Abstract
Kawasaki K, Akaike H, Miyauchi A, Ouchi K. Sivelestat relieves respiratory distress refractory to dexamethasone in all-trans retinoic acid syndrome: a report of two cases.
Treatment with all-trans retinoic acid (ATRA) improves the prognosis of patients with acute promyelocytic leukemia (APL), but ATRA syndrome may occur as a possible fatal side effect, especially in cases refractory to medication or involving pulmonary hemorrhage. We describe two patients with APL who suffered from intracranial hemorrhage. The first patient was a 16-yr-old girl who was treated with ATRA and then developed respiratory distress refractory to treatment with dexamethasone combined with anthracycline-cytarabine cytoreduction therapy. Treatment with Sivelestat, a small molecule inhibitor of neutrophil elastase, achieved rapid improvement in oxygenation and chest radiograph findings, and the patient has been in complete remission for 24 months. The second patient was a 10-yr-old boy in whom pulmonary hemorrhage developed following administration of ATRA, dexamethasone and cytoreduction therapy. Aspiration and administration of Sivelestat improved oxygenation and he remained stable. Hematological improvement was also achieved, but the patient died of brain dysfunction because of cerebral edema accompanied by intracranial bleeding. The two cases suggest that Sivelestat may be effective as an additional agent in the treatment of refractory ATRA syndrome, and, therefore, prospective randomized studies of treatment protocols are warranted.
Keywords: all-trans retinoic acid, acute promyelocytic leukemia, acute lung injury, respiratory distress, neutrophil elastase, elastase inhibitor
All-trans retinoic acid (ATRA) treatment improves the overall survival of patients with acute promyelocytic leukemia (APL) by reducing early deaths because of bleeding events and sepsis (1), but some patients receiving ATRA develop respiratory distress associated with pulmonary infiltration, consolidation, and ground-glass opacities on chest radiographs. This condition is known as ATRA syndrome (1, 2). To manage these symptoms, steroids and cytoreduction chemotherapy are administered based on the patient's leukocyte count (1, 2). Some patients, however, require prolonged mechanical ventilation (2) and occasionally ATRA syndrome can be fatal (3).
We describe two APL patients who were treated with ATRA and then suffered from respiratory distress that was not improved by standard agents. However, findings on chest radiographs and ventilation parameters improved following additional administration of Sivelestat, a small molecule inhibitor of neutrophil elastase.
Case 1
A 16-yr-old left-handed girl was admitted to our hospital because of menorrhagia and pancytopenia. The patient had been well a month earlier, when she had experienced a prolonged period and heavy menstrual flow. She consulted a clinic because of general fatigue, and was referred to this hospital.
She had a temperature of 37.5°C, a pulse rate of 110/min, a respiration rate of 40/min, and her blood pressure was 130/80 mmHg. The liver and spleen were palpable at 3 cm subcostally. No lymphadenopathy was found. Her white blood cell count (WBC) was 900 cells/μL, with 4% neutrophils, 81% lymphocytes, 1% monocytes, and 14% abnormal cells that contained folded nuclei, some granules and Auer rods. Her hemoglobin level was 4.7 g/dL, and her platelet count was 27 000 cells/μL. Prothrombin time (PT) and activated partial thromboplastin time (APTT) were 15.1 and 33.4 s, respectively, and the fibrinogen level was 71 mg/dL. Following diagnosis of acute myelogenous leukemia of subtype M3, ATRA was administered orally and gabexate mesilate (GM) intravenously. Two packs of erythrocytes were transfused.
The next morning the patient vomited and became drowsy, and a seizure accompanied by right hemiparesis developed. A CT scan of the head disclosed a left subcortical hematoma (Fig. 1A) and minor bleeding in the right pons. A hematectomy was performed, after which administration of ATRA was continued under mechanical ventilation. On hospital day 5, her WBC count increased to 15 000 cells/μL, at which time ATRA was discontinued, and administration of dexamethasone, daunorubicin, and cytarabine was initiated intravenously. Oxygen saturation decreased to 90% despite 60% oxygen being supplied, so FiO2 was increased to 1.0. A chest radiograph revealed an enlarged heart and bilateral diffuse ground-glass opacity (Fig. 1B). Another chest radiograph on the following day showed extensive consolidation (Fig. 1C). Her WBC count, however, decreased to 3700 cells/μL. After obtaining informed consent, Sivelestat was administered because oxygen saturation had decreased to 78% despite positive end-expiratory pressure (PEEP) being increased to 15 mm H2O and peak inspiratory pressure to 25 mm H2O. The next morning, PaO2 had improved to 278 mmHg, and a third chest radiograph revealed improvement of consolidation (Fig. 1D). Subsequently, FiO2, PEEP and peak inspiratory pressure were decreased gradually and mechanical ventilation was discontinued on hospital day 10. Sivelestat and dexamethasone were discontinued on day 12, and complete remission was achieved on day 35. The patient received 5 cycles of consolidation chemotherapy containing ATRA, and ATRA syndrome did not recur. The patient has now been in complete remission for 24 months.
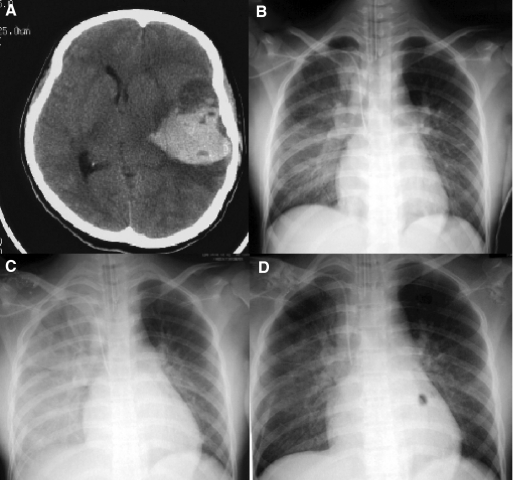
Fig. 1.
(A) A cranial CT-scan showing a high intensity lesion of 7 cm in diameter in the left subcortical region. (B–D) Serial chest radiographs showing progression and resolution of pulmonary infiltrates. (B) Five days after ATRA treatment, a chest radiograph showed bilateral diffuse ground-glass opacity, which was prominent in the right-middle to lower lung fields, and an enlarged cardiac silhouette. (C) Despite withdrawal of ATRA and administration of dexamethasone, daunorubicin and cytarabine, another radiograph on hospital day 6 revealed extensive consolidation. (D) After administration of Silvelestat, the consolidation had almost disappeared on the following morning.
Case 2
A 10-yr-old boy who had previously been healthy was admitted to our hospital with diarrhea and epistaxis. He presented to the emergency outpatient unit of the hospital with watery diarrhea. His father reported that the patient had suffered from repeated nasal bleeding and some eruptions had been noticed on his abdomen a few days earlier. The patient was a Brazilian of Japanese ancestry who had immigrated to Japan 6 months earlier.
Patient findings were as follows; temperature 37.4°C, pulse 92/min, respiration 22/min, and blood pressure 110/62 mmHg. Physical examination disclosed petechiae on the neck and abdomen. No lymphadenopathy or hepatosplenomegaly was found. His WBC count was 41 000 cells/μL, with 3% neutrophils, 7% lymphocytes, 0.5% monocytes, and 85% abnormal cells with bilobal and reniform nuclei and agranular cytoplasms. His hemoglobin level was 10.6 g/dL, his platelet count was 35 000 cells/μL, PT was 17.3 s, APTT was 25.4 s, the fibrinogen level was 50 mg/dL, and the concentration of fibrinogen degradation products was 26.6 μg/mL. The patient was given a platelet transfusion and treated with GM and fresh frozen plasma. On hospital day 4, a central venous catheter was introduced under anesthesia, and the patient subsequently became agitated and vomited. A chest radiograph taken at this time showed infiltration in the upper right lung field (Fig. 2A).
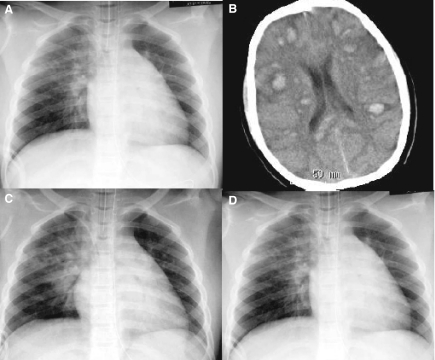
Fig. 2.
(A) A radiograph of the chest taken during recovery from general anesthesia, showing hazy perihilar and right upper zone opacities. (B) ATRA, dexamethasone, daunorubicin and cytarabine were administered on hospital day 7, and the patient then became drowsy. A computed tomography scan of the head disclosed multiple high intensity lesions of varying sizes in the brain parenchyma. (C) On hospital day 9, another radiograph showed new consolidations in the bilateral lower lung fields. (D) After administration of Silvelestat and suctioning of a bloody specimen using a bronchoscope, the consolidation diminished in thickness and oxygenation remained stable without suctioning.
On hospital day 7, a diagnosis of M3 variant APL was made, based on a RT-PCR analysis that showed a PML-RAR gene rearrangement. His WBC count was 116 000/μL. ATRA was commenced orally and cytarabine and daunorubicin were administered intravenously. Then, however, the patient vomited bloody specimens and became drowsy. A CT scan of the head revealed multiple sites of intraparenchymal bleeding and diffuse cerebral swelling, which was particularly prominent in the pons (Fig. 2B). Dexamethasone and mannitol were administered intravenously. Because SpO2 decreased to 80% with a 60% O2 supply, the patient was intubated and placed under mechanical ventilation. A bloody specimen was suctioned from the tracheal tube. Coffee-colored contents were also drained from an orogastric tube. Therefore, cimetidine was administered intravenously. Platelets and erythrocytes were transfused.
The next day, the bilateral pupils were enlarged and corneal reflexes were absent. Another CT scan of the head revealed deterioration of brain edema, and a chest radiograph on hospital day 9 disclosed new consolidations in the bilateral lower lung fields (Fig. 2C). Aspirated matter suctioned from an endotracheal tube became bloodier despite repeated transfusion of platelets and fresh frozen plasma. Administration of Sivelestat was commenced after obtaining informed consent from his parents, and the following day SpO2 suddenly fell to 80%. However, after suctioning with a tracheal endoscope, it increased to 95%. A chest radiograph on the following day showed reduced pulmonary infiltrates (Fig. 2D), and oxygenation remained stable without suctioning. However, blood flow in the carotid arteries could not be detected on a transcranial Doppler echogram, and his corneal reflexes remained absent on day 10. Electroencephalogram waves and auditory brainstem responses were flattened. Although a blood examination revealed a leukocyte count that had decreased to 4500/μL and coagulopathy was treated acceptably, the patient deteriorated further and died of brain dysfunction on day 13.
Discussion
Acute respiratory distress syndrome and acute lung injury (ARDS/ALI) is a critical illness syndrome consisting of acute impaired oxygenation and bilateral pulmonary infiltrates without left atrial hypertension, and arises from a variety of underlying insults (4). The clinical features of ARDS/ALI, rapid onset of respiratory failure in patients with risk factors, occasionally accompanied by involvement of other organs (4), are similar to those of ATRA syndrome (2, 3). In addition, several aspects of the radiologic manifestations of the two syndromes resemble each other, and include bilateral infiltration and pleural effusion (5). Case 1 was diagnosed as ATRA syndrome based on her clinical course, despite the development of intracranial hemorrhage. In case 2, the patient suffered from aspiration pneumonia, intracranial bleeding and pulmonary hemorrhage, making a diagnosis of ARDS/ALI possible. However, because diffuse alveolar hemorrhage in ATRA syndrome (6, 7) and rapid progression of ATRA syndrome in M3 variant APL (8) have been reported, the secondary pulmonary bleeding in case 2 might have been because of ATRA syndrome.
Neutrophil elastase is an enzyme that causes endothelial damage and increases permeability in ARDS/ALI (9). Sivelestat is a neutrophil elastase inhibitor that has been shown to be effective in animal models of ARDS/ALI. However, the efficacy of Sivelestat for ALI patients remains controversial: a Japanese phase III study reported good results (10), but Zeiher et al. found that Sivelestat combined with low tidal volume ventilation had no effect on 28-d all-cause mortality or ventilator-free days (11). Despite this uncertainty, we decided to use Sivelestat because our two patients were in serious respiratory distress that appeared to be refractory to other agents. From a safety perspective, double-blind placebo-controlled trials of ARDS/ALI have shown no difference in adverse events between Sivelestat-treated and control groups (10, 11). Respiratory functions and abnormalities on chest radiographs improved quickly after administration of Sivelestat in case 1.
Nicolls et al. (6) reported a case of ATRA syndrome with diffuse alveolar hemorrhage, in whom a lung biopsy revealed pulmonary capillaritis; i.e., infiltration of numerous neutrophils into the lung parenchyma. Since neutrophil elastase released from differentiated APL cells that have infiltrated the lung capillaries may be the main cause of damage to endothelial cells, the rapid improvement in respiratory function yielded by Sivelestat treatment may be because of its inhibitory effect on the enzyme. Pulmonary hemorrhage is another serious and potentially fatal condition that may not always be treatable with steroids and cytoreduction agents (6, 7), and prevention of lung hemorrhage by Sivelestat has been described in a rat model of cerulean-induced pancreatitis (12). In case 2, it is of interest that pulmonary bleeding refractory to transfusions of platelets and fresh frozen plasma was well controlled by introducing Sivelestat.
Intracranial hemorrhage is another life-threatening complication. Although coagulation disorders coexist frequently in APL, no significant differences in the incidence of early hemorrhagic death between subgroups administered heparin, anti-fibrinolytics and supportive therapy alone were reported in a large retrospective study (13). GM, a protease inhibitor, is used for coagulation disorders in Japan. The efficacy of GM for disseminated intravascular coagulation accompanying malignant neoplasms was described in preliminary report (14). Prospective studies of this agent are also warranted because reducing early death because of hemorrhage remains challenging.
In conclusion, we have reported on two patients with APL who suffered from intracranial hemorrhage and developed severe respiratory distress, with or without pulmonary hemorrhage. The respiratory condition was refractory to dexamethasone and anthracycline-cytarabine antineoplastic therapy, but Sivelestat achieved improvement in oxygenation and chest radiograph findings. Therefore, our results suggest that prospective studies are warranted to confirm the safety and efficacy of Sivelestat in the treatment of severe respiratory distress.
References
- 1.De Botton S, Coiteux V, Chevret S, et al. Outcome of childhood acute promyelocytic leukemia with all-trans-retinoic acid and chemotherapy. J Clin Oncol. 2004;22:1404–1412. doi: 10.1200/JCO.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 2.De Botton S, Dombret H, Sanz M, et al. The European APL Group. Incidence, clinical features, and outcome of all trans-retinoic acid syndrome in 413 cases of newly diagnosed acute promyelocytic leukemia. Blood. 1998;92:2712–2718. [PubMed] [Google Scholar]
- 3.Tallman MS, Andersen JW, Schiffer CA, et al. Clinical description of 44 patients with acute promyelocytic leukemia who developed the retinoic acid syndrome. Blood. 2000;95:90–95. [PubMed] [Google Scholar]
- 4.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 5.Jung JI, Choi JE, Hahn ST, Min CK, Kim CC, Park SH. Radiologic features of all-trans-retinoic acid syndrome. Am J Roentgenol. 2002;178:475–480. doi: 10.2214/ajr.178.2.1780475. [DOI] [PubMed] [Google Scholar]
- 6.Nicolls MR, Terada LS, Tuder RM, Prindiville SA, Schwarz MI. Diffuse alveolar hemorrhage with underlying pulmonary capillaritis in the retinoic acid syndrome. Am J Respir Crit Care Med. 1998;158:1302–1305. doi: 10.1164/ajrccm.158.4.9709085. [DOI] [PubMed] [Google Scholar]
- 7.Raanani P, Segal E, Levi I, Bercowicz M, Berkenstat H, Avigdor A, Perel A, Ben-Bassat I. Diffuse alveolar hemorrhage in acute promyelocytic leukemia patients treated with ATRA – a manifestation of the basic disease or the treatment. Leuk Lymphoma. 2000;37:605–610. doi: 10.3109/10428190009058513. [DOI] [PubMed] [Google Scholar]
- 8.Mahendra P, Harman K, Phillips M, Gunning K, Marcus RE. Rapid progression of ‘retinoic acid syndrome‘ in the hypogranular variant of acute promyelocytic leukaemia, despite treatment with dexamethasone and conventional chemotherapy. Clin Lab Haematol. 1994;16:371–374. doi: 10.1111/j.1365-2257.1994.tb00437.x. [DOI] [PubMed] [Google Scholar]
- 9.Moraes TJ, Chow CW, Downey GP. Proteases and lung injury. Crit Care Med. 2003;31:S189–S194. doi: 10.1097/01.CCM.0000057842.90746.1E. [DOI] [PubMed] [Google Scholar]
- 10.Tamakuma S, Ogawa M, Aikawa N, et al. Relationship between neutrophil elastase and acute lung injury in humans. Pulm Pharmacol Ther. 2004;17:271–279. doi: 10.1016/j.pupt.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 11.Zeiher BG, Artigas A, Vincent JL, Dmitrienko A, Jackson K, Thompson BT, Bernard G STRIVE Study Group. Neutrophil elastase inhibition in acute lung injury: results of the STRIVE study. Crit Care Med. 2004;32:1695–1702. doi: 10.1097/01.ccm.0000133332.48386.85. [DOI] [PubMed] [Google Scholar]
- 12.Guo L, Yamaguchi Y, Ikei S, Sugita H, Ogawa M. Neutrophil elastase inhibitor (ONO-5046) prevents lung hemorrhage induced by lipopolysaccharide in rat model of cerulein pancreatitis. Dig Dis Sci. 1995;40:2177–2183. doi: 10.1007/BF02209002. [DOI] [PubMed] [Google Scholar]
- 13.Rodeghiero F, Avvisati G, Castaman G, Barbui T, Mandelli F. Early deaths and anti-hemorrhagic treatments in acute promyelocytic leukemia. A GIMEMA retrospective study in 268 consecutive patients. Blood. 1990;75:2112–2117. [PubMed] [Google Scholar]
- 14.Umeki S, Adachi M, Watanabe M, Yaji S, Soejima R. Gabexate as a therapy for disseminated intravascular coagulation. Arch Intern Med. 1988;148:1409–1412. [PubMed] [Google Scholar]