Abstract
While chronic hypoglycaemia has been reported to increase unidirectional glucose transport across the blood-brain barrier (BBB) and to increase GLUT1 expression at the endothelium, the effect on steady-state brain d-glucose and brain glycogen content is currently unknown. Brain glucose and glycogen concentrations were directly measured in vivo using localized 13C magnetic resonance spectroscopy (MRS) following 12-14 days of hypoglycaemia. Brain glucose content was significantly increased by 48%, which is consistent with an increase in the maximal glucose transport rate, Tmax, by 58% compared with the sham-treated animals. The localized 13C NMR measurements of brain glucose were directly validated by comparison with biochemically determined brain glucose content after rapid focused microwave fixation (1.4 s at 4 kW). Both in vivo MRS and biochemical measurements implied that brain glycogen content was not affected by chronic hypoglycaemia, consistent with brain glucose being a major factor controlling brain glycogen content. We conclude that the increased glucose transporter expression in chronic hypoglycaemia leads to increased brain glucose content at a given level of glycaemia. Such increased brain glucose concentrations can result in a lowered glycaemic threshold of counter-regulation observed in chronic hypoglycaemia.
Keywords: chronic hypoglycaemia, glucose content, glucose transport kinetics, glycogen, localized 13C magnetic resonance sprectroscopy, rat brain
The maintenance of an adequate supply of glucose from blood across the blood-brain barrier (BBB) is important for generating the energy necessary to support normal brain function. It is generally accepted that glucose content is not rate-limiting for metabolism in the brain. When considering the transport steps, i.e. transport across the endothelial membranes constituting the BBB and cellular membranes inside the brain, transport of glucose across the BBB is considered rate-limiting (Gjedde 1980). Given the importance of glucose transport for normal brain function, an understanding of its physiology, mechanism and alterations are of crucial importance to the comprehension of the maintenance of normal brain function. For example, brain glucose transport/concentration has been reported to be increased in chronic hypoxia (Harik et al. 1994, 1995). The study of glucose transport has received considerable attention due to its putative implication in the cerebral mechanism of sensing plasma glucose levels (McCall 2004).
Glucose sensing is accomplished by specialized neurones (Routh 2003) and is impaired in diabetes as manifested, for example, by a progressive inability to appropriately release counter-regulatory hormones in response to antecedent (and recurring) hypoglycaemia (Dagogo-Jack et al. 1993; Evans et al. 2000; McCrimmon et al. 2003; Cryer 2004), which is considered a limiting factor for glycaemic control in Type I diabetes (Cryer 1994, 1999).
There are several mechanisms that may account for impaired glucose sensing in the brain as a result of hypoglycemia (Cryer 2005), one of which is an increase in glucose transport at the BBB (McCall et al. 1986), supported by changes in brain microvascular GLUT1 protein (Kumagai et al. 1995; Simpson et al. 1999).
Many elegant studies, including autoradiography (Gjedde and Diemer 1983), positron emission tomography (PET) (Herholz et al. 1989; Hasselbalch et al. 1999) and microdialysis (Borg et al. 1999; McCrimmon et al. 2003), have studied the mechanism of altered glucose sensing from the BBB glucose transport perspective, with mixed results. Over the past 10 years, we have developed in vivo 1H and 13C magnetic resonance spectroscopy (MRS) approaches to determine localized glucose transport kinetics, at steady state, from the measurement of brain glucose content in rodents (Choi et al. 2001, 2002) and humans (Gruetter et al. 1998; Seaquist et al. 2001; Criego et al. 2005). The unique capability of localized 13C MRS to assess brain carbohydrates permits the measurement of the metabolism and concentration of glucose and glycogen simultaneously, in a single subject, under physiological conditions. For example, we recently reported that brain glycogen, the single most concentrated storage of glucose equivalents in the brain, can be used as an emergency reservoir during acute hypoglycaemia (Choi et al. 2003). Furthermore, it was shown that supercompensation of brain glycogen occurred following a single episode of hypoglycaemia. This higher glycogen content was implicated in the syndrome of hypoglycaemia unawareness that occurs following a single episode of hypoglycaemia (Cryer 2001; Choi et al. 2003; Gruetter 2003; Oz et al. 2003). The rationale for the present study was that while brain glycogen is a significant source of glucose equivalents in experimental moderate hypoglycaemia (Choi et al. 2003), it is unlikely to sustain glucose supply deficits during chronic hypoglycaemia and thus, is not likely to explain the alterations in counter-regulation observed in, for example, insulinoma patients (Davis and Shamoon 1991) or patients with recurrent hypoglycaemia (Boyle et al. 1994).
We therefore sought to determine the effect of chronic hypoglycaemia on brain glucose concentrations at different steady-state plasma glucose levels and, concurrently, to assess the effect of chronic hypoglycaemia on brain glycogen concentration. We tested the specific hypothesis that the previously reported up-regulation of glucose transport and transporter expression in chronic hypoglycaemia is accompanied by increased brain glucose content in a manner consistent with these alterations in gene expression. Therefore, the purpose of this study was three-fold:first, to assess brain glucose content over a wide range of steady-state plasma glucose and to determine steady-state glucose transport kinetics in chronically hypoglycaemic rats using 13C MRS; second, to validate the in vivo NMR quantification of brain glucose using a standard biochemical assay; third, to determine the effect of chronic hypoglycaemia on total brain glycogen content.
Experimental procedures
Animal model of chronic hypoglycemia
All studies were performed according to the guidelines for the care and use of laboratory animals at the University of Minnesota and approved by the Institutional Animal Care and Use Committee (IACUC). Hypoglycaemia was induced in 18 male Sprague-Dawley rats (weight = 182 ± 18 g, mean ± SD) on day 0 by subcutaneous implantation of insulin pellets (6-8 IU/day; Linshin Canada Inc., Ontario, Canada) under light isoflurane anaesthesia (Simpson et al. 1999). On subsequent days, animals were considered hypoglycaemic as judged from morning plasma glucose values that were below 3mm, measured in spun-down plasma glucose samples obtained from tail bleeds (average plasma glucose 2.7 ± 0.2 mm, mean ± SD). An extra insulin pellet (2 IU/day) was administered to maintain hypoglycaemia. All glucose measurements were obtained using a glucose analyser (Analox GM7, Analox Instruments, Lunenburg, MA, USA). NMR studies were carried out on animals that had been in a hypoglycaemic condition for 12-14 days. A sham-treated group (average plasma glucose 10.7 ± 0.8 mm, mean ± SD, n = 13) received an equal number of blank pellets (Linshin Canada Inc.) that contained no insulin and were otherwise treated identically. In order to obtain brain glucose and glycogen contents at low plasma glucose concentrations without 13C glucose infusion, seven animals from each group were killed and biochemically analysed without further NMR studies, as described below.
All animals were on a regular light/dark cycle (06:00/18:00 hours) and had access to standard chow and water ad libitum daily, except for the sham-treated animals, which were fasted 16-18 h prior to the NMR and biochemical measurements. All NMR experiments and non-NMR biochemical measurements were started during the daylight cycle and were randomly distributed into two starting times at approximately 10:00 and 14:00 hours.
Animal preparation and handling
On the day of the NMR experiment, animals were anaesthetised with 2% isoflurane in a 2: 1 mixture of nitrous oxide (N2O) and oxygen (O2), and ventilated with a pressure-driven ventilator (Kent Scientific, Litchfield, CT, USA). Once vascular access was secured, anaesthesia was switched from isoflurane to α-chloralose, administrated with a 40 mg/kg bolus, followed 30 min later by a continuous intravenous infusion of approximately 26.7 mg/kg/h. A bolus of 99% enriched [1-13C]-d-glucose (20% weight/volume solution; Isotec Inc., Miamisburg, OH, USA and CIL Inc., Andover, MA, USA) was administered, followed by a variable rate infusion to achieve target steady-state plasma glucose values. Each animal was fixed stereotaxically with ear bars in a home-made holder, as described previously (Choi et al. 1999), and placed in the magnet. Body temperature was maintained at 37°C by warm water circulation (RTE-101 bath circulator, Thermo NESLAB, Portsmouth, NH, USA) based on feedback from a rectal temperature probe (Cole Palmer, Vernon Hills, IL, USA). Throughout the study, arterial blood was withdrawn at 25-35 min intervals. Arterial blood gases, i.e. PaO2, PaCO2 and pH were obtained with a blood gas analyser (Rapidlab 248, Bayer Corp., Newbury, UK) to maintain identical physiological conditions in both experimental groups, i.e. (values for the sham-treated group in parentheses, all values mean ± SD): rectal temperature 36.9 ± 0.2 (36.9 ± 0.3)°C; pH = 7.42 ± 0.03 (7.40 ± 0.04); PaCO2 = 40.8 ± 3.0 (39.7 ± 3.2) mmHg; and PaO2 = 155 ± 17 (153 ± 27) mmHg). Plasma glucose levels were determined as described above.
Immediately after the NMR experiment and after withdrawing a plasma sample, the animal was carefully handled and killed by focused microwave fixation irradiation of the entire brain (Gerling Applied Engineering Inc., Modesto, CA, USA) at 4 kW in 1.4 s (Kong et al. 2002). The brain, without cerebellum, was immediately excised from the skull and stored in a - 80°C freezer (Thermo Forma, Marietta, OH, USA), for a maximum of 4 weeks, for further biochemical assay of glucose and glycogen.
13C MRS methods
All experiments were performed in a 9.4 Tesla, 31 cm bore horizontal magnet (Magnex Scientific, Abingdon, UK), interfaced with an INOVA console (Varian, Palo Alto, CA, USA). A quadrature 1H radiofrequency (RF) 14 mm diameter coil and a linear polarized three-turn 11 mm diameter 13C RF coil (Adriany and Gruetter 1997; Choi et al. 2001) was used for MRI and MRS. FAST(EST) MAP (Fastaumatic shim technique using echo-planar signal read-out for mapping along projections) shimming (Choi et al. 2000; Gruetter and Tkac 2000) was applied to adjust B0 field homogeneity in a nominal approximate 440 μL volume of interest (Choi et al. 1999). Outer volume suppression was optimized for three-dimensional localization of the NMR signals to ensure elimination of signals from non-cerebral tissue (Choi et al. 1999).
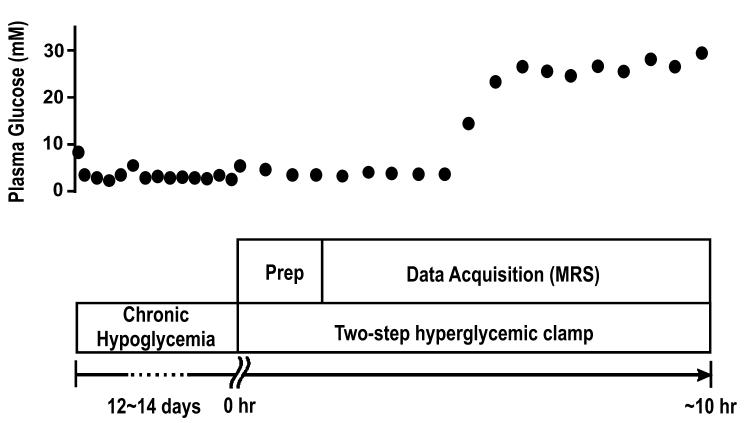
In order to obtain 13C detectable brain glucose signals, 99% [1-13C]-d-glucose (20% weight/volume) was infused intravenously and plasma glucose enrichment approached 99% 2 h after the start of infusion (Choi et al. 2001). Thereafter, 13C MRS data collection began. An example of the protocol is shown in Fig. 1. Brain glucose concentration was determined in spectra acquired after plasma glucose had been stable for at least 20 min, and when the 13C glucose signal varied by less than 10%.
Fig. 1.
An example of the experimental protocol used for chronically hypoglycaemic animals. On day 0, three insulin pellets (6 IU/day) were inserted to induce a hypoglycaemic condition (plasma glucose levels<3 mm), as described in Experimental procedures. On subsequent days, the hypoglycaemic condition was monitored with morning tail bleeds. Once plasma glucose rose above the target hypoglycaemic level, an extra insulin pellet (2 IU/day) was administrated. In this case, on day 5, the glucose level was slightly higher, and an extra insulin pellet was inserted. After the chronically hypoglycaemic episode, the animal was prepared at hour 0, immediately followed by a hyper-glycaemic clamp. In this case, the steady-state glycaemic level was maintained for 5 h. To reach a different steady-state glycaemic level, the infusion rate was adjusted accordingly.
Quantification of brain glucose signals
After each in vivo study, a reference phantom experiment identical to the in vivo measurement was conducted, for absolute quantification of 13C NMR signals of glucose and glycogen, using the external reference method (Choi et al. 2001). Briefly, signal intensities (signal area) were corrected for measured small differences in coil loading and Nuclear Overhauser Enhancement. The reference phantom contained 8 mm 99% enriched [1-13C]-d-glucose and 400 mm glucosyl units/L of natural abundance glycogen, whose concentrations were also enzymatically determined. The C1 signals of glucose were Lorentzian fitted using built-in spectrometer software, and the total intensity of both α and β glucose signals was determined. The brain glucose concentration ([Glc]) was calculated based on the following equation (Gruetter et al. 1998; Choi et al. 1999, 2001):
| (1) |
where I stands for the intensity of either glucose (Glc) or formic acid (FA) and 8 is the [1-13C]-d-glucose concentration (mm) in the phantom. The superscripts ‘invivo’ and ‘ref’ indicate measurements from in vivo animal brain and reference phantom studies, respectively.
Determination of glucose transport kinetics
The reversible Michaelis-Menten (ReMM) model of steady-state glucose transport kinetics was used to obtain kinetics parameters from the relation between plasma glucose and brain glucose, using the following expression (Gruetter et al. 1998) as in previous studies (Choi et al. 2001, 2002):
| (2) |
where G represents the glucose concentration in brain (μmol/g) or in plasma (mm), Vd = 0.77 mL/g is the physical distribution space of water in the brain, Tmax is the apparent maximum transport rate, CMRglc is the glucose metabolic rate and Kt is the apparent Michaelis-Menten constant. Consistent with the ReMM kinetic model, the relationship between plasma glucose and brain glucose at steady state is linear over a large range of plasma glucose concentrations (Gruetter et al. 1998). This has been observed in a number of studies in our laboratory (Choi et al. 2001, 2002; Seaquist et al. 2001) and by others (de Graaf et al. 2001). The brain glucose concentration at moderate hyperglycaemia is mostly influenced by the relative maximal glucose transport rate, Tmax/CMRglc (for a derivation see Appendix). For further analysis, CMRglc was assumed to be unchanged in chronic hypoglycaemia, based on several reports (Lucignani et al. 1987; Pelligrino et al. 1990; Segel et al. 2001) and on the fact that the measurement of brain glucose transport kinetics was performed at euglycaemia or above. The relative Tmax/CMRglc obtained was thus assumed to reflect mainly changes in Tmax.
The small amount of blood volume in rat brain, 3.4 mL/100 g (Shockley and LaManna 1988), potentially contributes to the total brain glucose signal from 13C MRS. When subtracting this potential contribution of blood glucose to the glucose signal based on the known plasma glucose level, brain glucose content was reduced by less than 8.5%, which did not affect the qualitative and quantitative findings of this study. Fitting of eqn 2 with origin 5.0 (OriginLab Corp., Northampton, MA, USA) was performed assuming a fixed Kt of 3.3 mm (Gruetter et al. 1998; Choi et al. 2001; Choi et al. 2002), as a Kt ranging from 1 to 6 mm did not affect the quantitative nature of the findings of this study, consistent with Kt having a minor influence on brain glucose content (see Appendix).
Brain tissue analysis
Extracted brain tissues were stored at) - 80°C for less than 4 weeks. On the day of the assay, frozen tissues were pulverized under liquid N2 using a mortar. The well mixed brain powder was separated into two, approximately 200 mg samples. Following extensive validation, the ‘direct’ HCl glycogen assay was applied (Cruz and Dienel 2002). Briefly, 500 μL 0.03 m HCl were added to each sample, well homogenized (25 kHz ultrasonic processor, Cole Parmer, Vernon Hills, IL, USA) and boiled for 45 min at 80-90°C in a water bath. From each sample, two identical 200 μL aliquots were taken and treated exactly the same, except that 5 μL of 5 mm sodium acetate was added to the ‘minus’ sample (to yield tissue glucose concentration) and 5 μL of amyloglucosidase (Roche Diagnostics Corp., Indianapolis, IN, USA) solution to the ‘plus’ sample (to yield total tissue glucosyl concentration). pH was adjusted in each sample to pH 5 by adding a small amount of either 1.1 m HCl or 3 m sodium acetate. The samples were incubated in parallel in a reciprocal water bath shaker (New Brunswick Scientific Co., Inc., Edison, NJ, USA) at 37°C for at least 2 h. Glucose levels were determined at room temperature (24°C) as described above, and the readouts were ensured to be in the linear range of the analyser. The glucose concentration of the brain extract ([Glc]brainextract, μmol/g wet weight) were calculated from glucose readouts ([Glc]readout) using the following equation:
| (3) |
where W represents the weight (g) from the different source, indicated by the respective subscript.
Statistics
All data are shown as mean ± SE. Difference between groups was detected using the Student’s unpaired two-tailed t-test.
Results
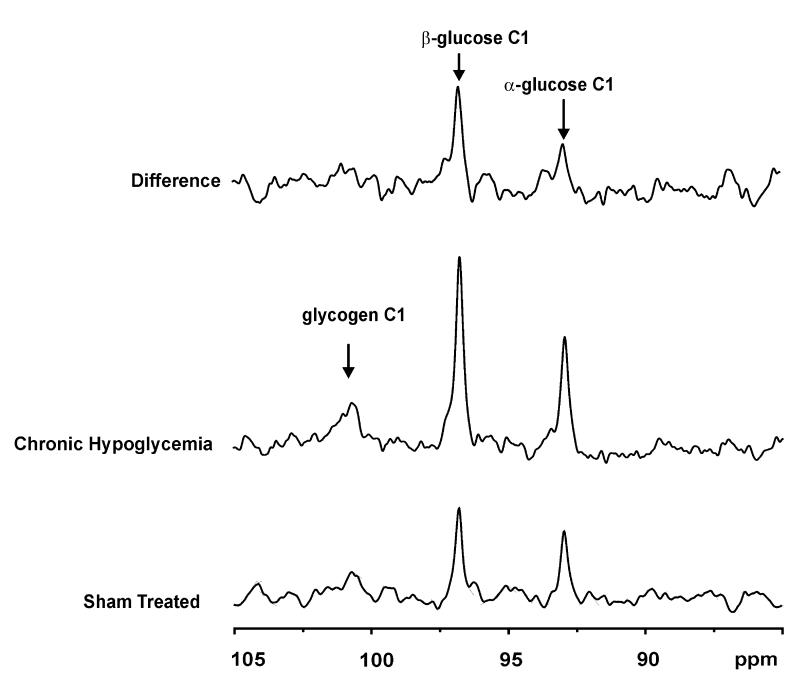
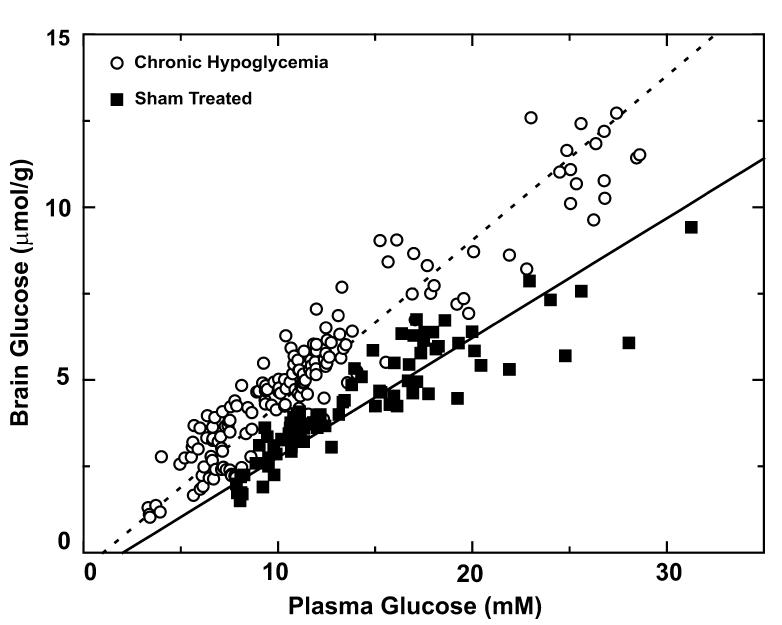
Comparing 13C NMR spectra obtained from two animals one each of the two respective experimental groups illustrated the profound effect of chronic hypoglycaemia on the brain [1-13C] glucose C1 signals, which were approximately 50% higher than those from sham-treated group (Fig. 2). It was of interest that the brain glycogen C1 signal was detected clearly after prolonged [1-13C] glucose infusion in both cases, with a less clear difference between the groups (Fig. 2, top). Over the entire range of steady-state plasma glucose levels measured, the corresponding brain glucose concentrations in chronically hypoglycaemic rat brain (open circles in Fig. 3) were significantly higher (p < 0.0001 determined from the best fit to the data) than the ones in the sham-treated group (closed squares in Fig. 3). To avoid large errors caused by the variation of brain glucose with plasma glucose, brain glucose concentrations were arranged into five groups, each defined to correspond to a plasma glucose range of 5 mm each, i.e. from 5 to 10 mm,10 to 15 mm, etc. Within each plasma glucose range, brain glucose in chronic hypoglycaemia was increased significantly (p < 0.005), with a concentration increase of 48 ± 7% (mean ± SE) when averaged over the entire plasma glucose range (Table 1).
Fig. 2.
Experimental difference in localized 13C glucose signals. The middle and lower spectra were acquired when plasma glucose levels were 12.2 mm in chronically hypoglycaemic, and 11.1 mm in shamtreated animals, respectively. The location and size of the volume of interest was the same. The vertical scale was adjusted for slight differences in loading factor, which amounted to less than 10%, and the line broadened to identical line widths of α-glucose C1. The top trace is the difference between the middle and bottom traces shown without rescaling. The arrows indicate the respective chemical shift of the compound indicated.
Fig. 3.
Brain glucose concentrations as a function of plasma glucose levels in the sham-treated (solid squares) and chronically hypoglycaemic animals (open circles). The best fit of the ReMM model (eqn 2) to the measurements in the sham-treated group resulted in a relative maximum transport rate of Tmax/CMRglc = 4.2 ± 0.2 between the chronically hypoglycaemic group (dashed line), and the sham-treated group (solid line) Tmax/CMRglc = 2.6 ± 0.1 (p < 0.0001).
Table 1.
Comparison of steady-state brain glucose content at certain plasma glucose ranges between chronic hypoglycaemia and sham-treated groups
| Plasma glucose range (mM) | 5-10 | 10-15 | 15-20 | 20-25 | 25-30 |
|---|---|---|---|---|---|
| Chronic hypoglycaemia (μmol/g) | 3.4 ± 0.9 (65)* | 5.3 ± 0.7 (65)* | 7.6 ± 1.0 (14)* | 10.1 ± 1.9 (6)* | 11.2 ± 1.0 (12)* |
| Sham-treated (μmol/g) | 2.5 ± 0.5 (18) | 3.9 ± 0.7 (39) | 5.5 ± 0.9 (25) | 6.2 ± 1.0 (7) | 6.9 ± 0.8 (3) |
All data are presented as mean ± SD. The number of measurements is listed in parentheses. For each specific plasma glucose range, the difference between the two groups was highly significant
(p < 0.005, unpaired t-test).
The steady-state brain glucose concentrations expressed as a function of plasma glucose was fitted with eqn 2. This resulted in a relative maximal transport rate of Tmax/CMRglc = 4.2 ± 0.2 in the chronically hypoglycaemic group (dashed line in Fig. 3) and Tmax/CMRglc = 2.6 ± 0.1 in the sham-treated group (solid line in Fig. 3), which represents a 58 ± 7% increase (p < 0.0001).
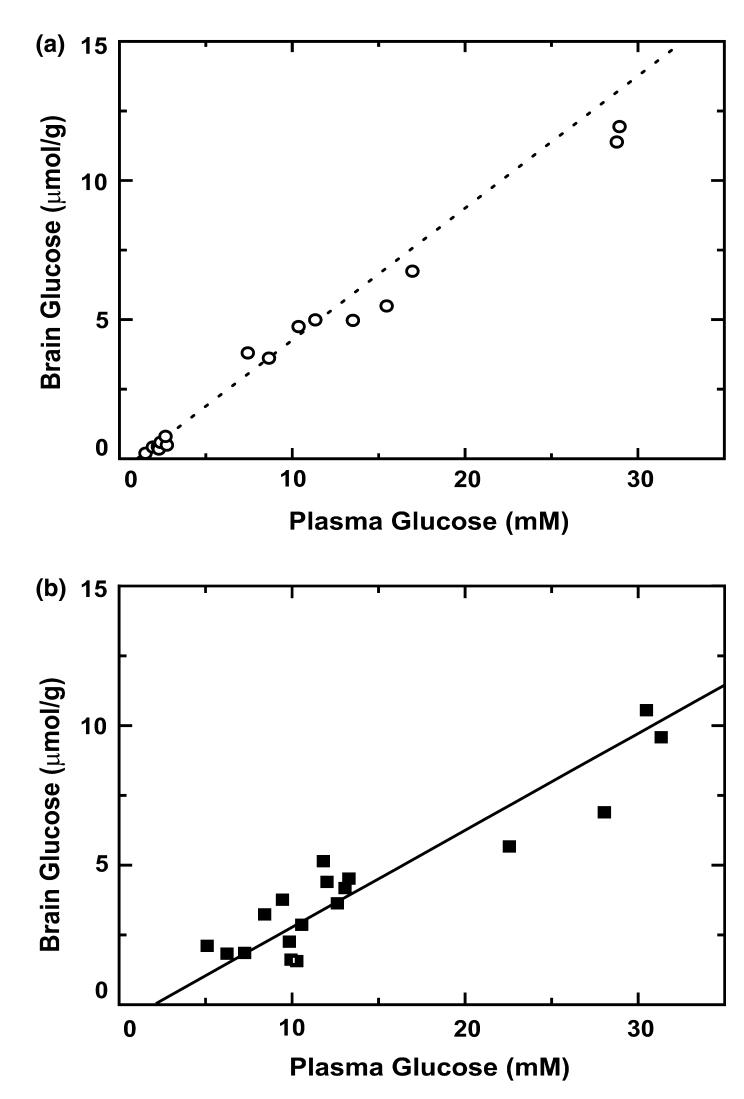
To validate the in vivo NMR quantification of brain glucose, brains were extracted using microwave fixation and assayed for tissue glucose, 16 from the chronic hypoglycaemia group and 18 from the sham-treated group. The ensuing quantification of brain glucose content was within 10% of that by NMR and not significantly different in both groups, evidenced by the overlap with the best fit to the NMR data (dashed line in Fig. 4a and solid line in Fig. 4b). Note the excellent agreement of the biochemical brain glucose quantification with the extrapolated NMR fit to low plasma glucose levels in the chronic hypoglycaemia group (Fig. 4a).
Fig. 4.
Comparison of the NMR measurements with the biochemical assay of brain glucose in both chronic hypoglycaemia (a) and shamtreated animals (b). The dashed line in (a) is identical to the dashed line in Fig. 3, and indicates the NMR quantification of brain glucose in chronic hypoglycaemia. The open circles represent the concurrent biochemical determination of glucose in the chronic hypoglycaemia group. Conversely, the solid line in (b) is the solid line from Fig. 3, and the solid squares are the respective biochemical determination of brain glucose.
Concurrent assessment of total tissue glycogen content after about 8 h of hyperglycaemia resulted in 6.9 ± 0.9 μmol glucosyl U/g (mean ± SD, n = 9) at 6.1 ± 3.6 μmol/g of brain glucose in the chronic hypoglycaemia group, and 6.0 ± 0.9 μmol glucosyl units/g (mean ± SD, n = 11) at 5.6 ± 2.5 μmol/g of brain glucose in the sham-treated group (p > 0.05). This was in overall agreement with the NMR observation of similar brain glycogen content (Fig. 2). In addition, total brain glycogen in chronic hypoglycaemia without glucose infusion was not significantly higher (p > 0.05) than in the sham-treated animals (Table 2).
Table 2.
Results of biochemical assay on total brain glycogen and brain glucose
| Group | Brain glycogen (μmol glucosyl U/g) | Brain glucose (μmol/g) | Plasma glucose (m M) |
|---|---|---|---|
| Chronic hypoglycaemia | 3.5 ± 0.6 | 0.9 ± 1.3 | 2.9 ± 2.2 |
| Sham-treated | 3.7 ± 0.6 | 2.1 ± 0.6 | 8.2 ± 2.0* |
Both chronically hypoglycaemic (n = 7) and sham-treated (n = 7) animals were killed shortly after chronic hypoglycaemia or sham-treated episode with no vascular access preparation, glucose infusion or NMR studies. Plasma glucose concentrations were determined with tail bleeds. All values are given as mean ± SD;
p < 0.05 (unpaired t-test). In this case, despite significant different plasma glucose levels (p = 0.0004), brain glucose (p = 0.057) and glycogen (p = 0.45) were not significantly different.
Discussion
The present study is the first to report elevated brain glucose content in chronic hypoglycaemia. From the brain glucose measurements, the observed 48% increase in brain glucose content was consistent with an estimated 58% increase in apparent Tmax. In contrast to the increased brain glucose content, brain glycogen levels were not substantially affected by chronic hypoglycaemia, directly evidenced by NMR and validated by biochemical measurements.
The increased brain glucose content in chronic hypoglycaemia could be visualized directly when comparing spectra obtained at similar plasma glucose levels from the two different groups (Fig. 2). The elevation of brain glucose was even more evident after quantifying brain glucose content over a larger range of steady-state plasma glucose levels (Table 1 and Fig. 3).
Quantification of brain glucose concentrations by 13C MRS has been verified in the past by comparing different NMR measures with each other (Choi et al. 2001). In the present study, we directly validated the NMR quantification with a standard biochemical assay of brain glucose, which showed that the accuracy of the NMR quantification was within 10% of that obtained by biochemical methods in both experimental groups (Figs 4a and b). Considering experimental inaccuracies of both methods, this was deemed an excellent agreement.
A comparison between brain glucose content as a function of plasma glucose concentration in the sham-treated animals, and a previous study using the same anaesthetic protocol (Choi et al. 2001), indicated that brain glucose concentrations from both groups were indistinguishable (data not shown). We therefore conclude that the treatment of the animal with the pellet implants, including the daily treatments such as tail bleeds under 2% isoflurane, was without noticeable effect on brain glucose content as such.
In the chronic hypoglycaemia group as well as the shamtreated group, the relationship between plasma and brain glucose at steady state was linear, within experimental limits, as in previous studies in the rat (Choi et al. 2001,2002) and human brain (Gruetter et al. 1998; de Graaf et al. 2001; Seaquist et al. 2001). Because a potential contribution from blood glucose to the NMR signal would be linear with plasma glucose, the linear nature of the relationship between blood and brain glucose was not affected by this potential NMR signal contribution from blood glucose. A change in blood volume of 1 mL/100 g affects the relative fraction of blood contribution to the brain glucose signal by less 2%. Thus, hypothetical blood volume changes are well below the observed increase in brain glucose content. Furthermore, a potential relative contribution of glucose in blood to the total signal was much less in chronic hypoglycaemia, due to the higher brain glucose content. When subtracting the blood contribution from the NMR quantification, both slopes were reduced by less than 5% and the change of the relative Tmax remained about 60%, thus not affecting our conclusion.
Recent studies using both PET (Hasselbalch et al. 1999) and 1H MRS (Seaquist et al. 2001) suggested that bulk brain glucose uptake is largely an insulin-independent process. Thus, the higher plasma insulin concentration in chronically hypoglycaemic animals was unlikely to affect our result or conclusion per se.
The derived glucose transport kinetics indicated a substantial Tmax/CMRglc increase in chronic hypoglycaemia (p < 0.0001) that was confirmed using standard biochemical measurements (Fig. 4). As shown in the Appendix (eqn A2), brain glucose content is mostly affected by changes in Tmax/CMRglc. CMRglc in the chronic hypoglycaemia group was likely the same as that in the shamtreated group because all NMR data were obtained (i) at euglycaemia or above, where the brain glucose concentration was not rate-limiting for metabolism, i.e. above the Km of hexokinase (50 μm) (Lucignani et al. 1987; McCall 2004), and (ii) under consistent anaesthetic conditions. The consistency of the anaesthetic regime was further supported by the brain glucose concentrations (when expressed as a function of plasma glucose) in the sham-treated group, being similar to those in previous studies from our laboratory using the same anaesthetic protocol. Therefore, we conclude that an increase in the apparent maximum transport rate, Tmax, accounts for most of the brain glucose content change.
Our study used a protocol identical to that of Simpson et al. (1999), which enabled a direct comparison of the increase in brain glucose content with the reported changes of the BBB glucose transporter protein. Simpson et al. reported specific changes in GLUT1 expression at the BBB but no effect on either the astrocytic 45 kDa GLUT1 or the neuronal GLUT3. This is not surprising, given that beyond the BBB, glucose is rather uniformly distributed in the brain’s aqueous phase (Pfeuffer et al. 2000). A plausible explanation for the lack of effect on the astrocytic and neuronal transporters is that in the chronic hypoglycaemia group, the average ambient brain glucose concentration seen by the cells beyond the BBB can be expected to be similar to the euglycaemic glucose concentration in the sham-treated group. The 58% increase in Tmax observed in the present study is consistent with the 52% increase of luminal GLUT1 in this model (Simpson et al. 1999), but almost twofold higher than the 23% increase in glucose transporter protein (GLUT1) at the BBB of the cortex (Simpson et al. 1999). These observations further support the notion that for transport of glucose into the brain, the BBB is likely to be rate-limiting. More specifically, our results imply that the density of GLUT1 transporters at the luminal membrane is rate-determining for transporting glucose into the brain. It remains to be determined whether the differential increase in glucose transporter is due to a redistribution of transporters between the luminal and ablumenal membrane of the brain endothelium, or whether it involves the presence of a cytoplasmic glucose transporter pool that, under normal conditions, is not physiologically active (Barros et al. 2005). This would be possible, e.g. by having a sequestration of GLUT1 at the BBB, similar to that of GLUT4 in the muscle. This scenario would be consistent with recent reports suggesting that acute regulation of brain glucose transport might be possible (Barros et al. 2005). To what extent this occurs, acutely or chronically, remains to be determined. Some other studies have also indicated a GLUT3 increase after only 7 (Duelli et al. 1999) or 8 days (Uehara et al. 1997) of chronic hypoglycaemia exposure. This suggests that GLUT3 is far more sensitive than GLUT1. However, the discrepancy would not affect our final conclusion that brain glucose increases mainly as a result of increased GLUT1 at the BBB.
The observed changes in brain glucose content are clearly ascribed to a consequence of altered GLUT1 gene expression and its protein product, which occurs only after long-term exposure (several days) to hypoglycaemia. However, with increasing frequency and depth, recurrent hypoglycaemia is likely to approximate the chronic hypoglycaemia protocol used here in terms of regulation of glucose transport. In practice, however, episodes of hypoglycaemia rarely last for hours, and impaired glucose sensing can also be elicited following a single episode of hypoglycaemia. Therefore, it is likely that other mechanisms can interfere with glucose sensing, such as alterations in the efficiency of intracellular glucose-sensing regulators, e.g. glucokinase and ATP-sensitive K+ channels (Levin et al. 2004). An additional potential mechanism is brain glycogen, an insulin-sensitive glucose reservoir, which has been shown to be altered following a single episode of hypoglycaemia (Gruetter 2003).
Whereas during and following acute hypoglycaemia brain glycogen concentrations are strongly affected (depletion followed by supercompensation) (Choi et al. 2003), in the present study brain glycogen content in the chronic hypoglycaemia group was similar to that in the control group (Table 2). This may be surprising, given the reported effects of lowered plasma glucose concentrations on brain glcyogen content (see Gruetter 2003; Brown 2004 and references therein). However, an identical brain glucose level in the two groups corresponds to two substantially different plasma glucose levels (Table 2, Fig. 3). This is evident when drawing a horizontal line in Fig. 3 and considering its intercept with the best fits (solid and dashed lines in Fig. 3). Thus, during chronic hypoglycaemia (12-14 days), the brain glucose concentration is well above the Km of hexokinase, providing adequate fuel for glucose phosphorylation despite the apparent mild hypoglycaemia. Therefore, the fact that glycogen was not depleted was not surprising. Note that the slightly higher glycogen signal in the chronic hypoglycaemia animal in Fig. 2 can be explained by a higher isotopic enrichment (data not shown). This supports the hypothesis that brain glucose content may have a major controlling effect on brain glycogen content (Gruetter 2003; Brown 2004), as was also recently reported for human liver phosphorylase (Ercan-Fang et al. 2002). As it is likely that the signal directly stimulating the altered expression of the glucose transporter is the ambient glucose concentration itself, the similarity of glucose content beyond the BBB in both experimental groups can explain the lack of effect of chronic hypoglycaemia on the astrocytic glucose transporter (the 45 kDa GLUT1) and the neuronal glucose transporter protein (GLUT3) (Simpson et al. 1999).
We conclude that chronic hypoglycaemia (12-14 days) has less effect on brain glycogen content than acute hypoglycaemia but increases the brain glucose concentration, most likely due to changes in BBB GLUT1 protein. Furthermore, such altered glucose transport and transporter activity could mimic a normal glucose environment in the brain under the mild hypoglycaemic conditions observed in the present study (Figs 2 and 3, Table 2). If such an upregulation also occurs in proximity to glucose-sensing neurones, this might contribute to diminished counter-regulation. These observations reinforce the notion that the brain is a glucose-sensing organ. An increased brain glucose content at a given plasma glucose concentration in chronic hypoglycaemia may thus contribute to impaired glucose sensing in chronic hypoglycaemia.
Acknowledgements
We thank Dr Tianwen Yue for excellent technical assistance. This work was supported by grants from Juvenile Diabetes Research Foundation International, NIH (R01 NS42005, P41 RR08079) and W. M. Keck Foundation.
Appendix
In this section, it is shown that the brain glucose concentration is a sensitive measure of the apparent maximal transport rate relative to the glucose metabolic rate, v = Tmax/CMRglc.
The change in brain glucose content with respect to changes in the apparent Michaelis-Menten constant, Kt, and v can be derived according to first-order error propagation theory, using first-order Taylor series expansion of eqn 2, by taking the first derivative and multiplying with the deviation in the respective parameter, i.e. the change in brain glucose concentration as a function of relative changes in v and Kt is given by
| (A 1) |
By dividing this expression of eqn 2, and assuming a plasma glucose of about 16 mm a Kt of about 3 mm (Choi et al. 2001) and v about 3 eqn A1 simplifies to a relative change in brain glucose content (Gbrain ∼ 5 μmol/g)
| (A 2) |
From this estimation it is clear that under the conditions of the current study, relative brain glucose concentration changes are 10-fold more sensitive to relative changes in Tmax than to relative changes in Kt.
Footnotes
The present address for Hongxia Lei and Rolf Gruetter is Laboratory for Functional and Metabolic Imaging (LIFMET), Ecole Polytechnique Federale de Lausanne, Station 6, CH-1015 Lausanne, Switzerland.
- BBB
- blood-brain barrier
- MRI
- magnetic resonance imaging
- MRS
- magnetic resonance spectroscopy
- PET
- positron emission tomography
- ReMM
- reversible Michaelis-Menten
References
- Adriany G, Gruetter R. A half-volume coil for efficient proton decoupling in humans at 4 tesla. J. Magn. Reson. 1997;125:178–184. doi: 10.1006/jmre.1997.1113. [DOI] [PubMed] [Google Scholar]
- Barros LF, Porras OH, Bittner CX. Why glucose transport in the brain matters for PET. Trends Neurosci. 2005;28:117–119. doi: 10.1016/j.tins.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Borg MA, Borg WP, Tamborlane WV, Brines ML, Shulman GI, Sherwin RS. Chronic hypoglycemia and diabetes impair counterregulation induced by localized 2-deoxy-glucose perfusion of the ventromedial hypothalamus in rats. Diabete. 1999;s:48–584. doi: 10.2337/diabetes.48.3.584. [DOI] [PubMed] [Google Scholar]
- Boyle PJ, Nagy RJ, O’Connor AM, Kempers SF, Yeo RA, Qualls C. Adaptation in brain glucose uptake following recurrent hypoglycemia. Proc. Natl Acad. Sci. USA. 1994;91:9352–9356. doi: 10.1073/pnas.91.20.9352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AM. Brain glycogen re-awakened. J. Neurochem. 2004;89:537–552. doi: 10.1111/j.1471-4159.2004.02421.x. [DOI] [PubMed] [Google Scholar]
- Choi IY, Tkac I, Ugurbil K, Gruetter R. Noninvasive measurements of [1-13C]glycogen concentrations and metabolism in rat brain in vivo. J. Neurochem. 1999;73:1300–1308. doi: 10.1046/j.1471-4159.1999.0731300.x. [DOI] [PubMed] [Google Scholar]
- Choi IY, Tkac I, Gruetter R. Single-shot, three-dimensional‘non-echo’localization method for in vivo NMR spectroscopy. Magn. Reson. Med. 2000;44:387–394. doi: 10.1002/1522-2594(200009)44:3<387::aid-mrm8>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Choi IY, Lee SP, Kim SG, Gruetter R. In vivo measurements of brain glucose transport using the reversible Michaelis-Menten model and simultaneous measurements of cerebral blood flow changes during hypoglycemia. J. Cereb. Blood Flow Metab. 2001;21:653–663. doi: 10.1097/00004647-200106000-00003. [DOI] [PubMed] [Google Scholar]
- Choi IY, Lei H, Gruetter R. Effect of deep pentobarbital anesthesia on neurotransmitter metabolism in vivo: on the correlation of total glucose consumption with glutamatergic action. J. Cereb. Blood Flow Metab. 2002;22:1343–1351. doi: 10.1097/01.WCB.0000040945.89393.46. [DOI] [PubMed] [Google Scholar]
- Choi IY, Seaquist ER, Gruetter R. Effect of hypoglycemia on brain glycogen metabolism in vivo. J. Neurosci. Res. 2003;72:25–32. doi: 10.1002/jnr.10574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criego AB, Tkac I, Kumar A, Thomas W, Gruetter R, Seaquist ER. Brain glucose concentrations in patients with type 1 diabetes and hypoglycemia unawareness. J. Neurosci. Res. 2005;79:42–47. doi: 10.1002/jnr.20296. [DOI] [PubMed] [Google Scholar]
- Cruz NF, Dienel GA. High glycogen levels in brains of rats with minimal environmental stimuli: implications for metabolic contributions of working astrocytes. J. Cereb. Blood Flow Metab. 2002;22:1476–1489. doi: 10.1097/01.WCB.0000034362.37277.C0. [DOI] [PubMed] [Google Scholar]
- Cryer PE. Banting lecture. Hypoglycemia: the limiting factor in the management of IDDM. Diabetes. 1994;43:1378–1389. doi: 10.2337/diab.43.11.1378. [DOI] [PubMed] [Google Scholar]
- Cryer PE. Hypoglycemia is the limiting factor in the management of diabetes. Diabetes Metab. Res. Rev. 1999;15:42–46. doi: 10.1002/(sici)1520-7560(199901/02)15:1<42::aid-dmrr1>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Cryer PE. Hypoglycemia-associated autonomic failure in diabetes. Am. J. Physiol. Endocrinol. Metab. 2001;281:E1115–E1121. doi: 10.1152/ajpendo.2001.281.6.E1115. [DOI] [PubMed] [Google Scholar]
- Cryer PE. Diverse causes of hypoglycemia-associated autonomic failure in diabetes. N Engl. J. Med. 2004;350:2272–2279. doi: 10.1056/NEJMra031354. [DOI] [PubMed] [Google Scholar]
- Cryer PE. Mechanisms of hypoglycemia-associated autonomic failure and its component syndromes in diabetes. Diabetes. 2005;54:3592–3601. doi: 10.2337/diabetes.54.12.3592. [DOI] [PubMed] [Google Scholar]
- Dagogo-Jack SE, Craft S, Cryer PE. Hypoglycemiaassociated autonomic failure in insulin-dependent diabetes mellitus. Recent antecedent hypoglycemia reduces autonomic responses to, symptoms of, and defense against subsequent hypoglycemia. J. Clin. Invest. 1993;91:819–828. doi: 10.1172/JCI116302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MR, Shamoon H. Deficient counter-regulatory hormone responses during hypoglycemia in a patient with insulinoma. J. Clin. Endocrinol. Metab. 1991;72:788–792. doi: 10.1210/jcem-72-4-788. [DOI] [PubMed] [Google Scholar]
- Duelli R, Staudt R, Duembgen L, Kuschinsky W. Increase in glucose transporter densities of Glut3 and decrease of glucose utilization in rat brain after one week of hypoglycemia. Brain Res. 1999;831:254–262. doi: 10.1016/s0006-8993(99)01463-8. [DOI] [PubMed] [Google Scholar]
- Ercan-Fang N, Gannon MC, Rath VL, Treadway JL, Taylor MR, Nuttall FQ. Integrated effects of multiple modulators on human liver glycogen phosphorylase a. Am. J. Physiol. Endocrinol. Metab. 2002;283:E29–E37. doi: 10.1152/ajpendo.00425.2001. [DOI] [PubMed] [Google Scholar]
- Evans ML, Pernet A, Lomas J, Jones J, Amiel SA. Delay in onset of awareness of acute hypoglycemia and of restoration of cognitive performance during recovery. Diabetes Care. 2000;23:893–897. doi: 10.2337/diacare.23.7.893. [DOI] [PubMed] [Google Scholar]
- Gjedde A. Rapid steady-state analysis of blood-brain glucose transfer in rat. Acta Physiol. Scand. 1980;108:331–339. doi: 10.1111/j.1748-1716.1980.tb06541.x. [DOI] [PubMed] [Google Scholar]
- Gjedde A, Diemer NH. Autoradiographic determination of regional brain glucose content. J. Cereb. Blood Flow Metab. 1983;3:303–310. doi: 10.1038/jcbfm.1983.45. [DOI] [PubMed] [Google Scholar]
- de Graaf RA, Pan JW, Telang F, Lee JH, Brown P, Novotny EJ, Hetherington HP, Rothman DL. Differentiation of glucose transport in human brain gray and white matter. J. Cereb. Blood Flow Metab. 2001;21:483–492. doi: 10.1097/00004647-200105000-00002. [DOI] [PubMed] [Google Scholar]
- Gruetter R. Glycogen: the forgotten cerebral energy store. J. Neurosci. Res. 2003;74:179–183. doi: 10.1002/jnr.10785. [DOI] [PubMed] [Google Scholar]
- Gruetter R, Tkac I. Field mapping without reference scan using asymmetric echo-planar techniques. Magn. Reson. Med. 2000;43:319–323. doi: 10.1002/(sici)1522-2594(200002)43:2<319::aid-mrm22>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Gruetter R, Ugurbil K, Seaquist ER. Steady-state cerebral glucose concentrations and transport in the human brain. J. Neurochem. 1998;70:397–408. doi: 10.1046/j.1471-4159.1998.70010397.x. [DOI] [PubMed] [Google Scholar]
- Harik SI, Behmand RA, LaManna JC. Hypoxia increases glucose transport at blood-brain barrier in rats. J. Appl. Physiol. 1994;77:896–901. doi: 10.1152/jappl.1994.77.2.896. [DOI] [PubMed] [Google Scholar]
- Harik SI, Lust WD, Jones SC, Lauro KL, Pundik S, LaManna JC. Brain glucose metabolism in hypobaric hypoxia. J. Appl. Physiol. 1995;79:136–140. doi: 10.1152/jappl.1995.79.1.136. [DOI] [PubMed] [Google Scholar]
- Hasselbalch SG, Knudsen GM, Videbaek C, Pinborg LH, Schmidt JF, Holm S, Paulson OB. No effect of insulin on glucose blood-brain barrier transport and cerebral metabolism in humans. Diabetes. 1999;48:1915–1921. doi: 10.2337/diabetes.48.10.1915. [DOI] [PubMed] [Google Scholar]
- Herholz K, Wienhard K, Pietrzyk U, Pawlik G, Heiss WD. Measurement of blood-brain hexose transport with dynamic PET: comparison of [18F]2-fluoro-2-deoxyglucose and [11C]O-methylglucose. J. Cereb. Blood Flow Metab. 1989;9:104–110. doi: 10.1038/jcbfm.1989.14. [DOI] [PubMed] [Google Scholar]
- Kong J, Shepel PN, Holden CP, Mackiewicz M, Pack AI, Geiger JD. Brain glycogen decreases with increased periods of wakefulness: implications for homeostatic drive to sleep. J. Neurosci. 2002;22:5581–5587. doi: 10.1523/JNEUROSCI.22-13-05581.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai AK, Kang YS, Boado RJ, Pardridge WM. Upregulation of blood-brain barrier GLUT1 glucose transporter protein and mRNA in experimental chronic hypoglycemia. Diabetes. 1995;44:1399–1404. doi: 10.2337/diab.44.12.1399. [DOI] [PubMed] [Google Scholar]
- Levin BE, Routh VH, Kang L, Sanders NM, Dunn-Meynell AA. Neuronal glucosensing: what do we know after 50 years? Diabetes. 2004;53:2521–2528. doi: 10.2337/diabetes.53.10.2521. [DOI] [PubMed] [Google Scholar]
- Lucignani G, Namba H, Nehlig A, Porrino LJ, Kennedy C, Sokoloff L. Effects of insulin on local cerebral glucose utilization in the rat. J. Cereb. Blood Flow Metab. 1987;7:309–314. doi: 10.1038/jcbfm.1987.68. [DOI] [PubMed] [Google Scholar]
- McCall AL. Cerebral glucose metabolism in diabetes mellitus. Eur. J. Pharmacol. 2004;490:147–158. doi: 10.1016/j.ejphar.2004.02.052. [DOI] [PubMed] [Google Scholar]
- McCall AL, Fixman LB, Fleming N, Tornheim K, Chick W, Ruderman NB. Chronic hypoglycemia increases brain glucose transport. Am. J. Physiol. 1986;251:E442–E447. doi: 10.1152/ajpendo.1986.251.4.E442. [DOI] [PubMed] [Google Scholar]
- McCrimmon RJ, Jacob RJ, Fan X, McNay EC, Sherwin RS. Effects of recurrent antecedent hypoglycaemia and chronic hyperglycaemia on brainstem extra-cellular glucose concentrations during acute hypoglycaemia in conscious diabetic BB rats. Dia-betologia. 2003;46:1658–1661. doi: 10.1007/s00125-003-1231-4. [DOI] [PubMed] [Google Scholar]
- Oz G, Henry PG, Seaquist ER, Gruetter R. Direct, noninvasive measurement of brain glycogen metabolism in humans. Neurochem. Int. 2003;43:323–329. doi: 10.1016/s0197-0186(03)00019-6. [DOI] [PubMed] [Google Scholar]
- Pelligrino DA, Segil LJ, Albrecht RF. Brain glucose utilization and transport and cortical function in chronic vs. acute hypoglycemia. Am. J. Physiol. 1990;259:E729–E735. doi: 10.1152/ajpendo.1990.259.5.E729. [DOI] [PubMed] [Google Scholar]
- Pfeuffer J, Tkac I, Gruetter R. Extracellular-intracellular distribution of glucose and lactate in the rat brain assessed noninvasively by diffusion-weighted 1H nuclear magnetic resonance spectroscopy in vivo. J. Cereb. Blood Flow Metab. 2000;20:736–746. doi: 10.1097/00004647-200004000-00011. [DOI] [PubMed] [Google Scholar]
- Routh VH. Glucosensing neurons in the ventromedial hypothalamic nucleus (VMN) and hypoglycemia-associated autonomic failure (HAAF) Diabetes Metab. Res. Rev. 2003;19:348–356. doi: 10.1002/dmrr.404. [DOI] [PubMed] [Google Scholar]
- Seaquist ER, Damberg GS, Tkac I, Gruetter R. The effect of insulin on in vivo cerebral glucose concentrations and rates of glucose transport/metabolism in humans. Diabetes. 2001;50:2203–2209. doi: 10.2337/diabetes.50.10.2203. [DOI] [PubMed] [Google Scholar]
- Segel SA, Fanelli CG, Dence CS, Markham J, Videen TO, Paramore DS, Powers WJ, Cryer PE. Blood-to-brain glucose transport, cerebral glucose metabolism, and cerebral blood flow are not increased after hypoglycemia. Diabete. 2001;s:50–1911. doi: 10.2337/diabetes.50.8.1911. [DOI] [PubMed] [Google Scholar]
- Shockley RP, LaManna JC. Determination of rat cerebral cortical blood volume changes by capillary mean transit time analysis during hypoxia, hypercapnia and hyperventilation. Brain Res. 1988;454:170–178. doi: 10.1016/0006-8993(88)90816-5. [DOI] [PubMed] [Google Scholar]
- Simpson IA, Appel NM, Hokari M, Oki J, Holman GD, Maher F, Koehler-Stec EM, Vannucci SJ, Smith QR. Bloodbrain barrier glucose transporter: effects of hypo- and hyperglycemia revisited. J. Neurochem. 1999;72:238–247. doi: 10.1046/j.1471-4159.1999.0720238.x. [DOI] [PubMed] [Google Scholar]
- Uehara Y, Nipper V, McCall AL. Chronic insulin hypoglycemia induces GLUT-3 protein in rat brain neurons. Am. J. Physiol. 1997;272:E716–E719. doi: 10.1152/ajpendo.1997.272.4.E716. [DOI] [PubMed] [Google Scholar]