Abstract
RATIONALE
The heterozygous reeler mouse has been proposed as a genetic mouse model of schizophrenia, based on several neuroanatomical and behavioral similarities between these mice and patients with schizophrenia. However, the effect of reelin haploinsufficiency on one of the cardinal symptoms of schizophrenia, the impairment of prefrontal cortex-dependent cognitive function, has yet to be determined.
OBJECTIVE
Here, we investigated multiple aspects of cognitive function in heterozygous reeler mice that are known to be impaired in schizophrenic patients.
METHODS
Heterozygous reeler mice were assessed for (1) cognitive flexibility in an instrumental reversal learning task; (2) impulsivity in an inhibitory control task; (3) attentional function in a three-choice serial reaction time task; and (4) working memory in a delayed matching-to-position task.
RESULTS
No differences were found between heterozygous reeler mice and wild-type littermate controls in any prefrontal-related cognitive measures. However, heterozygous reeler mice showed deficits in the acquisition of two operant tasks, consistent with a role for reelin in certain forms of learning.
CONCLUSIONS
These findings suggest that heterozygous reeler mice may not be an appropriate model for the core prefrontal-dependent cognitive deficits observed in schizophrenia, but may model more general learning deficits that are associated with many psychiatric disorders.
Keywords: Reelin, Prefrontal Cortex, Schizophrenia, Perseveration, Attention, Impulsivity, Working Memory
Introduction
Psychiatric and neurodevelopmental disorders are increasingly thought to involve alterations in synaptic connectivity, originating from a dysregulation of synapse formation, maintenance or plasticity. One candidate molecule is reelin, a large extracellular matrix protein that plays a critical role during brain development, but also continues to be expressed in adult cortex and hippocampus (D’Arcangelo et al. 1995; Alcantara et al. 1998). In the adult brain, reelin is secreted by GABAergic interneurons into the extracellular space surrounding dendrites, dendritic spines and axon boutons (Pappas et al. 2002). Given recent findings suggesting a role for reelin signaling in hippocampal LTP (Weeber et al. 2002) and synaptic protein translation (Dong et al. 2003), it has been argued that reelin might be important in synaptic plasticity, and that a reduction in extracellular reelin may alter synaptic structure and stability.
Levels of reelin mRNA and protein levels are decreased by 50% in the prefrontal cortex, hippocampus and cerebellum of schizophrenic patients (Impagnatiello et al. 1998; Guidotti et al. 2000), and this decrease has been proposed to result in psychosis vulnerability. In support of this hypothesis, heterozygous reeler mice display certain behavioral and anatomical abnormalities similar to those found in schizophrenic patients, including down-regulation of GAD67 expression, increased neuronal packing density and decreased spine density in frontal cortex (Liu et al. 2001), as well as deficits in prepulse inhibition of startle and olfactory learning (Tueting et al. 1999; Larson et al. 2003). Alterations in the mesolimbic dopamine system, consistent with the dopamine hypothesis of schizophrenia, have also been reported (Ballmaier et al. 2002). Based on these findings, the heterozygous reeler mouse was suggested to be a genetic mouse model for the study of psychosis vulnerability in schizophrenia (Liu et al. 2001) and for the development of new antipsychotic drugs (Costa et al. 2002;Carboni et al. 2004).
Two detailed studies of the behavioral phenotype of heterozygous reeler mice, however, have questioned the validity of this model (Salinger et al. 2003; Podhorna and Didriksen, 2004). No differences were observed in a wide range of tests, including sensory function, gait, social behavior, anxiety and sensorimotor gating, although a subsequent study reported deficits in contextual fear conditioning (Qiu et al. 2006). Moreover, very little is known about the effects of reelin haploinsufficiency on prefrontal-dependent cognitive control, the disruption of which is one of the hallmark features of schizophrenia (Elvevag and Goldberg, 2000; Volk and Lewis, 2002). Given the importance of developing reliable models of schizophrenia both for the understanding of the etiology of this disorder and as screens for novel antipsychotic treatments, a more extensive characterization of schizophrenia-related behaviors in these mice is essential.
Here, we tested heterozygous reeler mice for (1) cognitive flexibility; (2) inhibitory control; (3) attentional function; and (4) working memory. These measures are known to be dependent on the prefrontal cortex across multiple species including humans, non-human primates and rats (Robbins, 2000; Brown and Bowman, 2002; Royall et al. 2002; Miller et al. 2002), and while studies in mice have thus been less expansive, the literature available to date suggests at least a functional homology to rats in equivalent cognitive tests (Lidow et al. 2003; Greco et al. 2005; Izquierdo et al. 2006). In addition to these prefrontal-related tasks, two measures of hippocampal-dependent learning were included to help clarify previous conflicting findings.
Materials and Methods
Experimental animals
Heterozygous reeler breeding pairs (B6C3Fe a/a-Relnrl/+ strain) were obtained from the Jackson Laboratory (USA). All mice used in this study were Reln +/+ (wt) or +/− (het) littermates bred in our animal facility from heterozygous reeler breeders. Pups were genotyped by PCR as described (D’Arcangelo et al. 1996). Five sets of mice were used for the behavioral studies, one each for (1) locomotor activity; (2) reversal learning and working memory; (3) impulsivity; (4) attention; and (5) elevated plus maze, Morris water maze and fear conditioning. Each set of mice was 3 months old at the initiation of the training period. All mice were male, with the exception of the group used for the impulsivity study (wt = 4 male and 2 female; het = 5 male and 3 female). For behavioral studies involving instrumental behaviors motivated by food reinforcement, mice were food-restricted to 85–90% free-feeding body weight prior to the experiments. They were then given two days of magazine training, in which each entry into the food magazine was reinforced by one food pellet on a fixed time 15-sec schedule for 15 min or until 50 reinforcers were delivered. All experiments were performed during the light cycle between 7 a.m. and 7 p.m. All procedures were approved by the Yale University Animal Care and Use Committee (YACUC) and followed the NIH Guide for the Care and Use of Laboratory Animals.
Assessment of reelin levels
Prefrontal cortical and hippocampal samples from 6 mice of each genotype were prepared for protein analysis by standard immunoblotting procedures. Total protein (75 μg per sample) was run on 4–12% Novex gradient gels (Invitrogen, USA) and transferred to nitrocellulose membranes. Membranes were blocked in non-fat dry milk (Nestle, USA) and incubated in reelin G10 monoclonal antibody (Chemicon, USA) followed by secondary antibody (Gt anti-M-IRDye800, Rockland Immunochemicals, USA). Blots were scanned and quantified on the Odyssey Infrared Imager (LiCor Biosciences; USA).
Locomotor activity
Locomotor activity was measured using an automated Digiscan Micro-monitor system (Omnitech Electronics, USA), consisting of 16 pairs of photocells situated around the perimeter of a clear empty plastic cage (42 × 21 × 21 cm). Overall locomotor activity for each mouse (n=8 for wt, n=7 for het) was recorded in a 30 min session as number of photobeam breaks in 6 × 5-min bins.
Elevated plus maze
Elevated plus-maze was tested in a semi-lit room using standard equipment and procedures as previously described (Söderpalm and Engel, 1988). Animals (n=9 for wt, n=10 for het) were initially exposed to a novel environment for five minutes to stimulate exploratory behavior before being placed in the center of the maze and allowed to freely explore for 5 min. Entries into each arm, defined as placement of at least three paws placed into the arm for any length of time, were recorded by an observer blind to genotype and the data expressed as percentage time spent in the open arms.
Reversal learning
The reversal learning task was performed in operant conditioning chambers (Med Associates, USA) fitted with a food magazine, house light, stimulus light and tone generator, and three nose poke apertures. Mice (n=7 for wt, n=9 for het) were trained to perform a food-reinforced operant nosepoke response. One of the three illuminated nose poke holes was designated as active (left, center or right) in a pseudo-randomized and balanced manner for each genotype. A response in the active aperture (‘correct response’) resulted in delivery of a food pellet, accompanied by a 3 sec presentation of a stimulus light above the magazine and tone. A response in the other two apertures (‘incorrect response’) had no effect. The first 10 rewards in each session were delivered on a fixed ratio-1 (FR1) schedule; all subsequent rewards were delivered on a variable ratio-2 schedule (1, 2 or 3 correct responses were required to obtain reinforcement). Mice were tested for 15 min each day over 10 consecutive days of training, and the number of correct and incorrect responses recorded. On day 11, the position of the active nose poke hole was switched (1st reversal), and the nosepoke responses in the 15 min session were monitored for 4 daily sessions. On day 16, mice were subjected to a 2nd reversal. Based on the role for the prefrontal cortex in reversal learning (Dalley et al. 2004), mice with impaired prefrontal cortical function would be expected to show increased perseverative responding, i.e. increased responding at the previously reinforced aperture following reversal.
Inhibitory control
Response inhibition was measured as described (Bowers and Wehner, 2001) (n=6 for wt, n=8 for het) using the operant conditioning chambers described above. As before, one nose poke aperture was defined as active; the other two apertures were inactive. All three apertures were illuminated throughout the session. Training consisted of 4 phases: (1) Mice were trained on an operant conditioning task using a food-reinforced FR1 schedule until 25 correct responses were made in 30 min. (2) The schedule of reinforcement was then changed to FR3 and animals were tested daily until 25 correct responses were made in 30 min. (3) A 3 sec auditory stimulus was introduced on a variable interval 30-sec schedule. A correct response made during tone presentation resulted in delivery of a food pellet (rewarded response). Criterion was reached when 10 rewarded responses were made within 30 min. Responses made during the inter-stimulus periods or in the inactive apertures had no programmed consequences. (4) In the final phase, the auditory stimulus was preceded by a 1–10 sec randomly variable pre-stimulus period. A nose poke during this period resulted in resetting of the pre-stimulus timer, such that the auditory stimulus was delivered only if mice withheld responding for the entire length of the pre-stimulus period. Each mouse received daily 30 min test sessions for 10 consecutive days. The dependent variables measured in this phase included the efficiency ratio [(number of rewarded trials) / (number of correct nosepokes)] and the percentage of rewarded responses [(number of rewarded trials) / (total number of trials) * 100].
Attention
Attentional function was assessed using a three-choice serial reaction time task as described (Humby et al. 1999) in which mice (n=10 for both genotypes) were trained to attend to and respond to a visual stimulus randomly presented in one of three nosepoke apertures. Mice were placed into the operant conditioning chambers described above for one 30 min session each day. The first trial was initiated by making a head entry into the reinforcer magazine. After a delay period of 4 sec, a 32 sec visual stimulus was presented in one of the three nose poke apertures. A response in the active aperture (‘correct response’), resulted in delivery of a food pellet and initiation of the next trial. Anticipatory errors (responses made prior to delivery of the visual stimulus), commission errors (responses made in either of the non-illuminated apertures), and omission errors (failure to respond during the stimulus or the subsequent 5 sec holding period) resulted in an 8 sec timeout and initiation of the next trial. Response accuracy (i.e., the percentage of correct responses per session) was recorded for each session and mice were moved to the next stage of the procedure when they achieved > 80% correct responding and > 50 trials per session. The stimulus duration was then decreased progressively from 32 sec to 16, 8, 4, 2, 1 and finally 0.8 sec. Variables measured in this experiment included days required to reach the above criterion for each stimulus duration, as well as final performance at the 0.8 sec duration.
Delayed matching-to-position
Working memory was assessed using a delayed matching-to-position (DMTP) paradigm, modified from Estapé & Steckler (Estape and Steckler, 2002), using the operant conditioning chambers described above. Mice (n=5 for wt, n=7 for het) received DMTP training consisting of three acquisition phases and one test phase: (1) During the initial 60 min training sessions, each trial began with a 10 sec inter-trial interval (ITI), followed by illumination of one of two nose poke holes (left or right) in a pseudo-random order (sample phase). Following a correct response, mice were required to enter the reinforcer magazine that was located on the opposite side of the chamber to initiate the choice phase, in which a light was again presented in the previously illuminated aperture. A correct response in this aperture resulted in delivery of a food pellet and initiation of the next trial. An incorrect response in the non-illuminated aperture resulted in initiation of the next trial but did not result in food delivery. (2) The second training phase was identical to the first phase, except that both the active and the inactive aperture were illuminated during the choice phase, requiring the mouse to remember the location of the illuminated aperture presented in the sample phase. (3) In the final training phase, a time limit of 5 sec was introduced for the choice phase (limited hold). If the mouse failed to make a response during this period, the trial was terminated and recorded as an omission error. (4) For the test phase, a delay of 2, 5, 10 or 20 sec duration was introduced between the sample and choice phases, where the first magazine entry made after the end of the delay initiated the choice phase. There was no programmed consequence of responding during the delay phase. Each session consisted of 64 trials pseudo-randomized across the 4 delay periods and 2 nose poke positions. Mice were run on this procedure for 6 consecutive days, and results from days 4–6 were averaged and recorded as percentage of correct responses for each delay.
Morris water maze
The Morris water maze was performed in a round water-filled tank (100 cm in diameter, 24°C) containing a hidden platform (12 × 10.5 × 11cm, located 2 cm below water level) in a specific fixed location. At the beginning of a trial, mice (n=9 for wt, n=10 for het) were placed in the center of the tank, and they were required to swim to the platform in order to escape from the water. Latency to reach the platform for each trial was scored by an observer blind to genotype and averaged over trials per day. Each mouse received 8 daily training sessions, consisting of 4 trials per session, and the change in escape latency over time was recorded as a measure of spatial learning. On day 9, the platform was removed, and mice were allowed to explore the tank for 1 min. Time spent searching in the location previously occupied by the platform was recorded using the EthovisionPro Video Tracking System (Noldus Information Technology, USA).
Contextual fear conditioning
Contextual fear conditioning was performed in a chamber equipped with a house light and an electrified metal floor grid capable of providing foot shocks to the mice (Med Associates, USA). During training, mice (n=8 for wt, n=10 for het) received 3 foot shocks (1 sec, 0.4 mA) within a 3 min period with an ITI of 30–60 sec. In order to assess contextual fear, mice were returned to the same boxes 24 hours later. This context exposure was repeated at 48, 72 and 96 hours after training to assess extinction of the freezing response. Freezing was subsequently assessed by scoring behavior (immobile or not) every other second over a 3 minute trial by an observer blind to the experimental groups.
Results
Reelin levels in the prefrontal cortex and hippocampus of adult heterozygous reeler mice
To confirm that the levels of reelin protein are indeed decreased in the relevant brain areas of the heterozygous reeler mice, immunoblots were run on prefrontal cortical and hippocampal tissue from Reln +/+ and +/− mice. Levels of both the full-length 400 kDa reelin protein and the 180 kDa major N-terminal cleavage product were found to be decreased by ~50% in both prefrontal cortex and hippocampus (Figure 1A, Table 1, p < 0.01), in agreement with previous studies (Liu et al. 2001;Qiu et al. 2006).
Figure 1. General characterization of heterozygous reeler mice.
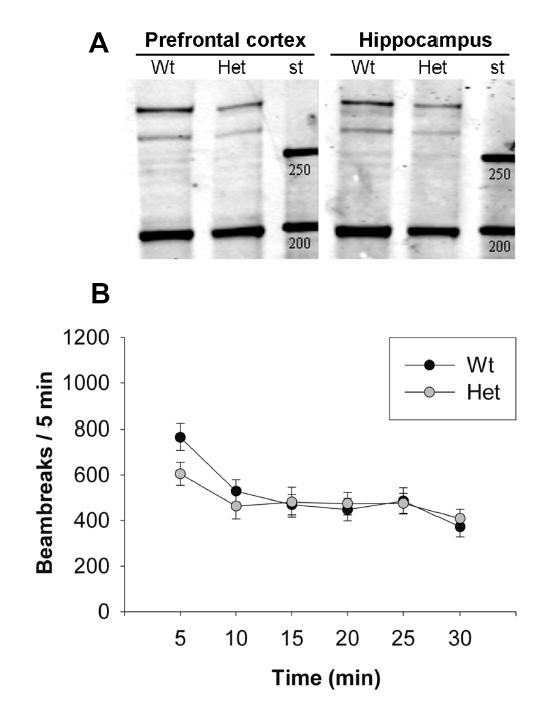
(A) Immunoblot of tissue from prefrontal cortex and hippocampus of adult wild-type and heterozygous reeler mice, immunoblotted with G10 monoclonal reelin antibody. (st) indicates standards of 200 and 250 kDa. Quantification of levels of both full-length reelin (400 kDa) and the major 180 kDa fragment is shown in table 1. Asterisks identify a significant effect of genotype (p < 0.05). (B) Analysis of locomotor activity in wt and het mice. Data are represented as number of beam breaks in each of 6 consecutive 5-min bins. No significant effect of genotype was observed.
Table 1.
Reelin levels and elevated plus maze performance in heterozygous reeler mice.
| WI
|
Het
|
|||
|---|---|---|---|---|
| Mean | SEM | Mean | SEM | |
| Reelin levels | ||||
| PFC 400 kDa | 100.0 | 8.4 | 48.7* | 6.7 |
| PFC 180 kDa | 100.0 | 12.7 | 52.3* | 3.8 |
| Hip 400 kDa | 100.0 | 12.8 | 42.7* | 7.1 |
| Hip 180 kDa | 100.0 | 2.8 | 57.9* | 2.5 |
| Elevated plus maze | ||||
| % Time in open | 17.5 | 3.2 | 14.6 | 1.5 |
| # Entries in open | 6.5 | 0.7 | 6.4 | 0.6 |
Locomotor activity and elevated plus maze performance in heterozygous reeler mice
The initial experiments evaluated the effect of reelin haploinsufficiency on baseline behaviors that may interfere with cognitive testing. In the locomotor activity test all mice showed a significant decrease in locomotor activity over time (Figure 1B; repeated measures ANOVA for time, F1,13 = 15.239, p < 0.05). However, the locomotor activity response observed over 30 min was not different between wildtype and heterozygous reeler mice (Figure 1B; repeated measures ANOVA for genotype, F1,13 < 1), although there was a trend towards a decrease in the initial period (repeated measures ANOVA for time × genotype, F1,13 = 2.182, p = 0.067). Similarly, the heterozygous reeler mice also showed no change in time spent in the open arm and entries into the open arm of an elevated plus maze (Table 1; ANOVA, F1,17 < 1) or in the number of total arm entries, a measure often used as an activity index (univariate ANOVA, F1,17 < 1, data not shown).
Cognitive flexibility in heterozygous reeler mice
Perseveration, or the lack of cognitive flexibility, is defined as contextually inappropriate and unintentional repetition of a response (Crider, 1997). Here, cognitive flexibility was assessed by measuring perseverative responding in a three-choice reversal learning task including a 10 day acquisition period, followed by two 5-day reversal phases. A significant effect of training day was observed for both groups in the acquisition and reversal phases (Figure 2A, C; repeated measures ANOVA, p < 0.05). However, no difference in correct (Figure 2C) or perseverative (Figure 2D) responses between genotypes was observed for either reversal (repeated measures ANOVA, F1,14 < 1 for both correct and perseverative responding in both reversals). Interestingly, however, there was a trend towards a deficit in the initial acquisition of the task (Figure 2A; repeated measures ANOVA genotype × day, F1,14 = 1.796, p = 0.075; genotype, F1,14 = 1.759, p = 0.206). Moreover, two heterozygous reelers were excluded from the reversals since they failed to acquire the task at all over 10 days. Including these two mice, a significant effect of genotype × day was observed (F1,16 = 2.710, p < 0.05; genotype, F1,16 = 3.481, p = 0.081).
Figure 2. Assessment of perseveration in heterozygous reeler mice.
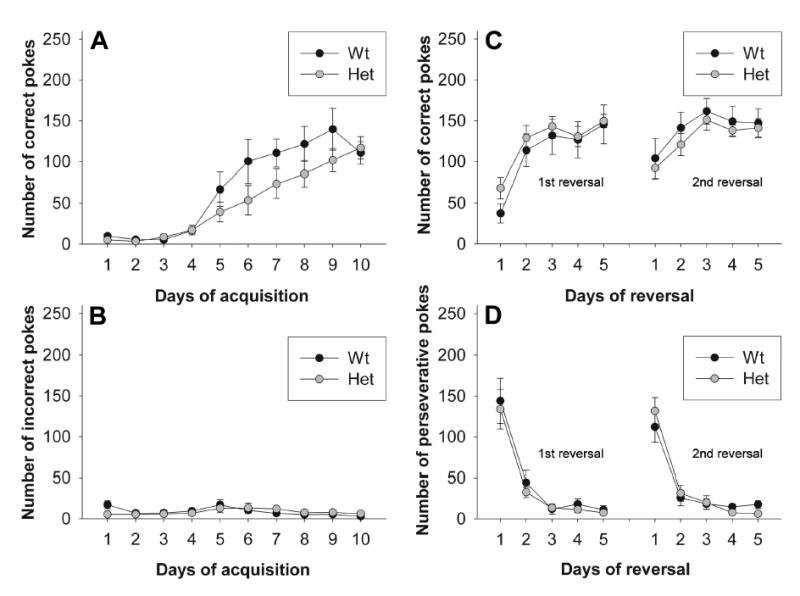
Perseveration was assessed using an instrumental reversal learning paradigm. (A, B) Acquisition phase of the instrumental learning paradigm. Results are reported as the number of correct (A) and incorrect (B) nosepokes per 15-min session. (C, D) Reversal phase of the instrumental learning paradigm. Results are reported as the number of correct (C) and perseverative (D) responses per 15-min session. No significant effect of genotype was observed, although there was a trend towards a deficit in correct responses in heterozygous reeler mice (p= 0.075).
Inhibitory control in heterozygous reeler mice
Impulsivity, defined as “actions that are poorly conceived, prematurely expressed, unduly risky, or inappropriate to the situation and that often result in undesirable outcomes” (Evenden, 1999), is a feature of many psychiatric disorders, including schizophrenia, substance abuse disorders and attention deficit/hyperactivity disorder. Here we focused on the inability to inhibit a nose poke response for a food reward until a go-signal was presented. The efficiency ratio, or number of nosepokes made per reward received, was used as a measure of inhibitory control. There was a significant increase in efficiency across days (repeated measures ANOVA for day, F1,12 = 3.309, p < 0.05), indicating that the mice learned to decrease responding prior to the onset of the auditory stimulus. In addition, the percentage of rewarded responses, i.e. the number of tones during which the mouse responded correctly, was used to measure the ability to learn to respond to the auditory stimulus across days. There was a significant increase in rewarded responses across days (repeated measures ANOVA for day, F1,12 = 4.062, p < 0.05), suggesting that the mice learned to associate the tone with the availability of reinforcement upon responding. Heterozygous reeler mice did not differ from wild-types in the measure of inhibitory control (Figure 3B, repeated measures ANOVA, F1,12 < 1). However, they showed a significant deficit in rewarded responses across days (Figure 3C, repeated measures ANOVA, genotype × day, F1,12 = 2.810, p < 0.05; genotype, F1,12 = 2.114, p = 0.172). There was no significant difference in total number of active responses (repeated measures ANOVA, genotype, F1,12 = 1.640, p = 0.225) or inactive responses (repeated measures ANOVA, genotype, F1,12 = 1.099, p = 0.315) across these 10 days (data not shown). However, a significant difference was observed in the first training phase (Figure 3A, ANOVA, F1,12 = 15.05, p < 0.05), but not in the subsequent phases.
Figure 3. Assessment of impulsivity in heterozygous reeler mice.
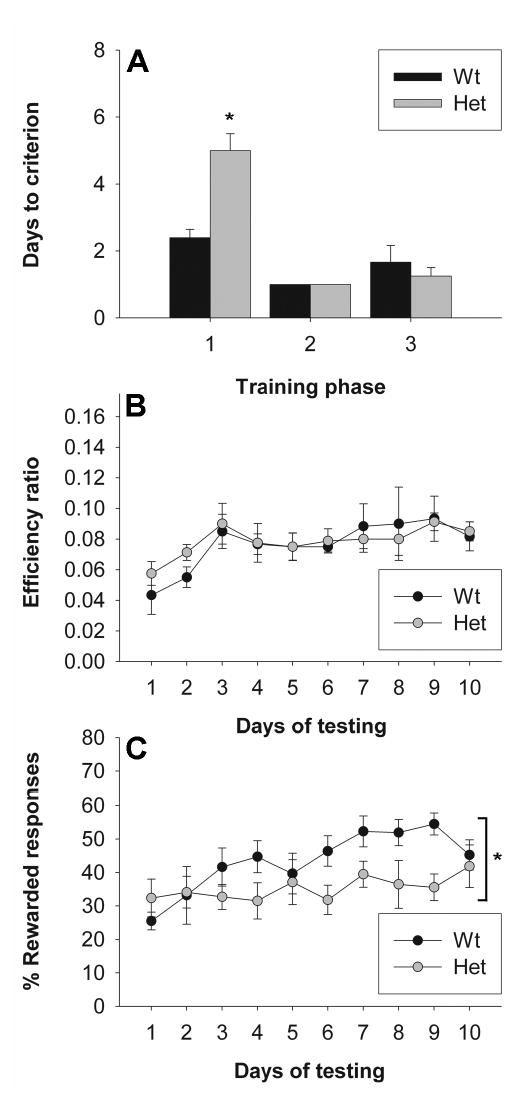
Impulsivity was assessed using an instrumental paradigm in which mice were required to withhold a nosepoke response until a go signal was delivered to receive a food reward. (A) Days to criterion for training phases 1, 2 and 3. (B) Efficiency ratio [(number of rewarded responses) / (number of active nosepokes)] as a measure of impulsivity. (C) Percentage of rewarded responses [(number of rewarded trials) / (total number of trials) * 100] as a measure of stimulus-reward learning. Asterisks identify a significant effect of genotype (p < 0.05).
Attentional function in heterozygous reeler mice
Attentional deficits are defined as the inability to exert attention in a selective manner while ignoring irrelevant information (Braver et al. 1999). Here, attention was measured using a modified version of the five-choice serial reaction time task as described (Humby et al. 1999). Response accuracy, i.e. the number of correct responses vs. incorrect responses and omission errors, is a measure of attentional function. In addition, the number of anticipatory responses provides an additional measure of inhibitory control. Heterozygous reeler mice did not show a deficit in acquisition of this task at any stage (Figure 4A, multivariate ANOVA, F1,18 < 1 for all stages), nor did they differ from wild-type littermate controls in the measures of attention and impulsivity (Figure 4B, multivariate ANOVA, F1,18 < 1 for correct, omission, anticipatory; F1,18 = 2.979, p=.104 for incorrect).
Figure 4. Assessment of attentional function in heterozygous reeler mice.
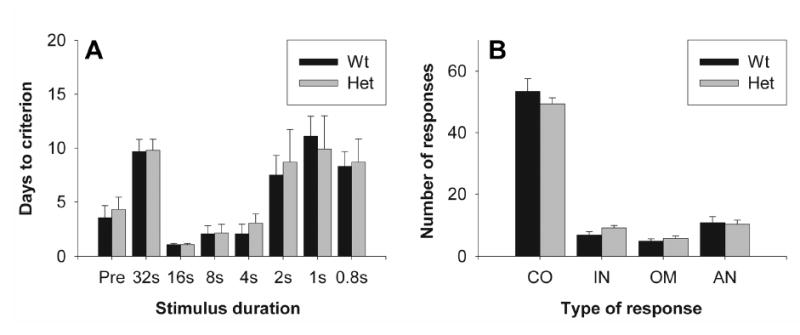
Attentional function was measured using a 3-choice serial reaction time task. (A) Days required to reach criterion for each of the training phases (pre-training, followed by stimulus durations of 32, 16, 8, 4, 2, 1 and 0.8 sec). (B) Number of correct (CO), incorrect (IN), omission (OM) and anticipatory (AN) responses across 30 min of test. No significant effect of genotype was observed.
Working memory in heterozygous reeler mice
Working memory, or the process of actively holding information ‘online’ to guide a response, is perhaps the best-studied function of the prefrontal cortex, and is known to be impaired in schizophrenia (Goldman-Rakic, 1994). Here, working memory was tested in the mice over a 2–20 sec delay. At the 2 sec delay, mice of both genotypes performed at ~80% accuracy, but as the delay period increased, accuracy decreased significantly until it reached almost chance levels, indicating an increasing working memory load for the mice (Figure 5; repeated measures ANOVA, F1,10 = 14.31, p < 0.05). No difference was observed between wild-type and heterozygous reeler mice in this test (repeated measures ANOVA, F1,10 < 1).
Figure 5. Assessment of working memory in heterozygous reeler mice.
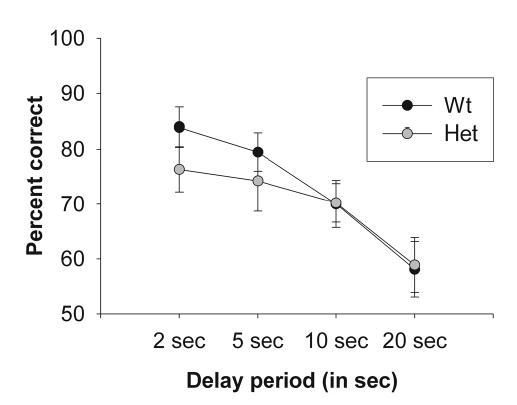
Working memory was assessed using an instrumental delayed-matching-to-position paradigm. Results are represented as percentage of correct responses at each delay period. No significant effect of genotype was observed.
Spatial and contextual learning and memory in heterozygous reeler mice
To determine whether the learning deficits described above generalized to other tasks of cognitive function, wild-type and heterozygous reeler mice were tested on two tasks of spatial and contextual memory, the Morris water maze (Figure 6A, B) and contextual fear conditioning (Figure 6C). In the Morris water maze, the latency to reach the platform decreased significantly over time (repeated measures ANOVA for days, F1,17 = 41.09, p < 0.05), but no differences were observed between genotypes (acquisition: repeated measures ANOVA for genotype, F1,17 < 1; probe trial: repeated measures ANOVA for genotype, F1,17 < 1). Likewise, mice showed a significant decrease in percentage of time spent freezing across four days of extinction testing following fear conditioning (repeated measures ANOVA for days, F1,17 = 8.40, p < 0.05), but no effect of genotype was observed (repeated measures ANOVA, F1,17 < 1). There was also no difference in baseline freezing prior to first shock (average 4.39%, univariate ANOVA for genotype, F1,17 < 1, data not shown).
Figure 6. Hippocampus-dependent learning in heterozygous reeler mice.
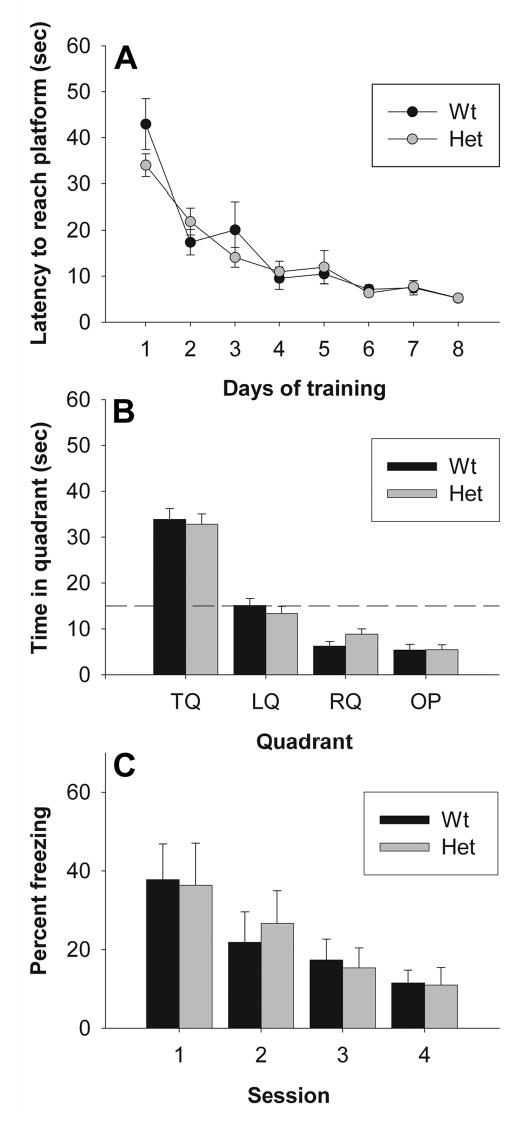
(A, B) Spatial learning and memory were assessed using a Morris water maze task. (A) Latency to reach the platform across 8 days of training. (B) Probe trial on day 9 of the Morris water maze. Results are expressed as time (in sec) spent in the target quandrant (TQ, which previously contained the platform), the opposite quadrant (OP), and the left (LQ) and right (RQ) quandrants relative to the previous location of the platform. The dashed line represents chance levels of performance. (C) Contextual fear conditioning in heterozygous reeler mice. Freezing is reported as percentage of time spent immobile over a 3 minute trial across 4 consecutive test days. No significant effect of genotype was observed.
Discussion
The present study sought to determine whether heterozygous reeler mice, proposed to be a genetic mouse model of schizophrenia, showed any of the prefrontal-related cognitive deficits that are the hallmark symptoms of schizophrenia. Heterozygous reeler mice and wild-type littermate controls were thus tested on cognitive flexibility, inhibitory control, attention and working memory. No differences were observed between wild-type and heterozygous reeler mice in any of these measures. However, heterozygous reeler mice showed deficits in the acquisition of several of the tasks, consistent with previous findings that reelin is involved in plasticity and learning (Larson et al. 2003; Qiu et al. 2006).
The association between reelin and schizophrenia originated from studies showing that reelin mRNA and protein levels are decreased 30–50% in the prefrontal cortex and other brain regions of schizophrenic patients (Impagnatiello et al. 1998; Guidotti et al. 2000). It was subsequently found that heterozygous reeler mice, which show a similar decrease in reelin, exhibit phenotypic traits resembling those found in schizophrenic patients. These included a down-regulation of GAD67-positive neurons in frontoparietal cortex, an increase of neuronal packing density and a decrease of dendritic spine density in frontoparietal cortex and hippocampus (Liu et al. 2001). Moreover, the mice showed disruptions in prepulse inhibition of startle (Tueting et al. 1999; Costa et al. 2002), increased anxiety on the elevated plus maze (Tueting et al. 1999) and an olfactory learning deficit (Larson et al. 2003). Consequently, the heterozygous reeler mouse was proposed as a genetic model for schizophrenia vulnerability and for screens of antipsychotic efficacy (Tueting et al. 1999; Liu et al. 2001; Costa et al. 2002). However, the findings presented here suggest strongly that, whereas reelin haploinsufficiency in the mouse may induce some of the anatomical prefrontal abnormalities observed in schizophrenia, it does not mimic any of the associated cognitive deficits seen in this disorder.
These discrepancies may be due to the fact that the circuitry affected by reelin is unrelated to that mediating the behavioral changes observed in schizophrenia. Alternatively, reelin haploinsufficiency alone may be enough to decrease GAD67 levels, but not to produce cognitive impairments associated with prefrontal dysfunction, and that additional factors are required to trigger obvious deficits in higher order cognitive functions. This would be particularly relevant in light of the ‘two-hit’ hypothesis of schizophrenia, where schizophrenia and possibly other psychiatric disorders are likely to arise from a combination of subtle abnormalities during prenatal development (genetic or environmental) and additional adverse events in childhood or early adulthood (Bayer et al. 1999). It should also be considered that the neural circuitry underlying executive function in primates on one hand and mice on the other hand may differ enough for equivalent molecular changes to have substantially different effects on behavioral output in the two species. In any case, it must be concluded from our data that the heterozygous reeler mouse cannot be used to study the core prefrontal-dependent cognitive deficits observed in schizophrenia. However, these data do not rule out a role for reelin in the human condition, nor do they preclude the development of other models of reelin involvement in prefrontal function.
In contrast to the lack of phenotype related to prefrontal-associated behaviors, heterozygous reeler mice were found to be impaired in the acquisition phase of two of the tasks. This finding is consistent with the fact that, independent of the proposed connection with schizophrenia, reelin has also been studied for its role in learning and plasticity. Reelin enhances LTP in hippocampal slices from wild-type mice, at least in part by increasing NMDAR function (Beffert et al. 2005; Chen et al. 2005), and mice lacking the reelin receptors ApoER2 and VLDLR show deficits in spatial learning and hippocampal LTP (Weeber et al. 2002; Beffert et al. 2005). In a recent study, heterozygous reeler mice also showed decreased contextual fear conditioning and impaired hippocampal LTP and LTD (Qiu et al. 2006). The findings presented in the current study suggest that reelin may also play a subtle role in non-hippocampal dependent plasticity and learning, consistent with previously reported deficits in olfactory discrimination learning (Larson et al. 2003). Thus, it may be that heterozygous reeler mice are a better model of learning deficits that are also associated with many psychiatric disorders than of the prefrontal cognitive symptoms related more specifically to schizophrenia. This hypothesis is consistent with reports of associations between reelin and a wide range of psychiatric and neurodevelopmental disorders, including bipolar disorder, major depression, autism and lissencephaly (Fatemi, 2001; D’Arcangelo, 2006).
In evaluating the heterozygous reeler mouse model, it is also important to note that there have been considerable discrepancies in the behavioral phenotype reported by different research groups. Following the initial description by Tueting et al. (Tueting et al. 1999), two groups conducted extensive characterizations of the heterozygous reeler phenotype (Salinger et al. 2003; Podhorna and Didriksen, 2004), in which they were unable to find any effects of reelin haploinsufficiency on tests of motor, social, anxiety-related, fear or learning behaviors. In particular, they were both unable to replicate the alterations in prepulse inhibition (PPI), a measure of sensorimotor gating that is abnormal in patients with schizophrenia and that formed the principal behavioral link between heterozygous reeler mice and schizophrenia (Tueting et al. 1999). In contrast, Qiu et al. (Qiu et al. 2006) reported a PPI deficit and impairments in contextual fear conditioning, but were not able to confirm the original report of increased anxiety on the elevated plus maze. In this study, we saw no changes in elevated plus maze performance or in contextual fear conditioning, but were able to identify subtle deficits in acquisition of instrumental responding. In contrast, no deficits were observed by Salinger et al. in a floorplate nose-poke task (Salinger et al. 2003). These inconsistencies between laboratories may result from differences in the age of the mice, training and testing protocols, rearing conditions, and genetic background, all of which can easily influence the outcome of behavioral experiments (Wahlsten et al. 2003; Lewejohann et al. 2006). This potential susceptibility to precise experimental conditions may reflect the fact that changes induced by reelin haploinsufficiency are either very specific or very subtle. A more powerful way of investigating the role of reelin in the adult brain may be to use mice that have reelin conditionally knocked out in a temporally and/or spatially restricted manner. This approach would allow complex behaviors to be assessed in the complete absence of reelin in adults, without introducing the significant confound of developmental deficits in the constitutive knockout.
In summary, there is growing evidence that reelin plays a role in plasticity in the adult brain, and that alterations in reelin signaling may contribute to cognitive deficits and psychiatric disorders. However, due to the subtle effects of reelin haploinsufficiency, the heterozygous reeler mouse does not appear to be the optimal model system for future studies. Instead, it will be critical to develop new approaches and tools to better define the role of reelin in postnatal brain function and dysfunction.
Acknowledgments
This work was supported by NIH grants MH 74866, MH 40899 and DA11717, as well as the Scottish Rite Schizophrenia Research Program.
References
- Alcantara S, Ruiz M, D’Arcangelo G, Ezan F, de Lecea L, Curran T, Sotelo C, Soriano E. Regional and Cellular Patterns of reelin mRNA Expression in the Forebrain of the Developing and Adult Mouse. J Neurosci. 1998;18:7779–7799. doi: 10.1523/JNEUROSCI.18-19-07779.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballmaier M, Zoli M, Leo G, Agnati LF, Spano P. Preferential alterations in the mesolimbic dopamine pathway of heterozygous reeler mice: an emerging animal-based model of schizophrenia. Eur J Neurosci. 2002;15:1197–1205. doi: 10.1046/j.1460-9568.2002.01952.x. [DOI] [PubMed] [Google Scholar]
- Bayer TA, Falkai P, Maier W. Genetic and non-genetic vulnerability factors in schizophrenia: the basis of the “Two hit hypothesis”. J Psychiatr Res. 1999;33:543–548. doi: 10.1016/s0022-3956(99)00039-4. [DOI] [PubMed] [Google Scholar]
- Beffert U, Weeber EJ, Durudas A, Qiu S, Masiulis I, Sweatt JD, Li WP, Adelmann G, Frotscher M, Hammer RE, Herz J. Modulation of Synaptic Plasticity and Memory by Reelin Involves Differential Splicing of the Lipoprotein Receptor Apoer2. Neuron. 2005;47:567–579. doi: 10.1016/j.neuron.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Bowers BJ, Wehner JM. Ethanol Consumption and Behavioral Impulsivity Are Increased in Protein Kinase Cgamma Null Mutant Mice. J Neurosci. 2001;21:180RC. doi: 10.1523/JNEUROSCI.21-21-j0004.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braver TS, Barch DM, Cohen JD. Cognition and control in schizophrenia: a computational model of dopamine and prefrontal function. Biol Psychiatry. 1999;46:312–328. doi: 10.1016/s0006-3223(99)00116-x. [DOI] [PubMed] [Google Scholar]
- Brown VJ, Bowman EM. Rodent models of prefrontal cortical function. Trends Neurosci. 2002;25:340–343. doi: 10.1016/s0166-2236(02)02164-1. [DOI] [PubMed] [Google Scholar]
- Carboni G, Tueting P, Tremolizzo L, Sugaya I, Davis J, Costa E, Guidotti A. Enhanced dizocilpine efficacy in heterozygous reeler mice relates to GABA turnover downregulation. Neuropharmacology. 2004;46:1070–1081. doi: 10.1016/j.neuropharm.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Chen Y, Beffert U, Ertunc M, Tang TS, Kavalali ET, Bezprozvanny I, Herz J. Reelin Modulates NMDA Receptor Activity in Cortical Neurons. J Neurosci. 2005;25:8209–8216. doi: 10.1523/JNEUROSCI.1951-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa E, Davis J, Pesold C, Tueting P, Guidotti A. The heterozygote reeler mouse as a model for the development of a new generation of antipsychotics. Curr Opin Pharmacol. 2002;2:56–62. doi: 10.1016/s1471-4892(01)00121-7. [DOI] [PubMed] [Google Scholar]
- Crider A. Perseveration in schizophrenia. Schizophr Bull. 1997;23:63–74. doi: 10.1093/schbul/23.1.63. [DOI] [PubMed] [Google Scholar]
- D’Arcangelo G. Reelin mouse mutants as models of cortical development disorders. Epilepsy Behav. 2006;8:81–90. doi: 10.1016/j.yebeh.2005.09.005. [DOI] [PubMed] [Google Scholar]
- D’Arcangelo G, Miao GG, Chen SC, Soares HD, Morgan JI, Curran T. A protein related to extracellular matrix proteins deleted in the mouse mutant reeler. Nature. 1995;374:719–723. doi: 10.1038/374719a0. [DOI] [PubMed] [Google Scholar]
- D’Arcangelo G, Miao GG, Curran T. Detection of the reelin breakpoint in reeler mice. Brain Res Mol Brain Res. 1996;39:234–236. doi: 10.1016/0169-328x(96)00046-0. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Cardinal RN, Robbins TW. Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neurosci Biobehav Rev. 2004;28:771–784. doi: 10.1016/j.neubiorev.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Dong E, Caruncho H, Liu WS, Smalheiser NR, Grayson DR, Costa E, Guidotti A. A reelin-integrin receptor interaction regulates Arc mRNA translation in synaptoneurosomes. Proc Natl Acad Sci U S A. 2003;100:5479–5484. doi: 10.1073/pnas.1031602100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elvevag B, Goldberg TE. Cognitive impairment in schizophrenia is the core of the disorder. Crit Rev Neurobiol. 2000;14:1–21. [PubMed] [Google Scholar]
- Estape N, Steckler T. Cholinergic blockade impairs performance in operant DNMTP in two inbred strains of mice. Pharmacol Biochem Behav. 2002;72:319–334. doi: 10.1016/s0091-3057(01)00747-x. [DOI] [PubMed] [Google Scholar]
- Evenden JL. Varieties of impulsivity. Psychopharmacology. 1999;146:348–361. doi: 10.1007/pl00005481. [DOI] [PubMed] [Google Scholar]
- Fatemi SH. Reelin mutations in mouse and man: from reeler mouse to schizophrenia, mood disorders, autism and lissencephaly. Mol Psychiatry. 2001;6:129–133. doi: 10.1038/sj.mp.4000129. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Working memory dysfunction in schizophrenia. J Neuropsychiatry Clin Neurosci. 1994;6:348–357. doi: 10.1176/jnp.6.4.348. [DOI] [PubMed] [Google Scholar]
- Greco B, Invernizzi RW, Carli M. Phencyclidine-induced impairment in attention and response control depends on the background genotype of mice: reversal by the mGLU2/3 receptor agonist LY379268. Psychopharmacology. 2005;179:68–76. doi: 10.1007/s00213-004-2127-9. [DOI] [PubMed] [Google Scholar]
- Guidotti A, Auta J, Davis JM, Di-Giorgi-Gerevini V, Dwivedi Y, Grayson DR, Impagnatiello F, Pandey G, Pesold C, Sharma R, Uzunov D, Costa E. Decrease in reelin and glutamic acid decarboxylase67 (GAD67) expression in schizophrenia and bipolar disorder: a postmortem brain study. Arch Gen Psychiatry. 2000;57:1061–1069. doi: 10.1001/archpsyc.57.11.1061. [DOI] [PubMed] [Google Scholar]
- Humby T, Laird FM, Davies W, Wilkinson LS. Visuospatial attentional functioning in mice: interactions between cholinergic manipulations and genotype. Eur J Neurosci. 1999;11:2813–2823. doi: 10.1046/j.1460-9568.1999.00701.x. [DOI] [PubMed] [Google Scholar]
- Impagnatiello F, Guidotti AR, Pesold C, Dwivedi Y, Caruncho H, Pisu MG, Uzunov DP, Smalheiser NR, Davis JM, Pandey GN, Pappas GD, Tueting P, Sharma RP, Costa E. A decrease of reelin expression as a putative vulnerability factor in schizophrenia. Proc Natl Acad Sci U S A. 1998;95:15718–15723. doi: 10.1073/pnas.95.26.15718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo A, Wiedholz LM, Millstein RA, Yang RJ, Bussey TJ, Saksida LM, Holmes A. Genetic and dopaminergic modulation of reversal learning in a touchscreen-based operant procedure for mice. Behav Brain Res. 2006 2006 Aug 10;171(2):181–8. doi: 10.1016/j.bbr.2006.03.029. [DOI] [PubMed] [Google Scholar]
- Larson J, Hoffman JS, Guidotti A, Costa E. Olfactory discrimination learning deficit in heterozygous reeler mice. Brain Res. 2003;971:40–46. doi: 10.1016/s0006-8993(03)02353-9. [DOI] [PubMed] [Google Scholar]
- Lewejohann L, Reinhard C, Schrewe A, Brandewiede J, Haemisch A, Gortz N, Schachner M, Sachser N. Environmental bias? Effects of housing conditions, laboratory environment and experimenter on behavioral tests. Genes Brain Behav. 2006;5:64–72. doi: 10.1111/j.1601-183X.2005.00140.x. [DOI] [PubMed] [Google Scholar]
- Lidow MS, Koh PO, Arnsten AF. D1 dopamine receptors in the mouse prefrontal cortex: Immunocytochemical and cognitive neuropharmacological analyses. Synapse. 2003;47:101–108. doi: 10.1002/syn.10143. [DOI] [PubMed] [Google Scholar]
- Liu WS, Pesold C, Rodriguez MA, Carboni G, Auta J, Lacor P, Larson J, Condie BG, Guidotti A, Costa E. Down-regulation of dendritic spine and glutamic acid decarboxylase 67 expressions in the reelin haploinsufficient heterozygous reeler mouse. Proc Natl Acad Sci U S A. 2001;98:3477–3482. doi: 10.1073/pnas.051614698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller E, Freedman D, Wallis J. The prefrontal cortex: categories, concepts and cognition. Phil Trans R Soc Lond B. 2002;357:1123–1136. doi: 10.1098/rstb.2002.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappas GD, Kriho V, Pesold C. Reelin in the extracellular matrix and dendritic spines of the cortex and hippocampus: a comparison between wild type and heterozygous reeler mice by immunoelectron microscopy. J Neurocytol. 2002;30:413–425. doi: 10.1023/a:1015017710332. [DOI] [PubMed] [Google Scholar]
- Podhorna J, Didriksen M. The heterozygous reeler mouse: behavioural phenotype. Behav Brain Res. 2004;153:43–54. doi: 10.1016/j.bbr.2003.10.033. [DOI] [PubMed] [Google Scholar]
- Qiu S, Korwek KM, Pratt-Davis AR, Peters M, Bergman MY, Weeber EJ. Cognitive disruption and altered hippocampus synaptic function in Reelin haploinsufficient mice. Neurobiol Learn Mem. 2006;85:228–242. doi: 10.1016/j.nlm.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Robbins TW. Chemical neuromodulation of frontal-executive functions in humans and other animals. Exp Brain Res. 2000;133:130–138. doi: 10.1007/s002210000407. [DOI] [PubMed] [Google Scholar]
- Royall DR, Lauterbach EC, Cummings JL, Reeve A, Rummans TA, Kaufer DI, LaFrance WC, Jr, Coffey CE. Executive Control Function: A Review of Its Promise and Challenges for Clinical Research. A Report from the Committee on Research of the American Neuropsychiatric Association. J Neuropsychiatry Clin Neurosci. 2002;14:377–405. doi: 10.1176/jnp.14.4.377. [DOI] [PubMed] [Google Scholar]
- Salinger WL, Ladrow P, Wheeler C. Behavioral phenotype of the reeler mutant mouse: effects of RELN gene dosage and social isolation. Behav Neurosci. 2003;117:1257–1275. doi: 10.1037/0735-7044.117.6.1257. [DOI] [PubMed] [Google Scholar]
- Söderpalm B, Engel JA. Biphasic effects of clonidine on conflict behavior: involvement of different alpha-adrenoceptors. Pharmacol Biochem Behav. 1988;30:471–477. doi: 10.1016/0091-3057(88)90482-0. [DOI] [PubMed] [Google Scholar]
- Tueting P, Costa E, Dwivedi Y, Guidotti A, Impagnatiello F, Manev R, Pesold C. The phenotypic characteristics of heterozygous reeler mouse. Neuroreport. 1999;10:1329–1334. doi: 10.1097/00001756-199904260-00032. [DOI] [PubMed] [Google Scholar]
- Volk DW, Lewis DA. Impaired prefrontal inhibition in schizophrenia: relevance for cognitive dysfunction. Physiol Behav. 2002;77:501–505. doi: 10.1016/s0031-9384(02)00936-8. [DOI] [PubMed] [Google Scholar]
- Wahlsten D, Metten P, Phillips TJ, Boehm SL, Burkhart-Kasch S, Dorow J, Doerksen S, Downing C, Fogarty J, Rodd-Henricks K, Hen R, McKinnon CS, Merrill CM, Nolte C, Schalomon M, Schlumbohm JP, Sibert JR, Wenger CD, Dudek BC, Crabbe JC. Different data from different labs: lessons from studies of gene-environment interaction. J Neurobiol. 2003;54:283–311. doi: 10.1002/neu.10173. [DOI] [PubMed] [Google Scholar]
- Weeber EJ, Beffert U, Jones C, Christian JM, Forster E, Sweatt JD, Herz J. Reelin and ApoE receptors cooperate to enhance hippocampal synaptic plasticity and learning. J Biol Chem. 2002;277:39944–39952. doi: 10.1074/jbc.M205147200. [DOI] [PubMed] [Google Scholar]


