Abstract
Previously we have shown that SV40 pseudovirions packaged in vitro (IVPs) are an efficient delivery system for super-coiled DNA plasmids of up to 17.7 kb, with or without SV40 sequences. RNA interference (RNAi) is a naturally occurring gene silencing mechanism mediated by small double-stranded RNA molecules (small interfering RNAs, siRNAs). This study demonstrates the first use of SV40 pseudovirions to deliver into human cells both principal types of RNAi effector molecules, plasmid expressed short hairpin RNAs (shRNAs) and synthetic siRNAs. We first established the ability of human lymphoblastoid cells to support RNAi using sequential transduction of .45 cells with packaged plasmid DNA expressing the green fluorescent protein (IVP-GFP), and an shRNA corresponding to the GFP (IVP-shGFP). SV40 mediates DNA transfer of nucleic acid to the cytoplasm, where RNAi-associated cleavage of mRNA principally occurs. Using SV40 pseudovirions, siRNA-mediated RNAi was observed in both .45 cells, following sequential transduction of IVP-GFP and IVP packaged siRNAs corresponding to GFP (IVP-siGFP), and in HeLa cells stably expressing a GFP transduced with IVP-siGFP. Our findings indicate that SV40 pseudovirions may be a useful addition to the delivery systems currently being used for the transfer of RNAi effector molecules.
INTRODUCTION
The delivery of circular DNA encapsidated in VP1, the SV40 major capsid protein, to form in vitro packaging pseudovirons (IVPs) is well established, and has been demonstrated as a highly efficient procedure. DNA that does not necessarily carry any SV40 sequences, up to 17.7 kb in size, can be introduced into a wide variety of human, murine and monkey tissues in vitro and in vivo (Kimchi-Sarfaty et al., 2002; Kimchi-Sarfaty et al., 2003; Kimchi-Sarfaty et al., 2004a; Kimchi-Sarfaty and Gottesman, 2004). The efficiency of DNA entry into cells is very high using IVPs. However, the level of transgene expression from plasmid DNA is often limited, partially because the delivered DNA remains located within the cytoplasmic compartment (Kimchi-Sarfaty et al., 2004b). Although this is a disadvantage for the expression of plasmid DNA, we speculated that this might be advantageous for the delivery of synthetic small interfering RNAs (siRNAs), an important mediator of the recently described gene silencing mechanism, RNA interference.
RNA interference (RNAi) is a sequence-specific, naturally occurring genesilencing mechanism mediated through the formation of an enzymatically active ribo-nucleoprotein termed the RNA-induced silencing complex (RISC). The RNA component of RISC is a single-stranded RNA species 21–23 nucleotides in length, which acts as a guide for RISC such that RNA transcripts with a cognate sequence to the RNA within RISC are cleaved in a position-dependent manner (Sontheimer, 2005). A number of studies have shown that RISC formation and mRNA transcript cleavage occur primarily within the cytoplasm (Martinez et al., 2002). A number of approaches have developed in recent years that allow for exploitation of this process principally through either the use of (1) synthetic small interfering RNAs (siRNAs), duplex RNA oligonucleotides that can be directly administered to cells, or (2) of plasmid-expressed short hairpin RNAs, or viral delivery which expresses these RNAs, that require intracellular processing by two endogenous RNAse III enzymes, Drosha and Dicer, to generate an siRNA. Both types of RNAi effector molecules are being actively applied to a very broad range of cell types including primary cells and are also being used in vivo as pre-clinical gene therapy studies have been reported (Caplen, 2004).
Typically, synthetic siRNAs have been delivered using cationic lipid or polyplex delivery systems. These methods are not able to efficiently deliver siRNAs into cells in suspension, such as lymphoblastoids or erythroleukemia cells. Short hairpin RNA expression cassettes have been adapted to be compatible with most plasmid and viral vector systems including retroviruses, adenoviruses, lentiviruses and adeno-associated viruses. As with the transfer of transgenes, all of these delivery systems require a significant degree of optimization and are often only useful in specific cell systems (Banan and Puri, 2004). Some viral vectors have the disadvantage of low titer, and also have a large genome size, which is difficult to manipulate. In addition, some are dependent on helper viruses or packaging cell lines, and some are not able to transduce non-dividing cells, or cells in suspension (Tiscornia et al., 2003; Izquierdo, 2005).
In this study, we demonstrate the use of SV40 in vitro pseudovirions to deliver plasmids expressing shRNAs and synthetic siRNAs. In both cases, RNAi was observed following transduction of human lymphoblastoid cells and HeLa cells. Our findings suggest that SV40 pseudovirions could be used to deliver RNAi effectors.
MATERIALS AND METHODS
Cell lines and cell culture
.45 human lymphoblastoid cells with high levels of MHC I were maintained in RPMI media (Invitrogen, Carlsbad, CA). HeLa cells and HeLa-GFP cells were maintained in DMEM media (Invitrogen). HeLa-GFP medium was supplemented with 1 mg/ml G418. All media were supplemented with 10% FBS (Hyclone, Logan, UT), 5 mM L-glutamine, 50 mg/ml penicillin, and 50 mg/ml streptomycin (Quality Biological, Gaithersburg, MD). Cell lines were kept at 37°C, in 5% CO2.
Transduction of Sf9 cells and preparation of nuclear extracts
A baculovirus construct expressing VP1, the SV40 main capsid protein, was added to 250 ml Sf9 cells with occasional mixing at a multiplicity of infection (MOI) of 10. Cells were harvested 4-5 days later. Nuclei were isolated using 10% NonidetTM-40 (NP40) (Sigma, St. Louis, MO, USA), 10 mM Hepes pH 7.9 (Cellgro, Herndon, VA, USA), 10 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA (all from Digene, Beltsville, MD, USA), 0.5 mM phenylmethylsulfonylfluoride (PMSF; Fluka, Basel, Switzerland), 1 mM Dithiothreitol (DTT) (Sigma) and Protease Inhibitor cocktail tablet (Roche, Mannheim, Germany). Nuclei were extracted using 20 mM Hepes pH 7.9, 0.4 M NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM PMSF, 1mM DTT and a protease inhibitor cocktail tablet (Roche). DTT, PMSF and the protease inhibitor cocktail were added to the buffer immediately before use (Sandalon et al., 1997; Sandalon and Oppenheim, 1997). Nuclear extract concentration was measured using the BCA Protein Assay protocol (Pierce, Rockford, Illinois). The nuclear extracts were stored for more than a year at −20°C.
RNAi effectors, plasmids and preparation of in vitro packaging vectors
Commercial or previously characterized RNAi effectors were utilized in this study. A short hairpin RNA corresponding to GFP expressed in pSilencer 3.1-H (Ambion, Austin, TX) – shGFP (under the promoter of pol II) was packaged into in vitro pseudovirions (IVPs) as previously described (Kimchi-Sarfaty et al., 2002) to generate IVP-shGFP. Briefly, packaging was performed using 100 μg of DNA, 100 μg of nuclear extract from Sf9 cells, in the presence of 5 mM ATP and 8 mM MgCl2 at 37°C for 6 hours, and 1 mM CaCl2 on ice for 1 hour (defined as one reaction). Mock reactions were empty in vitro packaging, or cells only. For GFP expression pEGFP-C1 (Clontech, Palo Alto, CA) was utilized, also encapsidated into IVPs (IVP-GFP). An FITC-labeled siRNA was used to examine the ability of the pseudovirions to deliver siRNA (5′- AAGCTGACCCTGAAGTTCATC-3′ and 5′-AAGCAGCACGACTTCTTCAAG-3′). The siRNA was labeled at the 3’ end which has previously been shown to have no effect on its silencing efficiency (IVP-siRNA.3’.Fl). Two different sequences corresponding to GFP were used as synthetic siRNAs, si-GFP-1, sense 5’ AAGCTGACCCTGAAGTTCATCT 3’, (induces over 80% decrease in eGFP expression in Hela cells expressing an eGFP transgene with a 2-hour half-life when assayed 48 hours after transfection with a cationic lipid; Caplen unpublished data), and siGFP-2, sense 5’GCAAGCTGACCCTGAAGTTCAT3’ which has been previously described (Caplen et al., 2001) and induces a 70% decrease in eGFP expression in Hela cells expressing an eGFP transgene with a 2-hour half-life when assayed 48 hours after transfection with a cationic lipid (Caplen unpublished data). 1–100 μg siRNA were used for the in vitro packaging (IVP-siGFP-1; IVP-siGFP-2).
In vitro pseudoviron transduction of cells
.45 cells in suspension were split using their growth media 24 hours prior to transduction, but were counted on the day of transduction, and were placed in 60 mm dishes. A reaction of in vitro-packaged SV40 vectors carrying the plasmid DNA or the RNAi, or empty SV40 vectors without DNA or RNAi as controls, was suspended in 340 μL of the growth medium. When cells were re-transduced immediately or 24 hours later with IVP-shGFP, we restricted the transduction volume of IVP-GFP to half of a reaction, and IVP-shGFP also to half of a reaction, as cells are not viable at levels above one reaction. Cells were transduced and placed on a rotary shaker at 30 rpm for 2.5 hours (at 37°C in 5% CO2). Post-infection, the dishes were supplemented with 4 ml of fresh growth medium. Transduction of HeLa cells was done similarly with the modification of counting 1–5 x 105 and plating the cells 24 hours prior to the transduction.
Transfection into HeLa cells
1–100 μg of siRNA-1 and/or siRNA-2 designed to inhibit GFP were introduced into 5 X 105 HeLa cells expressing GFP using Lipofectamine-Plus (Invitrogen) as described in the kit’s protocol, with minor modifications.
Detection of fluorescent-tagged siRNA and GFP gene expression
One to four days post-transduction, 2 x 105 cells were washed and suspended in 200 μl phosphate-buffered saline (PBS) (Invitrogen), 0.1% bovine albumin (BSA) (Sigma-Aldrich) at 4°C and analyzed by FACS (FL1) for fluorescence as previously described (Cormack et al., 1996). For confocal imaging, transduced cells were washed twice with PBS supplemented with 0.1% BSA, and fixed for 0.5 hour with 4% paraformaldehyde (PFA) (Sigma-Aldrich) and with additional fixation for 0.5 hour with 70% ethanol at room temperature. Then the cells were washed with PBS/0.1% BSA as before, dropped onto lysine-coated microscope slides (Erie Scientific Co., Portsmouth, NH), and allowed to dry. Fluorescent mounting medium (DAKO Corp., Carpinteria, CA) was used to affix a glass coverslip to the microscope slide, and the slides were stored in the absence of light at 4°C. Confocal fluorescent images were collected with a Bio-Rad MRC 1024 confocal scan head mounted on a Nikon Optihot microscope with a 60X planapochromat lens. Excitation at 488 nm was provided by a krypton-argon laser. The experiment was repeated 3 times.
RESULTS AND DISCUSSION
Establishment of RNAi in human lymphoblastoid .45 cells using the delivery of a plasmid expressed short hairpin RNA (shRNA)
SV40-in vitro pseudovirions (IVPs) can efficiently transfer plasmid DNA into a wide variety of cell lines in vitro and in vivo. We have previously reported that human lymphoblastoid .45 cells are efficiently transduced with in vitro-packaged plasmid DNA carrying the fluorescent marker gene GFP (IVP-GFP). To determine if these cells support RNAi, we transduced .45 cells with IVP-GFP and then subsequently treated these cells with IVPs encapsulating plasmid DNA expressing an shRNA corresponding to GFP (IVP-shGFP). Previous experiments (Kimchi-Sarfaty et al., 2002; Kimchi- Sarfaty et al., 2003) showed that .45 cells could be transduced with a maximum volume of one reaction as described in Materials and Methods (larger volumes are toxic to the cells). Therefore, the cells were transduced with a half reaction of IVP-GFP, and a half reaction of IVP-shGFP. As previously mentioned, the efficiency of the SV40 delivery system is very high, but the expression is relatively low. Thus, the expression detected after transduction with a half reaction is lower. IVP-shGFP was administered immediately following IVP-GFP transduction and also 24 hours after IVP-GFP transduction. GFP fluorescence was followed for 72 hours. Figure 1A demonstrates a complete shutdown of the GFP 3 days after transduction when the ratio of IVP-GFP to IVP-shGFP was 1:1, and Figure 1B shows the same phenomenon when the ratio is 1:7. Similar results were obtained when IVP-shGFP was added 24 hours post IVP-GFP transduction (data not shown).
FIG. 1.
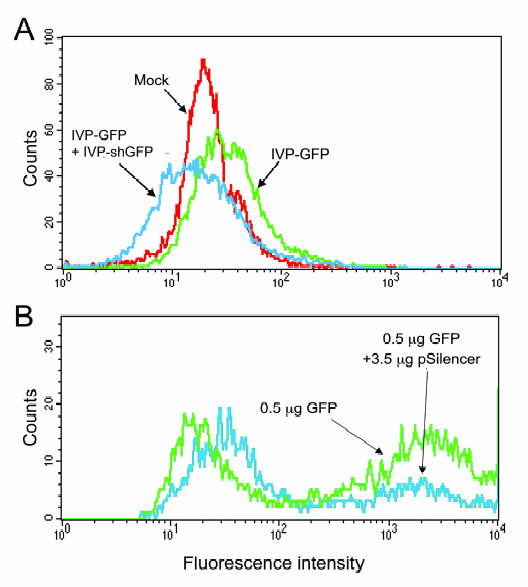
The induction of RNAi against GFP in the human lymphoblastoid .45 cell line using the pseudovirion delivery of plasmids expressing short hairpin RNAs. .45 human lymphoblastoid cells were transduced with a half reaction (A) or with 1/7 reaction (B) of GFP plasmid DNA packaged in vitro only (green). The same cells were transduced with GFP plasmid DNA packaged in vitro and then immediately with a half reaction (A) or with 6/7 reaction (B) IVP-shGFP pSilencer 3.1-H plasmid DNA packaged in vitro (blue), and were compared to mock, empty virion-transduced cells (red). The blue curve appears shifted to the left as compared to the green curve, indicating complete shutdown of the GFP expression.
Transduction of lymphoblastoid .45 cells with in vitro-packaged fluorescent-tagged siRNAs
One disadvantage of SV40 pseudovirions is a partial cellular localization within the cytoplasm that can reduce the expression from plasmid expression vectors that require nuclear localization (Kimchi-Sarfaty and Gottesman, 2004). However, cytoplasmic localization may be advantageous for synthetic siRNAs as this is a critical functional cellular compartment for RISC-mediated mRNA cleavage. In order to test the efficiency of delivery of synthetic siRNAs by SV40 pseudovirions, we studied the delivery of fluorescent-tagged siRNA to cells in suspension. These cells are very difficult to transduce using lipid-based transfection reagents. Up to 100% of the cells showed uptake, as can be demonstrated by FACS and confocal microscopy (Figure 2). The fluorescence intensity revealed by FACS was similar between 48 hours to 72 hours post-transduction, but gradually decreased to minimal fluorescence by day 6 after transduction (data not shown). The same experiment was performed on HeLa cells, with similar results (data not shown).
FIG. 2.
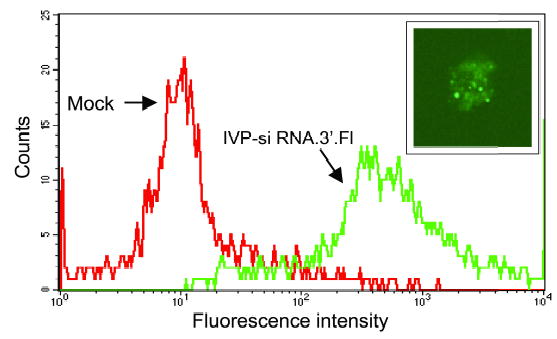
Uptake studies of IVP-encapsulated fluorescent-tagged siRNAs in .45 human lymphoblastoid cells. .45 human lymphoblastoid cells were transduced with fluorescenttagged siRNA packaged in vitro (IVP-siRNA.3’.Fl) (green) or empty particles (red). Cells were harvested three days post-transduction. The same cells were washed and fixed and were scanned by confocal microscopy. At the upper right is an example of a human lymphoblastoid cell expressing the fluorescent-tagged siRNA (X600).
SV40 pseudovirion-delivered siRNAs can mediate RNAi
To determine if the in vitro-packaged siRNAs can mediate RNAi, we packaged established sequences of siGFP (IVP-siGFPs), and delivered half of the in vitro-packaged reaction into 105 .45 cells following IVP-GFP transduction using half a reaction. Transduction with IVP-siGFP was performed either immediately or 24 hours after IVP-GFP transduction and revealed similar results. Two different sequences for siRNA were ecapsidated and tested (IVP-siGFP-1 and IVP-GFP-2). The use of each of the individual siRNAs reduced GFP expression by 50% (data not shown). The use of combined IVP-siGFPs resulted in no detectable GFP fluorescence for at least 3 days (Figure 3). At day 4, only low GFP expression was detected, which increased gradually up to day 6, when GFP expression completely recovered. The same experiment was performed on HeLa cells, with similar results (data not shown).
FIG 3.
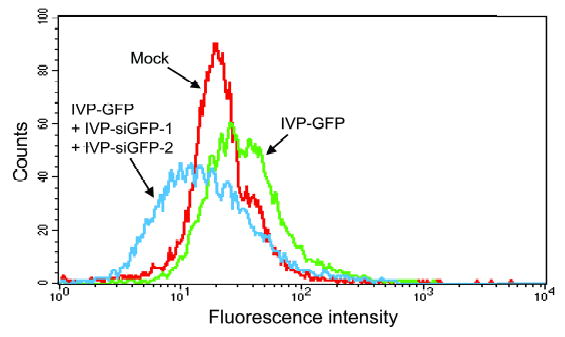
Delivery of GFP siRNAs into human lymphoblastoid cells using pseudovirions. Human lymphoblastoid .45 cells were transduced with a half reaction of GFP plasmid DNA packaged in vitro only (green). The same cells were then immediately transduced with a half reaction of IVPs mix containing two synthetic siRNAs corresponding to GFP (blue), and were compared to mock, non-transduced cells (red). Cells were harvested three days post transduction.
RNAi in HeLa-GFP cells transduced with SV40 in vitro-packaged plasmid expressed shRNAs or synthetic siRNAs.
HeLa cells stably expressing GFP were transduced with either IVP-shGFP or IVP-siGFP. RNAi, as measured by a reduction in GFP expression was observed with both RNAi effector molecules. IVP-siGFP was prepared with 1, 10, 25, 50 and 100 μg of siGFP. Only a limited dose response was seen (Figure 4) and so 1 μg was used routinely. These results may indicate that an even lower concentration than 1 μg of siGFP packaged and delivered by the SV40 pseudovirions would be sufficient to silence the reporter gene. The same experiment was performed on .45 cells and similar results were obtained--the packaging reaction included 1 μg of siGFP with 100 μg of nuclear extracts.
FIG. 4.
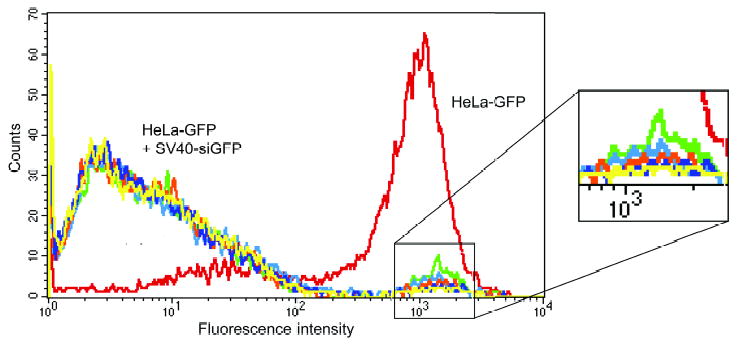
Dose response of siGFP packaged in vitro against HeLa cells stably expressing GFP. HeLa cells stably expressing GFP (red), were transduced with a mix of packaged siGFP IVP-siRNAs-1 and IVP-siRNA-2. The in vitro packaging reaction was prepared with different concentrations of the siGFP: 1 μg (green), 10 μg (light blue), 25 μg (orange), 50 μg (dark blue), 100 μg (yellow). Transducing with IVP containing these various concentrations resulted in complete elimination of GFP expression. Cells were analyzed three days post-transduction. The right side of the FACS figure is enlarged to better display the slight differences in efficiency of the various siGFP concentrations.
A comparison of the efficacy of cationic lipid transfected siRNA versus IVP delivered siRNA showed that the latter induced a complete cessation of GFP expression in the majority of the cells compared to the control, while lipid delivery induced only a log decrease in GFP expression (Figure 5).
FIG. 5.
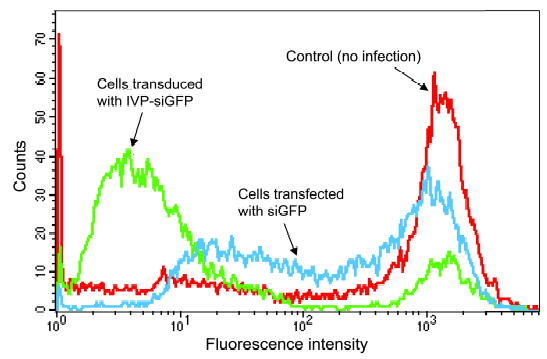
Expression of siRNA against HeLa cells stably expressing GFP delivered by SV40 pseudovirions and by lipid transduction. HeLa cells stably expressing GFP (red) were transduced with a full reaction of the mix of in vitro packaged siGFP-1 and siGFP- 2 via SV40 pseudovirions (green). The majority of the cells ceased GFP expression, while lipid-transfected siRNAs - siGFP-1 and siGFP-2 cells showed much higher expression (blue). Cells were analyzed three days post-transduction.
Earlier studies by Cordelier and colleagues (Cordelier et al., 2003) showed that it is possible to target the CCR5 chemokine receptor that functions as a coreceptor for most clinical strains of HIV, using rSV40-delivered siRNAs. They designed a DNA vector that expresses two different anti-CCR5 siRNAs, driven by the adenoviral VA1 polymerase III (pol III) promoter. Here, we report for the first time that the SV40 vectors are not limited to delivery of DNA; they are also capable of carrying RNA sequences. Previously, we have shown that the ses sequence from SV40 is not required for encapsidation, and no SV40 sequences are required for the in vitro packaging process (Kimchi-Sarfaty et al., 2003). Our results here are in agreement with those findings and may indicate that the VP1 from Sf9 cells tends to surround any nucleic acid molecule.
Other systems which deliver siRNA are often not as efficient as the SV40 system, do not always enter non-dividing cells, do not always transduce cells in suspension, and usually carry some of the viral genome, raising safety issues. The SV40 pseudovirions overcome these disadvantages and are shown in this study to deliver functional siRNA into cells.
Acknowledgments
We would like to thank George Leiman for his assistance with editing the manuscript and preparing the figures.
References
- BANAN M, PURI N. The ins and outs of RNAi in mammalian cells. Curr Pharm Biotechnol. 2004;5:441–450. doi: 10.2174/1389201043376643. [DOI] [PubMed] [Google Scholar]
- CAPLEN NJ. Gene therapy progress and prospects. Downregulating gene expression: the impact of RNA interference. Gene Ther. 2004;11:1241–1248. doi: 10.1038/sj.gt.3302324. [DOI] [PubMed] [Google Scholar]
- CAPLEN NJ, PARRISH S, IMANI F, FIRE A, MORGAN RA. Specific inhibition of gene expression by small double-stranded RNAs in invertebrate and vertebrate systems. Proc Natl Acad Sci U S A. 2001;98:9742–9747. doi: 10.1073/pnas.171251798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CORDELIER P, MORSE B, STRAYER DS. Targeting CCR5 with siRNAs: using recombinant SV40-derived vectors to protect macrophages and microglia from R5-tropic HIV. Oligonucleotides. 2003;13:281–294. doi: 10.1089/154545703322616961. [DOI] [PubMed] [Google Scholar]
- CORMACK BP, VALDIVIA RH, FALKOW S. FACS-optimized mutants of the green fluorescent protein (GFP) Gene. 1996;173:33–38. doi: 10.1016/0378-1119(95)00685-0. [DOI] [PubMed] [Google Scholar]
- IZQUIERDO M. Short interfering RNAs as a tool for cancer gene therapy. Cancer Gene Ther. 2005;12:217–227. doi: 10.1038/sj.cgt.7700791. [DOI] [PubMed] [Google Scholar]
- KIMCHI-SARFATY C, ALEXANDER NS, BRITTAIN S, ALI S, GOTTESMAN MM. Transduction of multiple cell types using improved conditions for gene delivery and expression of SV40 pseudovirions packaged in vitro. BioTechniques. 2004a;37:270–275. doi: 10.2144/04372RR04. [DOI] [PubMed] [Google Scholar]
- KIMCHI-SARFATY C, ARORA M, SANDALON Z, OPPENHEIM A, GOTTESMAN MM. High cloning capacity of in vitro packaged SV40 vectors with no SV40 virus sequences. Hum Gene Ther. 2003;14:167–177. doi: 10.1089/104303403321070865. [DOI] [PubMed] [Google Scholar]
- KIMCHI-SARFATY C, BEN-NUN-SHAUL O, RUND D, OPPENHEIM A, GOTTESMAN MM. In Vitro-Packaged SV40 Pseudovirions as Highly Efficient Vectors for Gene Transfer. Hum Gene Ther. 2002;13:299–310. doi: 10.1089/10430340252769815. [DOI] [PubMed] [Google Scholar]
- KIMCHI-SARFATY C, GOTTESMAN MM. SV40 pseudovirions as highly efficient vectors for gene transfer and their potential application in cancer therapy. Curr Pharm Biotechnol. 2004;5:451–458. doi: 10.2174/1389201043376670. [DOI] [PubMed] [Google Scholar]
- KIMCHI-SARFATY C, GARFIELD S, ALEXANDER NS, ALI S, CRUZ C, CHINNASAMY D, AND GOTTESMAN MM. The pathway of uptake of SV40 pseudovirions packaged in vitro: from MHC class I receptors to the nucleus. Gene Ther Mol Bio. 2004b;8:439–450. [Google Scholar]
- MARTINEZ J, PATKANIOWSKA A, URLAUB H, LUHRMANN R, TUSCHL T. Single-stranded antisense siRNAs guide target RNA cleavage in RNAi. Cell. 2002;110:563–574. doi: 10.1016/s0092-8674(02)00908-x. [DOI] [PubMed] [Google Scholar]
- SANDALON Z, DALYOT-HERMAN N, OPPENHEIM AB, OPPENHEIM A. In vitro assembly of SV40 virions and pseudovirions: vector development for gene therapy. Hum Gene Ther. 1997;8:843–849. doi: 10.1089/hum.1997.8.7-843. [DOI] [PubMed] [Google Scholar]
- SANDALON Z, OPPENHEIM A. Self-assembly and protein-protein interactions between the SV40 capsid proteins produced in insect cells. Virology. 1997;237:414–421. doi: 10.1006/viro.1997.8796. [DOI] [PubMed] [Google Scholar]
- SONTHEIMER EJ. Assembly and function of RNA silencing complexes. Nat Rev Mol Cell Biol. 2005;6:127–138. doi: 10.1038/nrm1568. [DOI] [PubMed] [Google Scholar]
- TISCORNIA G, SINGER O, IKAWA M, VERMA IM. A general method for gene knockdown in mice by using lentiviral vectors expressing small interfering RNA. Proc Natl Acad Sci U S A. 2003;100:1844–1848. doi: 10.1073/pnas.0437912100. [DOI] [PMC free article] [PubMed] [Google Scholar]


