Abstract
CCR4 is purported to be a Th type 2 (Th2) cell-biased receptor but its functional role is unclear. Recent studies suggest that chemokine receptor expression and function are more complex in vivo and raise doubts regarding restricted CCR4 expression by Th2 cells. To address these issues, we analyzed the role of CCR4 in highly polarized models of Th type 1 (Th1) and Th2 cell-mediated pulmonary granulomas, respectively, elicited by i.v. challenge of primed mice with either mycobacterial purified protein derivative or schistosomal egg Ag-coated beads. CCR4 agonists were expressed during both responses, correlating with a shift of CCR4+CD4+ T cells from blood to lungs. CCL22 dominated in draining nodes during the Th1 response. Analysis of CD4+ effector T cells revealed CCR4 expression and CCR4-mediated chemotaxis by both IFN-γ and IL-4 producers. Studies of CCR4 knockout (CCR4−/−) mice showed partial impairment of the local type-2 cytokine response and surprisingly strong impairment of the Th1 response with abrogated IFN-γ production during secondary but not primary challenge. Adoptive transfer indicated CCR4−/−CD4+ Th1 cell function was defective but this could not be reconstituted with wild-type (CCR4+/+) CD4+ T cells indicating involvement of another CCR4+ population. Coculture of CCR4+/+CD4+ T cells and CCR4−/− dendritic cells revealed intact IL-2 but impaired IFN-γ production, pointing to a role for CCR4+ dendritic cells in effector cell expression. Therefore, CCR4 is not Th2-restricted and was required for sustenance and expression of the Th1 effector/memory response to mycobacterial Ags.
The CD4+ effector T cell is a well-recognized component of the adaptive immune response (1). Among the subtypes of CD4+ T cells are Th type 1 (Th1)3 cells secreting IFN-γ and TNF-α and Th type 2 (Th2) cells secreting IL-4, IL-5, and IL-13, which participate in cell-mediated and allergic-type inflammatory responses, respectively (2). T cell migration through tissue microenvironments is thought to involve chemokine-chemokine receptor interactions (3). Studies of in vitro-generated populations suggested that Th1 and Th2 cells display differential expression of chemokine receptors (4). Th1 cells were reported to express CXCR3 and CCR5 expression whereas Th2 cells selectively expressed CCR3, CCR4, and CCR8 (5–11). Yet, recent in vivo studies suggest that patterns of chemokine receptor expression are more complex and flexible than in vitro polarization studies initially suggested. This has been especially the case with regard to the putative Th2 cell-restricted receptors CCR3, CCR4, and CCR8. For example, no reports have established an essential role for CCR3 in Th2 function, possibly due to receptor redundancy. In addition, cytokine-producing T cell subsets and their chemokine receptor expression are more diverse than originally envisioned. Recently, we demonstrated that during the murine adaptive response to parasite helminth egg Ag, CCR8 associated with a dominant IL-10-producing CD4+CD25+ T cell population, rather than a classic IL-4-producing CD4+ Th2 population (12).
CCR4, another chemokine receptor widely purported to be Th2 biased, has likewise become subject to debate. In support of a role in Th2 function, CCR4 or its ligands have been reported in asthmatic lungs and among peripheral blood T cells during atopic dermatitis (13–15). Contrary to this notion, CCR4 genetic deletion had no effect in a mouse model of Th2-dependent, OVA-elicited airway inflammation (16). In a detailed analysis of human peripheral blood T cells, CCR4 expression was detected on isolated human CD4+ memory T cells with Th1 as well as Th2 characteristics (17). CCR4 is also reportedly expressed by human peripheral blood CD4+CD25+ T regulatory cells (18). In addition, the specific ligands for CCR4, CCL17 (thymus and activation-regulated chemokine) and CCL22 (macrophage-derived chemokine) (19), reportedly induce migration of both Th2 and Th1 cells in vitro (20, 21). Finally, the receptor is not limited to T cells because CCR4 mRNA is reportedly expressed by platelets, NK cells, macrophages, and dendritic cells (DCs) (22–25). In view of these reports, it is clear that the role of CCR4 with regard to T cell function needs better definition.
To analyze the role of CCR4 in Th cell function, we tested the hypothesis that CCR4 is a selective receptor for Th2 cell function by directly comparing CCR4 manipulation in highly defined experimental models of pathogen Ag-elicited Th1 and Th2 cell-mediated granuloma formation. We have established that anamnestic Th1 and Th2 responses can be studied in mice by sensitizing with protein Ags of Mycobacterium bovis purified protein derivative (PPD) or Schistosoma mansoni eggs (schistosomal egg Ag (SEA)) followed by an i.v. challenge of agarose beads covalently coupled to the respective sensitizing Ags (26). In these models, the Ag-coated beads embolize to the lung where they induce granulomatous inflammation histologically similar to that generated by live infections; but unlike granulomas generated during the infection, the Ag-bead lesions are synchronized and can be compared in parallel. Our results refuted the base hypothesis. Comparison of type-1 and -2 granulomas revealed that the ligands CCL17 and CCL22 were produced in draining lymph nodes and challenged lungs during both responses and were associated with the accumulation of CCR4 transcripts in granulomatous lungs. Analysis of chemokine receptor transcripts among Ag-elicited, cytokine-secreting, effector CD4+ T cells from the lungs of mice undergoing type-1 and -2 immune responses indicated that both IFN-γ- and IL-4-secreting cells expressed transcripts for CCR4 and responded to CCR4 ligands. Correspondingly, CD4+ T cells isolated from type-1 and -2 lung granulomas by laser capture microdissection (LCM) exhibited comparable levels of CCR4 transcript expression. Thus, CCR4 was not Th2 restricted, rather CCR4+CD4+ effector cells were recruited during both type-1 and -2 granuloma formation. Functional analyses indicated that CCR4 −/− mice generated type-2 granulomas with partially impaired Th2-cytokine production in response to the Th2-eliciting S. mansoni egg Ags. Unexpectedly, Th1-mediated granuloma formation was compromised as was the recall response in draining lymphoid tissue. Adoptive transfer and in vitro analyses suggested that CCR4 mediates T cell-DC interactions were required for the survival or persistence of Th1 effector/memory cells as proposed by Baekkevold et al. (27) in a cutaneous hypersensitivity model. Our findings indicate that CCR4 participates in both Th1 and Th2 granulomatous responses and suggest that establishment of CD4+ Th1 effector/memory during type-1 pulmonary granuloma formation requires interaction of CCR4+ T cells and APCs.
Materials and Methods
Animals
Mice lacking the CCR4 gene (CCR4−/−) were provided by Dr. C. A. Power (Serono Pharmaceutical Research Institute, Geneva, Switzerland); they were generated as previously described and were bred onto a C57BL/6 background (16). Knockout status was confirmed by RT-PCR analysis using gene-specific primers and probes. CBA and C57BL/6 mice were obtained from The Jackson Laboratory. S. mansoni-infected Swiss outbred mice were obtained from Biomedical Research Institute. All mice were maintained under specific pathogen-free conditions and provided with food and water ad libitum. All animal studies have been approved by an institutional review board.
Induction of primary or secondary type-1 or -2 immune response
Secondary type 1 or 2 lung Ag-bead granulomas were generated as previously described (28). Briefly, mice were sensitized either s.c. with 20 μg of Mycobacterium bovis PPD (Department of Agriculture, Veterinary Division, Ames, IA) incorporated into 0.25 ml of CFA (Sigma-Aldrich) or i.p. with 3000 S. mansoni eggs in 0.5 ml of PBS. After 14 days, mice were challenged i.v. with 6000 Sepharose 4B beads covalently coupled to either PPD or soluble SEA (World Health Organization, Geneva, Switzerland). To generate a primary response, mice were challenged i.v. with Ag-coated beads without prior sensitization.
Flow cytometry
Abs used to identify murine cell populations included anti-CCR4 (Abcam), PE-conjugatedanti-CD3(BDBiosciences),andcytochrome-conjugatedanti-CD4 (eBioscience). FITC-conjugated anti-goat IgG (Vector Laboratories) was used as a secondary Ab. Isotype controls included goat IgG (Sigma-Aldrich), hamster IgG1, and rat IgG2b (BD Biosciences). After blocking with anti-CD16/CD32 (BD Biosciences) for 10 min, cells were stained with unconjugated Abs for 20 min on ice followed by the FITC-conjugated secondary Ab for an additional 20 min. Cells were washed with 2% FBS in PBS. Cells were stained with the remaining Abs for 20 min. A FACScan flow cytometer with CellQuest software (BD Biosciences) was used for data acquisition and analysis.
Granuloma and lymph node cell isolation and culture
Following perfusion with cold RPMI 1640 (JRH Biosciences), lungs, excluding trachea and major bronchi, and mediastinal lymph nodes were excised. Lungs were homogenized in a Waring blender. Intact granulomas were collected for culture or were digested with 1000 U/ml type IV collagenase to obtain individual cells for flow cytometry. Lymph nodes were teased into a single-cell suspension. Granulomas and cells were cultured in RPMI 1640 plus FBS at 1500 lesions/ml or 5 × 106 cells/ml in the presence or absence of 10 μg/ml PPD or SEA. Cultures were incubated at 37°C with 5% CO2 for 24 h. Supernatants were collected by centrifugation and measured by ELISA.
Laser capture microdissection
Following perfusion with cold RPMI 1640, lungs were inflated by injecting 1–2 ml of a 2:1 mixture of PBS and OCT into the trachea. Individual lobes were excised and snap frozen in OCT. Eight-micrometer-thick tissue sections were mounted onto glass slides. Slides were stained with biotinylated CD4+ Ab at 5 μg/ml (BD Pharmingen) for 2 min followed by streptavidin-peroxidase (Sigma-Aldrich) at 1:20 for 2 min and diaminobenzidine for 5 min (Vector Laboratories). Staining was followed by dehydration in 70, 95, 100% ethanol and xylene. LCM was performed using the PixCell IIe with CapSure Macro LCM Caps (Arcturus Bioscience). RNA was isolated using PicoPure RNA Isolation kits (Arcturus Bioscience) according to manufacturer’s instructions.
Enrichment of CD4+ T cell subpopulations
Single-cell suspensions were incubated with CD3+ or CD4+ T cell enrichment mixture (Stem Cell Technologies) and washed through a magnetic separation column according to manufacturer’s instructions. To isolate lung cytokine-secreting CD4+ populations MACS Cytokine Secretion Assay kits (Miltenyi Biotec) were used as previously described (12).
Chemotaxis assays
Type 1 and 2 anamnestic granulomas were elicited in wild-type mice. Lymph nodes were collected 3 days after bead challenge and cultured for 20 h with Ag stimulation. CD4+ T cells were isolated and resuspended in serum-free RPMI 1640. rCCL17 (R&D Systems) was warmed to 37°C and diluted to a concentration of 5 ng/ml in PBS. PBS was used as a control. A multiwell chemotaxis chamber system with a 5-μm polycarbonate filter was used (Neuro Probe). CCL17 was added to the bottom wells and the polycarbonate filter was placed between the bottom plate and top plate of the chamber assembly. Three hundred thousand CD4+ T cells were added to the top wells. The chamber was incubated for 2 h at 37°C in a 5% CO2, humidified atmosphere. Cells that migrated through the filter were collected and used for real-time PCR transcript analyses.
Cytokine measurement
Murine IL-2, -4, -5, -13, and IFN-γ were measured from cultured lymph node and granuloma supernatants by ELISA using commercially available reagents and standards (BD Pharmingen; R&D Systems; PeproTech). Sensitivities fell between 15 and 50 pg/ml.
Morphometry
Individual excised lung lobes were inflated and fixed with 10% buffered formalin for morphometric analysis. Granuloma area was measured in a blinded fashion in H&E-stained sections of paraffin-embedded lungs using computer-assisted morphometry. A minimum of 20 lesions was measured per lung.
Differential analyses of granulomas
Intact granulomas were collected as described above then digested in RPMI 1640 plus 1000 U/ml type IV collagenase for 25 min at 37°C. After washing, cells were resuspended at 2.5 × 106 cells/ml. A 200-cell differential analysis was performed on duplicate Wright-stained cytospin preparations of dispersed granulomas.
Real-time RT-PCR transcript analysis
RNA was isolated, reverse transcribed, and used for real-time PCR analyses as previously described (12). All primer-probe sets for mouse receptors, cytokine and chemokine genes were purchased commercially (Applied Biosystems), except for CCL22 which was developed using Primer Express Software (Applied Biosystems) and prepared by Operon Technologies. The primer and TaqMan probe sequences were as follows: primers: 5′-AGG CAG GTC TGG GTG AAG AAG CT-3′ and 5′-GG ATG GAG GTG AGT AAA GGT GGC-3′; probe: 5′-GGA GGA CCT GAT GAC CAT GGG TC-3′. Transcript levels, expressed as arbitrary units, were calculated as previously described (29).
Adoptive cell transfer
For adoptive transfer studies, donor CCR4+/+ and CCR4−/− mice were challenged with PPD or SEA Ag beads 14 days after sensitization. Draining lymph nodes, collected 4 days after bead challenge, were dispersed for CD4+ T cell isolation. Naive wild-type recipient mice received 1 × 106 CD4+ T cells i.v. followed by Ag beads on the next day. Recipients were sacrificed 4 days later.
T cell reconstitution
For CCR4−/− reconstitution studies, 1 × 106 CD4+ T cells isolated from axillary lymph nodes of PPD-sensitized CCR4+/+ mice were transferred i.v. into naive CCR4−/− mice. Recipient mice were challenged with PPD Ag beads on the following day and then were sacrificed 4 days postchallenge. Donor CD4+ T cells from PPD-sensitized CCR4−/− mice were used as a control.
CD4+ T cell and DC coculture
Axillary lymph nodes from CCR4 +/+ and CCR4−/− mice were collected 14 days after PPD sensitization. CD4+ and CD11c+ Microbeads (Miltenyi Biotec) were used to isolate cell populations according to manufacturer’s instructions. The enrichment method was confirmed by flow cytometry and cells were determined to be primarily CD11b+ myeloid DCs. CCR4+/+ or CCR4−/− CD11c+ DCs were cultured with CCR4+/+CD4+ T cells at a ratio of 1:10 plus 10 μg/ml PPD. Cultures were incubated for 70 h at 37°C, 5% CO2. Supernatants were collected by centrifugation and measured by ELISA.
Statistical analysis
ANOVA was used for multigroup comparisons and the Student t test was used for pairwise comparisons. Values of p < 0.05 were considered to indicate significant differences.
Results
CCR4 ligands are induced in lungs and draining lymph nodes during type-1 and -2 pulmonary granuloma formation
We first determined the potential involvement of CCR4 in hypersensitivity lung granuloma formation. To this end, transcript levels for the known CCR4 ligands, CCL17 and CCL22, were measured in Ag bead-challenged lungs and draining lymph nodes of wild-type mice with synchronized granuloma formation. Measurements were taken at days 0, 1, 2, 3, 4, and 8 postbead challenge for both type-1 (PPD Ag bead) and type-2 (SEA Ag bead) granuloma models. In lungs, both CCL17 and CCL22 transcripts were induced following bead challenge (Fig. 1A). CCL17 reached peak expression by days 1 or 2 in the models but was more dominant following SEA-bead challenge. CCL22 transcripts peaked later than CCL17 reaching maximum levels on day 3 in granulomatous lungs. Transcript levels dropped off thereafter. In draining lymph nodes, CCL17 and CCL22 displayed peak expression on day 1 after PPD-bead challenge, but did not reach peak expression until day 3 in the type-2 model (Fig. 1B). In addition, CCL22 transcripts were more dominant following PPD-bead than SEA-bead challenge. These findings suggested that CCR4 ligands participate in both types of responses and possibly functioned in granulomas and draining lymph nodes. We also examined tissue compartment expression of CCR4 ligands using LCM. A comparison transcript expression in T and B cell zones of lymph nodes revealed that CCL22 induction was limited largely to T cell zones with peak expression at 1–2 days after Ag challenge (data not shown). This is fully in accord with studies of Tang and Cyster (30).
FIGURE 1.
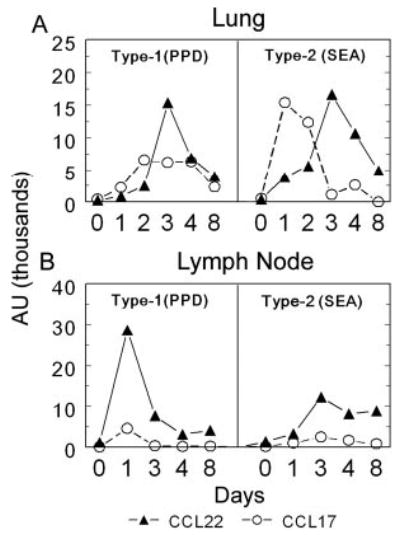
Time course of CCL17 and CCL22 mRNA expression in lungs and draining lymph nodes during type-1 (PPD) and type-2 (SEA) anamnestic granuloma formation. Lungs and draining lymph nodes were harvested before bead challenge (day 0) and on days 1, 2, 3, 4, and 8 post-Ag-bead challenge. A, Lungs; B, draining lymph nodes. Transcript expression was determined by real-time RT-PCR and expressed as arbitrary units. CCL17, ○; CCL22, ▴. Data are representative of three separate experiments.
CCR4 ligand expression during type-1 and -2 granuloma formation correlates with a blood-to-lung shift of CD4+ T cells expressing CCR4 transcripts
To determine the potential functional significance of CCR4 ligand expression, we compared the time course of CCR4 transcript expression among T cells in peripheral blood and whole lungs during synchronized granuloma formation. As shown in Fig. 2A, compared with naive mice (dashed line) there was a 2-fold increase of CCR4 transcripts among blood T cells in prechallenged sensitized mice (day 0). Following Ag-bead challenge, there was a distinct decrease in CCR4 transcripts among circulating T cells by 2–3 days corresponding to an increase in CCR4 transcripts in the challenged lungs (Fig. 2B, bars). This pattern was similar during both type-1 and -2 granuloma formation. These events correlated well with the time of CCR4 ligand expression in the lung as described above and were concomitant with the rapid growth period of lesions between 1 and 4 days (Fig. 2B, dashed lines). Once lesions achieved peak size (day 4), blood T cells displayed recovered levels of CCR4 transcripts. We next determined whether this shift was attributable to CD4+ T cell mobilization. To test this possibility, we analyzed CCR4 transcript expression among CD4+ T cells that were purified from the blood and lungs of naive, sensitized (day 0), and Ag-bead challenged sensitized mice (day 3). Similar to the whole CD3+ T cell population, sensitization increased the levels of CCR4 transcripts among circulating CD4+ T cells by ~3-fold compared with naive mice, but 3 days after either PPD or SEA Ag-bead challenge, transcripts decreased to naive levels in the blood (Fig. 2C) while increasing 2- to 3-fold in the granulomatous lungs (Fig. 2D, upper panel). The accumulation of CCR4+CD4+ T cells was confirmed by flow cytometric detection (Fig. 2D, lower panel, and E). These findings would be consistent with the enhanced lung mobilization of CCR4+CD4+ during both types-1 and -2 granuloma formation, but did not prove that CCR4 was required for mobilization.
FIGURE 2.
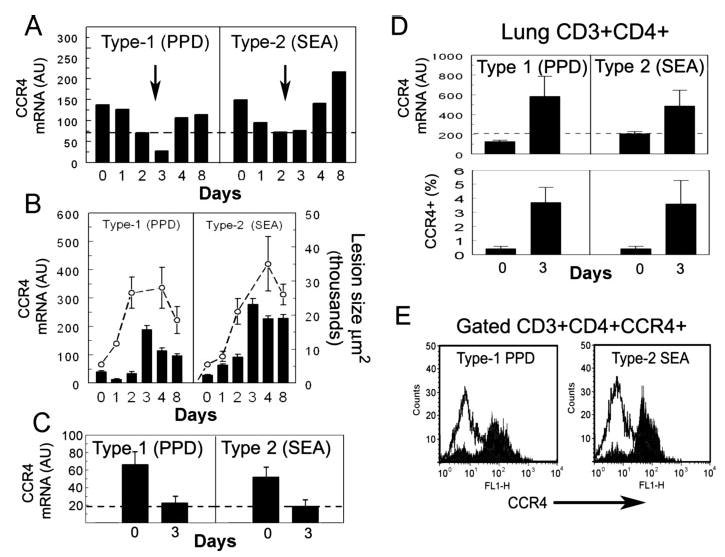
CCR4 expression among peripheral blood CD3+ and CD4+ T cells, whole lungs, and CD4+ lung T cells during type-1 (PPD) and type-2 (SEA) granulomatous responses. Blood and lungs were collected before bead challenge (day 0) and on days 1, 2, 3, 4, and 8 postbead challenge. T cells were isolated from blood and lung as described in Materials and Methods. A, Blood CD3+ T cell CCR4 transcript analysis; dashed line represents transcript levels among blood T cells of naive mice. Values were derived from the pooled blood of 10 mice/point. B, Whole lung analysis; dashed line shows growth of granulomas, bars show mean ± SD, CCR4 transcript levels measured in five to six individual mice per point. C, Blood, days 0 and 3, isolated CD4+ T cell CCR4 transcript analysis; bars show mean ± SD. Dashed lines indicates levels in naive mice CD4+ T cells were isolated from pooled blood and lungs of 5–10 mice before (day 0) and 3 days after challenge. Data are representative of three separate experiments. D, Lung, days 0 and 3, isolated CD4+ T cell CCR4 transcript and flow cytometric analyses. Bars show mean ± SD derived from 4 to 5 mice. E, Representative flow cytometric histograms showing surface expression of CCR4 on CD4+ T cells from granulomatous lungs, on day 3 after bead challenge. Solid area shows staining with CCR4 Ab. Open area is isotype control Ab.
It was noted that CCR4 mRNA signals were 10-fold higher among purified lung CD4+ T cells as compared with blood CD4+ T cells suggesting that CCR4+ transcripts were at higher incidence among lung CD4+ populations. This result could represent up-regulation of expression; however, because CCR4 is expressed by memory/effector but not naive T cells, our findings would be more consistent with organ-based recirculation of memory T cells as reported by others (31). Consequently, in the lung, naive CCR4-negative T cell populations would not dilute CCR4 transcripts to the same extent.
CD4+ T cells from both type-1 and -2 lung granulomas express CCR4 transcripts
The above studies suggested that CCR4+CD4+ T cells were mobilized to lungs with type-1 and -2 granulomas but did not establish whether those cells were simply marginated or were participating at sites of inflammation. To approach this question, individual lung lobes were collected from mice undergoing type-1 or -2 granuloma formation on day 4. Frozen tissue sections were cut and then stained with a CD4-specific Ab, dehydrated, and subjected to LCM (Fig. 3, A and B), We collected 100 individual CD4+ T cells from five granulomas (20 per lesion). Sufficient RNA was obtained to assess CCR4 and IL-4 transcripts using real-time RT-PCR. As shown in Fig. 3C, IL-4 expression was clearly more dominant in Th2- than Th1-mediated granulomas, confirming the polarized state of the response. However, Fig. 3D illustrates that CCR4 expression was not restricted to CD4+ cells from type-2 lesions, but was also expressed among CD4+ T cells from the type-1 lesions. Levels in control noninflamed lung tissue are represented by the dashed line. This result established that CCR4+CD4+ T cells were detectable at sites of granuloma formation and provided evidence that CCR4 expression might not be restricted to Th2 cells.
FIGURE 3.
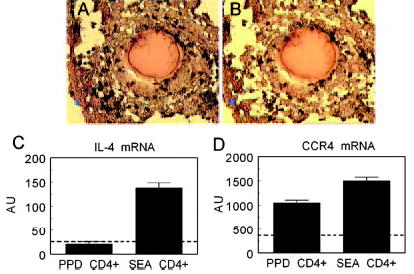
Lung granuloma CD4+ T cells isolated by LCM express CCR4 transcripts during both type-1 and -2 granuloma formation. Lungs were collected from mice on day 4 after PPD- or SEA-bead challenge. Frozen tissue sections were cut and stained with anti-CD4. LCM was used to excise ~100 CD4+ T cells from five individual granulomas (20 cells/granuloma). A, Sample of SEA-bead granuloma with CD4+ cells stained in brown. B, Same lesion after laser capture microdissection of CD4+ cells. C, IL-4 transcript expression among captured cells. D, CCR4 mRNA transcript expression among captured cells. Dashed lines indicate levels in excised noninflamed control lung tissue. Transcripts were measured by real-time RT-PCR. Bars are mean arbitrary units ± SD. Data are representative of three separate experiments.
Effector CD4+ T cells are enriched at sites of granuloma formation and CCR4 transcripts associate with both IL-4- and IFN-γ-producing cells
We further examined the relationship of CCR4 to cytokine-producing Th1 and Th2 effector cells by positively enriching for IL-4 and IFN-γ cytokine-secreting CD4+ T cells from the lungs of mice undergoing type-1 or -2 granuloma formation. Cytokine and chemokine receptor transcripts were measured by real-time RT-PCR. As seen in Fig. 4, A and B, insets, IFN-γ and IL-4 displayed expected polarization among the nonenriched CD4+ T cell populations in the type-1 (PPD) and type-2 (SEA) models, respectively. Compared with the nonenriched CD4+ T cells (ALL), IFN-γ-secreting cells could not be further enriched from the PPD-challenged lungs nor IL-4-secreting cells from SEA bead-challenged lungs. However, the enrichment method was successful because we achieved a 10-fold enrichment of IFN-γ producers from lungs with type-2 lesions and a 12-fold enrichment of IL-4-secreting cells from lungs with type-1 lesions, indicating that these populations were present as minor components among the dominant cytokine-secreting population (Fig. 4, A and B). The inability to further enrich the dominant cytokine-producing populations suggested that these populations were already enriched, likely because only Ag-specific CD4+ effector cells survived the overnight pre-enrichment culture period.
FIGURE 4.
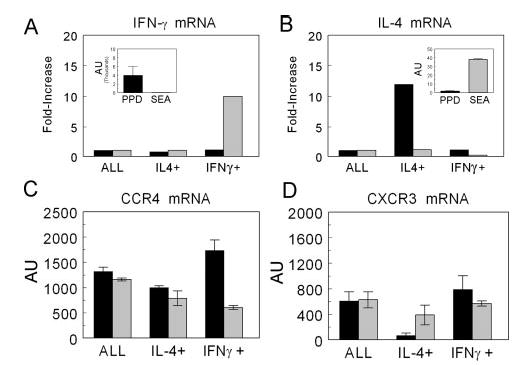
Purification of IFN-γ and IL-4 cytokine-producing CD4+ T cells from lungs during type-1 (PPD) and type-2 (SEA) granuloma formation. Anamnestic lung granulomas were induced in CBA/J mice and on day 3, lungs were collected, homogenized, and cultured overnight with Ag. CD4+ cytokine-secreting populations were isolated as described in Materials and Methods. Relative mRNA transcript levels were measured for IFN-γ (A) and IL-4 (B) with results expressed as the fold increase over the nonenriched CD4+ control (ALL). Insets, The expression in arbitrary units of the CD4+ nonenriched control demonstrates the initial cytokine polarization. Transcript levels of chemokine receptors were measured by real-time RT-PCR in the enriched populations. C, CCR4 transcripts. D, CXCR3 transcripts. Type-1 (PPD) response,█; type-2 (SEA) response,▒. Bars are mean arbitrary units ± SD derived from two separate experiments, five mice per experiment.
We next measured CCR4 and CXCR3 chemokine receptor transcripts among these populations. As shown in Fig. 4C, CCR4 transcripts were associated with both IL-4- and IFN-γ-enriched populations in both models. The chemokine receptor, CXCR3, purported to be associated with Th1 cells indeed showed a strong positive association with IFN-γ-producing cells and a negative association with IL-4 producers during PPD challenge (Fig. 4D). However, in the Th2 dominant model CXCR3 transcripts were detected among IL-4 and IFN-γ producers, indicating that CXCR3 was not absolutely Th1 restricted. These findings provided evidence that CCR4 was expressed among IFN-γ- and IL-4-secreting effector CD4+ T cell populations within granulomatous lungs. Thus, similar to reports for human T cells, mouse Th1 and Th2 cells display a more complicated chemokine receptor expression in vivo than that described for artificially generated populations (32).
Both IFN-γ- and IL-4-producing draining lymph node CD4+ T cells migrate in response to a CCR4 agonist
Having demonstrated CCR4 expression among both Th1 and Th2 populations generated during mycobacterial and schistosomal Ag-elicited immune responses, we next assessed the chemotactic response of the populations to a specific CCR4 agonist, CCL17. For these studies, CD4+ T cells were purified from reactive mediastinal lymph nodes during type-1 and -2 pulmonary granuloma formation and then used for chemotaxis assay. The migrated populations were collected and compared by RNA expression analysis for cytokines and chemokine receptors. As shown in Fig. 5, CCL17 was chemotactic for IFN-γ and IL-4/IL-13-producing cells in the mycobacterial and schistosomal Ag responses, respectively. In addition, there was enriched CCR4 transcript expression among the migrated populations. This result agreed with our previous expression analysis and indicated that CCR4 was likely functional on both Th1 and Th2 cells generated during challenge with strongly polarizing Ags.
FIGURE 5.
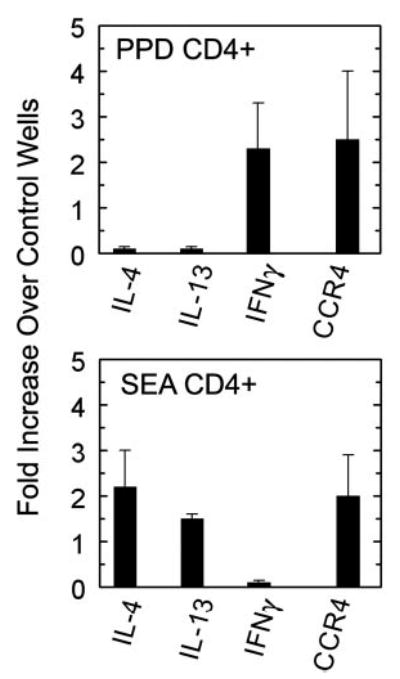
IFN-γ- and IL-4-producing CD4+ T cells induced during type-1 (PPD) and type-2 (SEA) granuloma formation migrate in response to CCL17. CD4+ T cells were isolated from draining lymph nodes collected 3 days after bead challenge. Using a multiwell chemotaxis chamber, 5 and 0 ng/ml CCL17 were added to bottom wells while 3 × 105 CD4+ T cells were added to the top wells. Cells that migrated were collected and analyzed for gene expression by real-time PCR analyses. Results are expressed as a fold increase of transcript expression over the control wells containing randomly migrated cells. Bars are means ± SD from two to three separate experiments.
CCR4−/− mice display impaired type-1 mycobacterial and partially impaired type-2 schistosomal Ag-elicited responses
The above studies provided strong circumstantial evidence that CCR4+CD4+ Th1 and Th2 cells were participating during type-1 and -2 Ag-bead lung granulomatous inflammation, but the functional contribution of CCR4 was not established. As part of a functional evaluation of CCR4, we next determined the effect of CCR4 gene knockout (CCR4−/−) on PPD and SEA Ag-bead granulomatous responses. CCR4−/− mice show no developmental abnormalities (16) and using flow cytometric analysis, we have confirmed that naive mice have normal proportions of T and B cells in lymphoid tissue (data not shown). Control (CCR4+/+) and knockout (CCR4−/−) mice were sensitized with either PPD in CFA or S. mansoni eggs and then lung granulomas were respectively elicited by i.v. injection of PPD or SEA Ag beads as described in Materials and Methods. Granuloma lesion sizes were assessed 4 days after bead challenge. As shown in Fig. 6A, PPD granuloma area was decreased by 25–30% in the CCR4-deficient mice. In contrast, type-2 granuloma size was not affected by CCR4 knockout. Differential analysis of dispersed granulomas showed no change in relative proportions of leukocyte populations other than a trend to fewer eosinophils in type-2 lesions, but this did not achieve statistical significance (Table I). Thus, CCR4 knockout caused a global reduction of the type-1 cellular response. To explore the possibility of accelerated kinetics in CCR4−/− mice, granulomas were examined on day 2 and these were likewise impaired in knockout mice compared with controls (data not shown).
FIGURE 6.
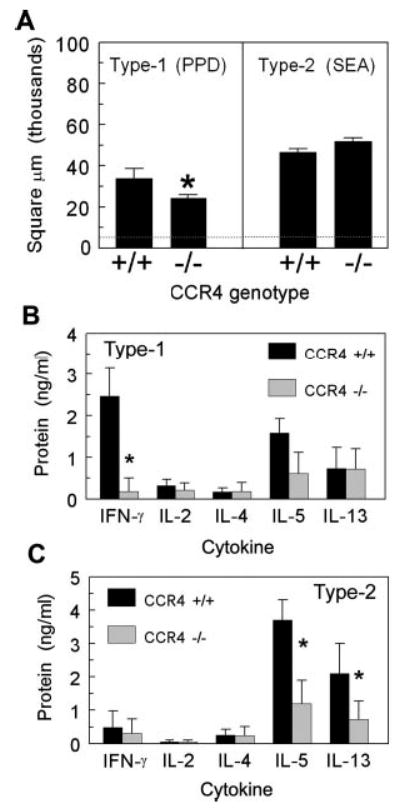
Type-1 and -2 granuloma formation and cytokine production in CCR4−/− mice. A, Granuloma cross-sectional area. Lesions were measured on day 4 after PPD or SEA-Ag bead challenge. The dashed line represents the average area of the bead alone. Bars are means ± SD derived from three separate experiments. B, Cytokine production by cultured type-1 (PPD) granulomas. C, Cytokine production by cultured type-2 (SEA) granulomas. On day 4, type-1 and -2 granulomas were isolated from lungs and cultured with Ag for 24 h. Supernatant cytokine levels were determined by ELISA. Bars are means ± SD from three separate experiments. *, p < 0.05 compared with CCR4+/+ mice.
Table I.
Differential leukocyte analysis of anamnestic mycobacterial and schistosomal Ag-bead granulomas from CCR4+/+ and CCR4−/− mice
| Challenge | Genotype | Lymphocytes (%) | Large Mononuclears (%) | Eosinophils (%) | Neutrophils (%) |
|---|---|---|---|---|---|
| Secondary PPD bead | CCR4+/+ | 22.5 ± 5.3 | 63.0 ± 6.7 | 2.2 ± 0.6 | 12.5 ± 6.6 |
| CCR4−/− | 19.3 ± 8.1 | 67.0 ± 2.6 | 1.7 ± 1.6 | 11.7 ± 4.2 | |
| Secondary SEA bead | CCR4+/+ | 24.0 ± 6.8 | 19.7 ± 1.5 | 28.8 ± 10.6 | 2.1 ± 0.6 |
| CCR4−/− | 23.0 ± 5.7 | 22.5 ± 5.3 | 24.0 ± 1.4 | 3.8 ± 1.1 |
To determine whether the impaired type-1 granuloma formation observed in CCR4−/− mice was related to changes in local cytokine production, we isolated and cultured intact granulomas from CCR4+/+ and CCR4−/− mice and then measured cytokines in supernatants. Fig. 6B shows that CCR4−/− mice had significantly reduced IFN-γ production at the lesion site. The other cytokines measured, IL-2, IL-4, IL-5, and IL-13, were comparable to control levels. Thus, at the site of the type-1 lesion, IFN-γ production was profoundly impaired consistent with a failure to recruit, generate, or support IFN-γ-producing effector cells. Interestingly, although overall lesion size and composition was largely unaffected during type-2 granuloma formation, cultured granulomas demonstrated partial reductions of the Th2 cytokines IL-5 and IL-13 suggesting that CCR4 was required for maximal effector cytokine production (Fig. 6C)
CCR4−/− CD4+ T cells fail to transfer type-1 hypersensitivity granuloma formation to naive recipients
To determine whether the defect in CCR4−/− mice was due to aberrant T cell function, CD4+ T cells were isolated from the draining lymph nodes of sensitized, Ag bead-challenged CCR4+/+ and CCR4−/− mice, then transferred to groups of naive CCR4+/+ mice that were subsequently challenged with Ag beads. Control groups received no donor cells. Granuloma formation and lymph node cytokine responses were assessed 4 days after challenge. As shown in Fig. 7A, CD4+ T cells from CCR4+/+ mice effectively transferred a secondary type-1 granulomatous response, whereas those from CCR4−/− mice failed to elicit a secondary response. In addition, the knockout CD4+ T cells failed to transfer a secondary draining lymph node cytokine response (Fig. 7B). In contrast, SEA-sensitized CD4+ T cells from CCR4−/− mice effectively transferred a secondary eosinophil-rich Th2 granuloma and a draining lymph node cytokine response. (Fig. 7, C and D). Differential analysis of dispersed lesions showed eosinophil percentages of 23.6 ± 5 and 36.5 ± 6.4 for CCR4+/+ and CCR4−/− transfers, respectively. Hence, only the type-1 adoptive response was compromised on adoptive transfer.
FIGURE 7.
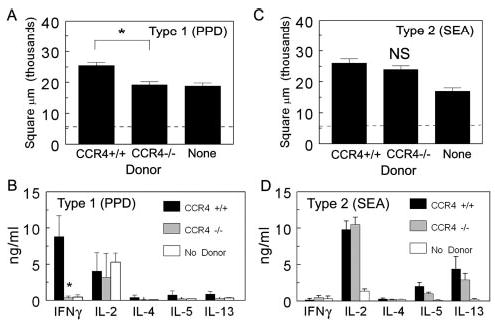
CCR4−/− CD4+ T cells fail to transfer type-1 granuloma formation to naive CCR4+/+ recipients. CD4+ T cells were isolated from PPD- or SEA-sensitized CCR4+/+ and CCR4−/− mice, then transferred as described in Materials and Methods. Four days later, lungs and draining lymph nodes were harvested. A and C, Granuloma cross-sectional areas, PPD and SEA models, respectively. Dashed line represents the area of the bead alone. B and D, Cytokine production by cultured draining lymph nodes derived from cell recipients and controls, PPD and SEA models, respectively. Bars are means ± SD derived from two separate experiments. *, p < 0.05 comparing mice that received CCR4−/− CD4+ T cells to those that received CCR4+/+ CD4+ T cells.
Primary mycobacterial Ag-elicited cytokine production is unimpaired but secondary elicitation is abrogated in draining lymph nodes of CCR4−/− mice
The above studies suggested that effector Th1 cells were impaired in CCR4−/− mice and this was possibly related to defective migration to sites of granuloma formation. However, it was also possible that CCR4−/− mice had impaired production or survival of effector T cells. To test this possibility, we examined the induction of cytokine-producing cells in draining lymph nodes following a primary and secondary challenge with PPD and SEA Ag beads. We previously showed that Ag beads evoke innate responses with committed cytokine-producing cells detectable in draining lymph nodes as early as 4 days after primary Ag-bead challenge (33). Therefore, we compared cytokine production in draining lymph node cultures from CCR4+/+ and CCR4−/− mice 4 days after primary challenge. In addition, we similarly examined the secondary response in groups of sensitized mice 4 days after challenge. Table II shows that during the primary response, CCR4−/− mice challenged with PPD or SEA beads displayed augmented IFN-γ or IL-2 levels. After primary PPD-bead challenge, knockout cultures had IFN-γ levels 3-fold above controls with trends to higher IL-2 production. After primary SEA-bead challenge IL-2 was 7-fold above controls with trends to higher IFN-γ levels. Levels of IL-4, IL-5, and IL-13 were comparable to controls. Thus, primary induction of cytokine-producing cells was not compromised in CCR4−/− mice nor was local innate granuloma formation (data not shown).
Table II.
Cytokine production by cultured draining lymph nodes from CCR4+/+ and CCR4−/−mice during primary and anamnestic mycobacterial and schistosomal Ag bead challenge
| Cytokine (ng/ml)
|
|||||||
|---|---|---|---|---|---|---|---|
| Challenge | Strain | Stimulus | IFN-γ | IL-2 | IL-4 | IL-5 | IL-13 |
| Primary PPD bead | CCR4+/+ | PPD | 0.6 ± 0.1 | 1.7 ± 0.8 | 0.3 ± 0.3 | 1.7 ± 0.9 | 2.9 ± 0.3 |
| CCR4−/− | PPD | 1.8 ± 0.5a | 4.7 ± 3.0 | 0.4 ± 0.6 | 1.3 ± 1.5 | 3.6 ± 0.7 | |
| Primary SEA bead | CCR4+/+ | SEA | 0.4 ± 0.4 | 1.0 ± 0.9 | 0.7 ± 0.6 | 1.8 ± 1.6 | 3.9 ± 3.2 |
| CCR4−/− | SEA | 0.8 ± 0.3 | 7.4 ± 4.4a | 0.5 ± 0.2 | 0.9 ± 0.9 | 3.9 ± 2.7 | |
| Secondary PPD bead | CCR4+/+ | PPD | 6.6 ± 3.5 | 1.7 ± 0.1 | 0.2 ± 0.1 | 4.2 ± 1.6 | 5.2 ± 1.4 |
| CCR4−/− | PPD | 1.0 ± 0.3a | 1.8 ± 0.6 | ND | 1.3 ± 0.6a | 0.8 ± 0.4a | |
| Secondary SEA bead | CCR4+/+ | SEA | 0.2 ± 0.3 | 2.6 ± 0.2 | 0.3 ± 0.3 | 13.4 ± 0.5 | 54.9 ± 2.1 |
| CCR4−/− | SEA | 0.7 ± 0.3 | 3.3 ± 0.3 | 3.5 ± 0.5a | 30.2 ± 0.4a | 126 ± 3.7a | |
Value of p < 0.05 compared to CCR4+/+ control.
In contrast to the primary response, upon secondary PPD-bead challenge CCR4−/− mice displayed profoundly (85%) impaired IFN-γ production as well as impaired IL-5 and IL-13 production in draining lymph node cultures. As expected, the secondary helminth Ag response showed strong Th2 polarization in CCR4+/+ controls and unlike the type-1 response, the profile was not compromised in knockout mice. Rather, levels of IL-4, IL-5, and IL-13 were enhanced. This result indicated that the mycobacterial Ag-elicited secondary response was more dependent on CCR4 than the schistosomal Ag-elicited response. Moreover, the impairment observed in the secondary PPD response must have been due to degradation of or an inability to support the anamnestic response to PPD. The observed augmentations during primary challenge also implied the presence of an underlying CCR4-dependent mechanism limiting the initial induction of the response. Thus, CCR4−/− mice displayed a paradoxical early accentuation but late impairment of the cytokine response following mycobacterial Ag challenge.
Wild-type CCR4+/+ CD4+ T cells fail to reconstitute normal type-1 granuloma formation when transferred to CCR4−/− mice
The above studies suggested that mycobacterial Ag-specific effector T cells were likely generated in CCR4−/− mice but could not be sustained or supported in CCR4−/− mice. Therefore, we next tested the capacity of the knockout tissue environment to permit expression of an effector T cell response to mycobacterial Ag challenge. To this end, we attempted reconstitution of CCR4−/− mice by adoptive cell transfer of wild-type CCR4+/+CD4+ T cells. Groups of naive CCR4+/+ and CCR4−/− recipient mice were i.v. administered purified CD4+ T cells from CFA-PPD-sensitized, CCR4+/+ donor mice. Recipients were subsequently challenged with PPD beads, then lungs and lymph nodes were examined. As shown in Fig. 8, CCR4+/+CD4+ T cells elicited the expected secondary response in wild-type recipients with augmented inflammation. In contrast, the response of knockout recipients resembled that of naive mice. This was likewise reflected in Ag-stimulated draining lymph node cultures prepared from knockout recipients in which all cytokines except IL-2 were reduced (Table III). Thus, the knockout environment would not support adoptive transfer of the PPD-elicited type-1 effector response, indicating dependence upon CCR4+ cells in the recipient.
FIGURE 8.
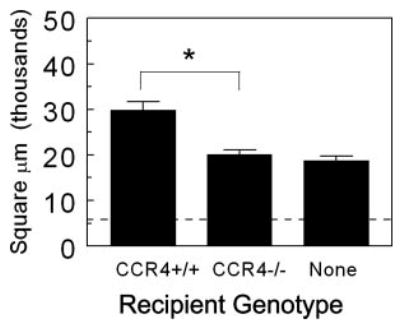
Wild-type CCR4+/+CD4+ T cells fail to transfer secondary type-1 mycobacterial Ag-elicited granuloma formation to CCR4−/− mice. PPD-sensitized CD4+ T cells were isolated from the axillary lymph nodes of donors. One million cells were administered i.v. to naive CCR4+/+ and CCR4−/− recipients. Recipients were challenged with PPD beads the following day. Granuloma cross-sectional area was measured 4 days after bead challenge. Data are representative of three separate transfer experiments. Dashed line indicates area occupied by bead alone. Bars are means ± SD. *, p < 0.05.
Table III.
Cytokine production by draining lymph node cultures following PPD-bead challenge of CCR4+/+ and CCR4−/− recipients of PPD-sensitized wild-type CD4+ T cells
| Cytokine (ng/ml)
|
||||||
|---|---|---|---|---|---|---|
| Donor | Recipient | IFN-γ | IL-2 | IL-4 | IL-5 | IL-13 |
| CCR4+/+ | CCR4+/+ | 3.5 ± 0.3 | 10.5 ± 0.1 | 0.1 ± 0.1 | 0.8 ± 0.1 | 5.7 ± 1.4 |
| CCR4+/+ | CCR4−/− | 0.5 ± 0.1a | 10.4 ± 0.1 | 0.1 ± 0.2 | 0.2 ± 0.4a | 0.6 ± 1.1a |
Value of p < 0.05 as compared to CCR4+/+ recipients.
Wild-type CD4+ T cells display impaired IFN-γ production when cultured with CCR4−/− DCs
The above results suggested that a CCR4-expressing non-CD4+ T cell population was required for an optimal PPD-elicited type-1 response. In this regard, APCs would be likely candidates because subpopulations of these cells are known to express CCR4 (34, 35). Studies of Tang and Cyster (30) have implicated CCR4 agonists in Ag presentation in lymph node T cell zones by facilitating the apposition of Ag-specific effector-memory CD4+ T cells and DC. In addition, Katou et al. (36) have provided evidence that CCR4 plays a role in the formation of DC-T cell clusters in lymph nodes and skin. As a possible means to test the role of CCR4 in T cell-DC interactions, we compared the in vitro capacity of sensitized CCR4+/+CD4+ T cells to produce cytokines in the presence of CCR4+/+ and CCR4−/− DCs purified from lymph nodes. As shown in Fig. 9, CD4+ T cells cocultured with CCR4−/− DCs displayed reduced IFN-γ production with about a 50% impairment when compared with culture with CCR4+/+ DCs. Consistent with the type-1 dominant response, IL-4 levels were negligible and unaffected by the DC population. Interestingly, IL-2, presumably produced in large part by naive CD4+ T cells, was augmented 5-fold in the same cultures. Thus, knockout DCs could support IL-2- but not IFN-γ-producing cells. Taken together, our in vivo and in vitro studies would support proposed models in which CCR4 is important to APC interactions with CD4+ effector/memory T cells. Moreover, the type-1 mycobacterial Ag-elicited response appears to be more dependent on these CCR4-mediated events than the type-2 or naive response.
FIGURE 9.
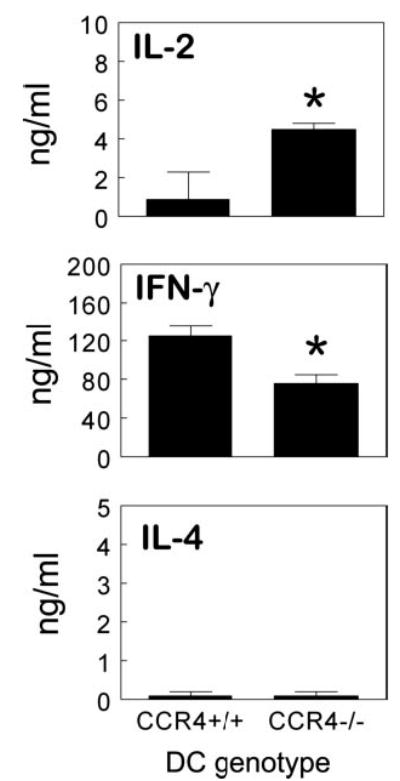
Sensitized wild-type CCR4+/+CD4+ T cells display diminished IFN-γ production when cultured with CCR4−/− DCs. CD4+ T cells and CD11c+ DCs were isolated from the axillary lymph nodes of PPD-sensitized donors. CCR4+/+CD4+ T cells were cultured with either CCR4+/+ or CCR4−/− DCs at a ratio of 10:1 for 72 h with PPD Ag. Cytokine levels were determined in culture supernatants by ELISA. Data are representative of two separate experiments, four to five mice per group. Bars are means ± SD. *, p < 0.05 comparing CCR4−/− to CCR4+/+ DC cultures.
Discussion
The contribution of CCR4 to adaptive immunity has yet to be fully elucidated and little is known regarding its role in pulmonary granuloma formation. CCR4 has been purported to be a Th2 cell-specific chemokine receptor and implicated as an important receptor in Th2 cell-mediated responses, based upon circumstantial evidence (13–15). The CCR4 ligands, CCL17 (TARC) and CCL22 (MDC) were shown to be expressed by airway epithelial cells and neutralization of the ligand CCL22 resulted in a reduction in airway hyperreactivity (13, 37). Contrarily, CCR4 gene knockout protected mice from endotoxin challenge, but had no effect on development and expression of a classical Th2-dependent murine model of allergic airway inflammation (16). In a Th2-dependent model of S. mansoni egg challenge, Jakubzick et al. (38) reported paradoxical findings with immune neutralization of CCL17 and CCL22 having different effects and resulting in both impaired and augmented responses. Thus, there is considerable confusion regarding the function of CCR4 and its ligands in T cell-mediated adaptive responses.
The present study attempted to systematically analyze the role of CCR4 in highly defined models of polarized Th1 (type-1) and Th2 (type-2) cell-mediated lung granuloma formation. This approach allows direct comparison of disparate responses under synchronized conditions. We first tested the hypothesis that CCR4 was selectively expressed during Th2 effector cell-mediated responses. Our results clearly disputed this hypothesis by demonstrating CCR4 expression among both Th1 and Th2 effector populations, similar to that reported in studies of human peripheral blood T cells (17, 32). Similarly, CXCR3 was also not restricted to Th1 cells as originally purported, but was detected among schistosome Ag-elicited IL-4-producing effector cells from inflamed lungs. Interestingly, CXCR3 did show biased expression among Th1 cells under conditions of mycobacterial Ag challenge, indicating that the nature of the antigenic stimulus may shape T cell chemokine receptor profiles. These observations emphasize the importance of evaluating T cell chemokine receptor expression under a variety of in vivo challenge conditions.
Our functional analysis demonstrated that CCR4 deletion caused partial impairment of the local Th2 effector cell-mediated response to S. mansoni egg Ags but did not affect the regional lymph node recall response. This observation might indicate differential effects on T effector and T central memory cells as former are thought to use CCR4 for peripheral tissue localization while the latter use CCR7 for lymphoid tissue recirculation Unexpectedly, CCR4 knockout also caused local impairments of lesion size and IFN-γ production in the mycobacterial Ag-elicited type-1 response. Further analysis demonstrated that sensitized CD4+ T cells from CCR4−/− mice were unable to transfer a type-1 response to naive CCR4+/+ recipients. We confirmed that the type-1 CD4+ T cell defect was not due to impaired primary induction of effector cells because cytokine-producing cells in draining lymph nodes were detected following primary challenge of CCR4 knockout mice. In fact, there appeared to be early accentuation of the primary Th1 effector response. These findings suggested that CCR4 was not required for afferent effector cell induction, but was required for the survival and or expression of Th1 effector/memory cells similar to that proposed for cutaneous T memory cells by Baekkevold et al. (27). That study showed defective establishment of Th1 cell memory in CCR4−/− mice in response to skin challenge, but, unlike our study, did not evaluate effects on the Th2 response. In humans, CCR4 is reportedly strongly expressed by cutaneous CLA+ T effector/memory cells associated with Th1-mediated inflammatory conditions (39). Our results indicate that CCR4 is likewise expressed by CD4+ T cells associated with interstitial lung inflammation and argue that CCR4 is broadly expressed by inflammatory effector/memory Th cells but is functionally more important to the pulmonary type-1 response. In this regard, we have recently demonstrated similar impairment of IFN-γ production in draining lymph nodes following airway infection with live M. bovis Bacille Calmette-Guérin (data not shown).
Since our results indicated a functional or quantitative defect in CCR4−/− CD4+ T cells in the type-1 response, it was surprising to find that wild-type CCR4+/+CD4+ T cells were unable to restore memory Th1 responses upon adoptive transfer to CCR4−/− mice. This finding demonstrated a defect in the environment of CCR4−/− mice that failed to permit expression and or expansion of memory/effector cells. We explored possible defects in the lung chemokine environment and an analysis of granulomatous lungs revealed that transcripts for CCL17 and CCL22 were reduced by 74 and 56%, respectively (data not shown), in the CCR4−/− mice with smaller granulomas. A similar defect in CCR4 ligand production was noted among peritoneal macrophages in endotoxin-challenged CCR4−/− mice (16), but because local chemokine production is cytokine amplified, this was possibly a secondary effect of impaired activation. Another explanation for the inability of the CCR4+/+CD4+ T cells to correct the defect in the CCR4−/− mice was the potential requirement for a second CCR4+ cell population. We hypothesized that Ag-presenting DCs may be involved as others and we have detected CCR4 expression among DCs (34, 35). It has been reported that CCL22 is chemotactic for myeloid DCs (40), promotes Ag-activated T cell binding to DCs (41), and permits formation of DC-T cell clusters in secondary lymphoid tissues and inflammatory sites (36). Indeed, our coculture studies demonstrated impaired IFN-γ production when CCR4+/+ T cells were cultured with CCR4−/− DCs, suggesting that CCR4 is necessary for optimal interaction of CD4+ T cells and APCs to promote an anamnestic Th1-mediated response to mycobacterial PPD. It has been proposed that persistent Ag presentation is required for survival/expansion of effector/memory CD4+ T cells (42). If such interactions are CCR4 dependent, it would explain the degradation of the Th1 effector/memory we observed in CCR4−/− mice. It would also explain the trend to augmented Th2 draining lymph node cytokine responses in these mice due to undermining of Th1 cross-regulatory effects.
Artificially generated human Th2 cells reportedly show biased CCR4 expression (6) and we have demonstrated a similar bias among in vitro-generated mouse Th2 cells (data not shown). Therefore, it was unexpected that CCR4 appeared to be playing a greater role in the establishment of Th1 than Th2 immune responses despite comparable CCR4 and CCR4 ligand expression. Similar to our findings, CCR4 knockout did not compromise a mouse airway allergy model (16) and CCR4 blockade in a guinea pig allergy model failed to inhibit the recruitment of inflammatory leukocytes to the lung, supporting the notion that Th2 cells can be recruited to the lung by CCR4-independent pathways (43). It is possible that Th2 cells are able to compensate for the lack of CCR4 with alternative chemokine receptors, such as CXCR4. CXCR4 was shown to be up-regulated by IL-4 and down-regulated by IFN-γ in vitro (44). Furthermore, Th2- but not Th1 cell-mediated responses were impaired when mice were systemically administered the selective CXCR4 antagonist, AMD3465 (45).
Another possible explanation for the differential effects of CCR4 knockout might be related to different migration patterns of Th1 and Th2 effector/memory cells. Currently, it is generally accepted that Th cells are composed of Th effector (high cytokine-secreting) and central memory T cell (low cytokine-secreting) pools. The latter population is considered necessary for long-term memory responses. The precise mechanisms for establishment of memory are unknown but could involve chemokine-dependent migration or activation events. The greater susceptibility we observed for the Th1 response would be consistent with reports demonstrating that Th1 are less efficient than Th2 cells in developing into a memory population (46). Th1 cells are also more susceptible to Fas-mediated apoptosis than Th2 cells (47). Moreover, Th1 memory/effector cells may require lymphoid organ or peripheral tissue migration to remain viable. In fact, Th1 and Th2 cells do show differences in their localization in secondary lymphoid tissue. Th2 cells migrate closer to the B cell follicles whereas Th1 cells are closer to central T cell zones (48). This corresponds well with our studies and those of others showing that CCL22 is predominantly expressed in T cell zones (30). Our findings would be consistent with a model in which Th1 and Th2 memory/effector cells rely on different chemokine receptors for localization, activation, and survival.
Taken together, our findings support the notion of flexibility and redundancy in chemokine receptor function with regard to T effector/memory cells. In addition, a number of novel findings are offered. The notion of CCR4 as a selective and critical functional receptor for Th2 cells is disputed at least in regard to pulmonary responses. Moreover, the greater CCR4 dependence of the Th1 cell-mediated anti-mycobacterial Ag response might indicate that CCR4 is required for sustaining an optimal Th1 memory response. Finally, we show that the sustenance of Th1 and Th2 memory has different mechanistic demands that might be exploited for therapeutic manipulations.
Acknowledgments
We thank Aron Pollack and Cynthia Brown for their expert histology support.
Footnotes
This work was supported by National Institutes of Health–National Institute of Allergy and Infectious Diseases Grant A143460 and in part by Department of Veterans Affairs and National Institutes of Health Grants HL31237 and HL35276. Schistosomal life stages or materials for this work were supplied through National Institutes of Health–National Institute of Allergy and Infectious Diseases Contract NO1-AI-55270.
Abbreviations used in this paper: Th1, Th type 1; Th2, Th type 2; DC, dendritic cell; PPD, purified protein derivative; SEA, schistosomal egg Ag; LCM, laser capture microdissection.
Disclosures The authors have no financial conflict of interest.
References
- 1.Santana MA, Rosenstein Y. What it takes to become an effector T cell: the process, the cells involved, and the mechanisms. J Cell Physiol. 2003;195:392–401. doi: 10.1002/jcp.10258. [DOI] [PubMed] [Google Scholar]
- 2.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 3.Campbell DJ, Debes GF, Johnston B, Wilson E, Butcher EC. Targeting T cell responses by selective chemokine receptor expression. Semin Immunol. 2003;15:277–286. doi: 10.1016/j.smim.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 4.O’Garra A, McEvoy LM, Zlotnik A. T-cell subsets: chemokine receptors guide the way. Curr Biol. 1998;8:R646–R649. doi: 10.1016/s0960-9822(07)00413-7. [DOI] [PubMed] [Google Scholar]
- 5.Qin S, Rottman JB, Myers P, Kassam N, Weinblatt M, Loetscher M, Koch AE, Moser B, Mackay CR. The chemokine receptors CXCR3 and CCR5 mark subsets of T cells associated with certain inflammatory reactions. J Clin Invest. 1998;101:746–754. doi: 10.1172/JCI1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonecchi R, Bianchi G, Bordignon PP, D’Ambrosio D, Lang R, Borsatti A, Sozzani S, Allavena P, Gray PA, Mantovani A, Sinigaglia F. Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s. J Exp Med. 1998;187:129–134. doi: 10.1084/jem.187.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loetscher P, Uguccioni M, Bordoli L, Baggiolini M, Moser B, Chizzolini C, Dayer JM. CCR5 is characteristic of Th1 lymphocytes. Nature. 1998;391:344–345. doi: 10.1038/34814. [DOI] [PubMed] [Google Scholar]
- 8.Sallusto F, Mackay CR, Lanzavecchia A. Selective expression of the eotaxin receptor CCR3 by human T helper 2 cells. Science. 1997;277:2005–2007. doi: 10.1126/science.277.5334.2005. [DOI] [PubMed] [Google Scholar]
- 9.Imai T, Nagira M, Takagi S, Kakizaki M, Nishimura M, Wang J, Gray PW, Matsushima K, Yoshie O. Selective recruitment of CCR4-bearing Th2 cells toward antigen-presenting cells by the CC chemokines thymus and activation-regulated chemokine and macrophage-derived chemokine. Int Immunol. 1999;11:81–88. doi: 10.1093/intimm/11.1.81. [DOI] [PubMed] [Google Scholar]
- 10.D’Ambrosio D, Iellem A, Bonecchi R, Mazzeo D, Sozzani S, Mantovani A, Sinigaglia F. Selective up-regulation of chemokine receptors CCR4 and CCR8 upon activation of polarized human type 2 Th cells. J Immunol. 1998;161:5111–5115. [PubMed] [Google Scholar]
- 11.Zingoni A, Soto H, Hedrick JA, Stoppacciaro A, Storlazzi CT, Sinigaglia F, D’Ambrosio D, O’Garra A, Robinson D, Rocchi M, et al. The chemokine receptor CCR8 is preferentially expressed in Th2 but not Th1 cells. J Immunol. 1998;161:547–551. [PubMed] [Google Scholar]
- 12.Freeman CM, Chiu BC, Stolberg VR, Hu J, Zeibecoglou K, Lukacs NW, Lira SA, Kunkel SL, Chensue SW. CCR8 is expressed by antigen-elicited, IL-10-producing CD4+CD25+ T cells, which regulate Th2-mediated granuloma formation in mice. J Immunol. 2005;174:1962–1970. doi: 10.4049/jimmunol.174.4.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Panina-Bordignon P, Papi A, Mariani M, Di Lucia P, Casoni G, Bellettato C, Buonsanti C, Miotto D, Mapp C, Villa A, et al. The C-C chemokine receptors CCR4 and CCR8 identify airway T cells of allergen-challenged atopic asthmatics. J Clin Invest. 2001;107:1357–1364. doi: 10.1172/JCI12655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nouri-Aria KT, Wilson D, Francis JN, Jopling LA, Jacobson MR, Hodge MR, Andrew DP, Till SJ, Varga EM, Williams TJ, et al. CCR4 in human allergen-induced late responses in the skin and lung. Eur J Immunol. 2002;32:1933–1938. doi: 10.1002/1521-4141(200207)32:7<1933::AID-IMMU1933>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 15.Yamamoto J, Adachi Y, Onoue Y, Adachi YS, Okabe Y, Itazawa T, Toyoda M, Seki T, Morohashi M, Matsushima K, Miyawaki T. Differential expression of the chemokine receptors by the Th1- and Th2-type effector populations within circulating CD4+ T cells. J Leukocyte Biol. 2000;68:568–574. [PubMed] [Google Scholar]
- 16.Chvatchko Y, Hoogewerf AJ, Meyer A, Alouani S, Juillard P, Buser R, Conquet F, Proudfoot AE, Wells TN, Power CA. A key role for CC chemokine receptor 4 in lipopolysaccharide-induced endotoxic shock. J Exp Med. 2000;191:1755–1764. doi: 10.1084/jem.191.10.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andrew DP, Ruffing N, Kim CH, Miao W, Heath H, Li Y, Murphy K, Campbell JJ, Butcher EC, Wu L. C-C chemokine receptor 4 expression defines a major subset of circulating nonintestinal memory T cells of both Th1 and Th2 potential. J Immunol. 2001;166:103–111. doi: 10.4049/jimmunol.166.1.103. [DOI] [PubMed] [Google Scholar]
- 18.Iellem A, Colantonio L, D’Ambrosio D. Skin-versus gut-skewed homing receptor expression and intrinsic CCR4 expression on human peripheral blood CD4+CD25+ suppressor T cells. Eur J Immunol. 2003;33:1488–1496. doi: 10.1002/eji.200323658. [DOI] [PubMed] [Google Scholar]
- 19.Imai T, Chantry D, Raport CJ, Wood CL, Nishimura M, Godiska R, Yoshie O, Gray PW. Macrophage-derived chemokine is a functional ligand for the CC chemokine receptor 4. J Biol Chem. 1998;273:1764–1768. doi: 10.1074/jbc.273.3.1764. [DOI] [PubMed] [Google Scholar]
- 20.Lieberam I, Forster I. The murine β-chemokine TARC is expressed by subsets of dendritic cells and attracts primed CD4+ T cells. Eur J Immunol. 1999;29:2684–2694. doi: 10.1002/(SICI)1521-4141(199909)29:09<2684::AID-IMMU2684>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 21.Schaniel C, Sallusto F, Ruedl C, Sideras P, Melchers F, Rolink AG. Three chemokines with potential functions in T lymphocyte-independent and -dependent B lymphocyte stimulation. Eur J Immunol. 1999;29:2934–2947. doi: 10.1002/(SICI)1521-4141(199909)29:09<2934::AID-IMMU2934>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 22.Boehlen F, Clemetson KJ. Platelet chemokines and their receptors: what is their relevance to platelet storage and transfusion practice? Transfus Med. 2001;11:403–417. doi: 10.1046/j.1365-3148.2001.00340.x. [DOI] [PubMed] [Google Scholar]
- 23.Belperio JA, Dy M, Murray L, Burdick MD, Xue YY, Strieter RM, Keane MP. The role of the Th2 CC chemokine ligand CCL17 in pulmonary fibrosis. J Immunol. 2004;173:4692–4698. doi: 10.4049/jimmunol.173.7.4692. [DOI] [PubMed] [Google Scholar]
- 24.Inngjerdingen M, Damaj B, Maghazachi AA. Human NK cells express CC chemokine receptors 4 and 8 and respond to thymus and activation-regulated chemokine, macrophage-derived chemokine, and I-309. J Immunol. 2000;164:4048–4054. doi: 10.4049/jimmunol.164.8.4048. [DOI] [PubMed] [Google Scholar]
- 25.Sallusto F, Lanzavecchia A. Mobilizing dendritic cells for tolerance, priming, and chronic inflammation. J Exp Med. 1999;189:611–614. doi: 10.1084/jem.189.4.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chensue SW, Warmington K, Ruth JH, Lukacs N, Kunkel SL. Mycobacterial and schistosomal antigen-elicited granuloma formation in IFN-γ and IL-4 knockout mice: analysis of local and regional cytokine and chemokine networks. J Immunol. 1997;159:3565–3573. [PubMed] [Google Scholar]
- 27.Baekkevold ES, Wurbel MA, Kivisakk P, Wain CM, Power CA, Haraldsen G, Campbell JJ. A role for CCR4 in development of mature circulating cutaneous T helper memory cell populations. J Exp Med. 2005;201:1045–1051. doi: 10.1084/jem.20041059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chensue SW, Lukacs NW, Yang TY, Shang X, Frait KA, Kunkel SL, Kung T, Wiekowski MT, Hedrick JA, Cook DN, et al. Aberrant in vivo T helper type 2 cell response and impaired eosinophil recruitment in CC chemokine receptor 8 knockout mice. J Exp Med. 2001;193:573–584. doi: 10.1084/jem.193.5.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chiu BC, Shang XZ, Stolberg VR, Komuniecki E, Chensue SW. Population analysis of CD4+ T cell chemokine receptor transcript expression during in vivo type-1 (mycobacterial) and type-2 (schistosomal) immune responses. J Leukocyte Biol. 2002;72:363–372. [PubMed] [Google Scholar]
- 30.Tang HL, Cyster JG. Chemokine up-regulation and activated T cell attraction by maturing dendritic cells. Science. 1999;284:819–822. doi: 10.1126/science.284.5415.819. [DOI] [PubMed] [Google Scholar]
- 31.Tietz W, Hamann A. The migratory behavior of murine CD4+ cells of memory phenotype. Eur J Immunol. 1997;27:2225–2232. doi: 10.1002/eji.1830270916. [DOI] [PubMed] [Google Scholar]
- 32.Kim CH, Rott L, Kunkel EJ, Genovese MC, Andrew DP, Wu L, Butcher EC. Rules of chemokine receptor association with T cell polarization in vivo. J Clin Invest. 2001;108:1331–1339. doi: 10.1172/JCI13543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chiu BC, Freeman CM, Stolberg VR, Hu JS, Komuniecki E, Chensue SW. The innate pulmonary granuloma: characterization and demonstration of dendritic cell recruitment and function. Am J Pathol. 2004;164:1021–1030. doi: 10.1016/S0002-9440(10)63189-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Penna G, Sozzani S, Adorini L. Cutting edge: selective usage of chemokine receptors by plasmacytoid dendritic cells. J Immunol. 2001;167:1862–1866. doi: 10.4049/jimmunol.167.4.1862. [DOI] [PubMed] [Google Scholar]
- 35.Chiu BC, Freeman CM, Stolberg VR, Hu JS, Zeibecoglou K, Lu B, Gerard C, Charo IF, Lira SA, Chensue SW. Impaired lung dendritic cell activation in CCR2 knockout mice. Am J Pathol. 2004;165:1199–1209. doi: 10.1016/S0002-9440(10)63380-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Katou F, Ohtani H, Nakayama T, Ono K, Matsushima K, Saaristo A, Nagura H, Yoshie O, Motegi K. Macrophage-derived chemokine (MDC/CCL22) and CCR4 are involved in the formation of T lymphocyte-dendritic cell clusters in human inflamed skin and secondary lymphoid tissue. Am J Pathol. 2001;158:1263–1270. doi: 10.1016/S0002-9440(10)64077-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gonzalo JA, Pan Y, Lloyd CM, Jia GQ, Yu G, Dussault B, Powers CA, Proudfoot AE, Coyle AJ, Gearing D, Gutierrez-Ramos JC. Mouse monocyte-derived chemokine is involved in airway hyperreactivity and lung inflammation. J Immunol. 1999;163:403–411. [PubMed] [Google Scholar]
- 38.Jakubzick C, Wen H, Matsukawa A, Keller M, Kunkel SL, Hogaboam CM. Role of CCR4 ligands, CCL17 and CCL22, during Schistosoma mansoni egg-induced pulmonary granuloma formation in mice. Am J Pathol. 2004;165:1211–1221. doi: 10.1016/S0002-9440(10)63381-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kunkel EJ, Boisvert J, Murphy K, Vierra MA, Genovese MC, Wardlaw AJ, Greenberg HB, Hodge MR, Wu L, Butcher EC, Campbell JJ. Expression of the chemokine receptors CCR4, CCR5, and CXCR3 by human tissue-infiltrating lymphocytes. Am J Pathol. 2002;160:347–355. doi: 10.1016/S0002-9440(10)64378-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Godiska R, Chantry D, Raport CJ, Sozzani S, Allavena P, Leviten D, Mantovani A, Gray PW. Human macrophage-derived chemokine (MDC), a novel chemoattractant for monocytes, monocyte-derived dendritic cells, and natural killer cells. J Exp Med. 1997;185:1595–1604. doi: 10.1084/jem.185.9.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu M, Fang H, Hwang ST. Cutting edge: CCR4 mediates antigen-primed T cell binding to activated dendritic cells. J Immunol. 2001;167:4791–4795. doi: 10.4049/jimmunol.167.9.4791. [DOI] [PubMed] [Google Scholar]
- 42.Obst R, van Santen HM, Mathis D, Benoist C. Antigen persistence is required throughout the expansion phase of a CD4+ T cell response. J Exp Med. 2005;201:1555–1565. doi: 10.1084/jem.20042521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Conroy DM, Jopling LA, Lloyd CM, Hodge MR, Andrew DP, Williams TJ, Pease JE, Sabroe I. CCR4 blockade does not inhibit allergic airways inflammation. J Leukocyte Biol. 2003;74:558–563. doi: 10.1189/jlb.0103030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Annunziato F, Cosmi L, Galli G, Beltrame C, Romagnani P, Manetti R, Romagnani S, Maggi E. Assessment of chemokine receptor expression by human Th1 and Th2 cells in vitro and in vivo. J Leukocyte Biol. 1999;65:691–699. doi: 10.1002/jlb.65.5.691. [DOI] [PubMed] [Google Scholar]
- 45.Hu JS, Freeman CM, Stolberg VR, Chiu B, Bridger GJ, Ficker SP, Lukacs NW, Chensue SW. AMD3465, a novel CXCR4 receptor antagonist, abrogates schistosomal Ag-elicited (type-2) pulmonary granuloma formation. Am J Pathol. 2006;169:424–432. doi: 10.2353/ajpath.2006.051234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu CY, Kirman JR, Rotte MJ, Davey DF, Perfetto SP, Rhee EG, Freidag BL, Hill BJ, Douek DC, Seder RA. Distinct lineages of TH1 cells have differential capacities for memory cell generation in vivo. Nat Immunol. 2002;3:852–858. doi: 10.1038/ni832. [DOI] [PubMed] [Google Scholar]
- 47.Zhang X, Brunner T, Carter L, Dutton RW, Rogers P, Bradley L, Sato T, Reed JC, Green D, Swain SL. Unequal death in T helper cell (Th)1 and Th2 effectors: Th1, but not Th2, effectors undergo rapid Fas/FasL-mediated apoptosis. J Exp Med. 1997;185:1837–1849. doi: 10.1084/jem.185.10.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Randolph DA, Huang G, Carruthers CJ, Bromley LE, Chaplin DD. The role of CCR7 in TH1 and TH2 cell localization and delivery of B cell help in vivo. Science. 1999;286:2159–2162. doi: 10.1126/science.286.5447.2159. [DOI] [PubMed] [Google Scholar]


