Abstract
Clinical evidence suggests that administration of Hypericum perforatum (Hp) extracts containing the photo-activated hypericin compounds may cause fewer skin photosensitization reactions than administration of pure hypericin. This study was conducted to determine whether the phototoxicity of hypericin in HaCaT keratinocytes could be attenuated by H. perforatum extracts and constituents. Two extracts, when supplemented with 20 μM hypericin: (1) an ethanol re-extraction of residue following a chloroform extraction (denoted ethanol(-chloroform)) (3.35 μM hypericin and 124.0 μM total flavonoids); and (2) a chloroform extract (hypericin and flavonoids not detected), showed 25% and 50% (p < 0.0001) less phototoxicity than 20 μM hypericin alone. Two H. perforatum constituents, when supplemented with 20 μM hypericin: (1) 10 μM chlorogenic acid; and (2) 0.25 μM pyropheophorbide, exhibited 24% (p < 0.05) and 40% (p < 0.05) less phototoxicity than 20 μM hypericin alone. The peroxidation of arachidonic acid was assessed as a measure of oxidative damage by photo-activated hypericin, but this parameter of lipid peroxidation was not influenced by the extracts or constituents. However α-tocopherol, a known antioxidant also did not influence the amount of lipid peroxidation induced in this system. These observations indicate that hypericin combined with H. perforatum extracts or constituents may exert less phototoxicity than pure hypericin, but possibly not through a reduction in arachidonic acid peroxidation.
Keywords: Hypericum perforatum, St. John’s Wort, HaCaT keratinocytes, Hypericin, Lipid peroxidation, Phototoxicity
Keywords: Abbreviations: Hp Hypericum perforatum, PPP pyropheophorbide
1. Introduction
Hypericum perforatum (St. John’s Wort) is an increasingly popular alternative to conventional medications used for the treatment of mild to moderate depression [1–3]. Aggressive analysis of this plant over the last three decades has revealed that it possesses several biological properties, including antidepressant, antiviral and antiproliferative activities [4–8]. Although H. perforatum (Hp) is most commonly used as an alternative to standard tricyclic antidepressants, other biological properties possessed by this plant have also been utilized for the treatment of cancer and viral infections [8–12]. Widespread use of Hp as an alternative medicine has raised concern regarding its safe use by the public [1,2]. In order to better understand the efficacy and safety of Hp herbal preparations, it has become imperative to identify and characterize the biologically active constituents composing this plant species and determine if agents in Hp are able to prevent the toxicity of key constituents such as hypericin.
Hp has been found to contain several classes of compounds common to most plants, including flavonoids, polyphenolics, porphyrins, and essential oils [1–4]. In addition to these compounds, two very active classes of constituents unique to Hypericum have also been identified, the phloroglucinols, consisting primarily of hyperforin and adhyperforin and the naphthodianthrones, hypericin and pseudohypericin [1–7]. Biological activities and toxicities possessed by this plant may also depend on the properties of several constituents collectively provoking a variety of physiological responses. An example of this may be found in studies that report the general absence of phototoxic side effects in human subjects administered Hp extracts [9,13] but moderate to severe phototoxicity in those administered pure hypericin [10,11].
The hypericin compounds produce a significant amount of reactive oxygen species upon photo-activation, which can cause damage to areas of the body exposed to light [12,14,15]. Due to the lipophilic nature of hypericin, it has been found to preferentially accumulate in cellular membranes as well as organelles, such as liposomes, endoplasmic reticulum, and the Golgi apparatus [16,17]. Many of the more common plant constituents present in Hp have been found to possess antioxidant activity, and thus it may be possible that the oxidative damage produced by hypericin can be mediated through ROS scavenging by these constituents present in preparations made from Hp plant material [4,18–21]. To determine whether the amount of oxidative damage inflicted by hypericin treatment upon light exposure could be reduced in the presence of Hp extract and individual constituents, a marker of lipid peroxidation was assessed. Peroxidation of arachidonic acid by free radicals causes the formation of isoprostanes which can be measured using an enzyme immunoassay procedure [22].
The general absence of light-sensitive toxicity in cultured fibroblast, keratinocytes, and colon cancer cells exhibited by several Hp extracts containing hypericin and pseudohypericin was previously reported [23]. These results raised questions about the impact these extracts and constituents were having on the known light-induced toxicity of the hypericin compounds. Therefore, the goal of this study was to determine whether the phototoxicity of pure hypericin in HaCaT keratinocytes could be attenuated when combined with Hp extracts and other pure constituents of Hp. The peroxidation of arachidonic acid was also assessed by measuring the formation of 8-isoprostane in the HaCaT cells to determine whether the reduced phototoxicity of pure hypericin in the presence of the extracts and constituents was associated with a reduction in this marker of oxidative stress.
2. Materials and methods
2.1. H. perforatum extracts and known constituents
Potential attenuation of the light-activated cytotoxicity of pure hypericin by H. perforatum (HP) extracts and several other known HP chemical constituents were assessed in HaCaT human keratinocytes. Three Hp extracts were chosen based on their chemical profiles as well as the differences in phototoxicity each exhibited in keratinocytes [23]. The dried plant material obtained from Frontier Herb® was harvested at the budding stage in Bulgaria in 1999, air dried and received at the Norway, Iowa warehouse in 2001. Detailed information on the cytotoxicity and quantification of pure compounds in these Hp extracts can be found in [23].
Six gram samples of dried plant material (obtained from Frontier Herb®, Norway, IA) were extracted by Soxhlet extraction for 6 h in either: (1) ethanol; (2) chloroform; or (3) the residue from the chloroform extraction was then extracted with ethanol. This sequentially extracted extract was prepared by Soxhlet extraction in chloroform, the chloroform extract was removed, the residue was evaporated to dryness prior to re-extraction in the same manner using ethanol and denoted ethanol(-chloroform). Once the final residues for all three extractions were evaporated to dryness and weighed, all material was dissolved in the minimum amount of dimethyl sulfoxide (DMSO) necessary to produce the extract stock solutions used for toxicity assessment. Note: extraction of the same amount of plant material in the three different solvents yielded different amounts of residue, so the concentration of each extract stock solution was different. This approach was used to compare the biological activity of material extracted by each solvent system from 6 g of dried Hp so that their relative importance in the plant could be assessed. Quantification of compounds by HPLC analysis in the extracts showed that the ethanol and ethanol(-chloroform) extracts contained 8.2–8.8 μM of the photo-activated hypericin and pseudohypericin compounds and 124–135 μM total flavonoids, but these compounds were not detected in the chloroform extract [23].
2.2. Fluorometric analysis
Fluorescence emission spectra were collected on the three Hp extracts at the concentration obtained upon extraction of the plant material, 1 and 10 μM pyropheophorbide, and 20 μM hypericin to illustrate the presence of hypericin and pyropheophorbide in the extracts, which compliments the detection of these compounds in the extracts using HPLC analysis [23]. Emission analysis was performed using a Spex Fluoromax™ flourometer (4 nm band-pass resolution) with an excitation wavelength of 410 nm. At 410 nm the molar extinction coefficient of hypericin is about 10,000 M−1 cm−1 [24] The extract stock solutions, hypericin, and pyropheophorbide, were dissolved in DMSO and added at 1% solvent concentration to phenyl-red free Dulbecco’s Modified Eagle’s Medium (Invitrogen, Carlsbad, CA) complexed with 10% FBS for analysis in 1 cm glass cuvettes. The chloroform extract and the most dilute concentration of pyropheophorbide (1 μM) were reassessed using a 3 mm cuvette with the same parameters in order to increase the signal–noise ratio.
2.3. Cell growth conditions
HaCaT human keratinocytes, generously provided by Dr. Tim Bowden’s lab (Arizona Cancer Center, University of Arizona), were grown in high glucose Dulbecco’s Modified Eagle’s Medium (4500 mg/L d-glucose) (Invitrogen, Carlsbad, CA) with 3.7 g/L sodium bicarbonate, supplemented with 100 UI/mL penicillin/streptomycin antibiotics and 10% fetal bovine serum. Incubation conditions were maintained at 37 °C, 70% humidity, and 5% CO2 in 75 cm2 flasks until approximately 80% confluent.
2.4. Celltiter96® aqueous one-solution cell proliferation assay
The ability of the Soxhlet Hp ethanol, ethanol(-chloroform), and chloroform extracts and several individual and combined pure compounds to reduce the phototoxicity of 2, 5, 10, 15, and 20 μM hypericin in the HaCaT keratinocytes was assessed using the Celltiter96® Aqueous One Solution Cell Proliferation Assay (Promega Corporation, Madison, WI). The HaCaT cells were plated in 48-well plates at a cell density of 10,000 cells/well and incubated for 16–18 h before treatment under restricted light exposure. Treatments were randomly assigned to wells within a plate with media and 1% DMSO used as solvent controls and 2, 5, 10, 15, and 20 μM hypericin as positive controls. Treatments consisted of either 1% of the highest concentration of extract stock solution obtained from extraction of 6 g of the dried plant material or dilutions of the extract stock solution alone or supplemented with 2, 5, 10, 15, or 20 μM pure hypericin in 10% FBS supplemented high glucose Dulbecco’s Modified Eagles Medium. In a similar manner the phototoxicity of several pure compounds was also assessed individually or in combination with additional hypericin. These treatment solutions consisted of the pure compounds individually supplemented with 2, 5, 10, 15, or 20 μM pure hypericin or the pure compounds combined together with an additional 15 μM hypericin at a 1% final solvent concentration in 10% FBS supplemented high glucose Dulbecco’s Modified Eagles Medium. Following treatment of the 48-well plates under restricted light conditions, each plate was exposed to ambient light provided by fluorescent light bulbs which supplied 5.2 ± 5% J/cm2 after 30 min of exposure at room temperature. Light exposure was measured using a Sper Scientific, Inc. Mini Environmental Quality Meter, Model 850070 (Scottsdale, AZ) and was always conducted on the same benchtop under the same bulbs. After light treatment, the plates were returned to 37 °C for 24 h in the dark. Fresh media and the Celltiter96® Aqueous One Solution dye were added to the plates after removal of the treatment solutions following the 24-h incubation period. The dye was allowed to incubate at 37 °C in the dark for 3 h and 15 min before transfer to 96-well plates and absorbance measurement on a Beckman plate reader at 490 nm, a wavelength found not to interfere with the photo-active hypericin compounds. A standard curve of cell densities was used in each experiment for the quantification of viable cells after treatment. Each treatment was normalized to the 1% DMSO solvent control and expressed as the percent cell survival. The relationship of hypericin concentration to predicted survival estimated at 0.25 μM intervals was assessed using the loess (LOcal regrESSion) function in R [25]. The predicted survival and standard error bands were plotted for ease of interpretation.
2.5. 8-Isoprostane enzyme immunoassay
8-Isoprostane (8-isoPGF2a) is a non-enzymatic family of eicosanoids that is a stable byproduct of the lipid peroxidation of arachidonic acid. A competitive enzyme immunoassay kit (Cayman Chemicals, Ann Arbor, MI) was used to detect the level of 8-isoPGF2a produced within the HaCaT keratinocytes upon treatment. The HaCaT cells were plated into 24-well plates at a cell density of 20,000 cells/well and incubated at 37 °C for 16–18 h to allow for attachment. Treatments were randomly assigned to the plates. Hydrogen peroxide (H2O2) (0.1, 1, and 100 mM) and hypericin (10, 15, and 20 μM) were used as positive controls for the formation of lipid peroxidation. The assay was performed using the concentrations of the ethanol and ethanol(-chloroform) extract stock solutions obtained upon plant extraction, diluted concentration of the chloroform extract, chlorogenic acid (10 and 50 μM), pyropheophorbide (0.25 and 0.5 μM), and α-tocopherol (1, 10 and 40 μM) all dissolved in DMSO. Extract, hypericin, pyropheophorbide, and α-tocopherol treatment solutions with or without the addition of either 20 μM hypericin or 100 mM H2O2 were prepared so the DMSO concentration was 1% in the FBS-supplemented cell culture media. Addition of the treatment solutions to the plates was performed at room temperature under restricted light conditions followed immediately by exposure to 30 min ambient light provided by fluorescent light bulbs (5.2 ± 5% J/cm2) and a 24-h incubation at 37 °C in the dark. This assay was also conducted by pre-incubating the cells with the extract stock solutions and pure compounds with and without α-tocopherol for 4 h at 37 °C in the dark. Following pre-incubation either 20 μM hypericin or 100 mM H2O2 was also added to the cells containing the extracts or compounds with or without α-tocopherol and immediately exposed to 30 min of ambient light (5.2 ± 5% J/cm2). After light treatment the cells were incubated in the dark for another 24 h at 37 °C. Following the 24-h treatment period, supernatants were collected from each treatment, centrifuged at 5,000 rpm for 10 min, and added to the enzyme immunoassay (EIA) buffer preparation provided in the kit at a 1:1 or a 1:2 dilution. Determination of 8-isoPGF2a levels in each treatment sample was conducted according to the kit protocol using the mouse anti-rabbit IgG coated plate provided in the kit and a standard curve of 8-isoprostane. Effects of extracts and treatments on 8-isoprostane concentrations were evaluated using one-way ANOVA, followed by comparisons between means using Tukey’s adjustment for multiple comparisons. Computations were done using proc glm in SAS [26].
Both assays used in this study, the Celltiter96® Aqueous One Solution Cell Proliferation Assay and 8-Isoprostane Enzyme Immunoassay, were chosen because all treatment solutions were removed prior to collection of the colorometric endpoints, ensuring the fluorometric properties of hypericin would not interfere with measurement.
3. Results
3.1. Fluorometric analysis of H. perforatum extracts, pure constituents, and pyropheophorbide
The emission spectra for the Hp ethanol extract stock solution (1161 μg/ml) revealed the presence of two peaks at 675 and 723 nm characteristic of porphyrin compounds, but the spectra for the chloroform extract stock solution (284 μg/ml) was much weaker and showed only one peak at 675 nm (Fig. 1A). To provide further evidence that these peaks resulted from a porphyrin compound, the emission spectra was also obtained for pure pyropheophorbide, a metabolite of porphyrin compounds commonly found in green plants [27]. Due to high background noise upon initial analysis, the chloroform extract and the 1 μM concentration of pyropheophorbide were repeated using a smaller cuvette in order to decrease the amount of noise and obtain a stronger signal (Fig. 1B). This second analysis showed both peaks unique to porphyrin compounds at 675 and 723 nm in both samples, verifying the presence of a porphyrin compound in the chloroform extract.
Fig. 1.
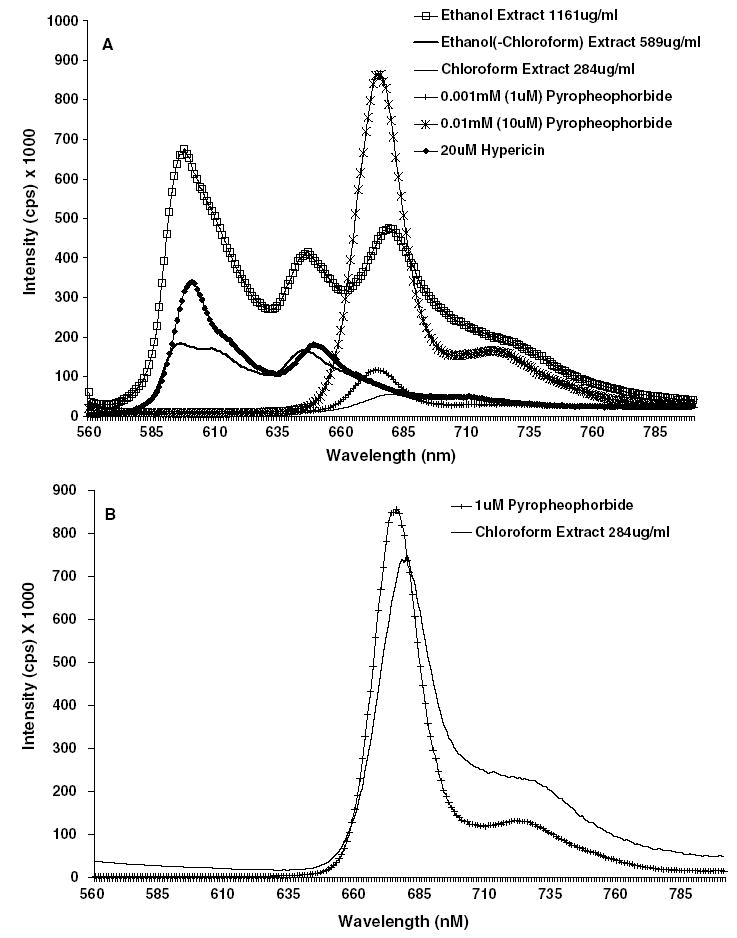
Emission spectra for treatments at 1% solvent (DMSO) concentration in phenyl-red free DMEM completed with 10% FBS using an excitation wavelength of 410 nm collected at 1 min intervals in a 1 cm cuvette. (A) The three Hp extracts and two concentrations of pyropheophorbide. (B) 1 μM pyropheophorbide and Hp chloroform extracts using a 3 mm cuvette.
3.2. Attenuation of hypericin phototoxicity by three H. perforatum extracts
Each extract was assessed for light-induced cytotoxicity with and without an additional 2, 5, 10, 15, and 20 μM hypericin and a statistical toxicity prediction model was then generated from this toxicity data using loess regression. The ethanol extract stock solution (diluted to 250 μg/ml) was assessed with and without an additional 2, 5, 10, 15, and 20 μM hypericin because at this concentration the ethanol extract alone was only toxic to approximately 50% of the cells (Fig. 2A). Higher concentrations of the ethanol extract stock solution (1161 and 500 μg/ml) were also unable to attenuate the toxicity of hypericin and exhibited more phototoxicity than the 250 μg/ml dilution, therefore only the data for the 250 μg/ml concentration of the ethanol extract was shown. The toxicity model generated from this cytotoxicity data showed that the ethanol extract exhibited significantly greater toxicity than hypericin alone, as shown by a greater reduction of percent cell survival in comparison with cells treated solely with hypericin, until reaching 17 μM hypericin, after which more than 80% of the cells were killed (Fig. 2A). The toxicity model obtained for the highest concentration of the ethanol(-chloroform) extract stock solution (589 μg/ml) assessed with and without 2, 5, 10, 15, and 20 μM hypericin showed significantly more phototoxicity when combined with up to 8.25 μM hypericin than either the extract or hypericin treatments alone, but the percent cell survival leveled off when combined with more than 8.25 μM hypericin (Fig. 2B). Dilutions of the ethanol(-chloroform) extract stock solution were also assessed, but the trend for reducing the phototoxicity of hypericin was lost at a lower concentration (200 μg/ml). The chloroform extract stock solution (diluted to 10 μg/ml) was combined with 2, 5, 10, 15, and 20 μM hypericin and the toxicity model obtained from this data showed that the phototoxicity of the extract combined with hypericin was similar to the phototoxicity of hypericin alone until reaching 12 μM hypericin (Fig. 2C). However, 10 μg/ml of the chloroform extract stock solution combined with more than 12 μM hypericin exhibited significantly less phototoxicity than the corresponding concentrations of hypericin alone (Fig. 2C). Higher concentrations of the chloroform extract stock solution (284, and 100 μg/ml) were too cytotoxic to determine whether the extract was able to reduce the phototoxicity of hypericin.
Fig. 2.
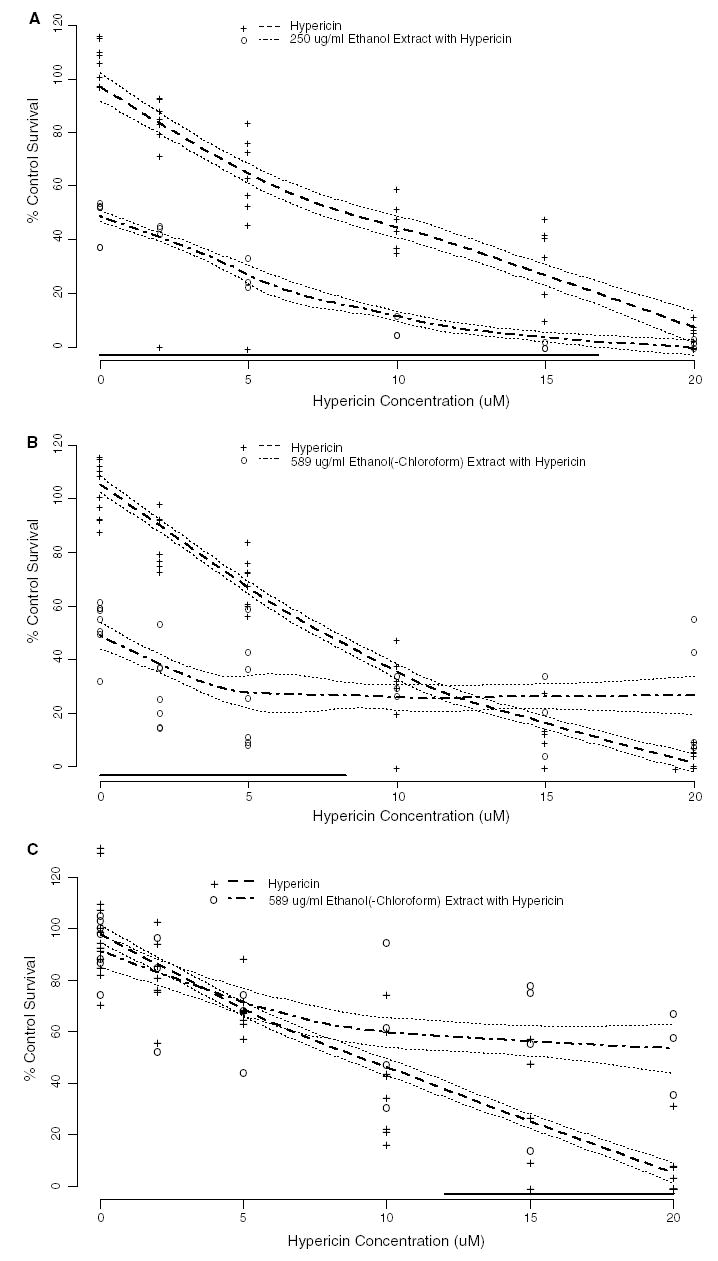
Phototoxicity (mean % control survival compared to vehicle control ± SE) of Hp extracts stock solutions at 1% concentration of the media treatment or dilutions of the extract stock solutions: (A) ethanol; (B) ethanol(-chloroform); (C) chloroform with and without increasing concentrations of additional hypericin on the HaCaT human keratinocytes (n = 3–7). The phototoxicity of the extracts supplemented with 2, 5, 10, 15, and 20 μM hypericin following exposure to 30 min of ambient light and 24-h incubation was used to generate a statistical model using loess regression. The dashed line gives the predicted phototoxicity for hypericin alone; the dot-dashed line gives the predicted phototoxicity for hypericin in combination with the extract. The dotted lines surrounding the predicted phototoxicity lines are ±one standard error bands. The solid line near the X-axis indicates those hypericin concentrations for which the predicted responses are significantly different (p < 0.05, using a Scheffé adjustment for multiple comparisons).
A second set of Hp extracts was prepared from the Frontier Herb® plant material in the same manner as the three extracts presented in this study and they were assessed for the ability to attenuate the phototoxicity of hypericin and each responded in a similar manner as the three extracts shown here (Fig. 2).
3.3. Attenuation of hypericin phototoxicity by individual and combined pure compounds identified within H. perforatum
The detection and quantification of five flavonoids, chlorogenic acid, hypericin, and pseudohypericin in Hp extracts was previously reported, as well as the individual cytotoxicity of these pure compounds in HaCaT human keratinocytes [23]. The concentration of each pure compound added to hypericin for this study was similar to what was quantified within the ethanol extracts. The phototoxicity of quercetin, isoquercitrin, hyperoside, chlorogenic acid, and pyropheophorbide individually supplemented with 2, 5, 10, 15, and 20 μM hypericin was assessed in HaCaT keratinocytes to determine whether any individual chemical was capable of reducing the phototoxicity of hypericin. Loess regression was also used to generate a predictive toxicity model from the phototoxicity data obtained with the individual constituents combined with pure hypericin. The toxicity model generated from the phototoxicity of each flavonoid supplemented with hypericin showed similar phototoxicity as hypericin alone (Fig. 3A). The toxicity model for 10 μM chlorogenic acid combined with up to 4.75 μM hypericin exhibited significantly more phototoxicity than up to 4.75 μM hypericin alone (Fig. 3B). However, 10 μM chlorogenic acid combined with more than 18 μM hypericin exhibited significantly less phototoxicity than hypericin alone (Fig. 3B). Both 0.25 and 0.5 μM pyropheophorbide showed similar phototoxicity as hypericin alone when combined with up to 14.5 and 16 μM hypericin, respectively, according to the toxicity model (Fig. 3C). Combined with more than 14.5 and 16 μM hypericin, pyropheophorbide exhibited significantly less phototoxicity than the respective hypericin concentrations alone (Fig. 3C).
Fig. 3.
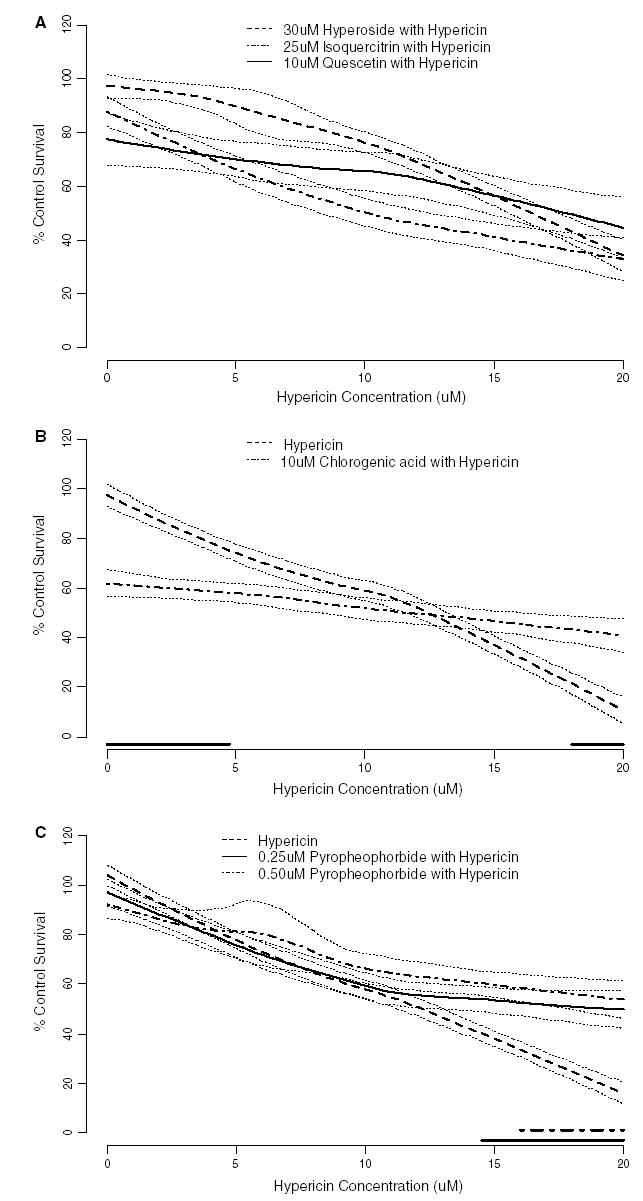
Phototoxicity (mean % control survival compared to vehicle control ± SE) of pure compounds identified within Hp preparations: (A) quercetin, isoquercitrin, and hyperoside; (B) chlorogenic acid; (C) pyropheophorbide with and without increasing concentrations of additional hypericin on the HaCaT human keratinocytes (n = 3–8). The phototoxicity of the compounds supplemented with 2, 5, 10, 15, and 20 μM hypericin following exposure to 30 min of ambient light and 24-h incubation was used to generate a statistical model using loess regression. The heavy lines give the predicted phototoxicity for hypericin alone or hypericin in combination with the extract, as indicated in each panel. The dotted lines surrounding the predicted phototoxicity lines are ± one standard error bands. The heavy lines near the X-axis indicates those hypericin concentrations for which the predicted responses are significantly different from the hypericin only predictions (p < 0.05, using a Scheffé adjustment for multiple comparisons). These are not shown for panel A because the predicted responses were never significantly different the appropriate hypericin control. For panel A mean % control survival for 10 μM hypericin alone was 67%, 45% and 72%; and for 20 μM hypericin alone was 17%, 11% and 13% in the experiments testing hypericin with quercetin, isoquercitrin, and hyperoside, respectively. Data points are not shown to avoid cluttering the figure.
The phototoxicity of combined 2 μM hypericin, 3 μM pseudohypericin, 15 μM quercetin, 25 μM isoquercitrin, 30 μM hyperoside, 15 μM quercitrin, 60 μM rutin, and 10 μM chlorogenic acid, was assessed with and without an additional 15 μM hypericin. The concentration of each compound assessed in this combination was based on the approximate concentration found to be present in the Hp ethanol extracts. The phototoxicity of the combined pure compounds without an additional 15 μM hypericin in the HaCaT keratinocytes resulted in 49.0% of the survival observed with the DMSO solvent control. However, the phototoxicity of the combined pure compounds upon addition of 15 μM hypericin resulted in 2.4% of the cell survival observed for the DMSO solvent control. In this study 15 μM hypericin alone exhibited 10.8% of the cell survival observed for the DMSO solvent control.
3.4. Influence of Hp extracts and pure constituents on the photo-induced lipid peroxidation of hypericin
Hypericin (20 μM) and H2O2 (100 mM) each produced a significant increase in the concentration of 8-isoprostane compared to the DMSO solvent control in the HaCaT human keratinocytes (Fig. 4A–D). Due to the potential presence of lipid components in the Hp extracts, cell-free analysis of each extract was conducted to assess interference with the assay. The Soxhlet ethanol extract was found to significantly interfere with the assay, so this extract was not included in the experiment. Although the ethanol(-chloroform) and chloroform extracts did not interfere with the assay, they each produced a significantly greater concentration of 8-isprostane than the DMSO control, p < 0.0001 and p < 0.05, respectively (Fig. 4A). The ethanol(-chloroform) extract supplemented with 20 μM hypericin also produced a significantly greater concentration of 8-isoprostane than 20 μM hypericin (p < 0.0001) (Fig. 4A). The chloroform extract supplemented with 20 μM hypericin produced a similar concentration of 8-isprostane as 20 μM hypericin alone (Fig. 4A). Chlorogenic acid (10, and 50 μM) alone produced a similar concentrations of 8-isoprostane as DMSO, but pyropheophorbide (0.25, and 0.5 μM) alone produced a significantly greater concentration than DMSO (p < 0.05) (Fig. 4A). Each concentration of chlorogenic acid and pyropheophorbide supplemented with 20 μM hypericin produced somewhat less 8-isoprostane than 20 μM hypericin alone, but neither was significantly different (Fig. 4A).
Fig. 4.
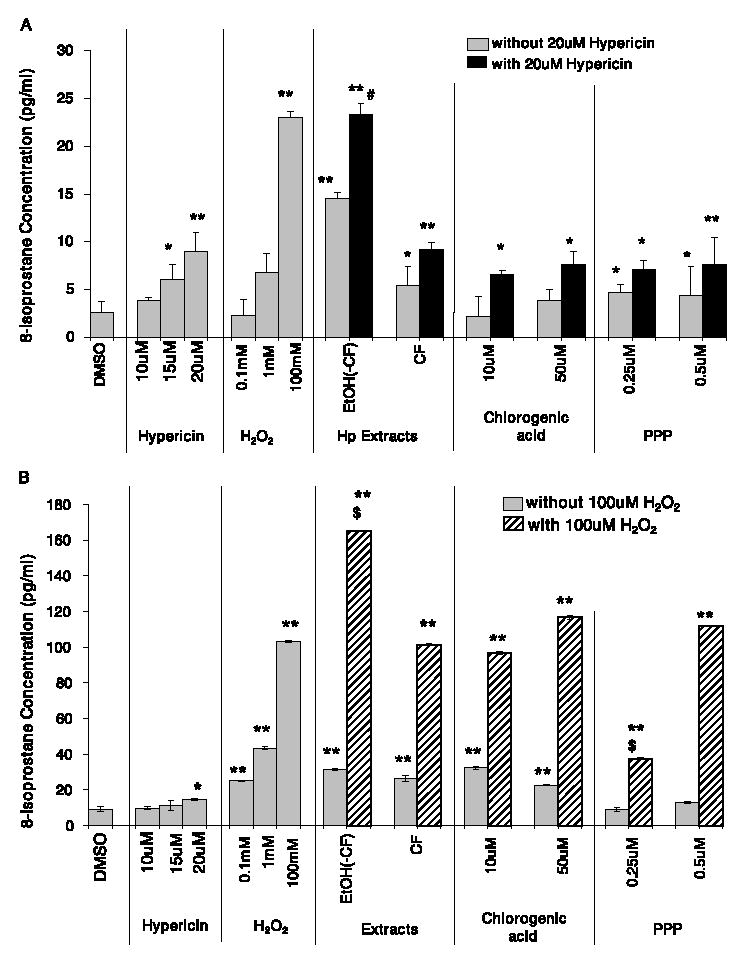
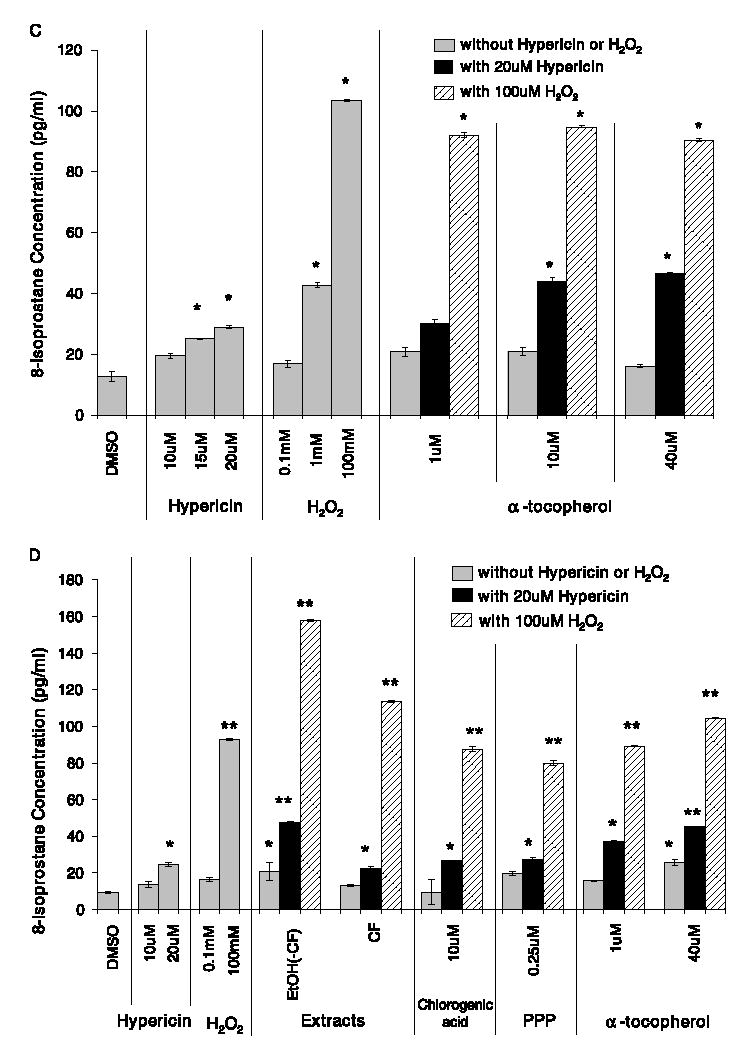
The concentration of 8-isoprostane (pg/ml) produced in the HaCaT human keratinocytes after treatment with Hp Soxhlet extracts or pure compounds: (A) with or without an additional 20 μM hypericin; (B) with or without an additional 100 mM hydrogen peroxide; (C) α-tocopherol with and without an additional 20 μM hypericin or 100 mM hydrogen peroxide; and (D) Hp extracts, pure compounds, and α-tocopherol pre-treated before the addition of 20 μM hypericin or 100 mM hydrogen peroxide and exposure to 30 min of ambient light (mean ± SE, n = 3). EtOH(-CF) = ethanol(-chloroform) extract; CF = chloroform extract; PPP = pyropheophorbide. **p < 0.0001, *p < 0.05 significantly different concentration of 8-isoprostane than solvent control. #p < 0.05 significantly different concentration of 8-isoprostane than 20 μM hypericin. $p < 0.05 significantly different concentration of 8-isoprostane than 100 mM hydrogen peroxide.
Upon supplementation with 100 mM H2O2, the ethanol(-chloroform) extract produced a significantly greater concentration of 8-isoprostane than 100 mM H2O2 alone (Fig. 4B). The chloroform extract and each concentration of chlorogenic acid (10, and 50 μM) supplemented with 100 mM H2O2 did not alter the concentration of 8-isoprostane produced compared to 100 mM H2O2 alone (Fig. 4B). Pyropheophorbide (0.25 μM) supplemented with 100 mM H2O2 significantly reduced the concentration of 8-isoprostane produced compared to 100 mM H2O2 alone, but 0.5 μM pyropheophorbide did not alter the 8-isoprostane levels compared to 100 mM H2O2 (Fig. 4B).
α-Tocopherol, a well-known membrane-bound antioxidant, produced similar concentrations of 8-isoprostane as the DMSO control (Fig. 4C). The concentration of 8-iso-prostane produced when 1, 10, or 40 μM α-tocopherol was supplemented with 20 μM hypericin or 100 mM H2O2 did not differ from the concentrations produced by hypericin or H2O2 alone (Fig. 4C).
Pre-incubation of the cells with the extracts, pure compounds, or α-tocopherol for 4 h prior to the addition of 20 μM hypericin or 100 mM H2O2 and light exposure did not change the amount of 8-isoprostane produced (Fig. 4D) compared to the studies in which they were not pre-incubated (Fig. 4A–C).
4. Discussion
H. perforatum (Hp) contains two unique classes of compounds, the hyperforins and hypericins, as well as many other constituents common to most plant species, such as flavonoids, phenolic acids, and porphyrins [1–3]. As mentioned previously, many of these compounds have been shown to possess antioxidant as well as cytotoxic properties [14–16,18–20]. The mechanism currently thought to be responsible for the toxicity of hypericin involves the production of singlet oxygen and superoxide radicals upon light-activation [4,28–30]. The production of ROS by hypericin upon light activation can cause oxidative damage leading to cell death [4,28–30].
Due to the photoactivation of toxic properties of hypericin, and the penetration of light through skin, keratinocyrtes are one of the most relevant cell culture models for the assessment of hypericin’s phototoxicity. The phototoxicity of hypericin has been shown to be dose- and light-dependent in various cell culture studies, inducing apoptosis at lower concentrations of hypericin and light energy, whereas higher concentrations tend to induce necrotic cell death [4,12,16]. The threshold concentration of hypericin and/or light energy required to induce apoptotic versus necrotic cell death in cultured cells seems to be dependent upon several factors, including pre-incubation time before irradiation, treatment in cell culture media versus PBS or DMSO and the type of cell line [4,17,29,31,32]. However, attenuation of hypericin’s bioactivity by Hp constituents has, not, to our knowledge, been previously reported. Studies suggest that cultured skin cells, such as fibroblasts and keratinocytes, tend to be more resistant to oxidative stress [33], indicating that they may also be more resistant to the phototoxic properties of hypericin.
The light-induced toxicity of hypericin and Hp extracts containing the hypericin compounds have also been studied in human clinical trials for treatment purposes as well as safety assessment [8–11]. Based on these studies, it seems as though hypericin administered via Hp extracts may be less toxic [8,9] than administration of pure hypericin [10,11].
The fewer phototoxic side effects observed in clinical trials that used Hp extracts containing hypericin compared to those that used pure hypericin may be due in part to the antioxidant properties of other Hp constituents, such as flavonoids, phenolic acids, and porphyrins. These constituents may be able to elicit cellular protection by reducing the amount of reactive oxygen species generated by photo-induced hypericin, thus decreasing oxidative damage. Therefore, the goal of this study was to determine whether the phototoxicity exhibited by pure hypericin on HaCaT human keratinocytes was reduced when added to Hp extracts or pure compounds. The three Hp extracts used in this study were chosen based on their previously reported differences in light-sensitive toxicity and identified pure compounds [23]. In the prior studies we conducted dilution studies with these extracts demonstrating their non-toxic concentrations [23]. Since the attenuation observed in our cell culture model occurred within the toxic concentration range of these extracts, these concentrations were studied in the research reported in this paper. To determine whether the Hp extracts and constituents were able to reduce the phototoxicity induced by hypericin, much greater concentrations of pure hypericin were used than would be present in the blood or skin of individuals consuming therapeutic doses of Hp extracts. Studies on the bioavailability of Hp constituents reported peak plasma concentrations of 0.0062 μM hypericin following administration of dietary supplement extracts containing 600 μg hypericin [34], blood concentrations with toxic intakes would be expected to be higher. The lowest concentration of hypericin used in this study, 2 μM, reduced HaCaT keratinocyte growth by approximately 15%. Therefore 2–20 μM hypericin was used to induce appreciable amounts of cell damage in the keratinocytes and to determine whether the Hp extracts and constituents were able to reduce the phototoxicity and lipid peroxidation induced by these high concentrations of hypericin.
Several concentrations of each extract stock solution were used for the assessment of hypericin phototoxicity attenuation, but only the most effective concentration was shown for each extract. Neither of the ethanol extracts (ethanol or ethanol(-chloroform)) were able to significantly reduce the phototoxicity of pure hypericin, but the chloroform extract was effective at reducing the phototoxicity of hypericin. Even though the highest concentration of the ethanol(-chloroform) extract stock solution did not significantly reduce the phototoxicity of hypericin, its ability to maintain about 20% cell survival at the highest concentrations of hypericin when hypericin alone was killing all the cells suggested that it may moderately attenuate the phototoxicity of hypericin. The phototoxicity of the chloroform extract stock solution diluted to 10 μg/ml combined with concentrations of hypericin greater than 12 μM pure hypericin was significantly reduced compared to the phototoxicity of the hypericin alone. This suggested that the compounds in the 10 μg/ml concentration of the chloroform extract were capable of reducing the cytotoxicity produced by photo-activated hypericin. This attenuation may have occurred by multiple mechanisms including scavenging the free radicals produced by hypericin or competing for light energy, reducing the occurrence of triplet state hypericin.
The chloroform extract did not contain detectable concentrations of any of the known pure compounds, but it contained a peak observed by fluorometry that was identified as a porphyrin compound [27]. Porphyrins, which are derivatives of chlorophyll, are the only natural compounds that display a primary fluorescence emission peak around 680 nm and a shoulder around 725 nm [27]. High concentrations of these compounds are used as agents in PDT because they generate ROS upon photo-activation, but lower concentrations have also been shown to possess anti-oxidant properties [4,33].
The phototoxicity of pure hypericin combined with two different concentrations of pyropheophorbide, a porphyrin compound known to be present in green plants [27] and detected in the Hp ethanol and chloroform extracts, was significantly reduced compared to hypericin alone. These results suggest that the attenuation observed with the chloroform Hp extract may be due to its porphyrin constituents. However, the moderate reduction in the phototoxicity of hypericin observed with the ethanol(-chloroform) extract (which did not contain the porphyrin compound, but contained most of the known pure compounds) versus the significant attenuation observed with the chloroform extract, suggest that there may be more than one class of compounds present within Hp extracts capable of reducing the phototoxicity of photo-activated hypericin in the HaCaT keratinocytes.
Therefore, the phototoxicity of several individual pure compounds identified in the Hp extracts and known to possess antioxidant activity was assessed with and without hypericin supplementation to determine whether any one compound was capable of reducing the phototoxicity of hypericin. Despite the known antioxidant activity of the flavonoids [21], those tested in this study did not individually influence the phototoxicity of hypericin. However, chlorogenic acid (10 μM) and pyropheophorbide (0.25, and 0.5 μM) were able to significantly reduce the amount of phototoxicity produced by higher concentrations of hypericin. The next step was to assess the phototoxicity of several pure compounds identified within the Hp extracts combined together with and without an additional 15 μM hypericin to determine whether the phototoxicity of hypericin could be reduced when in the presence of several pure compounds known to possess antioxidant activity. Despite the inability of most of the individual pure compounds to induce phototoxicity in the HaCaT keratinocytes, the pure compounds combined together reduced cell survival by 51%. Therefore, the pure compounds induce a greater amount of toxicity when in combination than they exhibited individually, suggesting a potential synergistic effect on their ability to reduce cancer cell survival. Even though this combination of pure compounds included 10 μM chlorogenic acid, which was able to significantly reduce the phototoxicity of higher concentrations of hypericin, the combination of pure compounds with 15 μM hypericin killed almost all of the cells, indicating that they were not able to reduce the phototoxicity of pure hypericin. The inability of the combined compounds, which included several compounds known to possess antioxidant activity, to reduce the phototoxicity of 15 μM hypericin provides further evidence that oxidative stress may not be the only mechanism of toxicity associated with hypericin.
Although all of the pure compounds assessed with hypericin were known to possess antioxidant properties, only two were capable of significantly reducing the phototoxicity of hypericin in the keratinocytes. Therefore, a parameter of lipid peroxidation was also assessed to determine whether the extracts and pure compounds were capable of influencing a marker of oxidative stress induced by either photo-activated hypericin or hydrogen peroxide.
None of the extracts or individual compounds assessed consistently reduced the amount of arachidonic acid peroxidation produced by 20 μM hypericin or 100 mM H2O2 in the HaCaT keratinocytes. The chloroform extract, chlorogenic acid and pyropheophorbide each significantly reduced the phototoxicity exhibited by supplemented hypericin, thus, it may be assumed that the mechanism of toxicity associated with this compound is not entirely dependent upon oxidative damage. However, the general inability of these compounds to significantly reduce the peroxidation of arachidonic acid produced by H2O2 may also demonstrate the complexity surrounding oxidative damage and indicate that a single parameter of lipid peroxidation may not adequately explain the oxidative stress being inflicted on the keratinocytes.
The inability of 1–40 μM α-tocopherol to significantly reduce the amount of arachidonic acid peroxidation induced by H2O2 may have been due to the excessive concentration of H2O2 used and a greater reduction may have been observed by using either a lower concentration of H2O2 or a higher concentration of α-tocopherol.
In summary, the data gathered in this study demonstrated the ability of at least one Hp extract and two pure constituents to reduce the phototoxicity exhibited by pure hypericin in HaCaT keratinocytes. Since hypericin is known to produce vast quantities of reactive oxygen species upon photo-activation, it could be hypothesized that the observed reduction in phototoxicity was associated with a reduction in oxidative damage. In this study, hypericin treatment was immediately followed by light exposure, making the cellular membrane the most likely target for oxidative damage. Therefore, a parameter of lipid peroxidation, the formation of 8-isoprostane from the peroxidation of arachidonic acid, was assessed. Despite the ability of the chloroform extract and chlorogenic acid to significantly reduce the phototoxicity exhibited by pure hypericin, they were unable to influence the amount of arachidonic peroxidation induced by hypericin. However, these treatments were also unable to reduce the amount of peroxidation induced by hydrogen peroxide, a compound commonly used to induce oxidative damage. The lower concentration of pyropheophorbide significantly reduced the amount of lipid peroxidation induced by hydrogen peroxide, but the response was not dose-dependent.
These observations suggest that the complex chemical mixtures present in Hp extracts may be intricately acting together to elicit biological activities, including phototoxicity. The ability of Hp extracts and pure constituents to reduce the phototoxicity exhibited by hypericin appears to support observations made from human clinical trials that indicate phototoxic skin reactions are less frequent upon administration of Hp extracts versus administration of pure hypericin. The contradictory phototoxicity and lipid peroxidation observations made in this study suggest that the toxicity of hypericin is complex and may involve more than just oxidative damage. However, oxidative stress is also extremely complicated and may not be fully explained by a single parameter of lipid peroxidation.
Acknowledgments
The authors thank Dr. Timothy Bowden for graciously providing the HaCaT human keratinocytes for this study and Lindsay Headley and Prasun Mukherjee for their assistance with the fluorometric analysis. The authors acknowledge the gift of the Hypericum plant material from Frontier Herb®, Norway, Iowa. This publication was made possible by grant number P01 ES012020 from the National Institute of Environmental Health Sciences (NIEHS) and the Office of Dietary Supplements (ODS), NIH. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIEHS, NIH.
References
- 1.Bilia AR, Gallori S, Vincieri FF. St. John’s Wort and depression: efficacy, safety and tolerability – an update. Life Sci. 2002;70:3077–3096. doi: 10.1016/s0024-3205(02)01566-7. [DOI] [PubMed] [Google Scholar]
- 2.Greeson JM, Sanford B, Monti DA. St. John’s Wort (Hypericum perforatum): a review of the current pharmacological, toxicological, and clinical literature. Psychopharmacology (Berl) 2001;153:402–414. doi: 10.1007/s002130000625. [DOI] [PubMed] [Google Scholar]
- 3.Barnes J, Anderson LA, Phillipson JD. St John’s Wort (Hypericum perforatum L.): a review of its chemistry, pharmacology and clinical properties. J Pharm Pharmacol. 2001;53:583–600. doi: 10.1211/0022357011775910. [DOI] [PubMed] [Google Scholar]
- 4.Dougherty TJ. Photodynamic therapy. Adv Exp Med Biol. 1985;193:313–328. doi: 10.1007/978-1-4613-2165-1_30. [DOI] [PubMed] [Google Scholar]
- 5.Butterweck V. Mechanism of action of St John’s Wort in depression: what is known? CNS Drugs. 2003;17:539–562. doi: 10.2165/00023210-200317080-00001. [DOI] [PubMed] [Google Scholar]
- 6.Laakmann G, Schule C, Baghai T, Kieser M. St. John’s Wort in mild to moderate depression: the relevance of hyperforin for the clinical efficacy. Pharmacopsychiatry. 1998;31(Suppl 1):54–59. doi: 10.1055/s-2007-979346. [DOI] [PubMed] [Google Scholar]
- 7.Lenard J, Rabson A, Vanderoef R. Photodynamic inactivation of infectivity of human immunodeficiency virus and other enveloped viruses using hypericin and rose bengal: inhibition of fusion and syncytia formation. Proc Natl Acad Sci USA. 1993;90:158–162. doi: 10.1073/pnas.90.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schempp CM, Simon-Haarhaus B, Heine A, Schopf E, Simon JC. In vitro and in vivo activation of hypericin with the incoherent light source PDT 1200 SOA (520–750 nm) and with solar simulated radiation (290–2500 nm) Photodermatol Photoimmunol Photomed. 1999;15(21):13–17. doi: 10.1111/j.1600-0781.1999.tb00046.x. [DOI] [PubMed] [Google Scholar]
- 9.Schempp CM, Winghofer B, Muller K, Schulte-Monting J, Mannel M, Schopf E, Simon JC. Effect of oral administration of Hypericum perforatum extract (St. John’s Wort) on skin erythema and pigmentation induced by UVB, UVA, visible light and solar simulated radiation. Phytother Res. 2003;17:141–146. doi: 10.1002/ptr.1091. [DOI] [PubMed] [Google Scholar]
- 10.Gulick RM, McAuliffe V, Holden-Wiltse J, Crumpacker C, Liebes L, Stein DS, Meehan P, Hussey S, Forcht J, Valentine FT. Phase I studies of hypericin, the active compound in St. John’s Wort, as an antiretroviral agent in HIV-infected adults. AIDS Clinical Trials Group Protocols 150 and 258. Ann Intern Med. 1999;130:510–514. doi: 10.7326/0003-4819-130-6-199903160-00015. [DOI] [PubMed] [Google Scholar]
- 11.Jacobson JM, Feinman L, Liebes L, Ostrow N, Koslowski V, Tobia A, Cabana BE, Lee D, Spritzler J, Prince AM. Pharmacokinetics, safety, and antiviral effects of hypericin, a derivative of St. John’s Wort plant, in patients with chronic hepatitis C virus infection. Antimicrob Agents Chemother. 2001;45:517–524. doi: 10.1128/AAC.45.2.517-524.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kubin A, Wierrani F, Burner U, Alth G, Grunberger W. Hypericin – the facts about a controversial agent. Curr Pharm Des. 2005;11(1):233–253. doi: 10.2174/1381612053382287. [DOI] [PubMed] [Google Scholar]
- 13.Schempp CM, Winghofer B, Langheinrich M, Schopf E, Simon JC. Hypericin levels in human serum and interstitial skin blister fluid after oral single-dose and steady-state administration of Hypericum perforatum extract (St. John’s Wort) Skin Pharmacol Appl Skin Physiol. 1999;12(5):299–304. doi: 10.1159/000066256. [DOI] [PubMed] [Google Scholar]
- 14.Park J, English DS, Wannemuehler Y, Carpenter S, Petrich JW. The role of oxygen in the antiviral activity of hypericin and hypocrellin. Photochem Photobiol. 1998;68(5):593–597. [PubMed] [Google Scholar]
- 15.Schey KL, Patat S, Chignell CF, Datillo M, Wang RH, Roberts JE. Photooxidation of lens alpha-crystallin by hypericin (active ingredient in St. John’s Wort) Photochem Photobiol. 2000;72:200–203. doi: 10.1562/0031-8655(2000)072<0200:polcbh>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 16.Piette J, Volanti C, Vantieghem A, Matroule JY, Habraken Y, Agostinis P. Cell death and growth arrest in response to photodynamic therapy with membrane-bound photosensitizers. Biochem Pharmacol. 2003;66:1651–1659. doi: 10.1016/s0006-2952(03)00539-2. [DOI] [PubMed] [Google Scholar]
- 17.Vantieghem A, Assefa Z, Vandenabeele P, Declercq W, Courtois S, Vandenheede JR, Merlevede W, WP de, Agostinis P. Hypericin-induced photosensitization of HeLa cells leads to apoptosis or necrosis. Involvement of cytochrome c and procaspase-3 activation in the mechanism of apoptosis. FEBS Lett. 1998;440:19–24. doi: 10.1016/s0014-5793(98)01416-1. [DOI] [PubMed] [Google Scholar]
- 18.Lu YH, Du CB, Liu JW, Hong W, Wei DZ. Neuroprotective effects of Hypericum perforatum on trauma induced by hydrogen peroxide in PC12 cells. Am J Chin Med. 2004;32:397–405. doi: 10.1142/S0192415X04002053. [DOI] [PubMed] [Google Scholar]
- 19.Benedi J, Arroyo R, Romero C, Martin-Aragon S, Villar AM. Antioxidant properties and protective effects of a standardized extract of Hypericum perforatum on hydrogen peroxide-induced oxidative damage in PC12 cells. Life Sci. 2004;75:1263–1276. doi: 10.1016/j.lfs.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 20.Zheng W, Wang SY. Antioxidant activity and phenolic compounds in selected herbs. J Agric Food Chem. 2001;49:5165–5170. doi: 10.1021/jf010697n. [DOI] [PubMed] [Google Scholar]
- 21.Kawaii S, Tomono Y, Katase E, Ogawa K, Yano M. Antiproliferative activity of flavonoids on several cancer cell lines. Biosci Biotechnol Biochem. 1999;63:896–899. doi: 10.1271/bbb.63.896. [DOI] [PubMed] [Google Scholar]
- 22.Lawson JA, Rokach J, FitzGerald GA. Isoprostanes: formation, analysis and use as indices of lipid peroxidation in vivo. J Biol Chem. 1999;274(21):24441–24444. doi: 10.1074/jbc.274.35.24441. [DOI] [PubMed] [Google Scholar]
- 23.Schmitt LA, Liu Y, Murphy PA, Birt DF. Evaluation of the light-sensitive cytotoxicity of Hypericum perforatum extracts, fractions, and pure compounds. J Agric Food Chem. 2006;54:2881–2890. doi: 10.1021/jf052344k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Das K, Halder M, Chowdhury PK, Park J, Alexeev Y, Gordon MS, Petrich JW. Hypericin and its perylene quinone analogs: probing structure, dynamics, and interactions with the environment. Adv Photochem. 2005;28:1–26. [Google Scholar]
- 25.Venables WN, Ripley BD. Springer; New York: 2002. Modern Applied Statistics With S. [Google Scholar]
- 26.SAS Institute, Inc. Cary NC: SAS Institute; 2005. SAS/STAT@User’s Guide, Version 9.0. online-documentation. [Google Scholar]
- 27.Ashby KD, Wen J, Chowdhury P, Casey TA, Rasmussen MA, Petrich JW. Fluorescence of dietary porphyrins as a basis for real-time detection of fecal contamination on meat. J Agric Food Chem. 2003;51(1):3502–3507. doi: 10.1021/jf0211736. [DOI] [PubMed] [Google Scholar]
- 28.Chen H, Tappel AL. Protection by vitamin E selenium, trolox C, ascorbic acid palmitate, acetylcysteine, coenzyme Q, beta-carotene, canthaxanthin, and (+)-catechin against oxidative damage to liver slices measured by oxidized heme proteins. Free Radic Biol Med. 1994;16(3):437–444. doi: 10.1016/0891-5849(94)90120-1. [DOI] [PubMed] [Google Scholar]
- 29.Bernd A, Simon S, Ramirez BA, Kippenberger S, Diaz AJ, Miquel J, Villalba Garcia JF, Pamies MD, Kaufmann R. Phototoxic effectsof Hypericumextract in cultures of human keratinocytescompared with those of psoralen. Photochem Photobiol. 1999;69:218–221. doi: 10.1562/0031-8655(1999)069<0218:peoeic>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 30.Wills NJ, Park J, Wen J, Kesavan S, Kraus GA, Petrich JW, Carpenter S. Tumor cell toxicity of hypericin and related analogs. Photochem Photobiol. 2001;74:216–220. doi: 10.1562/0031-8655(2001)074<0216:tctoha>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 31.Hopfner M, Maaser K, Theiss A, Lenz M, Sutter AP, Kashtan HLB, von Riecken EO, Zeitz M, Scherubl H. Hypericin activated by an incoherent light source has photodynamic effects on esophageal cancer cells. Int J Colorectal Dis. 2003;18:239–247. doi: 10.1007/s00384-002-0440-5. [DOI] [PubMed] [Google Scholar]
- 32.Leccia MT, Richard MJ, Joanny-Crisci F, Beani JC. UV-A1 cytotoxicity and antioxidant defence in keratinocytes and fibroblasts. Eur J Dermatol. 1998;8:478–482. [PubMed] [Google Scholar]
- 33.Zhang M, Zhang Z, Blessington D, Li H, Busch TM, Madrak V, Miles J, Chance B, Glickson JD, Zheng G. Pyropheophorbide 2-deoxyglucosamide: a new photosensitizer targeting glucose transporters. Bioconjug Chem. 2003;14(1):709–714. doi: 10.1021/bc034038n. [DOI] [PubMed] [Google Scholar]
- 34.Schultz HU, Schurer M, Bassler D, Weiser D. Investigation of the bioavailability of hypericin, pseudohypericin, hyperforin and the flavonoids quercetin and isoharmnetin following single and multiple oral dosing of a hypericum extract containing tablet. Arzneimittelf-orschung. 2005;55:15–22. doi: 10.1055/s-0031-1296820. [DOI] [PubMed] [Google Scholar]


