Abstract
The activity of NCX 972, a new molecule obtained by adding a nitric oxide (NO) moiety to metronidazole, was tested against six isolates of Entamoeba histolytica in xenic cultures. NCX 972 released NO into trophozoite cells and enhanced killing of amoebas in a dose- and time-dependent manner compared to metronidazole.
Entamoeba histolytica is a protozoan parasite causing amoebic colitis, dysentery and liver abscess. Transmission is by fecal-oral route, with cysts able to survive in the environment and trophozoites which penetrate the large intestine mucosa and cause tissue damage. Although the global prevalence of E. histolytica infections has been overestimated due to asymptomatic infections by the morphologically identical, noninvasive species Entamoeba dispar (2), this parasite is responsible for approximately 100,000 deaths per annum worldwide, being second in importance only to malaria among the pathogenic protozoa (23).
The first-line drugs for invasive amoebiasis are nitroimidazoles, with metronidazole (MTZ) being the drug of choice for efficacy and low cost (10). Although resistance has not yet been proven in E. histolytica strains isolated from patients, treatment failures following MTZ therapy have been documented (8). In contrast, resistance to this drug is common in bacteria and the protozoan flagellates Giardia intestinalis and Trichomonas vaginalis, and in vitro, trophozoites of E. histolytica readily adapt to therapeutic levels of MTZ (24). Hence, should resistance to MTZ become widespread, new amoebicidal compounds as well as the improvement of existing drugs will be needed.
Nitric oxide (NO) has been found to possess microbicidal effects against a number of pathogens, including DNA and RNA virus families (1), bacterial pathogens (13), and intracellular and extracellular protozoa (3, 4). Since 1992, NO has been demonstrated to be the major cytotoxic molecule released by activated macrophages for the in vitro cytotoxicity against E. histolytica (11). Later evidence suggests that NO is involved in host defense in vivo, this molecule being required for the control of amebic liver abscess in a murine model (19); and also, NO levels are increased in patients with acute intestinal amoebiasis (14).
Recently, novel NO-releasing drugs have been proposed for pharmaceutical uses. For example, NO-aspirin is currently tested as an anti-inflammatory and antithrombotic agent (22). In the literature there is no evidence of NO-releasing molecules being effective in treating amoebiasis. To investigate whether a newly synthesized MTZ derivative releasing NO (NCX 972) may be used for the treatment of amoebiasis, here we have compared the in vitro effect of MTZ and NCX 972 against E. histolytica.
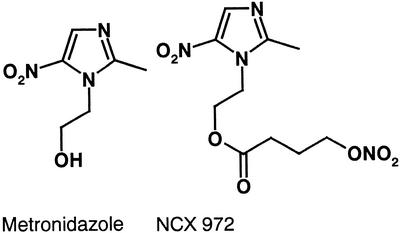
NCX 972 [4-(nitrooxy)butanoic acid 2-methyl-5-nitroimidazole-1-ethyl ester; molecular weight, 302.26] was from the Nicox Research Institute (Milan, Italy). MTZ (1-[2-hydroxyethyl]-2-methyl-5-nitroimidazole; molecular weight, 171.2) was purchased by Sigma (Milan) (Fig. 1).
FIG. 1.
Chemical structure of MTZ (1-[2-hydroxyethyl]-2-methyl-5-nitroimidazole) and NCX 972.
Pathogenic E. histolytica strains used (255, AX1, AX2, AX3, 128, and 735), as isolated from different areas of the world (Table 1), cloned and typed by isoenzyme electrophoresis and PCR (A. R. Sannella, M. Gramiccia, M. C. Angelici, G. Dettori, and E. Viani, Letter, J. Eukaryot. Microbiol. 45:137, 1998), were from the cryostabilate collection of Istituto Superiore di Sanità, Rome, Italy. For routine maintenance the strains were grown in tubes as xenic cultures in 2.5 ml of Robinson's medium supplemented with Escherichia coli bacteria (16). This medium, rather than an axenic culture medium (e.g., TYI-S-33 medium [18]), was chosen also for drug testing assays, in order to verify any possible interference between NCX 972 and common intestinal bacterial flora. Overgrowth of bacterial flora was controlled by 1 μg/ml penicillin-streptomycin mixture, a dosage proven nontoxic to amoeba cells.
TABLE 1.
Strains of E. histolytica used and activities of NCX 972 and MTZ after 48-h exposure
| E. histolytica strain | Origin | Mean % inhibition ± SEM caused by addition of:
|
|||
|---|---|---|---|---|---|
| NCX 972
|
MTZ
|
||||
| 5 μM | 20 μM | 5 μM | 20 μM | ||
| 255 | Zimbabwe | 46.1 ± 0.5 | 97.7 ± 0.1 | 28.6 ± 1.2 | 96.4 ± 1.2 |
| AX1 | Brazil | 47.6 ± 1.0 | 97.1 ± 0.2 | 25.1 ± 1.1 | 97.7 ± 0.8 |
| AX2 | Ecuador | 44.2 ± 0.6 | 97.6 ± 1.0 | 20.3 ± 1.0 | 97.8 ± 0.4 |
| AX3 | Italy | 36.0 ± 1.4 | 96.2 ± 0.8 | 19.7 ± 2.1 | 96.4 ± 0.2 |
| 128 | Mexico | 46.7 ± 2.1 | 100 | 27.3 ± 1.9 | 100 |
| 735 | India | 37.3 ± 0.9 | 97.8 ± 0.5 | 22.6 ± 1.5 | 97.8 ± 0.2 |
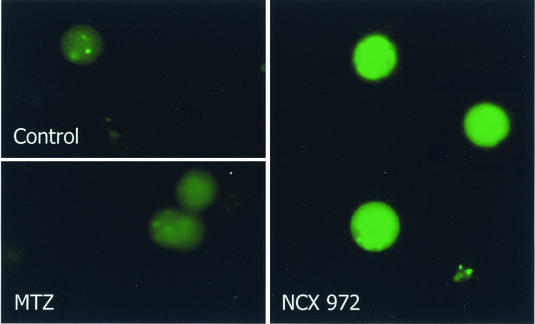
Firstly, we have observed that NCX 972 was able to release NO into amoebic trophozoites, as measured by using the DAF-2DA detection system (12). DAF-2DA is a dye that, upon binding an oxidized species of NO, results in irreversible DAF fluorescence, thus permitting identification of the source of local NO formation in living cells. This method has been recently used to demonstrate both the intracellular release of NO an NO-releasing derivative of aspirin (9), and the endogenous production of NO into cells (21).
Briefly, trophozoites of the 255 strain were loaded with 10 μM DAF-2DA for 15 min and then incubated with a 20- to 100-μM concentration of either NCX 972 or MTZ for 30 min. After washings, cells were plated onto glass micro slides, covered with coverslips and viewed with an Axioplan II microscope (Carl Zeiss, Jena, Germany) equipped with a cooled charge-coupled device camera SPOT (Diagnostic Instruments, Inc., Sterling Heights, Mich.). Images were digitally acquired and processed for densitometric determination at the single-cell level. Then, data were analyzed by one-way analysis of variance (ANOVA) followed by Bonferroni's multiple comparison test (GraphPad Prism Software, version 3.03).
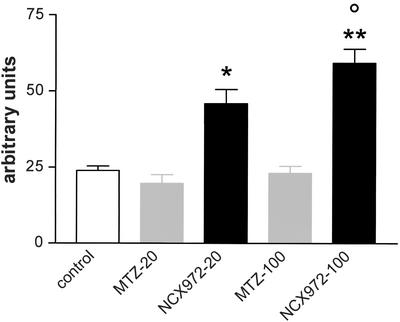
A basal low level of DAF fluorescence was found in untreated trophozoites (23.9 ± 1.5 arbitrary units; digital scale ranging from 0 to 255, minimum to maximum fluorescence intensity, respectively) as well as in cells treated for 30 min with 20 and 100 μM MTZ (19.7 ± 2.8 and 23.1 ± 2.3 arbitrary units, respectively) (Fig. 2) (data are presented as means ± standard errors of the means [SEM]). When the cells were treated with 20 and 100 μM NCX 972 for 30 min, DAF fluorescence was strongly enhanced above the background level (45.9 ± 4.7 and 59.2 ± 4.6 arbitrary units, respectively; P < 0.001) (Fig. 2).
FIG. 2.
Intracellular release of NO by NCX 972 in trophozoites of E. histolytica. Cells were preincubated with DAF-2DA and then treated with a 20- to 100-μM concentration of either MTZ or NCX 972 for 30 min. Images report DAF fluorescence of trophozoites untreated (control) or treated with 100 μM MTZ and 100 μM NCX 972. Data are expressed as arbitrary units. The gray density was calculated at the single-cell level (about 100 cells/treatment condition/experiment). Bars represent the mean ± SEM (error bars) from two separate experiments. One-way ANOVA followed by Bonferroni's test was used to determine the significant differences. Symbols: *, P < 0.001 between 20 μM NCX 972 and 20 μM MTZ; **, P < 0.001 between 100 μM NCX972 and 100 μM MTZ; °, P < 0.05 between 20 μM and 100 μM NCX 972 treatments. For further details see the text.
To compare the cytotoxic activities of NCX 972 and MTZ, two separate experiments were performed for each of the amoeba strains, and at each time point and concentration, viable trophozoites were counted from triplicate cultures with a phase-contrast microscope in 100 microscopic fields (magnification, ×200). Granular and rounded cells were regarded as dead. The mean viable cell count from treated cultures was expressed as percentage of the mean viable cell count from untreated cultures in each experiment (slightly modified from the method of Seifert et al. [18]).
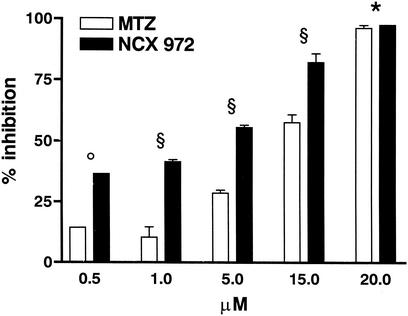
A 48-h dose-response assay using both drugs at concentrations from 0.5 to 20 μM was performed on the 255 strain (Fig. 3). These data were analyzed by two-way ANOVA with the F statistical test for independent effect of drug concentration and treatment (GraphPad Prism Software, version 3.03). As expected, MTZ produced a log-normal inhibition effect, with a 50% effective concentration of about 11 μM. Compared to MTZ, treatment with NCX 972 showed a significantly higher inhibitory activity, with a 50% effective concentration of about 3 μM (F, 161.0 [P < 0.0001]). Also differences in cell number among drug concentrations were significant by two-way ANOVA (F, 294.8 [P < 0.0001]). Notably, the efficacy of NCX 972 in killing E. histolytica trophozoites was significantly higher at low and mid drug concentrations, i.e., when MTZ possesses a suboptimal activity (e.g., 36.5% ± 0.1% versus 14.4% ± 0.2% at 0.5 μM; 41.5% ± 0.8% versus 10.4% ± 4.2% at 1 μM). On the contrary, at the highest concentration tested (20 μM), the difference between the activity of NCX 972 and MTZ was not significant (97.7% ± 0.1% versus 96.4% ± 1.2%). A similar inhibitory effect was obtained using 5 and 20 μM concentrations of NCX 972 or MTZ on further five E. histolytica strains (Table 1).
FIG. 3.
Activity of NCX 972 and MTZ against E. histolytica trophozoites. Drugs were added to the indicated concentrations, and the incubation was continued for 48 h. Data are expressed as percent inhibition and each bar represents the mean + SEM (error bars). Two-way ANOVA followed by Bonferroni's test was used to determine the significant differences. Symbols: °, P < 0.01 between 0.5 μM NCX 972 and 0.5 μM MTZ; §, P < 0.001 between 1 μM NCX972 and 1 μM MTZ, or 5 μM NCX972 and 5 μM MTZ, or 15 μM NCX972 and 15 μM MTZ; *, not significant. For further details see the text.
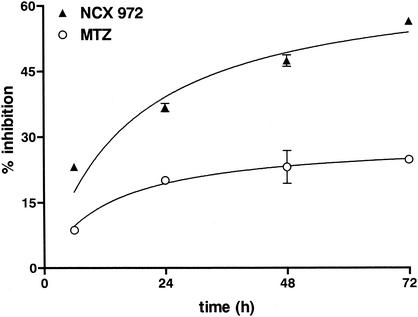
A time-dependent (6 to 72 h) effect of a sublethal concentration (5 μM) of NCX 972 or MTZ were also assayed on trophozoites of the 255 strain. Data were analyzed by two-way analysis of variance (ANOVA) with the F statistical test for independent effect of time and drug treatment followed by the Bonferroni's test. When compared with MTZ, treatment with NCX 972 showed an extremely significant quicker inhibitory activity (F, 51.7 [P < 0.0001]) (Fig. 4). However, the highest difference between NCX 972 and MTZ was observed after 72-h drug exposure (56.5% ± 0.1% versus 24.8% ± 0.7%; P < 0.001).
FIG. 4.
Time-dependent effect of NCX 972 and MTZ against E. histolytica trophozoites. Both drugs (5 μM) were incubated for 6 to 72 h. Data are expressed as percent inhibition and each point represents the mean ± SEM (error bars). Two-way ANOVA followed by Bonferroni's test was used to determine the significant differences: not significant between NCX 972 and MTZ at 6-h treatment; P < 0.01 between NCX 972 and MTZ at 24-h treatment; P < 0.001 between NCX972 and MTZ at 48-h or 72-h treatments. For further details see the text.
Since NO possesses antimicrobial activity (6), the possibility that the effect of NCX 972 was attributable to a killing of bacterial flora used as natural feed for trophozoites in the culture medium, has been also considered. To address this question, bacteria were counted by agar plating after 24-h exposure to NCX 972 or MTZ at different concentrations (1 to 20 μM). No significant differences were seen between number of E. coli colonies developed from untreated cultures and cultures treated with both drugs (data not shown).
Taken together, our data indicate that the NO-releasing MTZ derivative (NCX 972), especially at low doses, exerts an enhanced activity against E. histolytica.
Although the effect of NO on parasites has been well established (for reviews, see references 3 and 4), macromolecular targets have not been identified so far. Cysteine proteases from a number of intracellular parasitic protozoa (Leishmania infantum, Plasmodium falciparum, and Trypanosoma cruzi) have been reported to be inactivated by NO-mediated S-nitrosylation (5, 17; M. Colasanti, L. Salvati, G. Venturini, P. Ascenzi, and L. Gradoni, Letter, Trends Parasitol. 17:575, 2001). E. histolytica trophozoites release large amounts of cysteine proteases in extracellular environment, which significantly contribute to amebic virulence (7; for a review, see reference 15). Very recently, E. histolytica cysteine proteases have been found to be inhibited by S-nitroso-glutathione, a physiological NO donor (20). In addition, alcohol dehydrogenase-2, an enzyme required for the survival of this parasite, has been inactivated by the same NO donor (20). However, the possibility that under our conditions NO may affect other parasite molecular targets and/or enhance sensitivity of E. histolytica to MTZ should be taken into account.
In conclusion, NCX 972 displayed higher and faster activity than MTZ in killing E. histolytica and this effect appears to be correlated with NO release into parasite cells. These data support the concept that the addition of NO-releasing moiety to an established drug, such as MTZ, significantly improves its activity. Note that other drugs using the same NO-releasing approach have been found to be effective and safe, and are currently employed in clinical trials; also, they exert cytoprotective effects in the gastrointestinal mucosa (22).
Thus, although further studies are needed (including standard drug development processes), NO-releasing MTZ NCX 972 appears to be a promising lead compound to enhance efficacy of the present treatment and to reduce intestinal damage associated with invasive amoebiasis.
Acknowledgments
We thank Paolo Ascenzi for helpful discussion. We also thank Simona Sorino for technical assistance in the culture of the amoebae and Luisella Mattace for editorial assistance.
REFERENCES
- 1.Akaike, T., and H. Maeda. 2000. Nitric oxide and virus infection. Immunology 101:300-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anonymous. 1997. Entamoeba taxonomy. Bull. W. H. O. 75:291-292. [PMC free article] [PubMed] [Google Scholar]
- 3.Brunet, L. R. 2001. Nitric oxide in parasitic infections. Int. Immunopharmacol. 1:1457-1467. [DOI] [PubMed] [Google Scholar]
- 4.Clark, I. A., and K. A. Rockett. 1996. Nitric oxide and parasitic disease. Adv. Parasitol. 37:1-56. [DOI] [PubMed] [Google Scholar]
- 5.Colasanti, M., T. Persichini, G. Venturini, and P. Ascenzi. 1999. S-nitrosylation of viral proteins: molecular bases for antiviral effect of nitric oxide. IUBMB Life 48:25-31. [DOI] [PubMed] [Google Scholar]
- 6.De Groote, M. A., and F. C. Fang. 1995. NO inhibitions: antimicrobial properties of nitric oxide. Clin. Infect. Dis. 21(Suppl. 2):S162-S165. [DOI] [PubMed]
- 7.de Meester, F., E. Shaw, H. Scholze, T. Stolarsky, and D. Mirelman. 1990. Specific labeling of cysteine proteinases in pathogenic and nonpathogenic Entamoeba histolytica. Infect. Immun. 58:1396-1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dooley, C. P., and C. A. O'Morain. 1988. Recurrence of hepatic amebiasis after successful treatment with metronidazole. J. Clin. Gastroenterol. 10:339-342. [DOI] [PubMed] [Google Scholar]
- 9.Fiorucci, S., A. Mencarelli, R. Mannucci, E. Distrutti, A. Morelli, P. del Soldato, and S. Moncada. 2002. NCX-4016, a nitric oxide-releasing aspirin, protects endothelial cells against apoptosis by modulating mitochondrial function. FASEB J. 16:1645-1647. [DOI] [PubMed] [Google Scholar]
- 10.Freeman, C. D., N. E. Klutman, and K. C. Lamp. 1997. Metronidazole. A therapeutic review and update. Drugs 54:679-708. [DOI] [PubMed] [Google Scholar]
- 11.Lin, J. Y., and K. Chadee. 1992. Macrophage cytotoxicity against Entamoeba histolytica trophozoites is mediated by nitric oxide from L-arginine. J. Immunol. 148:3999-4005. [PubMed] [Google Scholar]
- 12.Lopez-Figueroa, M. O., H. E. Day, S. Lee, C. Rivier, H. Akil, and S. J. Watson. 2000. Temporal and anatomical distribution of nitric oxide synthase mRNA expression and nitric oxide production during central nervous system inflammation. Brain Res. 852:239-246. [DOI] [PubMed] [Google Scholar]
- 13.Nathan, C., and M. U. Shiloh. 2000. Reactive oxygen and nitrogen intermediates in the relationship between mammalian hosts and microbial pathogens. Proc. Natl. Acad. Sci. USA 97:8841-8848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pérez-Fuentes, R., M. del Carmen Sánchez-Guillén, M. Velázquez-Rojas, H. Salgado-Rosas, E. Torres-Rasgado, and P. Talamás-Rohana. 2000. Increased nitric oxide levels in patients with acute intestinal amebiasis. Arch. Med. Res. 31:S87-S88. [DOI] [PubMed] [Google Scholar]
- 15.Que, X., and S. L. Reed. 2000. Cysteine proteinases and the pathogenesis of amebiasis. Clin. Microbiol. Rev. 13:196-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robinson, G. L. 1968. The laboratory diagnosis of human parasitic amoebae. Trans. R. Soc. Trop. Med. Hyg. 62:285-294. [DOI] [PubMed] [Google Scholar]
- 17.Salvati, L., M. Mattu, M. Colasanti, A. Scalone, G. Venturini, L. Gradoni, and P. Ascenzi. 2001. NO donors inhibit Leishmania infantum cysteine proteinase activity. Biochim. Biophys. Acta 1545:357-366. [DOI] [PubMed] [Google Scholar]
- 18.Seifert, K., M. Duchene, W. H. Wernsdorfer, H. Kollaritsch, O. Scheiner, G. Wiedermann, T. Hottkowitz, and H. Eibl. 2001. Effects of miltefosine and other alkylphosphocholines on human intestinal parasite Entamoeba histolytica. Antimicrob. Agents Chemother. 45:1505-1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seydel, K. B., S. J. Smith, and S. L. Stanley, Jr. 2000. Innate immunity to amebic liver abscess is dependent on gamma interferon and nitric oxide in a murine model of disease. Infect. Immun. 68:400-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siman-Tov, R., and S. Ankri. 2003. Nitric oxide inhibits cysteine proteinases and alcohol dehydrogenase 2 of Entamoeba histolytica. Parasitol. Res. 89:146-149. [DOI] [PubMed] [Google Scholar]
- 21.Venturini, G., M. Colasanti, T. Persichini, E. Fioravanti, P. Ascenzi, L. Palomba, O. Cantoni, and G. Musci. 2002. Beta-amyloid inhibits NOS activity by subtracting NADPH availability. FASEB J. 16:1970-1972. [DOI] [PubMed] [Google Scholar]
- 22.Wallace, J. L., L. J. Ignarro, and S. Fiorucci. 2002. Potential cardioprotective actions of NO-releasing aspirin. Nat. Rev. Drug Discov. 1:375-382. [DOI] [PubMed] [Google Scholar]
- 23.Warhust, D. C. 1999. Amoebiasis, p. 548-559. In H. M. Gilles (ed.), Protozoal diseases. Arnold, London, United Kingdom.
- 24.Wassmann, C., A. Hellberg, E. Tannich, and I. Bruchhaus. 1999. Metronidazole resistance in the protozoan parasite Entamoeba histolytica is associated with increased expression of iron-containing superoxide dismutase and peroxiredoxin and decreased expression of ferredoxin 1 and flavin reductase. J. Biol. Chem. 274:26051-26056. [DOI] [PubMed] [Google Scholar]