Abstract
As the sole Ca2+ entry mechanism in a variety of non-excitable cells, store-operated calcium (SOC) influx plays an important role in Ca2+ signaling and many other cellular processes1–3. A calcium release-activated calcium (CRAC) channel in T lymphocytes is the best characterized SOC influx channel4–6 and is essential to the immune response, sustained activity of CRAC channels being required for gene expression and proliferation7–10. The molecular identity and the gating mechanism of SOC and CRAC channels have remained elusive. Previously, we identified Stim and the mammalian homolog STIM1 as essential components of CRAC channel activation in Drosophila S2 cells and human T lymphocytes11. Here, we show that expression of EF hand mutants of Stim or STIM1 activates CRAC channels constitutively without changing Ca2+ store content. By immunofluorescence, EM localization, and surface biotinylation we demonstrate that STIM1 migrates from ER-like sites to the plasma membrane upon depletion of the Ca2+ store. We propose that STIM1 functions as the missing link between Ca2+ store depletion and SOC influx, serving as a Ca2+ sensor that translocates upon store depletion to the plasma membrane to activate CRAC channels.
We previously characterized a CRAC channel in Drosophila S2 cells with biophysical properties closely similar to those in human T cells12. More recently, Stim was identified in an RNAi-based screen using the SERCA (Sarcoplasmic/Endoplasmic Reticulum Calcium ATPase) pump inhibitor thapsigargin (TG) to evoke SOC influx in Drosophila S2 cells11. Uniquely among the 170 candidate genes that were screened, including all trp-related genes, RNAi-mediated suppression of Stim inhibited Ca2+ influx evoked by TG. By single-cell [Ca2+]i imaging and patch clamp analysis, we confirmed a functional requirement for Stim, and for the human homolog STIM1, to mediate CRAC channel activity in S2 cells and in Jurkat T cells, respectively. Drosophila Stim and mammalian STIM1 (collectively referred to here as Stim1) are modular type I transmembrane proteins with an EF hand motif near the amino terminus located in the lumen of the ER or outside the cell (supplementary figure 1)13. Since Stim1 does not resemble any known ion channel, the presence of the EF hand motif and its localization suggested that Stim1 might function as a sensor of the ER Ca2+ store. According to this proposal, Ca2+ binding to the EF hand domain of Stim1 within the lumen of the Ca2+ store would keep CRAC channels in the plasma membrane closed.
To test this possibility, full length STIM1 and Stim cDNA were cloned from RBL cells and S2 cells and placed into appropriate expression vectors. Mutants in the EF hand region were prepared, two for STIM1 and four for Stim, on residues that are known to be critical for Ca2+ binding14,15. Overexpression of wild-type (WT) or mutant Stim1 following transient transfection was confirmed by western blot (supplementary figure 2). In single-cell Ca2+ imaging experiments, overexpression of WT Stim1 produced no significant difference in resting [Ca2+]i; TG-independent Ca2+ influx; or TG-evoked store release, compared to control blank-transfected cells (figure 1 for Jurkat; supplementary figure 3 for S2). A modest increase in TG-dependent (store-operated) Ca2+ influx was seen in Jurkat T cells (figure 1b) but not in S2 cells, consistent with the hypothesis that Stim1 by itself is not a functional CRAC channel. In contrast, Jurkat or S2 cells transfected with the EF hand mutants bore two severe phenotypes: resting [Ca2+]i and TG-independent Ca2+ influx were elevated from ~50 nM to more than 200 nM on average. Histograms of resting [Ca2+]i show that expression of EF hand mutants elevated resting [Ca2+]i to > 600 nM in many individual cells (supplementary figure 4). The Ca2+ release transient, obtained by addition of TG in zero-Ca2+ solution, was not changed (supplementary figure 5), demonstrating that the Ca2+ store content was not affected. To test if the high values of resting [Ca2+]i and enhanced TG-independent Ca2+ influx were caused by constitutively opened CRAC channels, 2-APB, SKF96365, and Gd3+ were applied as pharmacological tools that block CRAC channels in Jurkat and in S2 cells2,3,12,16–18. Each of these agents inhibited TG-evoked SOC influx in control cells and, at the same concentrations, all three inhibited both the high resting Ca2+ level and the enhanced TG-independent Ca2+ influx in Jurkat and S2 cells transfected with EF hand mutants (figure 1g-i; supplementary figure 3c). These results demonstrate that CRAC channels in Jurkat or S2 cells are constitutively opened by overexpression of Stim1 EF hand mutants.
Figure 1. Constitutive activation of CRAC channels by expression of EF hand mutant of Stim or STIM1.
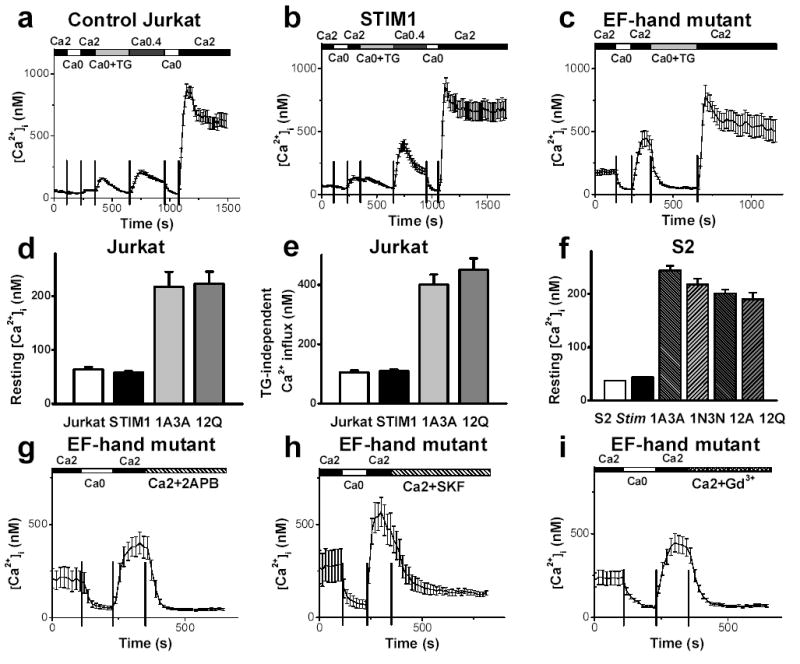
a–c, Average [Ca2+]i responses in blank-transfected Jurkat cells (n=59), Jurkat cells overexpressing WT STIM1 (n=29), and Jurkat cells overexpressing STIM1 EF12Q (n=18), respectively. Cells were bathed in solutions as indicated. d–f, Resting [Ca2+]i in Jurkat cells (from left to right: n=114, n=171, n=61, and n=65); TG-independent Ca2+ influx in Jurkat cells (n=114, n=136, n=61, and n=42); and resting [Ca2+]i in S2 cells (from left to right: n=392, n=568, n=425, n=149, n=316, and n=102). g–i, Effects of CRAC channel blockers 2-APB (50 μM), SKF96365 (20 μM) and Gd3+ (1 μM) on TG-independent Ca2+ influx in Jurkat cells. EF hand mutants: 12Q (g, n=16), 12Q (h, n=8), and 1A3A (i, n=28). Error bars: SEM.
In addition to affecting resting [Ca2+]i, expression of Stim EF hand mutants arrested the owth of S2 cells, whereas overexpressing WT Stim had a small effect, compared with S2 cells undergoing blank transfection (supplementary figure 6). The majority of S2 cells overexpressing Stim EF hand mutants were stained by annexin-V, an early marker for exposure of phosphatidylserine that occurs during apoptosis 19. The growth arrest and apoptosis were most likely triggered by abnormally high resting [Ca2+]i19–21. A similar but milder growth defect in Jurkat cells overexpressing STIM1 EF hand mutants was also observed.
How does STIM1 regulate the activity of CRAC channels? The subcellular localization of STIM1 was examined by immunofluorescence staining of Jurkat cells overexpressing either WT STIM1 or STIM1 EF hand mutants. In cells that overexpressed STIM1, most of the staining appeared within the ring of cytoplasm surrounding the nucleus, and very little was on the cell surface (figure 2a), consistent with a previous surface biotinylation study22. In contrast, EF hand mutant STIM1 proteins that induce constitutive activation of CRAC channels were located predominantly on the surface membrane (figure 2b). Comparison with co-transfected GFP helped to define STIM1 or EF hand mutant STIM1 at or below the cell surface. We reasoned that the altered localization of EF hand mutants to the plasma membrane could result from their inability to bind Ca2+ within the intracellular store. Thus, translocation of WT Stim1 from the Ca2+ store to the plasma membrane might represent a key event in the signaling pathway that activates CRAC channels. This hypothesis was supported by immunofluorescence staining of endogenous STIM1 in Jurkat T cells (figure 2c); and in RBL cells, resting T cells from human donors, nd PC12 cells (supplementary figure 7 and 8). In cells pretreated with 1 μM TG in zero-Ca2+ external solution to deplete the Ca2+ store without causing a large rise in cytosolic [Ca2+]i, hotspots of STIM1 staining were found at the cell surface, whereas a predominant cytosolic distribution of STIM1 staining was seen in control cells bathed in 2 mM Ca2+ Ringer solution. In the control condition with Ca2+ stores full, STIM1 co-localized with ER markers (SERCA2 and protein disulfide isomerase, PDI) in Jurkat and in RBL cells, respectively, but after store depletion STIM1 accumulated in hotspots at the surface and ER co-localization was less extensive (figure 2c; supplementary figure 8). STIM1 translocation kinetics were evaluated by fixing cells at different time points after treatment with TG or cyclopiazonic acid (CPA), another SERCA pump inhibitor that depletes ER Ca2+ stores and triggers SOC influx23 (figure 2d, supplementary figure 9a). When Ca2+-store depletion was initiated, STIM1 rapidly moved to the surface membrane, reaching near-maximal surface expression within ~5 min and remaining on the surface for at least 40 min. For comparison, inhibition of SERCA pumps activated CRAC currents with a somewhat more rapid time course in perforated patch recordings. Current begins to develop when STIM1 translocation is first observed, and reaches a peak at 160 ± 66 sec (mean ± S.D., n = 6 cells; Supplementary Figure 8b), when STIM1 translocation rate is maximal. Additional experiments, summarized in supplementary figure 9b-d, indicated that STIM1 surface translocation also occurred when [Ca2+]i was elevated accompanying store depletion by treatment with TG in 2 mM Ca2+ external solution, but did not occur in cells treated with zero-Ca2+ solution in the absence of TG, or in cells treated with 2 mM Ca2+ + DMSO used to dissolve TG. To summarize, depletion of intracellular Ca2+ stores is sufficient to trigger translocation of STIM1 proteins to the plasma membrane, suggesting a mechanistic link to CRAC channel activation.
Figure 2. Mutations in EF hand motif or store depletion induce STIM1 translocation to plasma membrane.
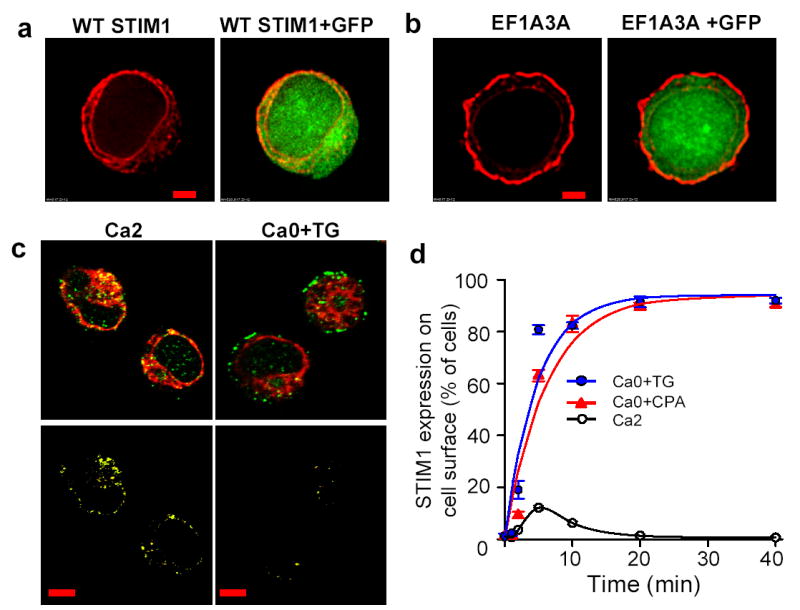
a,b, STIM1 (red) immunofluorescence staining of Jurkat cells transfected with WT STIM1 (a) or EF1A3A STIM1 (b), in 2 mM Ca2+ Ringer solution. GFP (green) was co-transfected to define the cytoplasmic region. Scale bar is 2 μm. c, STIM1 (green) and SERCA2 (red) immunofluorescence staining of Jurkat cells in 2 mM Ca2+ Ringer solution or 1 μM TG in zero-Ca2+ solution for 10 min. Note the separation of STIM1 at the cell surface (top right). Bottom panels: colocalization images (yellow) that depict pixels that contained both STIM1 and ER fluorescence. Note reduced olocalization caused by STIM1 translocation in TG-treated cells. Scale bar is 5 μm. d, Time course of STIM1 translocation triggered by 1 μM TG or 10 μM CPA, represented by the percentage of cells with strong surface fluorescence. An average of 315 cells was counted for each time point. Time constants: 4.7 min (TG), 6.1 min (CPA). Error bars: SEM.
The translocation of STIM1 was further confirmed by immuno-electron microscopy, using quantum dot-conjugated second antibodies following polyclonal anti-STIM1 antibodies to visualize STIM1 protein with single-molecule resolution. In control cells bathed in 2 mM Ca2+ Ringer, the majority of quantum dots were located in the lumen of smooth ER-like vesicular structures within the cytosol and occasionally at the cell surface (figure 3a–c). In contrast, in TG-treated cells Q-dots appeared in clusters on the plasma membrane (figure 3d-f). The STIM1 surface density, with or without Ca2+-store depletion, was determined by counting the number of quantum dots normalized to the linear distance along the plasma membrane. A 5-fold increase of STIM1 surface density was obtained following TG treatment to induce store depletion (figure 3g). Moreover, an increase in surface-accessible STIM1 was also detected by biotinylation (figure 3h, i).
Figure 3. Subcellular distribution of STIM1 before and after store depletion: immuno-electron microscopy and surface biotinylation.
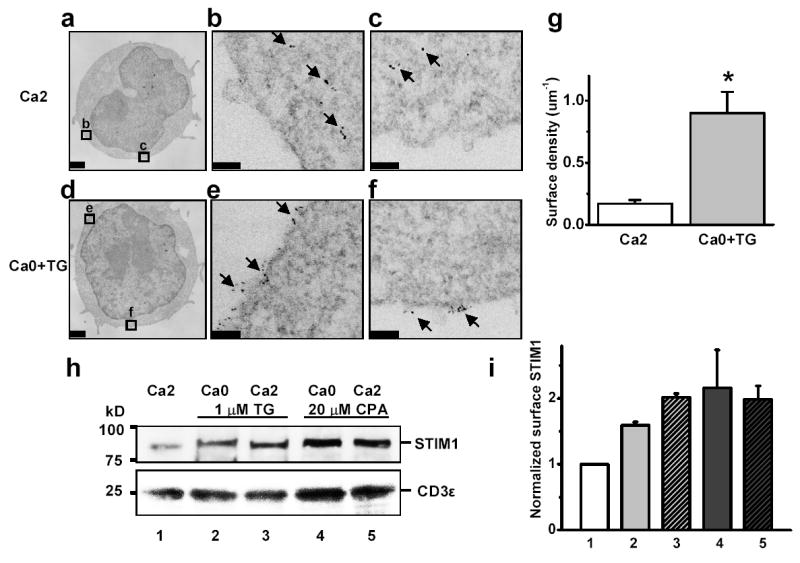
a, Quantum dot-labeled STIM1 in control Jurkat cells bathed in 2 mM Ca2+ Ringer solution. b,c, Enlargements of corresponding boxed areas in a. d, STIM1 in Jurkat cells pretreated with 1 μM TG in zero-Ca2+ solution for 15 min. e,f, Enlarged regions showing clustered STIM1 distribution on plasma membrane. Scale bar is 1 μm for a and d; 0.1 μm for b,c,e,f. g, STIM1 surface density, pooled data from control cells (n = 14) and store-depleted cells (n = 13); asterisk denotes P value < 0.0005 compared to controls. h, Jurkat cells were treated for 30 min as indicated. Biotinylated proteins were detected by antibodies to STIM1 (top) and CD3ɛ (bottom). i, Quantification of biotinylation experiments: 1.6–2.2 fold increase upon store depletion. Error bars: SEM.
The results can be summarized in two main conclusions: 1) CRAC channels are activated when Stim1 is unable to bind Ca2+ within the store, either following Ca2+ release or if the EF hand mutant is expressed; and 2) Stim1 translocates to the plasma membrane upon Ca2+ store depletion. Previously, we demonstrated that STIM1 also regulates SOC influx in HEK293 and SH-SY5Y cells11. In addition, while the current paper was in review, a report from Liou et al. showed that STIM1 plays a role in regulation of SOC influx in HeLa cells24. This group also reported redistribution (but not membrane insertion) of YFP-tagged STIM1 upon store depletion, but did not examine endogenous STIM1. By demonstrating translocation and membrane insertion of endogenous STIM1, our data suggest a crucial link to the functional role of STIM1. Based upon the findings, we propose a calcium sensor model to account for Stim1’s role in regulating SOC influx.
The proposed model represents a modification of a vesicle fusion hypothesis for CRAC channel activation, in which Stim1 is the element that translocates. As illustrated in figure 4, Stim1 is initially located in the membrane of the Ca2+ store, with its low-affinity EF hand inside the lumen sensing Ca2+ and stabilized by bound Ca2+ when the store is full. When the store is depleted by IP3-induced Ca2+ release or by inhibition of the SERCA pump, Ca2+ dissociates and Stim1 moves to the plasma membrane, presumably in vesicles that may insert into the membrane. Mutation of the EF hand mimics Ca2+ store depletion, initiating translocation and activation of CRAC channels. Once Stim1 is at the cell surface, it can activate the CRAC channel by: a) interacting with a putative pore-forming subunit; b) activating Ca2+ influx via conformational coupling through its coiled-coil domain; or c) assembling with additional Stim1 monomers and perhaps other components to form a unique functional Ca2+ channel. In addition to mechanistic insights, Stim1 may provide a biochemical handle to identify additional components of the translocation mechanism and the CRAC channel pore.
Figure 4. Models of STIM1 Function.
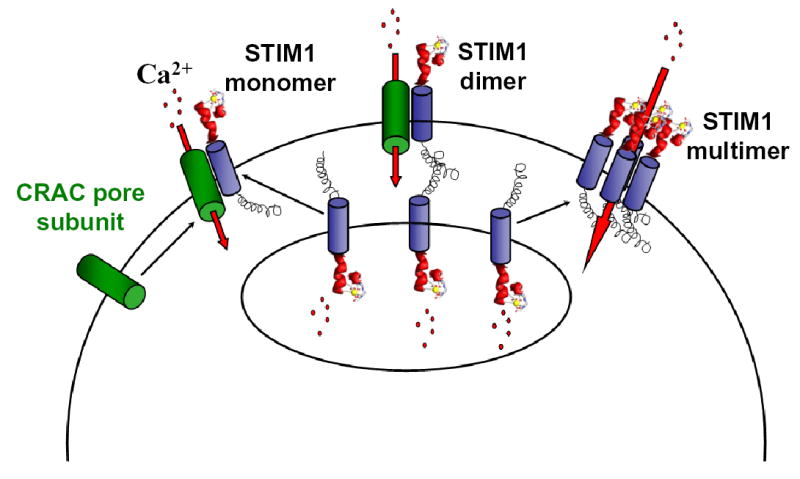
Upon store depletion, STIM1 located in the ER unbinds Ca2+ and translocates to the plasma membrane to activate CRAC channel subunits that are already in the plasma membrane (left), form junctions between the Ca2+ store and the plasma membrane (middle), or assemble to form functional CRAC channels (right).
METHODS
Cell culture and transfection
Drosophila S2 cells (Invitrogen) were propagated in Schneider’s medium (Invitrogen) supplemented with 12.5% FCS and 1% glutamine at 27° C. Cells were seeded at a density of 106 cells/ml and passed when the cells achieved a density of ~6 x 106 cells/ml. Jurkat T cells (ATCC) were maintained and propagated as detailed by the ATCC. RBL-2H3 cells nd PC12 cells were cultured in Eagle's MEM and DMEM, respectively, supplemented atmosphere at 37°C. Cells were ith 10% fetal bovine serum in 5% CO2-humidified assed twice weekly. Human peripheral T lymphocytes were isolated from the blood of healthy volunteers using CD3+ RosetteSep (StemCell Technologies) and Histopaque 1077 (Sigma) and cultured in RPMI supplemented with 10% fetal bovine serum. S2 cells and Jurkat cells were transfected (see clones described in Supplementary Information) using a Nucleofector (Amaxa) following the manufacturer's protocol. Forty-eight hours post-transfection, protein expression was confirmed by western blot, and cells were used for [Ca2+]i imaging, immunocytochemistry and apoptosis assays.
Antibodies and western blotting
Cell extracts were prepared by washing the cells with PBS and then extracting proteins with lysis buffer (in mM): 10 Tris, 3 CaCl2, 2 MgCl2, 2.5% NP40, pH 7.5. Protein concentration was determined using the Pierce BCA Protein Reagent Kit. The extract was prepared for SDS-PAGE analysis by addition of 4X sample buffer (Invitrogen). Samples were resolved by SDS-PAGE and analyzed by standard western blotting techniques.
STIM1 polyclonal antibodies to a C-terminal peptide (STIM1-CT
DNGSIGEETDSSPGRKKFPLKIFKKPLKK) were used at a dilution of 1:2500. Anti-GAPDH monoclonal antibodies were from Research Diagnostics and used at a dilution of 1:5000. Anti-V5/HRP monoclonal antibodies were from Invitrogen and used at a dilution of 1:5000. Proteins were detected by developing with the SuperSignal (Pierce) detection system.
Single-cell [Ca2+]i imaging
Ratiometric [Ca2+]i imaging experiments were performed on S2 and Jurkat cells using fura-2 as described11, using solution recipes indicated in Supplementary Information Table 1. Transfected cells were recognized by co-expressed EGFP, using filters to avoid contamination of fura-2 fluorescence by GFP fluorescence bleed-through25. Data were analyzed using Metafluor software (Universal Imaging) and OriginPro 7.5 software (OriginLab) and expressed as mean ± SEM.
Immunocytochemistry, light and electron microscopy
Stained cells were viewed under a confocal laser scanning microscope LSM510 META (Zeiss) or DeltaVision® RT Restoration Imaging System. Qdot-labeled cells were fixed in 2% glutaraldehyde for 10 minutes and post fixed in 1% osmium tetroxide for 20 minutes. Cells were then washed in distilled water and were dehydrated in ethanol and embedded in Durcupan ACM epoxy resin and subsequently prepared and viewed by electron microscopy (model: JEOL 2000FX).
Surface biotinylation
Cell surface STIM1 protein was detected by cell surface biotinylation and purification, according to manufacturer instructions (Pierce), using a modified protocol 22, as described in Supplementary Information. Data were quantified using ImageJ software (NIH) and normalized as mean ± SEM.
Perforated patch recording
CRAC current was activated by CPA or TG in RBL cells during perforated-patch recording with amphotericin B (0.2 mg/ml) in the pipette. See Supplementary Information for pipette and external solutions and recording protocols.
Acknowledgments
We thank Lu Forrest for assistance in cell culture; and Andy Yeromin, Olga Safrina and Sindy Wei for help with [Ca2+]i imaging. We thank George Chandy for use of molecular reagents and laboratory facilities; and Chris Hughes for providing access to the Amaxa Nucleofector. pAc5.1/EGFP was a kind gift from A. Kolski-Andreaco. We thank Dr. K. Knowlton and D. Summers-Torres (University of California, San Diego) for their help with deconvolution immunofluorescence microscopy. Confocal microscopy was performed at the Optical Biology Shared Resource, supported by the Developmental Biology Center and Cancer Center Support Grant (CA-62203) at UCI. The authors also wish to thank Paul J. DiGregorio, Gonul Velicelebi, Maria Lioudyno, Jim Hall, and Yun Li for helpful discussion. This work was supported by Grants from the National Institutes of Health (M.D.C. and M.H. Ellisman) and by a fellowship from the Pulmonary Hypertension Association (Y.Y).
References
- 1.Putney JW, Jr, Broad LM, Braun FJ, Lievremont JP, Bird GS. Mechanisms of capacitative calcium entry. J Cell Sci. 2001;114:2223–9. doi: 10.1242/jcs.114.12.2223. [DOI] [PubMed] [Google Scholar]
- 2.Putney JW., Jr Store-operated calcium channels: how do we measure them, and why do we care? Sci STKE. 2004;2004:pe37. doi: 10.1126/stke.2432004pe37. [DOI] [PubMed] [Google Scholar]
- 3.Lewis RS. Store-operated calcium channels. Adv Second Messenger Phosphoprotein Res. 1999;33:279–307. doi: 10.1016/s1040-7952(99)80014-7. [DOI] [PubMed] [Google Scholar]
- 4.Parekh AB, Putney JW., Jr Store-operated calcium channels. Physiol Rev. 2005;85:757–810. doi: 10.1152/physrev.00057.2003. [DOI] [PubMed] [Google Scholar]
- 5.Lewis RS, Cahalan MD. Mitogen-induced oscillations of cytosolic Ca2+ and transmembrane Ca2+ current in human leukemic T cells. Cell Regul. 1989;1:99–112. doi: 10.1091/mbc.1.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parekh AB, Penner R. Store depletion and calcium influx. Physiol Rev. 1997;77:901–30. doi: 10.1152/physrev.1997.77.4.901. [DOI] [PubMed] [Google Scholar]
- 7.Lewis RS. Calcium signaling mechanisms in T lymphocytes. Annu Rev Immunol. 2001;19:497–521. doi: 10.1146/annurev.immunol.19.1.497. [DOI] [PubMed] [Google Scholar]
- 8.Winslow MM, Neilson JR, Crabtree GR. Calcium signalling in lymphocytes. Curr Opin Immunol. 2003;15:299–307. doi: 10.1016/s0952-7915(03)00050-5. [DOI] [PubMed] [Google Scholar]
- 9.Feske S, Giltnane J, Dolmetsch R, Staudt LM, Rao A. Gene regulation mediated by calcium signals in T lymphocytes. Nat Immunol. 2001;2:316–24. doi: 10.1038/86318. [DOI] [PubMed] [Google Scholar]
- 10.Partiseti M, et al. The calcium current activated by T cell receptor and store depletion in human lymphocytes is absent in a primary immunodeficiency. J Biol Chem. 1994;269:32327–35. [PubMed] [Google Scholar]
- 11.Roos J, et al. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol. 2005;169:435–45. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yeromin AV, Roos J, Stauderman KA, Cahalan MD. A Store-operated Calcium Channel in Drosophila S2 Cells. J Gen Physiol. 2004;123:167–82. doi: 10.1085/jgp.200308982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams RT, et al. Identification and characterization of the STIM (stromal interaction molecule) gene family: coding for a novel class of transmembrane proteins. Biochem J. 2001;357:673–85. doi: 10.1042/0264-6021:3570673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beckingham K. Use of site-directed mutations in the individual Ca2(+)-binding sites of calmodulin to examine Ca2(+)-induced conformational changes. J Biol Chem. 1991;266:6027–30. [PubMed] [Google Scholar]
- 15.Nakayama S, Kretsinger RH. Evolution of the EF-hand family of proteins. Annu Rev Biophys Biomol Struct. 1994;23:473–507. doi: 10.1146/annurev.bb.23.060194.002353. [DOI] [PubMed] [Google Scholar]
- 16.Ross PE, Cahalan MD. Ca2+ influx pathways mediated by swelling or stores depletion in mouse thymocytes. J Gen Physiol. 1995;106:415–44. doi: 10.1085/jgp.106.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prakriya M, Lewis RS. Potentiation and inhibition of Ca(2+) release-activated Ca(2+) channels by 2-aminoethyldiphenyl borate (2-APB) occurs independently of IP(3) receptors. J Physiol. 2001;536:3–19. doi: 10.1111/j.1469-7793.2001.t01-1-00003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chung SC, McDonald TV, Gardner P. Inhibition by SK&F 96365 of Ca2+ current, IL-2 production and activation in T lymphocytes. Br J Pharmacol. 1994;113:861–8. doi: 10.1111/j.1476-5381.1994.tb17072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eray M, Matto M, Kaartinen M, Andersson L, Pelkonen J. Flow cytometric analysis of apoptotic subpopulations with a combination of annexin V-FITC, propidium iodide, and SYTO 17. Cytometry. 2001;43:134–42. doi: 10.1002/1097-0320(20010201)43:2<134::aid-cyto1028>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 20.Graber MN, Alfonso A, Gill DL. Ca2+ pools and cell growth: arachidonic acid induces recovery of cells growth-arrested by Ca2+ pool depletion. J Biol Chem. 1996;271:883–8. doi: 10.1074/jbc.271.2.883. [DOI] [PubMed] [Google Scholar]
- 21.Ferrari D, et al. Endoplasmic reticulum, Bcl-2 and Ca2+ handling in apoptosis. Cell Calcium. 2002;32:413–20. doi: 10.1016/s0143416002002014. [DOI] [PubMed] [Google Scholar]
- 22.Manji SS, et al. STIM1: a novel phosphoprotein located at the cell surface. Biochim Biophys Acta. 2000;1481:147–55. doi: 10.1016/s0167-4838(00)00105-9. [DOI] [PubMed] [Google Scholar]
- 23.Dolmetsch RE, Lewis RS. Signaling between intracellular Ca2+ stores and depletion-activated Ca2+ channels generates [Ca2+]i oscillations in T lymphocytes. J Gen Physiol. 1994;103:365–88. doi: 10.1085/jgp.103.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liou J, et al. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol. 2005;15:1235–41. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fanger CM, et al. Calcium-activated potassium channels sustain calcium signaling in T lymphocytes. Selective blockers and manipulated channel expression levels. J Biol Chem. 2001;276:12249–56. doi: 10.1074/jbc.M011342200. [DOI] [PubMed] [Google Scholar]


