Abstract
Background and Rationale
The reinstatement model is widely used animal model of relapse to drug addiction. However, the model’s validity is open to question.
Objective
We assess the reinstatement model in terms of criterion and construct validity.
Research highlights and Conclusions
We find that the reinstatement model has adequate criterion validity in the broad sense of the term, as evidenced by the fact that reinstatement in laboratory animals is induced by conditions reported to provoke relapse in humans. The model’s criterion validity in the narrower sense, as a medication screen, seems promising for relapse to heroin, nicotine, and alcohol. For relapse to cocaine, criterion validity has not yet established, primarily because clinical studies have examined medication’s effects on reductions in cocaine intake rather than relapse during abstinence. The model’s construct validity faces more substantial challenges and is yet to be established, but we argue that some of the criticisms of the model in this regard may have been overstated.
Keywords: Conditioned drug cues, Drug priming, Construct validity, Incubation, Criterion validity, Reinstatement, Relapse, Review, Stress
“All models are wrong, but some are useful.” —George Box, “Robustness in the strategy of scientific model building,” in Robustness in Statistics, R.L. Launer and G.N. Wilkinson, Editors. 1979, Academic Press: New York.
One of the main problems in the treatment of addiction is the high rate of relapse to drug use (Hunt et al. 1971; O'Brien 1997). Consequently, two major aims of preclinical research are (1) to elucidate the behavioral, environmental, and neural mechanisms underlying DRUG RELAPSE, and (2) to discover medications that will prevent relapse. The vast majority of studies on these topics (over 300 published papers since 2000) involve some version of a REINSTATEMENT model (Davis and Smith 1976; de Wit and Stewart 1981; Shaham et al. 2003; Stretch et al. 1971). In the self-administration version of the reinstatement model, which is based on an operant-conditioning paradigm, animals are trained to respond for drug infusions (or oral solutions in the case of alcohol), typically by pressing a lever; then, following EXTINCTION of the responding, non-reinforced pressing on the drug-associated lever is induced by drug priming injections (de Wit and Stewart 1981), drug cues (Crombag and Shaham 2002; Meil and See 1996; Weiss et al. 2000), or stressors (Shaham and Stewart 1995; Shalev et al. 2001b; Shepard et al. 2004). In the conditioned place preference (CPP) version of the reinstatement model, which is based on a classical-conditioning paradigm, CPP is induced by drug, extinguished, and then induced again by drug priming injections (Mueller and Stewart 2000) or stressors (Sanchez and Sorg 2001; Wang et al. 2006).
In this review, we assess the CRITERION VALIDITY and CONSTRUCT VALIDITY of the reinstatement model, referring primarily to the literature on heroin, alcohol, and cocaine, as these are the drugs that have been most extensively tested in this model (Bossert et al. 2005; Shaham et al. 2003; Shalev et al. 2002). We focus on reinstatement as a model of the overt behavior of relapse, although we also discuss the application of the model for the study of the subjective state of DRUG CRAVING. Table 1 provides a glossary of terms used in our review (CAPITAL LETTERS in the text). Before continuing with our assessment, we would like to point out that while two of the authors (YS and JS) have used the reinstatement model extensively in the past, the other two authors (DHE and KLP) are clinical researchers who have never used or plan to use the reinstatement model.
Table 1.
Glossary of terminology
| CONSTRUCT VALIDITY: A term refers to a similarity in the mechanisms underlying behavior in the model and behavior in the modeled condition. |
| CRITERION VALIDITY (often called predictive validity): A term refers to the extent to which laboratory-animal behavior induced by an experimental manipulation predicts human behavior induced by a similar event in the modeled condition. The concept is frequently narrowed, referring to a model’s ability to identify drugs with potential therapeutic value in humans. |
| DRUG CRAVING: An affective state that can be induced in human drug users by exposure to the self-administered drug, drug-associated cues or stress (O'Brien et al. 1992; Sinha et al. 2000). An unresolved issue in the addiction field is the degree to which craving is associated with drug relapse. |
| DRUG RELAPSE: A term used to describe the resumption of drug-taking behavior during periods of self-imposed or forced abstinence in humans (Wikler 1973). |
| EXTINCTION: The decrease in the frequency or intensity of learned responses after the removal of the unconditioned stimulus (e.g., food, drug) that has reinforced the learning (Pavlov 1927; Skinner 1953). In drug self-administration studies, extinction of lever responding is typically performed in the presence of contextual cues previously associated with drug availability (i.e., houselight) and responding leads to contingent presentations of a discrete cue (e.g., light, tone) previously paired with drug injections. In studies on discrete cue-induced reinstatement of drug seeking, extinction of lever (or nose-poke) responding is performed in the absence of the drug and a discrete cue (e.g., tone, light) that was previously paired with each drug infusion during training (See 2002). In conditioned place preference studies, extinction typically involves exposing rats (or mice) to the previously drug-paired context in the drug-free state (Shalev et al. 2002). |
| INCUBATION OF COCAINE CRAVING: A hypothetical process inferred from the findings of time-dependent increases in cue-induced cocaine seeking after withdrawal from cocaine self-administration in rats (Grimm et al. 2001; Lu et al. 2004b). |
| INCUBATION OF FEAR: A hypothetical process inferred from the findings of time-dependent increases in behavioral (e.g, freezing) or physiological (e.g., galvanic skin responses, heart rate) measures of anxiety and stress following exposure to cues previously paired with an aversive stimulus (e.g., footshock) (Eysenck 1968; Yehuda and Antelman 1993). |
| REINSTATEMENT: In the learning literature, reinstatement refers to the recovery of a learned response (e.g., lever-pressing behavior) that occurs when a subject is exposed non-contingently to the unconditioned stimulus (e.g., food) after extinction (Bouton and Swartzentruber 1991). In studies of reinstatement of drug seeking, reinstatement typically refers to the resumption of drug seeking after extinction following exposure to drugs, drug cues, or stressors (Shaham et al. 2003). |
| STRESS: A complex psychological construct that, despite many years of research (Cannon 1935; Selye 1956), has yet to be adequately operationally defined (Chrousos and Gold 1992; Cohen et al. 1982). In the context of animal models of psychiatric disorders, stress can be defined broadly as forced exposure to events or conditions that are normally avoided (Piazza and Le Moal 1998). In humans, the definition may be extended to incorporate cognitive and emotional responses—for example, “stress is a condition in which the environmental demands exceed the coping abilities of the individual” (Cohen et al. 1986). In laboratory animals, the precipitating events or conditions can be divided into two categories (Lu et al. 2003). The first category includes environmental events such as restraint, footshock, tail pinch, and defeat, events that are normally avoided by laboratory animals; also included in this category are “pharmacological stressors” (e.g., yohimbine; see text) that induce an avoidance response to an environment with which they have been paired.The second category includes food deprivation, social isolation, and maternal deprivation; each of these entails the removal of an environmental condition that is important for maintaining the animal’s normal physiological and psychological steady-state conditions, a state that the subject will attempt to ameliorate by seeking food, conspecific partners, or the dam. |
The criterion validity of the reinstatement model
Criterion validity: definition and importance
CRITERION VALIDITY (often called predictive validity), in its full sense, refers to the extent to which laboratory-animal behavior induced by an experimental manipulation predicts human behavior induced by a similar event in the modeled condition (Cronbach and Meehl 1955; Geyer and Markou 1995; Russell 1964). In the psychiatric literature, the concept is frequently narrowed, referring to a model’s ability to identify drugs with potential therapeutic value in humans (Geyer and Markou 1995; Markou et al. 1993; Sarter and Bruno 2002; Willner 1984). Some authors subdivide criterion validity into predictive, concurrent, and postdictive validity, depending on the temporal relationship between the predictor (i.e., findings in the model) and the external criterion (i.e., findings in the modeled condition) (Goldstein and Simpson 2002; Nunnally and Bernstein 1994). For example, if behavior in a model of anxiety were shown to be responsive to diazepam—long established as a prototypical anxiolytic drug—then the model would be said to possess postdictive validity, having retrospectively “predicted” an already known phenomenon. Authors have argued that the term predictive can still be used in such cases and that the logic of validation remains the same for all three temporal (predictive, concurrent, postdictive) conditions (Nunnally and Bernstein 1994). Nonetheless, we have chosen the term criterion validity over predictive validity to avoid the inadvertent implication that we are referring to the model’s prediction of subsequent findings in humans. Authors have generally agreed that a model must demonstrate criterion validity, especially in the narrow sense, in order to be useful (Geyer and Markou 1995; Katz and Higgins 2003; Markou et al. 1993; Sarter and Bruno 2002).
Assessment of criterion validity (full sense)
For the reinstatement model, criterion validity in the full sense is supported by the fact that reinstatement in laboratory animals (See 2002; Self and Nestler 1998; Shaham et al. 2003; Stewart 2000; Weiss 2005) is induced by conditions reported to provoke drug craving and relapse in humans: acute re-exposure to the drug (de Wit 1996), drug-associated cues (Childress et al. 1993), or STRESS (Sinha 2001). The main caveat regarding this point, at least in the case of heroin, cocaine, and alcohol, concerns not the model itself, but the unproven nature of the assumption that these factors truly induce relapse in humans (Epstein and Preston 2003). For these drugs, the idea that relapse results from acute exposure to drugs, drug cues, or stress during abstinence derives mostly from retrospective studies or from experimental manipulations of drug craving in a laboratory setting (Childress et al. 1992; Jaffe et al. 1989; Sinha 2001). Self-reports of craving (at least those obtained in a laboratory setting) only modestly predict real-life relapse (Carter and Tiffany 1999; Tiffany and Conklin 2000), and retrospective studies are subject to recall biases that limit interpretation (McKay et al. 2006). For example, retrospective but not prospective self-reports of stress have been associated with relapse to cocaine or heroin use (Hall et al. 1990; Wasserman et al. 1998). These findings could cast doubt on the clinical relevance of stress-induced reinstatement, but it is also possible that the time frame of the assessments was too long: stress levels were assessed only every several days. Shiffman and colleagues, who used electronic diaries and random prompting to assess relapse precipitants prospectively, reported that relapse was associated with increases in negative affect over a time course of hours (Shiffman et al. 1996; Shiffman and Waters 2004). (Negative affect is not identical to stress, but the two are closely related; Kassel et al. 2003.) Using the same techniques, Shiffman and colleagues also found that relapse was associated with real-life exposure to smoking-associated cues (Shiffman et al. 1996; Shiffman and Waters 2004). Finally, Shiffman and colleagues found that acute momentary exposure to nicotine (lapse) is positively associated with subsequent smoking relapse (Shiffman et al. 2006a), suggesting that drug priming-induced reinstatement in laboratory animals is potentially relevant to the human condition. However, it should be noted that in the human condition, lapse is a volitional act that is contingent on the individual’s behavior, while in reinstatement studies drug priming is given non-contingently by the experimenter (Stewart 2000).
Criterion validity (full sense): conclusions
Retrospective clinical evidence suggests that stimuli used to induce reinstatement in laboratory animals, such as acute exposure to stress, drug cues, or re-exposure to the self-administered drug, are relevant to the study of drug relapse in humans. Prospective clinical evidence from real-time assessment of relapse precipitants in smokers further supports the relevance of the model in humans. We are not familiar with studies incorporating real-time assessment of relapse precipitants with heroin, cocaine, or alcohol (McKay et al. 2006). If such studies support the assumption that drug relapse is caused by drug re-exposure, cues, and stress, they will help confirm the criterion validity of the reinstatement model.
Assessment of criterion validity (narrow pharmacological sense)
While most investigators in the addiction field probably agree that the reinstatement model has adequate criterion validity in the full sense, opinions differ concerning the model’s criterion validity in the narrow sense—its ability to identify drugs with potential therapeutic value in humans (Katz and Higgins 2003; O'Brien and Gardner 2005a). In discussing the potential evidence, it is important to note that clinical trials have very rarely been designed to assess relapse prevention in abstinent patients (Epstein and Preston 2003). Instead, the most commonly targeted outcome is decreases in ongoing drug intake (Vocci and Ling 2005). Thus, medication effects in most clinical trials may be more relevant for assessing the criterion validity of the drug self-administration procedure, a model widely used for assessing potential medications for drug addiction (Mello and Negus 1996), than for assessing the criterion validity of the reinstatement model. With this caveat in mind, we assess evidence for and against the pharmacological criterion validity of the reinstatement model.
For alcohol, unlike other drugs of abuse, clinical trials have been conducted specifically to test medications for relapse prevention in abstinent subjects. These trials have shown that the likelihood of relapse is modestly decreased by naltrexone (Latt et al. 2002; O'Malley et al. 1992; Streeton and Whelan 2001; Volpicelli et al. 1992) or acamprosate (Sass et al. 1996; Tempesta et al. 2000). The same drugs also prevent drug priming- and cue-induced reinstatement of alcohol seeking in rats (Bachteler et al. 2005; Ciccocioppo et al. 2003; Le et al. 1999). Thus, in the case of alcohol, the criterion validity of the reinstatement model seems to have received some empirical support. Full comparison between the reinstatement studies and the clinical trials is hampered by the fact that the drugs were administered acutely in the reinstatement studies and chronically in the clinical trials. A question for future research is whether acamprosate and naltrexone would remain effective in the reinstatement model following chronic treatment.
For opiates, the evidence is suggestive, but more preliminary. Naltrexone decreases relapse to heroin use in abstinent opioid abusers who have completed detoxification (Comer et al. 2006; Krupitsky et al. 2004; Shufman et al. 1994) and decreases heroin-priming-induced reinstatement in rats (Shaham and Stewart 1996). We are not familiar with studies specifically designed to assess the relapse-prevention effectiveness of methadone or buprenorphine, approved medications for opiate addiction (Dole et al. 1966; Johnson et al. 2000), but it is likely that relapse to heroin use is decreased by maintenance treatments with these drugs. Recently, Stewart and colleagues reported that methadone or buprenorphine prevents drug priming-induced reinstatement of heroin seeking (Leri et al. 2004; Sorge et al. 2005). These findings are particularly relevant in the context of the criterion validity of the reinstatement model because methadone or buprenorphine was given chronically via osmotic minipumps in an attempt to mimic the human condition of opiate maintenance (see also Shaham et al. (1996) for similar results with heroin given via osmotic minipumps). Interestingly, stress-induced reinstatement was not prevented by acute administration of naltrexone (Shaham and Stewart 1996) or chronic administration of opioids (Leri et al. 2004; Sorge et al. 2005), a finding that may offer a clue to the variability in clinical outcomes with opioid treatments. Finally, there is preliminary evidence that lofexidine (an alpha-2 adrenoceptor agonist) may help prevent relapse to opiate abuse (Sinha 2005). The sample size in the study of Sinha and colleagues is small, but if the finding is replicated, it would represent a novel treatment inspired by (rather than postdictively identified in) the reinstatement model: lofexidine and the similar drug clonidine have been shown to attenuate footshock-induced reinstatement of heroin, cocaine, or speedball (heroin-cocaine combination) seeking (Erb et al. 2000; Highfield et al. 2001; Shaham et al. 2000b). The lofexidine/speedball findings are particularly relevant to the human condition because lofexidine was given chronically before and during the tests for stress-induced reinstatement (Highfield et al. 2001).
For nicotine, there is some early evidence of correspondence between results from the reinstatement model and results from a clinical trial. The cannabinoid CB1 antagonist rimonabant (SR141716A) has been tested for relapse prevention in abstinent smokers; the available results suggest that it is effective (Fagerström and Balfour 2006). In rats, rimonabant administered acutely has been shown to attenuate cue-induced reinstatement of nicotine seeking (De Vries et al. 2005). Again, a question for future research is whether rimonabant would remain effective in the reinstatement model following chronic treatment.
For cocaine, the pharmacological criterion validity of the model is difficult to evaluate for two main reasons. First, despite decades of research, there are no effective medications for cocaine addiction (O'Brien and Gardner 2005b). Second, potential medications evaluated in the reinstatement model have not been tested in true relapse-prevention trials. Several of these potential medications, however, were tested in humans for their effect on cocaine intake or cocaine’s subjective effects, but none has yet been shown effective. For example, fluoxetine attenuates cue-induced reinstatement of cocaine seeking in rats (Burmeister et al. 2003), and SCH 31966 (ecopipam) attenuates priming-induced reinstatement of cocaine seeking in monkeys (Khroyan et al. 2000) and discriminative-cue-induced reinstatement of cocaine seeking in rats (Ciccocioppo et al. 2001). In contrast, in clinical trials, fluoxetine had no effect on cocaine use (Grabowski et al. 1995), and SCH 31966 had no effect on cocaine’s subjective effect or cocaine self-administration in laboratory settings (Haney et al. 2001; Nann-Vernotica et al. 2001); but see Romach et al., 1999 for different results). As mentioned above, however, we do not know the degree to which the criterion validity of the reinstatement model can be assessed in terms of results from human studies in which the dependent measure is ongoing drug intake (or subjective effects). In this regard, a large body of preclinical studies suggests that the neuronal mechanisms underlying reinstatement of cocaine seeking during forced abstinence differ from those that control ongoing cocaine self-administration (Kalivas and Volkow 2005; Shalev et al. 2002). In addition, Shiffman et al. (2006b) recently provided the first evidence for differential efficacy of a medication on drug use versus relapse. They found that whereas a high-dose nicotine patch had a modest effect on ongoing smoking and initial lapses among those who achieved abstinence, the pharmacological treatment had a profound effect on the progression to relapse among those who had lapsed.
A general issue relevant to the criterion validity of the reinstatement model is the effects of drugs used in agonist maintenance therapy in humans on reinstatement in laboratory animals. At issue here is that drugs used in agonist therapy in humans such as nicotine (Rose 1996), amphetamine (Grabowski et al. 2004), or opiate agonists (Jaffe 1990) reliably reinstate drug seeking in rats (De Vries et al. 1998; de Wit and Stewart 1983; Shaham et al. 1997a). Similarly, drugs such as methylphenidate (Schenk and Partridge 1999) or bromocriptine (Wise et al. 1990) induce cocaine seeking in the reinstatement model but have been used in clinical trials with cocaine abusers without evidence for increases in cocaine use (Gorelick and Wilkins 2006; Schubiner et al. 2002). This seeming discrepancy between the human condition and the reinstatement model can be explained by the pharmacokinetics associated with the mode of drug delivery. For example, replacement therapy with opiates and nicotine typically involves steady-state levels of drug that are achieved, for example, via daily oral ingestion of methadone or continuous transdermal absorption of nicotine. These forms of nicotine and methadone are not prone to abuse. In reinstatement studies, nicotine, opiates, or psychostimulants are administered by acute injections via the i.v., i.p. or s.c. routes (Shalev et al. 2002), paralleling the rapid-delivery modes of administration that are associated with drug abuse (Jaffe 1990; Johanson and Fischman 1989). Importantly, in several reinstatement studies in which opiate agonists were delivered chronically by osmotic minipumps, drug priming-induced reinstatement was attenuated (Leri et al. 2004; Shaham et al. 1996; Sorge et al. 2005), as would have been expected from the results of the clinical studies. A question for future research is whether chronic delivery of nicotine or amphetamine via osmotic minipumps would similarly decrease nicotine or cocaine seeking, respectively, in the reinstatement model.
Criterion validity (narrow pharmacological sense): conclusions
Although the pharmacological criterion validity of the reinstatement model is far from established, the model appears promising in the cases of alcohol, heroin, and nicotine. Acamprosate or naltrexone decreases alcohol relapse in humans and attenuates reinstatement of alcohol seeking induced by drug priming or cues in rats. Naltrexone decreases relapse to heroin use in abstinent humans and attenuates reinstatement of heroin seeking induced by drug priming in rats. Methadone and buprenorphine decrease heroin-priming-induced reinstatement in rats when the route of delivery (slow-release minipumps) mimics the conditions of clinical maintenance. Rimonabant decreases cue-induced reinstatement of nicotine seeking and appears to decrease relapse in smokers. As for cocaine, it has not been established that the model provides a useful screen for relapse-prevention medications. This state of affairs, however, is to a large degree because potential medications identified in reinstatement studies have not yet been assessed in clinical trials designed to assess relapse prevention in abstinence humans. In addition, several seeming discrepancies between findings from reinstatement studies and clinical trials may be accounted for by the route and timing of drug delivery. Finally, as discussed above, to the degree that the neuronal mechanisms underlying ongoing drug self-administration are different from those mediating relapse vulnerability during abstinence (Kalivas and Volkow 2005), discrepant results between reinstatement studies and clinical trials in which the main outcome measure is reduction in ongoing drug use intake should be interpreted with caution in the context of the model’s pharmacological criterion validity.
The construct validity of the reinstatement model
Construct validity: definition and importance
The term construct validity refers to a similarity in the mechanisms underlying behavior in the model and behavior in the modeled condition (Kornetsky 1977; McKinney and Bunney 1969; Sarter and Bruno 2002). There are differing opinions regarding its necessity. According to Geyer and Markou (1995): “There are only two criteria that a model must satisfy to establish its value in basic neurobiological research: reliability and predictive (i.e., criterion) validity. The satisfaction of other criteria, such as construct or discriminant validity, may have heuristic value; however, it is not essential.” In contrast, Sarter and Bruno (2002) argue that construct validity “represents the most important and a necessary component of the development and validation of an animal model in biological psychiatry.” Like Russell (1964), they warn that a model with criterion but not construct validity may identify the right medications “for the wrong reasons” and will fail to identify medications with novel mechanisms of action. We have discussed this concern in an earlier review (Epstein and Preston 2003). Though we believe that construct validity is desirable, it is important to keep in mind that very few of the widely used animal models of psychiatric disorders meet criteria for construct validity (Geyer and Markou 1995; Willner 1984; 1997). Nonetheless, we now address issues related to the construct validity of the reinstatement model. Rather than focus on findings with specific drug classes, we will frame our discussion in terms of general arguments against the model’s construct validity.
Assessment
There are four main issues to consider in assessing the construct validity of the reinstatement model, and each issue concerns a lack of homology between the model and the human condition: (1) the drug-free state occurs for different reasons in the model than in humans; (2) the contingencies involved in drug priming- and cue-induced reinstatement do not parallel those in human relapse; (3) stress-induced reinstatement relies mostly on footshock, which may not parallel the stressors encountered by humans; and (4) the time course of vulnerability to reinstatement of drug seeking is opposite that of relapse risk in humans. We address each of these issues in turn.
The drug-free state occurs for different reasons in the model than in humans
In the reinstatement model, rats cease to self-administer drug for only one reason: it is no longer available. In contrast, quit attempts among human drug abusers typically result from choices made in a complex, multi-operant environment in which the adverse consequences of ongoing drug use come to outweigh the reinforcing ones (Hughes 2002; Marlatt 1996). This is the clearest departure of the model from the human condition, as is frequently noted. There are, however, several issues that should be considered in this regard.
First, some drug-dependent patients have undergone formal extinction procedures in the context of treatment, and in those patients, reinstatement of conditioned physiological responses to drug-associated stimuli has been readily observed (O'Brien et al. 1986). What is unknown is whether the reinstated conditioned responses would have led to drug seeking in the patients’ daily environments; there may be only a modest relationship between cue reactivity in the laboratory and relapse in the drug environment (Carter and Tiffany 1999). In addition, extinction in humans may be fundamentally different from extinction in laboratory animals due to cognitive mediation of behavior in humans (Griffiths et al. 1980). Even if the process of extinction is the same across species, the fact remains that the extinction procedure was not the reason for abstinence in these patients; motivational factors had preceded it.
The second issue to consider is that homology in terms of motivational factors may be too much to ask. Ethnographic data suggest that cocaine abusers reassert control due largely to socially mediated contingencies that can be characterized as threats to the abusers’ “stake in conventional life” (Waldorf et al. 1992). We know of no attempts to model socially mediated contingencies in the context of any behavioral model of addiction, and we consider it unlikely that laboratory animals can learn to change their drug self-administration behavior in order to avoid distal adverse social consequences. This problem is not unique to the reinstatement model, it would apply to any animal model of addiction.
A third issue, less difficult to resolve, is whether the extinction operation has specific effects on subsequent responses to drug priming, cues, or stress—effects different even from those of forced abstinence. Extinction is an active learning process (Bouton 1993), and there is evidence that extinction of lever responding can alter brain neuroadaptations induced by prior exposure to cocaine self-administration (Self et al. 2004). In this regard, Fuchs (2006) argued that the neuroanatomical substrates of cue-induced reinstatement to cocaine seeking in rats seem to depend on whether the means of cessation was explicit extinction training or removal from the drug self-administration environment. Muscimol+baclofen inactivation of the dorsolateral caudate-putamen but not the basolateral amygdala or the dorsal prefrontal cortex decreased non-reinforced lever responding in an extinction test in rats that had been removed from the self-administration environment for two weeks without having undergone extinction of lever responding during the forced-abstinence period. In rats that had undergone extinction, muscimol+baclofen inactivation of the dorsolateral caudate-putamen attenuated both discrete-cue-induced- and contextual-cue-induced reinstatement, extending a previous finding (Vanderschuren et al. 2005). None of the rats that had undergone extinction of lever responding were given muscimol+baclofen inactivation of the basolateral amygdala or dorsal prefrontal cortex. Instead, citing historical data in which inactivation of those regions had attenuated discrete cue- or context-induced reinstatement (See 2005), Fuchs et al. concluded that “different neuronal substrates mediate cocaine seeking after abstinence versus extinction” (paper title).
However, the historical data involved regional inactivation with tetrodotoxin, not muscimol+baclofen. The use of different site-specific inactivation methods, however, can lead to different conclusions concerning the role of a given brain structure in reinstatement and behavior in general (Bossert et al. 2005). Particularly relevant here are previous findings from See and colleagues: while discrete-cue-induced reinstatement of cocaine seeking is not blocked by tetrodotoxin inactivation of the accumbens (with injections primarily aimed at the core) (Grimm and See 2000), it is blocked by muscimol+baclofen inactivation of the accumbens core (Fuchs et al. 2004). Thus, in the absence of the appropriate positive controls with muscimol+baclofen inactivation of the dorsal prefrontal cortex and basolateral amygdala for cue-induced reinstatement, it is premature to infer dissociation in the neuronal mechanisms underlying cue-induced drug seeking after abstinence versus extinction.
A fourth issue is that even if the dissociation were to be demonstrated, it would not address the crux of the validity question, which is not the difference between operant extinction and passive abstinence, but rather the difference between abstinence that is chosen and abstinence that is imposed entirely by uncontrollable circumstances. This question remains open and important. In terms of the human condition, enforced abstinence, while occurring less often, is not rare or irrelevant; it may accompany imprisonment or hospitalization, and is likely to be followed by relapse, even after long periods of time (Vaillant 1988). We know of no evidence that such relapse is functionally, mechanistically, or behaviorally different from relapse that follows volitional abstinence. In terms of modeling, there is now preliminary evidence that resumption of drug seeking occurs after drug priming and cues when drug seeking has been suppressed by means other than extinction. Based on earlier work (Smith and Davis 1974), Panlilio et al. (2003; 2005) suppressed ongoing drug self-administration through punishment (shock delivery contingent on lever pressing). They found that in rats that have undergone this punishment procedure, opiate (remifentanil) seeking resumed after a priming injection of heroin. Similarly, Katzir et al. (2006) assessed resumption of cocaine seeking rats, using a conflict procedure based on early work of Warden and colleagues (Jenkins et al. 1926; Warden 1931). After cocaine self-administration was eliminated by electrification of the grid near the lever, non-contingent exposure to cocaine priming injections or non-contingent exposure to a discrete tone-light cue led to resumption of lever responding. A question for future research is whether resumption of operant responding in these punishment and conflict models involves different neurobiological mechanisms from those that underlie reinstatement of responding after extinction.
The contingencies involved in drug priming- and cue-induced reinstatement do not parallel those in human relapse
The issue here is that during tests for reinstatement in laboratory animals, the drug priming injections are given non-contingently (de Wit and Stewart 1981), and exposure to discrete drug cues is contingent on the animal’s operant behavior (See 2002). In contrast, relapse episodes in humans typically involve contingent exposure to drugs or non-contingent exposure to drug-related cues (Everitt and Robbins 2000; Markou et al. 1999; Marlatt 1996; Marlatt 2002).
It is true that non-contingent priming is not identical to the self-initiated “lapse” (Shiffman 2005) it is intended to model (see above). However, in a variation of the reinstatement model, heroin seeking has now been shown to be reinitiated by acute exposure to the self-administered drug (Leri and Stewart 2002). With regard to cues, Grimm et al. (2000) reported that resumption of lever responding after extinction occurs only with contingent exposure to a discrete 5-sec tone-light cue that was previously paired with each cocaine infusion during training. However, in other variations of the reinstatement model, robust reinstatement of drug seeking was reported following non-contingent exposure to discriminative (McFarland and Ettenberg 1997; Weiss et al. 2000) or contextual cues (Bossert et al. 2006; Bossert et al. 2004; Crombag and Shaham 2002).
Stress-induced reinstatement relies mostly on footshock, which may not parallel the stressors encountered by humans
At issue here is that there is no human equivalent to intermittent footshock, the stressor most commonly used in reinstatement studies (Lu et al. 2003; Shaham et al. 2000a). Also, not all stressors can induce reinstatement (Shalev et al. 2002), and footshock itself is not effective outside of the environment in which drug self-administration occurred (Shalev et al. 2000) or outside a restricted range of shock intensity (Shaham 1996). Another commonly used laboratory stressor is food deprivation (Dallman et al. 1999), which also reinstates drug seeking (Carroll 1985; Shalev et al. 2006; Shalev et al. 2001b); this, too, is effective only under specific deprivation parameters (Highfield et al. 2002; Shalev et al. 2000) and experimental conditions (Carroll 1985). It is also unlikely that food deprivation is a major precipitant of human drug relapse, at least in Western societies.
In dealing with the generality of the findings of footshock-induced reinstatement, two main issues should be considered. The first issue is that there is now evidence that reinstatement in rats can be induced by other stressors. One approach has been to move “downstream,” directly inducing some physiological components of a stress response by administering a drug that induces stress-like responses in humans. For example, the alpha-2 adrenoceptor antagonist yohimbine, which induces stress-like symptoms in both humans and laboratory animals (Bremner et al. 1996a; 1996b), reliably reinstates drug seeking in both rats and monkeys (Le et al. 2005; Lee et al. 2004; Shepard et al. 2004). Similarly, the stress neurohormone corticotrophin-releasing factor (CRF) reinstates drug seeking after ventricular or site-specific brain injections (Erb et al. 2006; Erb and Stewart 1999; Le et al. 2002; Shaham et al. 1997b; Wang et al. 2005). Another approach has been to move “upstream”; for example, Ribeiro Do Couto et al. (2006) reported that social-defeat stress—regarded as an ethologically valid stressor (Miczek et al. 2002; Miczek et al. 1991)—reinstates CPP for morphine in mice at least as effectively as do common laboratory stressors such as tail pinch and restraint. In rats, Funk et al. (2005) reported that while social-defeat experience does not reinstate alcohol seeking, an odor cue paired with the defeat experience modestly reinstates this seeking.
The second issue is that the addiction field is not the only psychiatric discipline in which footshock stress has been used for research. In fact, the use of footshock stress helped enable many of the major breakthroughs over the last several decades in the understanding of the neurobiological, cognitive, and behavioral substrates of anxiety and depression; footshock was the stressor used in procedures such as conditioned fear, fear-potentiated startle, and learned helplessness (Davis et al. 2006; LeDoux 2000; Maier and Watkins 2005). One cautionary note is that the intensity of footshock in studies on footshock-induced reinstatement of drug seeking is typically lower than those used in animal models of depression, fear and anxiety (in which the shock parameters used result in freezing behavior). In our view, based on the past history of the successful use of the footshock stressor in other psychiatric fields, it may be premature to dismiss findings from studies of footshock-stress-induced reinstatement on the basis of there being no clear human analog for this manipulation.
The time course of vulnerability to reinstatement is opposite to that of relapse risk in humans
Another potential departure of the reinstatement model from the human condition is that the magnitude of the reinstatement response induced by drug priming (Lu et al. 2004a; Tran-Nguyen et al. 1998), footshock stress (Shalev et al. 2001a), and discrete cues (Grimm et al. 2001) does not decrease over time, as would have been predicted from the clinical literature that seems to show that the risk for relapse decreases as abstinence lengthens (Gilpin et al. 1997; Gossop et al. 1990; Higgins et al. 2000; McKay et al. 2001). In fact, in the case of cocaine cues, reinstatement responding increases over the first two months of withdrawal from the drug, a phenomenon termed INCUBATION OF COCAINE CRAVING (Grimm et al. 2001; Lu et al. 2004b). This incubation phenomenon is not limited to the reinstatement model; it is readily observed when rats are tested for the first time at different withdrawal periods in extinction in the presence of the drug-associated cues (Lu et al. 2004a; Lu et al. 2005) or in a procedure in which cues associated with cocaine, heroin, or sucrose are used as conditioned reinforcers for the acquisition of a new instrumental response (Di Ciano and Everitt 2004). Nevertheless, because incubation was first demonstrated in studies using the reinstatement model, we discuss below this phenomenon and its relevance to human relapse.
The main methodological factor to consider in discussing incubation’s potential role in relapse is that when a human drug addict relapses, there is no way to determine whether his or her vulnerability to relapse would have continued to increase beyond the time point when the relapse actually occurred; a survival curve would not show it. We attempt to illustrate this idea in Figure 1, a hypothetical depiction of three factors that might contribute to the rate of relapse: two risk factors (acute withdrawal and cue reactivity) and one protective factor (reflecting the finding that initial success in abstinence predicts long-term success (Higgins et al. 2000; Lussier et al. 2005). The top panel of Figure 1 shows the time course of each factor separately, and the bottom panel shows the individual and the combined effects of the factors, expressed as percent of users remaining abstinent. Thus, susceptibility to relapse due to withdrawal peaks within days of cessation, reflecting an early, sharp rise in withdrawal symptoms. Susceptibility to relapse due to cue reactivity rises gradually over a period of weeks or months (this is the hypothesized incubation effect), and eventually flattens again. In contrast, relapse susceptibility decreases as a function of duration of successful abstinence. The actual rate of relapse results from the combined effects of all three factors; we show this as a solid black line whose shape corresponds to empirically observed relapse curves for heroin, alcohol, and tobacco (Hunt et al. 1971).
Figure 1. Hypothetical depiction of the contribution of relapse and abstinence factors to an observed relapse survival curve.
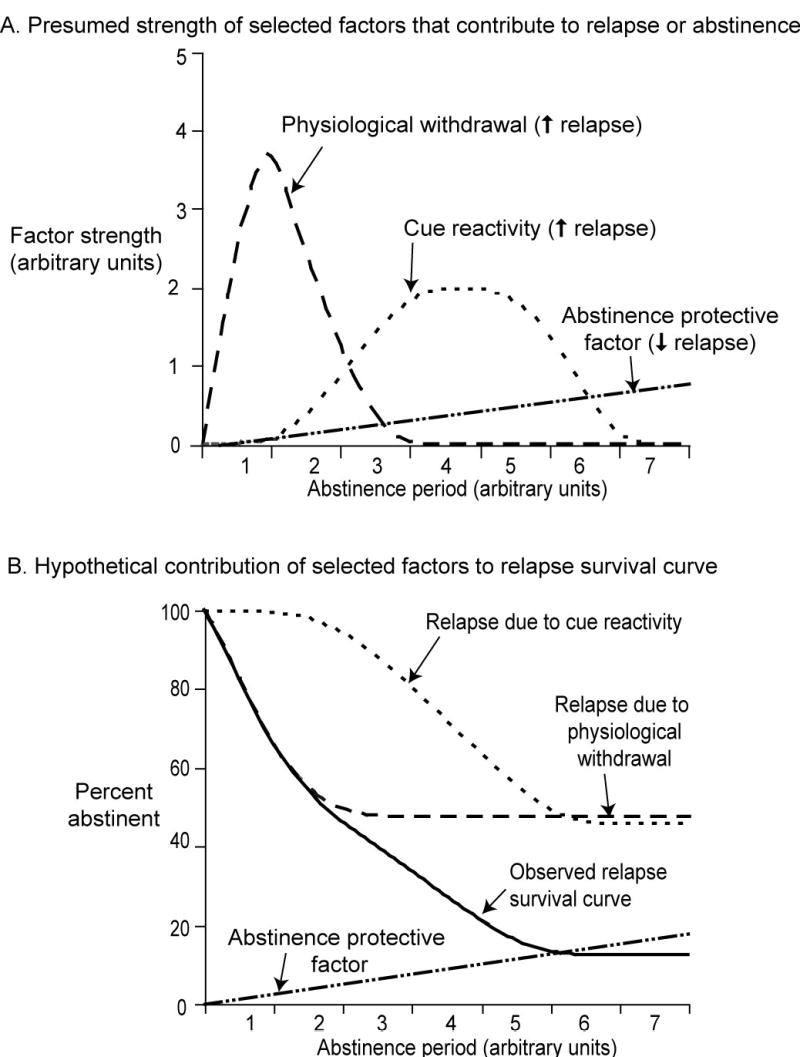
(A) Time course of strength of three factors hypothesized to influence relapse: physiological withdrawal (dashed line; early onset and offset), cue reactivity (dotted line; gradual onset and offset), and an abstinence protective factor (dotted-dashed line; gradual increase over time). (B) Relapse survival curve (percent abstinent): relapse due to each of two factors (physiological withdrawal, dashed line; cue reactivity, dotted line) was calculated for each time point by subtracting a constant proportion of the factor’s strength (shown above in A) from 100. The Observed Relapse Survival Curve (solid black line) was then calculated by adding relapse due to physiological withdrawal and cue reactivity, subtracting the abstinence protective factor (dotted-dashed line) and subtracting the result from 100.
This figure is an oversimplification, omitting unknown risk factors and protective factors. But an even greater oversimplification may be to conclude that the risk of relapse decreases as the duration of abstinence increases. As Figure 1 illustrates, real data on relapse rates may be compatible with the existence of the incubating risk factor originally suggested by reinstatement studies.
The existence of an incubating risk factor in humans is tentatively supported by studies of the time course of symptomatology during drug-use cessation. For example, out of 337 patients in a smoking-cessation study, 130 (39%) reported an increase in smoking urge and negative affect toward the end of the 50-day study period, well beyond the typical duration of the acute abstinence syndrome (Piasecki et al. 1998). We speculate that some of these late-emerging “withdrawal” symptoms reflect incubation of reactivity to smoking cues. Similarly, in cocaine-dependent patients, Kosten et al. (2005) found a “build-up” of abstinence symptomatology from weeks 2-5 of abstinence; relapse itself peaked during week 6.
One apparently contradictory finding is that, when smokers were randomized to 1 day, 7 days, or 14 days of paid abstinence followed by a cigarette self-administration session, smoking was chosen less frequently by the 14-day abstainers than by the 7-day or 1-day abstainers, despite the absence of any group difference in nicotine-withdrawal symptoms on the test day (Lussier et al. 2005). It seems clear that no incubation was detected in this study. One possible explanation is that incubation in humans requires more time to emerge (as is suggested by the findings of Piasecki et al. (1998). Another possible explanation is that the smokers, who had expressed no interest in long-term quitting at study intake, became more interested in maintaining their paid abstinent status as they achieved longer periods of success. This would have differentially affected behavior in the group randomized to 14-day abstinence. Future studies could incorporate repeated assessments of “interest in quitting” in order to explore this potential alternative interpretation of Lussier et al.’s data.
Finally, it is interesting to note that a seemingly similar “discrepancy” exists between preclinical and clinical research in the context of the well-established phenomenon of INCUBATION OF FEAR (Bindra and Cameron 1953; Brady 1951; Diven 1937; Eysenck 1968): relapse rates to anxiety-related disorders are lower with increasing duration of the patients' symptom-free periods (Yonkers et al., 2003), yet it has been demonstrated in both laboratory animals and humans that the responsiveness to anxiety- or stress-provoking stimuli can increase over time (Antelman et al. 2000; Davidson et al. 2004; Houston et al. 1999; Yehuda and Antelman 1993). In anxiety research, fear incubation is viewed as one of the basic mechanisms underlying anxiety-related disorders (Davidson et al. 2004; McFarlane 2000), and the different time courses of relapse rates and responsiveness to anxiety provoking stimuli are not considered to be necessarily contradictory. Rather, these different time courses may be due in part to the fact that in clinical studies, subjects are not randomly assigned to different symptom-free periods.
Reinstatement and craving
A final issue related to the construct validity of the reinstatement model is the model’s relevance to the study of drug craving. It has been suggested, by us and by others, that the model could be used to study the neuronal mechanisms underlying drug craving (Grimm et al. 2001; O'Brien 2005). However, craving is a problematic construct in the context of drug relapse. In humans, craving may not be the primary mediator of relapse (Tiffany and Carter 1998). (This issue can be assessed empirically in studies using real-time, in-the-field assessment that, to our knowledge, have yet to be performed.) In laboratory animals, craving cannot be directly assessed; it can only be inferred. The issues inherent in the concept of craving (or other motivational constructs related to addiction that can only be inferred from overt behavior but not directly measured, e.g., expectancy, impulsivity) and its measurement do not threaten the construct validity of the reinstatement model. Reinstatement clearly models overt drug-seeking behavior, so its utility and validity in that regard does not require a causal chain that includes craving. If, however, a causal chain were shown to exist between craving and relapse in the drug user’s daily environment—and if human laboratory findings were shown to predict vulnerability to this type of relapse—another line of evidence could be adduced to address the construct validity of the reinstatement model: the correspondence between the neuroanatomical substrates of reinstatement and the neuroanatomical correlates of human craving.
For example, activation of Fos (the protein product of the immediate early gene c-fos, a marker of neuronal activity; (Curran and Morgan 1995)) has been studied in cue-induced reinstatement of alcohol and cocaine seeking (Ciccocioppo et al. 2001; Neisewander et al. 2000); cue-induced reinstatement of cocaine seeking has also been studied electrophysiologically (Rebec and Sun 2005). Consistently, activated brain areas in these reinstatement studies include portions of prefrontal cortex, insular cortex, and the amygdala—brain areas also implicated in cue-induced craving for cocaine or alcohol in human neuroimaging studies (Childress et al. 1999; Garavan et al. 2000; Grant et al. 1996; Kilts et al. 2001; Maas et al. 1998; Wexler et al. 2001). These anatomical homologies may be somewhat crude, but they merit continued investigation. Another possible homology, at the behavioral level, is that reinstatement of cocaine seeking is attenuated by systemic injections of N-acetylcysteine that results in tonic increases in accumbens glutamate levels in rats (Baker et al. 2003); preliminary results suggest that the same drug attenuates cocaine craving in addicted humans (LaRowe et al. 2006). Again, the relevance of this work to the reinstatement model depends on the relevance of craving to relapse, and on the ecological relevance of human laboratory findings—all unknown.
Construct validity: conclusions
The construct validity of the reinstatement model has not yet been established. In some cases, this is because data on the relevant clinical phenomena are sparse or nonexistent (for example, assessment of relapse vulnerability after non-contingent exposure to a priming dose of drug during abstinence, or in subjects randomly assigned to different abstinence periods). While there is reasonable homology between reinstatement-associated brain activation in rats and craving-associated brain activation in a human laboratory setting, it has not been established that craving plays a causal role in relapse in drug users’ daily environments, so the relevance of the neuroanatomical homology is unknown. Finally, we would like to emphasize that uncertain construct validity is not unique to the reinstatement model; it is inevitable for any model of a psychiatric disorder with unknown etiology (Geyer and Markou 1995; Willner 1984).
Future prospects
The criterion validity of the reinstatement model will be judged on the basis of results of two types of future clinical work: natural-history studies designed to differentiate subtypes of relapse and to examine evidence for a causal role of situational craving in human relapse (Shiffman 2005; Shiffman et al. 1997), and medication trials in which the main outcome is relapse probability after a period of abstinence.
The construct validity of the reinstatement model—or at least the most contentious aspect of it—will be judged on the basis of findings from alternative models of relapse, models that eschew extinction in favor of other ways of suppressing self-administration of drug. In the strictest sense, such models cannot be called variants or extensions of the reinstatement model, because the original meaning of the term reinstatement explicitly incorporates the idea that a reinstated behavior is one that has been previously extinguished (Bouton and Swartzentruber 1991). As mentioned above, results from the reinstatement model generalize to the newer punishment and conflict models, at least in the case of drug priming and cues. These new models can be used to study the generality of findings obtained from the reinstatement model, and for exploring human relapse precipitants that are not readily manifested in this model. For example, Panlilio et al. (2005) reported that the anxiolytic drug lorazepam, which does not reinstate extinguished opiate seeking in the reinstatement model, induces resumption of lever responding in the punishment model. This finding may have clinical relevance because in cocaine-dependent humans, abuse of sedative/hypnotic drugs is associated with relapse to cocaine (McKay et al. 1999).
Another area in need of exploration is the assessment of relapse to drug seeking in a choice procedure in which non-drug rewards are available. To date, cocaine priming-induced resumption of drug seeking in a choice situation has been studied only on test days interspersed with days of ongoing self-administration (Gasior et al. 2004).
Finally, as data from imaging studies suggest that similar neuronal mechanisms are involved in reinstatement of drug seeking in laboratory animals and craving in humans (Kalivas and Volkow 2005), a critical issue for the validation of the construct validity of the reinstatement model is the degree to which craving induced by drug, cues and stress in the drug environment is related to drug relapse.
Acknowledgments
DE, KP, and YS receive their financial support from the Intramural Research Program of the NIH National Institute on Drug Abuse; JS receives her financial support from the Canadian Institutes of Health Research. We thank Roy Wise and one of the reviewers for critical comments on earlier versions of this paper.
References
- Antelman SM, Levine J, Gershon S. Time-dependent sensitization: the odyssey of a scientific heresy from the laboratory to the door of the clinic. Mol Psychiatry. 2000;5:350–356. doi: 10.1038/sj.mp.4000721. [DOI] [PubMed] [Google Scholar]
- Bachteler D, Economidou D, Danysz W, Ciccocioppo R, Spanagel R. The effects of acamprosate and neramexane on cue-induced reinstatement of ethanol-seeking behavior in rat. Neuropsychopharmacology. 2005;30:1104–1110. doi: 10.1038/sj.npp.1300657. [DOI] [PubMed] [Google Scholar]
- Baker DA, McFarland K, Lake RW, Shen H, Tang XC, Toda S, Kalivas PW. Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nat Neurosci. 2003;6:743–749. doi: 10.1038/nn1069. [DOI] [PubMed] [Google Scholar]
- Bindra D, Cameron L. Changes in experimentallly poroduced anxiety with the passage of time: incubation effect. J Exp Psychol. 1953;45:197–203. doi: 10.1037/h0055125. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Ghitza UE, Lu L, Epstein DH, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: An update and clinical implications. Eur J Pharmacol. 2005;526:36–50. doi: 10.1016/j.ejphar.2005.09.030. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Gray SM, Lu L, Shaham Y. Activation of group II metabotropic glutamate receptors in the nucleus accumbens shell attenuates context-induced relapse of heroin seeking. Neuropsychopharmacology. 2006;31:2197–3109. doi: 10.1038/sj.npp.1300977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Liu SY, Lu L, Shaham Y. A role of ventral tegmental area glutamate in contextual cue-induced relapse to heroin seeking. J Neurosci. 2004;24:10726–10730. doi: 10.1523/JNEUROSCI.3207-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME. Context, time, and memory retrieval in the interference paradigms of Pavlovian learning. Psychol Bull. 1993;114:80–99. doi: 10.1037/0033-2909.114.1.80. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Swartzentruber D. Sources of relapse after extinction in Pavlovian and instrumental learning. Clin Psychol Rev. 1991;11:123–140. [Google Scholar]
- Brady JV. The effect of electro-convulsive shock on a conditioned emotional response: the permanence of the effect. J Comp Physiol Psychol. 1951;44:507–511. doi: 10.1037/h0062743. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Krystal JH, Southwick SM, Charney DS. Noradrenergic mechanisms in stress and anxiety: I. preclinical studies. Synapse. 1996a;23:28–38. doi: 10.1002/(SICI)1098-2396(199605)23:1<28::AID-SYN4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Krystal JH, Southwick SM, Charney DS. Noradrenergic mechanisms in stress and anxiety: II.clinical studies. Synapse. 1996b;23:39–51. doi: 10.1002/(SICI)1098-2396(199605)23:1<39::AID-SYN5>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Burmeister JJ, Lungren EM, Neisewander JL. Effects of fluoxetine and d-fenfluramine on cocaine-seeking behavior in rats. Psychopharmacology. 2003;168:146–154. doi: 10.1007/s00213-002-1307-8. [DOI] [PubMed] [Google Scholar]
- Cannon WB. Stresses and strains of homeostasis. Am J Med Sci. 1935;189:1–14. [Google Scholar]
- Carroll ME. The role of food deprivation in the maintenance and reinstatement of cocaine-seeking behavior in rats. Drug Alcohol Dependence. 1985;16:95–109. doi: 10.1016/0376-8716(85)90109-7. [DOI] [PubMed] [Google Scholar]
- Carter BL, Tiffany ST. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999;94:327–340. [PubMed] [Google Scholar]
- Childress AR, Ehrman R, Rohsenow DJ, Robbins SJ, O'Brien CP. Classically conditioned factors in drug dependence. In: Lowinson JW, Luiz P, Millman RB, Langard G, editors. Substance abuse: A comprehensive textbook. Williams and Wilkins; Baltimore: 1992. pp. 56–69. [Google Scholar]
- Childress AR, Hole AV, Ehrman RN, Robbins SJ, McLellan AT, O'Brien CP. Cue reactivity and cue reactivity interventions in drug dependence. NIDA Res Monogr. 1993;137:73–95. [PubMed] [Google Scholar]
- Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O'Brien CP. Limbic activation during cue-induced cocaine craving. Am J Psychiatry. 1999;156:11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrousos GP, Gold PW. The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. JAMA. 1992;267:1244–1452. [PubMed] [Google Scholar]
- Ciccocioppo R, Lin D, Martin-Fardon R, Weiss F. Reinstatement of ethanol-seeking behavior by drug cues following single versus multiple ethanol intoxication in the rat: effects of naltrexone. Psychopharmacology. 2003;168:208–215. doi: 10.1007/s00213-002-1380-z. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Sanna PP, Weiss F. Cocaine-predictive stimulus induces drug-seeking behavior and neural activation in limbic brain regions after multiple months of abstinence: reversal by D(1) antagonists. Proc Natl Acad Sci (USA) 2001;98:1976–1981. doi: 10.1073/pnas.98.4.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen F, Horowitz MJ, Lazarus RS, Moos RH, Robbins LN, Rose RM, Rutter M. 1982. In: Eliott GR, Eisdorfer C, editors. Panel report on psychosocial and modifiers of stress. Stress and human health. Springer Publishing Co.; New York: pp. 147–188. [Google Scholar]
- Cohen S, Evans GW, Stokols D, Krantz DS. Behavior, health, and environmental stress. Plenum Press; Plenum Press: 1986. [Google Scholar]
- Comer SD, Sullivan MA, Yu E, Rothenberg JL, Kleber HD, Kampman K, Dackis C, O'Brien CP. Injectable, sustained-release naltrexone for the treatment of opioid dependence: a randomized, placebo-controlled trial. Arch Gen Psychiatry. 2006;63:210–8. doi: 10.1001/archpsyc.63.2.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombag HS, Shaham Y. Renewal of drug seeking by contextual cues after prolonged extinction in rats. Behav Neurosci. 2002;116:169–173. doi: 10.1037//0735-7044.116.1.169. [DOI] [PubMed] [Google Scholar]
- Cronbach LJ, Meehl PE. Construct validity in psychological tests. Psychol Bull. 1955;52:281–302. doi: 10.1037/h0040957. [DOI] [PubMed] [Google Scholar]
- Curran T, Morgan JI. Fos: an immediate-early transcription factor in neurons. J Neurobiol. 1995;26:403–12. doi: 10.1002/neu.480260312. [DOI] [PubMed] [Google Scholar]
- Dallman MF, Akana SF, Bhatnagar S, Bell ME, Choi S, Chu A, Horsley C, Levin N, Meijer O, Soriano LR, Strack AM, Viau V. Starvation: early signals, sensors, and sequelae. Endocrinology. 1999;140:4015–4023. doi: 10.1210/endo.140.9.7001. [DOI] [PubMed] [Google Scholar]
- Davidson JR, Stein DJ, Shalev AY, Yehuda R. Posttraumatic stress disorder: acquisition, recognition, course, and treatment. J Neuropsychiatry Clin Neurosci. 2004;16:135–147. doi: 10.1176/jnp.16.2.135. [DOI] [PubMed] [Google Scholar]
- Davis M, Myers KM, Chhatwal J, Ressler KJ. Pharmacological treatments that facilitate extinction of fear: relevance to psychotherapy. NeuroRx. 2006;3:82–96. doi: 10.1016/j.nurx.2005.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis WM, Smith SG. Role of conditioned reinforcers in the initiation, maintenance and extinction of drug-seeking behavior. Pavlovian J Biol Sci. 1976;11:222–236. doi: 10.1007/BF03000316. [DOI] [PubMed] [Google Scholar]
- De Vries TJ, de Vries W, Janssen MC, Schoffelmeer AN. Suppression of conditioned nicotine and sucrose seeking by the cannabinoid-1 receptor antagonist SR141716A. Behav Brain Res. 2005;161:164–168. doi: 10.1016/j.bbr.2005.02.021. [DOI] [PubMed] [Google Scholar]
- De Vries TJ, Schoffelmeer AN, Binnekade R, Mulder AH, Vanderschuren LJ. Drug-induced reinstatement of heroin- and cocaine-seeking behaviour following long-term extinction is associated with expression of behavioural sensitization. Eur J Neurosci. 1998;10:3565–3571. doi: 10.1046/j.1460-9568.1998.00368.x. [DOI] [PubMed] [Google Scholar]
- de Wit H. Priming effects with drugs and other reinforcers. Exp Clin Psychopharmacol. 1996;4:5–10. [Google Scholar]
- de Wit H, Stewart J. Reinstatement of cocaine-reinforced responding in the rat. Psychopharmacology. 1981;75:134–143. doi: 10.1007/BF00432175. [DOI] [PubMed] [Google Scholar]
- de Wit H, Stewart J. Drug reinstatement of heroin-reinforced responding in the rat. Psychopharmacology. 1983;79:29–31. doi: 10.1007/BF00433012. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Everitt BJ. Conditioned reinforcing properties of stimuli paired with self-administered cocaine, heroin or sucrose: implications for the persistence of addictive behaviour. Neuropharmacology. 2004;47(Suppl 1):202–213. doi: 10.1016/j.neuropharm.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Diven K. Certain determinants in the conditioning of anxiety reactions. J Psychol. 1937;3:291–308. [Google Scholar]
- Dole VP, Nyswander ME, Kreek MJ. Narcotic blockade. Arch Intern Med. 1966;118:304–309. [PubMed] [Google Scholar]
- Epstein DH, Preston KL. The reinstatement model and relapse prevention: a clinical perspective. Psychopharmacology. 2003;168:31–41. doi: 10.1007/s00213-003-1470-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb S, Hitchcott PK, Rajabi H, Mueller D, Shaham Y, Stewart J. Alpha-2 adrenergic agonists block stress-induced reinstatement of cocaine seeking. Neuropsychopharmacology. 2000;23:138–150. doi: 10.1016/S0893-133X(99)00158-X. [DOI] [PubMed] [Google Scholar]
- Erb S, Petrovic A, Yi D, Kayyali H. Central injections of CRF reinstate cocaine seeking in rats after postinjection delays of up to 3 h: an influence of time and environmental context. Psychopharmacology (Berl) 2006;187:112–120. doi: 10.1007/s00213-006-0392-5. [DOI] [PubMed] [Google Scholar]
- Erb S, Stewart J. A role for the bed nucleus of the stria terminalis, but not the amygdala, in the effects of corticotropin-releasing factor on stress-induced reinstatement of cocaine seeking. J Neurosci. 1999;19:RC35. doi: 10.1523/JNEUROSCI.19-20-j0006.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Second-order schedules of drug reinforcement in rats and monkeys: measurement of reinforcing efficacy and drug-seeking behaviour. Psychopharmacology. 2000;153:17–30. doi: 10.1007/s002130000566. [DOI] [PubMed] [Google Scholar]
- Eysenck HJ. A theory of the incubation of anxiety-fear responses. Behav Res Ther. 1968;6:309–321. doi: 10.1016/0005-7967(68)90064-8. [DOI] [PubMed] [Google Scholar]
- Fagerström K, Balfour DJ. Neuropharmacology and potential efficacy of new treatments for tobacco dependence. Expert Opin Investig Drugs. 2006;15:107–16. doi: 10.1517/13543784.15.2.107. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Branham RK, See RE. Different neural substrates mediate cocaine seeking after abstinence versus extinction training: a critical role for the dorsolateral caudate–putamen. J Neurosci. 2006;26:3584–88. doi: 10.1523/JNEUROSCI.5146-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Parker MC, See RE. Differential involvement of the core and shell subregions of the nucleus accumbens in conditioned cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology. 2004;176:459–465. doi: 10.1007/s00213-004-1895-6. [DOI] [PubMed] [Google Scholar]
- Funk D, Harding S, Juzytsch W, Le AD. Effects of unconditioned and conditioned social defeat on alcohol self-administration and reinstatement of alcohol seeking in rats. Psychopharmacology. 2005;183:341–349. doi: 10.1007/s00213-005-0194-1. [DOI] [PubMed] [Google Scholar]
- Garavan H, Pankiewicz J, Bloom A, Cho JK, Sperry L, Ross TJ, Salmeron BJ, Risinger R, Kelley D, Stein EA. Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. Am J Psychiatry. 2000;157:1789–1798. doi: 10.1176/appi.ajp.157.11.1789. [DOI] [PubMed] [Google Scholar]
- Gasior M, Paronis CA, Bergman J. Modification by dopaminergic drugs of choice behavior under concurrent schedules of intravenous saline and food delivery in monkeys. J Pharmacol Exp Ther. 2004;308:249–259. doi: 10.1124/jpet.103.052795. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Markou A. Psychopharmacology: the fourth generation of progress. Raven Press; New York: 1995. Animal models of psychiatric disorders; pp. 787–798. [Google Scholar]
- Gilpin EA, Pierce JP, Farkas AJ. Duration of smoking abstinence and success in quitting. J Natl Cancer Inst. 1997;89:572–526. doi: 10.1093/jnci/89.8.572. [DOI] [PubMed] [Google Scholar]
- Goldstein JM, Simpson JC. Validity: Definition and Applications to Psychiatric Research. In: Tsuang MT, Tohen M, editors. Textbook in Psychiatric Epidemiology. 2nd ed. Wiley-Liss; New York: 2002. pp. 149–163. [Google Scholar]
- Gorelick DA, Wilkins JN. Bromocriptine treatment for cocaine addiction: association with plasma prolactin levels. Drug Alcohol Depend. 2006;81:189–195. doi: 10.1016/j.drugalcdep.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Gossop M, Green L, Phillips G, Bradley B. Factors predicting outcome among opiate addicts after treatment. Br J Clin Psychol. 1990;29 ( Pt 2):209–216. doi: 10.1111/j.2044-8260.1990.tb00871.x. [DOI] [PubMed] [Google Scholar]
- Grabowski J, Rhoades H, Elk R, Schmitz J, Davis C, Creson D, Kirby K. Fluoxetine is ineffective for treatment of cocaine dependence or concurrent opiate and cocaine dependence: two placebo-controlled double-blind trials. J Clin Psychopharmacol. 1995;15:163–174. doi: 10.1097/00004714-199506000-00004. [DOI] [PubMed] [Google Scholar]
- Grabowski J, Rhoades H, Stotts A, Cowan K, Kopecky C, Dougherty A, Moeller FG, Hassan S, Schmitz J. Agonist-like or antagonist-like treatment for cocaine dependence with methadone for heroin dependence: two double-blind randomized clinical trials. Neuropsychopharmacology. 2004;29:969–981. doi: 10.1038/sj.npp.1300392. [DOI] [PubMed] [Google Scholar]
- Grant S, London ED, Newlin DB, Villemagne VL, Liu X, Contoreggi C, Phillips RL, Kimes AS, Margolin A. Activation of memory circuits during cue-elicited cocaine craving. Proc Natl Acad Sci (USA) 1996;93:12040–12045. doi: 10.1073/pnas.93.21.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths RR, Bigelow GE, Henningfield JE. Similarity in animal and human drug-taking behavior. In: Mello NK, editor. Advances in substance abuse, behavioral and biological research. JAI press; Greenwich: 1980. pp. 1–90. [Google Scholar]
- Grimm JW, Hope BT, Wise RA, Shaham Y. Incubation of cocaine craving after withdrawal. Nature. 2001;412:141–142. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Kruzich PJ, See RE. Contingent access to stimuli associated with cocaine self-administration is required for reinstatement of drug-seeking behavior. Psychobiology. 2000;28:383–386. [Google Scholar]
- Grimm JW, See RE. Dissociation of primary and secondary reward-relevant limbic nuclei in an animal model of relapse. Neuropsychopharmacology. 2000;22:473–479. doi: 10.1016/S0893-133X(99)00157-8. [DOI] [PubMed] [Google Scholar]
- Hall SM, Havassy BE, Wassermann DA. Commitment to abstinence and acute stress in relapse to alcohol, opiates and nicotine. J Counsel Clin Psychol. 1990;58:175–181. doi: 10.1037//0022-006x.58.2.175. [DOI] [PubMed] [Google Scholar]
- Haney M, Ward AS, Foltin RW, Fischman MW. Effects of ecopipam, a selective dopamine D1 antagonist, on smoked cocaine self-administration by humans. Psychopharmacology. 2001;155:330–337. doi: 10.1007/s002130100725. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Badger GJ, Budney AJ. Initial abstinence and success in achieving longer term cocaine abstinence. Exp Clin Psychopharmacol. 2000;8:377–386. doi: 10.1037//1064-1297.8.3.377. [DOI] [PubMed] [Google Scholar]
- Highfield D, Mead A, Grimm JW, Rocha BA, Shaham Y. Reinstatement of cocaine seeking in mice: effects of cocaine priming, cocaine cues and food deprivation. Psychopharmacology. 2002;161:417–424. doi: 10.1007/s00213-002-1047-9. [DOI] [PubMed] [Google Scholar]
- Highfield D, Yap J, Grimm J, Shalev U, Shaham Y. Repeated lofexidine treatment attenuates stress-induced, but not drug cues-induced reinstatement of a heroin-cocaine mixture (speedball) seeking in rats. Neuropsychopharmacology. 2001;25:320–331. doi: 10.1016/S0893-133X(01)00227-5. [DOI] [PubMed] [Google Scholar]
- Houston FP, Stevenson GD, McNaughton BL, Barnes CA. Effects of age on the generalization and incubation of memory in the F344 rat. Learn Mem. 1999;6:111–119. [PMC free article] [PubMed] [Google Scholar]
- Hughes JR. Is extinction in animals the same as abstinence in humans? Addiction. 2002;97:1219. doi: 10.1046/j.1360-0443.2002.00206.x. [DOI] [PubMed] [Google Scholar]
- Hunt WA, Barnett LW, Branch LG. Relapse rates in addiciton programs. J Clin Psychol. 1971;27:455–456. doi: 10.1002/1097-4679(197110)27:4<455::aid-jclp2270270412>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Jaffe JH. Drug addiction and drug abuse. In: Gilman AG, Rall TW, Nies AS, Taylor P, editors. Goodman & Gilman's the pharmacological basis of therapeutics. Pergamon Press; New York: 1990. pp. 522–573. [Google Scholar]
- Jaffe JH, Cascell NG, Kumor KM, Sherer MA. Cocaine-induced cocaine craving. Psychopharmacology. 1989;97:59–64. doi: 10.1007/BF00443414. [DOI] [PubMed] [Google Scholar]
- Jenkins TN, Warner LH, Warden CJ. Standard apparatus for the study of animal motivation. J Comp Psychol. 1926;6:361–382. [Google Scholar]
- Johanson CE, Fischman MW. The pharmacology of cocaine related to its abuse. Pharmacol Rev. 1989;41:3–52. [PubMed] [Google Scholar]
- Johnson RE, Chutuape MA, Strain EC, Walsh SL, Stitzer ML, Bigelow GE. A comparison of levomethadyl acetate, buprenorphine, and methadone for opioid dependence. N Engl J Med. 2000;343:1290–1297. doi: 10.1056/NEJM200011023431802. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Kassel JD, Stroud LR, Paronis CA. Smoking, stress, and negative affect: correlation, causation, and context across stages of smoking. Psychol Bull. 2003;129:270–304. doi: 10.1037/0033-2909.129.2.270. [DOI] [PubMed] [Google Scholar]
- Katz JL, Higgins ST. The validity of the reinstatement model of craving and relapse to drug use. Psychopharmacology. 2003;168:21–30. doi: 10.1007/s00213-003-1441-y. [DOI] [PubMed] [Google Scholar]
- Katzir AB-YN, Levy D, Shaham Y, Zangen A. A novel rat model to assess relapse to cocaine seeking. Soc Neurosci Abstr. 2006 in press. [Google Scholar]
- Khroyan TV, Barrett-Larimore RL, Rowlett JK, Spealman RD. Dopamine D1- and D2-like receptor mechanisms in relapse to cocaine- seeking behavior: effects of selective antagonists and agonists. J Pharmacol Exp Ther. 2000;294:680–687. [PubMed] [Google Scholar]
- Kilts CD, Schweitzer JB, Quinn CK, Gross RE, Faber TL, Muhammad F, Ely TD, Hoffman JM, Drexler KP. Neural activity related to drug craving in cocaine addiction. Arch Gen Psychiatry. 2001;58:334–41. doi: 10.1001/archpsyc.58.4.334. [DOI] [PubMed] [Google Scholar]
- Kornetsky C. Animal Models: Promises and Problems. In: Hanin I, Usdin E, editors. Animal Models in Psychiatry and Neurology. Pergamon Press; Oxford: 1977. pp. 1–7. [Google Scholar]
- Kosten TR, Kosten TA, Poling J, Oliveto A. “Incubation” of cocaine relapse during a disulfiram clinical trial. CPDD. Annual Meeting Abstracts; 2005. p. 90. [Google Scholar]
- Krupitsky EM, Zvartau EE, Masalov DV, Tsoi MV, Burakov AM, Egorova VY, Didenko TY, Romanova TN, Ivanova EB, Bespalov AY, Verbitskaya EV, Neznanov NG, Grinenko AY, O'Brien CP, Woody GE. Naltrexone for heroin dependence treatment in St. Petersburg, Russia. J Subst Abuse Treat. 2004;26:285–294. doi: 10.1016/j.jsat.2004.02.002. [DOI] [PubMed] [Google Scholar]
- LaRowe SD, Mardikian P, Malcolm R, Myrick H, Kalivas P, McFarland K, Saladin M, McRae A, Brady K. Safety and tolerability of N-acetylcysteine in cocaine-dependent individuals. Am J Addict. 2006;15:105–110. doi: 10.1080/10550490500419169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latt NC, Jurd S, Houseman J, Wutzke SE. Naltrexone in alcohol dependence: a randomised controlled trial of effectiveness in a standard clinical setting. Med J Aust. 2002;176:530–534. doi: 10.5694/j.1326-5377.2002.tb04550.x. [DOI] [PubMed] [Google Scholar]
- Le AD, Harding S, Juzytsch W, Fletcher PJ, Shaham Y. The role of corticotropin-releasing factor in the median raphe nucleus in relapse to alcohol. J Neurosci. 2002;22:7844–7849. doi: 10.1523/JNEUROSCI.22-18-07844.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le AD, Harding S, Juzytsch W, Funk D, Shaham Y. Role of alpha-2 adrenoceptors in stress-induced reinstatement of alcohol seeking and alcohol self-administration in rats. Psychopharmacology. 2005;179:366–373. doi: 10.1007/s00213-004-2036-y. [DOI] [PubMed] [Google Scholar]
- Le AD, Poulos CX, Harding S, Watchus W, Juzytsch W, Shaham Y. Effects of naltrexone and fluoxetine on alcohol self-administration and reinstatement of alcohol seeking induced by priming injections of alcohol and exposure to stress in rats. Neuropsychopharmacology. 1999;21:435–444. doi: 10.1016/S0893-133X(99)00024-X. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lee B, Tiefenbacher S, Platt DM, Spealman RD. Pharmacological blockade of alpha(2)-arenoceptors induces reinstatement of cocaine-seeking behavior in squirrel monkeys. Neuropsychopharmacology. 2004;29:686–693. doi: 10.1038/sj.npp.1300391. [DOI] [PubMed] [Google Scholar]
- Leri F, Stewart J. The consequences of different "lapses" on relapse to heroin seeking in rats. Exp Clin Psychopharmacol. 2002;10:339–349. doi: 10.1037//1064-1297.10.4.339. [DOI] [PubMed] [Google Scholar]
- Leri F, Tremblay A, Sorge RE, Stewart J. Methadone maintenance reduces heroin- and cocaine-induced relapse without affecting stress-induced relapse in a rodent model of poly-drug use. Neuropsychopharmacology. 2004;29:1312–1320. doi: 10.1038/sj.npp.1300435. [DOI] [PubMed] [Google Scholar]
- Lu L, Grimm JW, Dempsey J, Shaham Y. Cocaine seeking over extended withdrawal periods in rats: different time courses of responding induced by cocaine cues versus cocaine priming over the first 6 months. Psychopharmacology. 2004a;176:101–108. doi: 10.1007/s00213-004-1860-4. [DOI] [PubMed] [Google Scholar]
- Lu L, Grimm JW, Hope BT, Shaham Y. Incubation of cocaine craving after withdrawal: a review of preclinical data. Neuropharmacology. 2004b;47(Suppl 1):214–226. doi: 10.1016/j.neuropharm.2004.06.027. [DOI] [PubMed] [Google Scholar]
- Lu L, Hope BT, Dempsey J, Liu SY, Bossert JM, Shaham Y. Central amygdala ERK signaling pathway is critical to incubation of cocaine craving. NatNeurosci. 2005;8:212–219. doi: 10.1038/nn1383. [DOI] [PubMed] [Google Scholar]
- Lu L, Shepard JD, Scott Hall F, Shaham Y. Effect of environmental stressors on opiate and psychostimulant reinforcement, reinstatement and discrimination in rats: a review. Neurosci Biobehav Rev. 2003;27:457–491. doi: 10.1016/s0149-7634(03)00073-3. [DOI] [PubMed] [Google Scholar]
- Lussier JP, Higgins ST, Badger GJ. Influence of the duration of abstinence on the relative reinforcing effects of cigarette smoking. Psychopharmacology. 2005;181:486–495. doi: 10.1007/s00213-005-0008-5. [DOI] [PubMed] [Google Scholar]
- Maas LC, Lukas SE, Kaufman MJ, Weiss RD, Daniels SL, Rogers VW, Kukes TJ, Renshaw PF. Functional magnetic resonance imaging of human brain activation during cue-induced cocaine craving. Am J Psychiatry. 1998;155:124–126. doi: 10.1176/ajp.155.1.124. [DOI] [PubMed] [Google Scholar]
- Maier SF, Watkins LR. Stressor controllability and learned helplessness: the roles of the dorsal raphe nucleus, serotonin, and corticotropin-releasing factor. Neurosci Biobehav Rev. 2005;29:829–841. doi: 10.1016/j.neubiorev.2005.03.021. [DOI] [PubMed] [Google Scholar]
- Markou A, Arroyo M, Everitt BJ. Effects of contingent and non-contingent cocaine on drug-seeking behavior measured using a second-order schedule of cocaine reinforcement in rats. Neuropsychopharmacology. 1999;20:542–555. doi: 10.1016/S0893-133X(98)00080-3. [DOI] [PubMed] [Google Scholar]
- Markou A, Weiss F, Gold LH, Caine B, Schulteis G, Koob GF. Animal models of drug craving. Psychopharmacology. 1993;112:163–182. doi: 10.1007/BF02244907. [DOI] [PubMed] [Google Scholar]
- Marlatt AG. Models of relapse and relapse prevention: a commentary. Exp Clin Psychopharmacol. 1996;4:55–60. [Google Scholar]
- Marlatt GA. Do animal models provide a valid analogue for human drug lapse and relapse? Comment on Leri and Stewart (2002) Exp Clin Psychopharmacol. 2002;10:359–360. doi: 10.1037//1064-1297.10.4.359. [DOI] [PubMed] [Google Scholar]
- McFarland K, Ettenberg A. Reinstatement of drug-seeking behavior produced by heroin-predictive environmental stimuli. Psychopharmacology. 1997;131:86–92. doi: 10.1007/s002130050269. [DOI] [PubMed] [Google Scholar]
- McFarlane AC. Posttraumatic stress disorder: a model of the longitudinal course and the role of risk factors. J Clin Psychiatry. 2000;61(Suppl 5):15–20. 21–23. [PubMed] [Google Scholar]
- McKay JR, Alterman AI, Rutherford MJ, Cacciola JS, McLellan AT. The relationship of alcohol use to cocaine relapse in cocaine dependent patients in an aftercare study. J Stud Alcohol. 1999;60:176–180. doi: 10.15288/jsa.1999.60.176. [DOI] [PubMed] [Google Scholar]
- McKay JR, Franklin TR, Patapis N, Lynch KG. Conceptual, methodological, and analytical issues in the study of relapse. Clin Psychol Rev. 2006;26:109–127. doi: 10.1016/j.cpr.2005.11.002. [DOI] [PubMed] [Google Scholar]
- McKay JR, Merikle E, Mulvaney FD, Weiss RV, Koppenhaver JM. Factors accounting for cocaine use two years following initiation of continuing care. Addiction. 2001;96:213–225. doi: 10.1046/j.1360-0443.2001.9622134.x. [DOI] [PubMed] [Google Scholar]
- McKinney WT, Jr, Bunney WE., Jr Animal model of depression. I. Review of evidence: implications for research. Arch Gen Psychiatry. 1969;21:240–248. doi: 10.1001/archpsyc.1969.01740200112015. [DOI] [PubMed] [Google Scholar]
- Meil WM, See RE. Conditioned cued recovery of responding following prolonged withdrawal from self-administered cocaine in rats: an animal model of relapse. Behav Pharmacol. 1996;7:754–763. [PubMed] [Google Scholar]
- Mello NK, Negus SS. Preclinical evaluation of pharmacotherapies for treatment of cocaine and opioid abuse using drug self-administration procedures. Neuropsychopharmacology. 1996;14:375–424. doi: 10.1016/0893-133X(95)00274-H. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Fish EW, De Bold JF, De Almeida RM. Social and neural determinants of aggressive behavior: pharmacotherapeutic targets at serotonin, dopamine and gamma-aminobutyric acid systems. Psychopharmacology (Berl) 2002;163:434–458. doi: 10.1007/s00213-002-1139-6. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Thompson ML, Tornatzky W. Subordinates animals: behavioral and physiological adaptations and opioid tolerance. In: Brown MR, Koob GF, Rivier C, editors. Stress: neurobiology and neuroendocrinology. Marcel Dekker; New York: 1991. pp. 323–357. [Google Scholar]
- Mueller D, Stewart J. Cocaine-induced conditioned place preference: reinstatement by priming injections of cocaine after extinction. Behav Brain Res. 2000;115:39–47. doi: 10.1016/s0166-4328(00)00239-4. [DOI] [PubMed] [Google Scholar]
- Nann-Vernotica E, Donny EC, Bigelow GE, Walsh SL. Repeated administration of the D1/5 antagonist ecopipam fails to attenuate the subjective effects of cocaine. Psychopharmacology. 2001;155:338–347. doi: 10.1007/s002130100724. [DOI] [PubMed] [Google Scholar]
- Neisewander JL, Baker DA, Fuchs RA, Tran-Nguyen LT, Palmer A, Marshall JF. Fos protein expression and cocaine-seeking behavior in rats after exposure to a cocaine self-administration environment. J Neurosci. 2000;20:798–805. doi: 10.1523/JNEUROSCI.20-02-00798.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunnally JC, Bernstein IH. 3rd ed. McGraw-Hill; McGraw-Hill: 1994. Psychometric Theory. [Google Scholar]
- O'Brien CP, Gardner EL. Critical assessment of how to study addiction and its treatment: Human and non-human animal models. Pharmacol Ther. 2005a;108:18–58. doi: 10.1016/j.pharmthera.2005.06.018. [DOI] [PubMed] [Google Scholar]
- O'Brien CP. A range of research-based pharmacotherapies for addiction. Science. 1997;278:66–70. doi: 10.1126/science.278.5335.66. [DOI] [PubMed] [Google Scholar]
- O'Brien CP. Anticraving medications for relapse prevention: a possible new class of psychoactive medications. Am J Psychiatry. 2005;162:1423–1431. doi: 10.1176/appi.ajp.162.8.1423. [DOI] [PubMed] [Google Scholar]
- O'Brien CP, Childress AR, Mclellan TA, Ehrman R. Classical conditioning in drug dependent humans. Ann N Y Acad Sci. 1992;654:400–415. doi: 10.1111/j.1749-6632.1992.tb25984.x. [DOI] [PubMed] [Google Scholar]
- O'Brien CP, Ehrman RN, Ternes JW. Classical conditioning in human opioid dependence. In: Goldberg S, Stolerman I, editors. Behavioral analysis of drug dependence. Academic Press; Orlando: 1986. pp. 329–356. [Google Scholar]
- O'Brien CP, Gardner EL. Critical assessment of how to study addiction and its treatment: human and non-human animal models. Pharmacol Ther. 2005b;108:18–58. doi: 10.1016/j.pharmthera.2005.06.018. [DOI] [PubMed] [Google Scholar]
- O'Malley SS, Jaffe JH, Chang G, Schottenfeld RS, Meyer RE, Rounsaville BJ. Naltrexone and coping skills therapy for alcohol dependence: A controlled study. Arch Gen Psychiatry. 1992;49:881–887. doi: 10.1001/archpsyc.1992.01820110045007. [DOI] [PubMed] [Google Scholar]
- Panlilio LV, Thorndike EB, Schindler CW. Reinstatement of punishment-suppressed opioid self-administration in rats: an alternative model of relapse to drug abuse. Psychopharmacology. 2003;168:229–235. doi: 10.1007/s00213-002-1193-0. [DOI] [PubMed] [Google Scholar]
- Panlilio LV, Thorndike EB, Schindler CW. Lorazepam reinstates punishment-suppressed remifentanil self-administration in rats. Psychopharmacology. 2005;179:374–382. doi: 10.1007/s00213-004-2040-2. [DOI] [PubMed] [Google Scholar]
- Pavlov IP. Oxford University Press; Oxford University Press: 1927. Conditioned reflexes. [Google Scholar]
- Piasecki TM, Fiore MC, Baker TB. Profiles in discouragement: two studies of variability in the time course of smoking withdrawal symptoms. J Abnorm Psychol. 1998;107:238–51. doi: 10.1037//0021-843x.107.2.238. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Le Moal M. The role of stress in drug self-administration. Trends Pharmacol Sci. 1998;19:67–74. doi: 10.1016/s0165-6147(97)01115-2. [DOI] [PubMed] [Google Scholar]
- Rebec GV, Sun W. Neuronal substrates of relapse to cocaine-seeking behavior: role of prefrontal cortex. J Exp Anal Behav. 2005;84:653–666. doi: 10.1901/jeab.2005.105-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro Do Couto B, Aguilar MA, Manzanedo C, Rodriguez-Arias M, Armario A, Minarro J. Social stress is as effective as physical stress in reinstating morphine-induced place preference in mice. Psychopharmacology. 2006;185:459–470. doi: 10.1007/s00213-006-0345-z. [DOI] [PubMed] [Google Scholar]
- Romach MK, Glue P, Kampman K, Kaplan HL, Somer GR, Poole S, Clarke L, Coffin V, Cornish J, O'Brien CP, Sellers EM. Attenuation of the euphoric effects of cocaine by the dopamine D1/D5 antagonist ecopipam (SCH 39166) Arch Gen Psychiatry. 1999;56:1101–1106. doi: 10.1001/archpsyc.56.12.1101. [DOI] [PubMed] [Google Scholar]
- Rose JE. Nicotine addiction and treatment. AnnuRevMed. 1996;47:493–507. doi: 10.1146/annurev.med.47.1.493. [DOI] [PubMed] [Google Scholar]
- Russell RW. Little, Brown; Boston: 1964. Extrapolation from Animals to Man. In: Steinberg AH (ed) Animal Behaviour and Drug Action; pp. 410–418. [Google Scholar]
- Sanchez CJ, Sorg BA. Conditioned fear stimuli reinstate cocaine-induced conditioned place preference. Brain Res. 2001;908:86–92. doi: 10.1016/s0006-8993(01)02638-5. [DOI] [PubMed] [Google Scholar]
- Sarter M, Bruno JP. Biolotical psychiatry. Johns Willey & Sons Ltd.; Hoboken, NJ: 2002. Animal models in biological psychiatry; pp. 1–8. [Google Scholar]
- Sass H, Soyka M, Mann K, Zieglgansberger W. Relapse prevention by acamprosate. Results from a placebo-controlled study on alcohol dependence. Arch Gen Psychiatry. 1996;53:673–680. doi: 10.1001/archpsyc.1996.01830080023006. [DOI] [PubMed] [Google Scholar]
- Schenk S, Partridge B. Cocaine-seeking produced by experimenter-administered drug injections: dose-effect relationships in rats. Psychopharmacology. 1999;147:285–290. doi: 10.1007/s002130051169. [DOI] [PubMed] [Google Scholar]
- Schubiner H, Saules KK, Arfken CL, Johanson CE, Schuster CR, Lockhart N, Edwards A, Donlin J, Pihlgren E. Double-blind placebo-controlled trial of methylphenidate in the treatment of adult ADHD patients with comorbid cocaine dependence. Exp Clin Psychopharmacol. 2002;10:286–294. doi: 10.1037//1064-1297.10.3.286. [DOI] [PubMed] [Google Scholar]
- See RE. Neural substrates of conditioned-cued relapse to drug-seeking behavior. Pharmacol Biochem Behav. 2002;71:517–529. doi: 10.1016/s0091-3057(01)00682-7. [DOI] [PubMed] [Google Scholar]
- See RE. Neural substrates of cocaine-cue associations that trigger relapse. Eur J Pharmacol. 2005;526:140–146. doi: 10.1016/j.ejphar.2005.09.034. [DOI] [PubMed] [Google Scholar]
- Self DW, Choi KH, Simmons D, Walker JR, Smagula CS. Extinction training regulates neuroadaptive responses to withdrawal from chronic cocaine self-administration. Learn Mem. 2004;11:648–657. doi: 10.1101/lm.81404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Self DW, Nestler EJ. Relapse to drug-seeking: neural and molecular mechanisms. Drug Alcohol Depend. 1998;51:49–69. doi: 10.1016/s0376-8716(98)00065-9. [DOI] [PubMed] [Google Scholar]
- Selye H. McGraw-Hill; McGraw-Hill: 1956. The stress of life. [Google Scholar]
- Shaham Y. Effect of stress on opioid-seeking behavior: Evidence from studies with rats. Ann Behav Med. 1996;18:255–263. doi: 10.1007/BF02895287. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Adamson LK, Grocki S, Corrigall WA. Reinstatement and spontaneous recovery of nicotine-seeking in rats. Psychopharmacology. 1997a;130:396–403. doi: 10.1007/s002130050256. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Erb S, Stewart J. Stress-induced relapse to heroin and cocaine seeking in rats: a review. Brain Res Brain Res Rev. 2000a;33:13–33. doi: 10.1016/s0165-0173(00)00024-2. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Funk D, Erb S, Brown TJ, Walker CD, Stewart J. Corticotropin-releasing factor, but not corticosterone, is involved in stress-induced relapse to heroin-seeking in rats. J Neurosci. 1997b;17:2605–2614. doi: 10.1523/JNEUROSCI.17-07-02605.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, Highfield D, Delfs JM, Leung S, Stewart J. Clonidine blocks stress-induced reinstatement of heroin seeking in rats: an effect independent of the locus coeruleus noradrenergic neurons. Eur J Neurosci. 2000b;12:292–302. doi: 10.1046/j.1460-9568.2000.00899.x. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Rajabi H, Stewart J. Relapse to heroin-seeking under opioid maintenance: the effects of opioid withdrawal, heroin priming and stress. J Neurosci. 1996;16:1957–1963. doi: 10.1523/JNEUROSCI.16-05-01957.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology. 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Stewart J. Stress reinstates heroin self-administration behavior in drug-free animals: An effect mimicking heroin, not withdrawal. Psychopharmacology. 1995;119:334–341. doi: 10.1007/BF02246300. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Stewart J. Effects of opioid and dopamine receptor antagonists on relapse induced by stress and reexposure to heroin in rats. Psychopharmacology. 1996;125:385–391. doi: 10.1007/BF02246022. [DOI] [PubMed] [Google Scholar]
- Shalev U, Finnie PS, Quinn T, Tobin S, Wahi P. A role for corticotropin-releasing factor, but not corticosterone, in acute food-deprivation-induced reinstatement of heroin seeking in rats. Psychopharmacology. 2006;187:376–384. doi: 10.1007/s00213-006-0427-y. [DOI] [PubMed] [Google Scholar]
- Shalev U, Grimm JW, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacol Rev. 2002;54:1–42. doi: 10.1124/pr.54.1.1. [DOI] [PubMed] [Google Scholar]
- Shalev U, Highfield D, Yap J, Shaham Y. Stress and relapse to drug seeking in rats: studies on the generality of the effect. Psychopharmacology. 2000;150:337–346. doi: 10.1007/s002130000441. [DOI] [PubMed] [Google Scholar]
- Shalev U, Morales M, Hope B, Yap J, Shaham Y. Time-dependent changes in extinction behavior and stress-induced reinstatement of drug seeking following withdrawal from heroin in rats. Psychopharmacology. 2001a;156:98–107. doi: 10.1007/s002130100748. [DOI] [PubMed] [Google Scholar]
- Shalev U, Yap J, Shaham Y. Leptin attenuates food deprivation-induced relapse to heroin seeking. J Neurosci. 2001b;21:RC129. doi: 10.1523/JNEUROSCI.21-04-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard JD, Bossert JM, Liu SY, Shaham Y. The anxiogenic drug yohimbine reinstates methamphetamine seeking in a rat model of drug relapse. Biol Psychiatry. 2004;55:1082–1089. doi: 10.1016/j.biopsych.2004.02.032. [DOI] [PubMed] [Google Scholar]
- Shiffman S. Dynamic influences on smoking relapse process. J Pers. 2005;73:1715–1748. doi: 10.1111/j.0022-3506.2005.00364.x. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Ferguson SG, Gwaltney CJ. Immediate hedonic response to smoking lapses: relationship to smoking relapse, and effects of nicotine replacement therapy. Psychopharmacology. 2006a;184:608–618. doi: 10.1007/s00213-005-0175-4. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Hufford M, Hickcox M, Paty JA, Gnys M, Kassel JD. Remember that? A comparison of real-time versus retrospective recall of smoking lapses. J Consult Clin Psychol. 1997;65:292–300. doi: 10.1037/0022-006x.65.2.292.a. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Paty JA, Gnys M, Kassel JA, Hickcox M. First lapses to smoking: within-subjects analysis of real-time reports. J Consult Clin Psychol. 1996;64:366–379. doi: 10.1037//0022-006x.64.2.366. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Scharf DM, Shadel WG, Gwaltney CJ, Dang Q, Paton SM, Clark DB. Analyzing milestones in smoking cessation: illustration in a nicotine patch trial in adult smokers. J Consult Clin Psychol. 2006b;74:276–285. doi: 10.1037/0022-006X.74.2.276. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Waters AJ. Negative affect and smoking lapses: a prospective analysis. J Consult Clin Psychol. 2004;72:192–201. doi: 10.1037/0022-006X.72.2.192. [DOI] [PubMed] [Google Scholar]
- Shufman EN, Porat S, Witztum E, Gandacu D, Bar-Hamburger R, Ginath Y. The efficacy of naltrexone in preventing reabuse of heroin after detoxification. Biol Psychiatry. 1994;35:935–945. doi: 10.1016/0006-3223(94)91240-8. [DOI] [PubMed] [Google Scholar]
- Sinha R. How does stress increase risk of drug abuse and relapse. Psychopharmacology. 2001;158:343–359. doi: 10.1007/s002130100917. [DOI] [PubMed] [Google Scholar]
- Sinha R, Kimmerling A, Doebrick C, Kosten TR. Effects of lofexidine on stress-induced and cue-induced opioid craving and opioid abstinence rates: preliminary findings. Biol Psychiatry. 2006 doi: 10.1007/s00213-006-0640-8. (under review) [DOI] [PubMed] [Google Scholar]
- Sinha R, Fuse T, Aubin LR, O'Malley SS. Psychological stress, drug -related cues and cocaine craving. Psychopharnacology. 2000;152:140–148. doi: 10.1007/s002130000499. [DOI] [PubMed] [Google Scholar]
- Skinner BF. Free Press; Free Press: 1953. Science and human behavior. [Google Scholar]
- Smith SG, Davis WM. Punishment of amphetamine and morphine self-administration behavior. Psychol Rec. 1974;24:477–480. [Google Scholar]
- Sorge RE, Rajabi H, Stewart J. Rats maintained chronically on buprenorphine show reduced heroin and cocaine seeking in tests of extinction and drug-induced reinstatement. Neuropsychopharmacology. 2005;30:1681–1692. doi: 10.1038/sj.npp.1300712. [DOI] [PubMed] [Google Scholar]
- Stewart J. Pathways to relapse: the neurobiology of drug- and stress-induced relapse to drug-taking. J Psychiatry Neurosci. 2000;25:125–136. [PMC free article] [PubMed] [Google Scholar]
- Streeton C, Whelan G. Naltrexone, a relapse prevention maintenance treatment of alcohol dependence: a meta-analysis of randomized controlled trials. Alcohol Alcohol. 2001;36:544–552. doi: 10.1093/alcalc/36.6.544. [DOI] [PubMed] [Google Scholar]
- Stretch R, Gerber GJ, Wood SM. Factors affecting behavior maintained by response-contingent intravenous infusions of amphetamine in squirrel monkeys. Can J Physiol Pharmacol. 1971;49:581–589. doi: 10.1139/y71-075. [DOI] [PubMed] [Google Scholar]
- Tempesta E, Janiri L, Bignamini A, Chabac S, Potgieter A. Acamprosate and relapse prevention in the treatment of alcohol dependence: a placebo-controlled study. Alcohol Alcohol. 2000;35:202–209. doi: 10.1093/alcalc/35.2.202. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Carter BL. Is craving the source of compulsive drug use? J Psychopharmacol. 1998;12:23–30. doi: 10.1177/026988119801200104. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Conklin CA. A cognitive processing model of alcohol craving and compulsive alcohol use. Addiction. 2000;95(Suppl 2):S145–153. doi: 10.1080/09652140050111717. [DOI] [PubMed] [Google Scholar]
- Tran-Nguyen TL, Fuchs RA, Coffey GP, O'Dell LE, Baker DA, Neisewander JL. Time-dependent changes in cocaine-seeking behavior and dopamine overflow in the amygdala during cocaine withdrawal. Neuropsychopharmacology. 1998;19:48–59. doi: 10.1016/S0893-133X(97)00205-4. [DOI] [PubMed] [Google Scholar]
- Vaillant GE. What can long-term follow-up teach us about relapse and prevention of relapse in addiction? Br J Addict. 1988;83:1147–1157. doi: 10.1111/j.1360-0443.1988.tb03021.x. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Di Ciano P, Everitt BJ. Involvement of the dorsal striatum in cue-controlled cocaine seeking. J Neurosci. 2005;25:8665–8870. doi: 10.1523/JNEUROSCI.0925-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vocci F, Ling W. Medications development: Successes and challenges. Pharmacol Ther. 2005;108:94–108. doi: 10.1016/j.pharmthera.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Volpicelli JR, Anterman AI, Hayashida M, O'Brien CP. Naltrexone in the treatment of alcohol dependence. Arch Gen Psychiatry. 1992;49:876–880. doi: 10.1001/archpsyc.1992.01820110040006. [DOI] [PubMed] [Google Scholar]
- Waldorf D, Reinarman C, Murphy S. Temple University Press; Temple University Press: 1992. Cocaine Changes: The Experience of Using and Quitting. [Google Scholar]
- Wang B, Shaham Y, Zitzman D, Azari S, Wise RA, You ZB. Cocaine experience establishes control of midbrain glutamate and dopamine by corticotropin-releasing factor: a role in stress-induced relapse to drug seeking. J Neurosci. 2005;25:5389–5396. doi: 10.1523/JNEUROSCI.0955-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Fang Q, Liu Z, Lu L. Region-specific effects of brain corticotropin-releasing factor receptor type 1 blockade on footshock-stress- or drug-priming-induced reinstatement of morphine conditioned place preference in rats. Psychopharmacology. 2006;185:19–28. doi: 10.1007/s00213-005-0262-6. [DOI] [PubMed] [Google Scholar]
- Warden CJ. Columbia University Press; Columbia University Press: 1931. Animal motivation: experimental studies on the albino rat. [Google Scholar]
- Wasserman DA, Weinstein MG, Havassy BE, Hall SM. Factors associated with lapses to heroin use during methadone maintenance. Drug Alcohol Depend. 1998;52:183–192. doi: 10.1016/s0376-8716(98)00092-1. [DOI] [PubMed] [Google Scholar]
- Weiss F. Neurobiology of craving, conditioned reward and relapse. Curr Opin Pharmacol. 2005;5:9–19. doi: 10.1016/j.coph.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Weiss F, Maldonado-Vlaar CS, Parsons LH, Kerr TM, Smit DL, Ben-Shahar O. Control of cocaine-seeking behavior by drug-associated stimuli in rats: effects on recovery of extinguished operant-responding and extracellular dopamine levels in amygdala and nucleus accumbens. Proc Natl Acad Sci (USA) 2000;97:4321–4326. doi: 10.1073/pnas.97.8.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wexler BE, Gottschalk CH, Fulbright RK, Prohovnik I, Lacadie CM, Rounsaville BJ, Gore JC. Functional magnetic resonance imaging of cocaine craving. Am J Psychiatry. 2001;158:86–95. doi: 10.1176/appi.ajp.158.1.86. [DOI] [PubMed] [Google Scholar]
- Wikler A. Dynamics of drug dependence, implication of a conditioning theory for research and treatment. Arch Gen Psychiatry. 1973;28:611–616. doi: 10.1001/archpsyc.1973.01750350005001. [DOI] [PubMed] [Google Scholar]
- Willner P. The validity of animal models of depression. Psychopharmacology. 1984;83:1–16. doi: 10.1007/BF00427414. [DOI] [PubMed] [Google Scholar]
- Willner P. Validity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacology. 1997;134:319–329. doi: 10.1007/s002130050456. [DOI] [PubMed] [Google Scholar]
- Wise RA, Murray A, Bozarth MA. Bromocriptine self-administration and bromocriptine-reinstatement of cocaine-trained and heroin-trained lever pressing in rats. Psychopharmacology. 1990;100:355–360. doi: 10.1007/BF02244606. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Antelman SM. Criteria for rationally evaluating animal models of posttraumatic stress disorder. Biol Psychiatry. 1993;33:479–486. doi: 10.1016/0006-3223(93)90001-t. [DOI] [PubMed] [Google Scholar]
- Yonkers KA, Bruce SE, Dyck IR, Keller MB. Chronicity, relapse, and illness—course of panic disorder, social phobia, and generalized anxiety disorder: findings in men and women from 8 years of follow-up. Depress Anxiety. 2003;17:173–179. doi: 10.1002/da.10106. [DOI] [PubMed] [Google Scholar]


