Abstract
Objective
To examine the association of air pollutants with hospital admission for childhood asthma in Hong Kong.
Methods
Data on hospital admissions for asthma, influenza and total hospital admissions in children aged ≤18 years at all Hospital Authority hospitals during 1997–2002 were obtained. Data on daily mean concentrations of particles with aerodynamic diameter <10 μm (i. e. PM10) and <2.5 μm (i. e. PM2.5), nitrogen dioxide (NO2), sulphur dioxide (SO2), and ozone (O3) and data on meteorological variables were associated with asthma hospital admissions using Poisson's regression with generalized additive models for correction of yearly trend, temperature, humidity, day-of-week effect, holiday, influenza admissions and total hospital admission. The possibility of a lag effect of each pollutant and the interaction of different pollutants were also examined.
Results
The association between asthma admission with change of NO2, PM10, PM2.5 and O3 levels remained significant after adjustment for multi-pollutants effect and confounding variables, with increase in asthma admission rate of 5.64% (3.21–8.14) at lag 3 for NO2, 3.67% (1.52–5.86) at lag 4 for PM10, 3.24% (0.93–5.60) at lag 4 for PM2.5 and 2.63% (0.64–4.67) at lag 2 for O3. Effect of SO2 was lost after adjustment.
Conclusion
Ambient levels of PM10, PM2.5, NO2 and O3 are associated with childhood asthma hospital admission in Hong Kong.
Keywords: air pollution, asthma, children, Hong Kong, hospital admission
Introduction
There is substantial epidemiological evidence indicating a link between asthma morbidity including deterioration in lung functions, emergency department visits and hospital admission with outdoor air pollution levels [1]. Although it was generally believed that children are more vulnerable and susceptible than adults to air pollution exposure, [2] the PEACE project [3] which studied children aged 6–12 years in 28 regions of Europe, did not show any effects of particle matter, black smoke, sulphur dioxide (SO2) or nitrogen dioxide (NO2) on lung function, respiratory symptoms and bronchodilator use. The major limitation of the PEACE study has been the short observation period and it was suggested that increasing the length of the observation period, preferably including several seasons or years might provide better opportunities to tease out the relatively small effects of air pollution from the background noise in respiratory health indicators [4]. Hong Kong, one of the largest metropolitans in the world, is a good candidate for studying the health effects of air pollution with its dense population, detailed monitoring of air quality and hospital admissions. Since 1990s, there has been rapid decline in the air quality in Hong Kong attributed to vibrant economic activities in the region and nearby cities including Shenzhen and Guangzhou. In tune with the findings in PEACE, our previous prevalence study using International Study of Asthma and Related Allergies (ISAAC) methodology did not show a rise in prevalence of asthma in Hong Kong despite deteriorating air quality in past decades [5]. However, there was significant increase in prevalence of severe asthma symptoms including nocturnal awakenings by wheeze and nocturnal cough. We, therefore, performed a population-based time series analysis on daily hospital admission for asthma, a proxy for severe asthma attack in children less than 18 years old, with ambient air pollutants concentrations from 10 air quality-monitoring stations over Hong Kong. The result of the study may have important implication for health care policy in the region and may provide more evidences to support or dispute the acute effect of air pollutants in children with asthma.
Methods
Setting
Hong Kong is situated at the southeastern tip of China with a total area of 1102 km2 and a population of 6.816 million as of 2002. The population density is 6300 people/km2 and is one of the most densely populated cities in the world. Hong Kong's climate is sub-tropical, tending towards the temperate for nearly half the year. Temperatures can drop below 10°C in winter and exceed 31°C in summer. About 90% of the rainfall occurs between March and September. Air pollution derives mainly from motor vehicles, local power plants and industries in Guangdong because of the close proximity of these two regions.
Hospital admission data
In Hong Kong, the Hospital Authority manages a total of 28 517 hospital beds and accounts for 90% of all hospital admissions. Since 1995, all Hospital Authority inpatient data, including demographic characteristics, dates of admission and discharge, diagnoses and procedures on discharge using the International Classification of Diseases, 9th, Revision, Clinical Modification (ICD-9-CM), have been stored in a central-computerized database. We obtained data on daily hospital admissions for asthma (ICD-9-CM code 493) as primary diagnosis upon discharge, influenza (ICD-9-CM code 487) as primary diagnosis upon discharge for control of viral respiratory seasonal epidemics and the total hospital admissions in patients ≤18 years of age from all hospital authority hospitals from January 1997 to December 2002.
Data on air quality and weather
Data on five outdoor air pollutants including SO2, NO2, respirable suspended particulates (RSP) understood as particulate matter 10 and 2.5 μm in aerodynamic diameter (PM10 and PM2.5), respectively, and also ozone (O3) were obtained from the Environmental Protection Department, Hong Kong SAR [6]. There were nine stations for monitoring general air quality and it was increased to 11 stations in 2000 Further there are stations for roadside air quality across the territory. All stations are situated close, to residential areas, except the one in Tap Mum (Fig. 1), the data of which was excluded. The hourly concentration record of each air pollutant from each included station was retrieved and the daily mean of each air pollutant was calculated. Then the arithmetic mean of each air pollutant from all included stations was calculated. In each station, for O3 the 8-hourly (9:00–17:00 hours when O3 concentrations were highest) means were taken as daily data, if there were at least six valid hourly data each day, and for the other pollutants the 24-hourly means were used as daily data if there were at least 18 valid hourly data. When daily record of individual air pollutant from a particular station was considered to be invalid, the daily mean of that air pollutant was not included in mean calculation. In general, the concentration of gaseous pollutants and RSP are determined continuously by automatic analyzers. Manually operated high volume samplers using the gravimetric methods are also used regularly to measure the RSP. Meteorological data including mean temperature, humidity and atmospheric pressure were obtained from the Hong Kong observatory [7].
Fig. 1.
Location of the stations for monitoring air quality.
Statistical analysis
Pearson's correlation was used to determine the correlation between stations for daily concentrations of each air pollutant and so for correlation between daily concentrations of air pollutants and meteorological variables.
Semi-parametric Poisson's regression with generalized additive models for adjustment of over-dispersion using SAS version 8.02 was used to model the daily counts of asthma admissions. The core model included smooth function of the day of study, spline smooth functions of mean daily temperature and relative humidity, daily hospital admissions for influenza and total daily hospital admission and indicator variables for day of the week and holidays to avoid over control for the effect of the pollutants and to account for the over-dispersion for the variable and possible population changes during the study period.
Before adding the air pollutant variables into the model, the effects of temperature and humidity on day of admission and up to 5 days before admission (i.e. at lag 0, 1, 2, 3, 4 and 5) were investigated and modelled using minimization of Akaike's Information Criteria (AIC) and lack of over- or under-fitting in the residual correction for autocorrelation. Both mean daily temperature and relative humidity on day of admission (at lag 0) were selected in the subsequent model as they had the best-combined fit. After building up the core regression model for temperature and humidity-related hospital admissions, single pollutant was entered into the regression, and the effects of the pollutant on the day of admission and the previous 5 days (i.e. at lag 0, 1, 2, 3, 4 and 5) were examined to account for potential delays in disease incidence after important exposures. Multi-pollutant models were run for pollutants that were significant in the single pollutant analysis, and the lag that had the strongest univariate effect was tested. The result was expressed as per cent increase with 95% confidence intervals (95% CI) in daily admission with each increment of an inter-quartile range (IQR) change of each pollutant.
Results
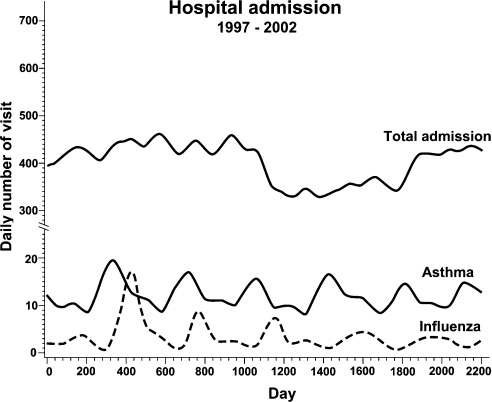
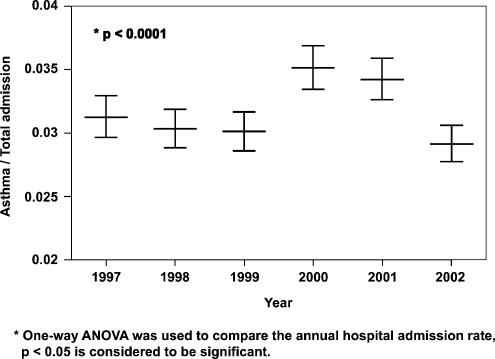
Table 1 summarizes data for hospital admissions, and for meteorological and pollution variables. There were 879 384 total hospital admissions, 26 663 asthma admissions and 5821 influenza admissions for children ≤18 years of age recorded with the respective daily average admission of 401, 12.1 and 2.7 over the 6-year study period. Standardized annual total hospital admission, admission for asthma and influenza were about 999, 31 and 7 per 10 000 children ≤18 years, respectively, based on population figures [8]. There was a decreasing trend in annual total hospital admission until year 2002, while annual asthma admission rate remained fairly constant over these 6 years (Fig. 2), resulting in an increasing contribution of asthma admission to total hospital admission (P < 0.001) (Fig. 3).
Table 1.
Summary of environmental variables and daily asthma hospital admission data in Hong Kong, 1997–2002
| Percentiles | |||||
|---|---|---|---|---|---|
| Pollutant variable | Mean±SD | 25th | 50th | 75th | IQR |
| Environmental variables | |||||
| Temperature (°C) | 23.7 ± 4.8 | 20.0 | 24.8 | 27.8 | 7.8 |
| Humidity (%) | 78.1 ± 10.0 | 74 | 79 | 85 | 11 |
| Rainfall mm | 7.4 ± 23.5 | 0 | 0 | 2.3 | 2.3 |
| Pollutant variable (μg/m3) | |||||
| PM10 (24 h) | 56.1 ± 24.2 | 37.3 | 51.1 | 70.7 | 33.4 |
| PM2.5 (24 h) | 45.3 ± 16.2 | 33.4 | 43.0 | 54.0 | 20.6 |
| SO2 (24 h) | 17.7 ± 10.7 | 10.6 | 15.2 | 21.7 | 11.1 |
| NO2 (24 h) | 64.7 ± 20.9 | 49.7 | 63.5 | 76.8 | 27.1 |
| O3 (8 h) | 28.6 ± 16.0 | 15.9 | 25.4 | 38.9 | 23 |
| Hospital daily admission (number) | 401 ± 90.7 | 332 | 404 | 468 | 136 |
| Asthma daily hospital admission (number) | 12.1 ± 5.4 | 8 | 11 | 15 | 7 |
| Influenza daily hospital admission (number) | 2.7 ± 4.3 | 0 | 1 | 3 | 3 |
Observation period was 2191 days, total, hospital admission was 879 384, asthma admission was 26 663 and influenza admission was 5821.
PM10, aerodynamic diameter <10 μm; PM2.5, aerodynamic diameter <2.5 μm; SO2, sulphur dioxide; NO2, nitrogen dioxide; O3, ozone.
Fig. 2.
Spline smooth of total hospital admission, asthma admission and influenza admission from years 1997 to 2002.
Fig. 3.
Mean ratio with 95% confidence interval (95% CI) of asthma admission to total hospital admission from 1997 to 2002.
Table 2 shows the correlations among the air pollution and weather variables. There was a marked seasonal variation in ambient concentrations of PM10, NO2 and O3, all of which were lowest in warm season (April–September) and highest in cool season (October–March). In contrast, there is no significant seasonal fluctuation of SO2. Strong correlations were observed between PM10 and PM2.5 (r = 0.90), NO2 (r = 0.78), O3 (r = 0.48) and SO2 (r = 0.37); between SO2 and NO2 (r = 0.49), and between NO2 and O3 (r = 0.35). The correlation of PM2.5 to other pollutants was similar to the correlation of PM10 with these pollutants The pattern of correlations remained similar across seasons. About 70% of PM10 is in the fraction of PM2.5, and the main source is motor vehicle exhaust in Hong Kong [9]. The between-station correlations were high for all air pollutants with mean (range) 0.93 (0.85–0.97) for PM10, 0.78 (0.53–0.94) for NO2, 0.82 (0.57–0.93) and 0.61 (0.53–0.94) for SO2.
Table 2.
Pearson's correlation coefficients among environmental variables, Hong Kong 1997–2002
| Temperature | Humidity | Rainfall | PM10 | PM2.5 | SO2 | NO2 | |
|---|---|---|---|---|---|---|---|
| Whole year | |||||||
| Humidity (%) | 0.22 | ||||||
| Rainfall (mm) | 0.12 | 0.35 | |||||
| PM10 (μg/m3) | −0.33 | −0.48 | −0.27 | ||||
| PM2.5 (μg/m3) | −0.21 | −0.35 | −0.24 | 0.90 | |||
| SO2 (μg/m3) | 0.13 | −0.15 | −0.09 | 0.37 | 0.47 | ||
| NO2 (μg/m3) | −0.38 | −0.37 | −0.20 | 0.78 | 0.75 | 0.49 | |
| O3 (μg/m3) | −0.09 | −0.40 | −0.20 | 0.48 | 0.36 | −0.17 | 0.35 |
| Cold season (October–March) | |||||||
| Humidity (%) | 0.15 | ||||||
| Rainfall (mm) | 0.03 | 0.27 | |||||
| PM10 (μg/m3) | 0.01 | −0.44 | −0.24 | ||||
| PM2.5 (μg/m3) | 0.05 | −0.29 | −0.21 | 0.90 | |||
| SO2 (μg/m3) | 0.01 | −0.18 | −0.12 | 0.53 | 0.59 | ||
| NO2 (μg/m3) | 0.00 | −0.33 | −0.18 | 0.69 | 0.70 | 0.69 | |
| O3 (μg/m3) | 0.27 | −0.36 | −0.15 | 0.33 | 0.21 | −0.24 | 0.08 |
| Warm season (April–September) | |||||||
| Humidity (%) | −0.32 | ||||||
| Rainfall (mm) | −0.16 | 0.46 | |||||
| PM10 (μg/m3) | −0.18 | −0.38 | −0.23 | ||||
| PM2.5 (μg/m3) | −0.03 | −0.28 | −0.21 | 0.89 | |||
| SO2 (μg/m3) | 0.23 | −0.21 | −0.13 | 0.39 | 0.48 | ||
| NO2 (μg/m3) | −0.31 | −0.25 | −0.12 | 0.80 | 0.74 | 0.51 | |
| O3 (μg/m3) | −0.30 | −0.43 | −0.20 | 0.60 | 0.47 | −0.11 | 0.52 |
PM10, aerodynamic diameter <10 μm; PM2.5, aerodynamic diameter <2.5 μm; SO2, sulphur dioxide; NO2, nitrogen dioxide; O3, ozone.
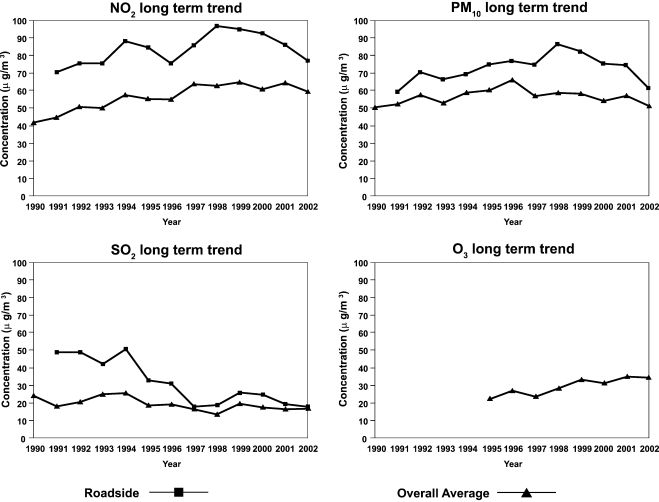
There has been a noticeable change in ambient decline in air quality in Hong Kong since 1990s (Fig. 4). SO2 concentration has decreased significantly after the introduction of fuel quality restriction in 1990. The 1997 annual averages were about 40–64% lower than that before enforcement of fuel restriction. The roadside concentration dropped to 18 μg/m3 in 2002. The overall NO2 level was over 60 μg/m3, while the roadside NO2 level persistently exceeded the permissible limit of 80 μg/m3 over all these years. Although there was a decreasing trend of (RSP) over the study period, the roadside RSP trend remained well above the permissible limit of 55 μg/m3. The recognition of the steep rise in the overall O3 average in the past few years (76.5% from 1997 to 1999) was enabled by the addition of seven stations in O3 monitoring. Nevertheless, data from old stations, which have been monitoring O3 since 1990 also revealed a slow but steady rising trend in the last decade.
Fig. 4.
Long-term trends of four pollutants studied.
Table 3 summarizes the results of the single-pollutant analysis for asthma admission up to 5 lag days. All the five pollutants studied were associated with an increase in daily asthma admission. An increase in IQR of daily mean concentration of PM10 (33.4 μg/m3), PM2.5 (20.6 μg/m3) and NO2 (27.1 μg/m3) were associated with increase in daily asthma admission on the day of admission and all the 5 days before admission (lags 1–5). The most significant increase in asthma admission was 9.08% (95% CI 7.26–10.93) per IQR change of NO2 level 3 days before admission (lag 3). The corresponding increase in asthma admission were 7.45% (5.58–9.35) per IQR change of PM10 on lag 4, 6.59% (4.51–8.72) per IQR change of PM2.5 on lag 4, 5.97% (4.10–7.89) per IQR change of O3 (23.0 μg/m3) on lag 3 and 1.46% (0.19–2.74) per IQR change of SO2 (11.1 μg/m3) on lag 5.
Table 3.
Percentage increase in daily hospital admissions for asthma for age <=18 year old per interquartile range (IQR) increase of pollutants at different lags, Hong Kong 1997–2002
| Lag | PM10; IQR: 33.4 μg/m3 | PM2.5; IQR: 20.6 μg/m3 | SO2; IQR: 11.1 μg/m3 | NO2; IQR: 27.1 μg/m3 | O3; IQR: 23.0 μg/m3 |
|---|---|---|---|---|---|
| 0 | 4.97 (2.96–7.03) | 5.10 (2.95–7.30) | −1.57 (−2.87–−0.26) | 4.37 (2.51–6.27) | 2.34 (0.40–4.31) |
| 1 | 5.71 (3.78–7.68) | 5.00 (2.88–7.16) | −1.77 (−3.06–−0.46) | 5.88 (4.00–7.70) | 4.59 (2.71–6.51) |
| 2 | 6.40 (4.51–8.32) | 4.83 (2.75–6.95) | −1.15 (−2.42–0.14) | 7.19 (5.37–9.04) | 5.97 (4.10–7.89)* |
| 3 | 7.25 (5.38–9.16) | 4.83 (2.78–6.93) | 0.82 (−0.45–2.11) | 9.08 (7.26–10.93)* | 3.87 (2.02–5.75) |
| 4 | 7.45 (5.58–9.35)* | 6.59 (4.51–8.72)* | 1.40 (0.13–2.69) | 7.64 (5.84–9.48) | 2.41 (0.58–4.26) |
| 5 | 5.96 (4.11–7.85) | 5.24 (3.18–7.34) | 1.46 (0.19–2.74)* | 6.40 (4.60–8.22) | 0.86 (−0.94–2.7) |
Data presented as percentage increase (95% confidence interval). PM10: aerodynamic diameter <10 μm; PM2.5,aerodynamic diameter <2.5 μm; SO2, sulphur dioxide; NO2, nitrogen dioxide; O3, ozone.
Highest percentage change after control for the mean daily temperature, relative humidity, daily hospital admissions for influenza, total daily hospital admission and indicator variables for day of the week and holidays.
Table 4 shows that association between asthma admission with change of NO2, PM10, PM2.5 and O3 levels remained significant after adjustment for multi-pollutants effect and confounding variables, with increase in asthma admission rate of 5.64% (3.21–8.14) at lag 3 for NO2, 3.67% (1.52–5.86) at lag 4 for PM10, 3.24% (0.93–5.60) at lag 4 for PM2.5 and 3.76% (0.47–6.26) at lag 2 for O3. Effect of SO2 was lost after adjustment. Owing to the multi-collinearity of PM10 and PM2.5, they were separately put into the multi-pollutants model. For the other pollutants, the result is similar when putting either PM10 or PM2.5, and only the set of results when PM10 was included in the multi-pollutants model is presented.
Table 4.
Percentage increase in daily asthma hospital admission per inter-quartile range (IQR) increase of pollutants in single- and multi-pollutant models, Hong Kong 1997–2002
| Variable | Lag | Single-pollutant model* | Multi-pollutant model† |
|---|---|---|---|
| PM10 | 4 | 7.45 (5.58–9.35) | 3.67 (1.52–5.86)‡ |
| PM2.5 | 4 | 6.59 (4.51–8.72) | 3.24 (0.93–5.60)‡ |
| SO2 | 5 | 1.46 (0.19–2.74) | 0.81 (−0.75–2.4) |
| NO2 | 3 | 9.08 (7.26–10.93) | 5.64 (3.21–8.14) |
| O3 | 2 | 5.97 (4.10–7.89) | 3.76 (0.47–6.26) |
Data presented as percentage increase (95% confidence interval). PM10, aerodynamic diameter <10 μm; PM2.5, aerodynamic diameter <2.5 μm; SO2, sulphur dioxide; NO2, nitrogen dioxide; O3, ozone.
Single pollutant model results for the most significant single lag day.
Estimates from regression models containing five pollutants simultaneously.
Owing to the multi-collinearity of PM10 and PM2.5, they are separately put into the multi-pollutant model.
Discussion
Our study showed that ambient level of both particulates (PM10, and PM2.5) and gaseous pollutants (NO2 and O3) are associated with childhood asthma hospital admission in Hong Kong. The effects of these four pollutants were independent as the associations remained significant after adjustment in the multi-pollutant model. The validity of our study is supported by long study period, reliable central-computerized source of hospital admission data for over 90% of the population and good air-quality monitoring system of international standard.
There have been much debate on the appropriate statistical methods in analysing the effect of air pollution with different heath outcomes and comparing the results across different regions. There are several important approaches, e.g. the National Mortality and Morbidity Air Pollution Study (NMMAPS) in the United States focused on the 20 largest cities during 1987–1994 [10, 11], and Air Pollution and Health: a European Approach (APHEA) 1 and 2 in European countries [10, 12]. It was later discovered that there was a problem in statistical model of the NMMAPS [13], which led to an overestimation of the effect reported although qualitative conclusions did not change. APHEA 1 attempted to standardize the statistical method for comparison across different European cities using Poisson's time series models and combine the city-specific estimates of the effect of air pollution using meta-analysis. Parametric approach in modelling seasonality and weather was used, but it was subjected to potential biases [14]. Nevertheless, non-parametric smoothing function, however, does not perform well when there are a large number of independent variables in the model and this could be one of the reasons for lacking significant association between ambient air pollutants and asthma hospital admission in some of the previous studies. We adopted the generalized additive model in our data analysis which is similar to that of APHEA 2 [15]. It allows the adjustment of both parametric and non-parametric data in the same model. We included several important confounders in our model. Susceptibility to epidemics of respiratory infections and the effects of school holidays are important considerations for asthma admission in children. The increase in traffic across the border between Hong Kong and mainland China may lead to a fluctuation in population size during holidays. Birth rate has also been dropping in Hong Kong over the past decade. Thus, in addition to adjustment for day of the study, day of the week and calendar holidays, total hospital admissions for children ≤18 years old were also considered as a proxy for the population denominator. Our recent study showed that influenza is an important cause of hospitalization among children in Hong Kong, with rates exceeding those reported for temperate regions [16] and have influenza epidemic has been included in our model. We did not take exposure of pollens into account as suggested by other studies [17, 18] because pollenosis is uncommon and there is no official data available in our locality.
Information bias related to accuracy and completeness of data on hospital admissions for asthma could account for inconsistencies of the result among different studies or across different age groups even within the same study. We obtained data from a central computerized database. The computerized system was launched in all hospitals under the Hospital Authority in Hong Kong since 1995 and the data became complete and reliable since 1997. We restricted our study population to all subjects younger than 18 years old. All patients under this age have been managed in paediatric wards in Hong Kong since 1997, a change in practice that has been adopted in many other developed countries. The difference between vulnerability and susceptibility of children and adult to air pollution exposure has been reviewed in detail [2]. Moreover, the relative lower contribution of asthma admission to total admission for respiratory diseases in adults and elderly would render less power to detect the associations of asthma admission with air pollutants when the studies were carried out for whole age group with same period of time [15, 19]. A recent study from Atlanta, USA [20] showed that the association for paediatric asthma emergency visits in relation to PM10 was stronger than those for adult asthma visits. We attempted to include the longest possible duration of observation period but this could only be extended up to year 2002 as the hospital admission for children was greatly affected by the SARS in 2003. A post-priori calculation of our sample size of 2191 days of observation showed that it could detect a low correlation rate of r = 0.06 (95% CI 0.02–0.10) between air pollutants and asthma admission at 80% power and 5% of α.
Biological plausibility of respirable particulates on respiratory morbidity was reviewed [21]. However, there were few studies actually showing significant association of respirable particulates with asthma admission, particularly in children (Table 5). Some of these studies [17,23–28] showed significant association of asthma admission in children with ambient NO2 level but none except early Ontario study [23] showed significant association with particulate matter. Particulate matter was considered as a surrogate for other pollutants with no contribution of its own [29]. The apparent lack of effect of the studies could be attributed to the use of black smokes [17, 24–26] and PM13 [27] instead of PM10 in their study. Using a newer statistical model and use of data on PM10, APHEA 2 was able to show that PM10 was positively associated with increased number of admissions for asthma in children aged 0–14 years [15]. Nevertheless, there was substantial heterogeneity in results among the participating cities and the association between asthma admissions and PM10 was lost after inclusion of NO2. Our study used a model similar to that of APHEA 2 but we also adjusted for the population change using total hospital admission as a proxy and included admissions of children aged above 14 to below 18 years old. This would provide a more complete and reliable estimation of both numerators and denominators in calculating the possible association. Our study showed a strong association of PM10 and PM2.5 with asthma admission and the effect remained significant after adjustment in the multi-pollutants model. This argued against particulate matter as a surrogate only. A recent systematic review of panel studies showed an adverse effect of particulate air pollution that is greater for PM2.5 than for PM10 on lung function and symptoms of children with asthma [30]. However, there have not been any panel studies or time series studies of the effect of PM2.5 on hospital admission for asthma in children. To the best of our knowledge, our study is the first to show such a relationship.
Table 5.
Time series population based studies* of short term effect of air pollutants on asthma hospital admission in children
| Author | Year | Place | Months | Age | NO2 | SO2 | Particles | O3 | Remarks | Statis method |
|---|---|---|---|---|---|---|---|---|---|---|
| Bates [22] | 74, 76–83 | Ontario | 1,2,7,8 | 0–14 | Sign (winter) | NS | Sign | NS | Change in ICD code in 1979 | Pearson correlation |
| Burnett et al. [23] | 83–88 | Ontario | 1–12 | 2–34 | NA | Sign | NA | Sign | Only two pollutants measured | Pearson correlation |
| Sunyer et al. [24] | 86–92 | Helsinki | 1–12 | 0–14 | NS | NS | NS (BS) | NS | APHEA1 | |
| London | 1–12 | 0–14 | Sign | Sign | NS (BS) | NS | ||||
| Paris | 1–12 | 0–14 | NS | Sign | NS (BS) | NS | ||||
| Anderson et al. [17] | 87–92 | London | 1–12 | 0–14 | Sign | Sign | NS (BS) | NS | APHEA1 | |
| Morgan et al. [25] | 90–94 | Sydney | 1–12 | 1–14 | Sign | NS | NS (BSP) | NS | Similar to APHEA 1 | |
| Petroeschevsky et al. [26] | 87–94 | Brisbane | 1–12 | 0–14 | Sign | NS | Sign neg (BSP) | Sign | Sign assoc | APHEA 1 |
| Between GI disease | ||||||||||
| And SO2 | ||||||||||
| Fusco et al. [27] | 95–97 | Rome | 1–12 | 0–14 | Sign | NS | NS (PM13) | NS | Sign assoc with CO | Similar to APHEA 2 |
| Atkinson et al. [15] | 94–96 | Barcelona | 1–12 | 0–14 | NA | NA | NS (PM10) | NA | APHEA 2 | |
| 92–94 | Birmingham | Sign (PM10) | ||||||||
| 92–94 | London | NS (PM10) | ||||||||
| 90–97 | Milan | Sign (TSP) | ||||||||
| 92–95 | The Netherlands | NS (PM10) | ||||||||
| 92–96 | Paris | NS (PM13) | ||||||||
| 95–97 | Rome | NS (TSP) | ||||||||
| 94–96 | Stockholm | NS (PM10) | ||||||||
| Summary | Sign (PM10)† | |||||||||
| Barnett et al. [28] | 98–01 | Brisbane | 1–12 | 0–14 | Sign | NS | Sign (PM10)‡ | NS | Cross-over study | |
| Christchurch | ||||||||||
| Melbourne | ||||||||||
| Perth | ||||||||||
| Syndey |
NO2, nitrogen dioxide; O3, Ozone; SO2, sulphur dioxide; CO, carbon monoxide; TSP: total suspended particles; BS, black smoke; BSP, particles measured by nephelometry (air heated to 70°C to control for humidity and the light scattering of dry particles is measured); PM10, PM13, particles with an aerodynamic diameter of less than 10 and 13 μm; Sign, significant; NS, not significant; NA, data not available.
Only studies with separate result in children were included for comparison.
Pooled estimates in single pollutant model; effect lost after inclusion of NO2, SO2, CO but not O3 in two pollutants model.
Effect lost after matching on NO2.
NO2 has been shown to activate oxidant pathways, enhance airway responses to inhaled allergens in asthmatic individuals and impair function of alveolar macrophages and epithelial cells. Exposure to NO2 occurs both indoors from gas cooking appliances and outdoors, mainly from vehicle exhausts. Epidemiological studies showed conflicting results of the effect of NO2 with some showed an association between indoor exposure and respiratory symptoms in children whereas other failed to confirm it. Living close to main road is shown to be a risk factor for wheezing illness in children [31]. Another well-designed prospective study from Southampton showed that high personal exposure to NO2, at levels within air quality standard in the week before the start of a respiratory viral infection is associated with asthma exacerbation in children [32]. In Hong Kong, the roadside NO2 and RSP level has persistently exceeded the permissible limits in the past 10 years which could be attributed to the incessant increase in road traffic [8] and trapping of polluted air including vehicle exhausts by the city's tall buildings, the so-called canyon effect. Many residential areas are situated in close proximity to busy road traffic. As outdoor particles readily penetrate indoors, we believed that PM measured at outdoor fixed sites would correlate closely with personal exposure. In addition, exposure misclassification from using central regional PM data should have biased towards the null findings. A recent panel study in children with asthma in fact showed that ambient-generated component of PM2.5 exposure has stronger association than indoor-generated component with increase in exhaled nitric oxide, a marker of airway inflammation [33]. All these factors helped to explain the relative strong association of particulate matter and NO2 with hospital admission for asthma in our study when compared with studies from other cities.
Exposure to O3 can increase respiratory symptoms and lead to acute hospital admissions for asthma. Ambient O3 is formed by a series of complicated photochemical reactions of oxygen, nitrogen oxides and volatile organic compounds under sunlight. Nitric oxide emissions from motor vehicles have scavenging effect on O3 and areas with heavy traffic flow normally have lower O3 levels. This is reflected in ambient O3 level of Hong Kong where much higher O3 level is detected in rural than urban stations. O3 provocation studies showed that effective dose depends on concentration, duration of exposure and degree of exercise. The close proximity of most residential areas to heavy road traffic and the relative lack of outdoor activities in children in Hong Kong might explain the less marked effect of O3 on asthma admission when compared with that of PM10 and NO2.
The restrictions on sulphur content of fuel in Hong Kong in July 1990 led to immediate improvement in air quality and immediate and long-term beneficial health effects [34–36]. Absence of association of SO2 with asthma admission was attributed to low ambient SO2 level over these years. Our study added evidence of health benefits related to this industrial fuel intervention.
In conclusion, our study showed that ambient levels of PM10, PM2.5, NO2 and O3 in our locality were associated with childhood asthma hospital admission in Hong Kong. We speculate that the increasing prevalence of severe asthma symptoms in our previous ISAAC study [5] and the rising contribution of asthma admission to total hospital admission in children over the study period could be related to the worsening air pollution in our population. This called for the attention of health policy makers towards the ever-rising air pollutants level in Hong Kong. Our study also suggested that age of target population and the indigenous causes attributing to ambient air pollutants level are important factors to be considered when studying the effect of air pollutants on respiratory health and added supporting evidence to the acute effect of air pollutants including PM10, PM2.5, NO2 and O3 on children with asthma.
References
- 1.D'Amato G, Liccardi G, D'Amato M, Holgate S. Environmental risk factors and allergic bronchial asthma. Clin Exp Allergy. 2005;35:1113–24. doi: 10.1111/j.1365-2222.2005.02328.x. [DOI] [PubMed] [Google Scholar]
- 2.WHO Regional Office for Europe. The effects of air pollution on children's health and development: a review of evidence. 2004.
- 3.Roemer W, Hoek G, Brunekreef B, Haluszka J, Kalandidi A, Pekkanen J. Daily variations in air pollution and respiratory health in a multicentre study: the PEACE project. Pollution effects on asthmatic children in Europe. Eur Respir J. 1998;12:1354–61. doi: 10.1183/09031936.98.12061354. [DOI] [PubMed] [Google Scholar]
- 4.Roemer W, Hoek G, Brunekreef B. Pollution effects on asthmatic children in Europe, the PEACE study. Clin Exp Allergy. 2000;30:1067–75. doi: 10.1046/j.1365-2222.2000.00851.x. [DOI] [PubMed] [Google Scholar]
- 5.Lee SL, Wong W, Lau YL. Increasing prevalence of allergic rhinitis but not asthma among children in Hong Kong from 1995 to 2001 (Phase 3 International Study of Asthma and Allergies in Childhood) Pediatr Allergy Immunol. 2004;15:72–8. doi: 10.1046/j.0905-6157.2003.00109.x. [DOI] [PubMed] [Google Scholar]
- 6.Air quality in Hong Kong 2002. Environmental Protection Department 2002; 2005 (04-05-2005) http://www.epd.gov.hk/epd/english/environmentinhk/air/air_quality/aq_annualrpt.html.
- 7.Extract of meteorological observations for Hong Kong. Hong Kong Observatory 2005; 2005 (21-4-2005) http://www.hko.gov.hk/wxinfo/pastwx/extract.htm.
- 8.Hong Kong in Figures 2002. Census and Statistics Department, Hong Kong 2002; 2005 (21-05-2005) http://www.info.gov.hk/censtatd/eng/hkstat/index2.html.
- 9.Chow JC, Watson JG, Kohl SD, Gonzi MP. Measurements and Validation for the Twelve Month Particulate Matter Study in Hong Kong. 2002. http://www.epd.gov.hk/epd/english/environmentinhk/air/studyrpts/air_studyrpts.html.
- 10.Daniels MJ, Dominici F, Samet JM, Zeger SL. Estimating particulate matter-mortality dose–response curves and threshold levels: an analysis of daily time-series for the 20 largest US cities. Am J Epidemiol. 2000;152:397–406. doi: 10.1093/aje/152.5.397. [DOI] [PubMed] [Google Scholar]
- 11.Samet JM, Dominici F, Curriero FC, Coursac I, Zeger SL. Fine particulate air pollution and mortality in 20 U.S. cities, 1987–1994. N Engl J Med. 2000;343:1742–9. doi: 10.1056/NEJM200012143432401. [DOI] [PubMed] [Google Scholar]
- 12.Katsouyanni K, Schwartz J, Spix C, et al. Short term effects of air pollution on health: a European approach using epidemiologic time series data: the APHEA protocol. J Epidemiol Community Health. 1996;50(Suppl. 1):S12–8. doi: 10.1136/jech.50.suppl_1.s12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. 2002–2003 Annual Report. Health Effects Institute 2002; 2005(21 April, 2005). http://www.healtheffects.org/Pubs/annualreport2002-2003.pdf.
- 14.Schwartz J, Spix C, Touloumi G, et al. Methodological issues in studies of air pollution and daily counts of deaths or hospital admissions. J Epidemiol Commun Health. 1996;50(Suppl. 1):S3–11. doi: 10.1136/jech.50.suppl_1.s3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Atkinson RW, Anderson HR, Sunyer J, et al. Acute effects of particulate air pollution on respiratory admissions: results from APHEA 2 project. Air pollution and health: a European approach. Am J Respir Crit Care Med. 2001;164:1860–6. doi: 10.1164/ajrccm.164.10.2010138. [DOI] [PubMed] [Google Scholar]
- 16.Chiu SS, Lau YL, Chan KH, Wong WH, Peiris JS. Influenza-related hospitalizations among children in Hong Kong. N Engl J Med. 2002;347:2097–103. doi: 10.1056/NEJMoa020546. [DOI] [PubMed] [Google Scholar]
- 17.Anderson HR, Ponce de Leon A, Bland JM, Bower JS, Emberlin J, Strachan DP. Air pollution, pollens, and daily admissions for asthma in London 1987–92. Thorax. 1998;53:842–8. doi: 10.1136/thx.53.10.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galan I, Tobias A, Banegas JR, Aranguez E. Short-term effects of air pollution on daily asthma emergency room admissions. Eur Respir J. 2003;22:802–8. doi: 10.1183/09031936.03.00013003. [DOI] [PubMed] [Google Scholar]
- 19.Anderson HR, Atkinson RW, Bremner SA, Marston L. Particulate air pollution and hospital admissions for cardiorespiratory diseases: are the elderly at greater risk? Eur Respir J. 2003;40(Suppl. 1):39s–46s. doi: 10.1183/09031936.03.00402203. [DOI] [PubMed] [Google Scholar]
- 20.Peel JL, Tolbert PE, Klein M, et al. Ambient air pollution and respiratory emergency department visits. Epidemiology. 2005;16:164–74. doi: 10.1097/01.ede.0000152905.42113.db. [DOI] [PubMed] [Google Scholar]
- 21.Brunekreef B, Holgate ST. Air pollution and health. Lancet. 2002;360:1233–42. doi: 10.1016/S0140-6736(02)11274-8. [DOI] [PubMed] [Google Scholar]
- 22.Bates DV, Sizto R. Air pollution and hospital admissions in Southern Ontario: the acid summer haze effect. Environ Res. 1987;43:317–31. doi: 10.1016/s0013-9351(87)80032-4. [DOI] [PubMed] [Google Scholar]
- 23.Burnett RT, Dales RE, Raizenne ME, et al. Effects of low ambient levels of ozone and sulfates on the frequency of respiratory admissions to Ontario hospitals. Environ Res. 1994;65:172–94. doi: 10.1006/enrs.1994.1030. [DOI] [PubMed] [Google Scholar]
- 24.Sunyer J, Spix C, Quenel P, et al. Urban air pollution and emergency admissions for asthma in four European cities: the APHEA project. Thorax. 1997;52:760–5. doi: 10.1136/thx.52.9.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morgan G, Corbett S, Wlodarczyk J. Air pollution and hospital admissions in Sydney, Australia, 1990 to 1994. Am J Public Health. 1998;88:1761–6. doi: 10.2105/ajph.88.12.1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petroeschevsky A, Simpson RW, Thalib L, Rutherford S. Associations between outdoor air pollution and hospital admissions in Brisbane, Australia. Arch Environ Health. 2001;56:37–52. doi: 10.1080/00039890109604053. [DOI] [PubMed] [Google Scholar]
- 27.Fusco D, Forastiere F, Michelozzi P, et al. Air pollution and hospital admissions for respiratory conditions in Rome, Italy. Eur Respir J. 2001;17:1143–50. doi: 10.1183/09031936.01.00005501. [DOI] [PubMed] [Google Scholar]
- 28.Barnett AG, Williams GM, Schwartz J, et al. Air pollution and child respiratory health: a case-crossover study in Australia and New Zealand. Am J Respir Crit Care Med. 2005;171:1272–8. doi: 10.1164/rccm.200411-1586OC. [DOI] [PubMed] [Google Scholar]
- 29.Moolgavkar SH, Luebeck EG. A critical review of the evidence on particulate air pollution and mortality. Epidemiology. 1996;7:420–8. doi: 10.1097/00001648-199607000-00014. [DOI] [PubMed] [Google Scholar]
- 30.Ward DJ, Ayres JG. Particulate air pollution and panel studies in children: a systematic review. Occup Environ Med. 2004;61:e13. doi: 10.1136/oem.2003.007088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Venn AJ, Lewis SA, Cooper M, Hubbard R, Britton J. Living near a main road and the risk of wheezing illness in children. Am J Respir Crit Care Med. 2001;164:2177–80. doi: 10.1164/ajrccm.164.12.2106126. [DOI] [PubMed] [Google Scholar]
- 32.Chauhan AJ, Inskip HM, Linaker CH, et al. Personal exposure to nitrogen dioxide (NO2) and the severity of virus-induced asthma in children. Lancet. 2003;361:1939–44. doi: 10.1016/S0140-6736(03)13582-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koenig JQ, Mar TF, Allen RW, et al. Pulmonary effects of indoor- and outdoor-generated particles in children with asthma. Environ Health Perspect. 2005;113:499–503. doi: 10.1289/ehp.7511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peters J, Hedley AJ, Wong CM, et al. Effects of an ambient air pollution intervention and environmental tobacco smoke on children's respiratory health in Hong Kong. Int J Epidemiol. 1996;25:821–8. doi: 10.1093/ije/25.4.821. [DOI] [PubMed] [Google Scholar]
- 35.Hedley AJ, Wong CM, Thach TQ, Ma S, Lam TH, Anderson HR. Cardiorespiratory and all-cause mortality after restrictions on sulphur content of fuel in Hong Kong: an intervention study. Lancet. 2002;360:1646–52. doi: 10.1016/s0140-6736(02)11612-6. [DOI] [PubMed] [Google Scholar]
- 36.Wong CM, Lam TH, Peters J, et al. Comparison between two districts of the effects of an air pollution intervention on bronchial responsiveness in primary school children in Hong Kong. J Epidemiol Commun Health. 1998;52:571–8. doi: 10.1136/jech.52.9.571. [DOI] [PMC free article] [PubMed] [Google Scholar]