Abstract
Spontaneous coronary artery dissection is a rare but potentially life-threatening event associated with the peripartum period. We present a case of postpartum multivessel spontaneous coronary artery dissection diagnosed by conventional angiography and monitored with computed tomographic coronary angiography. The patient was initially managed medically and later received a coronary stent.
A 39-year-old Caucasian woman who had given birth to triplets a week earlier presented with sharp, pressure-like chest pain that radiated to her left arm and was associated with generalized weakness, shortness of breath, diaphoresis, and tingling/numbness of her left hand. The chest pain lasted 45 minutes and was precipitated by a domestic dispute. She had preterm labor at 28 weeks of gestation, for which she was hospitalized, and ultimately developed preeclampsia at 33 weeks. Her triplets were delivered via an uncomplicated cesarean section the day after preeclampsia was diagnosed.
The patient's past medical history was otherwise unremarkable with no underlying hypertension, hypercholesterolemia, or diabetes mellitus. She did not abuse drugs or tobacco and had no personal or family history of Marfan syndrome, vasculitis, or connective tissue disorders. On admission, her blood pressure was 160/74 mm Hg, and she had mild peripheral edema. Her chest radiograph was unremarkable, and routine laboratory results were significant for anemia with a hematocrit of 29.3%. A chest computed tomography (CT) scan was negative for pulmonary emboli. Her admission electrocardiogram showed normal sinus rhythm with a 1-mm ST depression in lateral leads. The troponin I level was initially 0.9 ng/mL and later peaked at 10.4 ng/mL. The patient was started on metoprolol, aspirin, clopidogrel, intravenous heparin, and nitroglycerin.
Coronary angiography and a ventriculogram demonstrated an ejection fraction of 55% with mild inferior-apical and posterior- lateral hypokinesis (Figure 1). The left anterior descending coronary artery had a dissection that extended to midvessel with an 80% minimal diameter stenosis (Figure 2a). The circumflex artery had a large dissection (Figure 2b) that extended to the distal circumflex with involvement of the first obtuse marginal artery. The right coronary artery had dissections in both the posterior descending and posterior lateral artery (Figure 2c). Given the diffuse involvement of the dissections and fear of further propagation of the dissection with percutaneous coronary intervention (PCI) or grafting into a false lumen with bypass surgery, the patient was managed with continued medical therapy. She was eventually discharged home on aspirin 325 mg daily, metoprolol 50 mg twice daily, isosorbide mononitrate 60 mg daily, clopidogrel 75 mg daily, lisinopril 10 mg daily, and atorvastatin 80 mg daily.
Figure 1.
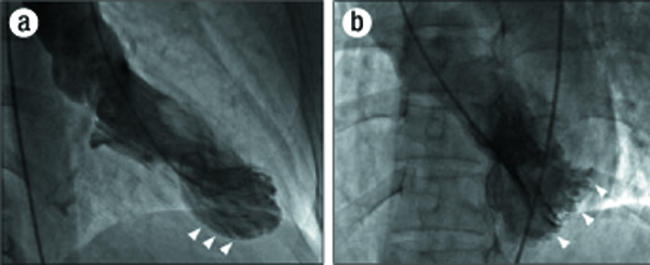
Left ventriculograms at end systole: (a) in right anterior oblique projection with inferior-apical hypokinesis (arrowheads); (b) in left anterior oblique projection with posterior-lateral hypokinesis (arrowheads).
Figure 2.
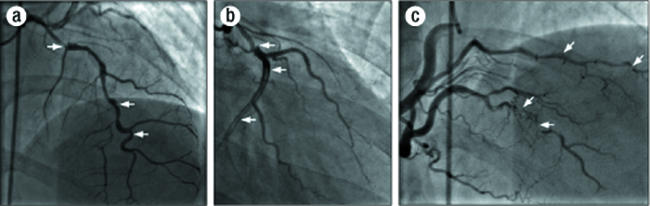
Index angiogram: (a) of left anterior descending artery with dissections (arrows); (b) of circumflex artery with dissections (arrows); (c) of right coronary artery with dissections in posterior descending artery and posterior lateral artery (arrows).
The patient had minimal symptoms until approximately 6 weeks postpartum, when she developed worsening angina pectoris and was readmitted for repeat cardiac catheterization. Angiography demonstrated marked improvement in the dissections initially seen in the left anterior descending artery and distal branches of the right coronary artery. Unfortunately, the dissection seen in the circumflex artery was unchanged and was thought to be the cause of the patient's symptoms. A coronary CT angiogram showed the continued dissection in the proximal and mid circumflex artery (Figure 3). The decision was made to continue medical management and repeat a cardiac catheterization at 6 months postpartum.
Figure 3.
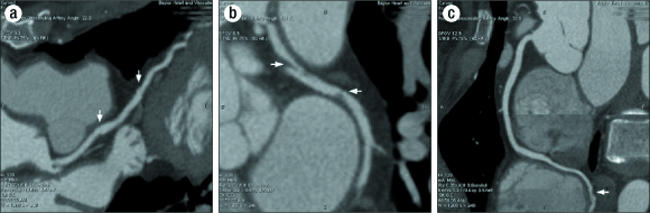
6-week CT angiogram: (a) of left anterior descending artery with dissections (arrows); (b) of circumflex artery with dissections (arrows); (c) of right coronary artery with dissections in posterior descending artery (arrow).
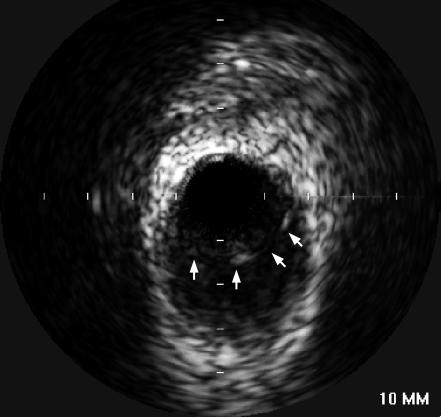
The patient did well over the intervening months with stable exertional angina pectoris. The 6-month angiogram showed complete resolution of the dissections in the right coronary artery and a nearly complete resolution in the left anterior descending artery (Figure 4a, 4c). However, the dissection in the circumflex artery was persistent with significant luminal narrowing (Figure 4b), as also demonstrated by intravascular ultrasound (Figure 5). Given the elapsed time from the initial event, the risk of worsening the dissection with PCI was felt to be significantly reduced, and the circumflex artery was successfully repaired with two overlapping 3.0 ? 33-mm Cypher sirolimus-eluting stents and a distal 2.0 ? 12-mm Minivision stent (Figure 6). The proximal vessel was dilated with a 3.5-mm postdilation balloon. The patient is doing well clinically and remains free of angina 1 year after PCI.
Figure 4.
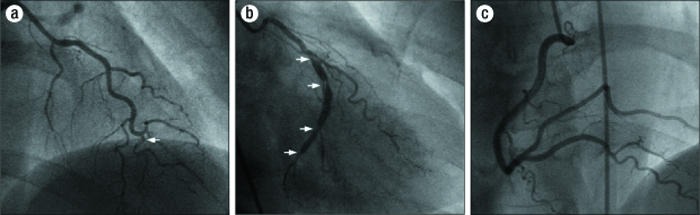
6-month angiogram: (a) of left anterior descending artery with improved dissections (arrow); (b) of circumflex artery with dissections (arrows); (c) of right coronary artery with resolved dissections in posterior descending artery and posterior lateral artery.
Figure 5.
Intravascular ultrasound of mid circumflex artery showing dissection (arrows).
Figure 6.
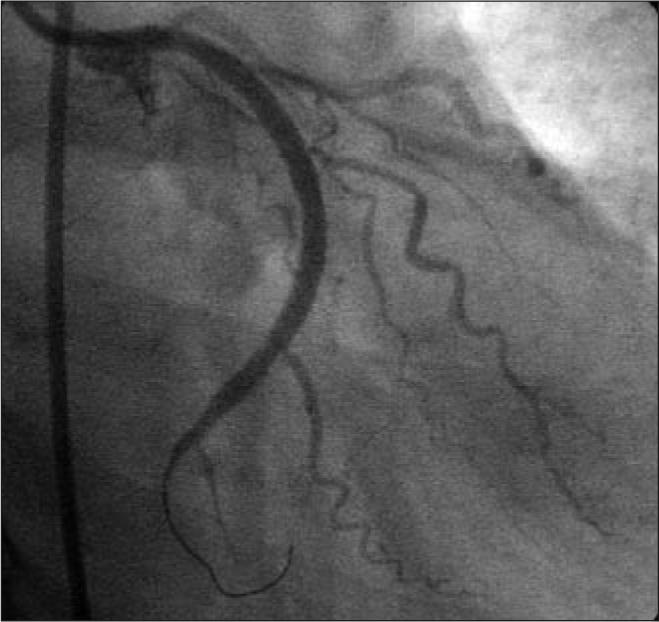
Angiogram of circumflex artery 6 months after stent deployment.
While spontaneous coronary artery dissection (SCAD) is well recognized as a rare cause of chest pain, acute coronary syndrome, and sudden cardiac death, its optimal treatment is not established. We present a case of postpartum multivessel spontaneous coronary artery dissection initially managed medically with double antiplatelet, statin, beta-blocker, angiotensin- converting enzyme inhibitor, and nitrate therapy followed by late coronary stenting. Moreover, this is the first reported case with multivessel SCAD confirmed by coronary CT angiography.
SCAD is three times more likely to occur in women than in men and is often associated with the peripartum period, defined as the 9 months of pregnancy and up to 3 months postpartum. Twenty-five percent of SCAD cases occur in the peripartum period. About 80% of pregnancy-related SCAD cases present in the 3 months following delivery (1). The median time for dissection to occur is approximately 13 days postpartum (2). A definitive cause of the association between SCAD and pregnancy is unknown, but it is hypothesized that the hormonal and hemodynamic changes that occur during pregnancy and the immediate postpartum period play a role. SCAD is felt to be precipitated by weakening of the tunica media by progesterone (3). Additionally, the increased blood volume and cardiac output during this period increase the shear stress on the wall of the coronary vessel. The validity of this hormonal hypothesis is strengthened by data suggesting that oral contraceptives can increase the risk of SCAD as well (4).
Other hypotheses have been explored. One of these theories proposes that eosinophils migrate into the arterial wall and lead to an intimal-medial separation. This was the reasoning behind adding the anti-inflammatory effects of atorvastatin to our medical regimen. However, some have challenged this theory, arguing that eosinophilic migration occurs in response to the dissection rather than inciting the cause. Nevertheless, the dissection causes a “false lumen,” which develops a hematoma that limits the flow of blood through the “true lumen,” leading to myocardial ischemia and infarction (5).
With a mortality rate around 70%, most SCAD diagnoses are made at autopsy (6). Patients presenting to a hospital facility typically have their definite diagnosis made by traditional coronary angiography. One published case reported the use of intravascular ultrasound to show the luminal dissection characteristic of SCAD (7). We believe that new CT angiography technology can be applied to the diagnosis and monitoring of SCAD.
CT coronary angiography is an evolving technology that allows evaluation of the coronary vessels without the complications of cardiac arrhythmias, myocardial infarctions, coronary dissections, and femoral artery injuries that can occur with traditional coronary angiography. Recent studies have shown that CT angiography has approximately 99% sensitivity and 96% specificity for detecting significant coronary stenosis (8). However, the sensitivity for detection of coronary artery dissection is unknown. Moreover, increased heart rates and obesity decrease the effectiveness of this modality. In our patient, CT angiography did an excellent job of showing multivessel coronary artery dissections (Figure 3). In future cases, CT angiography can be used to diagnose and monitor SCAD until resolution or until the timing is optimal for traditional coronary angiography and PCI.
There is great debate over how SCAD should be treated. The lack of consensus is not surprising since only several hundred cases have been reported in the literature and the majority of cases are diagnosed at autopsy. The rarity of this condition makes it difficult to study SCAD in randomized controlled trials. Most data we have are from single case reports. However, a recent article from Switzerland systematically reviewed the literature and identified 16 women with SCAD. The authors found that 10 of 16 patients were initially treated with medical management. In 50% of the women treated with medical management, the dissections resolved. The remaining 50% of patients had persistent or progressive dissections and required intervention (2).
Conservative management or medical management includes treatment with heparin, aspirin, nitrates, and blood pressure control; glycoprotein IIb/IIIa inhibitors have been recently added to this regimen. It is theorized that aspirin, clopidogrel, and glycoprotein IIb/IIIa inhibitors help decrease the thrombus formation in the false lumen, allowing for more normal flow through the true lumen (9). However, thrombolytics have been shown to potentially worsen the dissection in some cases by causing increased bleeding in the false lumen, therefore further limiting blood flow through the true lumen (10).
If medical management fails, then stenting of the true lumen can serve to open the vessel and return adequate blood flow to the cardiac tissue. Blood flow can also be restored by surgically bypassing the affected vessels. Additionally, there have been case reports of patients requiring the use of left ventricular assist devices as a bridge to cardiac transplantation when medical management, PCI, and bypass surgery have proven unsuccessful. There are case reports of successful treatment of SCAD with all of the above-mentioned interventions; however, it is difficult to access the failure rate since not all cases of success or failure are reported. Velusamy and associates documented a case of a worsening dissection secondary to stenting of the true lumen. This is believed to be due to a “tendency for the dissection to more readily propagate due to the lack of fibrous tissue in these non-atherosclerotic arteries” during the stenting procedure (5).
In conclusion, early recognition and diagnosis of SCAD is important given the high mortality associated with this condition. Once diagnosed, the ultimate therapy will depend on the patient's clinical condition. When the patient is hemodynamically stable, an initial treatment strategy of medical management can delay or eliminate the need for mechanical intervention by giving the damaged coronary endothelium an opportunity to heal. In addition, patients can be monitored by CT coronary angiography, as this noninvasive modality can decrease the risk of complications associated with traditional coronary angiography and furthermore eliminate the tendency to stent prematurely.
References
- 1.Hinojal YC, Di Stefano S, Florez S, Martinez G, de la Fuente L, Casquero E, Gualis J. Spontaneous coronary dissection during postpartum: etiology and controversies in management. Ital Heart J. 2004;5(7):563–565. [PubMed] [Google Scholar]
- 2.Maeder M, Ammann P, Drack G, Rickli H. Pregnancy-associated spontaneous coronary artery dissection: impact of medical treatment. Case report and systematic review. Z Kardiol. 2005;94(12):829–835. doi: 10.1007/s00392-005-0302-6. [DOI] [PubMed] [Google Scholar]
- 3.Madu EC, Kosinski DJ, Wilson WR, Burket MW, Fraker TD, Jr, Ansel GM. Two-vessel coronary artery dissection in the peripartum period. Case report and literature review. Angiology. 1994;45(9):809–816. doi: 10.1177/000331979404500909. [DOI] [PubMed] [Google Scholar]
- 4.Heefner WA. Dissecting hematoma of the coronary artery. A possible complication of oral contraceptive therapy. JAMA. 1973;223(5):550–551. [PubMed] [Google Scholar]
- 5.Velusamy M, Fisherkeller M, Keenan ME, Kiernan FJ, Fram DB. Management of dissection and other complications: spontaneous coronary artery dissection in a young woman precipitated by retching. J Invasive Cardiol. 2002;14(4):198–201. [PubMed] [Google Scholar]
- 6.Hamilos MI, Kochiadakis GE, Skalidis EI, Igoumenidis NE, Chrysostomakis SI, Vardakis CE, Vardas PE. Acute myocardial infarction in a patient with spontaneous coronary artery dissection. Hellenic J Cardiol. 2003;44(5):348–351. [Google Scholar]
- 7.Iyisoy A, Kursaklioglu H, Kose S, Ozturk C, Amasyali B, Demirtas E. Spontaneous intimal dissection in a patient with post-infarct angina: identification with intravascular ultrasound and treatment with coronary stenting. Jpn Heart J. 2003;44(4):557–564. doi: 10.1536/jhj.44.557. [DOI] [PubMed] [Google Scholar]
- 8.Pugliese F, Mollet NR, Runza G, van Mieghem C, Meijboom WB, Malagutti P, Baks T, Krestin GP, deFeyter PJ, Cademartiri F. Diagnostic accuracy of non-invasive 64-slice CT coronary angiography in patients with stable angina pectoris. Eur Radiol. 2006;16(3):575–582. doi: 10.1007/s00330-005-0041-0. [DOI] [PubMed] [Google Scholar]
- 9.Choi JW, Davidson CJ. Spontaneous multivessel coronary artery dissection in a long-distance runner successfully treated with oral antiplatelet therapy. J Invasive Cardiol. 2002;14(11):675–678. [PubMed] [Google Scholar]
- 10.Chen S, Duan B. Treatment of spontaneous coronary artery dissection: report of two cases. Chin Med J (Engl) 2000;113(12):1150–1152. [PubMed] [Google Scholar]