nonspecific IgG in the UVB-exposed human epidermis.
Figure 5.
Time course of the expression of prepro-ET-1 mRNA transcripts in human epidermis after UVB irradiation. Epidermal sheets after UVB exposure were obtained by suction blister biopsy, as detailed in Materials and Methods, followed by real-time quantitative RT-PCR analysis of ET-1 mRNA transcripts. The expression of ET-1 transcripts in UVB-exposed epidermis was normalized against the expression of GAPDH. The values reported represent means ± SD from two to eight volunteers. Symbols for circles, triangles, and squares correspond to each volunteer. White isosceles triangles represent the average of volunteers. *, P < 0.05.
Figure 6.
UVB irradiation increases the production of ET-1 in human epidermis as revealed by immunohistochemistry. A: Immunostaining of nonexposed human epidermis with anti-ET-1. B: Immunostaining of nonexposed human epidermis with nonspecific IgG. C: Immunostaining of UVB-exposed human epidermis with anti-ET-1. D: Immunostaining of UVB-exposed human epidermis with nonspecific IgG. Scale bars, 70 μm.
UVB Exposure Elicits a Significant Decrease in c-KIT mRNA and Protein Expression
Real-time quantitative RT-PCR analysis of UV-irradiated human skin demonstrated that the expression of c-KIT mRNA transcripts significantly decreased at day 3, but returned to the nonirradiated control level by days 5 to 10 (Figure 7). Consistent with the gene expression, Western blotting revealed that the expression of KIT protein was significantly decreased at day 3 and returned to the nonirradiated control level at day 5 (Figure 8).
Figure 7.
Time course of the expression of c-KIT mRNA transcripts in human epidermis after UVB irradiation. Epidermal sheets after UVB exposure were obtained by suction blister biopsy, as detailed in Materials and Methods, followed by real-time quantitative RT-PCR analysis of c-kit transcripts. The expression of c-kit transcripts in UVB-exposed epidermis was normalized against the expression of GAPDH. The values reported represent means ± SD from two to six volunteers. Symbols for circles, triangles, and squares correspond to each volunteer. White isosceles triangles represent the average of volunteers. *, P < 0.05.
Figure 8.
The expression of c-kit protein in human epidermis decreases after UVB irradiation. A: Western blotting. B: Densitometric analysis. Epidermal sheets after UVB exposure were obtained by suction blister biopsy, followed by Western blotting analysis of c-kit protein as detailed in Materials and Methods. The bands show two representatives of Western blotting analyses that were repeated four times at 3 days and three times at 5 days with similar results. **, P < 0.01. The values reported represent means ± SD from four or three experiments.
The Level of ETBR mRNA and Protein Is Significantly Increased in Human Epidermis after UVB Exposure
Real-time quantitative RT-PCR analysis of UVB-exposed human epidermis revealed that the expression of ETBR mRNA transcripts was significantly increased by day 10 after UVB irradiation compared with the nonexposed human epidermis (Figure 9). This expression of ETBR transcripts is preceded by the enhanced expression of SCF and parallels the up-regulated expression of ET-1 and the appearance of pigmentation. Western blotting analysis revealed a significantly increased expression of ETBR protein at day 10 (Figure 10).
Figure 9.
Time course of the expression of ETBR mRNA transcripts in human epidermis after UVB irradiation. Epidermal sheets after UVB irradiation were obtained by suction blister biopsy, as detailed in Materials and Methods, followed by real-time quantitative RT-PCR analysis of ETBR transcripts. The expression of ETBR transcripts in UVB-exposed epidermis was normalized against the expression of GAPDH. The values reported represent means ± SD from two to eight volunteers. Symbols for circles, triangles, and squares correspond to each volunteer. White isosceles triangles represent the average of volunteers. *, P < 0.05.
Figure 10.
ETBR protein expression is increased in human epidermis after UVB exposure. A: Western blotting. B: Densitometric analysis. Epidermal sheets after UVB exposure were obtained by suction blister biopsy, followed by Western blotting analysis of ETBR protein as detailed in Materials and Methods. The bands show two representatives of Western blotting analyses that were repeated three times with similar results. *, P < 0.05. The values reported represent means ± SD from three experiments.
SCF Up-Regulates the Expression of ETBR in Human Melanocytes
To clarify whether SCF/c-kit signaling enhances the ET-1/ETBR linkage in the later phase of UVB-induced melanogenesis, we examined the effect of SCF on ETBR protein expression in cultured human melanocytes. When human melanocytes in culture were incubated with human recombinant SCF, ETBR protein expression increased by day 1 as assessed by Western blotting, with a peak at day 2 (Figure 11). In addition, the ligand-binding assay revealed that the binding of 125I-labeled ET-1 to ETBR increased significantly in human melanocytes 2 days after incubation with SCF (Figure 12).
Figure 11.
SCF stimulates the expression of ETBR protein in cultured human melanocytes. A: Western blotting. B: Densitometric analysis. Each band shows a representative result of Western blotting analyses for ETBR that were repeated four times with similar results. The membrane fraction of human melanocytes after incubation with SCF (10 nmol/L) was solubilized in RIPA buffer on the indicated days. Ten μg of protein of each extract were electrophoresed and analyzed as described in Materials and Methods. **, P < 0.01.
Figure 12.
SCF enhances the binding of ET-1 to ETBR in cultured human melanocytes. The values reported represent means ± SD from six independent experiments. Subconfluent melanocytes in 24-well plates were incubated with 10 nmol/L 125I-ET-1 as described in Materials and Methods. *, P < 0.05.
IL-1α Enhances the Expression of SCF in Human Keratinocytes
To clarify whether SCF expression is up-regulated by the primary inflammatory cytokines, IL-1α and/or TNF-α, we examined their effects on SCF protein expression by cultured human keratinocytes using Western blotting analysis. When human keratinocytes were incubated with IL-1α at concentrations ranging from 0.05 to 0.5 nmol/L, membrane-bound SCF protein expression was significantly stimulated in a dose-dependent manner (Figure 13). In contrast, TNF-α had only a slight effect.
Figure 13.
The expression of membrane-bound SCF protein by cultured human keratinocytes is significantly increased by IL-1α. Human keratinocytes after incubation with IL-1α or TNF-α at concentrations ranging from 0.05 to 0.5 nmol/L for 48 hours were solubilized in Nonidet P-40/SDS buffer. Ten μg of protein of each extract were electrophoresed and analyzed as described in Materials and Methods. Each band shows a representative result of Western blotting analyses that were repeated three times with similar results. **, P < 0.01.
Discussion
In this study, for the first time, we have found that the expression of SCF mRNA transcripts increases remarkably in UVB-exposed human epidermis during the early phase of UVB-induced pigmentation. In contrast, the increased expression of ET-1 and ETBR mRNA transcripts are synchronized during a later phase of UVB-induced pigmentation. These findings strongly suggest that SCF/c-kit signaling between keratinocytes and melanocytes is predominantly involved in an early phase of UVB-induced pigmentation, whereas ET-1/ETBR signaling is associated with the later phase, both factors playing central and coordinated roles in UVB-induced human pigmentation. This is in agreement with our previous work that showed that the inhibition of UVB-induced pigmentation by antibodies to c-kit was complete at day 6 but not at day 10 during UVB-induced pigmentation in brownish guinea pig skin,18 an ideal model for human skin because of the presence of functional melanocytes in the epidermis.65 Because the expression of tyrosinase and the subsequently induced skin pigmentation are preceded by the expression of SCF and has a pattern similar in time course to the expression of ET-1 and ETBR, it seems likely that SCF predominantly contributes to melanocyte proliferation, whereas ET-1 plays a major role in stimulating melanogenesis including tyrosinase expression. Consistent with this, proliferation and melanogenesis assays in cultured human melanocytes have shown that whereas ET-1 can act as both a mitogen and a melanogen, SCF is considered only a mitogen.7
One of the most important issues addressed in this study is how SCF and ET-1 act on melanocytes leading to the up-regulation of melanogenesis after UVB irradiation. Most SCF in human epidermis has been reported to be expressed as the membrane-bound type, which is not released into intercellular spaces between keratinocytes.66,67 It has also been suggested that the membrane-bound form of SCF is expressed on the plasma membrane of keratinocytes and activates the c-kit receptor persistently without c-kit internalization and degradation within melanocytes.68 Consistent with this, our previous study on UVB-induced human pigmentation demonstrated that the membrane-bound form, but not the soluble form, of SCF was significantly increased in the UVB-exposed epidermis.18 Although it has not been completely elucidated how membrane-bound SCF produced by surrounding keratinocytes might interact with KIT on neighboring melanocytes to transduce signaling, our previous evidence that a monoclonal antibody to KIT can abolish UVB-stimulated proliferation and melanogenesis of epidermal melanocytes strongly suggests that membrane-bound SCF produced by keratinocytes can directly interact with the SCF receptor on melanocytes to activate melanocyte function.
In this study, although KIT protein expression in cultured human melanocytes was significantly decreased 2 days after treatment with human recombinant SCF (the soluble form of SCF, data not shown), the expression of KIT protein remains unchanged 5 days after UVB exposure while its transcription and protein expression were down-regulated at day 3 after irradiation and returned to the control nonirradiated level at day 5. A similar lack of responsiveness of c-kit mRNA transcripts by overexpressed membrane-bound-type SCF occurs in lentigo senilis in which there is no change in the expression of c-kit transcripts in the epidermis despite the fact that SCF transcripts are significantly up-regulated in the lesional pigmented epidermis.55 In contrast, the significance of KIT protein in stimulating or maintaining the melanogenesis of melanocytes is evidenced by the fact that in one hypopigmentary disorder, vitiligo vulgaris, despite the increased expression of SCF in the lesional epidermis, the marked deficiency in KIT protein on melanocytes leads to the complete loss of pigmentation.69
In relation to the mechanism(s) involved in the different time courses of expression of SCF and prepro-ET-1 mRNAs after UVB radiation, it has been speculated that the expression of prepro-ET-1 mRNA is regulated by an autocrine system in which UVB exposure first stimulates the release of the primary inflammatory cytokine, IL-1α, which in turn binds to the IL-1α receptor on keratinocytes, resulting in the transduction of a signal that leads to the expression of prepro-ET-1 mRNA.11 This hypothesized autocrine system is supported by the following two findings: the exogenous addition of IL-1α caused cultured human keratinocytes to release ET-1 and the exogenous addition of an antibody to IL-1α immediately after the exposure of cultured human keratinocytes to UVB completely abolished the release of ET-1 to the conditioned medium. In this study, we found that the exogenous application of IL-1α at concentrations ranging from 0.05 to 0.5 nmol/L to cultured human keratinocytes markedly up-regulates the expression of membrane-bound SCF protein within 48 hours, whereas TNF-α increases it only slightly. Thus, although further study using a neutralizing antibody will be required to be a conclusive, it seems likely that the increased expression of SCF mRNA transcripts in cultured human keratinocytes after UVB exposure is mediated in part through an autocrine-cytokine network resembling the pathway of prepro-ET-1 expression in UVB-exposed human keratinocytes. However, based on the quick response to UVB radiation, which leads to the increased expression of SCF mRNA transcripts, it is conceivable that there is another UVB-mediated mechanism in which UVB-induced membrane injury triggers unidentified regulatory elements leading to the activation of transcription factors regulating SCF expression. On the other hand, it is probable that there is no direct stimulatory pathway by UVB radiation for the increase in prepro-ET-1 expression, providing the autocrine interaction with a longer lag time before the expression. Further, in IL-1α-exposed human keratinocytes, the onset of ET-1 secretion into the medium is much later (3 to 4 days after the treatment)11 than the increase in SCF protein (2 days after the treatment). In addition to the relatively late expression of prepro-ET-1 mRNA transcripts, biological processes that consume time must be required for the final secretion of ET-1 by human keratinocytes. These processes include gene expression, protein synthesis, and modification of prepro-ET-1. ET isopeptides are first expressed as corresponding ∼200-residue inactive prepropolypeptides (preproendothelins). After removal of their signal peptides during their early processing, the propeptides are presumably cleaved by furin, a prohormone convertase of the constitutive secretory pathway, at pairs of basic amino acids to yield the intermediate Big ETs.70 Big ETs are then further cleaved by an endopeptidase termed endothelin-converting enzyme (ECE)71 at Trp21-(Val22/ILe22) to produce biologically active 21-residue peptide ETs.23 Recently, we reported that ECE-1α is a major isoform in human keratinocytes and that it plays a constitutive role in the processing and secretion of ET-1 by human keratinocytes.72 Collectively, these findings suggest the possibility that IL-1α-mediated signal-transducing mechanisms that lead to the expression of prepro-ET-1 and SCF mRNAs differ completely in the time sequence with distal regulatory elements that remain to be identified. Compared with the expression of membrane-bound-type SCF protein, the production and secretion of active ET-1 is further delayed because of its complex processing by proteolytic enzymes.
The preferential role of the early expression of SCF protein before the expression of ET-1 in stimulating melanogenesis in melanocytes may be substantiated by our results that the exogenous addition of soluble type SCF stimulates the expression of ETBR protein in cultured human melanocytes, resulting in accelerating the binding of ET-1 to its receptor on melanocytes. The similar cross-reaction between SCF and ET-1 cascades is also observed in the lesional pigmented epidermis of lentigo senilis in which the expression of SCF and ET-1 is markedly and simultaneously accentuated at the gene and protein levels in concert with the significantly increased expression of ETBR mRNA.52,55
Although the expression pattern of SCF and ET-1 in response to UVB radiation does not differ between in vitro and in vivo experiments, indicating that a direct UVB effect on keratinocytes mainly occurs even in the epidermis, there are some differences in the expression pattern of KIT protein between the two different situations. These differences suggest that the increased expression of known or unidentified cytokines including SCF and ET-1 in keratinocytes may regulate the expression of this receptor (KIT protein) in melanocytes in the UVB-exposed epidermis although a precise in vivo interaction between keratinocyte-derived cytokines and melanocytes remains unclear until several experiments on UVB effects would be performed using co-culture system of keratinocytes and melanocytes.
In conclusion, our results suggest that as mitogens and as melanogens for human melanocytes, SCF/c-kit signaling and ET-1/ETBR signaling are involved in the biological mechanism of UVB-induced human pigmentation at an early phase and at a later phase, respectively. These findings provide a new insight into the complex networks in the epidermis underlying UVB induction of human skin pigmentation. Most importantly, our results improve the fundamental understanding of regulatory mechanisms underlying melanogenesis as well as the cutaneous melanocyte homeostasis in many pigmentary disorders.
Acknowledgments
We thank S. Izawa for technical assistance.
Footnotes
Address reprint requests to Genji Imokawa, Ph.D., Kao Biological Science Laboratories, 2606 Akabane, Ichikai-machi Haga, Tochigi 321-3497 Japan. E-mail: imokawag@dream.ocn.ne.jp.
References
- Rosdahl IK, Szabo G. Mitotic activity of epidermal melanocytes in UV-irradiated mouse skin. J Invest Dermatol. 1978;70:143–148. doi: 10.1111/1523-1747.ep12258559. [DOI] [PubMed] [Google Scholar]
- Imokawa G, Mishima Y. Loss of melanogenic properties in tyrosinase induced by glycosylation inhibitor within malignant melanoma cells. Cancer Res. 1982;42:1994–2002. [PubMed] [Google Scholar]
- Mishima Y, Imokawa G. Selective aberration and pigment loss in melanosomes of malignant melanoma cells in vitro by glycosylation inhibitors: premelanosomes as glycoprotein. J Invest Dermatol. 1983;81:106–114. doi: 10.1111/1523-1747.ep12542192. [DOI] [PubMed] [Google Scholar]
- Okazaki K, Uzuka M, Morikawa F, Toda K, Seiji M. Transfer mechanism of melanosomes in epidermal cell culture. J Invest Dermatol. 1976;67:541–547. doi: 10.1111/1523-1747.ep12664554. [DOI] [PubMed] [Google Scholar]
- Halaban R, Langdon R, Birchall N, Cuono C, Baird A, Scott G, Moellmann G, McGuire J. Basic fibroblast growth factor from human keratinocytes is a natural mitogen for melanocytes. J Cell Biol. 1988;107:1611–1619. doi: 10.1083/jcb.107.4.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdel-Malek ZA, Swope VB, Suzuki I, Akcali C, Harriger MD, Boyce ST, Urabe K, Hearing VJ. Mitogenic and melanogenic stimulation of normal human melanocytes by melanotropic peptides. Proc Natl Acad Sci USA. 1995;92:1789–1793. doi: 10.1073/pnas.92.5.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imokawa G, Yada Y, Kimura M. Signaling mechanisms of endothelin-induced mitogenesis in human melanocytes. Biochem J. 1996;314:305–312. doi: 10.1042/bj3140305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imokawa G, Kobayashi T, Miyagishi M, Higashi K, Yada Y. The role of endothelin-1 in epidermal hyperpigmentation and signaling mechanisms of mitogenesis and melanogenesis. Pigment Cell Res. 1997;10:218–228. doi: 10.1111/j.1600-0749.1997.tb00488.x. [DOI] [PubMed] [Google Scholar]
- Tada A, Suzuki I, Im S, Davis MB, Cornelius J, Babcock G, Nordlund JJ, Abdel-Malek ZA. Endothelin-1 is a paracrine growth factor that modulates melanogenesis of human melanocytes and participates in their responses to ultraviolet radiation. Cell Growth Differ. 1998;9:575–584. [PubMed] [Google Scholar]
- Yada Y, Higuchi K, Imokawa G. Effects of endothelins on signal transduction and proliferation in human melanocytes. J Biol Chem. 1991;266:18352–18357. [PubMed] [Google Scholar]
- Imokawa G, Yada Y, Miyagishi M. Endothelins secreted from human keratinocytes are intrinsic mitogens for human melanocytes. J Biol Chem. 1992;267:24675–24680. [PubMed] [Google Scholar]
- Imokawa G, Miyagishi M, Yada Y. Endothelin-1 as a new melanogen: coordinated expression of its gene and the tyrosinase gene in UVB-exposed human epidermis. J Invest Dermatol. 1995;105:32–37. doi: 10.1111/1523-1747.ep12312500. [DOI] [PubMed] [Google Scholar]
- Yohn JJ, Morelli JG, Walchack SJ, Rundell KB, Norris DA, Zamora MR. Cultured human keratinocytes synthesize and secrete endothelin-1. J Invest Dermatol. 1993;100:23–26. doi: 10.1111/1523-1747.ep12349932. [DOI] [PubMed] [Google Scholar]
- Schauer E, Trautinger F, Köck A, Schwarz A, Bhardwaj R, Simon M, Ansel JC, Schwarz T, Luger TA. Proopiomelanocortin-derived peptides are synthesized and released by human keratinocytes. J Clin Invest. 1994;93:2258–2262. doi: 10.1172/JCI117224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty AK, Funasaka Y, Slominski A, Ermak G, Hwang J, Pawelek J, Ichihashi M. Production and release of proopiomelanocortin (POMC) derived peptides by human melanocytes and keratinocytes in culture: regulation by UVB. Biochim Biophys Acta. 1996;1313:130–138. doi: 10.1016/0167-4889(96)00063-8. [DOI] [PubMed] [Google Scholar]
- Wintzen M, Gilchrest BA. Proopiomelanocortin gene product regulation in keratinocytes. J Invest Dermatol. 1996;106:673–678. doi: 10.1111/1523-1747.ep12345496. [DOI] [PubMed] [Google Scholar]
- Hedley SJ, Gawkrodger DJ, Weetman AP, MacNeil S. Alpha-MSH and melanogenesis in normal human adult melanocytes. Pigment Cell Res. 1998;11:45–56. doi: 10.1111/j.1600-0749.1998.tb00710.x. [DOI] [PubMed] [Google Scholar]
- Hachiya A, Kobayashi A, Ohuchi A, Takema Y, Imokawa G. The paracrine role of stem cell factor/c-kit signaling in the activation of human melanocytes in ultraviolet B-induced pigmentation. J Invest Dermatol. 2001;116:578–586. doi: 10.1046/j.1523-1747.2001.01290.x. [DOI] [PubMed] [Google Scholar]
- Roméro-Gaillet C, Aberdam E, Clément M, Ortonne J-P, Ballotti R. Nitric oxide produced by ultraviolet-irradiated keratinocytes stimulates melanogenesis. J Clin Invest. 1997;99:635–642. doi: 10.1172/JCI119206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funasaka Y, Chakraborty AK, Hayashi Y, Komoto M, Ohashi A, Nagahama M, Inoue Y, Pawelek J, Ichihashi M. Modulation of melanocyte-stimulating hormone receptor expression on normal human melanocytes: evidence for a regulatory role of ultraviolet B, interleukin-1alpha, interleukin-1beta, endothlin-1 and tumor necrosis factor-alpha. Br J Dermatol. 1998;139:216–224. doi: 10.1046/j.1365-2133.1998.02357.x. [DOI] [PubMed] [Google Scholar]
- Sirsjo A, Karlsson M, Gidlof A, Rollman O, Torma H. Increased expression of inducible nitric oxide synthase in psoriatic skin and cytokine-stimulated keratinocytes. Br J Dermatol. 1996;134:643–648. doi: 10.1111/j.1365-2133.1996.tb06963.x. [DOI] [PubMed] [Google Scholar]
- Imokawa G, Kobayashi T, Miyagishi M. Intracellular signaling mechanisms leading to synergistic effects of endothelin-1 and stem cell factor on proliferation of cultured human melanocytes: cross-talk via trans-activation of the tyrosine kinase C-kit receptor. J Biol Chem. 2000;275:33321–33328. doi: 10.1074/jbc.M004346200. [DOI] [PubMed] [Google Scholar]
- Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, Yazaki Y, Goto K, Masaki T. A novel peptide vasoconstrictor, endothelin, is produced by vascular endothelium and modulates smooth muscle Ca2+ channels. Nature. 1988;322:411–415. doi: 10.1097/00004872-198812040-00056. [DOI] [PubMed] [Google Scholar]
- Inoue A, Yanagisawa M, Kimura S, Kasuya Y, Miyauchi T, Goto K, Masaki T. The human preproendothelin-1 gene. Complete nucleotide sequence and regulation of expression. Proc Natl Acad Sci USA. 1989;86:2863–2867. [Google Scholar]
- Sakurai T, Yanagisawa M, Takuwa Y, Miyazaki H, Kimura S, Goto K, Masaki T. Cloning of a cDNA encoding a non-isopeptide-selective subtype of the endothelin receptor. Nature. 1990;348:732–735. doi: 10.1038/348732a0. [DOI] [PubMed] [Google Scholar]
- Arai H, Nakao K, Takaya K, Hosoda K, Ogawa Y, Nakanishi S, Immura H. The human endothelin B receptor gene. Structural organization and chromosomal assignment. J Biol Chem. 1993;268:3463–3470. [PubMed] [Google Scholar]
- Sakamoto A, Yanagisawa M, Sawamura T, Enoki T, Ohtani T, Sakurai T, Nakao K, Toyo-Oka T, Masaki T. Distinct subdomains of human endothelin receptor determine their selectivity to endothelin A selective antagonist and endothelin B selective agonist. J Biol Chem. 1993;268:8547–8553. [PubMed] [Google Scholar]
- Brenner BM, Troy JL, Ballermann BJ. Endothelium-dependent vascular responses. Mediators and mechanisms. J Clin Invest. 1989;84:1373–1378. doi: 10.1172/JCI114309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resink TJ, Scott-Burden T, Buhler ER. Enhanced responsiveness to angiotensin II in vascular smooth muscle cells from spontaneously hypertensive rats is not associated with alterations in protein kinase C. Biochem Biophys Res Commun. 1989;158:279–286. [Google Scholar]
- Reynolds EE, Mok LL, Kurokawa S. Phorbol ester dissociates endothelin-stimulated phosphoinositide hydrolysis and arachidonic acid release in vascular smooth muscle cells. Biochem Biophys Res Commun. 1989;160:868–873. doi: 10.1016/0006-291x(89)92515-1. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Miyazaki H, Kondoh M, Masuda Y, Kimura S, Yanagisawa M, Masaki T, Murakami K. Two distinct types of endothelin receptors are present on chick cardiac membranes. Biochem Biophys Res Commun. 1989;161:1252–1259. doi: 10.1016/0006-291x(89)91377-6. [DOI] [PubMed] [Google Scholar]
- Matsui Y, Zsebo KM, Hogan BLM. Embryonic expression of a hematopoietic growth factor encoded by the Sl locus and the ligand for c-kit. Nature. 1990;347:667–669. doi: 10.1038/347667a0. [DOI] [PubMed] [Google Scholar]
- Orr-Urtreger A, Avivi A, Zimmer Y, Givol D, Yarden Y, Lonai P. Developmental expression of c-kit, a proto-oncogene encoded by the W locus. Development. 1990;109:911–923. doi: 10.1242/dev.109.4.911. [DOI] [PubMed] [Google Scholar]
- Bernstein A, Forrester L, Reith AD, Dubreuil P, Rottapel R. The murine W/c-kit and steel loci and the control of hematopoiesis. Semin Hematol. 1991;28:138–142. [PubMed] [Google Scholar]
- Williams DE, de Vries P, Namen AE, Widmer MB, Lyman SD. The steel factor. Dev Biol. 1992;151:368–376. doi: 10.1016/0012-1606(92)90176-h. [DOI] [PubMed] [Google Scholar]
- Besmer P, Manova K, Duttlinger R, Huang EJ, Packer A, Gyssler C, Bachvarova R. The kit-ligand (steel factor) and its receptor c-kit/W: pleiotropic roles in gametogenesis and melanogenesis. Development. 1993;119:125–137. [PubMed] [Google Scholar]
- Galli SJ, Zsebo KM, Geissler EN. The kit ligand, stem cell factor. Adv Immunol. 1993;55:1–96. doi: 10.1016/s0065-2776(08)60508-8. [DOI] [PubMed] [Google Scholar]
- Halaban R, Moellmann G. White mutants in mice shedding light on humans. J Invest Dermatol. 1993;100:176S–185S. [PubMed] [Google Scholar]
- Nishikawa S, Kusakabe M, Yoshinaga K, Ogawa M, Hayashi S, Kunisada T, Era T, Sakakura T, Nishikawa S-I. In utero manipulation of coat color formation by a monoclonal anti-c-kit antibody: two distinct waves of c-kit-dependency during melanocyte development. EMBO J. 1991;10:2111–2118. doi: 10.1002/j.1460-2075.1991.tb07744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okura M, Maeda H, Nishikawa S, Mizoguchi M. Effects of monoclonal anti-c-kit antibody (ACK2) on melanocytes in newborn mice. J Invest Dermatol. 1995;105:322–328. doi: 10.1111/1523-1747.ep12319939. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Hayashi S, Shultz LD, Yamamura K, Nishikawa S, Nishikawa S-I, Kunisada T. Neural and skin cell specific expression pattern conferred by steel factor regulatory sequence in transgenic mice. Dev Dyn. 1996;207:222–232. doi: 10.1002/(SICI)1097-0177(199610)207:2<222::AID-AJA10>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Giebel LB, Spritz RA. Mutation of the KIT (mast/stem-cell growth factor receptor) protooncogene in human piebaldism. Proc Natl Acad Sci USA. 1991;88:8696–8699. doi: 10.1073/pnas.88.19.8696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spritz RA, Giebel LB, Holmes SA. Dominant negative and loss of function mutations of the c-kit (mast/stem-cell growth factor receptor) protooncogene in human piebaldism. Am J Hum Genet. 1992;50:261–269. [PMC free article] [PubMed] [Google Scholar]
- Ezoe K, Holmes SA, Ho I, Bennett CP, Bolognia JL, Brueton L, Burn J, Falabella R, Gatto EM, Ishii N, Moss C, Pittelkow MR, Thompson E, Ward KA, Spritz RA. Novel mutations and deletions of the KIT (steel factor receptor) gene in human piebaldism. Am J Hum Genet. 1995;56:58–66. [PMC free article] [PubMed] [Google Scholar]
- Puffenberger EG, Hosoda K, Washington SS, Nakao K, deWit D, Yanagisawa M, Chakravarti A. A missense mutation of the endothelin-B receptor gene in multigenic Hirschsprung’s disease. Cell. 1994;79:1257–1266. doi: 10.1016/0092-8674(94)90016-7. [DOI] [PubMed] [Google Scholar]
- Amiel J, Attie T, Jan D, Pelet A, Edery P, Bidaud C, Lacombe D, Tam P, Simeoni J, Flori E, Nihoul-Fekete C, Munnich A, Lyonnet S. Heterozygous endothelin receptor B (EDNRB) mutations in isolated Hirschsprung disease. Hum Mol Genet. 1996;5:255–257. doi: 10.1093/hmg/5.3.355. [DOI] [PubMed] [Google Scholar]
- Tachibana M, Takeda K, Nobujuni Y, Urabe K, Long JE, Meyers KA, Aaronson SA, Miki T. Ectopic expression of MITF, a gene for Waardenburg syndrome type 2, converts fibroblasts to cells with melanocyte characteristics. Nat Genet. 1996;14:50–54. doi: 10.1038/ng0996-50. [DOI] [PubMed] [Google Scholar]
- Read AP, Newton VE. Waardenburg syndrome. J Med Genet. 1997;34:656–665. doi: 10.1136/jmg.34.8.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolotto C, Busca R, Abbe P, Bille K, Aberdam E, Ortonne JP, Ballotti R. Different cis-acting elements are involved in the regulation of TRP1 and TRP2 promoter activities by cyclic AMP: pivotal role of M boxes (GTCATGTGCT) and of microphthalmia. Mol Cell Biol. 1998;18:694–702. doi: 10.1128/mcb.18.2.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemesath TJ, Price ER, Takemoto C, Badalian T, Fisher DE. MAPK links the transcription factor microphthalmia to c-kit signaling in melanocytes. Nature. 1998;391:289–301. doi: 10.1038/34681. [DOI] [PubMed] [Google Scholar]
- Teraki E, Tajima S, Manaka I, Kawashima M, Miyagishi M, Imokawa G. Role of endothelin-1 in hyperpigmentation in seborrhoeic keratosis. Br J Dermatol. 1996;135:918–923. doi: 10.1046/j.1365-2133.1996.d01-1095.x. [DOI] [PubMed] [Google Scholar]
- Kadono S, Manaka I, Kawashima M, Kobayashi T, Imokawa G. The role of the epidermal endothelin cascade in the hyperpigmentation mechanism of lentigo senilis. J Invest Dermatol. 2001;116:571–577. doi: 10.1046/j.1523-1747.2001.01296.x. [DOI] [PubMed] [Google Scholar]
- Shishido E, Kadono S, Manaka I, Kawashima M, Imokawa G. The mechanism of epidermal hyperpigmentation in dermatofibroma is associated with stem cell factor and hepatocyte growth factor expression. J Invest Dermatol. 2001;117:627–633. doi: 10.1046/j.0022-202x.2001.01440.x. [DOI] [PubMed] [Google Scholar]
- Okazaki M, Yoshimura K, Suzuki Y, Uchida G, Kitano Y, Harii K, Imokawa G. The mechanism of epidermal hyperpigmentation in café-au-lait macules of neurofibromatosis type 1 (von Recklinghausen’s disease) may be associated with dermal fibroblast-derived stem cell factor and hepatocyte growth factor. Br J Dermatol. 2003;148:689–697. doi: 10.1046/j.1365-2133.2003.05283.x. [DOI] [PubMed] [Google Scholar]
- Hattori H, Kawashima M, Ichikawa Y, Imokawa G. The epidermal stem cell factor is over-expressed in lentigo senilis: implication for the mechanism of hyperpigmentation. J Invest Dermatol. 2004;122:1256–1265. doi: 10.1111/j.0022-202X.2004.22503.x. [DOI] [PubMed] [Google Scholar]
- Furukawa F, Huff JC, Weston WL, Norris DA. Serum-free serial culture of adult human keratinocytes from suction blister roof epidermis. J Invest Dermatol. 1987;89:460–463. doi: 10.1111/1523-1747.ep12460904. [DOI] [PubMed] [Google Scholar]
- Martin FH, Suggs S, Langley KE, Lu HS, Ting J, Okino KH, Morris CF, McNiece IK, Jacobsen FW, Mendiaz EA, Birkett NC, Smith KA, Johnson MJ, Parker VP, Flores JC, Patel AC, Fisher EF, Erjavec HO, Herrera CJ, Wypych J, Sachdev RK, Pope JA, Leslie I, Wen D, Lin C-H, Cupples RL, Zsebo KM. Primary structure and functional expression of rat and human stem cell factor DNAs. Cell. 1990;63:203–211. doi: 10.1016/0092-8674(90)90301-t. [DOI] [PubMed] [Google Scholar]
- Yarden Y, Kuang WJ, Yang-Feng T, Coussens L, Munemitsu S, Dull TJ, Chen E, Schlessinger J, Francke U, Ullrich A. Human proto-oncogene c-kit: a new cell surface receptor tyrosine kinase for an unidentified ligand. EMBO J. 1987;6:3341–3351. doi: 10.1002/j.1460-2075.1987.tb02655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh Y, Yanagisawa M, Ohkubo S, Kimura C, Kosaka T, Inoue A, Ishida N, Mitsui Y, Onda H, Fujino M, Masaki T. Cloning and sequence analysis of cDNA encoding the precursor of a human endothelium-derived vasoconstrictor peptide, endothelin: identity of human and porcine endothelin. FEBS Lett. 1988;231:440–444. doi: 10.1016/0014-5793(88)80867-6. [DOI] [PubMed] [Google Scholar]
- Vane J. Endothelins come home to roost. Nature. 1990;348:673. doi: 10.1038/348673a0. [DOI] [PubMed] [Google Scholar]
- Shibahara S, Tomita Y, Tagami H, Muller RM, Cohen T. Molecular basis for the heterogeneity of human tyrosinase. Tohoku J Exp Med. 1988;156:403–414. doi: 10.1620/tjem.156.403. [DOI] [PubMed] [Google Scholar]
- Arcari P, Martinelli R, Salvatore F. The complete sequence of a full length cDNA for human liver glyceraldehyde-3-phosphate dehydrogenase. Evidence for multiple mRNA species. Nucleic Acids Res. 1984;12:9179–9189. doi: 10.1093/nar/12.23.9179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein I, Lehner A, Rieck P, Klein K, Brandl E, Wagner M. Comparison of different approaches to quantify Staphylococcus aureus cells by real-time quantitative PCR and application of this technique for examination of cheese. Appl Environ Microbiol. 2001;67:3122–3126. doi: 10.1128/AEM.67.7.3122-3126.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lev S, Yarden Y, Givol D. Dimerization and activation of the kit receptor by monovalent and bivalent binding of the stem cell factor. J Biol Chem. 1992;267:15970–15977. [PubMed] [Google Scholar]
- Imokawa G, Kawai M, Mishima Y, Motegi I. Differential analysis of experimental hypermelanosis induced by UVB, PUVA, and allergic contact dermatitis using a brownish guinea pig model. Arch Dermatol Res. 1986;278:352–362. doi: 10.1007/BF00418162. [DOI] [PubMed] [Google Scholar]
- Longley BJ, Morganroth GS, Tyrrell L, Ding T, Gerson DM, Williams DE, Halaban R. Altered metabolism of mast-cell growth factor (c-kit ligand) in cutaneous mastocytosis. N Engl J Med. 1993;328:1302–1307. doi: 10.1056/NEJM199305063281803. [DOI] [PubMed] [Google Scholar]
- Hamann K, Haas N, Grabbe J, Czarnetzki BM. Expression of stem cell factor in cutaneous mastocytosis. Br J Dermatol. 1995;133:203–208. doi: 10.1111/j.1365-2133.1995.tb02616.x. [DOI] [PubMed] [Google Scholar]
- Longley BJ, Carter EL. SCF-KIT pathway in human epidermal melanocyte homeostasis. J Invest Dermatol. 1999;113:139–140. doi: 10.1046/j.1523-1747.1999.00643.x. [DOI] [PubMed] [Google Scholar]
- Kitamura R, Tsukamoto K, Harada K, Shimizu A, Shimada S, Kobayashi T, Imokawa G. Mechanisms underlying the dysfunction of melanocytes in vitiligo epidermis: role of SCF/KIT protein interactions and its downstream effector, MITF-M. J Pathol. 2004;202:463–475. doi: 10.1002/path.1538. [DOI] [PubMed] [Google Scholar]
- Laporte S, Denault JB, D’Orléans-Juste P, Leduc R. Presence of furin mRNA in cultured bovine endothelial cells and possible involvement of furin in the processing of the endothelin precursor. J Cardiovasc Pharmacol. 1993;22:S7–S10. doi: 10.1097/00005344-199322008-00004. [DOI] [PubMed] [Google Scholar]
- Xu D, Emoto N, Giaid A, Slaughter C, Kaw S, deWit D, Yanagisawa M. ECE-1: a membrane-bound metalloprotease that catalyzes the proteolytic activation of Big endothelin-1. Cell. 1994;78:473–485. doi: 10.1016/0092-8674(94)90425-1. [DOI] [PubMed] [Google Scholar]
- Hachiya A, Kobayashi T, Takema Y, Imokawa G. Biochemical characterization of endothelin-converting enzyme-1α in cultured skin-derived cells and its postulated role in the stimulation in human epidermis. J Biol Chem. 2002;277:5395–5403. doi: 10.1074/jbc.M105874200. [DOI] [PubMed] [Google Scholar]