Abstract
Brazilian patients with benign prostatic hyperplasia were randomised in a 12-week, double-blind, double-dummy study to receive doxazosin gastrointestinal therapeutic system (GITS) 4 mg q.i.d. (n = 82) or tamsulosin 0.4 q.i.d. (n = 83). Primary endpoints were the absolute and percentage change from baseline in symptoms measured by International Prostate Symptom Score (IPSS). Secondary endpoints included IPSS, quality-of-life (QOL) question from the IPSS, and questions 6 and 7 of the Sexual Function Abbreviated Questionnaire (SFAQ) at weeks 4 and 12. Doxazosin GITS and tamsulosin improved IPSS with no significant differences between groups at week 12. During weeks 4–8, tamsulosin-treated patients demonstrated a slower improvement (p < 0.001) in IPSS than doxazosin GITS-treated patients. The proportion of satisfied patients was observed earlier with doxazosin GITS (p = 0.006) vs. tamsulosin. At week 12, the proportion of patients with little or no difficulty at ejaculation (Q6 of SFAQ) was higher in the doxazosin GITS group (p = 0.019). Both treatments were well tolerated.
Keywords: Doxazosin, tamsulosin, benign prostatic hyperplasia, international prostate symptom score, prostate, ejaculation, gastrointestinal therapeutic system
Introduction
Benign prostatic hyperplasia (BPH) is a non-malignant enlargement of the prostate that can cause bladder obstruction leading to lower urinary tract symptoms (LUTS) as the increased prostatic mass compresses the urethra and inhibits urinary flow (1). The incidence of BPH increases proportionately with advancing age, with more than 70% of men older than 70 years having histologic evidence of BPH (2).
Selective α1-adrenoceptor antagonists are considered the first line of standard pharmacologic treatment for patients with BPH (3). These agents reduce urethral pressure (3) and inhibit smooth muscle tone in the prostate and lower urinary tract (4) by interrupting the motor sympathetic adrenergic nerve supply to the prostate, reducing the pressure, and improving the LUTS and urinary function in patients with BPH (5).
Although all of the currently available α1-adrenoceptor antagonists have demonstrated efficacy in the treatment of BPH, each is unique in its affinity for various receptor subtypes (α1A, α1B and α1D) (3) and, thus, the potential for both beneficial and adverse events (AEs). Doxazosin and tamsulosin, both α1-adrenoceptor antagonists, have been shown to provide the relief of symptomatic BPH with once-daily dosing. The efficacy of doxazosin, a long-acting, selective α1-adrenoceptor antagonist, has been demonstrated by improvements in urinary flow rate and symptomatic measures (e.g. nocturia, hesitancy, urgency and weak stream) (6,7). Recently, a controlled-release formulation, doxazosin gastrointestinal therapeutic system (GITS), has been shown to enhance the pharmacokinetic profile and drug delivery rate, minimising the fluctuations and extending the time to peak serum concentration compared with the doxazosin standard formulation (8). Doxazosin GITS eliminates the need for titration by starting at an effective dose of 4 mg q.i.d. (8). Previous studies have shown doxazosin GITS to be effective in reducing the symptoms of BPH when compared with placebo (9) and tamsulosin (10,11) or alfuzosin (7). Tamsulosin, shown to be effective for the relief of symptomatic BPH, also provides once-daily treatment in a modified-release capsule and requires no titration (12). However, unlike doxazosin, tamsulosin has been associated with abnormal ejaculation in 10–30% of patients in long-term studies (13,14).
The current clinical goal of therapy for patients with BPH is to reduce the symptoms, with minimal AEs. The goal of this study was to compare the efficacy of the new formulation of doxazosin 4 mg q.i.d. with tamsulosin 0.4 mg q.i.d. for symptom improvement, changes in quality of life (QOL) and in sexual function during 12 weeks of treatment.
Materials and methods
Study Design
This trial was a multicenter, randomised, double-blind, double-dummy study comprised of two phases: a 2-week washout phase and a 12-week active treatment phase. The study was conducted in 10 Brazilian research centers from 13 November 2001 to 30 September 2002. The trial was carried out in compliance with the Declaration of Helsinki and with local laws and regulations relevant to the use of new and non-approved therapeutic agents in patients.
Patients
All participating patients provided written informed consent. Patients eligible for this clinical trial included male ambulatory patients ≥50 years of age with a diagnosis of BPH confirmed by digital rectal examination and ultrasound, patients with International Prostate Symptom Score (IPSS) >12, and patients able to receive oral treatment with α1-adrenoceptor antagonists.
Those ineligible for this trial included patients with a previous history of urological surgery (prostate, bladder or urethra) and/or urine retention or catheter in the urinary duct that, by the investigator's sole discretion, may need catheterisation within the next 3 months; patients with prostate cancer, infection of the urinary tract or prostate-specific antigen (PSA) >5 ng/ml; those with a clinical history suggesting serious cardiac or hepatic insufficiency, hypotension or blood pressure >180/110 mmHg; those already receiving treatment for BPH; patients with a clinical history of oesophagus or intestinal duct obstruction; those receiving therapeutic drugs that may interfere with study drugs (androgens, antiandrogens, diuretics, cholinergics, anticholinergics and phytotherapy) within the previous 6 months; and patients with a history of alcohol or drug abuse, concurrent serious disease or malignancy or significant psychological problems.
Study Methods
The initial visit (week 2) included the collection of baseline information, such as demographics, medical history, physical examination, measurement of blood pressure and pulse rate, assessment of the Qmax and urine volume, PSA test and prostate ultrasound examination. Patients also answered the IPSS questionnaire, including the question on QOL.
During the 12-week treatment phase, patients were randomised to receive either 4-mg doxazosin GITS plus tamsulosin placebo q.i.d. or 0.4-mg tamsulosin plus doxazosin placebo q.i.d.. Subsequent visits (weeks 0, 4, 8 and 12) also included benign prostatic hyperplasia impact index (BII) on QOL, questions one through five and 15 of the International Index of Erectile Function (IIEF), and questions six and seven of the Sexual Function Abbreviated Questionnaire (SFAQ). Additionally, Qmax, urine volume, blood pressure and pulse rate were measured, and all AEs were recorded.
Efficacy Assessments
Primary efficacy endpoints were the absolute and percentage change from baseline as measured by the IPSS at final evaluation. Secondary efficacy endpoints included IPSS behaviour over the length of the study (weeks 0, 4, 8 and 12), the responses to the QOL question, the BII, the IIEF and SFAQ. Safety parameters included AEs and assessment of vital signs.
Statistical Methods
The intent-to-treat (ITT) population included the patients who took at least one dose of study medication and had both a baseline measurement and at least one measure of efficacy variable analysed after the start of treatment. Results were considered statistically significant at p ≤ 0.05. All tests were two-tailed, and the sas® (Statistical Analysis System–SAS Institute, Cary, NC, USA) system was used for statistical calculations. Comparison of the primary efficacy variables was made using an ancova model with baseline values as covariate for absolute change from baseline and an anova model for the percentage change from baseline. Secondary variables were analysed using the repeated measures anova, adjusted for baseline values, assuming an unstructured covariance matrix.
Results
Patients
Of the 194 patients screened, 165 were randomised to treatment. All randomised patients received study drug: 82 patients received doxazosin GITS and 83 received tamsulosin (Table 1). At baseline, a medical history of hypertension (blood pressure >140/90 mmHg) was recorded in 19 patients from the doxazosin GITS group and 20 patients from the tamsulosin group. In the doxazosin GITS group, 65 patients completed the study. Fifteen patients violated the protocol, and two were discontinued because of AEs. In the tamsulosin group, 71 patients completed the study. Seven patients violated the protocol, four were discontinued because of AEs, and one patient withdrew informed consent during the treatment period. Analyses were conducted on the ITT population of 158 patients (76 in the doxazosin GITS group and 82 in the tamsulosin group), excluding the seven patients who had no postbaseline efficacy data.
Table 1.
Baseline characteristics of the evaluated population
| Characteristic (n = 158) | Doxazosin GITS (n = 82) | Tamsulosin (n = 83) |
|---|---|---|
| Age (years), mean ± SD | 62.6 (6.8) | 61.7 (7.6) |
| Weight (kg), mean ± SD | 74.7 ± 12.1 | 74.9 ± 13.1 |
| Height (cm), mean ± SD | 169.8 ± 7.2 | 168.8 ± 6.2 |
| Race, n (%) | ||
| White | 66 (80.5) | 72 (86.7) |
| Black | 11 (13.4) | 11 (13.3) |
| Mixed race | 4 (4.9) | 0 |
| Asian | 1 (1.2) | 0 |
GITS, gastrointestinal therapeutic system; SD, standard deviation.
Efficacy Assessments
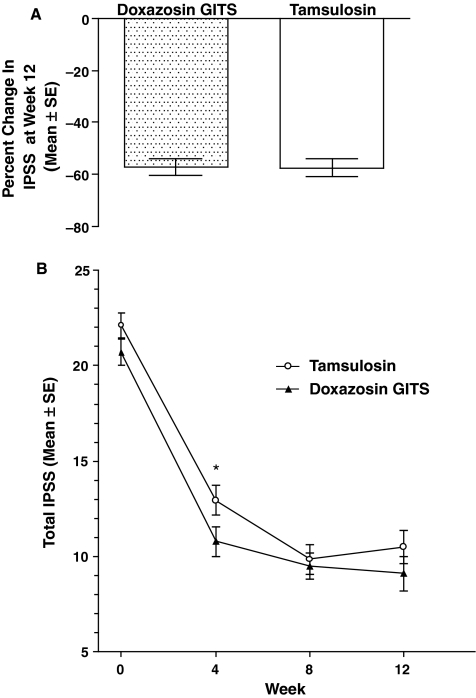
The primary endpoint was the total amount and percent change in IPSS at the final visit (week 12). The total IPSS showed significant improvements from baseline in both groups (p = 0.001), as shown in Figure 1A. The difference in IPSS change between the two groups was not significant (p = 0.759) at endpoint. IPSS values were similar at weeks 4, 8 and 12 in patients receiving doxazosin GITS (Figure 1B). IPSS values in patients receiving tamsulosin decreased significantly between weeks 4 and 8 (p < 0.0001).
Figure 1.
Effect of treatment on IPSS. (A) Percent change ± SD in total IPSS at week 12. (B) Mean ± SE of IPSS at baseline, weeks 4, 8, and 12. *p < 0.01 tamsulosin week 4 vs. tamsulosin week 8. GITS = gastrointestinal therapeutic system; IPSS, International Prostate Symptom Score
When patients were asked, ‘If you were to stay with your current urinary situation, how would you feel?’ the proportion of satisfied patients in the doxazosin GITS group did not vary over the study period (p = 0.262). In contrast, the responses of patients in the tamsulosin group changed significantly from weeks 4–8 (p = 0.006), suggesting that the number of satisfied patients in the doxazosin GITS group increased earlier (Table 2). Although the response with tamsulosin at week 12 was numerically greater than the response with doxazosin, there was no statistically significant difference between the two groups.
Table 2.
Estimated proportion (%±SE) of patients satisfied with their current condition based on the quality-of-life question
| Group | Visit | Satisfied patients (% ± SE) |
|---|---|---|
| Doxazosin GITS | Week 4 | 42.57 ± 5.75 |
| Week 8 | 48.12 ± 5.88 | |
| Week 12 | 53.06 ± 5.75 | |
| Tamsulosin | Week 4 | 31.33 ± 5.27 |
| Week 8 | 47.53 ± 5.71 | |
| Week 12 | 56.59 ± 5.50 |
GITS, gastrointestinal therapeutic system; SE, standard error.
The BII scores decreased significantly during the study period in patients receiving both doxazosin GITS and tamsulosin (p = 0.001), with no differences between the groups (p = 0.674) (Table 3). Although the mean values for Qmax and urine volume were larger for tamsulosin at week 12 than for doxazosin, differences between the two groups were not significant for Qmax (p = 0.526) or urine volume (p =0.057; Table 3).
Table 3.
BII, Qmax, and urine volume during the trial (mean ± SD)
| Group | Visit | BII | Qmax(ml/s) | Urine volume (ml) |
|---|---|---|---|---|
| DOX GITS | Baseline | 5.85 ± 2.55 (n = 82) | 11.50 ± 5.63 (n = 76) | 230.34 ± 111.89 (n = 76) |
| Week 4 | 3.43 ± 2.89 (n = 74) | 13.40 ± 7.94 (n = 71) | 223.61 ± 121.33 (n = 71) | |
| Week 8 | 3.10 ± 2.78 (n = 70) | 13.01 ± 5.57 (n = 67) | 228.65 ± 127.56 (n = 67) | |
| Week 12 | 2.47 ± 2.67 (n = 75) | 12.98 ± 6.33 (n = 72) | 200.06 ± 107.33 (n = 72) | |
| Tamsulosin | Baseline | 6.11 ± 2.65 (n = 82) | 11.55 ± 6.50 (n = 78) | 193.19 ± 124.42 (n = 78) |
| Week 4 | 3.56 ± 2.82 (n = 78) | 13.48 ± 9.27 (n = 74) | 236.06 ± 149.25 (n = 74) | |
| Week 8 | 2.80 ± 2.86 (n = 75) | 13.78 ± 6.57 (n = 71) | 256.65 ± 157.45 (n = 71) | |
| Week 12 | 2.43 ± 2.83 (n = 81) | 13.68 ± 7.56 (n = 72) | 245.79 ± 142.74 (n = 72) |
BII, benign prostatic hyperplasia impact index; Qmax, maximum urine flow rate; DOX, doxazosin; GITS, gastrointestinal therapeutic system; SD, standard deviation.
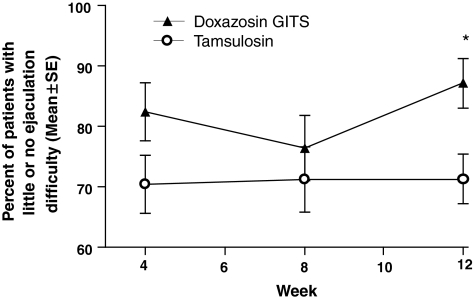
Patients were asked question 6 of the SFAQ: ‘In the past 30 days, how much difficulty have you had ejaculating when you have been sexually stimulated?’ The proportion of patients that answered ‘little difficulty’ or ‘no difficulty’ to this question was significantly higher (p = 0.018) in the doxazosin GITS group (87.14%) compared with those in the tamsulosin group (71.33%) at the last visit (week 12) (Figure 2). Patients were also asked question 7 of the SFAQ: ‘In the past 30 days, how much did you consider the amount of semen you ejaculate to be a problem for you?’ The proportion of satisfied patients did not vary significantly between the two groups (p = 0.109 for doxazosin GITS; p = 0.658 for tamsulosin). There were no significant differences between the doxazosin GITS group and tamsulosin group for IIEF scores (p = 0.156; Table 4).
Figure 2.
Percent of patients (mean ± SE) with no or little difficulty in ejaculation (question 6 of the Sexual Function Abbreviated Questionnaire). *p = 0.018 vs. tamsulosin. GITS, gastrointestinal therapeutic system
Table 4.
IIEF Score during the trial (mean ± SD)
| Group | Visit | IIEF |
|---|---|---|
| DOX GITS | Baseline | 17.95 ± 8.84 (n = 82) |
| Week 4 | 18.86 ± 9.16 (n = 74) | |
| Week 8 | 17.37 ± 9.67 (n = 70) | |
| Week 12 | 18.25 ± 10.15 (n = 75) | |
| Tamsulosin | Baseline | 17.76 ± 9.20 (n = 82) |
| Week 4 | 18.36 ± 9.06 (n = 78) | |
| Week 8 | 18.64 ± 8.96 (n = 75) | |
| Week 12 | 19.81 ± 9.28 (n = 80) |
IIEF, international index of erectile function; DOX, doxazosin; GITS, gastrointestinal therapeutic system; SD, standard deviation.
Safety Assessments
Among the patients that received doxazosin GITS, 20.7% (17) experienced at least 1 AE. In the tamsulosin group, 26.5% (22) reported at least one AE. No significant difference was found with regard to the proportion of patients that experienced at least one AE in the two groups (p = 0.383). In the doxazosin GITS group, the most frequently reported AEs are shown in Table 5. More patients in the doxazosin group reported dizziness and headache (3; 3.7%) than in the tamsulosin group (2; 2.4%). More patients in the tamsulosin group reported abnormal ejaculation (4; 4.8%) than in the doxazosin group (2; 2.4%). One patient (1.2%) in the tamsulosin group reported retrograde ejaculation.
Table 5.
Adverse events (AEs) occurring in ≥2% of patients*
| AEs | Doxazosin GITS (n = 82) | Tamsulosin (n = 83) |
|---|---|---|
| Number of patients with events, n (%) | 17 (21) | 22 (27) |
| Dizziness | 3 (3.7) | 2 (2.4) |
| Headache | 3 (3.7) | 2 (2.4) |
| Abnormal ejaculation | 2 (2.4) | 4 (4.8) |
| Chest pain | 2 (2.4) | 1 (1.2) |
| Decreased libido | 2 (2.4) | 0 |
| Insomnia | 2 (2.4) | 0 |
| Asthenia | 1 (1.2) | 2 (2.4) |
| Flu syndrome | 0 | 2 (2.4) |
| Constipation | 0 | 2 (2.4) |
Safety population included all patients that received at least one dose of study medication. GITS, gastrointestinal therapeutic system.
The number of patients who discontinued because of AEs included four from the tamsulosin group and two from the doxazosin GITS group. One patient from the doxazosin GITS group experienced a serious AE, unstable angina, which was considered related to the study drug. Two patients from the tamsulosin group experienced serious AEs: one patient had precordial pain related to the study drug; the other patient had a cardiac valve disorder and symptoms of myocardial infarction, which were considered unrelated to the study drug.
Discussion
The characteristics of various α1-adrenoceptor antagonists have been compared in numerous placebo-controlled clinical trials and have been shown to have similar efficacy in the management of BPH, improving the total symptom score by approximately 30–45% (1). The current study showed similar results, with both the doxazosin GITS group and the tamsulosin group showing the significant improvements in total IPSS from baseline (p = 0.001).
Patients receiving the tamsulosin in the current study experienced a reduction in IPSS more slowly than those receiving doxazosin GITS. This finding is supported by similar results in other trials showing that doxazosin GITS produced improvements in symptoms early in treatment (9,15). Also, these data are consistent with a recent report demonstrating that doxazosin GITS was more effective in improving the BPH symptoms than tamsulosin after 4 weeks of treatment (10).
Each of the α-blocking agents is considerably different in terms of subtype binding and pharmacodynamics, which may relate to their differences in potential for AEs (16). Tamsulosin, for example, has been reported to result in abnormal ejaculation with a frequency as high as 10% with a dose of 0.4-mg q.i.d. and 26% with a dose of 0.8-mg q.i.d. (13). In contrast, doxazosin GITS did not cause abnormal ejaculation according to Kirby et al. (11). In the current study, the proportion of patients that reported ‘little difficulty’ or ‘no difficulty’ with ejaculation when sexually stimulated was significantly higher (p = 0.018) in the doxazosin GITS group (87.14%) compared with those in the tamsulosin group (71.33%) at the last visit (week 12). Only two (2.4%) of patients receiving doxazosin GITS reported abnormal ejaculation, compared with four (4.8%) in the tamsulosin group. None of the patients in the doxazosin GITS group reported retrograde ejaculation, compared with one (1.2%) in the tamsulosin group. We believe that the more rapid onset and reduced incidence of sexual side effects observed with doxazosin treatment are clinically significant and will probably improve compliance.
The general population of men with BPH is older, and they often have concomitant diseases that require multiple prescription drugs (17). Because doxazosin is also indicated for hypertension, treatment with doxazosin GITS is especially efficient in hypertensive patients with BPH symptoms, yet normotensive patients experience no important reductions in blood pressure (7,18). Tamsulosin does not reduce the blood pressure in a clinically significant manner (14).
The recently published Medical Therapy of Prostatic Symptoms (MTOPS) trial demonstrated that doxazosin significantly reduced the overall risk of clinical progression of BPH over 4 years when compared with placebo (19). Clinical progression in MTOPS was defined as worsening of symptoms, acute urinary retention, incontinence, urinary tract infection or renal insufficiency (19).
In this study, unstable angina occurred in one of 82 patients taking doxazosin. In large clinical studies with doxazosin, including those lasting over a period of 4 years, AEs were generally mild to moderate. Although reports of angina have occurred during doxazosin use (20), it is not distinguishable from symptoms of the underlying disease that might have occurred without doxazosin use.
Conclusions
Doxazosin GITS and tamsulosin were similarly effective in the treatment of symptomatic BPH after 12 weeks of treatment and both treatments were well tolerated. Improvement in symptoms was significantly greater at 4 weeks in patients receiving doxazosin GITS vs. those receiving tamsulosin. Additionally, the proportion of patients reporting little or no difficulty in ejaculation was greater in patients treated with doxazosin GITS than patients treated with tamsulosin.
Author disclosures
The authors have no direct or indirect commercial financial incentive associated with publishing this study.
Acknowledgments
Financial support was provided by Pfizer Inc.
References
- 1.Djavan B, Marberger M. A meta-analysis on the efficacy and tolerability of alpha1-adrenoceptor antagonists in patients with lower urinary tract symptoms suggestive of benign prostatic obstruction. Eur Urol. 1999;36:1–13. doi: 10.1159/000019919. [DOI] [PubMed] [Google Scholar]
- 2.Berry SJ, Coffey DS, Walsh PC, et al. The development of human benign prostatic hyperplasia with age. J Urol. 1984;132:474–9. doi: 10.1016/s0022-5347(17)49698-4. [DOI] [PubMed] [Google Scholar]
- 3.Debruyne FM. Alpha blockers: are all created equal. Urology. 2000;56:20–2. doi: 10.1016/s0090-4295(00)00744-5. [DOI] [PubMed] [Google Scholar]
- 4.Chapple C. Selective alpha 1-adrenoceptor antagonists in benign prostatic hyperplasia: rationale and clinical experience. Eur Urol. 1996;29:129–44. [PubMed] [Google Scholar]
- 5.Tammela T. Benign prostatic hyperplasia. Practical treatment guidelines. Drugs Aging. 1997;10:349–66. doi: 10.2165/00002512-199710050-00004. [DOI] [PubMed] [Google Scholar]
- 6.Fawzy A, Braun K, Lewis GP, et al. Doxazosin in the treatment of benign prostatic hyperplasia in normotensive patients: a multicenter study. J Urol. 1995;154:105–9. [PubMed] [Google Scholar]
- 7.De Reijke TM, Klarskov P. Comparative efficacy and tolerability of two a1-Adrenoreceptor antagonists, doxazosin and alfuzosin, in patients with lower urinary tract symptoms due to benign prostatic enlargement. BJU Int. 2004;93:757–62. doi: 10.1111/j.1464-410X.2003.04720.x. [DOI] [PubMed] [Google Scholar]
- 8.Chung M, Vashi V, Puente J, et al. Clinical pharmacokinetics of doxazosin in a controlled-release gastrointestinal therapeutic system (GITS) formulation. Br J Clin Pharmacol. 1999;48:678–87. doi: 10.1046/j.1365-2125.1999.00067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andersen M, Dahlstrand C, Hoye K. Double-blind trial of the efficacy and tolerability of doxazosin in the gastrointestinal therapeutic system, doxazosin standard, and placebo in patients with benign prostatic hyperplasia. Eur Urol. 2000;38:400–9. doi: 10.1159/000020315. [DOI] [PubMed] [Google Scholar]
- 10.Kirby RS, Quinn S, Mallen S, et al. Doxazosin controlled release vs tamsulosin in the management of benign prostatic hyperplasia: an efficacy analysis. Int J Clin Pract. 2004;58:6–10. doi: 10.1111/j.1368-5031.2004.0031.x. [DOI] [PubMed] [Google Scholar]
- 11.Kirby RS. A randomized, double-blind crossover study of tamsulosin and controlled-release doxazosin in patients with benign prostatic hyperplasia. BJU Int. 2003;91:41–4. doi: 10.1046/j.1464-410x.2003.03077.x. [DOI] [PubMed] [Google Scholar]
- 12.Abrams P, Schulman C, Vaage S. Tamsulosin, a selective α1c-adrenoceptor antagonist: a randomized controlled trial in patients with benign prostatic “obstruction” (symptomatic BPH) Br J Urol. 1995;76:325–36. doi: 10.1111/j.1464-410x.1995.tb07709.x. [DOI] [PubMed] [Google Scholar]
- 13.Lepor H. Long-term evaluation of tamsulosin in benign prostatic hyperplasia: placebo-controlled, double-blind extension of phase III trial. Tamsulosin Investigator Group. Urology. 1998;51:901–6. doi: 10.1016/s0090-4295(98)00127-7. [DOI] [PubMed] [Google Scholar]
- 14.Narayan P, Lepor H. Long-term, open-label, phase III multicenter study of tamsulosin in benign prostatic hyperplasia. Urology. 2001;57:466–70. doi: 10.1016/s0090-4295(00)01042-6. [DOI] [PubMed] [Google Scholar]
- 15.Kirby RS, Andersen M, Gratzke P, et al. A combined analysis of double-blind trials of the efficacy and tolerability of doxazosin-gastrointestinal therapeutic system, doxazosin standard and placebo in patients with benign prostatic hyperplasia. BJU Int. 2001;87:192–200. doi: 10.1046/j.1464-410x.2001.02032.x. [DOI] [PubMed] [Google Scholar]
- 16.Roehrborn CG, Schwinn DA. Alpha1-adrenergic receptors and their inhibitors in lower urinary tract symptoms and benign prostatic hyperplasia. J Urol. 2004;171:1029–35. doi: 10.1097/01.ju.0000097026.43866.cc. [DOI] [PubMed] [Google Scholar]
- 17.Jimenez-Garcia R, Anegon M, Esteban J, et al. Safety and effectiveness of replacing standard doxazosin with doxazosin in the gastrointestinal therapeutic system (GITS) formulation in elderly hypertensive patients. Int J Clin Pract. 2003;57:267–72. [PubMed] [Google Scholar]
- 18.Lund-Johansen P, Kirby RS. Effect of doxazosin GITS on blood pressure in hypertensive and normotensive patients: a review of hypertension and BPH studies. Blood Press. 2003;12:5–13. doi: 10.1080/08038020310000078. [DOI] [PubMed] [Google Scholar]
- 19.McConnell JD, Roehrborn CG, Bautista OM, et al. The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N Engl J Med. 2003;349:2387–98. doi: 10.1056/NEJMoa030656. [DOI] [PubMed] [Google Scholar]
- 20.Pfizer A. Cardura XL (Doxazosin Mesylate Extended Release Tablets) New York, NY, USA: Pfizer Inc; 2006. [Google Scholar]