Abstract
In the event of a bioterrorism attack using smallpox virus, there currently is no approved drug for the treatment of infections with this virus. We have reported previously that (S)-1-[3-hydroxy-2-(phosphonomethoxy)propyl]cytosine (HPMPC) (also known as cidofovir [CDV]) has good activity against poxvirus infections; however, a major limitation is the requirement for intravenous administration. Two related acyclic nucleoside phosphonates (ANPs), adefovir (PMEA) and tenofovir (PMPA), are active against human immunodeficiency virus or hepatitis B virus but do not have activity against the orthopoxviruses. Therefore, we have evaluated a number of analogs and potential oral prodrugs of these three compounds for their ability to inhibit the replication of vaccinia virus or cowpox virus in tissue culture cells. The most-active compounds within the CDV series were (S)-HPMPA and (butyl l-alaninyl) cyclic HPMPC, with 50% effective concentrations (EC50s) from 4 to 8 μM, compared with 33 to 43 μM for CDV. Although PMEA itself was not active, adefovir dipivoxil {bis[(pivaloyl)oxymethyl] PMEA} and bis(butyl l-alaninyl) PMEA were active against both viruses, and bis(butyl l-alaninyl) PME-N6-(cyclopropyl)DAP and (isopropyl l-alaninyl)phenyl PME-N6-(cyclopropyl)DAP were the most active compounds tested, with EC50s of 0.1 to 2.6 μM. In the PMPA series, none of the analogs tested had significantly better activity than PMPA itself. These data indicate that a number of these ANP derivatives have activity against vaccinia virus and cowpox virus in vitro and should be evaluated for their efficacies in animal models.
The success of the acyclic nucleoside phosphonate analogs (ANPs) as broad-spectrum antiviral agents with potent and selective activity in vitro and in vivo is the result of selective interactions of their diphosphate metabolite, which acts as both a competitive inhibitor and an alternative substrate with the viral DNA polymerase. The prototype compounds for this class of agents are (S)-1-[3-hydroxy-2-(phosphonomethoxy)propyl]cytosine (HPMPC) (also known as cidofovir [CDV]), 9-[2-(phosphonomethoxy)ethyl]adenine (PMEA) (also known as adefovir), and (R)-9-[2-(phosphonomethoxy) propyl]adenine (PMPA) (also known as tenofovir). CDV, which is the best-known member of this class of compounds, and its cyclic ester cHPMPC have potent and prolonged in vitro and in vivo activity against several herpesviruses, including herpes simplex virus types 1 and 2, varicella-zoster virus, cytomegalovirus, and Epstein-Barr virus (16, 17, 28). CDV is currently approved for treatment of cytomegalovirus retinitis in patients with AIDS (26, 32). In addition, CDV is a potent inhibitor of poxvirus replication in vitro (24, 25, 35) and has been shown to be very effective against both vaccinia virus (VV) and cowpox virus (CV) infections in animal models (7, 8, 19, 29, 33, 34; D. C. Quenelle, D. J. Collins, and E. R. Kern, submitted for publication). An investigational new drug application was approved in 2001 for the emergency treatment of smallpox. Potential nephrotoxicity (27) and poor oral bioavailability (14), however, may limit its widespread use in the event of a smallpox outbreak. The inhibitory effect of another ANP, PMEA, against retro-, herpes- and hepadnaviruses has been demonstrated (18, 36). The low oral bioavailability in animals and humans (14) of this compound, which is a common feature of ANPs, led to the development of an oral prodrug, adefovir dipivoxil [bis(pivaloyl)oxymethyl PMEA], which was recently approved for treatment of hepatitis B (M. B. McClellan, Letter, JAMA 288:2112, 2002). PMPA, another ANP, has demonstrated antiviral activity against retroviruses and hepadnaviruses (3, 18), with low cytotoxicity in a variety of human cell types (13). An oral prodrug of PMPA, tenofovir disoproxil fumarate {bis[(isopropoxycarbonyl)oxymethyl] PMPA}, which was approved in 2001 for the treatment of AIDS, has demonstrated potent antiviral efficacy and a favorable safety profile in these patients (5).
The effectiveness of this class of compounds as antiviral agents and the continuing need to discover and develop compounds which may prove useful against orthopoxvirus infections have led to our evaluation of a number of ANPs and their cyclic ester prodrugs for their ability to inhibit the replication of VV and CV in tissue culture cells. Neutralization of the negative charges on the phosphonyl function in ANPs by substitution with a lipophilic group(s) generally enhances ANP permeation through cellular membrane and oral bioavailability. For this purpose, the alkyloxycarbonylphenyl prodrugs of cHPMPC (30), lipophilic alkoxyalkyl esters of cHPMPC (6, 24), and bis(amidate) and aryl ester amidate prodrugs of ANPs (2, 11, 25) were investigated. In the latter two groups, phosphonamidates derived from alkyl l-alanine exhibit potent activities. The purpose of our studies was to determine the activity of a variety of the prodrugs of CDV, PMEA, or PMPA and/or related substituted ANPs against two orthopoxviruses, VV and CV. In our laboratory, a plaque reduction assay was utilized to determine the inhibitory activity (50% effective concentration [EC50]), while a neutral-red uptake assay was used for assessing compound cytotoxicity (50% cytotoxic concentration [CC50]). The selectivity index (SI), which expresses the activity of a compound by taking into account both its efficacy and cytotoxicity, was determined for a number of these compounds. The results of these studies will hopefully provide information regarding new active compounds that may also be active orally and could be candidates for development as new therapeutic agents for poxvirus infections.
MATERIALS AND METHODS
Compounds.
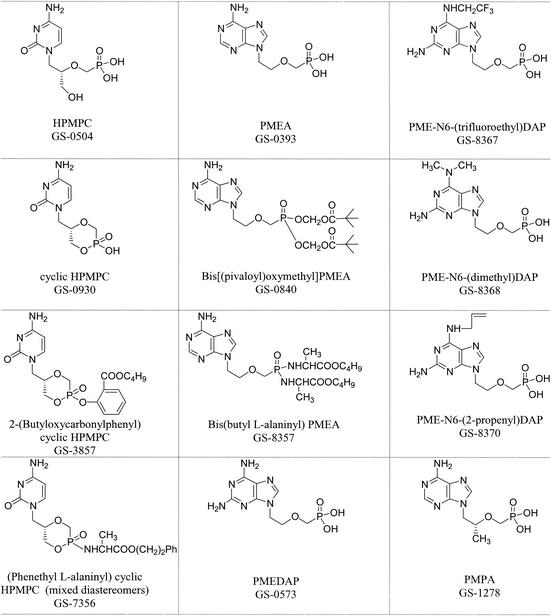
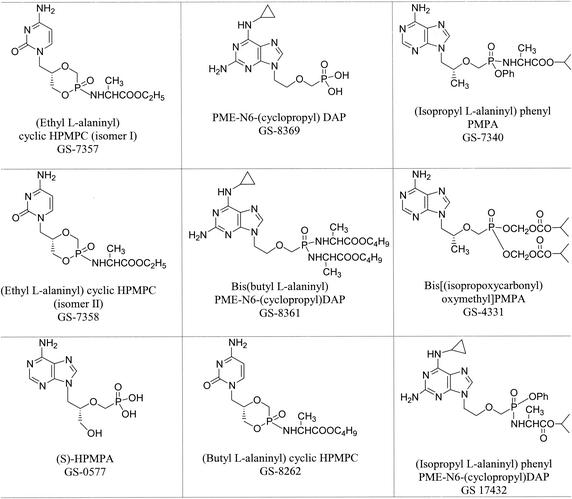
All compounds were provided by Gilead Sciences, Foster City, Calif., through the Antiviral Substances Program, National Institute of Allergy and Infectious Diseases, National Institutes of Health (Bethesda, Md.). They were prepared by procedures described elsewhere (2, 11, 12, 24, 30). N6-substituted PMEDAPs were prepared according to the method of Holý et al. (22). The chemical structures and descriptive names are presented in Fig. 1.
FIG. 1.
Chemical structures and descriptive names of ANPs.
Virus pools, media, and cells.
VV strain Copenhagen and CV strain Brighton stock pools were obtained from John Huggins of U.S. Army Medical Research Institute of Infectious Diseases, Frederick, Md. These pools had been prepared in Vero cells and were diluted in our laboratory to prepare the working stocks. VV strains WR and NYC were obtained from the American Type Culture Collection, Manassas, Va., and were propagated in human foreskin fibroblasts (HFF). These cells were prepared as primary cultures from freshly obtained newborn human foreskins (UAB or Brookwood Hospital, Birmingham, Ala.) as soon as possible after circumcision. Vero cells were obtained from the American Type Culture Collection. Culture medium for both cell lines was Eagle's minimal essential medium (EMEM) containing 10% fetal bovine serum (FBS) and standard concentrations of l-glutamine, penicillin, and gentamicin.
Plaque reduction assay (efficacy).
Two days (HFF) or 1 day (Vero) prior to use, cells were plated into six-well plates and incubated at 37°C with 5% CO2 and 90% humidity. On the day of assay, the drug was made up at twice the desired concentration in 2× EMEM with 10% FBS and diluted serially 1:5 in 2× EMEM to provide final concentrations of drug ranging from 100 to 0.032 μg/ml. The virus to be used was diluted in EMEM containing 10% FBS to a desired concentration which would give 20 to 30 plaques per well. The medium was then aspirated from the wells, and 0.2 ml of virus was added to each well in triplicate, with 0.2 ml of medium being added to drug and cell control wells. The plates were incubated for 1 h with shaking every 15 min. After the incubation period, an equal amount of 1% agarose was added to an equal volume of each drug dilution. The drug-agarose mixture was added to each well in 2-ml volumes, and the plates were incubated for 3 days, after which the cells were stained with a 0.02% solution of neutral red in phosphate-buffered saline (PBS). After a 5- to 6-h incubation period, the stain was aspirated and plaques were counted using a stereomicroscope at a magnification of ×10. The MacSynergy II (version 1) computer program was used to calculate the 50% effective concentration (EC50) value.
Neutral-red uptake assay (toxicity).
Twenty-four hours prior to assay, HFF were plated into 96-well plates at a concentration of 2.5 × 104 cells per well. After 24 h, the medium was aspirated and 125 μl of each drug concentration in EMEM with 2% FBS was added to the first row of wells and then diluted serially 1:5 using the Beckman BioMek Liquid Handling System. Final drug concentrations ranged from 100 to 0.032 μg/ml. The plates were incubated for 7 days in a CO2 incubator at 37°C, the medium-drug mixture was aspirated, 200 μl of 0.01% neutral red in PBS was added to each well, and the plates were incubated for 1 h. The dye was aspirated and the cells were washed with PBS using a Nunc plate washer. After removing the PBS, 200 μl of 50% ethanol-1% glacial acetic acid (in H2O) was added to each well. The plates were placed on a rotary shaker for 15 min, and the optical densities were read at 540 nm on a Bio-tek plate reader. The concentration of drug that reduced cell viability by 50% (CC50) was calculated using the software indicated previously.
RESULTS
In order to identify compounds with oral activity and reduced toxicity, we evaluated a number of ANPs that are either derivatives or prodrugs of the parent drugs CDV, PMEA, or PMPA. In these studies we evaluated the compounds for efficacy against VV and CV infections in vitro and also determined their cytotoxicity for human cells. In the first series of experiments, we determined the activity of a variety of CDV analogs (Table 1). CDV, cyclic CDV (cHPMPC) and 2-(butyloxycarbonyl) phenyl cHPMPC had similar EC50s and SI values for both VV and CV in HFF. As indicated from previous studies (16, 35), (S)-HPMPA was active against VV and, as presented in this study, was also effective against CV, exhibiting the most activity in this series, with EC50s of 3.5 and 5.0 μM and SI values of 77 and 54 for VV and CV, respectively. Both diastereomers of (ethyl l-alaninyl) cHPMPC (isomers I and II) showed virtually indistinguishable results, whereas (phenethyl l-alaninyl) cHPMPC (mixture of diastereomers) and (butyl l-alaninyl) cHPMPC were five- to sevenfold more active than HPMPC. All compounds were nontoxic or only slightly toxic in HFF at the concentrations tested.
TABLE 1.
Efficacies and cytotoxicities of HPMPC and related ANPs against VV and CV in HFF
| Compound | GS no.a | CC50 (μM)b | VV
|
CV
|
||
|---|---|---|---|---|---|---|
| EC50 (μM)b | SIc | EC50 (μM) | SI | |||
| HPMPC (CDV) | GS 0504 | 278 ± 9.2 | 33 ± 9.1 | 8.4 | 43 ± 2.5 | 6.5 |
| cHPMPC | GS 0930 | >302 ± 0 | 38 ± 11 | >7.9 | 48 ± 8.0 | >6.3 |
| (S)-HPMPA | GS 0577 | 269 ± 21 | 3.5 ± 2.8 | 77 | 5.0 ± 4.7 | 54 |
| 2-(Butyloxycarbonyl)phenylcHPMPC | GS 3857 | >213 ± 22 | 32 ± 13 | >6.7 | 34 ± 4.2 | >6.3 |
| (Butyl-l-alaninyl)cHPMPC | GS 8262 | >153 ± 57 | 4.6 ± 0.8 | >33 | 8.4 ± 5.3 | >18 |
| (Phenethyl-l-alaninyl)cHPMPC (mixed diastereomers) | GS 7356 | 207 ± 18 | 7.1 ± 0.3 | 29 | 6.8 ± 1.8 | 30 |
| (Ethyl-l-alaninyl)cHPMPC (isomer I) | GS 7357 | >276 ± 23 | 15 ± 13 | >18 | 27 ± 6.5 | >10 |
| (Ethyl-l-alaninyl)cHPMPC (isomer II) | GS 7358 | >278 ± 0 | 15 ± 11 | >19 | 26 ± 3.5 | >11 |
GS, Gilead Sciences.
Values are the means of two or more assays ± standard deviation.
SI = CC50/EC50.
Although PMEA was not active against VV or CV, all other related ANP prodrugs tested exhibited activity against VV as seen in Table 2. Bis[(pivaloyl)oxymethyl] PMEA (adefovir dipivoxil) and a bis(butyl l-alaninyl) PMEA were both active, with EC50s of 4.4 to 13 μM. Similarly, the 2,6-diaminopurine analog of PMEA (PMEDAP) was inactive, whereas N6-cyclopropyl (GS 8369), N6-(2-propenyl) (GS 8370), N6-(trifluoroethyl) (GS 8367), and N6-(dimethyl) (GS 8368) derivatives were efficacious against VV, but less active against CV. Of particular interest were the results obtained with bis(butyl l-alaninyl) PME-N6-(cyclopropyl)DAP (GS 8361) and (isopropyl l-alaninyl) phenyl PME-N6-(cyclopropyl)DAP (GS 17432). Antiviral activity was greatest for bis(butyl l-alaninyl) PME-N6-(cyclopropyl)DAP (GS 8361) (EC50 of 0.08 μM for VV and 0.26 μM for CV) with some cytotoxicity (CC50 of 49 μM) but had SI values of 613 for VV and 189 for CV. The ANP (isopropyl l-alaninyl) phenyl PME-N6-(cyclopropyl)DAP (GS 17432) was very active against both VV and CV, with a lesser degree of cytotoxicity, and SI values of >190 against VV and >80 against CV. Not unexpectedly, the compounds with the best antiviral activities were generally the most toxic.
TABLE 2.
Efficacies and cytotoxicities of PMEA and related ANPs against VV and CV in HFF
| Compound | GS no.a | CC50 (μM)b | VV
|
CV
|
||
|---|---|---|---|---|---|---|
| EC50 (μM)b | SIc | EC50 (μM) | SI | |||
| PMEA | GS 0393 | >366 ± 0 | >366 ± 0 | — | >366 ± 0 | — |
| Bis[(pivaloyl)oxymethyl]PMEA | GS 0840 | 117 ± 27 | 5.1 ± 0.7 | 23 | 13 ± 8.8 | 9.0 |
| Bis (butyl-l-alaninyl)PMEA | GS 8357 | 100 ± 27 | 4.4 ± 0.2 | 23 | 10 ± 8.2 | 10 |
| PMEDAP | GS 0573 | >339 ± 12 | 204 ± 15 | 1.7 | >347 ± 0 | — |
| PME-N6-(cyclopropyl)DAP | GS 8369 | >263 ± 59 | 23 ± 6.9 | >11 | 28 ± 13 | >9.4 |
| Bis(butyl-l-alaninyl)PME-N6-(cyclopropyl)DAP | GS 8361 | 49 ± 33 | 0.08 ± 0.01 | 613 | 0.26 ± 0.2 | 189 |
| (Isopropyl-l-alaninyl)phenyl-PME-N6-(cyclopropyl)DAP | GS 17432 | >209 ± 69 | 1.1 ± 0.3 | >190 | 2.6 ± 1.9 | >80 |
| PME-N6-(trifluoroethyl)DAP | GS 8367 | >270 ± 0 | 42 ± 11 | >6.4 | >270 ± 0 | — |
| PME-N6-(dimethyl)DAP | GS 8368 | >316 ± 0 | 35 ± 1.6 | >9.0 | 53 ± 8.3 | >6.0 |
| PME-N6-(2-propenyl)DAP | GS 8370 | >305 ± 0 | 25 ± 0.9 | >12 | 115 ± 78 | >2.7 |
GS, Gilead Sciences.
Values are the means of two or more assays ± standard deviation.
SI = CC50/EC50.
PMPA and its oral prodrug tenofovir disoproxil fumarate {bis[(isopropoxycarbonyl)oxymethyl] PMPA}, were both inactive against VV and CV replication. In this case, the analogous prodrug (isopropyl l-alaninyl) phenyl PMPA was only marginally active, with SI values of >6.1 and >1.4 for VV and CV, respectively (Table 3).
TABLE 3.
Efficacies and cytotoxicities of PMPA and related ANPs against VV and CV in HFF
| Compound | GS no.a | CC50 (μM) | VV
|
CV
|
||
|---|---|---|---|---|---|---|
| EC50 (μM) | SIb | EC50 (μM) | SIb | |||
| PMPA | GS 1278 | >300 | >300 | — | >300 | — |
| Bis[(isopropoxycarbonyl)oxymethyl]PMPA | GS 4331 | >157.4 | >157.4 | — | >157.4 | — |
| (Isopropyl-l-alaninyl)phenyl PMPA | GS 7340 | >143 | 23.5 | >6.1 | 98.9 | >1.4 |
GS, Gilead Sciences.
SI = CC50/EC50.
In the event that VV Copenhagen is not a representative VV strain for antiviral evaluation, active compounds were also tested against two additional strains of VV (Table 4). This could not be done for CV since Brighton is the only strain available. The compounds CDV, (S)-HPMPA, bis(butyl l-alaninyl) PMEA (GS 8357), bis(butyl l-alaninyl) PME-N6-(cyclopropyl)DAP (GS 8361), and, to a slightly lesser extent, PME-N6-(dimethyl)DAP (GS 8368) and PME-N6-(2-propenyl)DAP (GS 8370) had essentially identical EC50s for all three strains of VV. For the remaining compounds, EC50s were comparable for at least two strains, most often the WR and NYC strains. Interestingly, in several of these instances, the EC50 was lower for the Copenhagen strain, lending credence to the use of this strain in screening assays.
TABLE 4.
Activities of ANPs against VV in HFF
| Compound | GS no.a | EC50 (μM) for VV strain:
|
||
|---|---|---|---|---|
| Copenhagen | WR | NYC | ||
| HPMPC (CDV) | GS 0504 | 33 | 32 | 36 |
| cHPMPC | GS 0930 | 38 | 46 | 16 |
| (S)-HPMPA | GS 0577 | 3.5 | 7.6 | 5.9 |
| 2-(Butyloxycarbonyl)phenyl-cHPMPC | GS 3857 | 32 | 11 | 24 |
| (Phenethyl l-alaninyl)cHPMPC (mixed diastereomers) | GS 7356 | 7.1 | 15 | 16 |
| (Ethyl-l-alaninyl)cHPMPC (isomer I) | GS 7357 | 15 | 27 | 27 |
| (Ethyl-l-alaninyl)cHPMPC (isomer II) | GS 7358 | 15 | 31 | 28 |
| Bis[(pivaloyl)oxymethyl]PMEA | GS 0840 | 5.1 | 18.5 | 23 |
| Bis(butyl-l-alaninyl)PMEA | GS 8357 | 4.4 | 4.4 | 5.1 |
| PMEDAP | GS 0573 | 204 | 128 | 122 |
| PME-N6-(cyclopropyl)DAP | GS 8369 | 23 | 18 | 12 |
| Bis(butyl-l-alaninyl)PME-N6-(cyclopropyl)DAP | GS 8361 | 0.08 | 0.12 | 0.14 |
| PME-N6-(trifluoroethyl)DAP | GS 8367 | 42 | 38 | 50 |
| PME-N6-(dimethyl)DAP | GS 8368 | 35 | 22 | 34 |
| PME-N6-(2-propenyl)DAP | GS 8370 | 25 | 23 | 16 |
GS, Gilead Sciences.
Selected compounds were also tested in Vero cells for comparison purposes since our testing is performed in HFF, while Vero cells appear to be the host cells of choice in other laboratories. Similar results were seen for all compounds tested in both cell lines. Compounds found to be active or inactive in HFF were found to be correspondingly active or inactive in VERO cells (data not presented). We have reported previously similar results with a series of other antiviral agents (24), which further supports the use of human cells in evaluating antiviral compounds.
DISCUSSION
The cessation of routinely vaccinating the population against smallpox after the global eradication of that disease more than 20 years ago (9) has left the population vulnerable to the deliberate use of smallpox as a biological weapon or to an unanticipated spread of an indigenous agent like monkeypox virus to other parts of the world (10, 23). The potential threat of such occurrences has led to the search for antiviral therapy that could be effective and deployed rapidly. Compounds such as ribavirin, interferon, and idoxuridine, which have been used to treat such diseases as hepatitis C, respiratory syncytial virus in infants, multiple myeloma, and herpes simplex virus infections of the inner eyelid and corneas (20, 21, 31), have been identified as having some activity against poxviruses (15, 19). Unfortunately, none of these compounds are good candidates for further development and use against poxvirus infections for a variety of reasons, including lack of clear efficacy, toxicity, or availability. Consequently, there is a continued need to develop new and better modes of therapy for poxvirus infection.
Currently, the drug of choice for the treatment of orthopoxvirus infections is CDV. It has relatively good activity against all poxviruses tested including monkeypox virus and variola virus (1) and also has good activity in animal models using VV and CV (7, 8, 19, 29, 33, 34; D. J. Collins, D. C. Quenelle, and E. R. Kern, Program Abstr. 14th Int Conf. Antivir. Res. 50:A70, 2001; Quenelle et al., submitted). A noted limitation to the use of CDV is the potential for nephrotoxicity observed in some patients during the treatment of cytomegalovirus retinitis. Toxicity may be of lesser consequence for poxvirus infections, however, due to infrequent dosing over a short duration. Of more probable importance is the necessity of giving the drug intravenously because of its lack of activity after oral administration due to poor absorption. However, recent studies suggest that this drug is very effective when administered by aerosol to animals before or after infection with aerosolized CV (8). These data indicate that aerosolized CDV may be useful for prophylaxis or early postexposure treatment of orthopoxvirus infections.
Since the major limitation to the benefit of CDV for the use in the emergency treatment of smallpox is its lack of oral activity, new investigations have focused on chemically modifying CDV so that it has better oral absorption, distribution, and penetration into critical target organs. One such approach has been to prepare ether lipid analogs of CDV or cyclic CDV. These analogs have been shown to increase antiviral activity to poxviruses (24) and herpesviruses (6) several fold. In addition, the analogs are effective orally in rodents and have superior tissue distribution over CDV (K. L. Winegarden, S. L. Ciesla, K. A. Aldern, J. R. Beadle, and K. Y. Hostetler, Program Abstr. 15th Int. Conf. Antivir. Res. 53:A67, 2002).
An alternative approach used in these studies was to determine the activity of other ANPs as well as a series of their prodrugs synthesized to enhance their oral absorption. The best compound identified in our studies was adefovir dipivoxil. It was recently approved for use in hepatitis B virus infections and is available commercially. Importantly, it is active when given orally and appears to be relatively nontoxic (4). Although it is quite active against the orthopoxviruses in vitro, it still needs to be evaluated in murine and nonhuman primate models of poxvirus infections. Some other compounds identified in these studies as potential antiviral agents for poxvirus infections are the prodrugs of cHPMPC and PMEDAP. In particular, bis(butyl-l-alaninyl)PME-N6-(cyclopropyl)DAP (GS 8361) was the most active of all the phosphonate nucleotides in tissue culture that we have tested. Animal model studies to determine efficacy as well as its metabolic and pharmacokinetic properties need to be carried out on these and other compounds before their potential for use in treatment of poxvirus infections in humans is known.
The results of these studies indicate that many of the nucleoside phosphonates have potent and selective activity against orthopoxvirus infections. In particular, adefovir dipivoxil, which is active and orally bioavailable and is already approved for use in humans, should be considered a high priority for further evaluation as a treatment for smallpox and complications of VV vaccination.
Acknowledgments
These studies were supported by Public Health Service contract NO1-AI-85347 from the Antiviral Research Branch, NIAID, NIH, to the University of Alabama at Birmingham (E.R.K.), and, as a part of research project of the Institute of Organic Chemistry and Biochemistry (Prague, Czech Republic) (4055905), it was supported by the program of targeted projects of the Academy of Sciences of the Czech Republic (S4055109) (A.H.).
REFERENCES
- 1.Baker, R. O., M. Bray, and J. W. Huggins. 2002. Potential antiviral therapeutics for smallpox, monkeypox and other orthopoxvirus infections. Antivir. Res. 57:13-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ballatore, C., C. McGuigan, E. De Clercq, and J. Balzarini. 2001. Synthesis and evaluation of novel amidate prodrugs of PMEA and PMPA. Bioorg. Med. Chem. Lett. 11:1053-1056. [DOI] [PubMed] [Google Scholar]
- 3.Balzarini, J., S. Aquaro, C.-F. Perno, M. Witvrouw, A. Holý, and E. De Clercq. 1996. Activity of the (R)-enantiomers of 9-(2-phosphonylmethoxypropyl)-adenine and 9-(2-phosphonylmethoxypropyl)-2,6-diaminopurine against human immunodeficiency virus in different human cell systems. Biochem. Biophys. Res. Commun. 219:337-341. [DOI] [PubMed] [Google Scholar]
- 4.Barditch-Crovo, P., J. Toole, C. W. Hendrix, K. C. Cundy, D. Ebeling, H. S. Jaffe, and P. S. Lietman. 1997. Anti-human immunodeficiency virus (HIV) activity, safety, and pharmacokinetics of adefovir dipivoxil (9-[2-(bis-pivaloyloxymethyl)-phosphonyl-methoxyethyl]adenine) in HIV-infected patients. J. Infect. Dis. 176:406-413. [DOI] [PubMed] [Google Scholar]
- 5.Barditch-Crovo, P., S. G. Deeks, A. Collier, S. Safrin, D. F. Coakley, M. Miller, B. P. Kearney, R. L. Coleman, P. D. Lamy, J. O. Kahn, I. McGowan, and P. S. Lietman. 2001. Phase I/II trial of the pharmacokinetics, safety, and antiretroviral activity of tenofovir disoproxil fumarate in human immunodeficiency virus-infected adults. Antimicrob. Agents Chemother. 45:2733-2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beadle, J. R., C. Hartline, K. A. Aldern, N. Rodriguez, E. Harden, E. R. Kern, and K. Y. Hostetler. 2002. Alkoxyalkyl esters of cidofovir and cyclic cidofovir exhibit multiple-log enhancement of antiviral activity against cytomegalovirus and herpesvirus replication in vitro. Antimicrob. Agents Chemother. 46:2381-2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bray, M., M. Martinez, D. F. Smee, D. Kefauver, E. Thompson, and J. W. Huggins. 2000. Cidofovir protects mice against lethal aerosol or intranasal cowpox virus challenge. J. Infect. Dis. 181:10-19. [DOI] [PubMed] [Google Scholar]
- 8.Bray, M., M. Martinez, D. Kefauver, M. West, and C. Roy. 2002. Treatment of aerosolized cowpox virus infection in mice with aerosolized cidofovir. Antivir. Res. 54:129-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Breman, J. G., and I. Arita. 1980. The confirmation and maintenance of smallpox eradication. N. Engl. J. Med. 303:1263-1273. [DOI] [PubMed] [Google Scholar]
- 10.Breman, J. G., and D. A. Henderson. 1998. Poxvirus dilemmas—monkeypox, smallpox, and biologic terrorism. N. Engl. J. Med. 339:556-559. [DOI] [PubMed] [Google Scholar]
- 11.Chapman, H., M. Kernan, E. Prisbe, J. Rohloff, M. Sparacino, T. Terhorst, and R. Yu. 2001. Practical synthesis, separation, and stereochemical assignment of the PMPA pro-drug GS-7340. Nucleosides Nucleotides Nucleic Acids 20:621-628. [DOI] [PubMed] [Google Scholar]
- 12.Chapman, H., M. Kernan, J. Rohloff, M. Sparacino, and T. Terhorst. 2001. Purification of PMPA amidate prodrugs by SMB chromatography and x-ray crystallography of the diastereomerically pure GS-7340. Nucleosides Nucleotides Nucleic Acids 20:1085-1090. [DOI] [PubMed] [Google Scholar]
- 13.Cihlar, T., G. Birkus, D. E. Greenwalt, and M. J. M. Hitchcock. 2002. Tenofovir exhibits low cytotoxicity in various human cell types: comparison with other nucleoside reverse transcriptase inhibitors. Antivir. Res. 54:37-45. [DOI] [PubMed] [Google Scholar]
- 14.Cundy, K. C. 1999. Clinical pharmacokinetics of the antiviral nucleotide analogues cidofovir and adefovir. Clin. Pharmacokinet. 36:127-143. [DOI] [PubMed] [Google Scholar]
- 15.De Clercq, E., M. Luczak, D. Shugar, P. F. Torrence, J. A. Waters, and B. Witkop. 1976. Effect of cytosine, arabinoside, iododeoxyuridine, ethyldeoxyuridine, thiocyanatodeoxyuridine, and ribavirin on tail lesion formation in mice infected with vaccinia virus. Proc. Soc. Exp. Biol. Med. 151:487-490. [DOI] [PubMed] [Google Scholar]
- 16.De Clercq, E., T. Sakuma, M. Baba, R. Pauwels, J. Balzarini, I. Rosenberg, and A. Holý. 1987. Antiviral activity of phosphonylmethoxyalkyl derivatives of purine and pyrimidines. Antivir. Res. 8:261-272. [DOI] [PubMed] [Google Scholar]
- 17.De Clercq, E. 1993. Antivirals for the treatment of herpesvirus infections. J. Antimicrob. Chemother. 32:121-132. [DOI] [PubMed] [Google Scholar]
- 18.De Clercq, E. 1997. Acyclic nucleoside phosphonates in the chemotherapy of DNA virus and retrovirus infections. Intervirology 40:295-303. [DOI] [PubMed] [Google Scholar]
- 19.De Clercq, E. 2001. Vaccinia virus inhibitors as a paradigm for the chemotherapy of poxvirus infections. Clin. Microbiol. Rev. 14:382-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gorschlüter, M., C. Ziske, A. Glasmacher, and I. G. H. Schmidt-Wolf. 2001. Current clinical and laboratory strategies to augment the efficacy of immunotherapy in multiple myeloma. Clin. Cancer Res. 7:2195-2204. [PubMed] [Google Scholar]
- 21.Guerguerian, A.-M., M. Gauthier, M. H. Lebel, C. A. Farrell, and J. Lacroix. 1999. Ribavirin in ventilated respiratory syncytial virus bronchiolitis. Am. J. Respir. Crit. Care Med. 160:829-834. [DOI] [PubMed] [Google Scholar]
- 22.Holý, A., I. Votruba, E. Tloustová, and M. Masojídková. 2001. Synthesis and cytostatic activity of N-[2-(phosphonomethoxy)alkyl] derivatives of N6-substituted adenines, -2,6-diaminopurines and related compounds. Collect. Czech. Chem. Commun. 66:1545-1592. [Google Scholar]
- 23.Hutin, Y. J. F., R. J. Williams, P. Malfait, R. Pebody, V. N. Loparev, S. L. Ropp, M. Rodriguez, J. C. Knight, F. K. Tshioko, A. S. Khan, M. V. Szczeniowski, and J. J. Esposito. 2001. Outbreak of human monkeypox, Democratic Republic of Congo, 1996 to 1997. Emerg. Infect. Dis. 7:434-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kern, E. R., C. Hartline, E. Harden, K. Keith, N. Rodriguez, J. R. Beadle, and K. Y. Hostetler. 2002. Enhanced inhibition of orthopoxvirus replication in vitro by alkoxyalkyl esters of cidofovir and cyclic cidofovir. Antimicrob. Agents Chemother. 46:991-995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kern, E. R. 2003. In vitro activity of potential anti-poxvirus agents. Antivir. Res. 57:35-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lalezari, J. P., R. J. Stagg, B. D. Kuppermann, G. N. Holland, F. Kramer, D. V. Ives, M. Youle, M. R. Robinson, W. L. Drew, and H. S. Jaffe. 1997. Intravenous cidofovir for peripheral cytomegalovirus retinitis in patients with AIDS. A randomized, controlled trial. Ann. Intern. Med. 126:257-263. [DOI] [PubMed] [Google Scholar]
- 27.Meier, P., S. Dautheville-Guibal, P. M. Ronco, and J. Rossert. 2002. Cidofovir-induced end-stage renal failure. Nephrol. Dial. Transplant. 17:148-149. [DOI] [PubMed] [Google Scholar]
- 28.Naesens, L., R. Snoeck, G. Andrei, J. Balzarini, J. Neyts, and E. De Clercq. 1997. HPMPC (cidofovir), PMEA (adefovir) and related acyclic nucleoside phosphonate analogues: a review of their pharmacology and clinical potential in the treatment of viral infections. Antivir. Chem. Chemother. 8:1-23. [Google Scholar]
- 29.Neyts, J., and E. DeClercq. 1993. Efficacy of (S)-1-(3-hydroxy-2-phosphonyl methoxypropyl)cytosine for the treatment of lethal vaccinia virus infections in severe combined immune deficiency (SCID) mice. J. Med. Virol. 41:242-246. [DOI] [PubMed] [Google Scholar]
- 30.Oliyai, R., M. N. Arimilli, R. J. Jones, and W. A. Lee. 2001. Pharmacokinetics of salicylate ester prodrugs of cyclic HPMPC in dogs. Nucleosides Nucleotides Nucleic Acids 20:1411-1414. [DOI] [PubMed] [Google Scholar]
- 31.Reichard, O., J. Andersson, R. Schvarcz, and O. Weiland. 1991. Ribavirin treatment for chronic hepatitis C. Lancet 337:1058-1061. [DOI] [PubMed] [Google Scholar]
- 32.Safrin, S., J. Cherrington, and H. S. Jaffe. 1999. Cidofovir. Review of current and potential clinical uses. Adv. Exp. Med. Biol. 458:111-120. [PubMed] [Google Scholar]
- 33.Smee, D. F., K. W. Bailey, M. Wong, and R. W. Sidwell. 2000. Intranasal treatment of cowpox virus respiratory infections in mice with cidofovir. Antivir. Res. 47:171-177. [DOI] [PubMed] [Google Scholar]
- 34.Smee, D. F., K. W. Bailey, and R. W. Sidwell. 2001. Treatment of lethal vaccinia virus respiratory infections in mice with cidofovir. Antivir. Chem. Chemother. 12:71-76. [DOI] [PubMed] [Google Scholar]
- 35.Snoeck, R., A. Holý, C. Dewolf-Peeters, J. Van Den Oord, E. De Clercq, and G. Andrei. 2002. Antivaccinia activities of acyclic nucleoside phosphonate derivatives in epithelial cells and organotypic cultures. Antimicrob. Agents Chemother. 46:3356-3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yokota, T., S. Mochizuki, K. Konno, S. Mori, S. Shigeta, and E. De Clercq. 1991. Inhibitory effects of selected antiviral compounds on human hepatitis B virus DNA synthesis. Antimicrob. Agents Chemother. 35:394-397. [DOI] [PMC free article] [PubMed] [Google Scholar]