Abstract
AMP-activated protein kinase (AMPK) is a key cellular sensor of reduced energy supply that is activated by increases in the cellular ratio of AMP/ATP. Phenformin and 5-aminoimida-zole-4-carboxamide riboside (AICAR) are two drugs widely used to activate AMPK experimentally. In both differentiated hippocampal neurons and neuroblastoma SH-SY5Y cells we found that these two agents not only activated AMPK, but conversely greatly reduced the activating Ser/Thr phosphorylation of Akt. This blockade of Akt activity consequently lowered the inhibitory serine-phosphorylation of its substrates, glycogen synthase kinase-3a/b (GSK3a/b). An inhibitor of AMPK (Compound C) did not block dephosphorylation of Akt and GSK3. Thus, both drugs widely used to activate AMPK also caused dephosphorylation of Akt and of GSK3. The mechanism for Akt dephosphorylation caused by phenformin, but not AICAR, was due to inhibition of growth factor-induced signaling that leads to Akt phosphorylation. Stimulation of muscarinic receptors with carbachol in SH-SY5Y cells also activated AMPK and transiently caused dephosphorylation of Akt. These findings show that Akt dephosphorylation often occurs concomitantly with AMPK activation when cells are treated with phenformin or AICAR, indicating that these drugs do not only affect AMPK but also cause a coordinated inverse regulation of AMPK and Akt.
Keywords: Akt, Protein kinase B, AMPK, AMP-activated kinase, GSK3, AICAR, Phenformin
1. Introduction
The responses of enzymes that sense cellular stress critically influences cell fate, which can range from adaptation and recovery to debilitation and death. AMP-activated protein kinase (AMPK) is one of these crucial stress-sensing enzymes, which is conveyed by its sensitivity to AMP. Stressful or pathological conditions that provoke ATP depletion cause increases in the amount of AMP bound to AMPK [1,2]. AMP binding to AMPK allosterically increases its activity and, more importantly, facilitates the activating phosphorylation of AMPK on threonine-172, which is mediated by LKB1 [3,4], and inhibits its dephosphorylation, thus efficiently activating AMPK by multiple mechanisms [2]. Experimentally, two drugs are widely used to specifically activate AMPK, 5-aminoimida-zole-4-carboxamide riboside (AICAR) and phenformin. AICAR is an adenosine analog that is easily taken up by cells and then is rapidly phosphorylated to form 5-aminoimidazole-4-carboxamide-1-d-ribofuranosyl-5′-monophosphate, which mimics the activating effects of AMP on AMPK [5-7]. In contrast, the mechanism by which phenformin activates AMPK is still unclear [1,2]. Like several other key enzymes that are activated by cell stress, AMPK can promote responses to aid cellular recovery and survival following ATP depletion. Thus, AMPK promotes catabolism to enhance ATP synthesis and reduces anabolism to spare ATP utilization. Although these actions of AMPK support cell survival [8-13], activation of AMPK also has been reported to promote apoptotic cell death [14-21].
Akt and GSK3 also are important enzymes that regulate many cellular functions in physiological as well as pathological conditions. Akt is activated by dual phosphorylation on Thr308 and Ser473 which is often a downstream consequence of phosphatidylinositol 3-kinase (PI3K) activated by growth factor receptor signaling cascades or cellular stress [22]. Among the most prevalent targets of Akt are the two isoforms of GSK3 which are inhibited by Akt-mediated phosphorylation of an N-terminal serine, serine-9 in GSK3b or serine-21 in GSK3a [23,24]. This coupling of Akt and GSK3 leads to inverse changes in their activities, when Akt is activated by phosphorylation it maintains GSK3 in a serine-phosphorylated inhibited state, and decreases in Akt activity lead to dephosphorylation and activation of GSK3.
While examining the effects of treatments that activate AMPK we noted concomitant changes in the phosphorylation states of Akt and GSK3. The results show in two neuronal model systems, mouse differentiated immortalized hippocampal cells and human neuroblastoma SH-SY5Y cells, that in addition to activating AMPK, dephosphorylation of Akt and GSK3 also occurred after treatment with either phenformin and AICAR, but by different mechanisms.
2. Materials and methods
2.1. Cells and treatments
Human neuroblastoma SH-SY5Y cells were grown in RPMI 1640 medium containing 10% horse serum (Life Technologies, Gaithersburg, MD), 5% fetal clone II (Hyclone, Logan, UT), 2 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin (Life Technologies) in humidified, 37 °C chambers with 5% CO2. Immortalized hippocampal neurons [25] (generously provided by Dr. M.F. Mehler, Albert Einstein College of Medicine) were differentiated by incubation for 2-3 days at 39 °C in Neurobasal media containing B27 supplement [26] prior to experimental manipulations. Where indicated, cells were treated with 10 mM phenformin, 3 mM 5-aminoimidazole-4-carboxamide riboside (AICAR), 20 mM LiCl, 300 μM carbachol (Sigma, St. Louis, MO), 40 μM Compound C (generously provided by Dr. Gaochao Zhou, Merck Research Laboratories, Rahway, NJ), or 50 ng/ml insulin-like growth factor-1 (IGF-1) (Serologicals, Purchase, NY).
2.2. Immunoblotting
Cells were washed twice with PBS, and lysed in lysis buffer (20 mM Tris, pH 7.5, 150 mM NaCl, 2 mM EDTA, 2 mM EGTA, 1 mM sodium orthovanadate, 100 μM phenylmethylsulfonyl fluoride, 10 μg/ml leupeptin, 10 μg/ml aprotinin, 5 μg/ml pepstatin, 50 mM NaF, 1 nM okadaic acid and 0.5% NP-40). The lysates were sonicated for 10 s on ice, centrifuged at 16,000 × g for 15 min, and supernatants were collected. Protein concentrations were determined using the bicinchoninic method (Pierce, Rockford, IL). Cell lysates were mixed with Laemmli sample buffer and placed in a boiling water bath for 5 min. Proteins (10-20 μg) were resolved in 7.5% SDS-polyacrylamide gels, and transferred to nitrocellulose. Blots were probed with antibodies to phospho-Ser9-GSK3b, phospho-Ser21-GSK3a, phospho-Tyr279/216-GSK3a/b, total GSK3a/b, phospho-Thr308-Akt, phospho-Ser473-Akt, total Akt, phospho-Ser79-acetyl-coenzyme A carboxylase (ACC), phospho-Thr172-AMPK, or total AMPK (Cell Signaling Technology, Beverly, MA). Immunoblots were developed using horseradish peroxidase-conjugated goat anti-mouse or goat anti-rabbit IgG (Bio-Rad Laboratories, Hercules, CA), followed by detection with enhanced chemiluminescence.
2.3. Enzyme activities
Akt activity was measured after immunoprecipitation of Akt from 100 μg protein, using a non-radioactive Akt activity assay kit according to the manufacturer’s instructions (Cell Signaling Technology). GSK3b activity was measured as described previously [27] after immunoprecipitation of GSK3b from 100 μg protein. Immobilized immune complexes were washed twice with lysis buffer and twice with kinase buffer (10 mM MOPS, pH 7.4, 1 mM EDTA, 10 mM magnesium acetate, 20 mM magnesium chloride, 1 mM dithiothreitol, 1 μg/ml aprotinin, 1 mM benzamidine, 50 mM b-glycerophosphate, 0.1 mM okadaic acid, 1 mM sodium orthovanadate, and 0.5 mM NaF). Kinase activity was measured by mixing immunoprecipitates with 30 ml of kinase buffer containing 125 mMATP,1.4 mCi [g-32P]-ATP, and 0.1 μg/ml recombinant tau protein (Panvera, Madison, WI). The samples were incubated at 30 °C for 15 min, and 25 mlof Laemmlisample buffer was added to each sample to stop the reaction. Samples were placed in a boiling water bath for 5 min, and proteins were separated in 7.5% SDS-polyacrylamide gels. The gels were vacuum-dried, exposed to a phosphoscreen overnight, and quantitated using a PhosphorImager (Molecular Dynamics, Sunnyvale, CA). The efficiencies of immunoprecipitations were determined by immunoblotting with appropriate antibodies.
3. Results
3.1. Phenformin treatment activates AMPK and reduces the phosphorylation of Akt and GSK3
To test if activators of AMPK have a regulatory influence on Akt and GSK3, differentiated hippocampal neurons were treated with the AMPK activator phenformin and the regulatory phosphorylations of Akt and GSK3 were measured using immunoblot analyses with phospho-specific antibodies to Akt or to each of the two isoforms of GSK3. Treatment with 10 mM phenformin caused a rapid, time-dependent increase in the phosphorylation of Ser79-ACC (Fig. 1A), a well-characterized substrate of AMPK [28] that is widely used as a detector of AMPK activation [29-33]. The phenformin-induced increase in phospho-Ser79-ACC was evident within 10 min of treatment and was maintained for 120 min. Phenformin treatment also increased the level of phospho-Thr172-AMPK, confirming the activation of AMPK, whereas the level of AMPK protein did not change although its migration became more diffuse with the appearance of a slower migrating band after phenformin treatment (Fig. 1A). These results are consistent with several studies that have reported that phosphorylation of ACC is a sensitive marker of AMPK activity, often more sensitive even than increased levels of phospho-Thr172-AMPK [29-33], and verify that phenformin treatment activated AMPK in differentiated hippocampal neurons.
Fig. 1.
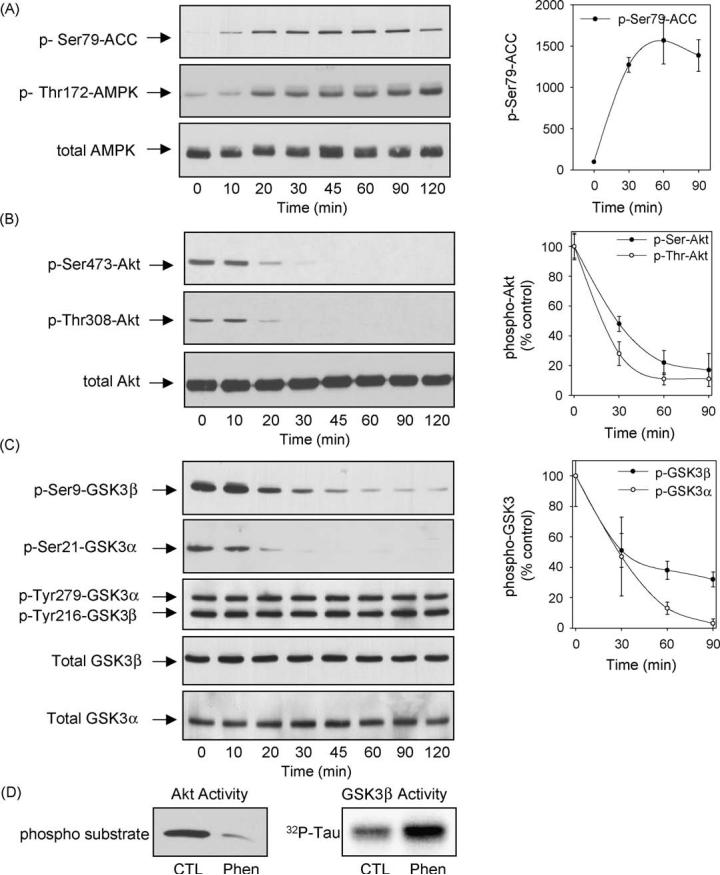
Treatment with phenformin activated AMPK and dephosphorylated Akt and GSK3 in immortalized differentiated mouse hippocampal neurons. Differentiated mouse hippocampal neurons were treated with 10 mM phenformin for 0, 10, 20, 30, 45, 60, 90, and 120 min, and cell lysates were immunoblotted for (A) phospho-Ser79-ACC, phospho-Thr172-AMPK, total AMPK; (B) phospho-Ser473-Akt, phospho-Thr308-Akt, total Akt; and (C) phospho-Ser9-GSK3b, phospho-Ser21-GSK3a, phospho-Tyr279/216-GSK3a/b, and total GSK3b and GSK3a. (D) Akt and GSK3b activities were measured in samples from controls (CTL) and cells treated with phenformin for 60 min. Immunoblots shown are representative of two experiments which produced similar results and quantitative values are means W S.E.M., n = 3-4 separate experiments.
Phenformin treatment of differentiated hippocampal neurons also caused large changes in the phosphorylation levels of Akt and GSK3. Activation of Akt is mediated by dual phosphorylation on Thr308 and Ser473. Phenformin (10 mM) treatment caused a time-dependent decrease in the phosphorylation of Akt at both sites (Fig. 1B). Phenformin treatment did not alter the total level of Akt except after the longer treatment times where there was a modest decline. Considering the reduced phosphorylation of Akt caused by phenformin treatment we also examined the serine-phosphorylation of the two isoforms of GSK3 which are known to be substrates of Akt [34]. Phenformin treatment caused decreases in the inhibitory serine-phosphorylation of both GSK3 isoforms with a time course similar to the decreased phosphorylation of Akt (Fig. 1C). The tyrosine phosphorylation levels and total amounts of both GSK3 isoforms were unchanged by phenformin treatment (Fig. 1C). In accordance with the reduced phosphorylation levels of Akt and GSK3b, the activity of Akt decreased and the activity of GSK3b increased following phenformin treatment (Fig. 1D), confirming that the phosphorylation levels of Akt and GSK3 reflect their enzymatic activities. Thus, phenformin treatment of differentiated hippocampal neurons caused reduced phosphorylation of Akt and consequently reduced serine-phosphorylation of GSK3.
To test if these effects of phenformin were evident in proliferating cells which are most commonly used in studies of AMPK, we used another neuronal model system, proliferating human neuroblastoma SH-SY5Y cells. The responses to phenformin were much slower in SH-SY5Y cells than in differentiated hippocampal neurons. Treatment with 10 mM phenformin increased the phosphorylation of ACC after 2 h and it remained highly phosphorylated for 5 h although with some diminution in intensity at the longer treatment times (Fig. 2A). A similar, though weaker, increase in phospho-Thr172-AMPK also occurred after phenformin treatment, while the total protein level of AMPK remained constant. These results verify that phenformin treatment caused a long-lasting activation of AMPK in SH-SY5Y cells. Substantial reductions in the phosphorylation of Akt on both Ser473 and Thr308 were evident after phenformin treatment in a time-dependent manner (Fig. 2B). Matching the reduced phosphorylation of Akt, the phosphorylation of Ser9-GSK3b and Ser21-GSK3a were greatly reduced following treatment with phenformin (Fig. 2C). The tyrosine-phosphorylated and total protein levels of GSK3b and GSK3a initially were unchanged before diminishing after 5 h of treatment.
Fig. 2.
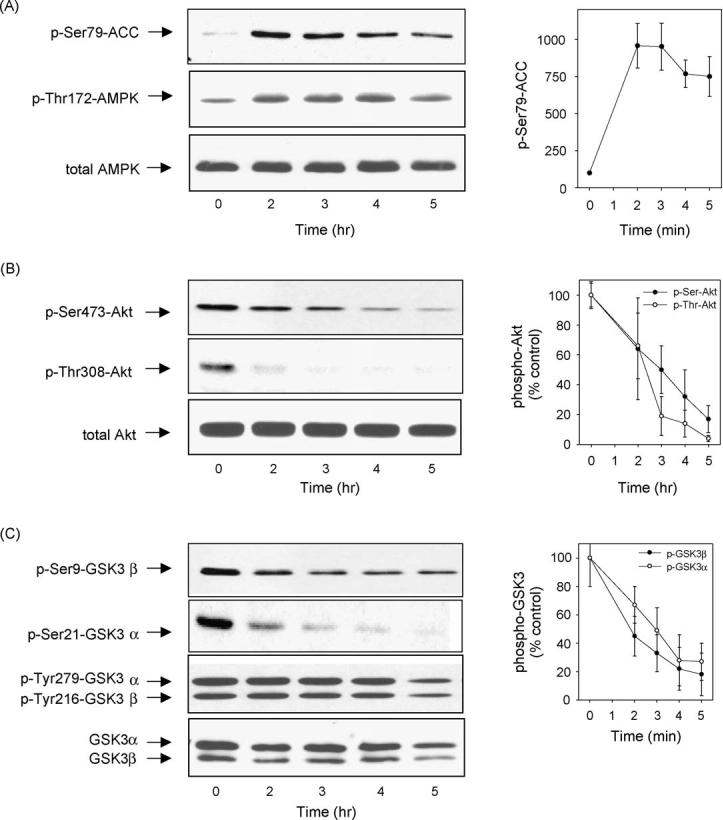
Phenformin treatment caused activation of AMPK and dephosphorylation of Akt and GSK3 in SH-SY5Y cells. SH-SY5Y cells were treated with 10 mM phenformin for 0, 2, 3, 4, and 5 h, and cell lysates were immunoblotted for (A) phospho-Ser79-ACC, phospho-Thr172-AMPK, total AMPK; (B) phospho-Ser473-Akt, phospho-Thr308-Akt, total Akt; and(C) phospho-Ser9-GSK3b, phospho-Ser21-GSK3a, phospho-Tyr279/216-GSK3a/b, and total GSK3a/b. Immunoblots shown are representative of two experiments which produced similar results and quantitative values are means W S.E.M., n = 3-4 separate experiments.
To test if phenformin reduced Akt phosphorylation by inhibiting intracellular signaling leading to its activation, the phosphorylation of Akt induced by IGF-1 was examined. IGF-1 stimulates receptors coupled to activation of PI3K which produces 3-phosphoinositides that activate kinases responsible for mediating the activation-associated phosphorylation of Akt on Thr308 and Ser473. Differentiated hippocampal neurons that had been preincubated in B27-free media to reduce basal Akt phosphorylation were stimulated with IGF-1 (50 ng/ml) with or without a 2 h pretreatment with 10 mM phenformin. IGF-1 treatment caused a rapid and sustained increase in the levels of phospho-Ser473-Akt and phospho-Thr308-Akt in control differentiated hippocampal neurons (Fig. 3A). However, pretreatment with phenformin greatly diminished the phosphorylation of Akt induced by IGF-1 treatment. The effect of phenformin on Akt phosphorylation induced by IGF-1 also was tested in SH-SY5Y cells that had been preincubated in serum-free media. IGF-1 treatment caused a rapid increase in the levels of phospho-Ser473-Akt and phospho-Thr308-Akt (Fig. 3B), and phenformin pretreatment largely blocked IGF-1-induced phosphorylation of Akt at both sites (Fig. 3B). These results demonstrate that treatment with phenformin inhibits growth factor-induced phosphorylation of Akt.
Fig. 3.
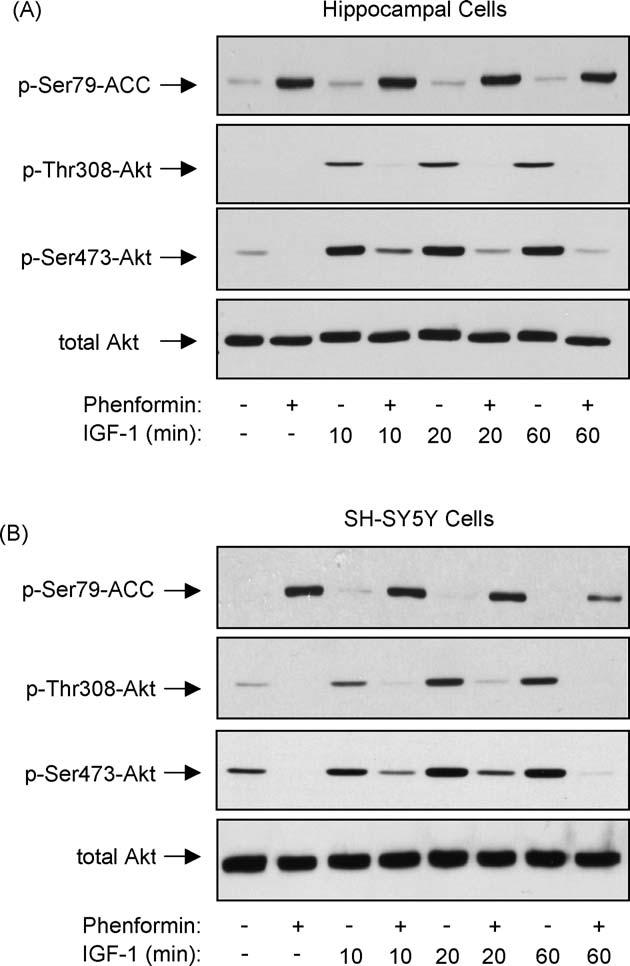
Phenformin blocks IGF-1-induced activation of Akt. (A) Differentiated mouse hippocampal neurons or (B) SH-SY5Y cells were incubated overnight in media without B27 or serum, respectively. Cells were pretreated with 10 mM phenformin for 2 h and then stimulated with 50 ng/ml IGF-1 for 0, 10, 20, or 60 min. Cell lysates were immunoblotted for phospho-Ser79-ACC, phospho-Thr308-Akt, phospho-Ser473-Akt, and total Akt. Immunoblots shown are representative of two experiments which produced similar results.
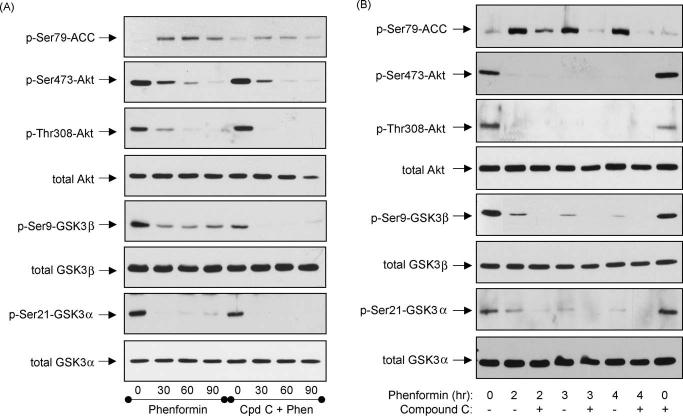
To test if AMPK activation by phenformin was responsible for the dephosphorylation of Akt and GSK3, cells were treated with the selective AMPK inhibitor Compound C [35].Forthese experiments, differentiated hippocampal neurons were treated with a higher concentration of Compound C (40 mM) than the generally used 10-20 mM concentrations [35-38] because in preliminary concentration-response experiments the lower concentrations of Compound C only slightly inhibited AMPK in these cells (data not shown). Pretreatment with 40 mM Compound C reduced the phenformin-induced increase in the phosphorylation of ACC at Ser79 (Fig. 4A). However, Compound C treatment did not block the phenformin-induced decreases in the phosphorylation of Akt or GSK3, but tended to increase these dephosphorylations, especially of GSK3b (Fig. 4A). Equivalent effects also were observed in SH-SY5Y cells where 40 mM Compound C reduced the phenformin-induced increase in phospho-Ser79-ACC but did not attenuate the dephosphorylation of Akt or GSK3 caused by phenformin treatment (Fig. 4B). Thus, phenformin treatment not only caused activation of AMPK but also caused dephosphorylation of Akt and GSK3 by a mechanism that was not blocked by the AMPK inhibitor Compound C, indicating that an AMPK-independent effect contributes to this response to phenformin.
Fig. 4.
AMPK inhibition did not block phenformin-induced dephosphorylation of Akt and GSK3. (A) Differentiated mouse hippocampal neurons were treated 40 mM Compound C for 1 h prior to treatment with 10 mM phenformin for 0, 30, 60, or 90 min. (B) SH-SY5Y cells were treated with 40 mM Compound C for 1 h prior to treatment with 10 mM phenformin for 2, 3, or 4 h. Cell lysates were immunoblotted for phospho-Ser79-ACC, phospho-Ser473-Akt, phospho-Thr308-Akt, total Akt, phospho-Ser9-GSK3b, phospho-Ser21-GSK3a, total GSK3b, and total GSK3a. Immunoblots shown are representative of two experiments, which produced similar results.
3.2. AICAR treatment activates AMPK and reduces the phosphorylation of Akt and GSK3
To further examine the link between AMPK activation and the phosphorylation levels of Akt and GSK3, we tested the effects of another activator of AMPK, AICAR. Treatment of differentiated hippocampal neurons with 3 mM AICAR activated AMPK within 1 h as indicated by increased phosphorylation levels of ACC and of AMPK, and this was maintained over a sustained period of time (Fig. 5A). At early times (1 and 2 h) after AICAR treatment when AMPK was activated there was neither a decrease in the phosphorylation of Akt(instead it slightly increased)(Fig. 5B) nor in the serine-phosphorylation of either GSK3 isoform (Fig. 5C). However, with longer treatment times AICAR caused decreases in the phosphorylation of Akt on Ser473 and Thr308 and reduced phospho-Ser9-GSK3b and phospho-Ser21-GSK3a levels (Fig. 5C). AICAR treatment did not change the total level of Akt or GSK3. These results demonstrate that AICAR, as well as phenformin, caused dephosphorylation of Akt and GSK3, but this occurred with a time course that was delayed compared with AMPK activation suggesting that AICAR regulated the phosphorylations of Akt and GSK3 independently of its effects on AMPK. Unfortunately, Compound C cannot be used in conjunction with AICAR because it blocks the uptake of AICAR into cells [39] so we could not test directly if blocking AMPK activity with Compound C reduced the AICAR-induced dephosphorylation of Akt and GSK3.
Fig. 5.
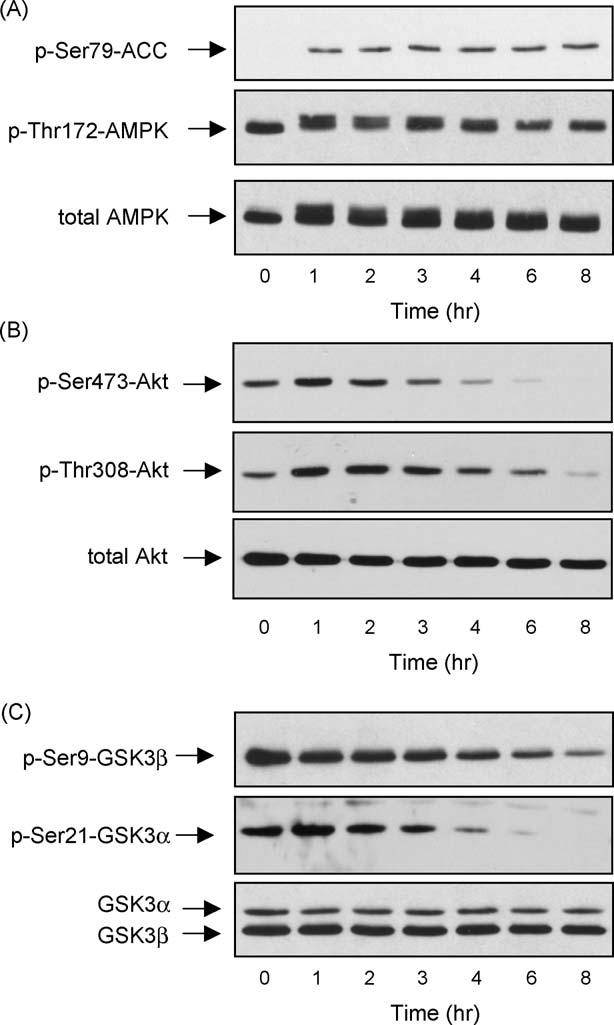
Treatment with AICAR activated AMPK and dephosphorylated Akt and GSK3 in differentiated mouse hippocampal neurons. Differentiated mouse hippocampal neurons were treated with 3 mM AICAR for 0, 1, 2, 3, 4, 6, or 8 h, and cell lysates were immunoblotted for (A) phospho-Ser79-ACC, phospho-Thr172-AMPK, total AMPK; (B) phospho-Ser473-Akt, phospho-Thr308-Akt, total Akt; and (C) phospho-Ser9-GSK3b, phospho-Ser21-GSK3a, and total GSK3a/b. Immunoblots shown are representative of two experiments, which produced similar results.
To test if AICAR inhibited growth factor-mediated signaling to Akt phosphorylation as did phenformin, differentiated hippocampal neurons were stimulated with IGF-1 with or without pretreatment with AICAR. In contrast to the inhibitory effect of phenformin, pretreatment with AICAR did not inhibit IGF-1-induced phosphorylation of Akt (Fig. 6A). A similar outcome was obtained with SH-SY5Y cells, in which IGF-1 treatment increased the dual phosphorylation of Akt and this was unaffected by pretreatment with AICAR (Fig. 6B). Thus, while AICAR shared with phenformin the properties of causing AMPK activation and dephosphorylation of Akt and GSK3, only phenformin, not AICAR, blocked IGF-1-induced Akt phosphorylation. These results indicate that although Akt dephosphorylation occurs with both phenformin and AICAR, the results with IGF-1 indicate that different actions of phenformin and AICAR account for inhibition of Akt phosphorylation.
Fig. 6.
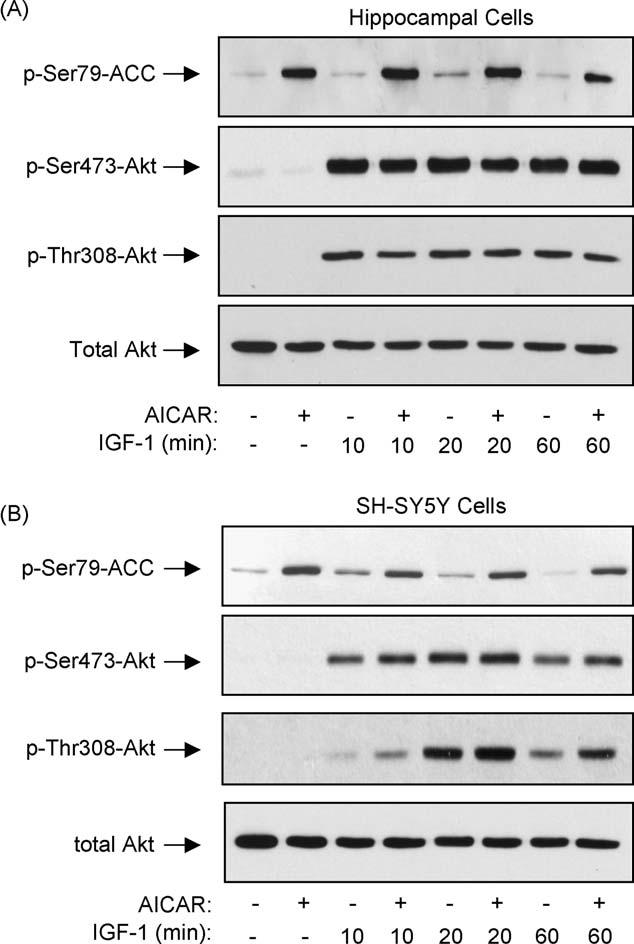
AICAR treatment did not block IGF-1-induced activation of Akt. (A) Differentiated mouse hippocampal neurons or (B) SH-SY5Y cells were incubated overnight in media without B27 or serum, respectively. Cells were pretreated with 3 mM AICAR for (A) 6 h, or (B) 2 h, and then stimulated with 50 ng/ml IGF-1 for 0, 10, 20, or 60 min. Cell lysates were immunoblotted for phospho-Ser79-ACC, phospho-Ser473-Akt, phospho-Thr308-Akt, and total Akt. Immunoblots shown are representative of two experiments which produced similar results.
3.3. Muscarinic receptor stimulation with carbachol induced AMPK activation
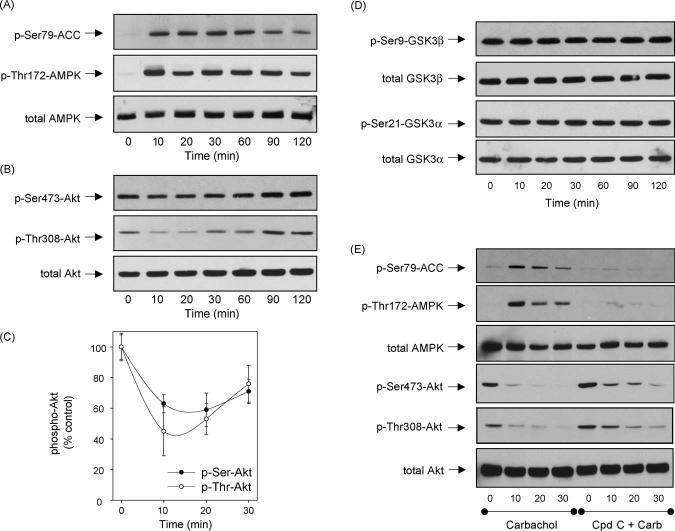
In addition to drugs that directly activate AMPK, we tested if activation of a receptor-coupled signaling pathway known to activate AMPK also modulated Akt and GSK3 phosphorylation. Stimulation of muscarinic receptors with the cholinergic agonist carbachol previously was reported to activate AMPK in rat parotid acinar cells [40]. Since SH-SY5Y cells endogenously express muscarinic receptors, predominantly of the M3 subtype coupled to the phosphoinositide signal transduction system [41], we tested if AMPK was activated by muscarinic receptor stimulation in SH-SY5Y cells and if it caused dephosphorylation of Akt and GSK3. Treatment with carbachol increased the phosphorylation of AMPK and its substrate, ACC, within 10 min, and this was maintained up to 60 min followed by a gradual decrease in phosphorylation between 90 and 120 min after treatment (Fig. 7A). This confirms that muscarinic receptor stimulation activates AMPK. Carbachol treatment caused a transient decrease in the phosphorylation of Akt (Fig. 7B and C), but this rebounded towards control levels after 30 min even while AMPK was highly activated. In contrast to all other treatments that dephosphorylated Akt, there was no change in the phosphorylation of either of the two isoforms of GSK3 (Fig. 7D). This may be due to activation of protein kinase C which is known to be activated by the phosphoinositide signal transduction system and to phosphorylate Ser9 of GSK3b [42]. Pretreatment with 40 mM Compound C strongly inhibited AMPK activation following carbachol treatment, and partially attenuated the dephosphorylation of Akt (Fig. 7E), indicating that the dephosphorylation of Akt was a parallel process to activation of AMPK.
Fig. 7.
Carbachol-induced AMPK activation. (A-D) SH-SY5Y human neuroblastoma cells were treated with 300 mM carbachol for 0, 10, 20, 30, 60, 90, or 120 min, or (E) were pretreated with 40 mM Compound C for 1 h prior to treatment with carbachol for 0, 10, 20, or 30 min. Cell lysates were immunoblotted for (A, E) phospho-Ser79-ACC, phospho-Thr172-AMPK, total AMPK; (B, C, E) phospho-Ser473-Akt, phospho-Thr308-Akt, total Akt; and (D) phospho-Ser9-GSK3b, total GSK3b, phospho-Ser21-GSK3a, and total GSK3a. Immunoblots shown are representative of at least two experiments with similar results and quantitative values are means W S.E.M., n = 3 separate experiments.
4. Discussion
AMPK is a critical cellular sensor of reduced energy levels. In order to investigate its physiological effects, AMPK is often experimentally activated by either of two agents, phenformin or AICAR. These two drugs activate AMPK by different mechanisms, with AICAR structurally mimicking the endogenous activator AMP, while several different mechanisms involving activation of an AMPK-phosphorylating kinase have been proposed for the effect of phenformin and its analog metformin [43-48]. In non-proliferating differentiated hippocampal neurons and in proliferating neuroblastoma SH-SY5Y cells, the present study found that both phenformin and AICAR not only activated AMPK, but also greatly reduced the phosphorylation of Akt on its regulatory Ser/Thr sites. Attenuation of AMPK with the selective inhibitor Compound C did not block Akt dephosphorylation induced by phenformin or muscarinic receptor activation, and the time courses of AMPK activation and Akt dephosphorylation were markedly different following treatment with AICAR or carbachol. Taken together these findings indicate that parallel pathways are activated by each of these agents which concomitantly activate AMPK and dephosphorylate Akt, and consequently GSK3. These results indicate that actions ascribed to AMPK following phenformin or AICAR treatment may be influenced by the concomitant modulatory actions of these drugs on Akt and GSK3. AMPK and Akt generally have opposing roles on cellular metabolism. AMPK is activated when AMP levels increase in conjunction with decreased ATP levels, and activated AMPK inhibits anabolic processes and promotes catabolism in order to minimize ATP utilization while promoting ATP production [1,2]. Akt, on the other hand, generally promotes anabolic cellular functions that utilize ATP, such as proliferation and cell growth [22], although Akt may share with AMPK the ability to promote ATP synthesis by different mechanisms [49]. Thus, the combined effects of AMPK activation and Akt inhibition caused by phenformin and AICAR may accentuate the outcomes that have been ascribed to their activating effects on AMPK.
The mechanistic basis of the dephosphorylation of Akt was found to differ between phenformin and AICAR. Dephosphorylation of Akt by phenformin treatment was due to blockade of intracellular signaling leading to Akt phosphorylation. This was evident because IGF-1-induced Akt phosphorylation, an outcome of receptor-mediated activation of PI3K, was largely blocked by phenformin. In contrast to the mechanism of action of phenformin, AICAR reduced Akt phosphorylation by another mechanism because activation of Akt by IGF-1 was unimpeded by AICAR treatment. A recent report also found Akt to be dephosphorylated following AICAR treatment in C6 glioma cells [50], further verifying our conclusion that this is a robust effect and it is not cell type specific. However, in those cells it was shown to be through inactivation of PI3K, whereas our results indicated signaling from the IGF-1 receptor through PI3K to Akt was not impaired by AICAR. Thus, the inhibitory mechanism of AICAR requires further exploration to be defined, but it may involve activation of phosphatases that are known to dephosphorylate Akt [22]. Alternatively, inhibition of other kinases may be involved in the effects of AICAR because it was recently reported that AICAR inhibited the serine-9 phosphorylation of GSK3b induced by co-treatment with a phorbol ester activator of protein kinase C plus the calcium ionophore ionomycin [51]. Overall, the differential effects of phenformin and AICAR on IGF-1-induced Akt phosphorylation indicated that the two drugs which commonly activated AMPK caused Akt dephosphorylation by different mechanisms.
These results extend recent studies of an analog of phenformin, metformin, in which mixed results have been obtained in evaluations of its effects on Akt phosphorylation. Treatment with metformin increased insulin-induced Akt phosphorylation in cultured HepG2 cells [52] and HGL5 cells[53], as well as in vivo in rat heart [54]. In contrast, metformin reduced Akt phosphorylation stimulated by interleukin-1b or by high glucose [55], whereas Akt phosphorylation was unaltered by metformin treatment in H4IIE cells [56] and in vivo in diabetic muscle [57-59]. These mixed results with metformin indicate that context (i.e., cell type and stimulus) as well as possible multiple targets of metformin cause this diversity of effects on Akt phosphorylation. Whether or not such a variable outcome occurs with phenformin awaits further studies, but in our examination of two different cell types, and with both basal and IGF-1 stimulation, phenformin consistently reduced Akt phosphorylation.
Compound C was developed as a selective inhibitor of AMPK[35] and it has been used in several studies to decipher cellular effects of AMPK [36-38]. Inhibition of AMPK by Compound C was evident in both hippocampal neurons and SH-SY5Y cells by its reduction of the phenformin-induced phosphorylation of ACC, a prototypical AMPK substrate commonly used as an indicator of AMPK activation [28]. Compound C also reduced carbacholinduced activation of AMPK in SH-SY5Y cells. Although activation of AMPK induced by phenformin or by carbachol was reduced by Compound C, there was little reduction in the dephosphorylation of Akt. These results demonstrate that dephosphorylation of Akt is not dependent on AMPK activation, although it is interesting that Akt dephosphorylation consistently occurs concomitantly with AMPK activation, indicating an inverse regulation of these two kinases.
As mentioned above, in addition to using direct activators of AMPK, we also examined the effect of AMPK activation on Akt phosphorylation induced by stimulation of an intracellular signaling pathway coupled to muscarinic receptors. A previous report showed that plasma membrane receptors coupled to the phosphoinositide signal transduction system through the G-proteins Gq and G11 activate AMPK [60]. More specifically, stimulation of Gq/11-coupled muscarinic receptors by carbachol was shown to activate AMPK in rat parotid acinar cells [40]. Since SH-SY5Y cells endogenously express muscarinic receptors, predominantly of the M3 subtype coupled to the Gq/11-mediated phosphoinositide signaling cascade [41],we tested if activation of AMPK through this pathway caused dephosphorylation of Akt and GSK3. We confirmed that muscarinic receptor stimulation caused a rapid and robust increase in AMPK activation, however it only slightly and transiently decreased Akt phosphorylation and there was no dephosphorylation of GSK3. The control of serine-phosphorylation of GSK3 following carbachol treatment likely reflects a complex interaction between the action of Akt and the muscarinic receptor-induced activation of protein kinase C through the phosphoinositide signal transduction system since GSK3 can be phosphorylated on the regulatory serines by protein kinase C as well as by Akt [61]. The lack of activation of GSK3 following muscarinic receptor stimulation also is consistent with previous reports that muscarinic receptor activation does not increase GSK3-mediated phosphorylation of its substrates, which actually has been reported to decrease following muscarinic receptor stimulation [62].
Overall, our results demonstrate that two drugs commonly used to activate AMPK, phenformin and AICAR, also caused the dephosphorylation of Akt and of GSK3. Thus, results obtained with these two drugs need to be interpreted cautiously since the Akt/GSK3 signaling pathway has many outcomes that overlap with those following AMPK activation and these drugs caused a consistent inverse relationship in their effects on AMPK and Akt.
Acknowledgements
We thank Dr. Gaochao Zhou, Merck Research Laboratories, for the gift of Compound C, and Dr. M. F. Mehler, Albert Einstein College of Medicine, for the hippocampal neuronal cell line. This research was supported by grants from the National Institutes of Health.
References
- [1].Carling D. The AMP-activated protein kinase cascade—a unifying system for energy control. Trends Biochem Sci. 2004;29:18–24. doi: 10.1016/j.tibs.2003.11.005. [DOI] [PubMed] [Google Scholar]
- [2].Hardie DG. The AMP-activated protein kinase pathway— new players upstream and downstream. J Cell Sci. 2004;117:5479–87. doi: 10.1242/jcs.01540. [DOI] [PubMed] [Google Scholar]
- [3].Hawley SA, Boudeau J, Reid JL, Mustard KJ, Udd L, Makela TP, et al. Complexes between the LKB1 tumor suppressor, STRAD a/b and MO25a/b are upstream kinases in the AMP-activated protein kinase cascade. J Biol. 2003;2:28. doi: 10.1186/1475-4924-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Woods A, Johnstone SR, Dickerson K, Leiper FC, Fryer LG, Neumann D, et al. LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr Biol. 2003;13:2004–8. doi: 10.1016/j.cub.2003.10.031. [DOI] [PubMed] [Google Scholar]
- [5].Corton JM, Gillespie JG, Hawley SA, Hardie DG. 5-Aminoimidazole-4-carboxamide ribonucleoside. A specific method for activating AMP-activated protein kinase in intact cells? Eur J Biochem. 1995;229:558–65. doi: 10.1111/j.1432-1033.1995.tb20498.x. [DOI] [PubMed] [Google Scholar]
- [6].Henin N, Vincent MF, Gruber HE, Van den Berghe G. Inhibition of fatty acid and cholesterol synthesis by stimulation of AMP-activated protein kinase. FASEB J. 1995;9:541–6. doi: 10.1096/fasebj.9.7.7737463. [DOI] [PubMed] [Google Scholar]
- [7].Hardie DG. Minireview: the AMP-activated protein kinase cascade: the key sensor of cellular energy status. Endocrinology. 2003;144:5179–83. doi: 10.1210/en.2003-0982. [DOI] [PubMed] [Google Scholar]
- [8].Stefanelli C, Stanic I, Bonavita F, Flamigni F, Pignatti C, Guarnieri C, et al. Inhibition of glucocorticoid-induced apoptosis with 5-aminoimidazole-4-carboxamide ribonucleoside, a cell-permeable activator of AMP-activated protein kinase. Biochem Biophys Res Commun. 1998;243:821–6. doi: 10.1006/bbrc.1998.8154. [DOI] [PubMed] [Google Scholar]
- [9].Blazquez C, Geelen MJ, Velasco G, Guzman M. The AMP-activated protein kinase prevents ceramide synthesis de novo and apoptosis in astrocytes. FEBS Lett. 2001;489:149–53. doi: 10.1016/s0014-5793(01)02089-0. [DOI] [PubMed] [Google Scholar]
- [10].Culmsee C, Monnig J, Kemp BE, Mattson MP. AMP-activated protein kinase is highly expressed in neurons in the developing rat brain and promotes neuronal survival following glucose deprivation. J Mol Neurosci. 2001;17:45–58. doi: 10.1385/JMN:17:1:45. [DOI] [PubMed] [Google Scholar]
- [11].Ido Y, Carling D, Ruderman N. Hyperglycemia-induced apoptosis in human umbilical vein endothelial cells: inhibition by the AMP-activated protein kinase activation. Diabetes. 2002;51:159–67. doi: 10.2337/diabetes.51.1.159. [DOI] [PubMed] [Google Scholar]
- [12].Kobayashi H, Ouchi N, Kihara S, Walsh K, Kumada M, Abe Y, et al. Selective suppression of endothelial cell apoptosis by the high molecular weight form of adiponectin. Circ Res. 2004;94:e27–31. doi: 10.1161/01.RES.0000119921.86460.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Russell RR, III, Li J, Coven DL, Pypaert M, Zechner C, Palmeri M, et al. AMP-activated protein kinase mediates ischemic glucose uptake and prevents postischemic cardiac dysfunction, apoptosis, and injury. J Clin Invest. 2004;114:495–503. doi: 10.1172/JCI19297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Meisse D, Van de Casteele M, Beauloye C, Hainault I, Kefas BA, Rider MH, et al. Sustained activation of AMP-activated protein kinase induces c-Jun N-terminal kinase activation and apoptosis in liver cells. FEBS Lett. 2002;526:38–42. doi: 10.1016/s0014-5793(02)03110-1. [DOI] [PubMed] [Google Scholar]
- [15].Campas C, Lopez JM, Santidrian AF, Barragan M, Bellosillo B, Colomer D, et al. Acadesine activates AMPK and induces apoptosis in B-cell chronic lymphocytic leukemia cells but not in T lymphocytes. Blood. 2003;101:3674–80. doi: 10.1182/blood-2002-07-2339. [DOI] [PubMed] [Google Scholar]
- [16].Kefas BA, Cai Y, Ling Z, Heimberg H, Hue L, Pipeleers D, et al. AMP-activated protein kinase can induce apoptosis of insulin-producing MIN6 cells through stimulation of c-Jun-N-terminal kinase. J Mol Endocrinol. 2003;30:151–61. doi: 10.1677/jme.0.0300151. [DOI] [PubMed] [Google Scholar]
- [17].Kefas BA, Heimberg H, Vaulont S, Meisse D, Hue L, Pipeleers D, et al. AICA-riboside induces apoptosis of pancreatic beta cells through stimulation of AMP-activated protein kinase. Diabetologia. 2003;46:250–4. doi: 10.1007/s00125-002-1030-3. [DOI] [PubMed] [Google Scholar]
- [18].Kefas BA, Cai Y, Kerckhofs K, Ling Z, Martens G, Heimberg H, et al. Metformin-induced stimulation of AMP-activated protein kinase in beta-cells impairs their glucose responsiveness and can lead to apoptosis. Biochem Pharmacol. 2004;68:409–16. doi: 10.1016/j.bcp.2004.04.003. [DOI] [PubMed] [Google Scholar]
- [19].Garcia-Gil M, Pesi R, Perna S, Allegrini S, Giannecchini M, Camici M, et al. 5′-Aminoimidazole-4-carboxamide riboside induces apoptosis in human neuroblastoma cells. Neuroscience. 2003;117:811–20. doi: 10.1016/s0306-4522(02)00836-9. [DOI] [PubMed] [Google Scholar]
- [20].Jung JE, Lee J, Ha J, Kim SS, Cho YH, Baik HH, et al. 5-Aminoimidazole-4-carboxamide-ribonucleoside enhances oxidative stress-induced apoptosis through activation of nuclear factor-kB in mouse Neuro 2a neuroblastoma cells. Neurosci Lett. 2004;354:197–200. doi: 10.1016/j.neulet.2003.10.012. [DOI] [PubMed] [Google Scholar]
- [21].Saitoh M, Nagai K, Nakagawa K, Yamamura T, Yamamoto S, Nishizaki T. Adenosine induces apoptosis in the human gastric cancer cells via an intrinsic pathway relevant to activation of AMP-activated protein kinase. Biochem Pharmacol. 2004;67:2005–11. doi: 10.1016/j.bcp.2004.01.020. [DOI] [PubMed] [Google Scholar]
- [22].Lawlor MA, Alessi DR. PKB/Akt: a key mediator of cell proliferation, survival and insulin responses? J Cell Sci. 2001;114:2903–10. doi: 10.1242/jcs.114.16.2903. [DOI] [PubMed] [Google Scholar]
- [23].Frame S, Cohen P, Biondi RM. A common phosphate binding site explains the unique substrate specificity of GSK3 and its inactivation by phosphorylation. Mol Cell. 2001;7:1321–7. doi: 10.1016/s1097-2765(01)00253-2. [DOI] [PubMed] [Google Scholar]
- [24].Grimes CA, Jope RS. The multi-faceted roles of glycogen synthase kinase-3b in cellular signaling. Prog Neurobiol. 2001;65:391–426. doi: 10.1016/s0301-0082(01)00011-9. [DOI] [PubMed] [Google Scholar]
- [25].Mehler MF, Rozental R, Dougherty M, Spray DC, Kessler JA. Cytokine regulation of neuronal differentiation of hippocampal progenitor cells. Nature. 2003;362:62–5. doi: 10.1038/362062a0. [DOI] [PubMed] [Google Scholar]
- [26].Brewer GJ, Torricelli JR, Evege EK, Price PJ. Optimized survival of hippocampal neurons in B27-supplemented Neurobasal, a new serum-free medium combination. J Neurosci Res. 1993;35:567–76. doi: 10.1002/jnr.490350513. [DOI] [PubMed] [Google Scholar]
- [27].Watcharasit P, Bijur GN, Zmijewski JW, Song L, Zmijewska A, Chen X, et al. Direct, activating interaction between glycogen synthase kinase-3b and p53 after DNA damage. Proc Natl Acad Sci USA. 2002;99:7951–5. doi: 10.1073/pnas.122062299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Carlson CA, Kim KH. Regulation of hepatic acetyl coenzyme A carboxylase by phosphorylation and dephosphorylation. J Biol Chem. 1973;248:378–80. [PubMed] [Google Scholar]
- [29].Hutber CA, Hardie DG, Winder WW. Electrical stimulation inactivates muscle acetyl-CoA carboxylase and increases AMP-activated protein kinase. Am J Physiol. 1997;272:E262–6. doi: 10.1152/ajpendo.1997.272.2.E262. [DOI] [PubMed] [Google Scholar]
- [30].Winder WW. Energy-sensing and signaling by AMP-activated protein kinase in skeletal muscle. J Appl Physiol. 2001;91:1017–28. doi: 10.1152/jappl.2001.91.3.1017. [DOI] [PubMed] [Google Scholar]
- [31].Longnus SL, Wambolt RB, Parsons HL, Brownsey RW, Allard MF. 5-Aminoimidazole-4-carboxamide 1-β-d-ribofuranoside (AICAR) stimulates myocardial glycogenolysis by allosteric mechanisms. Am J Physiol Regul Integr Comp Physiol. 2003;284:R936–44. doi: 10.1152/ajpregu.00319.2002. [DOI] [PubMed] [Google Scholar]
- [32].Al-Khalili L, Krook A, Zierath JR, Cartee GD. Prior serum- and AICAR-induced AMPK activation in primary human myocytes does not lead to subsequent increase in insulin-stimulated glucose uptake. Am J Physiol Endocrinol Metab. 2004;287:E553–7. doi: 10.1152/ajpendo.00161.2004. [DOI] [PubMed] [Google Scholar]
- [33].Pencek RR, Shearer J, Camacho RC, James FD, Lacy DB, Fueger PT, et al. 5-Aminoimidazole-4-carboxamide-1-β-d-ribofuranoside causes acute hepatic insulin resistance in vivo. Diabetes. 2005;54:355–60. doi: 10.2337/diabetes.54.2.355. [DOI] [PubMed] [Google Scholar]
- [34].Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–9. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- [35].Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–74. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Wu X, Motoshima H, Mahadev K, Stalker TJ, Scalia R, Goldstein BJ. Involvement of AMP-activated protein kinase in glucose uptake stimulated by the globular domain of adiponectin in primary rat adipocytes. Diabetes. 2003;52:1355–63. doi: 10.2337/diabetes.52.6.1355. [DOI] [PubMed] [Google Scholar]
- [37].Al-Khalili L, Chibalin AV, Yu M, Sjodin B, Nylen C, Zierath JR, et al. MEF2 activation in differentiated primary human skeletal muscle cultures requires coordinated involvement of parallel pathways. Am J Physiol Cell Physiol. 2004;286:C1410–6. doi: 10.1152/ajpcell.00444.2003. [DOI] [PubMed] [Google Scholar]
- [38].McCullough LD, Zeng Z, Li H, Landree LE, McFadden J, Ronnett GV. Pharmacological inhibition of AMP-activated protein kinase provides neuroprotection in stroke. J Biol Chem. 2005;280:20493–502. doi: 10.1074/jbc.M409985200. [DOI] [PubMed] [Google Scholar]
- [39].Fryer LG, Parbu-Patel A, Carling D. Protein kinase inhibitors block the stimulation of the AMP-activated protein kinase by 5-amino-4-imidazolecarboxamide riboside. FEBS Lett. 2002;531:189–92. doi: 10.1016/s0014-5793(02)03501-9. [DOI] [PubMed] [Google Scholar]
- [40].Soltoff SP. Evidence that tyrphostins AG10 and AG18 are mitochondrial uncouplers that alter phosphorylation-dependent cell signaling. J Biol Chem. 2004;279:10910–8. doi: 10.1074/jbc.M305396200. [DOI] [PubMed] [Google Scholar]
- [41].Lambert DG, Ghataorre AS, Nahorski SR. Muscarinic receptor binding characteristics of a human neuroblastoma SK-N-SH and its clones SH-SY5Y and SH-EP1. Eur J Pharmacol. 1989;165:71–7. doi: 10.1016/0014-2999(89)90771-1. [DOI] [PubMed] [Google Scholar]
- [42].Jope RS, Johnson GVW. The glamour and gloom of glycogen synthase kinase-3 (GSK3) Trends Biochem Sci. 2004;29:95–102. doi: 10.1016/j.tibs.2003.12.004. [DOI] [PubMed] [Google Scholar]
- [43].El-Mir MY, Nogueira V, Fontaine E, Averet N, Rigoulet M, Leverve X. Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I. J Biol Chem. 2000;275:223–8. doi: 10.1074/jbc.275.1.223. [DOI] [PubMed] [Google Scholar]
- [44].Fryer LG, Parbu-Patel A, Carling D. The anti-diabetic drugs rosiglitazone and metformin stimulate AMP-activated protein kinase through distinct signaling pathways. J Biol Chem. 2002;277:25226–32. doi: 10.1074/jbc.M202489200. [DOI] [PubMed] [Google Scholar]
- [45].Hawley SA, Gadalla AE, Olsen GS, Hardie DG. The antidiabetic drug metformin activates the AMP-activated protein kinase cascade via an adenine nucleotide-independent mechanism. Diabetes. 2002;51:2420–5. doi: 10.2337/diabetes.51.8.2420. [DOI] [PubMed] [Google Scholar]
- [46].Sakamoto K, Goransson O, Hardie DG, Alessi DR. Activity of LKB1 and AMPK-related kinases in skeletal muscle: effects of contraction, phenformin, and AICAR. Am J Physiol Endocrinol Metab. 2004;287:E310–7. doi: 10.1152/ajpendo.00074.2004. [DOI] [PubMed] [Google Scholar]
- [47].Sakamoto K, McCarthy A, Smith D, Green KA, Grahame Hardie D, Ashworth A, et al. Deficiency of LKB1 in skeletal muscle prevents AMPK activation and glucose uptake during contraction. EMBO J. 2005;24:1810–20. doi: 10.1038/sj.emboj.7600667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Zou MH, Kirkpatrick SS, Davis BJ, Nelson JS, Wiles WG, IV, Schlattner U, et al. Activation of the AMP-activated protein kinase by the anti-diabetic drug metformin in vivo. Role of mitochondrial reactive nitrogen species. J Biol Chem. 2004;279:43940–51. doi: 10.1074/jbc.M404421200. [DOI] [PubMed] [Google Scholar]
- [49].Bijur GN, Jope RS. Dynamic regulation of mitochondrial Akt and GSK3b. J Neurochem. 2003;87:1427–35. doi: 10.1046/j.1471-4159.2003.02113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Rattan R, Giri S, Singh AK, Singh I. 5-Aminoimidazole-4-carboxamide-1-β-d-ribofuranoside inhibits cancer cell proliferation in vitro and in vivo via AMP-activated protein kinase. J Biol Chem. 2005;280:39582–93. doi: 10.1074/jbc.M507443200. [DOI] [PubMed] [Google Scholar]
- [51].Jhun BS, Oh YT, Lee JY, Kong Y, Yoon KS, Kim SS, et al. AICAR suppresses IL-2 expression through inhibition of GSK-3 phosphorylation and NF-AT activation in Jurkat T cells. Biochem Biophys Res Commun. 2005;332:339–46. doi: 10.1016/j.bbrc.2005.04.126. [DOI] [PubMed] [Google Scholar]
- [52].Tzatsos A, Kandror KV. Nutrients suppress phosphatidylinositol 3-kinase/Akt signaling via raptor-dependent mTOR-mediated insulin receptor substrate 1 phosphorylation. Mol Cell Biol. 2006;26:63–76. doi: 10.1128/MCB.26.1.63-76.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Sonntag B, Gotte M, Wulfing P, Schuring AN, Kiesel L, Greb RR. Metformin alters insulin signaling and viability of human granulosa cells. Fertil Steril. 2005;84:1173–9. doi: 10.1016/j.fertnstert.2005.04.043. [DOI] [PubMed] [Google Scholar]
- [54].Longnus SL, Segal en C, Giudicelli J, Sajan MP, Farese RV, Van Obberghen E. Insulin signalling downstream of protein kinase B is potentiated by 5′AMP-activated protein kinase in rat hearts in vivo. Diabetologia. 2005;48:2591–601. doi: 10.1007/s00125-005-0016-3. [DOI] [PubMed] [Google Scholar]
- [55].Isoda K, Young JL, Zirlik A, Macfarlane LA, Tsuboi N, Gerdes N. Metformin inhibits proinflammatory responses and nuclear factor-kB in human vascular wall cells. Arterioscler Thromb Vasc Biol. 2006;26:611–7. doi: 10.1161/01.ATV.0000201938.78044.75. [DOI] [PubMed] [Google Scholar]
- [56].Morioka K, Nakatani K, Matsumoto K, Urakawa H, Kitagawa N, Katsuki A, et al. Metformin-induced suppression of glucose-6-phosphatase expression is independent of insulin signaling in rat hepatoma cells. Int J Mol Med. 2005;15:449–52. [PubMed] [Google Scholar]
- [57].Kim Y-B, Ciaraldi TP, Kong A, et al. Troglitazone, but not metformin, restores insulin-stimulated phosphoinositide 3-kinase activity and increases p100b protein levels in skeletal muscle of type 2 diabetic humans. Diabetes. 2002;51:443–8. doi: 10.2337/diabetes.51.2.443. [DOI] [PubMed] [Google Scholar]
- [58].Karlsson HK, Hallsten K, Bjornholm M, Tsuchida H, Chibalin AV, Virtanen KA, et al. Effects of metformin and rosiglitazone treatment on insulin signaling and glucose uptake in patients with newly diagnosed type 2 diabetes: a randomized controlled study. Diabetes. 2005;54:1459–67. doi: 10.2337/diabetes.54.5.1459. [DOI] [PubMed] [Google Scholar]
- [59].Luna V, Casauban L, Sajan MP, Gomez-Daspet J, Powe JL, Miura A, et al. Metformin improves atypical protein kinase C activation by insulin and phosphatidylinositol-3,4,5-(PO4)3in muscle of diabetic subjects. Diabetologia. 2006;49:375–82. doi: 10.1007/s00125-005-0112-4. [DOI] [PubMed] [Google Scholar]
- [60].Kishi K, Yuasa T, Minami A, Yamada M, Hagi A, Hayashi H, et al. AMP-activated protein kinase is activated by the stimulations of Gq-coupled receptors. Biochem Biophys Res Commun. 2000;276:16–22. doi: 10.1006/bbrc.2000.3417. [DOI] [PubMed] [Google Scholar]
- [61].Goode N, Hughes K, Woodgett JR, Parker PJ. Differential regulation of glycogen synthase kinase-3b by protein kinase C isotypes. J Biol Chem. 1992;267:16878–82. [PubMed] [Google Scholar]
- [62].Forlenza OV, Spink JM, Dayanandan R, Anderton BH, Olesen OF, Lovestone S. Muscarinic agonists reduce tau phosphorylation in non-neuronal cells via GSK-3b inhibition and in neurons. J Neural Transm. 2000;107:1201–12. doi: 10.1007/s007020070034. [DOI] [PubMed] [Google Scholar]